Chronic–Progressive Dopaminergic Deficiency Does Not Induce Midbrain Neurogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Immunohistochemistry
2.3. Quantitative Histology and Statistics
3. Results
3.1. Degree of Dopaminergic Deficiency
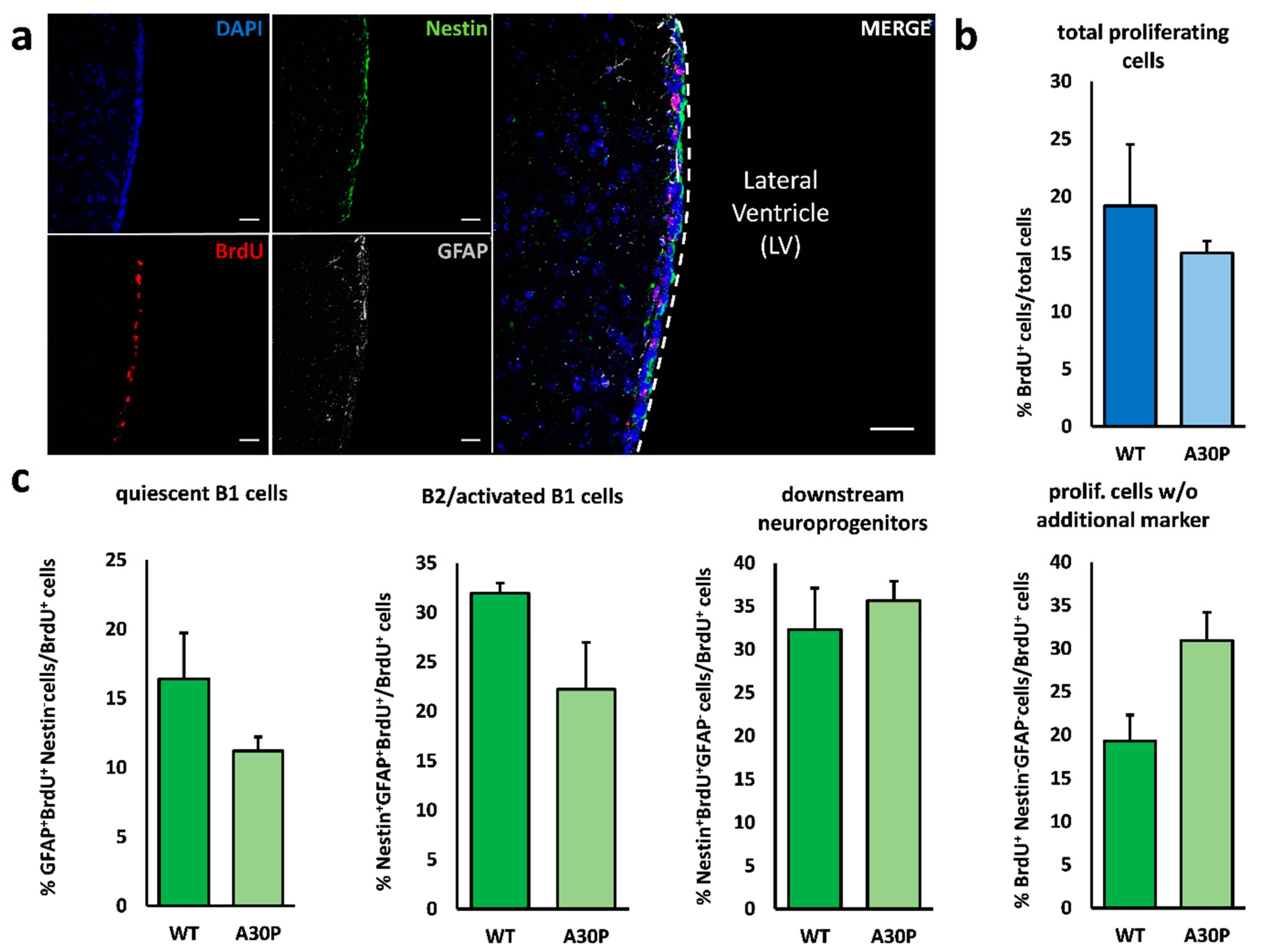
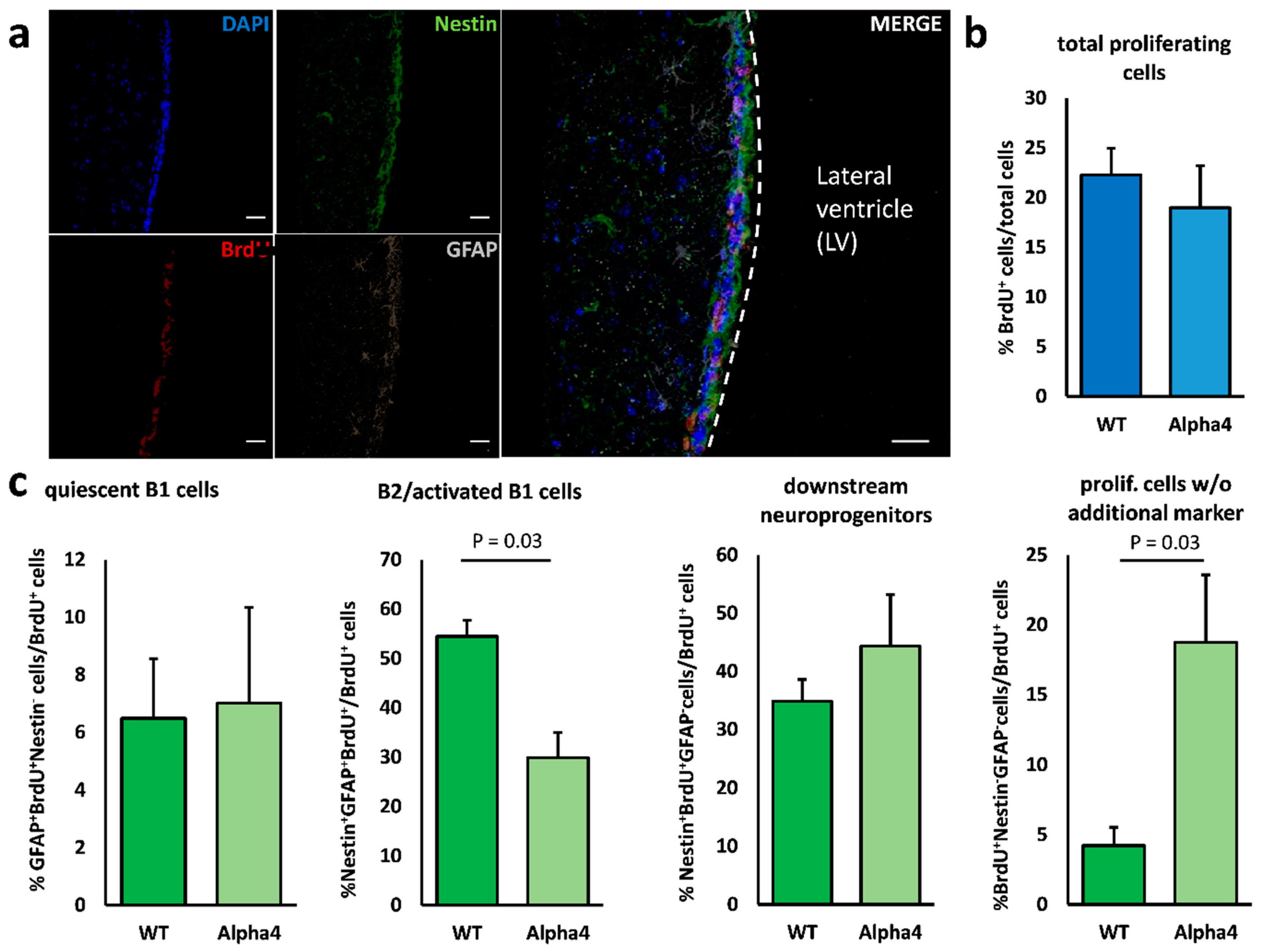
3.2. Minor Alterations in V–SVZ Neurogenesis
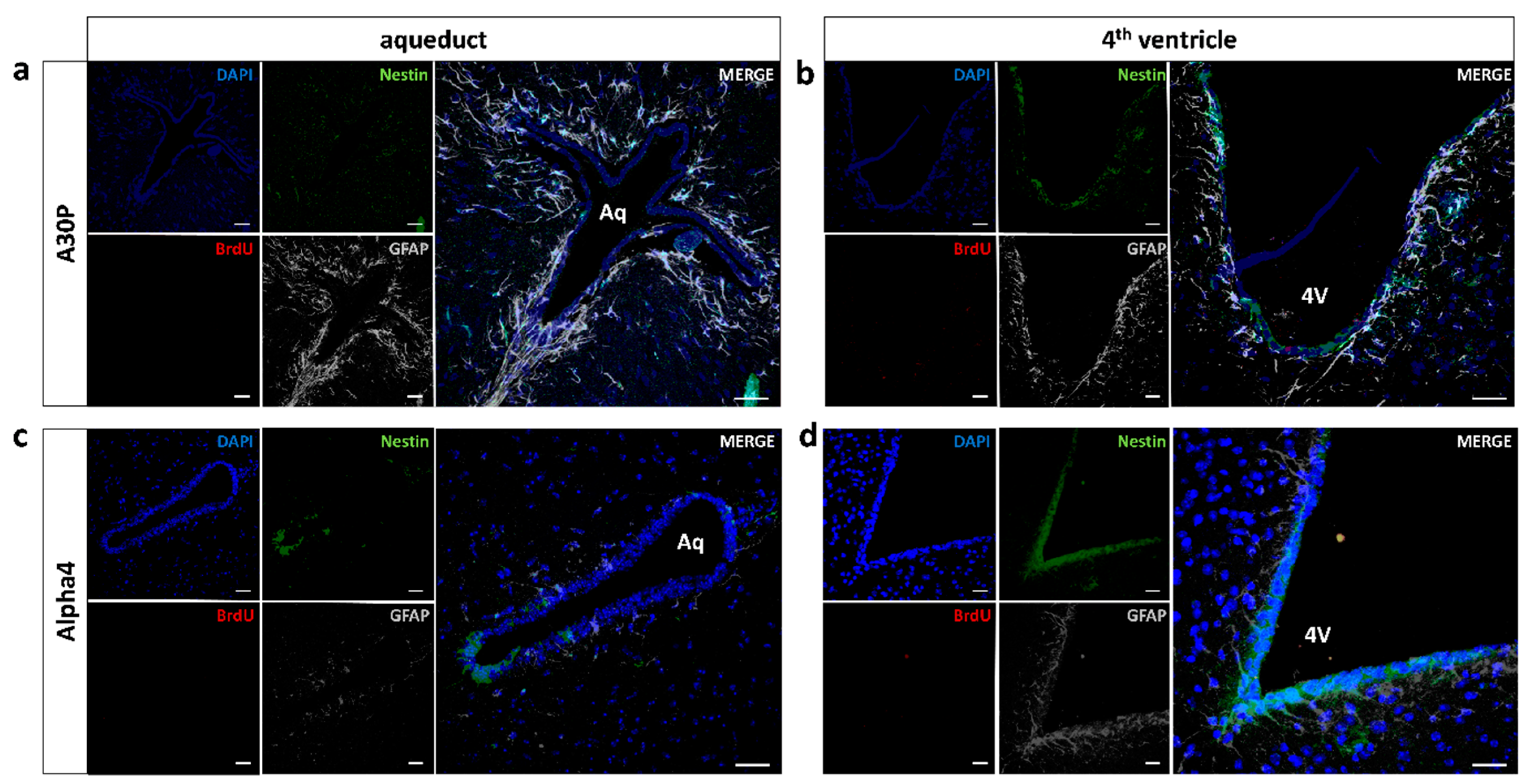
3.3. No Induction of Mid/Hindbrain Neurogenic Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erkkinen, M.G.; Kim, M.O.; Geschwind, M.D. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb. Perspect Biol. 2018, 10. [Google Scholar] [CrossRef]
- Lang, A.E.; Espay, A.J. Disease Modification in Parkinson’s Disease: Current Approaches, Challenges, and Future Considerations. Mov. Disord. 2018, 33, 660–677. [Google Scholar] [CrossRef]
- Liu-Seifert, H.; Schumi, J.; Miao, X.; Tian, Y.; Rabbia, M.; Andersen, S.W.; Wilson, S.; Li, W.; Entsuah, R. Disease Modification in Alzheimer’s Disease: Current Thinking. Ther. Innov. Regul. Sci. 2020, 54, 396–403. [Google Scholar] [CrossRef]
- Luskin, M.B. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron 1993, 11, 173–189. [Google Scholar] [CrossRef]
- Palmer, T.D.; Takahashi, J.; Gage, F.H. The adult rat hippocampus contains primordial neural stem cells. Mol. Cell Neurosci. 1997, 8, 389–404. [Google Scholar] [CrossRef]
- Markakis, E.A.; Palmer, T.D.; Randolph-Moore, L.; Rakic, P.; Gage, F.H. Novel neuronal phenotypes from neural progenitor cells. J. Neurosci. 2004, 24, 2886–2897. [Google Scholar] [CrossRef]
- Kokoeva, M.V.; Yin, H.; Flier, J.S. Neurogenesis in the hypothalamus of adult mice: Potential role in energy balance. Science 2005, 310, 679–683. [Google Scholar] [CrossRef]
- Hermann, A.; Storch, A. Endogenous regeneration in Parkinson’s disease: Do we need orthotopic dopaminergic neurogenesis? Stem Cells 2008, 26, 2749–2752. [Google Scholar] [CrossRef]
- van den Berge, S.A.; van Strien, M.E.; Hol, E.M. Resident adult neural stem cells in Parkinson’s disease—The brain’s own repair system? Eur. J. Pharmacol. 2013, 719, 117–127. [Google Scholar] [CrossRef]
- Doetsch, F. The glial identity of neural stem cells. Nat. Neurosci. 2003, 6, 1127–1134. [Google Scholar] [CrossRef]
- Doetsch, F.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 1997, 17, 5046–5061. [Google Scholar] [CrossRef] [PubMed]
- Winner, B.; Desplats, P.; Hagl, C.; Klucken, J.; Aigner, R.; Ploetz, S.; Laemke, J.; Karl, A.; Aigner, L.; Masliah, E.; et al. Dopamine receptor activation promotes adult neurogenesis in an acute Parkinson model. Exp. Neurol. 2009, 219, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Hoglinger, G.U.; Rizk, P.; Muriel, M.P.; Duyckaerts, C.; Oertel, W.H.; Caille, I.; Hirsch, E.C. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat. Neurosci. 2004, 7, 726–735. [Google Scholar] [CrossRef] [PubMed]
- van den Berge, S.A.; van Strien, M.E.; Korecka, J.A.; Dijkstra, A.A.; Sluijs, J.A.; Kooijman, L.; Eggers, R.; De Filippis, L.; Vescovi, A.L.; Verhaagen, J.; et al. The proliferative capacity of the subventricular zone is maintained in the parkinsonian brain. Brain A J. Neurol. 2011, 134, 3249–3263. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Momma, S.; Delfani, K.; Carlen, M.; Cassidy, R.M.; Johansson, C.B.; Brismar, H.; Shupliakov, O.; Frisen, J.; Janson, A.M. Evidence for neurogenesis in the adult mammalian substantia nigra. Proc. Natl. Acad. Sci. USA 2003, 100, 7925–7930. [Google Scholar] [CrossRef] [PubMed]
- Hermann, A.; Suess, C.; Fauser, M.; Kanzler, S.; Witt, M.; Fabel, K.; Schwarz, J.; Höglinger, G.U.; Storch, A. Rostro-caudal gradual loss of cellular diversity within the periventricular regions of the ventricular system. Stem Cells 2009, 27, 928–941. [Google Scholar] [CrossRef]
- Weselek, G.; Keiner, S.; Fauser, M.; Wagenführ, L.; Müller, J.; Kaltschmidt, B.; Brandt, M.D.; Gerlach, M.; Redecker, C.; Hermann, A.; et al. Norepinephrine is a negative regulator of the adult periventricular neural stem cell niche. Stem Cells 2020. [Google Scholar] [CrossRef]
- Frielingsdorf, H.; Schwarz, K.; Brundin, P.; Mohapel, P. No evidence for new dopaminergic neurons in the adult mammalian substantia nigra. Proc. Natl. Acad. Sci. USA 2004, 101, 10177–10182. [Google Scholar] [CrossRef]
- Lie, D.C.; Dziewczapolski, G.; Willhoite, A.R.; Kaspar, B.K.; Shults, C.W.; Gage, F.H. The adult substantia nigra contains progenitor cells with neurogenic potential. J. Neurosci. 2002, 22, 6639–6649. [Google Scholar] [CrossRef]
- Van Kampen, J.M.; Robertson, H.A. A possible role for dopamine D3 receptor stimulation in the induction of neurogenesis in the adult rat substantia nigra. Neuroscience 2005, 136, 381–386. [Google Scholar] [CrossRef]
- Van Kampen, J.M.; Eckman, C.B. Dopamine D3 receptor agonist delivery to a model of Parkinson’s disease restores the nigrostriatal pathway and improves locomotor behavior. J. Neurosci. 2006, 26, 7272–7280. [Google Scholar] [CrossRef]
- Shan, X.; Chi, L.; Bishop, M.; Luo, C.; Lien, L.; Zhang, Z.; Liu, R. Enhanced de novo neurogenesis and dopaminergic neurogenesis in the substantia nigra of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease-like mice. Stem Cells 2006, 24, 1280–1287. [Google Scholar] [CrossRef]
- Yoshimi, K.; Ren, Y.R.; Seki, T.; Yamada, M.; Ooizumi, H.; Onodera, M.; Saito, Y.; Murayama, S.; Okano, H.; Mizuno, Y.; et al. Possibility for neurogenesis in substantia nigra of parkinsonian brain. Ann. Neurol. 2005, 58, 31–40. [Google Scholar] [CrossRef]
- Worlitzer, M.M.; Viel, T.; Jacobs, A.H.; Schwamborn, J.C. The majority of newly generated cells in the adult mouse substantia nigra express low levels of Doublecortin, but their proliferation is unaffected by 6-OHDA-induced nigral lesion or Minocycline-mediated inhibition of neuroinflammation. Eur. J. Neurosci. 2013, 38, 2684–2692. [Google Scholar] [CrossRef]
- Aponso, P.M.; Faull, R.L.; Connor, B. Increased progenitor cell proliferation and astrogenesis in the partial progressive 6-hydroxydopamine model of Parkinson’s disease. Neuroscience 2008, 151, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Ungerstedt, U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur. J. Pharmacol. 1968, 5, 107–110. [Google Scholar] [CrossRef]
- Chiueh, C.C.; Markey, S.P.; Burns, R.S.; Johannessen, J.N.; Pert, A.; Kopin, I.J. Neurochemical and behavioral effects of systemic and intranigral administration of N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in the rat. Eur. J. Pharm. 1984, 100, 189–194. [Google Scholar] [CrossRef]
- Hisahara, S.; Shimohama, S. Toxin-induced and genetic animal models of Parkinson’s disease. Parkinsons Dis. 2010, 2011, 951709. [Google Scholar] [CrossRef]
- Kahle, P.J.; Neumann, M.; Ozmen, L.; Muller, V.; Jacobsen, H.; Schindzielorz, A.; Okochi, M.; Leimer, U.; van Der Putten, H.; Probst, A.; et al. Subcellular localization of wild-type and Parkinson’s disease-associated mutant alpha-synuclein in human and transgenic mouse brain. J. Neurosci. 2000, 20, 6365–6373. [Google Scholar] [CrossRef]
- Marxreiter, F.; Nuber, S.; Kandasamy, M.; Klucken, J.; Aigner, R.; Burgmayer, R.; Couillard-Despres, S.; Riess, O.; Winkler, J.; Winner, B. Changes in adult olfactory bulb neurogenesis in mice expressing the A30P mutant form of alpha-synuclein. Eur. J. Neurosci. 2009, 29, 879–890. [Google Scholar] [CrossRef]
- Labarca, C.; Schwarz, J.; Deshpande, P.; Schwarz, S.; Nowak, M.W.; Fonck, C.; Nashmi, R.; Kofuji, P.; Dang, H.; Shi, W.; et al. Point mutant mice with hypersensitive alpha 4 nicotinic receptors show dopaminergic deficits and increased anxiety. Proc. Natl. Acad. Sci. USA 2001, 98, 2786–2791. [Google Scholar] [CrossRef] [PubMed]
- Orb, S.; Wieacker, J.; Labarca, C.; Fonck, C.; Lester, H.A.; Schwarz, J. Knockin mice with Leu9’Ser alpha4-nicotinic receptors: Substantia nigra dopaminergic neurons are hypersensitive to agonist and lost postnatally. Physiol. Genom. 2004, 18, 299–307. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schwarz, J.; Schwarz, S.C.; Dorigo, O.; Stützer, A.; Wegner, F.; Labarca, C.; Deshpande, P.; Gil, J.S.; Berk, A.J.; Lester, H.A. Enhanced expression of hypersensitive alpha4* nAChR in adult mice increases the loss of midbrain dopaminergic neurons. FASEB J. 2006, 20, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Filali, M.; Lalonde, R. Neurobehavioral Anomalies in the Pitx3/ak Murine Model of Parkinson’s Disease and MPTP. Behav. Genet 2016, 46, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Golmohammadi, M.G.; Blackmore, D.G.; Large, B.; Azari, H.; Esfandiary, E.; Paxinos, G.; Franklin, K.B.; Reynolds, B.A.; Rietze, R.L. Comparative analysis of the frequency and distribution of stem and progenitor cells in the adult mouse brain. Stem Cells 2008, 26, 979–987. [Google Scholar] [CrossRef]
- Weiss, S.; Dunne, C.; Hewson, J.; Wohl, C.; Wheatley, M.; Peterson, A.C.; Reynolds, B.A. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J. Neurosci. 1996, 16, 7599–7609. [Google Scholar] [CrossRef]
- Hermann, A.; Maisel, M.; Wegner, F.; Liebau, S.; Kim, D.W.; Gerlach, M.; Schwarz, J.; Kim, K.S.; Storch, A. Multipotent neural stem cells from the adult tegmentum with dopaminergic potential develop essential properties of functional neurons. Stem Cells 2006, 24, 949–964. [Google Scholar] [CrossRef]
- Ekmark-Lewén, S.; Lindström, V.; Gumucio, A.; Ihse, E.; Behere, A.; Kahle, P.J.; Nordström, E.; Eriksson, M.; Erlandsson, A.; Bergström, J.; et al. Early fine motor impairment and behavioral dysfunction in (Thy-1)-h[A30P] alpha-synuclein mice. Brain Behav. 2018, 8, e00915. [Google Scholar] [CrossRef]
- Neumann, M.; Kahle, P.J.; Giasson, B.I.; Ozmen, L.; Borroni, E.; Spooren, W.; Müller, V.; Odoy, S.; Fujiwara, H.; Hasegawa, M.; et al. Misfolded proteinase K-resistant hyperphosphorylated alpha-synuclein in aged transgenic mice with locomotor deterioration and in human alpha-synucleinopathies. J. Clin. Investig. 2002, 110, 1429–1439. [Google Scholar] [CrossRef]
- Bennett, L.; Yang, M.; Enikolopov, G.; Iacovitti, L. Circumventricular organs: A novel site of neural stem cells in the adult brain. Mol Cell Neurosci 2009, 41, 337–347. [Google Scholar] [CrossRef]
- Magavi, S.S.; Leavitt, B.R.; Macklis, J.D. Induction of neurogenesis in the neocortex of adult mice. Nature 2000, 405, 951–955. [Google Scholar] [CrossRef]
- Kim, W.R.; Chun, S.K.; Kim, T.W.; Kim, H.; Ono, K.; Takebayashi, H.; Ikenaka, K.; Oppenheim, R.W.; Sun, W. Evidence for the spontaneous production but massive programmed cell death of new neurons in the subcallosal zone of the postnatal mouse brain. Eur. J. Neurosci. 2011, 33, 599–611. [Google Scholar] [CrossRef]
- Zhang, X.M.; Anwar, S.; Kim, Y.; Brown, J.; Comte, I.; Cai, H.; Cai, N.N.; Wade-Martins, R.; Szele, F.G. The A30P α-synuclein mutation decreases subventricular zone proliferation. Hum. Mol. Genet. 2019, 28, 2283–2294. [Google Scholar] [CrossRef]
- Lindquist, R.A.; Guinto, C.D.; Rodas-Rodriguez, J.L.; Fuentealba, L.C.; Tate, M.C.; Rowitch, D.H.; Alvarez-Buylla, A. Identification of proliferative progenitors associated with prominent postnatal growth of the pons. Nat. Commun. 2016, 7, 11628. [Google Scholar] [CrossRef]
- Martens, D.J.; Seaberg, R.M.; van der Kooy, D. In vivo infusions of exogenous growth factors into the fourth ventricle of the adult mouse brain increase the proliferation of neural progenitors around the fourth ventricle and the central canal of the spinal cord. Eur. J. Neurosci. 2002, 16, 1045–1057. [Google Scholar] [CrossRef]
- Fauser, M.; Weselek, G.; Hauptmann, C.; Markert, F.; Gerlach, M.; Hermann, A.; Storch, A. Catecholaminergic innervation of periventricular neurogenic regions of the developing mouse brain. Front. Neuroanat. 2020, 14, 558435. [Google Scholar] [CrossRef]
- Delaville, C.; Deurwaerdère, P.D.; Benazzouz, A. Noradrenaline and Parkinson’s disease. Front. Syst. Neurosci. 2011, 5, 31. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fauser, M.; Pan-Montojo, F.; Richter, C.; Kahle, P.J.; Schwarz, S.C.; Schwarz, J.; Storch, A.; Hermann, A. Chronic–Progressive Dopaminergic Deficiency Does Not Induce Midbrain Neurogenesis. Cells 2021, 10, 775. https://doi.org/10.3390/cells10040775
Fauser M, Pan-Montojo F, Richter C, Kahle PJ, Schwarz SC, Schwarz J, Storch A, Hermann A. Chronic–Progressive Dopaminergic Deficiency Does Not Induce Midbrain Neurogenesis. Cells. 2021; 10(4):775. https://doi.org/10.3390/cells10040775
Chicago/Turabian StyleFauser, Mareike, Francisco Pan-Montojo, Christian Richter, Philipp J. Kahle, Sigrid C. Schwarz, Johannes Schwarz, Alexander Storch, and Andreas Hermann. 2021. "Chronic–Progressive Dopaminergic Deficiency Does Not Induce Midbrain Neurogenesis" Cells 10, no. 4: 775. https://doi.org/10.3390/cells10040775
APA StyleFauser, M., Pan-Montojo, F., Richter, C., Kahle, P. J., Schwarz, S. C., Schwarz, J., Storch, A., & Hermann, A. (2021). Chronic–Progressive Dopaminergic Deficiency Does Not Induce Midbrain Neurogenesis. Cells, 10(4), 775. https://doi.org/10.3390/cells10040775






