Abstract
In view of the current and expected future rise in atmospheric CO2 concentrations, we examined the effect of elevated CO2 on photoinhibition of photosystem I (PSI) under fluctuating light in Arabidopsis thaliana. At 400 ppm CO2, PSI showed a transient over-reduction within the first 30 s after transition from dark to actinic light. Under the same CO2 conditions, PSI was highly reduced after a transition from low to high light for 20 s. However, such PSI over-reduction greatly decreased when measured in 800 ppm CO2, indicating that elevated atmospheric CO2 facilitates the rapid oxidation of PSI under fluctuating light. Furthermore, after fluctuating light treatment, residual PSI activity was significantly higher in 800 ppm CO2 than in 400 ppm CO2, suggesting that elevated atmospheric CO2 mitigates PSI photoinhibition under fluctuating light. We further demonstrate that elevated CO2 does not affect PSI activity under fluctuating light via changes in non-photochemical quenching or cyclic electron transport, but rather from a rapid electron sink driven by CO2 fixation. Therefore, elevated CO2 mitigates PSI photoinhibition under fluctuating light at the acceptor rather than the donor side. Taken together, these observations indicate that elevated atmospheric CO2 can have large effects on thylakoid reactions under fluctuating light.
1. Introduction
Photosynthetic organisms absorb light energy to drive photosynthetic electron flow and CO2 assimilation. In linear electron flow (LEF), electrons are transferred from photosystem II (PSII) to photosystem I (PSI), and ultimately to NADP+, producing NADPH. This electron flow is coupled to the formation of proton motive force that powers the regeneration of ATP. In cyclic electron flow (CEF) around PSI, electrons are transported from ferredoxin into the plastoquinone pool, generating ATP without producing NADPH. PSI and PSII work co-operatively to form ATP and NADPH, which is essential for the primary metabolism. Once PSII is photoinhibited, as indicated by the decrease in the maximum quantum yield of PSII (Fv/Fm), LEF would be suppressed [1]. Once PSI is photodamaged, as indicated by the decrease in the maximum photo-oxidizable P700 (Pm), both LEF and CEF are depressed, which will affect CO2 fixation and impair plant growth [2,3,4,5,6].
Photosynthetic organisms are often exposed to dynamic fluctuations in light intensity when grown in the field [7,8,9]. Under such fluctuating light (FL) conditions, an abrupt increase in light intensity will lead to an immediate rise in light absorption, triggering electron flow from PSII to PSI [10,11,12]. Meanwhile, CO2 assimilation has a much slower kinetics than electron flow from PSII [7,13,14]. Therefore, NADPH cannot be immediately consumed by CO2 assimilation. Because the pool size of NADPH is relatively small, such an imbalance between electron flow and primary metabolism leads to an increase in the NADPH/NADP+ ratio. The lack of NADP+ restricts LEF and thus induces the accumulation of excited states at PSI, resulting in the generation of reactive oxygen species (ROS) in PSI [15,16]. Because the ROS produced in PSI cannot be immediately scavenged by antioxidant system [17], FL can cause selective photodamage to PSI [16,18,19].
Accordingly, photosynthetic organisms employ several alternative electron transport routes to protect PSI under FL [20,21,22]. In Arabidopsis (A. thaliana), the proton gradient regulation 5 (pgr5) is seedling-lethal when grown under FL, as a consequence of uncontrolled PSI photoinhibition due to a defect in CEF [23]. Therefore, CEF is essential for PSI photoprotection under FL in angiosperms [16,24,25]. During CEF, electrons from ferredoxin are transferred to plastoquinone, generating a ΔpH without reducing NADP+ [24,26,27,28]. In response to a sudden increase in irradiance, CEF first rises before gradually decreasing and reaching a constant rate [29,30,31,32]. The initial stimulation of CEF facilitates the rapid formation of ΔpH [29], slowing down the electron flow at the cytochrome (Cyt) b6/f complex and increasing the ATP/NADPH production ratio [33,34]. CEF therefore protects PSI under FL at both electron donor and acceptor sides [16]. Once ΔpH reaches a sufficient level, CEF activity decreases to a steady state to avoid over-acidification of the thylakoid lumen, thus optimizing the tradeoff between photosynthesis and photoprotection [29,35]. Therefore, CEF plasticity plays an important role in sustaining photosynthesis under FL.
One of the main drivers of global climate change, atmospheric CO2 concentrations are expected to continue increasing in the near future. Higher atmospheric CO2 may lead to a rise in intercellular and chloroplast CO2 concentrations, which will boost the rate of CO2 assimilation and plant growth [36,37]. However, the maintenance of a high level of PSI activity is essential for optimal photosynthesis. Once PSI photoinhibition occurs under FL, the rate of CO2 assimilation under higher atmospheric CO2 conditions will also suffer [3]. Therefore, the predicted positive effects of elevated atmospheric CO2 on crop yield are not only a reflection of the rate of CO2 fixation, but are also likely linked to light reactions. However, how elevated atmospheric CO2 affects light reactions under FL is largely known.
Theoretically, an elevated concentration of atmospheric CO2 will raise the photosynthetic induction rate when transitioning to high light [38,39], increasing the NADPH consumption rate and leading to an increase in the NADP+/NADPH ratio. Consequently, elevated atmospheric CO2 concentrations might facilitate electron flow from PSI to NADP+, with the potential to alleviate the over-reduction of PSI under FL. However, it is unclear whether elevated atmospheric CO2 will in fact break the imbalance between light and dark reactions of photosynthesis and alleviate PSI photoinhibition under FL. In the present study, we measured the chlorophyll fluorescence and PSI signals under FL at 400 and 800 ppm CO2 concentrations in Arabidopsis leaves. The aims of this study were to (1) assess the effect of elevated CO2 on the redox state of PSI under FL and (2) examine whether elevated CO2 can mitigate PSI photoinhibition under FL.
2. Materials and Methods
2.1. Plant Materials
Arabidopsis (Arabidopsis thaliana) wild-type plants grown in a greenhouse (light intensity~100 μmol photons m−2 s−1, 12-h photoperiod, 25 °C, 60% humidity, 400 ppm CO2) for 6–8 weeks after germination. We used fully expanded but not senescent leaves for photosynthetic measurements.
2.2. Measurement of P700 Redox Kinetics
After incubation in darkness for 60 min, we used a Dual-PAM 100 measuring system (Heinz Walz, Effeltrich, Germany) to record the P700 redox kinetics following the transition from darkness to a light intensity of 1809 μmol photons m−2 s−1 for 20 s at 400 or 800 ppm CO2. All photosynthetic measurements were conducted in a phytotron. The air temperature was set to 25 °C; the relative humidity was set to 60%; the CO2 concentrations were set to 60% and 400 or 800 ppm.
2.3. PSI and PSII Measurements
After dark adaptation for 15 min, plants were illuminated at 272 μmol photons m−2 s−1 for 10 min to activate photosynthesis. Afterward, plants were exposed to FL alternating between LL (59 μmol photons m−2 s−1) and HL (1809 μmol photons m−2 s−1), and the changes in PSI and PSII parameters were recorded using a Dual-PAM 100 measuring system. The quantum yield of PSI photochemistry (Y(I)), the quantum yield of PSI non-photochemical energy dissipation due to donor side limitation (Y(ND)), and the quantum yield of non-photochemical energy dissipation due to acceptor side limitation (Y(NA)) were calculated with the following formulas [40]: Y(I) = (Pm′ − P)/Pm, Y(ND) = P/Pm and Y(NA) = (Pm − Pm′)/Pm.
The PSII parameters were calculated with another three formulas [41,42]: Y(II) = (Fm′ − Fs)/Fm′, Y(NO) = Fs/Fm and NPQ = (Fm − Fm′)/Fm′. Y(II) was the effective quantum yield; Y(NO) was the quantum yield of non-regulated energy dissipation in PSII; NPQ was the non-photochemical quenching in PSII. Fs was the steady state after light adaptation. Fm and Fm′ represented the maximum fluorescence after dark and light adaptation, respectively. Fm was measured after dark-adaptation for 15 min. The photosynthetic electron transport rate through PSI (or PSII) was calculated as: ETRI (or ETRII) = PPFD × Y(I) (or Y(II)) × 0.84 × 0.5, where PPFD is the photosynthetic photon flux density, and the light absorption of incident irradiance is assumed to be 0.84.
2.4. Statistical Analysis
All results are displayed as mean values of five individual experiments. t-tests were used to determine the significant differences between different treatments (α = 0.05).
3. Results
3.1. Elevated Atmospheric CO2 Affects the PSI Redox State after Transition from Dark to Light
After transition from darkness to 1809 μmol photons m−2 s−1, P700 became gradually re-oxidized in 20 s when measured in 800 ppm CO2 (Figure 1). However, we failed to observe a similar re-oxidation of P700 in 400 ppm CO2 (Figure 1). The rapid re-oxidation of P700 after transition from darkness to light is attributed to the outflow of electrons from PSI to downstream electron acceptors [43,44,45]. Because photo-reduction of O2 mediated by flavodiiron proteins and water–water cycle are not observed in A. thaliana, the difference in P700 redox kinetics between 400 and 800 ppm CO2 suggested that elevated atmospheric CO2 accelerates the outflow of electrons from PSI to NADP+, probably due to the increased rate of CO2 fixation.
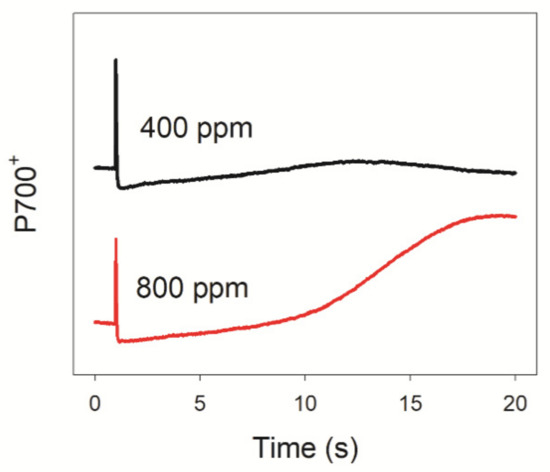
Figure 1.
Changes in P700 redox kinetics after transition from darkness to actinic light (1809 μmol photons m−2 s−1) in 400 and 800 ppm atmospheric CO2. Data are shown as mean values of five leaves from five individual plants.
During photosynthetic induction at a moderate light of 272 μmol photons m−2 s−1, the quantum yield of PSI photochemistry (Y(I)) was enhanced at 800 ppm CO2 (Figure 2A). The quantum yield of PSI non-photochemical energy dissipation due to the donor side limitation (Y(ND)) remained at a low level for 30 s upon transfer from dark to light when measured in 400 ppm CO2 (Figure 2B). This led to the over-reduction of PSI, as indicated by the high value of PSI acceptor side limitation (Y(NA)) at the same time point (Figure 2C). By comparison, Y(ND) had already almost reached its maximal value within 30 s in 800 ppm CO2 (Figure 2B), resulting in a correspondingly low Y(NA) value (Figure 2C). Therefore, elevated atmospheric CO2 concentrations significantly affected the redox state of PSI during transition from darkness to actinic light. By contrast, elevated atmospheric CO2 had minor effects on electron flow from PSII (ETRII) and non-photochemical quenching (NPQ) within the first 30 s after transition from darkness to light (Figure 2D,E). Thus, we concluded that the effects of elevated CO2 concentration on PSI redox state are not caused by electron flow from PSII or the formation of a ΔpH. Instead, an increase in CO2 concentration raises the rate of CO2 fixation and thus facilitates electron transfer from PSI to NADP+, which in turn alleviates the over-reduction of PSI.

Figure 2.
Changes in PSI and PSII parameters after transition from darkness to 272 μmol photons m−2 s−1 in Arabidopsis leaves, measured in 400 and 800 ppm CO2. (A) Y(I), the quantum yield of PSI photochemistry; (B) Y(ND), the quantum yield of PSI non-photochemical energy dissipation due to the donor side limitation; (C) Y(NA), the quantum yield of PSI non-photochemical energy dissipation due to the acceptor side limitation; (D) ETRII, electron transport rate through PSII; (E) NPQ, non-photochemical quenching in PSII. Data are shown as means ± SD (n = 5). Asterisk indicates a significant different between 400 and 800 ppm.
3.2. Elevated Atmospheric CO2 Affect PSI and PSII Performances Differently after Transition from LL to HL
Next, we examined the effect of elevated atmospheric CO2 on photosynthetic performances after transition from LL (59 μmol photons m−2 s−1) to HL (1809 μmol photons m−2 s−1). The value of quantum yield of PSI photochemistry (Y(I)) under LL was slightly higher in 800 ppm CO2 than in 400 ppm CO2 (Figure 3A). After transition from LL to HL, Y(I) did not differ between 400 and 800 ppm CO2 (Figure 3A). However, within the first 10 s after transition from LL to HL, Y(ND) was significantly higher in 800 ppm CO2 than in 400 ppm CO2 (Figure 3B). This rapid oxidation of PSI in 800 ppm CO2 alleviated the over-reduction of PSI electron carriers under FL (Figure 3C). In contrast to PSI, PSII performance under FL did not change significantly as a function of atmospheric CO2 concentration (Figure 4). Indeed, the effective quantum yield of PSII, Y(II), first decreased and then gradually rose upon a sudden transition from LL to HL, as expected (Figure 4A). Meanwhile, NPQ rapidly increased upon transfer to HL (Figure 4B), suggesting the gradual formation of a ΔpH. After transition from LL to HL, the quantum yield of non-regulatory energy dissipation in PSII (Y(NO)) increased sharply before undergoing a rapid drop (Figure 4C). Furthermore, the redox state of the plastoquinone pool of PSII (qP) did not differ significantly between 400 and 800 ppm (Figure 4D).
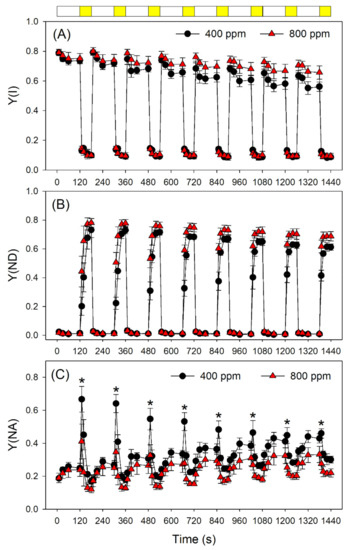
Figure 3.
Changes in PSI parameters during fluctuating light alternating between 59 and 1809 μmol photons m−2 s−1 in Arabidopsis leaves, measured in 400 and 800 ppm CO2. (A) Y(I), the quantum yield of PSI photochemistry; (B) Y(ND), the quantum yield of PSI non-photochemical energy dissipation due to the donor side limitation; (C) Y(NA), the quantum yield of PSI non-photochemical energy dissipation due to the acceptor side limitation. Data are shown as means ± SD (n = 5). White bars indicate low light (59 μmol photons m−2 s−1); yellow bars indicate high light (1809 μmol photons m−2 s−1). Asterisk indicates a significant different between 400 and 800 ppm.
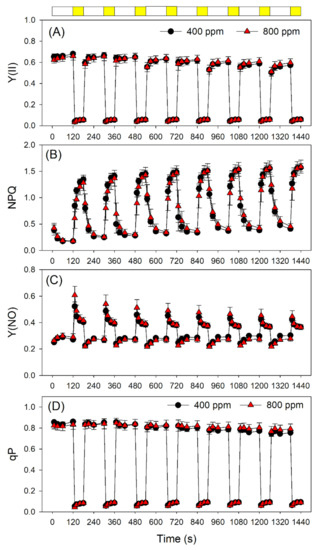
Figure 4.
Changes in PSII parameters during fluctuating light alternating between 59 and 1809 μmol photons m−2 s−1 in Arabidopsis leaves measured in 400 and 800 ppm CO2. (A) Y(II), the effective quantum yield of PSII photochemistry; (B) NPQ, non-photochemical quenching in PSII; (C) Y(NO), the quantum yield of non-regulatory energy dissipation in PSII; (D) the redox state of the plastoquinone pool of PSII (qP). Data are shown as means ± SD (n = 5). White bars indicate low light (59 μmol photons m−2 s−1); yellow bars indicate high light (1809 μmol photons m−2 s−1).
At LL, the ETRI/ETRII ratio was close to 1 when measured at 400 and 800 ppm (Figure 5A). Within the first 10 s after the transition from LL to HL, the ETRI/ETRII ratio was very high in exposed leaves (Figure 5A). Such an increase in the ETRI/ETRII ratio indicated that CEF was stimulated after transition to HL. Furthermore, we noticed that the change in ETRI/ETRII ratio under FL largely correlated with the PSI acceptor side limitation (Figure 5B). Once PSI was over-reduced, CEF was stimulated to help the rapid formation of ΔpH. Once the over-reduction of PSI has been relaxed, CEF activity decreased to the steady state. Therefore, CEF plays an important role in the regulation of photosynthetic rates under fluctuating light.
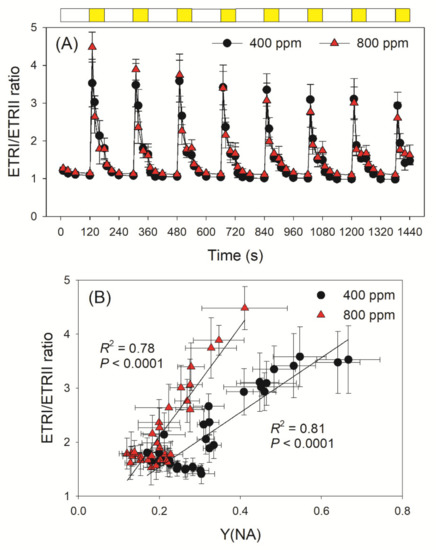
Figure 5.
Changes in ETRI/ETRII ratio under fluctuating light and its relationship to Y(NA) in high-light phases. (A) Change in the ratio of ETRI/ETRII during fluctuating light alternating between 59 and 1809 μmol photons m−2 s−1 in Arabidopsis leaves measured in 400 and 800 ppm CO2; (B) Relationship between the ETRI/ETRII ratio and Y(NA) during the high light phase of fluctuating light treatments. Data are shown as means ± SD (n = 5). White bars indicate low light (59 μmol photons m−2 s−1); yellow bars indicate high light (1809 μmol photons m−2 s−1).
PSI redox state under FL is determined by donor- and acceptor-side regulation. To further explore the effect of elevated atmospheric CO2 on PSI redox state under FL, we examined the relationships between NPQ, Y(ND) and Y(NA) after transition from LL to HL for 10 s. Irrespective of the CO2 concentration, NPQ10s was positively correlated to Y(ND)10s but was negatively correlated to Y(NA)10s (Figure 6). These results indicated that an increase in ΔpH facilitates the oxidation of PSI and thus prevents an over-reduction of PSI. Meanwhile, the same value of NPQ10s was accompanied by a higher Y(ND)10s and a lower Y(NA)10s in 800 ppm CO2 (Figure 6), suggesting that elevated atmospheric CO2 affects the PSI redox state under FL mainly at acceptor side rather than at donor side.
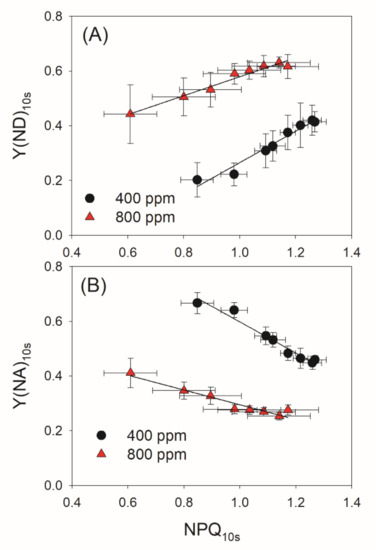
Figure 6.
Relationships between NPQ and PSI redox state after transition from low to high light for 10 s in the eight cycles of low/high light. (A) Relationship between NPQ10s and Y(ND)10s; (B) Relationship between NPQ10s and Y(NA)10s. Data are prepared by combining the different time points of Figure 3 and Figure 4.
3.3. Elevated Atmospheric CO2 Concentrations Mitigates PSI Photoinhibition under FL
After FL treatment, we measured Fv/Fm and Pm to evaluate PSI and PSII photoinhibition. Irrespective of the CO2 concentration, PSI photoinhibition under FL was more evident than that of PSII (Figure 7A). In addition, elevated atmospheric CO2 did not affect the extent of PSII photoinhibition but alleviated PSI photoinhibition (Figure 7A). After FL treatment, Pm decreased by 25% and 16% in 400 and 800 ppm CO2, respectively, relative to Pm values before FL (Figure 7A). The stronger PSI photoinhibition seen in 400 ppm CO2 was mainly caused by the higher Y(NA) within the first 20 s after the transition from LL to HL (Figure 7B). A higher Y(NA) represents a stronger over-reduction of PSI, which leads to the generation of ROS in PSI and thus causes PSI photoinhibition.
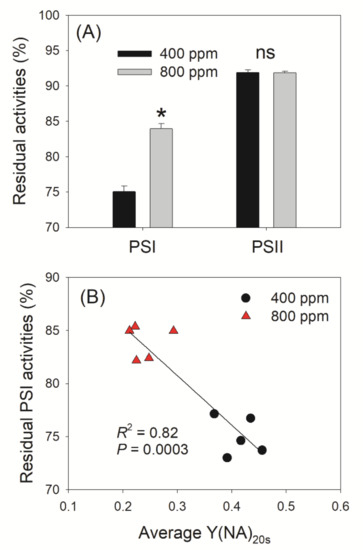
Figure 7.
Effect of elevated CO2 concentration of photoinhibition under fluctuating light and its relationship to redox state of PSI. (A) Changes in Pm and Fv/Fm after exposure to fluctuating light for 24 min. Fv/Fm and Pm represent photosystem PSII and PSI activity, respectively; (B) Relationship between residual PSI activity and the mean value of Y(NA) after transition to high light for 20 s (Y(NA)20s). Data are shown as means ± SD (n = 5). Asterisk indicates a significant different between 400 and 800 ppm.
4. Discussion
Plants usually undergo FL under natural field conditions [7,14]. Following a sudden increase in illumination, the corresponding rapid rise in light absorption and electron flow from PSII cannot be immediately consumed by the CO2 fixation machinery, which displays much slower kinetics than ETRII [9,31,32,46]. As a result, excited states in PSI cannot be immediately transported to NADP+, leading to the over-reduction of PSI electron carriers and inducing the production of ROS within PSI [15,16,47]. However, ROS cannot be immediately scavenged by the antioxidant system, thereby causing photodamage to PSI [17]. A higher atmospheric CO2 concentration, such as that resulting from industrial activities and behind the global climate change crisis, will facilitate CO2 fixation after transition from LL to HL [13]. Therefore, we hypothesized that elevated atmospheric CO2 might mitigate PSI photoinhibition under FL.
To test this hypothesis, we measured the chlorophyll fluorescence and P700 signals under FL conditions in 400 and 800 ppm CO2. We documented that an elevated CO2 concentration significantly affected the redox state of PSI after any increase in light intensity. Upon transition from darkness to high light (1809 μmol photons m−2 s−1) for 20 s, P700 was highly reduced in 400 ppm CO2 but was re-oxidized in 800 ppm CO2 (Figure 1). Similarly, after transition from darkness to 272 μmol photons m−2 s−1 for 30 s, leaves showed a low value of Y(ND) in 400 ppm CO2, causing PSI to be over-reduced (Figure 2). By contrast, over the same time frame, leaves showed a high Y(ND) value in 800 ppm CO2, indicating that the higher CO2 concentration prevents PSI over-reduction (Figure 2). Furthermore, after transition from LL to HL for 20 s, the over-reduction of PSI was alleviated by the elevated CO2 concentration (Figure 3). When P700 is highly oxidized, the probability of electron donation from P700 to O2 is suppressed. Therefore, oxidation of P700 can prevent the production of ROS within PSI and thus protect PSI against excess light energy [48]. Consistently, the extent of PSI photoinhibition under FL was mainly determined by the over-reduction of PSI within the first 20 s after exposure to HL (Figure 7). Therefore, the elevated atmospheric CO2 concentration significantly contributed to preventing PSI photoinhibition under FL in Arabidopsis leaves.
PSI photoinhibition occurs only when electrons transferred to PSI cannot be immediately transported to downstream electron acceptors [10,49,50]. The PSI redox state under FL can be regulated at the donor and acceptor sides. On the donor-side regulation, the ΔpH-dependent photosynthetic control at the Cyt b6/f complex slows electron transfer from PSII to PSI, thus decreasing the excitation pressure on PSI [22,24,33,51]. Upon a sudden transition from LL to HL, the transient stimulation of CEF facilitates the rapid formation of a ΔpH, which prevents uncontrolled photoinhibition of PSI under FL conditions [18,29,47]. The impairment of CEF strongly accelerates PSI photoinhibition under FL, leading to the death of pgr5 seedlings under FL [16,18,23]. In agreement, we also observed the transient stimulation of CEF upon transition from LL to HL in both 400 and 800 ppm CO2 (Figure 6), indicating that CEF plays a major role in photoprotection for PSI under FL irrespective of CO2 concentration. In addition, either PSII photoinhibition or minimizing the activity of the oxygen-evolving complex can restrict electron flow to PSI and thus protect PSI at donor side [49,52,53]. Indeed, minimizing the activity of the oxygen-evolving complex can rescue the phenotype of pgr5 plants under FL [52]. In this study, we observed that NPQ induction under FL is not influenced by an elevated CO2 concentration (Figure 4B), suggesting that higher CO2 levels hardly affected the formation of ΔpH under FL. Furthermore, the CEF stimulation under FL remained unchanged by the CO2 concentrations employed here (Figure 5). Therefore, the effect of elevated CO2 on the redox state of PSI under FL cannot be explained by regulation on the donor side. As shown in Figure 6, the relationships between NPQ, Y(ND) and Y(NA) under FL were altered by the elevated atmospheric CO2 concentration, suggesting that elevated atmospheric CO2 concentration influenced the PSI redox state under FL at the acceptor side rather than at the donor side.
The acceptor-side regulation of PSI is mainly attributed to electron transport from PSI to downstream electron acceptors. In photosynthetic organisms, three pathways are responsible for the regulation of PSI on the acceptor side [24,43]: (1) electron transport from PSI to NADP+; (2) the Mehler reaction (water-water cycle); (3) O2 photoreduction mediated by flavodiiron proteins (Flvs). Flvs are present in photosynthetic organisms ranging from cyanobacteria to gymnosperms but appear to have been lost in angiosperms during evolution [15,43,54]. In addition, the activity of the water–water cycle in angiosperms is species-dependent [30,45,46]. In Arabidopsis leaves, the P700 redox kinetics upon transition from darkness to light indicated that the activity of the water–water cycle is negligible (Figure 1). Therefore, the rapid oxidation of PSI under FL under elevated CO2 is likely to be attributed to accelerated electron transfer from PSI to NADP+. The electron transfer from PSI to NADP+ is largely affected by the NADP+/NADPH ratio. At elevated CO2 concentrations, CO2 fixation under FL increases [38], raising the rate of NADPH consumption. Under such conditions, the NADP+/NADPH ratio increases, facilitating electron transfer from PSI to NADP+ and thus alleviating the over-reduction of PSI electron carriers. Therefore, elevated CO2 affects the PSI redox state under FL through the acceptor-side regulation.
Improving photosynthesis under FL is a critical and timely target for crop improvement under field conditions [9,13,14,55]. Maintaining high photosynthetic rates requires the avoidance of photoinhibition [6,56,57,58]. However, FL-induced photoinhibition of PSI can significantly affect CO2 assimilation [5]. Therefore, diminishing the extent of PSI photoinhibition under FL is an effective way to improve photosynthesis under natural FL. Raising CO2 concentrations can significantly boost crop yields and is thought to be attributed to enhanced photosynthetic rates [36,39]. Furthermore, we discovered here that elevated CO2 can significantly alter the PSI redox state under FL and thus mitigate FL-induced photoinhibition of PSI, which in turn safeguards high rates of photosynthesis. Therefore, in addition to dark reactions, elevated CO2 concentrations can have large effects on thylakoid reactions under FL conditions.
Author Contributions
Conceptualization, W.H. and S.-B.Z.; methodology, W.H. and S.-L.T.; validation, W.H. and S.-B.Z.; formal analysis, S.-L.T.; investigation, S.-L.T.; resources, X.H. and W.-Q.L.; data curation, S.-L.T. and W.H.; writing—original draft preparation, W.H.; writing—review and editing, S.-B.Z.; supervision, S.-B.Z.; project administration, W.H.; funding acquisition, W.H. and S.-B.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant 31971412), Project for Construction of International Flower Technology Innovation Center and Achievement Industrialization (2019ZG006) and the Project for Innovation Team of Yunnan Province.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Acevedo-Siaca, L.G.; Coe, R.; Wang, Y.; Kromdijk, J.; Quick, W.P.; Long, S.P. Variation in photosynthetic induction between rice accessions and its potential for improving productivity. New Phytol. 2020, 227, 1097–1108. [Google Scholar] [CrossRef] [Green Version]
- Allahverdiyeva, Y.; Suorsa, M.; Tikkanen, M.; Aro, E.M. Photoprotection of photosystems in fluctuating light intensities. J. Exp. Bot. 2015, 66, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Ananyev, G.; Kolber, Z.S.; Klimov, D.; Falkowski, P.G.; Berry, J.A.; Rascher, U.; Martin, R.; Osmond, B. Remote sensing of heterogeneity in photosynthetic efficiency, electron transport and dissipation of excess light in Populus deltoides stands under ambient and elevated CO2 concentrations, and in a tropical forest canopy, using a new laser-induced fluoresc. Glob. Chang. Biol. 2005, 11, 1195–1206. [Google Scholar] [CrossRef]
- Armbruster, U.; Correa Galvis, V.; Kunz, H.H.; Strand, D.D. The regulation of the chloroplast proton motive force plays a key role for photosynthesis in fluctuating light. Curr. Opin. Plant. Biol. 2017, 37, 56–62. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M.; Kunderlikova, K.; Allakhverdiev, S.I. High temperature specifically affects the photoprotective responses of chlorophyll b-deficient wheat mutant lines. Photosynth. Res. 2016, 130, 251–266. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M.; Kunderlikova, K.; Sytar, O.; Shao, H.; Kalaji, H.M.; Allakhverdiev, S.I. Low PSI content limits the photoprotection of PSI and PSII in early growth stages of chlorophyll b-deficient wheat mutant lines. Photosynth. Res. 2015, 125, 151–166. [Google Scholar] [CrossRef]
- De Souza, A.P.; Wang, Y.; Orr, D.J.; Carmo-Silva, E.; Long, S.P. Photosynthesis across African cassava germplasm is limited by Rubisco and mesophyll conductance at steady state, but by stomatal conductance in fluctuating light. New Phytol. 2020, 225, 2498–2512. [Google Scholar] [CrossRef] [Green Version]
- Ferroni, L.; Živčak, M.; Sytar, O.; Kovár, M.; Watanabe, N.; Pancaldi, S.; Baldisserotto, C.; Brestič, M. Chlorophyll-depleted wheat mutants are disturbed in photosynthetic electron flow regulation but can retain an acclimation ability to a fluctuating light regime. Environ. Exp. Bot. 2020, 178, 104156. [Google Scholar] [CrossRef]
- Gerotto, C.; Alboresi, A.; Meneghesso, A.; Jokel, M.; Suorsa, M.; Aro, E.-M.; Morosinotto, T. Flavodiiron proteins act as safety valve for electrons in Physcomitrella patens. Proc. Natl. Acad. Sci. USA 2016, 113, 12322–12327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrickson, L.; Furbank, R.T.; Chow, W.S. A simple alternative approach to assessing the fate of absorbed light energy using chlorophyll fluorescence. Photosynth. Res. 2004, 82, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yang, Y.-J.; Zhang, S.-B. Photoinhibition of photosystem I under fluctuating light is linked to the insufficient ΔpH upon a sudden transition from low to high light. Environ. Exp. Bot. 2019, 160, 112–119. [Google Scholar] [CrossRef]
- Huang, W.; Yang, Y.J.; Zhang, S.B. The role of water-water cycle in regulating the redox state of photosystem I under fluctuating light. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, S.-B.; Liu, T. Moderate photoinhibition of photosystem II significantly affects linear electron flow in the shade-demanding plant Panax notoginseng. Front. Plant. Sci. 2018, 9, 637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilík, P.; Pavlovič, A.; Kouřil, R.; Alboresi, A.; Morosinotto, T.; Allahverdiyeva, Y.; Aro, E.M.; Yamamoto, H.; Shikanai, T. Alternative electron transport mediated by flavodiiron proteins is operational in organisms from cyanobacteria up to gymnosperms. New Phytol. 2017, 214, 967–972. [Google Scholar] [CrossRef] [Green Version]
- Jokel, M.; Johnson, X.; Peltier, G.; Aro, E.M.; Allahverdiyeva, Y. Hunting the main player enabling Chlamydomonas reinhardtii growth under fluctuating light. Plant., J. 2018, 94, 822–835. [Google Scholar] [CrossRef] [Green Version]
- Kimura, H.; Hashimoto-Sugimoto, M.; Iba, K.; Terashima, I.; Yamori, W. Improved stomatal opening enhances photosynthetic rate and biomass production in fluctuating light. J. Exp. Bot. 2020, 71, 2339–2350. [Google Scholar] [CrossRef]
- Kono, M.; Noguchi, K.; Terashima, I. Roles of the cyclic electron flow around PSI (CEF-PSI) and O2-dependent alternative pathways in regulation of the photosynthetic electron flow in short-term fluctuating light in Arabidopsis thaliana. Plant. Cell Physiol. 2014, 55, 990–1004. [Google Scholar] [CrossRef] [Green Version]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of Q A redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Kreslavski, V.D.; Strokina, V.V.; Pashkovskiy, P.P.; Balakhnina, T.I.; Voloshin, R.A.; Alwasel, S.; Kosobryukhov, A.A.; Allakhverdiev, S.I. Deficiencies in phytochromes A and B and cryptochrome 1 affect the resistance of the photosynthetic apparatus to high-intensity light in Solanum lycopersicum. J. Photochem. Photobiol. B Biol. 2020, 210, 111976. [Google Scholar] [CrossRef] [PubMed]
- Munekage, Y.N.; Genty, B.; Peltier, G. Effect of PGR5 impairment on photosynthesis and growth in Arabidopsis thaliana. Plant. Cell Physiol. 2008, 49, 1688–1698. [Google Scholar] [CrossRef] [Green Version]
- Nakano, H.; Yamamoto, H.; Shikanai, T. Contribution of NDH-dependent cyclic electron transport around photosystem I to the generation of proton motive force in the weak mutant allele of pgr5. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 369–374. [Google Scholar] [CrossRef]
- Nawrocki, W.J.; Bailleul, B.; Cardol, P.; Rappaport, F.; Wollman, F.A.; Joliot, P. Maximal cyclic electron flow rate is independent of PGRL1 in Chlamydomonas. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, W.J.; Bailleul, B.; Picot, D.; Cardol, P.; Rappaport, F.; Wollman, F.A.; Joliot, P. The mechanism of cyclic electron flow. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Pearcy, R.W. Sunflecks and photosynthesis in plant canopies. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1990, 41, 421–453. [Google Scholar] [CrossRef]
- Qiao, M.-Y.; Zhang, Y.-J.; Liu, L.-A.; Shi, L.; Ma, Q.-H.; Chow, W.S.; Jiang, C.-D. Do rapid photosynthetic responses protect maize leaves against photoinhibition under fluctuating light? Photosynth. Res. 2020. [Google Scholar] [CrossRef]
- Sakoda, K.; Yamori, W.; Shimada, T.; Sugano, S.S.; Hara-Nishimura, I.; Tanaka, Y. Higher stomatal density improves photosynthetic induction and biomass production in Arabidopsis under fluctuating light. Front. Plant. Sci. 2020, 11, 1308. [Google Scholar] [CrossRef]
- Salter, W.T.; Merchant, A.M.; Richards, R.A.; Trethowan, R.; Buckley, T.N. Rate of photosynthetic induction in fluctuating light varies widely among genotypes of wheat. J. Exp. Bot. 2019, 70, 2787–2796. [Google Scholar] [CrossRef]
- Schreiber, U.; Klughammer, C. Non-photochemical fluorescence quenching and quantum yields in PS I and PS II: Analysis of heat-induced limitations using Maxi-Imaging- PAM and Dual-PAM-100. PAM Appl. Notes 2008, 1, 15–18. [Google Scholar] [CrossRef]
- Shikanai, T.; Yamamoto, H. Contribution of cyclic and pseudo-cyclic electron transport to the formation of proton motive force in chloroplasts. Mol. Plant. 2017, 10, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Shimakawa, G.; Miyake, C. What quantity of photosystem I is optimum for safe photosynthesis? Plant. Physiol. 2019, 179, 1479–1485. [Google Scholar] [CrossRef]
- Slattery, R.A.; Walker, B.J.; Weber, A.P.M.; Ort, D.R. The impacts of fluctuating light on crop performance. Plant. Physiol. 2018, 176, 990–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Yang, Y.-J.; Huang, W. The water-water cycle is more effective in regulating redox state of photosystem I under fluctuating light than cyclic electron transport. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148235. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, S.-B.; Liu, T.; Huang, W. Decreased photosystem II activity facilitates acclimation to fluctuating light in the understory plant Paris polyphylla. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148135. [Google Scholar] [CrossRef] [PubMed]
- Suorsa, M.; Jarvi, S.; Grieco, M.; Nurmi, M.; Pietrzykowska, M.; Rantala, M.; Kangasjarvi, S.; Paakkarinen, V.; Tikkanen, M.; Jansson, S.; et al. PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant. Cell 2012, 24, 2934–2948. [Google Scholar] [CrossRef] [Green Version]
- Suorsa, M.; Rossi, F.; Tadini, L.; Labs, M.; Colombo, M.; Jahns, P.; Kater, M.M.; Leister, D.; Finazzi, G.; Aro, E.-M.; et al. PGR5-PGRL1-dependent cyclic electron transport modulates linear electron transport rate in Arabidopsis thaliana. Mol. Plant. 2016, 9, 271–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takagi, D.; Amako, K.; Hashiguchi, M.; Fukaki, H.; Ishizaki, K.; Goh, T.; Fukao, Y.; Sano, R.; Kurata, T.; Demura, T.; et al. Chloroplastic ATP synthase builds up a proton motive force preventing production of reactive oxygen species in photosystem I. Plant. J. 2017, 91, 306–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takagi, D.; Ishizaki, K.; Hanawa, H.; Mabuchi, T.; Shimakawa, G.; Yamamoto, H.; Miyake, C. Diversity of strategies for escaping reactive oxygen species production within photosystem I among land plants: P700 oxidation system is prerequisite for alleviating photoinhibition in photosystem I. Physiol. Plant. 2017, 161, 56–74. [Google Scholar] [CrossRef] [PubMed]
- Takagi, D.; Takumi, S.; Hashiguchi, M.; Sejima, T.; Miyake, C. Superoxide and singlet oxygen produced within the thylakoid membranes both cause photosystem I photoinhibition. Plant. Physiol. 2016, 171, 1626–1634. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.-L.; Huang, J.-L.; Zhang, F.-P.; Zhang, S.-B.; Huang, W. Photosystem I photoinhibition induced by fluctuating light depends on background low light irradiance. Environ. Exp. Bot. 2021, 181, 104298. [Google Scholar] [CrossRef]
- Tan, S.-L.; Liu, T.; Zhang, S.-B.; Huang, W. Balancing light use efficiency and photoprotection in tobacco leaves grown at different light regimes. Environ. Exp. Bot. 2020, 175, 104046. [Google Scholar] [CrossRef]
- Tan, S.-L.; Yang, Y.-J.; Huang, W. Moderate heat stress accelerates photoinhibition of photosystem I under fluctuating light in tobacco young leaves. Photosynth. Res. 2020, 144, 373–382. [Google Scholar] [CrossRef]
- Tan, S.-L.; Yang, Y.-J.; Liu, T.; Zhang, S.-B.; Huang, W. Responses of photosystem I compared with photosystem II to combination of heat stress and fluctuating light in tobacco leaves. Plant. Sci. 2020, 292, 110371. [Google Scholar] [CrossRef]
- Tikkanen, M.; Aro, E.M. Integrative regulatory network of plant thylakoid energy transduction. Trends Plant. Sci. 2014, 19, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, M.; Grieco, M.; Nurmi, M.; Rantala, M.; Suorsa, M.; Aro, E.-M. Regulation of the photosynthetic apparatus under fluctuating growth light. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3486–3493. [Google Scholar] [CrossRef] [Green Version]
- Tikkanen, M.; Mekala, N.R.; Aro, E.-M. Photosystem II photoinhibition-repair cycle protects Photosystem I from irreversible damage. Biochim. Biophys. Acta Bioenerg. 2014, 1837, 210–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, S.; Yamamoto, H.; Suzuki, Y.; Yamori, W.; Shikanai, T.; Makino, A. Flavodiiron protein substitutes for cyclic electron flow without competing CO2 assimilation in rice. Plant. Physiol. 2018, 176, 1509–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, B.J.; Strand, D.D.; Kramer, D.M.; Cousins, A.B. The response of cyclic electron flow around photosystem I to changes in photorespiration and nitrate assimilation. Plant. Physiol. 2014, 165, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, H.; Shikanai, T. PGR5-dependent cyclic electron flow protects photosystem I under fluctuating light at donor and acceptor sides. Plant. Physiol. 2019, 179, 588–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, H.; Takahashi, S.; Badger, M.R.; Shikanai, T. Artificial remodelling of alternative electron flow by flavodiiron proteins in Arabidopsis. Nat. Plants 2016, 2, 16012. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; Kusumi, K.; Iba, K.; Terashima, I. Increased stomatal conductance induces rapid changes to photosynthetic rate in response to naturally fluctuating light conditions in rice. Plant. Cell Environ. 2020, 43, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; Makino, A.; Shikanai, T. A physiological role of cyclic electron transport around photosystem I in sustaining photosynthesis under fluctuating light in rice. Sci. Rep. 2016, 6, 20147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.-J.; Ding, X.-X.; Huang, W. Stimulation of cyclic electron flow around photosystem I upon a sudden transition from low to high light in two angiosperms Arabidopsis thaliana and Bletilla striata. Plant. Sci. 2019, 287, 110166. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Tan, S.-L.; Huang, J.-L.; Zhang, S.-B.; Huang, W. The water-water cycle facilitates photosynthetic regulation under fluctuating light in the epiphytic orchid Dendrobium officinale. Environ. Exp. Bot. 2020, 180, 104238. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Zhang, S.-B.; Huang, W. Photosynthetic regulation under fluctuating light in young and mature leaves of the CAM plant Bryophyllum pinnatum. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-J.; Zhang, S.-B.; Wang, J.-H.; Huang, W. Photosynthetic regulation under fluctuating light in field-grown Cerasus cerasoides: A comparison of young and mature leaves. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 148073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xu, J.; Bao, M.; Yan, D.; Beer, S.; Beardall, J.; Gao, K. Elevated CO2 concentration alleviates UVR-induced inhibition of photosynthetic light reactions and growth in an intertidal red macroalga. J. Photochem. Photobiol. B Biol. 2020, 213, 112074. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Kunderlikova, K.; Sytar, O.; Allakhverdiev, S.I. Repetitive light pulse-induced photoinhibition of photosystem I severely affects CO2 assimilation and photoprotection in wheat leaves. Photosynth. Res. 2015, 126, 449–463. [Google Scholar] [CrossRef]
- Zlobin, I.E.; Kartashov, A.V.; Pashkovskiy, P.P.; Ivanov, Y.V.; Kreslavski, V.D.; Kuznetsov, V.V. Comparative photosynthetic responses of Norway spruce and Scots pine seedlings to prolonged water deficiency. J. Photochem. Photobiol. B Biol. 2019, 201, 111659. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).