Abstract
Duchenne muscular dystrophy (DMD) is a muscular disease characterized by progressive muscle degeneration. Life expectancy is between 30 and 50 years, and death is correlated with cardiac or respiratory complications. Currently, there is no cure, so there is a great interest in new pharmacological targets. Sirtuin1 (SIRT1) seems to be a potential target for DMD. In muscle tissue, SIRT1 exerts anti-inflammatory and antioxidant effects. The aim of this study is to summarize all the findings of in vivo and in vitro literature studies about the potential role of SIRT1 in DMD. A systematic literature search was performed according to PRISMA guidelines. Twenty-three articles satisfied the eligibility criteria. It emerged that SIRT1 inhibition led to muscle fragility, while conversely its activation improved muscle function. Additionally, resveratrol, a SIRT1 activator, has brought beneficial effects to the skeletal, cardiac and respiratory muscles by exerting anti-inflammatory activity that leads to reduced myofiber wasting.
1. Introduction
Duchenne muscular dystrophy (DMD) is an X-linked muscular disease with an incidence of 1 in 3500 male births worldwide. It is characterized by dystrophin deficiency due to a mutation in the gene encoding for this protein. Dystrophin is a member of the sarcolemmal dystrophin glycoprotein complex (DGC) which connects the intracellular actin cytoskeleton and the extracellular matrix of skeletal muscle [1,2]. Dystrophin deficiency leads to an alteration of the DGC resulting in a cascade of several events, such as membrane instability, chronic inflammation, muscle degeneration, and myofiber necrosis [1,2,3]. As muscle regeneration processes decrease, muscle fibers are gradually replaced by connective and adipose tissue [4]. Muscle weakness generally begins around the age of three and degenerates until the loss of the ability to walk before the age of thirteen. Unfortunately, life expectancy is between 30 and 50 years and patients usually die because of cardiac and/or respiratory complications [1,5]. Nowadays, there is no cure for DMD, the only approved therapy is the use of glucocorticoids (GCs) such as prednisone, prednisolone and deflazacort, which are able to slow down the muscle decline [6,7]. However, long-term use of these anti-inflammatory agents may cause several side effects. Currently there is an unmet need for innovative therapeutic approaches. Some of the experimental molecular-based strategies, used to modify the DMD gene product, include exone skipping or suppression of premature stop codons. However, these approaches seem to be effective only in a limited number of patients; for this reason, it is necessary to focus on pharmacological strategies applicable in all DMD patients [8,9,10].
Interestingly, several studies have shown that reducing muscular inflammation ameliorates both muscle function and morphology, suggesting that inflammation should be a good therapeutic target [11,12].
Regarding inflammation, an emerging and innovative therapeutic target for DMD seems to be the activation of Sirtuin1 (SIRT1).
SIRT1 belongs to a class of NAD+-dependent class III histone/deacetylase proteins, the sirtuins [13]. These enzymes modulate the activity of different nuclear and cytoplasmatic proteins, which in turn regulate several cellular processes [14]. Under stressful conditions, when NAD+ levels increase, sirtuin transcription appears to be activated, and this is the reason why these enzymes are considered cell survival mediators. Sirtuins, and especially the SIRT1 isoform, promote longevity by delaying cellular aging, inhibit apoptosis, regulate the cell cycle, the glucose homeostasis and the insulin secretion, and are involved in inflammation, oxidative stress and mitochondrial biogenesis [15,16,17,18].
Due to its role in the physiological processes, SIRT1 plays a key role in various diseases, such as diabetes, obesity, cancer, neurodegenerative and cardiovascular diseases, and age-related pathologies [19].
SIRT1 is found both in the nucleus and in the cytosol, and exerts its action by deacetylating histone and non-histone proteins, such as the tumor suppressor p53, the forkhead box O (FOXO) family of transcription factors, the hypoxia-inducible factor 1-alpha (HIF-1α), the peroxisome proliferator-activated receptor gamma (PPARγ), the cofactor of PPARγ (PGC-1α), Ku70 (a protein involved in DNA repair), and the nuclear factor NF-kB [20,21].
SIRT1 is expressed in different tissues, and we focus on its role in skeletal muscle, where it deacetylates and activates PGC-1α. The activated form of PGC-1α controls mitochondrial biogenesis and homeostasis, improves muscle resistance, enhances muscle fiber-type switching, and decreases the process of muscle wasting during aging due to its ROS scavenging activity (Figure 1) [22,23,24,25,26,27,28,29]. Hence, the activation of the SIRT1-PGC-1α axis in skeletal muscle ameliorates the phenotype of the X-linked recessive, muscle wasting disease DMD [30].
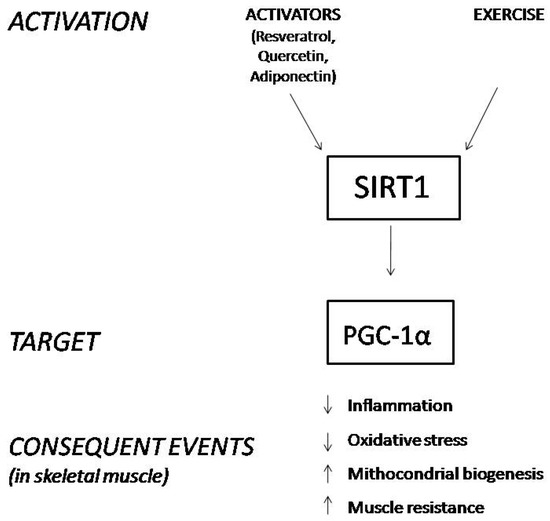
Figure 1.
Schematic representation of the consequences derived from SIRT1 stimulation in skeletal muscle.
Since the modulation of SIRT1 leads to several beneficial effects, attention has also been paid to activators of this protein, such as resveratrol and quercetin. Both of them are polyphenols, a class of flavonones that have been largely studied for their anti-inflammatory properties, for their positive effects in reducing oxidative stress and ameliorating cardiac diseases [31,32,33]. Some recent findings suggest that polyphenols may alleviate muscular dystrophic pathologies by activating the SIRT1/PGC-1α axis [34]. Additionally, adiponectin (ApN), a hormone secreted by adipocytes under normal conditions, exerts anti-inflammatory properties in dystrophic muscles by activating the SIRT1-PGC-1α pathway [2,35].
In this systematic review, we aimed to summarize and highlight all the findings concerning the role of SIRT1 as a potential therapeutic target in DMD.
2. Materials and Methods
This systematic review was conducted according to the preferred reporting items for systematic review (PRISMA) guidelines [36]. We selected all the necessary data based on the eligibility criteria regarding the role of SIRT1 in Duchenne muscular dystrophy.
2.1. Study Design
Nowadays, the elective therapy for Duchenne muscular dystrophy is the use of glucocorticoids, which are unfortunately associated with several side effects. Therefore, we were interested in new possible drug targets that could be exploited for the treatment of this pathology, specifically on a novel hypothetical target, SIRT1, a protein belonging to the sirtuin family. The aim of our research was to collect the information currently available regarding the role of the SIRT1 enzyme in improving the pathophysiology of Duchenne muscular dystrophy, including the effects that its activation brings to skeletal muscles. As the main cause of death of DMD patients appears to be cardiac and/or respiratory failure, we also evaluated the studies concerning the action of SIRT1 in cardiac and respiratory tissues of dystrophic animal models.
2.2. Eligibility Criteria
Initially, we defined the eligibility criteria for the inclusion of the studies in this systematic review by analyzing all the original research papers currently present in different databases, describing the correlation between SIRT1 and DMD. Since the activation of SIRT1 is involved in cell survival processes, we wondered how this protein and its activators can slow down the normal degeneration of the muscle tissue in DMD, so we studied the possible mechanism behind SIRT1-mediated muscle regeneration. We extrapolated data concerning the composition of muscle fibers, myofiber damage, force resistance and fatigue resistance. SIRT1 is also involved in inflammatory and oxidative processes, so we investigated the metabolites involved, as well as the mitochondrial activity and damage. Due to cardiomyopathy, the main cause of death in DMD is heart failure, so we searched for the benefits of SIRT1 activation and its activators in dystrophic hearts and their role as cardioprotective agents. Considering that respiratory failure is another primary cause of death in patients with muscular dystrophies, we also assessed the effects of SIRT1 activators in respiratory function.
We included and considered eligible all the studies (in vivo and in vitro) carried out in dystrophic animal models, or in cell cultures of dystrophic animals and humans, which:
- -
- evaluated the expression of SIRT1 in muscle, cardiac and respiratory tissues of dystrophic models;
- -
- demonstrated the effect of SIRT1 activation in the mentioned tissues;
- -
- evaluated the effect of SIRT1 activators in Duchenne muscular dystrophy;
- -
- explained the potential mechanisms of action of SIRT1 in improving the pathophysiology of DMD.
We excluded and considered as non-eligible all articles that, although concerning potential new therapies for Duchenne muscular dystrophy, did not include SIRT1 as a molecular target. Furthermore, reviews were excluded from this study, selecting only original experimental studies.
Our search includes all the eligible articles in English regarding in vitro and in vivo animal studies.
2.3. Literature Search and Selection of Articles
We searched PubMed, Embase and Cochrane databases using different keywords in order to identify all the studies concerning the hypothetical correlation between SIRT1 and DMD. The keywords used during the search were: “Sirtuin and Duchenne Muscular Dystrophy”, “Sirtuin and Muscular Dystrophy”, “SIRT1 and Muscular Dystrophy”, “SIRT1 and Duchenne Muscular Dystrophy”, “SIRT2 and Muscular Dystrophy”, “SIRT3 and Muscular Dystrophy”, “SIRT4 and Muscular Dystrophy”, “SIRT5 and Muscular Dystrophy”, “SIRT6 and Muscular Dystrophy”, “SIRT7 and Muscular Dystrophy”, “SIRT1 activators and Duchenne Muscular Dystrophy”, “Resveratrol and Duchenne Muscular Dystrophy”, “Resveratrol and Muscular Dystrophy”, “Quercetin and Duchenne Muscular Dystrophy”, “SIRT1 inhibitor and Duchenne Muscular Dystrophy” and “SIRT1 and utrophin”. Initially, all the papers were screened based on their title and the abstract. After this first selection phase, we read and analyzed the full text of the selected studies to verify that they satisfied the eligibility criteria. The PRISMA diagram below shows the steps followed during our literature search and evaluation (Figure 2).
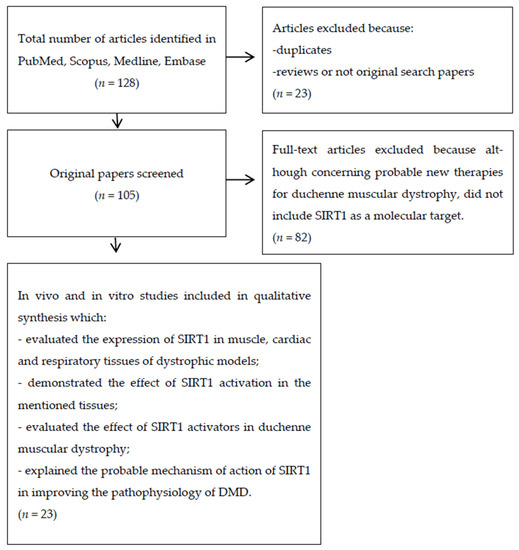
Figure 2.
Prisma flow diagram represents the steps we followed during the literature search and article selection for this systematic review.
2.4. Data Extraction
We identified one hundred and twenty-eight papers, of which twenty-three studies were included in this systematic review.
3. Results
3.1. Overview of Literature Search Results
As shown in Figure 2, after a first literature search, we identified a total of 128 articles. We excluded 23 articles and eliminated duplicates and other studies not eligible for different reasons, like reviews and/or articles not corresponding to the main argument (SIRT1 pathway and its implication in DMD). Finally, we systematically reviewed twenty-three studies.
3.2. Description of Articles Included in the Systematic Review
In total, we included twenty three articles for further analysis. Some articles reported both in vivo and in vitro studies, so we grouped the experiments according to the methodology used, respectively: twenty-two in vivo animal experimental studies, and seven in vitro experimental studies, among which five were in vitro animal studies and two were in vitro human studies.
3.2.1. Main Outcomes Obtained by In Vivo Animal Studies
In Table 1, we summarize all the endpoints resulting from in vivo animal studies. In all the experiments, mdx mice, which have a mutation that leads muscle cells to produce a small, nonfunctional dystrophin protein, were used as an animal model of Duchenne muscular dystrophy. Fujiwara et al. observed pathological features similar to mice with muscular dystrophies in skeletal muscle-specific SIRT1 knockout mice (SIRT1-MKO) [37]. Moreover, Hulmi et al. highlighted an increase in phosphorylated SIRT1 (p-SIRT1), and a decrease in total SIRT1 in mdx mice when compared to wild type [38]. This evidence suggests the importance of SIRT1 activation in improving muscle function. Other studies have also shown the key role of SIRT1 activation in DMD disease; in particular, Chalkiadaki et al. elucidated how SIRT1 overexpression may influence the skeletal muscle properties of mdx mice. Initially, they generated SIRT1 transgenic mice, a mice model with higher SIRT1 muscle level, and then they observed that this modification resulted in a fiber shift from fast-to-slow twitch, which prevents muscular atrophy and dystrophy [39]. Moreover, they crossed SIRT1 muscle transgenic mice to mdx mice, and once again a notable improvement in the muscular parameters and performance was noted [39]. Hence, SIRT1 activation appears to be crucial for muscle fibers’ composition, mitochondrial genes and atrophy [39]. Different methods have been used in studies to increase SIRT1 expression or its activation, and to date, one of the most promising SIRT1 activators that can improve the clinical conditions in mdx mice seems to be resveratrol at a dose of 100 mg/kg/day [40,41]. Further studies are needed to affirm the effectiveness of SIRT1 activation as a new therapeutic target in DMD patients, and below we summarize the effect of the treatments in skeletal muscles, cardiac tissue and diaphragm.

Table 1.
Overview of the characteristics of in vivo animal studies included in the systematic review.
Effects of SIRT1 Activation in Skeletal Muscle
A total of nine studies elucidated the role of SIRT1 activation in improving skeletal muscle properties and function of mdx mice. Two basic activators were used in the various experiments: resveratrol and quercetin, respectively, at different doses and for different periods of treatment.
Capogrosso et al. studied the effect of some natural compounds, such as resveratrol, taurine and apocynin, in ameliorating DMD pathophysiology, compared to α-methyl prednisolone (PDN), a corticosteroid largely used in this disease [8].The treatment lasted 4–5 weeks and the administration of these natural activators of SIRT1/PGC-1α pathway, in particular resveratrol at a dose of 100 mg/kg5 days/week, led to a reduction in superoxide anion production and to decreased plasma levels of creatine kinase and lactate dehydrogenase, hence muscle force is increased [8]. The study of Gordon et al. analyzed the effect of resveratrol in reducing muscle inflammation [41]. It was found that resveratrol, by increasing SIRT1 expression, decreased immune cells infiltration, reduced macrophage infiltration, enhanced PGC-1α activity, and increased utrophin levels (a dystrophin analog) [41]. These effects were observed for a short treatment period of only 10 days [41].
However, long-term treatment of resveratrol has also shown positive effects in improving the pathophysiology of DMD. In fact, it was found that daily oral intake or intraperitoneal injection (i.p.) for several weeks in mdx mice contributed to the preservation of muscle function and muscle mass [43,45,53]. These beneficial effects appear to be attributed to the activation of SIRT1 with consequent fiber shift from fast-to-slow twitch, which, as mentioned before, prevents muscular dystrophies [40,55].The optimal dose of resveratrol treatment in most of the experiments is shown to be 100 mg/kg/day [8,40,41,53,55].
Quercetin also proved to be a good activator of SIRT1 with beneficial effects against Duchenne muscular dystrophy. A long-term quercetin dietary intake prevents 50% loss of specific tension and fatigue resistance in skeletal muscle of mdx mice, as demonstrated by Spaulding et al. [51].
Another compound that exerts positive effects in skeletal muscle is adiponectin, a hormone with anti-inflammatory properties [35]. To prove its effectiveness in mdx mice, Abou-Samra et al. generated mdx-ApN mice by crossing mdx mice with mice overexpressing ApN [35]. mdx-ApN mice showed decreased muscle damage and enhanced muscle force compared to mdx mice; the effect seems to be due to SIRT1 activation, that in turn promotes the downregulation of inflammatory genes and the upregulation of utrophin [35].
Effects of SIRT1 Activation in Cardiac Tissue
Considering that cardiomyopathy is the main cause of death in DMD, different studies elucidated the role of SIRT1 activators in ameliorating cardiac function of dystrophic hearts. The SIRT1 activators used in these studies are, once again, resveratrol and quercetin.
Kuno et al. demonstrated that a long-term dietary intake of resveratrol for 32 weeks [42,44] or 56 weeks [46] inhibited hypertrophy and fibrosis in cardiac tissue of mdx mice, resulting in the maintenance of cardiac functions [42,44]. Overall, these data suggest that resveratrol improves mdx mouse survival.
Quercetin also appears to relieve cardiomyopathies in mdx mice. Ballman et al. proved that a 0.2% enriched quercetin diet administered for 6, 8 or 12 months prevented cardiac dysfunction of dystrophic hearts by decreasing the inflammatory markers and cardiac tissue damage, and by increasing mitochondrial biogenesis and utrophin expression [34,52,54].
Effects of SIRT1 Activation in Diaphragm
From the studies analyzed for the preparation of this systematic review, it emerged that the activation of SIRT1 has positive effects also in the diaphragm muscle, thus improving respiratory function, which generally fails in patients with DMD. A long-term intake of 0.2% enriched quercetin diet, administered for 6 months, lead to an increase in the number of muscle fibers and reduced fibrotic area, but fails to increase utrophin levels, suggesting that maybePGC-1α/SIRT1 pathway is only partially activated in diaphragm muscle [50]. In fact, Selsby et al. highlighted that a prolonged administration of quercetin for 12 months improved the respiratory function only for the first 6 months [47]. The insensitivity to the treatment seems to be a result of the decreased SIRT1 activity in mdx mice [47].
3.2.2. Main Outcomes Obtained by In Vitro Studies
In Table 2, we report a schematic summary of the main endpoints resulting from in vitro studies. We identified five different studies conducted in animal cells where the experiments were performed in two different cell lines: myoblast cells and cardiomyocytes, respectively. We also analyzed two in vitro human studies, and in this case, the experiments were conducted in human myotubes.

Table 2.
Overview of the characteristics of in vitro animal and human studies included in the systematic review.
SIRT1 Effects in C2C12 Myoblast Cells
Three studies examined the role of SIRT1 in myoblast cells; in particular, Fujiwara et al. observed that SIRT1 inhibition in C2C12 myoblast cells inhibits membrane resealing after injury [37]. In fact, they proved that cells treatment with a SIRT1 inhibitor, or Sirt1-silencing RNA (siRNA), alters the fusion of intracellular vesicles to the injured membranes, thus preventing their repairing mechanisms [37]. Furthermore, beneficial effects were observed from the activation of SIRT1 in myoblastic cells. Hori et al. demonstrated that the treatment of C2C12 cells with transforming growth factor-β1 (TGF-β1) led to increased reactive oxygen species (ROS) levels and fibronectin production [43]. However, resveratrol pretreatment of cells, followed byTGF-β1 treatment, reverses all these effects through SIRT1 activation, resulting in a containment of oxidative damage [43]. In C2C12 myoblast cells, resveratrol also promotes mitophagy processes of damaged mitochondria containing high superoxide species levels, resulting overall in reduced inflammation [45].
SIRT1 Effects in Cardiomyocytes
Two in vitro studies were performed in cardiomyocytes, since heart failure is one of the main causes of death in DMD patients. Progressive muscle degeneration leads to the replacement of muscle tissue with connective or adipose tissue, which loses its contractile function [4]. Kuno et al. analyzed how SIRT1 may exert its beneficial effect in cardiomyocytes. SIRT1 activation via resveratrol treatment promotes autophagy and mitophagy, leading overall to decreased ROS accumulation in heart [46], and hypertrophy inhibition through pro-hypertrophic co-activator p300 downregulation [44]. p300 protein is generally upregulated in mdx hearts and induces cardiomyocyte hypertrophy and tissue fibrosis [44]. Overall, these data confirm the efficacy of resveratrol in activating SIRT1, and consequently in relieving or slowing down the degradation process of cardiac muscle tissue in mdx mice. However, further studies are certainly needed before translating these results in DMD patients.
Main Outcomes Obtained by In Vitro Human Studies
In Table 2, we also report some evidence regarding the implication of SIRT1 pathway in human cells. Two experiments were performed in human myotubes. Cell cultures have been treated with adiponectin (ApN), a hormone with anti-inflammatory properties that has shown to have beneficial effects in mdx mice [35], and for this reason its activity has also been tested in human cells.
Lecompte et al. reported the effects of ApN following an inflammatory event in human myotubes of DMD and control patients [2]. Interestingly ApN activates the receptor AdipoR1, and consequently the AMPK-SIRT1-PGC-1α axis, leading to a cascade of biochemical events that ultimately result in the downregulation of two pro-inflammatory markers: tumor necrosis factor alpha (TNFα) and interleukin-17A (IL-17A); the upregulation of interleukin-6 (IL-6) with anti-inflammatory activity, and the upregulation of utrophin [2].
Samra et al. also elucidated the role of ApN in human myotube cells and showed that ApN treatment led to the downregulation of the nuclear factor kappa B (NF-κB), and the upregulation of utrophin [35]. To highlight the role of SIRT1 in this mechanism, they used silencing RNA encoding for AdipoR1, SIRT1 and PGC-1α and observed that the beneficial effects of ApN disappeared, thus clarifying the importance of the SIRT1-PGC-1α axis for the anti-inflammatory action of ApN [35].
Overall, these studies demonstrate the effectiveness of ApN, through AMPK-SIRT1-PGC-1α activation in reducing muscle inflammation.
4. Discussion
This systematic review includes 23 original papers, both animal (in vivo and in vitro), and in vitro human studies. The main aim of our systematic review is to assess the correlation between Duchenne muscular dystrophy and the enzyme SIRT1. We identified different studies and considered all the outcomes obtained. We found of relevant interest the information regarding the effects due to the lack of SIRT1 activity.
Fujiwara in his study found that SIRT1 knockout mice, characterized by suppressed SIRT1 expression, showed similar characteristics to dystrophic mice, such as reduced exercise capacity, muscle inflammation and muscle fragility [37]. In fact, in SIRT1 knockout mice high serum levels of some muscle injury markers such as CK and LDH, necrotic fibers and inflammatory changes were observed. Altered parameters are shown in C2C12 cell cultures of myoblasts too, where reduced SIRT1 activity led to a failure in repairing damaged cells membranes after injury, due to muscular membrane fragility caused by genetic mutations of the dystroglycan complex [37]. Conversely, by activating the PGC-1α/SIRT1 pathway, several beneficial effects have been observed. In fact, SIRT1 overexpression in mdx muscles leads to a fiber shift from fast to slow twitch, and fibers with a smaller size, which results in an increase in oxidative fibers [39]. The expression levels of different fibers type markers were evaluated, such as troponin slow, a marker of oxidative fibers, which is increased by SIRT1 overexpression. SIRT1 overexpression also affects the expression of myosin heavy chain isoforms MHC-I and MHC-2 (markers of oxidative fibers too) that seem to be increased, while the expression of MHC-2B isoform (marker of fast-twitch fibers) is reduced [39]. These findings are correlated with an increased expression of PGC-1α, which in turn is positively activated by SIRT1 deacetylation. PGC-1α overexpression or activation promotes mitochondrial biogenesis and oxidative fibers as well. Overall, these conditions reverse the characteristics of dystrophic muscles, ameliorating the disease and improving muscle function [39].
The active form of SIRT1 is the phosphorylated one [38] and its activation is determined by various circumstances. For this reason, we reviewed different papers that showed the effect of some exogenous agents that can enhance the expression/activity of SIRT1, improving DMD pathology. Resveratrol, a natural compound with antioxidant properties [40], is the main activator used in various studies and its excellent efficacy has been observed in the skeletal muscles, in the cardiac tissue, and at a respiratory level as well.
In skeletal muscles, the treatment of mdx mice with resveratrol leads to a reduction in superoxide anion production, creatine kinase plasma level and lactate dehydrogenase plasma level [8,45], all parameters of muscle inflammation. Its long-term dietary intake helps in the reduction in myofiber wasting and in the enhancement of muscular maturation [45]. Muscle mass and its composition are also improved: myofiber loss is reduced [43], myofibroblast cells are reduced [43] and the expression of slow, oxidative fibers is promoted [40]. Additionally, resveratrol implementation of dystrophic animal models drastically improves muscle inflammation: immune cell infiltration was reduced, macrophage infiltration was decreased, the expression of anti-inflammatory markers such as the IL-6 gene was increased, and ROS levels were decreased, all compared to untreated dystrophic mice [41,43].
Resveratrol treatment also promotes the PGC-1α signaling enhancing phenotype in mdx mice and improves utrophin gene expression [41]. The effect of resveratrol is dose-dependent and the most effective one seems to be 100 mg/kg/day [40,41].
Since the main cause of death in patients with DMD is cardiomyopathy, we also assessed the studies that demonstrated the efficacy of activating SIRT1 in heart. Resveratrol treatment of dystrophic mice also improves the cardiomyopathy of dystrophic hearts. In fact, resveratrol treatment suppressed cardiac hypertrophy of dystrophic mice: the heart weight of treated mdx mice is decreased compared to untreated mdx mice; the heart weight/body weight of untreated mdx mice is increased compared to control, but resveratrol reverses the increase [44]. Resveratrol treatment preserves also the cardiac function demonstrated by the downregulation of p300 protein levels in treated mdx mice [44] and by the increase in the mitophagy process that promotes the damaged mitochondrial deletion [46].
Similar evidence was observed in cultured cells of C2C12 myoblast cells, where resveratrol treatment decreased ROS levels and fibronectin synthesis [33], and promoted mitophagy [51]; and in cardiomyocytes where it downregulated p300 activity and promoted FoxO3a activity in the nucleus of cells leading overall to a decreased ROS accumulation in heart [46].
However, interesting results were also observed with quercetin, another natural SIRT1 activator. In quercetin-treated mdx mice, physical activity is increased compared to the untreated ones and they show a better muscle profile with an increase in muscle fibers, a reduction in fibers with centralized nuclei, an increased muscle mass, a reduction in immune cell infiltration, and a decrease in fibrotic area [51]. Muscle function is also affected by quercetin treatment: loss of specific tension and fatigue resistance were prevented [51].
Furthermore, quercetin treatment results in a reduction in the pathological remodeling of dystrophic hearts. The heart weight of untreated dystrophic mice is increased compared to control, but quercetin lowers the value [52]. The dietary intake of quercetin also lead to a reduction in the amount of fibronectin in mdx mice compared to the untreated ones [34], to a decrease in inflammatory marker levels, and to an increase in the protein level of PGC-1α, suggesting an improved mitochondrial biogenesis and reduced cardiac inflammation and damage [34,52,54].The positive effects of quercetin were observed also in ameliorating respiratory function by increasing muscle fibers, reducing fibrotic area and decreasing immune cell infiltration in diaphragm [50]. However, these effects seemed to be transient, so further examinations are needed [47]. In fact, respiratory frequency was increased compared to untreated mdx mice and was similar to control mice for the first 6–8 months of age, but beyond the 8th month there was not any improvement. Other parameters of respiratory function are also affected by quercetin treatment, such as tidal volume, minute ventilation, peak inspiratory flow, and peak expiratory flow, which are increased compared to untreated mdx mice [47].
The beneficial effects deriving from the activation of SIRT1 in animal models of DMD prompted the authors to study the effects that this activation may have on human cells too. We collected two studies performed on human myotubes and the cell cultures were treated with adiponectin, a hormone with anti-inflammatory properties [35]. In dystrophic myotubes ApN levels are decreased and its complementation leads to a downregulation of the pro-inflammatory marker TNFα, to an upregulation of the anti-inflammatory IL-6, and to an upregulation of utrophin A, a dystrophin analogue [2,35]. These positive effects of ApN are abrogated by siRNA silencing of genes encoding for AdipoR1, SIRT1, or PGC-1α suggesting that SIRT1/PGC-1α axis plays a crucial role in the anti-inflammatory activity of ApN [35].
Although further research is needed to clarify the molecular mechanisms underlying the protective role of SIRT1 in DMD, we propose SIRT1 as a novel hypothetical therapeutic target for patients with muscular dystrophies.
5. Conclusions
Muscular dystrophies are hereditary muscular disorders characterized by a progressive loss of muscle composition and function. Duchenne muscular dystrophy is one of the most common and severe forms, and is caused by a mutation in the gene encoding for dystrophin protein. Dystrophin deficiency leads to an alteration of the dystrophin glycoprotein complex resulting in a multitude of molecular alteration that ultimately lead to muscle degeneration and myofiber necrosis [1,2,3]. To date, the only effective therapy is represented by glucocorticoids administrations, which on the other hand are associated with multiple side effects. Therefore, the identification of new pharmacological targets represents an excellent prospect of current interest.
SIRT1, a NAD+-dependent histone/protein deacetylase, aroused considerable interest in us due its role in different cellular processes, including stress resistance, inflammatory processes and cell survival. SIRT1 is expressed in different tissues, and we focus on its role in skeletal muscle since experimental studies have shown that SIRT1 activation provides beneficial effects in the dystrophin-deficient mdx mouse. SIRT1 promotes the deacetylation, and consequently the activation of PGC-1α, which in its active form controls mitochondrial biogenesis and homeostasis through mitophagy and autophagy processes, improves muscle resistance, enhances muscle fiber-type switching, and decreases the process of muscle wasting due to its ROS scavenging activity and due to its role in promoting pro-inflammatory markers level.
However, the main causes of death of DMD patients are cardiac and respiratory arrest, and we found the role of SIRT1 also interesting in cardiac and respiratory muscle tissue. At the cardiac level, the activation of the SIRT1-PGC-1α axis inhibits cardiac hypertrophy, characteristic of dystrophic models, and improves cardiac function.
Respiratory function is also enhanced by SIRT1 activation, but in this case, the effect is transient, so further studies are needed to elucidate its role in respiratory muscles.
In fact, from the experimental studies collected in the literature, the techniques used to implement SIRT1 activity were the generation of transgenic mice which show SIRT1 overexpression, or the use of specific SIRT1 activators such as resveratrol (especially effective at the dose of 100 mg/kg/day) and quercetin.
The beneficial effects deriving from SIRT1 activation have been found in human cells too. In this case, adiponectin, a hormone with anti-inflammatory properties, was performed to increase SIRT1 activity, leading to downregulation of the pro-inflammatory marker TNFα, to an upregulation of the anti-inflammatory IL-6 and to an upregulation of utrophin A, a dystrophin analogue [35]. These findings lead the research to move further by using SIRT1 as a molecular target in DMD.
However, all these findings taken together are only the beginning of a major study necessary to prove the real efficacy of SIRT1 as a new pharmacological target in the prevention of muscle degeneration in DMD patients, but they can still be considered a good starting point.
Author Contributions
Conceptualization, M.H., B.Z., E.D. and E.P.; methodology, M.H., B.Z., E.D. and E.P.; software, E.D., M.H., B.Z. and E.P.; validation, M.H., B.Z., E.D. and E.P.; formal analysis, E.D., M.H., B.Z. and E.P.; investigation, E.D., M.H., B.Z., E.D. and E.P.; resources, E.D., M.H., B.Z. and E.P.; data curation, M.H., B.Z., E.D. and E.P.; writing—original draft preparation, E.D., M.H., B.Z. and E.P.; writing—review and editing, M.H., B.Z., E.D. and E.P.; visualization, M.H., B.Z., E.D. and E.P.; supervision, M.H. and B.Z.; project administration, M.H. and B.Z. All authors have read and agreed to the published version of the manuscript.
Funding
Catholic University Our Lady of Good Counsel, Department for Chemical-Toxicological and Pharmacological Evaluation of Drugs, Rruga Dritan Hoxha, Tirana 1000, Albania.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article, and in the reference section.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hoxha, M. Duchenne Muscular Dystrophy: Focus on Arachidonic Acid Metabolites. Biomed. Pharmacother. 2019, 110, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Lecompte, S.; Abou-Samra, M.; Boursereau, R.; Noel, L.; Brichard, S.M. Skeletal Muscle Secretome in Duchenne Muscular Dystrophy:A Pivotal Anti-Inflammatory Role of Adiponectin. Cell. Mol. Life Sci. 2017, 74, 2487–2501. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Guzman, O.R.; Rodriguez-Cruz, M.; Escobar Cedillo, R.E. Systemic Inflammation in Duchenne Muscular Dystrophy: Association with Muscle Function and Nutritional status. Biomed. Res. Int. 2015, 2015, 891972. [Google Scholar] [CrossRef] [PubMed]
- Deconinnck, N.; Dan, B. Pathophysiology of Duchenne Muscular Dystrophy: Current Hypotheses. Pediatr. Neurol. 2007, 36, 1–7. [Google Scholar] [CrossRef]
- Kuno, A.; Horio, Y. Review Article SIRT1: A Novel Target for the Treatment of Muscular Dystrophies. Oxidative Med. Cell. Longev. 2016, 2016, 6714686. [Google Scholar] [CrossRef]
- Ljubiic, V.; Burt, M.; Jasmin, B.J. The Therapeutic Potential of Skeletal Muscle Plasticity in Duchenne Muscular Dystrophy: Phenotypic Modifiers as Pharmacologic Targets. FASEB J. 2014, 28, 548–568. [Google Scholar] [CrossRef]
- Bushby, K.; Finkel, R.; Birnkrant, D.J.; Case, L.E.; Clemens, P.R.; Cripe, L.; Kaul, A.; Kinnett, K.; McDonald, C.; Pandya, S.; et al. Diagnosis and Management of Duchenne Muscular Dystrophy, Part1: Diagnosis, and Pharmacological and Psychosocial Management. Lancet Neurol. 2010, 9, 77–93. [Google Scholar] [CrossRef]
- Capogrosso, R.F.; Cozzoli, A.; Mantuanoa, P.; Camerino, G.M.; Massari, A.M.; Sblendorio, V.T.; deBellis, M.; Tamma, R.; Giustino, A.; Nico, B.; et al. Assessment of Resveratrol, Apocynin and Taurine on Mechanical-Metabolic Uncoupling and Oxidative Stressina Mouse Model of Duchenne Muscular Dystrophy: A Comparison with the Gold Standard—Methyl Prednisolone. Pharm. Res. 2016, 106, 101–113. [Google Scholar] [CrossRef]
- Malik, V.; Rodino-Klapac, L.R.; Mendell, J.R. Emerging Drugs for Duchenne Muscular Dystrophy. Exprt. Opin. Emerg. Drugs 2012, 17, 261–277. [Google Scholar] [CrossRef]
- DeLuca, A. Pre-Clinical Drug Testsin the Mdx Mouseasa Model of Dystrophinopathies: An Overview. Acta Myol. 2012, 31, 40–47. [Google Scholar]
- Tidball, J.G.; Wehling-Henricks, M. Damage and Inflammation in Muscular Dystrophy: Potential Implications and Relationships with Autoimmune Myositis. Curr. Opin. Rheumatol. 2005, 17, 707. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Kumar, A. Therapeutic Targeting of Signaling Pathways in Muscular Dystrophy. J. Mol. Med. 2010, 88, 155. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule Activators of Sirtuins Extend Saccharomyces Cerevisiae Lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Chang, H.C.; Guarente, L. SIRT1 and Other Sirtuinsin Metabolism. Trends Endocrinol. Metab. 2014, 25, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Wierman, M.B.; Smith, J.S. Yeast Sirtuins and the Regulation of Aging. FEMS Yeast Res. 2013, 25, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Houtkooper, R.H.; Pirinen, E.; Auwerx, J. Sirtuins as Regulators of Metabolism and Healthspan. Nat. Rev. Mol. Cell Biol. 2012, 13, 225–238. [Google Scholar] [CrossRef]
- Baur, J.A.; Ungvari, Z.; Minor, R.K.; Le Couteur, D.G.; deCabo, R. Are Sirtuins Viable Targets for Improving Healthspan and Lifespan? Nat. Rev. Drug Discov. 2012, 11, 443–461. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by ActivatingSIRT1and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Granchi, C.; Minutolo, F. Activators of Sirtuin-1and their Involvmentin Cardioprotection. Med. Chem. 2018, in press. [Google Scholar]
- Rahman, S.; Islam, R. Mammalian Sirt1: Insights on Ist Biological Functions. Cell Comm. Signal. 2011, 9, 11. [Google Scholar] [CrossRef]
- North, B.; Verdin, E. Sirtuins: Sir2-Related NAD-Dependent Protein Deacetylases. Genome Biol. 2004, 5, 224. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.D.; Yang, D.I.; Lin, T.K.; Shaw, F.Z.; Liou, C.W.; Chuang, Y.C. Roles of Oxidative Stress, Apoptosis, PGC-1a and Mitochondrial Biogenesis in Cerebral Ischemia. Int. J. Mol. Sci. 2011, 12, 7199–7215. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wu, H.; Tarr, P.T.; Zhang, C.Y.; Wu, Z.; Boss, O. Transcriptional Co-Activator PGC-1a Drives the Formation of Slow-Twitch Muscle Fibres. Nature 2002, 418, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Canto, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK Regulates Energy Expenditure by Modulating NAD+Metabolism and SIRT1Activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Canto, C.; Jiang, L.Q.; Deshmukh, A.S.; Mataki, C.; Coste, A.; Lagouge, M.; Zierath, J.R.; Auwerx, J. Interdependence of AMPK and SIRT1 for Metabolic Adaptation to Fasting and Exercise in Skeletal Muscle. Cell Metab. 2010, 11, 213–219. [Google Scholar] [CrossRef]
- Gerhart-Hines, Z.; Rodgers, J.T.; Bare, O.; Lerin, C.; Kim, S.H.; Mostoslavsky, R.; Alt, F.W.; Wu, Z.; Puigserver, P. Metabolic Control of Muscle Mitochondrial Function and Fatty Acid Oxidation through SIRT1/PGC-1alpha. EMBO J. 2007, 26, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms Controlling Mitochondrial Biogenesis and Respiration through the Thermogenic coactivator PGC-1α. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Sandri, M.; Lin, J.; Handschin, C.; Yang, W.; Arany, Z.P.; Lecker, S.H.; Goldberg, A.L.; Spiegelman, B.M. PGC-1alpha Protects Skeletal Muscle from Atrophy by Suppressing Foxo3ActionandAtrophyspecificGeneTranscription. Proc. Natl. Acad. Sci. USA 2006, 103, 16260–16265. [Google Scholar] [CrossRef]
- Wenz, T.; Rossi, S.G.; Rotundo, R.L.; Spiegelman, B.M.; Moraes, C.T. Increased Muscle PGC-1alpha Expression Protects from Sarcopenia and Metabolic Disease during Aging. Proc. Natl. Acad. Sci. USA 2009, 106, 20405–20410. [Google Scholar] [CrossRef]
- Handschin, C.; Kobayashi, Y.M.; Chin, S.; Seale, P.; Campbell, K.P.; Spiegelman, B.M. PGC-1alpha Regulates the Neuromuscular Junction Program and Ameliorates Duchenne Muscular Dystrophy. Genes Dev. 2007, 21, 770–783. [Google Scholar] [CrossRef]
- Boots, A.W.; Wilms, L.C.; Swennen, E.L.; Kleinjans, J.C.; Bast, A.; Haenen, G.R. In Vitro and Ex Vivo Anti-Inflammatory Activity of Quercetin in Healthy volunteers. Nutrition 2008, 24, 703–710. [Google Scholar] [CrossRef]
- Han, J.J.; Hao, J.; Kim, C.H.; Hong, J.S.; Ahn, H.Y.; Lee, Y.S. Quercetin Prevents cardiac Hypertrophy Induced by Pressure Overloadin Rats. J. Vet. Med. Sci. 2009, 71, 737–743. [Google Scholar] [CrossRef]
- Vásquez-Garzón, V.R.; Arellanes-Robledo, J.; García-Román, R.; Aparicio-Rautista, D.I.; Villa-Treviño, S. Inhibition of Reactive Oxygen Species and Pre-Neoplastic Lesions by Quercetin through an Antioxidant defense Mechanism. Free Radic. Res. 2009, 43, 128–137. [Google Scholar] [CrossRef]
- Ballmann, C.; Denney, T.S.; Beyers, R.J.; Quindry, T.; Romero, M.; Amin, R.; Selsby, J.T.; Quindry, J.C. Lifelong Quercetin Enrichment and Cardioprotection in Mdx/Utrn+/−Mice. J. Physiol. Heart Circ. Physiol. 2017, 312, H128–H140. [Google Scholar] [CrossRef] [PubMed]
- Abou-Samra, M.; Lecompte, S.; Schakman, O.; Noel, L.; Many, M.C.; Gailly, P.; Brichard, S.M. Involvement of Adiponectin in the Pathogenesis of Dystrophinopathy. Skelet. Muscle 2015, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies that Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, D.; Iwahara, N.; Sebori, R.; Hosoda, R.; Shimohama, S.; Kuno, A.; Horio, Y. SIRT1 Deficiency Interferes with Membrane Resealing after Cell Membrane Injury. PLoS ONE 2019, 14, e0218329. [Google Scholar] [CrossRef]
- Hulmi, J.J.; Hentilä, J.; De Ruisseau, K.C.; Oliveira, B.M.; Papaioannou, K.G.; Autio, R.; Kujala, U.M.; Ritvos, O.; Kainulainen, H.; Korkmaz, A.; et al. Effects of Muscular Dystrophy, Exercise and Blocking Activin Receptor IIB Ligand so the Unfolded Protein Response and Oxidative Stress. Free Radic. Biol. Med. 2016, 99, 308–322. [Google Scholar] [CrossRef]
- Chalkiadaki, A.; Igarashi, M.; Nasamu, A.S.; Knezevic, J.; Guarante, L. Muscle-Specific SIRT1 Gain-of-Function Increases Slow Twitch Fibers and Ameliorates Pathophysiology in a Mouse Model of Duchenne Muscular Dystrophy. PLoS Genet. 2014, 10, e1004490. [Google Scholar] [CrossRef] [PubMed]
- Ljubicic, V.; Burt, M.; Lunde, J.A.; Jasmin, B.J. Resveratrol Induces Expression of the Slow, Oxidative Phenotype in Mdx Mouse Muscle Together with Enhanced Activity of the SIRT1-PGC-1Axis. Am. J. Physiol. Cell Physiol. 2014, 307, C66–C82. [Google Scholar] [CrossRef]
- Gordon, B.S.; Delgado Diaz, D.C.; Kostek, M.C. Resveratrol Decreases Inflammation and Increases Utrophin Gene Expression in the Mdx Mouse Model of Duchenne Muscular Dystrophy. Clin. Nutr. 2013, 32, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Kuno, A.; Tanno, M.; Horio, Y. The Effects of Resveratrol and SIRT1Activation on Dystrophic Cardiomyopathy. Ann. N. Y. Acad. Sci. 2015, 1348, 46–54. [Google Scholar] [CrossRef]
- Hori, Y.S.; Kuno, A.; Hosoda, R.; Tanno, M.; Miura, T.; Shimamoto, K.; Horio, Y. Resveratrol Ameliorates Muscular Pathology in the Dystrophic Mdx Mouse, a Model for Duchenne Muscular Dystrophy. J. Pharm. Exp. 2011, 338, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Kuno, A.; Hori, Y.S.; Hosoda, R.; Tanno, M.; Miura, T.; Shimamoto, K.; Horio, Y. Resveratrol Improves Cardiomyopathy in Dystrophin-deficient Mice through SIRT1 Protein-mediated Modulation of p300Protein. J. Biol. Chem. 2013, 288, 5963–5972. [Google Scholar] [CrossRef] [PubMed]
- Sebori, R.; Kuno, A.; Hosoda, R.; Hayashi, T.; Horio, Y. Resveratrol Decreases Oxidative Stress by Restoring Mitophagy and Improves the Pathophysiology of Dystrophin-Deficient mdx Mice. Oxidative Med. Cell. Longev. 2018, 29, 9179270. [Google Scholar] [CrossRef]
- Kuno, A.; Hosoda, R.; Sebori, R.; Hayashi, T.; Sakuragi, T.; Tanabe, M.; Horio, Y. Resveratrol Ameliorates Mitophagy Disturbance and Improves Cardiac Pathophysiology of Dystrophin deficient mdx Mice. Sci. Rep. 2018, 8, 15555. [Google Scholar] [CrossRef]
- Selsby, J.T.; Ballmann, C.G.; Spaulding, H.R.; Ross, J.V.; Quindry, J.C. Oral Quercetin Administration Transiently Protects Respiratory Function in Dystrophin-Deficient Mice. J. Physiol. 2016, 594, 6037–6053. [Google Scholar] [CrossRef] [PubMed]
- Camerino, G.M.; Cannone, M.; Giustino, A.; Massari, A.M.; Capogrosso, R.F.; Cozzoli, A.; de Luca, A. Gene Expressionin Mdx Mouse Muscle in Relation to Age and Exercise: Aberrant Mechanical–Metabolic Coupling and Implications for Pre-Clinical Studies in Duchenne Muscular Dystrophy. Hum. Mol. Genet. 2014, 21, 5720–5732. [Google Scholar] [CrossRef] [PubMed]
- Ljubicic, V.; Khogali, S.; Renaud, J.M.; Jasmin, B.J. Chronic AMPK Stimulation Attenuates Adaptive Signaling in Dystrophic Skeletal Muscle. Am. J. Physiol. Cell Physiol. 2012, 302, C110–C121. [Google Scholar] [CrossRef]
- Hollinger, K.; Shanely, R.A.; Quindry, J.C.; Selsby, J.T. Long-Term Quercetin Dietary Enrichment Decreases Muscle Injury in Mdx Mice. Clin. Nutr. 2015, 34, 515–522. [Google Scholar] [CrossRef]
- Spaulding, H.R.; Ballmann, C.G.; Quindry, J.C.; Selsby, J.T. Long-Term Quercetin Dietary Enrichment Partially Protects Dystrophic Skeletal Muscle. PLoS ONE 2016, 11, e0168293. [Google Scholar] [CrossRef] [PubMed]
- Ballmann, C.; Denney, T.; Beyers, R.J.; Quindry, T.; Romero, M.; Selsby, J.T.; Quindry, J.C. Long Term Dietary Quercetin Enrichment as a Cardioprotective Counter measure in Mdx Mice. Exp. Physiol. 2017, 102, 635–649. [Google Scholar] [CrossRef]
- Gordon, B.S.; Delgado-Diaz, D.C.; Carson, J.; Fayad, R.; Wilson, L.B.; Kostek, M.C. Resveratrol Improves Muscle Function but Not Oxidative Capacity in Young Mdx Mice. Can. J. Physiol. Pharm. 2014, 92, 243–251. [Google Scholar] [CrossRef]
- Ballmann, C.; Hollinger, K.; Selsby, J.T.; Amin, R.; Quindry, J.C. Histological and Biochemical Outcomes of Cardiac Pathologyinmdx Mice with Dietary Quercetin Enrichment. Exp. Physiol. 2015, 100, 12–22. [Google Scholar] [CrossRef]
- Selsby, J.T.; Morine, K.J.; Pendrak, K.; Barton, E.R.; Sweeney, H.L. Rescue of Dystrophic Skeletal MusclebyPGC-1aInvolves a Fast to Slow Fiber Type Shift in the mdx Mouse. PLoS ONE 2012, 7, e30063. [Google Scholar] [CrossRef] [PubMed]
- Morselli, E.; Mariño, G.; Bennetzen, M.V. Spermidine and Resveratrol Induce Autophagy by Distinct Pathways Convergingonthe Acetylproteome. J. Cell Biol. 2011, 192, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Breuss, J.M.; Atanasov, A.G.; Uhrin, P. Resveratrol and Its Effects on the Vascular System. Int. J. Mol. Sci. 2019, 20, 1523. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).