Abstract
The kidney is an important organ for the maintenance of Ca2+ homeostasis in the body. However, disruption of Ca2+ homeostasis will cause a series of kidney diseases, such as acute kidney injury (AKI), chronic kidney disease (CKD), renal ischemia/reperfusion (I/R) injury, autosomal dominant polycystic kidney disease (ADPKD), podocytopathy, and diabetic nephropathy. During the progression of kidney disease, Ca2+ signaling plays key roles in various cell activities such as necrosis, apoptosis, eryptosis and autophagy. Importantly, there are complex Ca2+ flux networks between the endoplasmic reticulum (ER), mitochondria and lysosomes which regulate intracellular Ca2+ signaling in renal cells and contribute to kidney disease. In addition, Ca2+ signaling also links the crosstalk between various cell deaths and autophagy under the stress of heavy metals or high glucose. In this regard, we present a review of Ca2+ signaling in cell death and crosstalk with autophagy and its potential as a therapeutic target for the development of new and efficient drugs against kidney diseases.
1. Introduction
Through gradual evolutionary development, Calcium has become one of the most important metal elements in living organisms. With a wide variety of biological functions in living creatures, calcium ions (Ca2+) are involved in almost every process from birth to death [1].
Ca2+ is primarily stored in bones in the form of CaPO3 (hydroxyapatite), where it plays a structural role and also can be dissolved to serve as a source of Ca2+ in the blood [2]. In addition, Ca2+ is a ubiquitous, multifunctional signaling molecule that controls a wide variety of life processes including muscle contraction, neuronal delivery, hormone secretion, organelle communication, cell movement, fertilization, and cell growth. Because of the critical role of Ca2+ in these vital activities, cellular Ca2+ concentration is strictly regulated, and dysfunction of cellular Ca2+ homeostasis is closely associated with a great number of diseases such as heart and hepatic diseases, aging, and type 2 diabetes, as well as certain cancers [3,4,5].
The kidney is an indispensable organ for maintaining body Ca2+ homeostasis; the Ca2+ signaling system in kidney cells regulates cellular processes and decides cell fate, including cell proliferation, apoptosis, necrosis and autophagy, all of which are associated with kidney disease [6,7]. Ca2+ homeostasis has a certain influence on renal function and the occurrence and development of a series of nephropathies including acute kidney injury (AKI), chronic kidney disease (CKD), renal ischemia/reperfusion (I/R) injury, autosomal dominant polycystic kidney disease (ADPKD), podocytopathy, and diabetic nephropathy, which will be discussed in the following sections.
Based on the significance of Ca2+ signaling in the regulation of cell fate in the context of kidney disease, this review focuses on cell death determined by Ca2+ signaling in kidney cells and erythrocytes. We present a general overview of Ca2+ signaling in kidney disease, as well as some types of cell death that are regulated by Ca2+ signaling. In addition, targets of Ca2+ signaling to prevent kidney disease are discussed based on the available data.
2. Relationship between Ca2+ Signaling and Various Forms of Cell Death in Kidney Cells
Ca2+ not only regulates the normal life activities of cells and organisms, but has also been found to play an important role in the regulation of various cell deaths. Early death of necrotic cells is associated with intracellular Ca2+ overload. It has also been found that apoptosis is controlled by Ca2+ signaling, and other cell activity, including cell death of erythrocytes (eryptosis), has also been shown to be regulated by Ca2+. Moreover, Ca2+ signaling is an important mediator of autophagy, which plays dual roles in cell survival and death. The numerous Ca2+ channels on cell membranes, endoplasmic reticulum (ER), and mitochondria, as well as alterations in lysosomes, modulate cytosolic Ca2+ levels associated with cell necrosis, apoptosis, eryptosis and autophagy. We searched for original research articles from the past two decades using the keywords “Ca2+” and “kidney disease” or “kidney injury”, and “necrosis”, or “apoptosis”, or “eryptosis”, or “autophagy”, and/or “endoplasmic reticulum (ER)”, and/or “mitochondrial”, and/or “lysosome” in the PubMed database, then sorted by “best match” with the “summary” format. For review articles, we referenced the information as mentioned in the study by Aromataris et al., such as by number of databases sourced and searched, number of studies, type and country of origin of the studies included in each review, and method of synthesis/analysis employed to synthesize the evidence [8].
2.1. Ca2+ Mediates Necrosis in Kidney Injury
Necrosis is early cell death associated with intracellular Ca2+ overload, and has been found to be regulated by intracellular Ca2+ release and/or extracellular Ca2+ influx. Methylglyoxal, a physiological glucose metabolite, induces necrotic cell death in Madin-Darby canine kidney (MDCK) renal tubular cells by stimulating both extracellular Ca2+ influx and ER Ca2+ release [9]. Oxidant tert-butyl hydroperoxide (TBHP) causes ER lipid peroxidation and Ca2+ release in isolated rabbit kidney cortex microsomes, and TBHP-induced necrotic cell death is reduced by an ER Ca2+ reuptake pump inhibitor, thapsigargin [10]. Bothrops leucurus venom increases cytosolic Ca2+ in a concentration-dependent manner, participating predominantly in necrotic cell death, which contributes to the acute renal failure and nephrotoxicity in the isolated perfused kidney and cultured renal tubular epithelia [11]. This suggests that intracellular Ca2+ release mediates necrotic cell death induced by stress in kidney cells.
Transient receptor potential ankyrin 1 (TRPA1), a redox-sensing Ca2+-influx channel, is upregulated in the renal tubules of patients with acute tubular necrosis, which is significantly associated with high incidence of renal function recovery [12]. Internalization of stone-forming calcium crystals such as calcium phosphate (CaP), calcium oxalate (CaOx), and CaP + CaOx in renal tubular cells via endocytosis promotes necrosis by store-operated Ca2+ entry (SOCE), which causes prolonged Ca2+ entry and leads to a continuous rise in intracellular Ca2+ levels [13]. This shows that the stimulation of Calcium-Sensing Receptor (CaSR) by melamine causes a sustained rise in intracellular Ca2+ levels, leading to enhanced ROS production and a dose-dependent increase in apoptotic and necrotic cell death in the porcine renal proximal tubule cell line, LLC-PK1 [14]. However, the activation of CaSR by L-ornithine can protect from H2O2-induced necrosis in proximal tubular cells and reduce subsequent acute kidney injury (AKI), which is mediated by transient receptor potential canonical (TRPC)-dependent receptor-operated Ca2+ entry [15]. Due to this nephroprotective role of L-ornithine, it can be a useful treatment to reverse kidney diseases. These results suggest that the Ca2+-influx channels which mediate Ca2+ entry have dual roles in renal epithelial cells. Sustained extracellular Ca2+ entry can contribute to renal cell necrosis, while modulation of appropriate extracellular Ca2+ entry may protect against kidney injury.
In addition, Na+/Ca2+ exchange is one of the main factors in reversal of intracellular Ca2+ overload in animals with ischemia/reperfusion injury and contrast-induced acute kidney injury [16,17]. KB-R7943, an inhibitor of Na+/Ca2+ exchange, significantly attenuates renal damage and Ca2+ deposition in necrotic tubular epithelium, suggesting that KB-R7943 may be an effective therapeutic agent for acute kidney injury. The use of chemotherapy with ifosfamide is limited by the nephrotoxicity caused by its metabolite chloroacetaldehyde. Chloroacetaldehyde induces sustained elevation of intracellular free Ca2+ by inhibiting the Na+/Ca2+ exchanger, which contributes to necrotic rather than apoptotic cell death and finally leads to nephrotoxicity [18]. Both KB-R7943 and chloroacetaldehyde contribute to the inhibition of Na+-dependent extrusion of intracellular Ca2+; however, the former did not induce nephrotoxicity, which might due to different Ca2+ load levels in the kidney cells.
2.2. Ca2+ Signaling-Mediated Apoptosis Contributes to Kidney Disease
Apoptosis, or autonomous programmed cell death, is an important process for the maintenance of the stability of the internal environment, and helps an organism better adapt to its living environment. In kidney injury, apoptosis usually occurs in association with necrosis, and Ca2+ signaling acts as an important regulator of apoptosis. Ca2+ overload leads to necrotic or apoptotic death in renal ischemia–reperfusion (I/R) injury [19]. Sustained ER Ca2+ release induces ER stress and oxidative stress and leads to apoptosis in glomerular mesangial cells, which contributes to the progression of CKD [20]. CaSR is a pleiotropic receptor capable of regulating Ca2+ homeostasis, and plays important roles in kidney cells and cancers [21]. Activation of adiponectin receptors by AdipoRon and activation of CaSR by cinacalcet increases intracellular Ca2+ levels, which inhibits apoptosis in the kidneys induced by high glucose and ameliorates glomerular endothelial cell and podocyte injury in type 2 diabetes-associated diabetic nephropathy [22,23]. This suggests that regulation of cytosolic Ca2+ can control the apoptosis of kidney cells.
2.2.1. Endoplasmic Reticulum (ER) Ca2+ Signaling Mediates Apoptosis in Kidney Disease
It is well known that the ER is the largest pool of Ca2+ in cells, and it plays an important role in regulating intracellular Ca2+ balance; however, its dysregulation causes cell apoptosis. The ER Ca2+ homeostasis is mainly controlled by two Ca2+ releasing channels, inositol 1,4,5-trisphosphate receptor (IP3R) and ryanodine receptor (RyR), and one Ca2+ reuptake channel, sarco/endoplasmic reticulum Ca2+-ATPase (SERCA). Dysfunctions of these ER Ca2+ channels cause a variety of kidney diseases, including ischemia/reperfusion (I/R)-induced tubular injury, autosomal dominant polycystic kidney disease (ADPKD), podocytopathy, and diabetic nephropathy [24]. It is reported that I/R induces renal tubule apoptosis when activation of IP3R starts a cascade of Ca2+ release from the ER store [25]. In addition, inhibition of the phosphorylation of IP3R1 at S2681 increases IP3-induced Ca2+ release in cystic cells, which contributes to increased apoptosis in ADPKD [26]. Stressed podocytes in the ER undergo phosphorylation of RyR2 at S2808, causing ER Ca2+ efflux through leaky RyR2 which in turn activates cytosolic protease calpain 2, leading to podocyte injury and podocyte apoptosis [27]. The evidence implies that significant reductions in SERCA2 activity and expression in the kidney contribute to the development of diabetic nephropathy through ER stress-mediated apoptotic pathways [28]. These results suggest that Ca2+ channels in the ER play crucial roles in maintaining intracellular Ca2+ homeostasis in kidney cells, and that it is for this reason that renal cell apoptosis and kidney dysfunction contribute to the development of kidney disease.
2.2.2. ER-Mitochondrial Ca2+ Signaling Mediates Apoptosis in Kidney Disease
Mitochondria represent another intracellular Ca2+ store, and Ca2+ signaling mediates the crosstalk between the ER and the mitochondria. Ca2+ released by IP3R under ER stress is sequestered by mitochondria for mitochondrial respiration and ATP production, and for regulating cell survival. Mitochondrial release of calpain or caspase-3 promotes the change of ER Ca2+ channels, including cleavage IP3R or oxidizing RyR2. These changes may dramatically increase mitochondrial and cytosolic Ca2+ levels and augment apoptotic cell death [6]. In addition, cytochrome c released by mitochondria can bind to IP3R, thereby blocking its functional inhibition and increasing the cytoplasmic Ca2+ concentration. The sustained increase of Ca2+ level feeds back to enhance cytochrome c release and amplification of apoptotic signals [29]. ER-mitochondrial Ca2+ signaling plays a key role in the increased apoptosis found in kidney disease. In diabetic nephropathic mice the impaired activity and expression of SERCA2 causes ER Ca2+ depletion, which triggers mitochondria-mediated apoptotic pathways [28]. Downregulation of mitochondrial Ca2+ channel polycystin 2 enhances the expression of ER-mitochondrial tethering protein mitofusin 2, which increases ER-mediated mitochondrial Ca2+ transfer and apoptosis and thereby contributes to ADPKD [30]. In bilateral I/R injury kidney, ER protein sigma-1 receptor (Sig-1R) dissociates from BiP and binds to ER Ca2+ release channel IP3R3 at ER–mitochondrial contact sites, while stabilized IP3R3 prolongs Ca2+ signaling to the mitochondria and enhances apoptosis [31]. These results suggest that there is a complex regulating Ca2+ signaling between the ER and mitochondria which is associated with apoptosis and plays an important role in kidney disease.
2.3. Ca2+ Mediates Eryptosis in Kidney Injury
Eryptosis, also known as suicidal erythrocyte death, is characterized by cell shrinkage and phosphatidylserine (PS) exposure at the erythrocyte surface. Eryptotic cells are phagocytosed and thus rapidly cleared from circulating blood. Eryptosis is the key event in eliciting renal anemia associated with CKD and anemia in end stage renal disease [32]. The mycotoxin ochratoxin A, an agent responsible for endemic Balkan nephropathy, is known to trigger anemia with suicidal cell death or eryptosis of erythrocytes by Ca2+-entry and increased cytosolic Ca2+ levels [33]. In addition, the uremic toxin indoxyl sulfate also induces suicidal erythrocyte death by stimulating extracellular Ca2+ entry, which may contribute to anemia in end stage renal disease [34,35]. Vanadate VO43− intoxication in the kidney also induces eryptosis through increased cytosolic Ca2+ levels, which could contribute to the development of anemia in chronic renal failure [36]. A vitamin D-rich diet in mice can augment the stimulation of PS exposure and cell shrinkage without significantly modifying the Ca2+ concentration in freshly drawn erythrocytes, suggesting that the effect of vitamin D treatment is presumably not effective in stimulating Ca2+-entry [37]. These studies indicate that dramatic Ca2+ entry or increasing cytosolic Ca2+ levels could contribute to eryptosis, which consequently causes kidney disease.
2.4. Ca2+ Signaling Regulates Autophagy in Kidney Diseases
Macroautophagy/autophagy, an evolutionarily conserved process in eukaryotes, plays an important role in intracellular material recycling. In the process of autophagy, some damaged organelles and harmful proteins are wrapped by the autophagosomes with a double membrane structure, then sent into lysosomes or vacuoles for degradation and reuse [38]. Ca2+, as an important messenger molecule that regulates cell death, is also involved in the regulation of autophagy [39].
It has been suggested that intracellular Ca2+ signaling mediates autophagy in renal tubular cells. An in vivo study has shown that the key regulator of the autophagy pathway, mTOR, is involved in tubular repair after AKI [40]. In conditionally immortalized proximal tubular epithelial cells (ciPTEC) generated from an ADPKD1 patient, activation of CaSR increased intracellular Ca2+ release and decreased mTOR activity [41]. Increased Ca2+ influx in renal proximal tubular cells inhibits mTOR-dependent autophagy, thereby rendering cells more susceptible to death [42]. This indicates that the mTOR-dependent autophagy regulated by intracellular Ca2+ release or Ca2+ influx controls the development of kidney disease.
The canonical transient receptor potential channel 6 (TRPC6), a major Ca2+ influx channel in renal cells, plays an important role in such renal diseases as diabetic nephropathy, immune-mediated kidney disease, renal fibrosis, glomerular disease and CKD [43]. An in vitro study in renal proximal tubular cells showed that the cytoprotective role of autophagy was suppressed by TRPC6-mediated Ca2+ influx [42]. The same study showed that TRPC6 knockout promoted autophagy flux and alleviated tubular apoptosis upon renal I/R, a major cause of AKI [44]. The transient receptor potential non-selective cation channel, subfamily M, member 3 (TRPM3) is another Ca2+ channel that conducts Ca2+ flux to regulating autophagy. Increased expression of TRPM3 leads to Ca2+ influx and stimulates autophagy through the CAMKK2/AMPK/ULK1 pathway, which promotes the growth of clear cell renal cell carcinoma (ccRCC) [45]. These results suggest that Ca2+ channels on the cell membrane mediate Ca2+ influx and suppress autophagy, which may contribute to kidney injury or diseases.
In addition, intracellular modulators of Ca2+ could mediate autophagy, and play important roles in kidney disease. Stromal interaction molecule 1 (STIM1) is a key regulator of ER Ca2+ which can activate store-operated Ca2+ entry (SOCE) upon sensing ER Ca2+ depletion. In podocytes cultured in the serum of diabetic nephrotic rats, silencing STIM1 can reverse decreased autophagy and increased epithelial–mesenchymal transition (EMT) by restoring Ca2+ homeostasis [46]. In podocyte cells of ADPKD, autophagy is defective and EMT is enhanced in cystic kidneys, which could be reversed by reducing ER Ca2+ release to silence high levels of STIM1 [47,48,49]. In epithelial kidney cells of ADPKD patients, autophagy is inhibited because of the lack of interaction between BECN1 and mutant PKD2 (encoding polycystin 2, PC2), which prolongs the increase in intracellular Ca2+ levels [50]. The apolipoprotein L (APOL) could function as an ion channel in intracellular membranes and be involved in regulating programmed cell death. APOL1 variants interfere with autophagy by antagonizing Ca2+-dependent binding of APOL3 to neuronal calcium sensor 1 (NCS-1) and interacting with PI4-kinase IIIB in podocytes, which aggravates kidney inflammation [51]. The roles of these intracellular modulators of Ca2+ suggests that there are also complex networks of intracellular Ca2+-regulated autophagy in kidney disease.
2.5. Lysosomal Ca2+ Signaling in Kidney Diseases
Lysosomal Ca2+ contributes to autophagy and is important for lysosomal degradation. Lysosomal degradation is mediated by a class of Ca2+-activated proteases, Calpains (CAPNs), on lysosomes [52]. Lack of PKD1 impairs lysosomal acidification in a CAPN protease-dependent manner, and loss-of-function mutations in PKD1 or PKD2 result in ADPKD [53]. However, in cystinotic ciPTEC lines lysosomal Ca2+ stores are unaffected, while some extracellular agonist-induced Ca2+ responses may contribute to disease pathogenesis [54]. Therefore, whether lysosomal Ca2+ signaling contributes to the development of kidney diseases also depends on renal cell type.
In addition, intracellular Ca2+ distribution between the ER, mitochondria and lysosomes may affect the function of lysosomal degradation. The mitochondria contact the ER membrane through the mitochondria-associated membrane (MAM), which provides communication between these two organelles and mediates Ca2+ transfer from the ER to the mitochondria. Moreover, MAM-mediated ER–mitochondria Ca2+ crosstalk regulates apoptosis and autophagy, which contributes to the pathogenesis of kidney disease [55]. It has been shown that Ca2+ redistribution from the ER to the mitochondria regulates apoptosis and autophagy and contributes to lead-induced nephrotoxicity in primary rat proximal tubular cells [56,57]. Along with the Ca2+ release channel IP3R and the Ca2+ reuptake pump SERCA, the ER delivers cytosolic Ca2+ to the lysosomes in “piston-like” fashion [58]. Ca2+ uptake by lysosomes is speculated to regulate lysosomal pH [59]. The lysosomal acidic environment is mainly regulated by lysosome V-ATPase, and has been supposed to be regulated by a Ca2+/H+ exchange, although this has not been identified in mammalian cells [60,61]. In hepatic cell HepG2, Cd induced Ca2+ release from the ER store to the cytosol, which caused disruption of the lysosomal acidic environment, resulting in degradation. However, the Cd-disrupted lysosomal pH was restored by pretreatment with the IP3R inhibitor 2-APB and the SERCA activator CDN1163 [62,63]. This suggests that Ca2+ mediates interaction between the ER and mitochondria and lysosomes, and modulates lysosomal function.
It has also been suggested that Ca2+ efflux from the ER regulates lysosomal Ca2+ levels. The ER Ca2+ channel-like protein transmembrane BAX inhibitor motif containing 6 (TMBIM6) not only mediates ER stress response and apoptosis, but also regulates the local release of Ca2+ through the lysosomal transient receptor potential mucolipin 1 (TRPML1) channel, triggering autophagy induction in the kidney in starved mice [64]. In addition, lysosomal TRPML1 also regulates mitochondria–lysosome contact and promotes Ca2+ transfer from lysosome to mitochondria to modulate mitochondrial homeostasis [65]. Moreover, TRPML1-mediated lysosomal Ca2+ release regulates transcription factor EB (TFEB), which transcriptionally regulates the expression of TRPML1 as well as expression of other autophagic and lysosomal genes [66,67]. In primary proximal tubular cells, Cd induces lysosomal dysfunction via TFEB-dependent lysosomal degradation, which leads to persistent activation of Nrf2 and causes kidney injury [68,69,70]. In addition, in the study of a knockout mouse strain (Asah1fl/fl/PodoCre) with a podocyte-specific deletion of the α subunit of acid ceramidase, lysosomal Ca2+ release through TRPML1 channel was inhibited in the podocytes, which may show a contribution to the development of podocytopathy and associated nephrotic syndrome [71]. These results suggest that lysosomal Ca2+ is regulated in the intracellular Ca2+ store system, which may play important roles in the progression of kidney disease.
3. Ca2+ Signaling Links Cell Death and Autophagy in Kidney Cells
The relation of cell death and autophagy is complex and occasionally contradictory, but it is critical to cell fate. Intriguingly, Ca2+ signaling acts as a bridge linking these two types of cellular activities [72,73,74,75,76]. Ca2+ promote cell proliferation and survival through release of IP3R by the ER; Ca2+ is subsequently transferred to mitochondria to activate mitochondrial metabolism. Mitochondrial Ca2+ homeostasis dysfunction results in mitochondrial degradation by autophagy via activation of AMPK. Mitochondrial Ca2+ overload causes production of reactive oxygen species (ROS) and release of cytochrome c, which eventually leads to cell apoptosis [39,77,78]. Therefore, Ca2+ signaling and Ca2+ subcellular homeostasis may determine the balance between cell survival, apoptosis and autophagy.
3.1. Induced Autophagy Promotes Cell Death
In some cases, induced autophagy promotes cell death. In the renal fibrosis model of unilateral ureteral obstruction (UUO) in mice, persistent autophagy in kidney proximal tubules was observed. Pharmacological inhibition of autophagy and kidney proximal tubule-specific knockout of autophagy-related 7 (PT-ATG7 KO) suppressed tubular atrophy, apoptosis, nephron loss, and interstitial macrophage infiltration in these mice [79]. This suggests that persistent induction of autophagy in kidney proximal tubules promotes renal interstitial fibrosis during UUO. In addition, influx of extracellular Ca2+ triggered by Trichokonin VI, an antimicrobial peptide, induces autophagy and apoptosis in hepatocellular carcinoma cells. Moreover, siRNA knockdown of autophagy related gene (ATG5) reduces cell apoptosis [73]. This shows that Cd induces the mitochondrial-derived autophagic cell death of hepatocytes in a dose-dependent manner. By suppressing Cd-induced autophagic cell death, melatonin has a hepatoprotective effect in Cd-exposed mice [80]. In mouse spleen and human B cells, Cd induces vacuole membrane protein 1 (VMP1)-mediated autophagy via elevation of intracellular Ca2+, which contributes to apoptosis [81]. In RAW264.7 mouse monocytes, Cd induced autophagy and ER-mediated apoptosis; however, pharmacological and genetic inhibition of autophagy suppressed Cd-induced apoptosis. Moreover, treatment with Ca2+ chelators completely restored cell viability and inhibited Cd-induced apoptosis and autophagy [82]. In porcine kidney cell LLC-PK1, the autophagy mediator calpain induced necrosis before apoptosis by increasing intracellular Ca2+ levels in high-glucose conditions [83]. In addition, Ca2+ also plays important roles in ferroptosis [84], a type of autophagy-dependent cell death [85] which has recently been shown to have implications in diverse kidney diseases [86]. These studies suggest that intracellular Ca2+ signaling-mediated autophagy may promote cell death and contribute to kidney disease.
3.2. Inhibition of Autophagy Promotes against Cell Death
In some cases, induced autophagy has protective roles against cell death. It has been shown that high glucose promotes autophagy flux in podocyte cultures and induces LC3B-II expression in podocytes in diabetic mice. Specifically, deletion of ATG5 in podocytes resulted in accelerated diabetes-induced podocytopathy with a leaky glomerular filtration barrier and glomerulosclerosis. Furthermore, the endothelial-specific deletion of ATG5 also resulted in capillary rarefaction and accelerated diabetic nephropathy. Thus, endothelial cell and podocyte autophagy synergistically protect from diabetes-induced glomerulosclerosis [87]. In mouse mesangial cells (MES-13), Cd induced autophagy and apoptosis by inducing ER Ca2+ release through IP3R [88]. In addition, Cd induced the expression of LC3B-II but reduced the expression of sequestosome-1 (p62) in rat mesangial cells. When autophagy is disrupted either by gene knockout or RNA silencing, cell viability is decreased and increased pro-caspase-3 cleavage indicates the initiation of apoptotic cell death [89]. These results suggest that induced autophagy may protect against nephrotoxicity.
However, initial autophagic protection will switch to disruption of autophagic flux, resulting in cell death in renal cells [61]. In other words, inhibition of autophagy contributes to cell death. The autophagy inhibitor 3-methyladenine exacerbates Cd-caused germ cell apoptosis, which is relieved by the autophagy inducer rapamycin. More importantly, loss of ATG5 in Sertoli cells aggravates Cd-triggered germ cell apoptosis. This suggests that autophagy in Sertoli cells protects against Cd-induced germ cell apoptosis in mouse testes [90]. In human placental trophoblasts and mouse placenta, it has also been shown that activation of autophagy inhibits Cd-triggered apoptosis [91]. As an important excretory organ, the kidney is the main accumulation target of toxins such as heavy metals [92,93]. Previous studies have shown that Cd induces kidney injury and apoptosis via long-term inhibition of autophagy flux [94]. In vitro studies also show that inhibition of autophagy flux can aggravate cell apoptosis; Ca2+ signaling may link these two cell activities. In mouse renal tubular cells, Cd-inhibited autophagy flux aggravated apoptosis by inducing elevation of Ca2+ level [95]. In primary rat proximal tubular cells, Cd and lead (Pb)-inhibited autophagic degradation aggravated apoptotic death [96], which could be due to the redistribution of subcellular Ca2+ between the ER, cytosol and mitochondria [97,98]. Activation of CaSR can promote cell proliferation, and protects against Cd-induced renal tubular cell apoptosis through competing PLC-IP3-Ca2+ signaling [95]. Restoring the Ca2+-mediated autophagy process can protect against heavy metal-induced renal cell cytotoxicity and kidney injury [94,99,100]. These results suggest that elevation of cytosolic Ca2+ level-mediated inhibition of autophagy may aggravate apoptosis and contribute to kidney injury.
4. Targeted Ca2+ Signaling for Therapy of Kidney Diseases
As described above, the Ca2+ microdomains regulating apoptosis, necrosis and autophagy contribute to the development of kidney disease. Under these conditions, Ca2+ signaling determines the fate of kidney cells and the progression of disease. In view of this, therapeutic strategies have to consider whether to target a specific microdomain of Ca2+ signaling in kidney disease.
Firstly, ER Ca2+ signaling-mediated apoptotic pathways in kidney disease could be considered as potential therapeutic targets (Figure 1). Regulating ER stress in kidney cells may provide a therapeutic target in acute kidney injury triggered by renal ischemia reperfusion and cisplatin nephrotoxicity [101]. Pretreatment with trans-4,5-dihydroxy-1,2-dithiane (DTTox) enhances expression of the ER stress markers glucose-regulated protein 78 (GRP78) and GRP94 and protects against increased cellular Ca2+ levels and cell death in LLC-PK1 renal epithelial cells [102], as well as protecting the kidneys from nephrotoxic injury in vivo [103]. Treatment with tauroursodeoxycholic acid (TUDCA) prevents advanced glycation end product (AGE)-induced apoptosis of mouse podocytes in diabetic nephropathy by blocking an ER Ca2+ release-mediated apoptotic pathway [104]. By knocking down STIM1 levels, it can reduce Ca2+ release and restore intracellular Ca2+ homeostasis, which decreases PC2 protein levels in PC1-null proximal tubule cells and inhibits cyst growth in ADPKD [49].
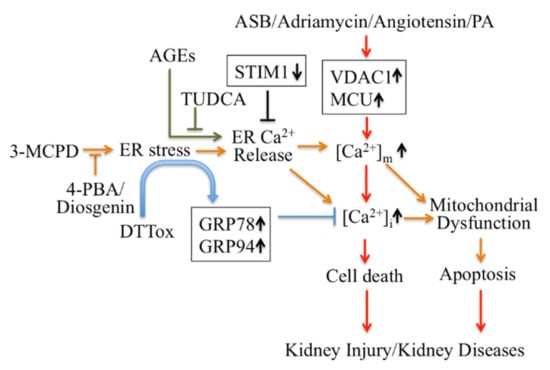
Figure 1.
Targeting Ca2+ signaling regulating cell death for therapy of kidney diseases. 4-PBA: 4-phenylbutyric acid; AGEs: advanced glycation end products; ASB: andrographolide sodium bisulfite; DTTox: trans-4,5-dihydroxy-1,2-dithiane; GRP: glucose-regulated protein; PA: Palmitic acid PCV2: Porcine Circovirus Type 2; TUDCA: tauroursodeoxycholic acid; VDAC1: voltage-dependent anion channel.
Secondly, modulators targeting mitochondrial Ca2+ mediate apoptotic pathways and may also be treatments for kidney disease therapy (Figure 1). Inhibition of ER stress by 4-phenylbutyric acid (4-PBA) and Diosgenin mitigates ER-associated mitochondrial apoptosis by maintaining Ca2+ homeostasis and mitochondrial dynamics, which ameliorates 3-MCPD-induced kidney injury [105,106]. In addition, 4-PBA also decreases Porcine Circovirus Type 2 (PCV2) infection-induced apoptosis by decreasing the cytosolic and mitochondrial Ca2+ load in porcine kidney PK-15 cells [107]. Mitochondrial outer membrane-located voltage-dependent anion channel (VDAC1) acts as gatekeeper for Ca2+ distribution between the mitochondria, cytosol and ER. Proteomic identification showed VDAC1 to be one the mitochondrial targets involved in andrographolide sodium bisulfite (ASB)-induced nephrotoxicity in a rat model [108]. Palmitic acid (PA) induced apoptosis through disruption of calcium homeostasis in mice podocytes [109,110]. In addition, Palmitic acid induced a continual increase in autophagy, ER stress, and apoptosis in primary cultured proximal tubular cells, and markedly upregulated VDAC1, which is associated with mitochondrial damage in HK-2 cells and may contribute to tubular injuries in obesity-related kidney disease [111]. In Adriamycin- or angiotensin II-treated rats, expression of VDAC1 and mitochondrial calcium uniporter (MCU) were upregulated; these mediate podocyte apoptosis by facilitating mitochondrial Ca2+ overload. However, MCU inhibitors can protect podocytes from apoptosis and proteinuria induced by Adriamycin or angiotensin II [112]. This suggests that regulating mitochondrial Ca2+ may also be a potential target for some stress-induced nephropathies.
Thirdly, Ca2+ signaling-mediated autophagy provides new therapeutic ways to treat kidney disease (Figure 2). PCV2 induces autophagy via the CaMKKβ-AMPK pathway in pig kidney PK-15 cells by increasing cytosolic Ca2+ [113]. Activation of vitamin D receptors can restore defective autophagy through the Ca2+-CAMKKβ-AMPK pathway in renal tubular epithelial cells in streptozotocin-induced diabetic mice [114]. Ca2+ signaling is involved in AMPK-mediated autophagy, and plays a role in coordinating cellular survival and kidney function. A selective activator of AMPK (A769662) reduced intracellular Ca2+ by activation of SERCA in vascular smooth muscle, which produces vasodilation in human intrarenal arteries [115]. This suggests that activation of AMPK and SERCA might be therapeutic targets in kidney diseases. Podocyte apoptosis induced by diabetes or high glucose and progression of diabetic nephropathy are prevented by astragaloside IV, which attenuates SERCA2-dependent ER stress and induces AMPKα-promoted autophagy [116]. Saikosaponin-d (SSd), a SERCA inhibitor, suppresses excess ER Ca2+ reuptake and cell proliferation in ADPKD cells by inducing autophagy through the CaMKKβ-AMPK-mTOR signaling pathway, which indicates that SSd might be a potential treatment for ADPKD and that SERCA might be a novel target for ADPKD therapy [117]. It has been reported that AMPK activation restored the defective autophagy in high glucose-induced HK-2 cells [114]. Activators of CaSR such as cinacalcet have renoprotective effects in high glucose-treated human glomerular endothelial cells, murine podocytes and diabetic mice. Cinacalcet decreases oxidative stress and apoptosis and increases autophagy by increasing intracellular Ca2+ level through activation of the CaMKKβ-LKB1-AMPK pathway in glomerular endothelial cells and podocytes in the kidney [23]. Taken together, Ca2+ signaling-mediated autophagy might be a potential target in therapy for metabolic disease-associated kidney diseases.
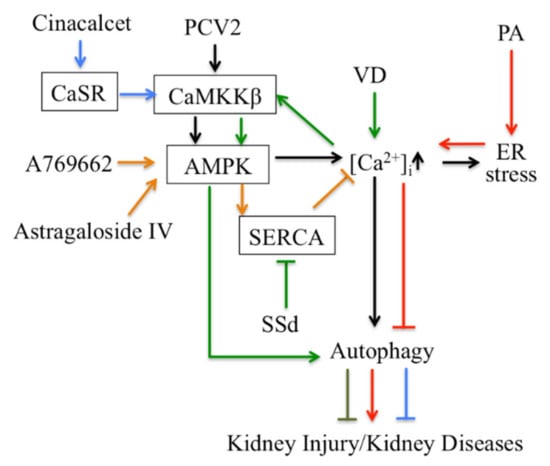
Figure 2.
Targeting autophagy regulated by Ca2+ signaling for therapy in kidney disease. CaSR: Calcium sensing receptor; PA: Palmitic acid; PCV2: Porcine Circovirus Type 2; SSd: Saikosaponin-d; VD: vitamin D.
5. Conclusions
Taken together, the kidney plays a key role in regulating Ca2+ homeostasis in the body; thus, disruption of Ca2+ homeostasis will cause a series of kidney diseases. Regulation of Ca2+ signaling in kidney cells decides cell fate in a way that is closely associated with kidney disease. Ca2+ microdomains including the ER, mitochondria and lysosomes regulate the various modes of cell death, such as necrosis, apoptosis and eryptosis, which when disrupted contributes to the overall development of kidney disease. Moreover, interplay between the Ca2+ microdomains also mediates the crosstalk between these forms of cell death and autophagy. Based on the roles of the Ca2+ microdomains in regulating cell fate in kidney cells, targeting these Ca2+ signaling may lead to novel therapeutic strategies against kidney diseases.
Author Contributions
B.N., C.G., H.S. and J.G. discussed the topic of this article. B.N., C.G. wrote the draft of the manuscript. A.K. and K.L. participated in interpretation of the literature. Y.X., H.S. and J.G. revised the review. All authors revised the draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (31600952 and 31271272) and the Start-Up Research Funding of Jiangsu University for Distinguished Scholars (4111330006).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ermak, G.; Davies, K.J. Calcium and oxidative stress: From cell signaling to cell death. Mol. Immunol. 2002, 38, 713–721. [Google Scholar] [CrossRef]
- Pozzan, T.; Rizzuto, R.; Volpe, P.; Meldolesi, J. Molecular and cellular physiology of intracellular calcium stores. Physiol. Rev. 1994, 74, 595–636. [Google Scholar] [CrossRef]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef]
- Guerrero-Hernandez, A.; Verkhratsky, A. Calcium signalling in diabetes. Cell Calcium 2014, 56, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Anderson, M.E. Mechanisms of altered Ca²⁺ handling in heart failure. Circ. Res. 2013, 113, 690–708. [Google Scholar] [CrossRef] [Green Version]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [Green Version]
- Aromataris, E.; Fernandez, R.; Godfrey, C.M.; Holly, C.; Khalil, H.; Tungpunkom, P. Summarizing systematic reviews: Methodological development, conduct and reporting of an umbrella review approach. Int. J. Evid.-Based Healthc. 2015, 13, 132–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jan, C.R.; Chen, C.H.; Wang, S.C.; Kuo, S.Y. Effect of methylglyoxal on intracellular calcium levels and viability in renal tubular cells. Cell. Signal. 2005, 17, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Eaddy, A.C.; Cummings, B.S.; McHowat, J.; Schnellmann, R.G. The role of endoplasmic reticulum Ca2+-independent phospholipase a2γ in oxidant-induced lipid peroxidation, Ca2+ release, and renal cell death. Toxicol. Sci. 2012, 128, 544–552. [Google Scholar] [CrossRef] [Green Version]
- De Morais, I.C.; Torres, A.F.; Pereira, G.J.; Pereira, T.P.; De Menezes, R.R.P.B.; Mello, C.P.; Jorge, A.R.C.; Bindá, A.H.; Toyama, M.H.; Monteiro, H.S.; et al. Bothrops leucurus venom induces nephrotoxicity in the isolated perfused kidney and cultured renal tubular epithelia. Toxicon 2013, 61, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.K.; Wu, C.L.; Su, T.C.; Kou, Y.R.; Kor, C.T.; Lee, T.S.; Tarng, D.C. Renal Tubular TRPA1 as a Risk Factor for Recovery of Renal Function from Acute Tubular Necrosis. J. Clin. Med. 2019, 8, 2187. [Google Scholar] [CrossRef] [Green Version]
- Gombedza, F.C.; Shin, S.; Kanaras, Y.L.; Bandyopadhyay, B.C. Abrogation of store-operated Ca(2+) entry protects against crystal-induced ER stress in human proximal tubular cells. Cell Death Discov. 2019, 5, 124. [Google Scholar] [CrossRef] [Green Version]
- Yiu, A.J.; Ibeh, C.L.; Roy, S.K.; Bandyopadhyay, B.C. Melamine induces Ca(2+)-sensing receptor activation and elicits apoptosis in proximal tubular cells. Am. J. Physiol. Cell Physiol. 2017, 313, C27–C41. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.; Gombedza, F.C.; Bandyopadhyay, B.C. l-ornithine activates Ca(2+) signaling to exert its protective function on human proximal tubular cells. Cell. Signal. 2020, 67, 109484. [Google Scholar] [CrossRef]
- Yamashita, J.; Ogata, M.; Takaoka, M.; Matsumura, Y. KB-R7943, a selective Na+/Ca2+ exchange inhibitor, protects against ischemic acute renal failure in mice by inhibiting renal endothelin-1 overproduction. J. Cardiovasc. Pharmacol. 2001, 37, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yang, D.; Jia, R.; Tan, J. Na+/Ca2+ exchange inhibitor, KB-R7943, attenuates contrast-induced acute kidney injury. J. Nephrol. 2013, 26, 877–885. [Google Scholar] [CrossRef]
- Benesic, A.; Schwerdt, G.; Mildenberger, S.; Freudinger, R.; Gordjani, N.; Gekle, M. Disturbed Ca2+-signaling by chloroacetaldehyde: A possible cause for chronic ifosfamide nephrotoxicity. Kidney Int. 2005, 68, 2029–2041. [Google Scholar] [CrossRef] [Green Version]
- Pittas, K.; Vrachatis, D.A.; Angelidis, C.; Tsoucala, S.; Giannopoulos, G.; Deftereos, S. The Role of Calcium Handling Mechanisms in Reperfusion Injury. Curr. Pharm. Des. 2018, 24, 4077–4089. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Gava, A.L.; Zhang, D.; Gao, B.; Krepinsky, J.C. Follistatin Protects Against Glomerular Mesangial Cell Apoptosis and Oxidative Stress to Ameliorate Chronic Kidney Disease. Antioxid. Redox Signal. 2019, 31, 551–571. [Google Scholar] [CrossRef]
- Tuffour, A.; Kosiba, A.A.; Zhang, Y.; Peprah, F.A.; Gu, J.; Shi, H. Role of the calcium-sensing receptor (CaSR) in cancer metastasis to bone: Identifying a potential therapeutic target. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188528. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lim, J.H.; Kim, M.Y.; Kim, E.N.; Yoon, H.E.; Shin, S.J.; Choi, B.S.; Kim, Y.S.; Chang, Y.S.; Park, C.W. The Adiponectin Receptor Agonist AdipoRon Ameliorates Diabetic Nephropathy in a Model of Type 2 Diabetes. J. Am. Soc. Nephrol. JASN 2018, 29, 1108–1127. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.H.; Kim, H.W.; Kim, M.Y.; Kim, T.W.; Kim, E.N.; Kim, Y.; Chung, S.; Kim, Y.S.; Choi, B.S.; Kim, Y.S.; et al. Cinacalcet-mediated activation of the CaMKKβ-LKB1-AMPK pathway attenuates diabetic nephropathy in db/db mice by modulation of apoptosis and autophagy. Cell Death Dis. 2018, 9, 270. [Google Scholar] [CrossRef]
- Park, S.J.; Li, C.; Chen, Y.M. Endoplasmic Reticulum Calcium Homeostasis in Kidney Disease: Pathogenesis and Therapeutic Targets. Am. J. Pathol. 2021, 191, 256–265. [Google Scholar] [CrossRef]
- Wu, D.; Chen, X.; Ding, R.; Qiao, X.; Shi, S.; Xie, Y.; Hong, Q.; Feng, Z. Ischemia/reperfusion induce renal tubule apoptosis by inositol 1,4,5-trisphosphate receptor and L-type Ca2+ channel opening. Am. J. Nephrol. 2008, 28, 487–499. [Google Scholar] [CrossRef]
- Szado, T.; Vanderheyden, V.; Parys, J.B.; De Smedt, H.; Rietdorf, K.; Kotelevets, L.; Chastre, E.; Khan, F.; Landegren, U.; Söderberg, O.; et al. Phosphorylation of inositol 1,4,5-trisphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proc. Natl. Acad. Sci. USA 2008, 105, 2427–2432. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J.; Kim, Y.; Yang, S.M.; Henderson, M.J.; Yang, W.; Lindahl, M.; Urano, F.; Chen, Y.M. Discovery of endoplasmic reticulum calcium stabilizers to rescue ER-stressed podocytes in nephrotic syndrome. Proc. Natl. Acad. Sci. USA 2019, 116, 14154–14163. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Cao, A.; Chu, S.; Wang, Y.; Zang, Y.; Mao, X.; Wang, H.; Wang, Y.; Liu, C.; Zhang, X.; et al. Astragaloside IV Attenuates Podocyte Apoptosis Mediated by Endoplasmic Reticulum Stress through Upregulating Sarco/Endoplasmic Reticulum Ca(2+)-ATPase 2 Expression in Diabetic Nephropathy. Front. Pharmacol. 2016, 7, 500. [Google Scholar] [CrossRef] [Green Version]
- Boehning, D.; Patterson, R.L.; Sedaghat, L.; Glebova, N.O.; Kurosaki, T.; Snyder, S.H. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat. Cell Biol. 2003, 5, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Kuo, I.Y.; Brill, A.L.; Lemos, F.O.; Jiang, J.Y.; Falcone, J.L.; Kimmerling, E.P.; Cai, Y.; Dong, K.; Kaplan, D.L.; Wallace, D.P.; et al. Polycystin 2 regulates mitochondrial Ca(2+) signaling, bioenergetics, and dynamics through mitofusin 2. Sci. Signal. 2019, 12, eaat7397. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Su, T.P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 2007, 131, 596–610. [Google Scholar] [CrossRef] [Green Version]
- Dias, G.F.; Grobe, N.; Rogg, S.; Jörg, D.J.; Pecoits-Filho, R.; Moreno-Amaral, A.N.; Kotanko, P. The Role of Eryptosis in the Pathogenesis of Renal Anemia: Insights From Basic Research and Mathematical Modeling. Front. Cell Dev. Biol. 2020, 8, 598148. [Google Scholar] [CrossRef] [PubMed]
- Jilani, K.; Lupescu, A.; Zbidah, M.; Abed, M.; Shaik, N.; Lang, F. Enhanced apoptotic death of erythrocytes induced by the mycotoxin ochratoxin A. Kidney Blood Press. Res. 2012, 36, 107–118. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Abed, M.; Voelkl, J.; Lang, F. Triggering of suicidal erythrocyte death by uremic toxin indoxyl sulfate. BMC Nephrol. 2013, 14, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, M.S.; Langer, H.; Abed, M.; Voelkl, J.; Lang, F. The uremic toxin acrolein promotes suicidal erythrocyte death. Kidney Blood Press. Res. 2013, 37, 158–167. [Google Scholar] [CrossRef]
- Föller, M.; Sopjani, M.; Mahmud, H.; Lang, F. Vanadate-induced suicidal erythrocyte death. Kidney Blood Press. Res. 2008, 31, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Lang, E.; Jilani, K.; Bissinger, R.; Rexhepaj, R.; Zelenak, C.; Lupescu, A.; Lang, F.; Qadri, S.M. Vitamin D-Rich Diet in Mice Modulates Erythrocyte Survival. Kidney Blood Press. Res. 2015, 40, 403–412. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, K.; Kong, A.; Zhou, Y.; Chen, D.; Gu, J.; Shi, H. Dysregulation of autophagy acts as a pathogenic mechanism of non-alcoholic fatty liver disease (NAFLD) induced by common environmental pollutants. Ecotoxicol. Environ. Saf. 2021, 217, 112256. [Google Scholar] [CrossRef] [PubMed]
- La Rovere, R.M.; Roest, G.; Bultynck, G.; Parys, J.B. Intracellular Ca(2+) signaling and Ca(2+) microdomains in the control of cell survival, apoptosis and autophagy. Cell Calcium 2016, 60, 74–87. [Google Scholar] [CrossRef]
- Livingston, M.J.; Dong, Z. Autophagy in acute kidney injury. Semin. Nephrol. 2014, 34, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Di Mise, A.; Tamma, G.; Ranieri, M.; Centrone, M.; van den Heuvel, L.; Mekahli, D.; Levtchenko, E.N.; Valenti, G. Activation of Calcium-Sensing Receptor increases intracellular calcium and decreases cAMP and mTOR in PKD1 deficient cells. Sci. Rep. 2018, 8, 5704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, X.; Xiao, H.; Zhang, Y.; Zeng, X.; Huang, M.; Chen, X.; Birnbaumer, L.; Liao, Y. Transient receptor potential channel 6 knockdown prevents apoptosis of renal tubular epithelial cells upon oxidative stress via autophagy activation. Cell Death Dis. 2018, 9, 1015. [Google Scholar] [CrossRef] [PubMed]
- Hall, G.; Wang, L.; Spurney, R.F. TRPC Channels in Proteinuric Kidney Diseases. Cells 2019, 9, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, X.; Huang, M.; Zeng, X.; Zhang, Y.; Sun, A.; Wu, Q.; Zhu, L.; Zhao, H.; Liao, Y. The Role of TRPC6 in Renal Ischemia/Reperfusion and Cellular Hypoxia/Reoxygenation Injuries. Front. Mol. Biosci. 2021, 8, 698975. [Google Scholar] [CrossRef]
- Hall, D.P.; Cost, N.G.; Hegde, S.; Kellner, E.; Mikhaylova, O.; Stratton, Y.; Ehmer, B.; Abplanalp, W.A.; Pandey, R.; Biesiada, J.; et al. TRPM3 and miR-204 establish a regulatory circuit that controls oncogenic autophagy in clear cell renal cell carcinoma. Cancer Cell 2014, 26, 738–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, J.; Wu, D.; Zhao, L.; Zou, W.; Shen, W.; Tu, Q.; He, Q. Effect of autophagy and stromal interaction molecule 1 on podocyte epithelial-mesenchymal transition in diabetic nephropathy. Int. J. Clin. Exp. Pathol. 2018, 11, 2450–2459. [Google Scholar]
- Boletta, A. Emerging evidence of a link between the polycystins and the mTOR pathways. PathoGenetics 2009, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Di Giovanni, V.; He, N.; Wang, K.; Ingram, A.; Rosenblum, N.D.; Pei, Y. Systems biology of autosomal dominant polycystic kidney disease (ADPKD): Computational identification of gene expression pathways and integrated regulatory networks. Hum. Mol. Genet. 2009, 18, 2328–2343. [Google Scholar] [CrossRef] [Green Version]
- Yanda, M.K.; Liu, Q.; Cebotaru, V.; Guggino, W.B.; Cebotaru, L. Role of calcium in adult onset polycystic kidney disease. Cell. Signal. 2019, 53, 140–150. [Google Scholar] [CrossRef]
- Peña-Oyarzun, D.; Rodriguez-Peña, M.; Burgos-Bravo, F.; Vergara, A.; Kretschmar, C.; Sotomayor-Flores, C.; Ramirez-Sarmiento, C.A.; De Smedt, H.; Reyes, M.; Perez, W.; et al. PKD2/polycystin-2 induces autophagy by forming a complex with BECN1. Autophagy 2021, 17, 1714–1728. [Google Scholar] [CrossRef]
- Pays, E. The function of apolipoproteins L (APOLs): Relevance for kidney disease, neurotransmission disorders, cancer and viral infection. FEBS J. 2021, 288, 360–381. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Saido, T.C.; Sorimachi, H. Calpain research for drug discovery: Challenges and potential. Nat. Rev. Drug Discov. 2016, 15, 854–876. [Google Scholar] [CrossRef] [PubMed]
- Peintner, L.; Venkatraman, A.; Waeldin, A.; Hofherr, A.; Busch, T.; Voronov, A.; Viau, A.; Kuehn, E.W.; Köttgen, M.; Borner, C. Loss of PKD1/polycystin-1 impairs lysosomal activity in a CAPN (calpain)-dependent manner. Autophagy 2021, 17, 2384–2400. [Google Scholar] [CrossRef]
- Ivanova, E.A.; Elmonem, M.A.; Bongaerts, I.; Luyten, T.; Missiaen, L.; van den Heuvel, L.P.; Levtchenko, E.N.; Bultynck, G. Ca(2+) signalling in human proximal tubular epithelial cells deficient for cystinosin. Cell Calcium 2016, 60, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Yang, W.; Sun, L. Mitochondria-Associated Endoplasmic Reticulum Membranes (MAMs) and Their Prospective Roles in Kidney Disease. Oxidative Med. Cell. Longev. 2020, 2020, 3120539. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Z.K.; Wang, Z.Y.; Yang, D.B.; Liu, Z.P.; Wang, L. Mitochondrial permeability transition and its regulatory components are implicated in apoptosis of primary cultures of rat proximal tubular cells exposed to lead. Arch. Toxicol. 2016, 90, 1193–1209. [Google Scholar] [CrossRef]
- Song, X.B.; Liu, G.; Liu, F.; Yan, Z.G.; Wang, Z.Y.; Liu, Z.P.; Wang, L. Autophagy blockade and lysosomal membrane permeabilization contribute to lead-induced nephrotoxicity in primary rat proximal tubular cells. Cell Death Dis. 2017, 8, e2863. [Google Scholar] [CrossRef]
- Atakpa, P.; Thillaiappan, N.B.; Mataragka, S.; Prole, D.L.; Taylor, C.W. IP(3) Receptors Preferentially Associate with ER-Lysosome Contact Sites and Selectively Deliver Ca(2+) to Lysosomes. Cell Rep. 2018, 25, 3180–3193.e7. [Google Scholar] [CrossRef] [Green Version]
- López-Sanjurjo, C.I.; Tovey, S.C.; Prole, D.L.; Taylor, C.W. Lysosomes shape Ins(1,4,5)P3-evoked Ca2+ signals by selectively sequestering Ca2+ released from the endoplasmic reticulum. J. Cell Sci. 2013, 126, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Forster, C.; Kane, P.M. Cytosolic Ca2+ homeostasis is a constitutive function of the V-ATPase in Saccharomyces cerevisiae. J. Biol. Chem. 2000, 275, 38245–38253. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.K.; Probst, S.; Santoyo-Sánchez, M.P.; Al-Hamdani, W.; Diebels, I.; von Sivers, J.K.; Kerek, E.; Prenner, E.J.; Thévenod, F. Initial autophagic protection switches to disruption of autophagic flux by lysosomal instability during cadmium stress accrual in renal NRK-52E cells. Arch. Toxicol. 2017, 91, 3225–3245. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ji, X.; Dai, S.; Liu, H.; Yan, D.; Zhou, Y.; Gu, J.; Shi, H. Cadmium induced redistribution of cholesterol by upregulating ABCA1 and downregulating OSBP. J. Inorg. Biochem. 2018, 189, 199–207. [Google Scholar] [CrossRef]
- Kong, A.; Zhang, Y.; Ning, B.; Li, K.; Ren, Z.; Dai, S.; Chen, D.; Zhou, Y.; Gu, J.; Shi, H. Cadmium induces triglyceride levels via microsomal triglyceride transfer protein (MTTP) accumulation caused by lysosomal deacidification regulated by endoplasmic reticulum (ER) Ca(2+) homeostasis. Chem.-Biol. Interact. 2021, 348, 109649. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, G.H.; Bhattarai, K.R.; Lee, M.S.; Back, S.H.; Kim, H.R.; Chae, H.J. TMBIM6 (transmembrane BAX inhibitor motif containing 6) enhances autophagy through regulation of lysosomal calcium. Autophagy 2021, 17, 761–778. [Google Scholar] [CrossRef] [Green Version]
- Peng, W.; Wong, Y.C.; Krainc, D. Mitochondria-lysosome contacts regulate mitochondrial Ca(2+) dynamics via lysosomal TRPML1. Proc. Natl. Acad. Sci. USA 2020, 117, 19266–19275. [Google Scholar] [CrossRef]
- Medina, D.L.; Di Paola, S.; Peluso, I.; Armani, A.; De Stefani, D.; Venditti, R.; Montefusco, S.; Scotto-Rosato, A.; Prezioso, C.; Forrester, A.; et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol. 2015, 17, 288–299. [Google Scholar] [CrossRef] [Green Version]
- Sardiello, M.; Palmieri, M.; di Ronza, A.; Medina, D.L.; Valenza, M.; Gennarino, V.A.; Di Malta, C.; Donaudy, F.; Embrione, V.; Polishchuk, R.S.; et al. A gene network regulating lysosomal biogenesis and function. Science 2009, 325, 473–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.Y.; Fan, R.F.; Yang, D.B.; Zhang, D.; Wang, L. Puerarin reverses cadmium-induced lysosomal dysfunction in primary rat proximal tubular cells via inhibiting Nrf2 pathway. Biochem. Pharmacol. 2019, 162, 132–141. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.F.; Zhang, D.; Wang, Z.Y.; Wang, L. Quercetin alleviates Cadmium-induced autophagy inhibition via TFEB-dependent lysosomal restoration in primary proximal tubular cells. Ecotoxicol. Environ. Saf. 2021, 208, 111743. [Google Scholar] [CrossRef]
- Fan, R.F.; Tang, K.K.; Wang, Z.Y.; Wang, L. Persistent activation of Nrf2 promotes a vicious cycle of oxidative stress and autophagy inhibition in cadmium-induced kidney injury. Toxicology 2021, 464, 152999. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Huang, D.; Bhat, O.M.; Poklis, J.L.; Zhang, A.; Zou, Y.; Kidd, J.; Gehr, T.W.B.; Li, P.L. Abnormal podocyte TRPML1 channel activity and exosome release in mice with podocyte-specific Asah1 gene deletion. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158856. [Google Scholar] [CrossRef]
- Smaili, S.S.; Pereira, G.J.; Costa, M.M.; Rocha, K.K.; Rodrigues, L.; do Carmo, L.G.; Hirata, H.; Hsu, Y.T. The role of calcium stores in apoptosis and autophagy. Curr. Mol. Med. 2013, 13, 252–265. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, T.; Sun, L.; Luo, Y.; Liu, D.H.; Xie, S.T.; Song, X.Y.; Wang, G.F.; Chen, X.L.; Zhou, B.C.; et al. Calpain, Atg5 and Bak play important roles in the crosstalk between apoptosis and autophagy induced by influx of extracellular calcium. Apoptosis Int. J. Program. Cell Death 2013, 18, 435–451. [Google Scholar] [CrossRef]
- Mammano, F.; Bortolozzi, M. Ca(2+) signaling, apoptosis and autophagy in the developing cochlea: Milestones to hearing acquisition. Cell Calcium 2018, 70, 117–126. [Google Scholar] [CrossRef]
- Zhou, X.; Hao, W.; Shi, H.; Hou, Y.; Xu, Q. Calcium homeostasis disruption—A bridge connecting cadmium-induced apoptosis, autophagy and tumorigenesis. Oncol. Res. Treat. 2015, 38, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Kosiba, A.A.; Wang, Y.; Chen, D.; Wong, C.K.C.; Gu, J.; Shi, H. The roles of calcium-sensing receptor (CaSR) in heavy metals-induced nephrotoxicity. Life Sci. 2020, 242, 117183. [Google Scholar] [CrossRef] [PubMed]
- Decuypere, J.P.; Monaco, G.; Bultynck, G.; Missiaen, L.; De Smedt, H.; Parys, J.B. The IP(3) receptor-mitochondria connection in apoptosis and autophagy. Biochim. Biophys. Acta 2011, 1813, 1003–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wacquier, B.; Combettes, L.; Van Nhieu, G.T.; Dupont, G. Interplay Between Intracellular Ca(2+) Oscillations and Ca(2+)-stimulated Mitochondrial Metabolism. Sci. Rep. 2016, 6, 19316. [Google Scholar] [CrossRef]
- Livingston, M.J.; Ding, H.F.; Huang, S.; Hill, J.A.; Yin, X.M.; Dong, Z. Persistent activation of autophagy in kidney tubular cells promotes renal interstitial fibrosis during unilateral ureteral obstruction. Autophagy 2016, 12, 976–998. [Google Scholar] [CrossRef] [Green Version]
- Pi, H.; Xu, S.; Reiter, R.J.; Guo, P.; Zhang, L.; Li, Y.; Li, M.; Cao, Z.; Tian, L.; Xie, J.; et al. SIRT3-SOD2-mROS-dependent autophagy in cadmium-induced hepatotoxicity and salvage by melatonin. Autophagy 2015, 11, 1037–1051. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Wang, Y.; Liu, Y.; Shi, M.; Yin, L.; Hou, Y.; Zhou, Y.; Wong, C.K.C.; Chen, D.; Guo, Z.; et al. Inhibition of Autophagy Alleviates Cadmium-Induced Mouse Spleen and Human B Cells Apoptosis. Toxicol. Sci. 2019, 170, 109–122. [Google Scholar] [CrossRef]
- So, K.Y.; Lee, B.H.; Oh, S.H. The critical role of autophagy in cadmium-induced immunosuppression regulated by endoplasmic reticulum stress-mediated calpain activation in RAW264.7 mouse monocytes. Toxicology 2018, 393, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Harwood, S.M.; Allen, D.A.; Raftery, M.J.; Yaqoob, M.M. High glucose initiates calpain-induced necrosis before apoptosis in LLC-PK1 cells. Kidney Int. 2007, 71, 655–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, P.; van Leyen, K.; Dey, P.N.; Honrath, B.; Dolga, A.; Methner, A. The role of Ca(2+) in cell death caused by oxidative glutamate toxicity and ferroptosis. Cell Calcium 2018, 70, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Liu, J.; Kang, R.; Klionsky, D.J.; Kroemer, G.; Tang, D. Ferroptosis is a type of autophagy-dependent cell death. Semin. Cancer Biol. 2020, 66, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kang, S.W.; Joo, J.; Han, S.H.; Shin, H.; Nam, B.Y.; Park, J.; Yoo, T.H.; Kim, G.; Lee, P.; et al. Characterization of ferroptosis in kidney tubular cell death under diabetic conditions. Cell Death Dis. 2021, 12, 160. [Google Scholar] [CrossRef]
- Lenoir, O.; Jasiek, M.; Hénique, C.; Guyonnet, L.; Hartleben, B.; Bork, T.; Chipont, A.; Flosseau, K.; Bensaada, I.; Schmitt, A.; et al. Endothelial cell and podocyte autophagy synergistically protect from diabetes-induced glomerulosclerosis. Autophagy 2015, 11, 1130–1145. [Google Scholar] [CrossRef]
- Wang, S.H.; Shih, Y.L.; Ko, W.C.; Wei, Y.H.; Shih, C.M. Cadmium-induced autophagy and apoptosis are mediated by a calcium signaling pathway. Cell. Mol. life Sci. CMLS 2008, 65, 3640–3652. [Google Scholar] [CrossRef] [PubMed]
- Fujishiro, H.; Liu, Y.; Ahmadi, B.; Templeton, D.M. Protective effect of cadmium-induced autophagy in rat renal mesangial cells. Arch. Toxicol. 2018, 92, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.X.; Zhu, H.L.; Shi, X.T.; Nan, Y.; Liu, W.B.; Dai, L.M.; Xiong, Y.W.; Yi, S.J.; Cao, X.L.; Xu, D.X.; et al. Autophagy in Sertoli cell protects against environmental cadmium-induced germ cell apoptosis in mouse testes. Environ. Pollut. 2021, 270, 116241. [Google Scholar] [CrossRef]
- Zhu, H.L.; Xu, X.F.; Shi, X.T.; Feng, Y.J.; Xiong, Y.W.; Nan, Y.; Zhang, C.; Gao, L.; Chen, Y.H.; Xu, D.X.; et al. Activation of autophagy inhibits cadmium-triggered apoptosis in human placental trophoblasts and mouse placenta. Environ. Pollut. 2019, 254, 112991. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Sun, X.; Kong, A.; Ma, H.; Xie, Y.; Cheng, D.; Wong, C.K.C.; Zhou, Y.; Gu, J. Cadmium induces epithelial-mesenchymal transition and migration of renal cancer cells by increasing PGE2 through a cAMP/PKA-COX2 dependent mechanism. Ecotoxicol. Environ. Saf. 2021, 207, 111480. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Jiang, T.; Yuan, X.; Ren, Z.; Tuffour, A.; Liu, H.; Zhou, Y.; Gu, J.; Shi, H. Nephrotoxicity Profile of Cadmium Revealed by Proteomics in Mouse Kidney. Biol. Trace Elem. Res. 2021, 199, 1929–1940. [Google Scholar] [CrossRef]
- Gu, J.; Ren, Z.; Zhao, J.; Peprah, F.A.; Xie, Y.; Cheng, D.; Wang, Y.; Liu, H.; Chu Wong, C.K.; Zhou, Y.; et al. Calcimimetic compound NPS R-467 protects against chronic cadmium-induced mouse kidney injury by restoring autophagy process. Ecotoxicol. Environ. Saf. 2020, 189, 110052. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Dai, S.; Liu, Y.; Liu, H.; Zhang, Y.; Ji, X.; Yu, F.; Zhou, Y.; Chen, L.; Tse, W.K.F.; et al. Activation of Ca(2+)-sensing receptor as a protective pathway to reduce Cadmium-induced cytotoxicity in renal proximal tubular cells. Sci. Rep. 2018, 8, 1092. [Google Scholar] [CrossRef] [Green Version]
- Chu, B.X.; Fan, R.F.; Lin, S.Q.; Yang, D.B.; Wang, Z.Y.; Wang, L. Interplay between autophagy and apoptosis in lead(II)-induced cytotoxicity of primary rat proximal tubular cells. J. Inorg. Biochem. 2018, 182, 184–193. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.K.; Jiao, P.; Zhou, X.P.; Yang, D.B.; Wang, Z.Y.; Wang, L. Redistribution of subcellular calcium and its effect on apoptosis in primary cultures of rat proximal tubular cells exposed to lead. Toxicology 2015, 333, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, Z.F.; Wang, Z.Y.; Wang, L. Role of subcellular calcium redistribution in regulating apoptosis and autophagy in cadmium-exposed primary rat proximal tubular cells. J. Inorg. Biochem. 2016, 164, 99–109. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.Y.; Zhou, X.P.; Liu, Z.P.; Song, X.B.; Wang, Z.Y.; Wang, L. Cadmium disrupts autophagic flux by inhibiting cytosolic Ca(2+)-dependent autophagosome-lysosome fusion in primary rat proximal tubular cells. Toxicology 2017, 383, 13–23. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yang, H.; Wang, M.G.; Yang, D.B.; Wang, Z.Y.; Wang, L. Trehalose protects against cadmium-induced cytotoxicity in primary rat proximal tubular cells via inhibiting apoptosis and restoring autophagic flux. Cell Death Dis. 2017, 8, e3099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, M.; Shu, S.; Guo, C.; Tang, C.; Dong, Z. Endoplasmic reticulum stress in ischemic and nephrotoxic acute kidney injury. Ann. Med. 2018, 50, 381–390. [Google Scholar] [CrossRef]
- Liu, H.; Bowes, R.C., 3rd; van de Water, B.; Sillence, C.; Nagelkerke, J.F.; Stevens, J.L. Endoplasmic reticulum chaperones GRP78 and calreticulin prevent oxidative stress, Ca2+ disturbances, and cell death in renal epithelial cells. J. Biol. Chem. 1997, 272, 21751–21759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asmellash, S.; Stevens, J.L.; Ichimura, T. Modulating the endoplasmic reticulum stress response with trans-4,5-dihydroxy-1,2-dithiane prevents chemically induced renal injury in vivo. Toxicol. Sci. 2005, 88, 576–584. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Liu, C.P.; Xu, K.F.; Mao, X.D.; Lu, Y.B.; Fang, L.; Yang, J.W.; Liu, C. Effect of taurine-conjugated ursodeoxycholic acid on endoplasmic reticulum stress and apoptosis induced by advanced glycation end products in cultured mouse podocytes. Am. J. Nephrol. 2008, 28, 1014–1022. [Google Scholar] [CrossRef]
- Zhong, Y.; Jin, C.; Han, J.; Zhu, J.; Liu, Q.; Sun, D.; Xia, X.; Peng, X. Inhibition of ER stress attenuates kidney injury and apoptosis induced by 3-MCPD via regulating mitochondrial fission/fusion and Ca(2+) homeostasis. Cell Biol. Toxicol. 2021, 37, 795–809. [Google Scholar] [CrossRef]
- Zhong, Y.; Jin, C.; Han, J.; Zhu, J.; Liu, Q.; Sun, D.; Xia, X.; Zhang, Y.; Peng, X. Diosgenin Protects Against Kidney Injury and Mitochondrial Apoptosis Induced by 3-MCPD Through the Regulation of ER Stress, Ca(2+) Homeostasis, and Bcl2 Expression. Mol. Nutr. Food Res. 2021, 65, e2001202. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, R.; Geng, S.; Shan, Y.; Li, X.; Fang, W. Porcine Circovirus Type 2 Induces ORF3-Independent Mitochondrial Apoptosis via PERK Activation and Elevation of Cytosolic Calcium. J. Virol. 2019, 93, e01784-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, W.M.; Yuan, T.J.; Xu, J.D.; Gu, L.L.; Liang, P.; Lu, H. Proteomic identification of mitochondrial targets involved in andrographolide sodium bisulfite-induced nephrotoxicity in a rat model. Environ. Toxicol. Pharmacol. 2015, 40, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Cao, A.; Liu, H.; Guo, H.; Zang, Y.; Wang, Y.; Wang, Y.; Wang, H.; Yin, P.; Peng, W. Calcium Uptake via Mitochondrial Uniporter Contributes to Palmitic Acid-Induced Apoptosis in Mouse Podocytes. J. Cell. Biochem. 2017, 118, 2809–2818. [Google Scholar] [CrossRef]
- Zang, Y.; Liu, S.; Cao, A.; Shan, X.; Deng, W.; Li, Z.; Wang, H.; Wang, Y.; Wang, L.; Peng, W. Astragaloside IV inhibits palmitic acid-induced apoptosis through regulation of calcium homeostasis in mice podocytes. Mol. Biol. Rep. 2021, 48, 1453–1464. [Google Scholar] [CrossRef]
- Kong, Y.; Zhao, X.; Qiu, M.; Lin, Y.; Feng, P.; Li, S.; Liang, B.; Zhu, Q.; Huang, H.; Li, C.; et al. Tubular Mas receptor mediates lipid-induced kidney injury. Cell Death Dis. 2021, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Guan, N.; Ren, Y.L.; Wei, Q.J.; Tao, Y.H.; Yang, G.S.; Liu, X.Y.; Bu, D.F.; Zhang, Y.; Zhu, S.N. IP(3)R-Grp75-VDAC1-MCU calcium regulation axis antagonists protect podocytes from apoptosis and decrease proteinuria in an Adriamycin nephropathy rat model. BMC Nephrol. 2018, 19, 140. [Google Scholar] [CrossRef]
- Gu, Y.; Qi, B.; Zhou, Y.; Jiang, X.; Zhang, X.; Li, X.; Fang, W. Porcine Circovirus Type 2 Activates CaMMKβ to Initiate Autophagy in PK-15 Cells by Increasing Cytosolic Calcium. Viruses 2016, 8, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, A.; Yi, B.; Han, H.; Yang, S.; Hu, Z.; Zheng, L.; Wang, J.; Liao, Q.; Zhang, H. Vitamin D-VDR (vitamin D receptor) regulates defective autophagy in renal tubular epithelial cell in streptozotocin-induced diabetic mice via the AMPK pathway. Autophagy 2021, 1–14. [Google Scholar] [CrossRef]
- Rodríguez, C.; Contreras, C.; Sáenz-Medina, J.; Muñoz, M.; Corbacho, C.; Carballido, J.; García-Sacristán, A.; Hernandez, M.; López, M.; Rivera, L.; et al. Activation of the AMP-related kinase (AMPK) induces renal vasodilatation and downregulates Nox-derived reactive oxygen species (ROS) generation. Redox Biol. 2020, 34, 101575. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wang, Y.; Zhang, X.; Zang, Y.; Zhang, Y.; Wang, L.; Wang, H.; Wang, Y.; Cao, A.; Peng, W. Astragaloside IV protects against podocyte injury via SERCA2-dependent ER stress reduction and AMPKα-regulated autophagy induction in streptozotocin-induced diabetic nephropathy. Sci. Rep. 2017, 7, 6852. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.; Xu, D.; Gu, J.; Xue, C.; Yang, B.; Fu, L.; Song, S.; Liu, D.; Zhou, W.; Lv, J.; et al. Saikosaponin-d inhibits proliferation by up-regulating autophagy via the CaMKKβ-AMPK-mTOR pathway in ADPKD cells. Mol. Cell. Biochem. 2018, 449, 219–226. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).