Abstract
This review integrates current knowledge on how greenhouse conditions regulate the nutritional quality and shelf life of tomato, cucumber, and sweet pepper. Preharvest environmental factors jointly shape fruit composition, firmness, and storage performance through their control of photosynthesis, assimilate partitioning, and structural stability. Across all variables, light intensity and fruit temperature emerge as the dominant determinants of overall quality and shelf life potential. Relative air humidity (RH), irrigation regime, and nutrient balance primarily affect firmness, water loss, and physiological disorders, while CO2 enrichment, shading, and mineral or biostimulant inputs exert secondary yet consistent effects. Comparative evaluation shows that tomato is most sensitive to temperature and RH, cucumber to water status and epidermal stress, and sweet pepper to radiation for color and antioxidant development. These distinctions confirm that no single climatic optimization can be universally applied, and management must therefore target species-specific physiological constraints to sustain both nutritional excellence and storage performance. Major knowledge gaps remain, particularly regarding the combined effects of interacting environmental drivers and the integration of physiological responses with postharvest behavior. Future research should adopt multifactorial designs and predictive modeling to support climate-smart greenhouse strategies that optimize quality and storability under variable growing conditions.
1. Introduction
Preharvest environmental conditions strongly influence both the nutritional composition and storability of vegetables [1,2]. In greenhouse cultivation, the microclimate [i.e., temperature, light, relative air humidity (RH), CO2 concentration, and the supply of water and nutrients] can be precisely regulated. Although these parameters are typically adjusted to maximize yield, they also affect fruit physiology and biochemical composition, and thereby determine postharvest performance [3,4]. Understanding how controlled environmental factors shape fruit metabolism and structure is therefore essential for achieving both productivity and product integrity. Consumer acceptance further depends on aroma-related volatile organic compounds (VOCs), which are also influenced by preharvest climate but are beyond the primary scope of this review.
Tomato (Solanum lycopersicum L.), cucumber (Cucumis sativus L.) and sweet pepper (Capsicum annuum L.) were selected as model crops because they dominate greenhouse vegetable production globally. These species differ markedly in fruit physiology [5,6,7], offering an opportunity to explore how similar cultivation environments lead to distinct nutritional and structural outcomes. Their wide genetic diversity, continuous year-round cultivation, and sensitivity to environmental variation make them ideal indicators for assessing the effects of microclimate management on fruit quality and shelf life [8,9,10].
Only studies conducted under greenhouse conditions were included, ensuring that the evaluated responses reflect the complex but controllable environments encountered in commercial production. Climate-chamber studies were excluded because they do not capture the combined and fluctuating stresses typical of real cultivation systems, while field studies were omitted due to limited capacity for environmental manipulation. Priority was placed on recent literature (the last five years), complemented by landmark earlier studies where relevant. By focusing on greenhouse experiments, this review evaluates responses to directly controlled environmental drivers [2,11], enabling mechanistic interpretation and facilitating the translation of findings into practical cultivation guidelines.
Despite extensive studies on individual environmental factors, a mechanistic, cross-species synthesis linking preharvest microclimate to postharvest outcomes remains incomplete. The objective of this work is to integrate current evidence on how greenhouse cultivation conditions influence the nutritional, structural and physiological quality of tomato, cucumber and sweet pepper fruits, both at harvest and during storage. The review aims to identify the most decisive environmental factors, quantify their relative impact across species, and outline management strategies that improve nutritional value, enhance fruit resilience and prolong shelf life. By linking cultivation environment with fruit physiology, the present study contributes to the design of climate-smart greenhouse systems that sustain high yield and superior postharvest performance.
2. Concepts and Metrics
2.1. Nutritional Quality: Definitions, Compounds, and Analytical Endpoints
The nutritional quality of greenhouse-grown vegetables reflects the concentration and balance of biochemical constituents with health-promoting and sensory properties [12,13]. These include soluble carbohydrates, organic acids, vitamins, pigments, phenolic compounds, and minerals [12,13]. Their concentrations are strongly influenced by environmental factors, regulating primary and secondary metabolism [12,13]. Quantification is typically achieved through physicochemical analyses such as refractometry [soluble solids (°Brix)], titration (acidity), spectrophotometric assays (pigments and antioxidants), and chromatographic or mass spectrometric methods (detailed profiling).
In tomato, nutritional quality is shaped by sweetness, acidity, and color (Table 1) [14,15]. Soluble sugars and organic acids contribute substantially to flavor [15,16], while carotenoids, primarily lycopene and β-carotene, define red coloration and respond to light and temperature [15,17]. Ascorbic acid (vitamin C), phenolics and minerals [e.g., potassium (K) and calcium (Ca)] contribute to antioxidant capacity and tissue stability [14,17,18]. Analytical endpoints include total soluble solids, titratable acidity (TA), lycopene, ascorbic acid, and phenolics [15,19].
In cucumber, nutritional quality is characterized by high water content (WC), moderate levels of reducing sugars, and low concentrations of acids and secondary metabolites (Table 1) [20]. The main determinants of quality are crisp texture, refreshing flavor, and the maintenance of chlorophyll and green coloration [20,21]. Ascorbic acid and phenolics are present at low levels but respond to environmental cues such as light or mild stress [20,22,23]. Nutritional assessment focuses on soluble solids, firmness, ascorbic acid, and total chlorophyll, reflecting both compositional and textural traits [20,24].
Sweet pepper is rich in ascorbic acid and carotenoids, which accumulate during ripening (Table 1) [23,25]. Ascorbic acid concentrations can exceed most vegetables, while carotenoids (e.g., capsanthin, capsorubin, and β-carotene) contribute to pigmentation and antioxidant potential [23,25,26]. Phenolics and flavonoids enhance the total antioxidant capacity [23,26]. Analytical endpoints include ascorbic acid, total carotenoids, phenolics, and soluble solids [23]. Because these metabolites are responsive to temperature, light, and nutrient balance during growth, their accumulation provides a sensitive indicator of how preharvest conditions affect nutritional quality [2,25].

Table 1.
Key physiological and compositional traits determining nutritional quality and shelf life (SL) in greenhouse-grown tomato, cucumber, and sweet pepper. ascorbic acid = vitamin C; a*, green (–) → red (+) axis; b*, blue (–) → yellow (+) axis; β-carotene, beta-carotene; °Brix, soluble solids content; FW, fresh weight; RH, relative air humidity; T, temperature; TA, titratable acidity; WC, water content. Arrows indicate direction of projected change (↓ decrease). Definitions of physiological disorders are provided in Supplementary Table S1.
Table 1.
Key physiological and compositional traits determining nutritional quality and shelf life (SL) in greenhouse-grown tomato, cucumber, and sweet pepper. ascorbic acid = vitamin C; a*, green (–) → red (+) axis; b*, blue (–) → yellow (+) axis; β-carotene, beta-carotene; °Brix, soluble solids content; FW, fresh weight; RH, relative air humidity; T, temperature; TA, titratable acidity; WC, water content. Arrows indicate direction of projected change (↓ decrease). Definitions of physiological disorders are provided in Supplementary Table S1.
| Trait Category | Tomato | Cucumber | Sweet Pepper | Key References |
|---|---|---|---|---|
| Ripening physiology | Climacteric; strong ethylene & respiration peaks; ripening continues after harvest | Non-climacteric; quality fixed at harvest; high respiration | Non-climacteric; biochemical color transition (green → red/yellow); no ethylene burst | [6,27,28,29] |
| Dominant quality determinants | Soluble sugars, organic acids, lycopene + β-carotene, ascorbic acid, phenolics | WC, firmness, chlorophyll, ascorbic acid (moderate), phenolics (low) | Ascorbic acid (very high), carotenoids (capsanthin, capsorubin, β-carotene), phenolics/flavonoids | [14,24,25] |
| Typical analytical endpoints | °Brix, TA, lycopene, ascorbic acid, phenolics | °Brix, firmness, ascorbic acid, chlorophyll, color (a*/b*) | °Brix, ascorbic acid, total carotenoids, total phenolics, antioxidant capacity | [20,23,24] |
| Major nutritional compounds affected by environment | Sugars, acids, carotenoids, ascorbic acid, phenolics | Sugars, chlorophyll, ascorbic acid, firmness-related polysaccharides | Ascorbic acid, carotenoids, phenolics; influenced by light & nutrient balance | [15,22,30] |
| Primary SL limitations | Softening, water loss, cracking, blotchy ripening, decay | Rapid turgor loss, yellowing, chilling injury, decay | Shriveling, firmness loss, color fading, chilling injury, decay | [31,32,33] |
| Key kinetic/end-point indicators | Firmness ↓ (20–30%), % weight loss, lycopene stability, visible defects | % weight loss, firmness retention, color stability, absence of pitting | % weight loss, firmness decline, retention of ascorbic acid & carotenoids, visual shrivel index | [34,35,36] |
| Cuticle & epidermis | Moderately thick cuticle; prone to micro-cracking under RH fluctuations | Thin epidermis with stomata; waxy but permeable; high transpiration | Thick pericarp with robust cuticle; permeability modulated by light & cultivar | [37,38,39] |
| Anatomical/structural factors affecting storability | Locular gel tissue promotes softening when cell walls weaken | High surface-to-volume ratio; limited barrier to water loss | Multi-layered pericarp confers mechanical strength & ↓ desiccation | [39,40,41] |
| Typical storage T threshold | 10–12 °C (below: chilling injury) | 10–12 °C (high sensitivity to chilling) | 7–10 °C (moderate chilling sensitivity) | [21,31,42] |
| Indicative nutritional highlights (per 100 g FW) | Ascorbic acid 20–40 mg; lycopene 3–10 mg | Ascorbic acid 10–15 mg; low carotenoids | Ascorbic acid 80–150 mg; carotenoids 2–6 mg | [20,23,43] |
| Overall postharvest resilience | Moderate; manageable with careful ripeness & RH control | Low; highly perishable even under optimal storage | Relatively high; strong tissues but sensitive to low T | [21,44,45] |
2.2. Shelf Life: Operational Definition, Kinetic Endpoints, and Disorder Metrics
Shelf life represents the duration during which harvested produce maintains acceptable commercial, nutritional, and sensory attributes [46]. It is driven by the postharvest environment and the physiological state of the product at harvest [46]. Operationally, shelf life is assessed through kinetic endpoints such as weight loss, firmness decline, color change, decay incidence, or quality degradation beyond a defined threshold of marketability [47,48].
In tomato, shelf life is governed by the rate of softening and the evolution of color and flavor, processes closely linked to ethylene production and respiration (Table 1) [28,33,40]. Firmness loss reflects underlying cell-wall disassembly processes, while excessive transpiration accelerates wrinkling and decay [40]. The onset of physiological disorders (e.g., blotchy ripening or postharvest cracking) is often traced back to preharvest imbalances in temperature, RH, or Ca nutrition [7,18,49]. Definitions of all physiological disorders treated in this review are provided in Supplementary Table S1. Quantitative metrics include the time required to reach a 20–30% firmness reduction, percentage weight loss, lycopene and acidity stability, and the incidence of visible defects [33,50].
Cucumber fruit exhibits one of the shortest shelf lives among greenhouse vegetables due to its high WC and delicate epidermis (Table 1) [21,32]. Postharvest deterioration is characterized by rapid loss of turgor, yellowing of the peel, and the appearance of chilling injury symptoms when stored below 10–12 °C [21,33,35]. Surface stomata further promote transpiration and infection under high RH [38,41]. Shelf life evaluation in cucumber therefore emphasizes water loss rate, firmness retention, color stability, and the absence of pitting or decay [35,41]. The influence of preharvest environment is particularly evident through its effects on cuticle formation, fruit epidermal strength, and stomatal density [2,38,41].
In sweet pepper, shelf life is determined by firmness retention, color stability, and the development of shriveling or decay (Table 1) [31,34]. Water loss is the principal cause of quality decline, while chilling injury may occur when storage temperatures drop below 7 °C [31,34,45]. Fruits that develop under optimal light and Ca nutrition tend to have thicker pericarp walls and stronger cuticular barriers, which slow postharvest softening and desiccation [8,39]. Kinetic indicators commonly include weight loss percentage, firmness decline, visual scoring for shriveling, and retention of ascorbic acid and carotenoids over storage time [34]. These metrics provide an integrated view of both physical and biochemical stability.
2.3. Crop Biology Relevant to Postharvest Behavior
The biological characteristics of each crop largely explain their contrasting responses to preharvest and postharvest environments.
Tomato is a climacteric fruit that exhibits a pronounced respiratory and ethylene peak during ripening (Table 1) [28], allowing ripening to continue after harvest. Harvest maturity therefore strongly influences postharvest performance. Its relatively thick cuticle provides moderate protection against dehydration, although susceptibility to cracking and physiological disorders such as blossom-end rot (BER) remains high under fluctuating RH or Ca deficiency [18,37]. The internal gel-rich locular tissue further accelerates softening when cell wall integrity declines [40].
Cucumber is non-climacteric, and its quality is largely fixed at harvest (Table 1) [27,29]. The fruit epidermis is thin and partially covered by stomata, resulting in high transpirational water loss and extreme sensitivity to low-temperature injury [38,41]. The cuticular layer is smooth and waxy but not sufficiently thick to prevent rapid desiccation under low RH [38]. Because cucumber fruits continue to respire intensely, even small imbalances in temperature or RH accelerate senescence, color loss, and microbial decay.
Sweet pepper, although non-climacteric, undergoes marked biochemical transformations during ripening, including the degradation of chlorophyll and the accumulation of carotenoids and ascorbic acid (Table 1) [6,25]. The fruit wall is composed of a thick pericarp that contributes to firmness and water retention [8,39]. The cuticle is relatively well developed, but its permeability can be influenced by light exposure, cultivar, and nutrient regime during cultivation [39,51]. Postharvest longevity therefore depends heavily on the pericarp and the mechanical or physiological stress encountered during growth and harvest [2,7,8].
Understanding these biological principles is essential for interpreting species-specific responses and for contextualizing the pathways summarized in Figure 1.
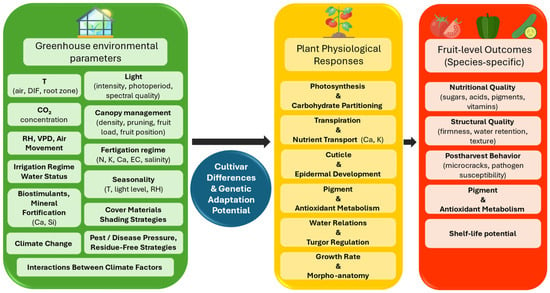
Figure 1.
Conceptual framework illustrating the cascade from greenhouse environmental parameters to physiological processes and fruit-level outcomes determining quality and shelf life. Ca, calcium; CO2, carbon dioxide; DIF, day–night temperature difference; EC, electrical conductivity; K, potassium; N, nitrogen; RH, relative air humidity; Si, silicon; T, temperature; VPD, vapor pressure deficit.
3. Environmental Factors During Greenhouse Cultivation
3.1. Air Temperature
Temperature is one of the most influential factors determining fruit quality and shelf life in greenhouse vegetables [2,11]. It controls development and metabolism. The optimal thermal window for quality is narrower than for growth. Even moderate deviations affect nutritional and structural traits [2,45,52].
In tomato, optimal fruit-development temperatures are 18–24 °C, with day temperatures ~25 °C and night temperatures ~17 °C [53,54]. When means exceed 28–30 °C, lycopene synthesis declines and fruit shows orange coloration from β-carotene accumulation (Table 2) [55,56,57]. Elevated night temperatures accelerate respiration, reduce acidity, and promote softening, shortening shelf life [56,58,59]. Low night temperatures delay ripening and increase blotchy coloration [7,60]. The day–night temperature difference (DIF) is important. A positive DIF improves color development and firmness, whereas small or negative DIFs, common in poorly ventilated summer greenhouses, produce softer fruit with reduced soluble solids [57,58]. Maintaining temperature uniformity within the canopy is essential, as canopy gradients cause uneven ripening and inconsistent quality.

Table 2.
Effects of environmental factors during greenhouse cultivation on major quality traits and shelf life (SL) in tomato. B, blue (spectra); BER, blossom-end rot; C, carbon; Ca, calcium; CO2, carbon dioxide; DIF, day–night temperature difference; DLI, daily light integral; DM, dry matter; EC, electrical conductivity of the nutrient solution; IPM, integrated pest management; K, potassium; N, nitrogen; R, red (spectra); RH, relative air humidity; Si, silicon; T, temperature; TSS, total soluble solids; VPD, vapor pressure deficit; °Brix, soluble solids content. Arrows indicate direction of projected change (↑ increase, ↓ decrease). Definitions of physiological disorders are provided in Supplementary Table S1.
Cucumber requires a warmer thermal environment than tomato for sustained growth, with optimal fruit-setting temperatures ~25–28 °C during the day and 18–20 °C at night [98,99]. High mean temperatures accelerate fruit enlargement but can reduce firmness and increase curvature, especially under high RH (Table 3) [99,100]. Excessive heat impairs chlorophyll retention, producing paler fruit [95,100,101]. Night temperatures below 15 °C slow growth and induce irregular shape and weak epidermal development [99,102]. Large DIF amplitudes favor elongation and epidermal reinforcement, whereas small DIFs promote soft texture and greater postharvest water loss [99,103]. The thin pericarp makes cucumber highly sensitive to short heat or chilling episodes during expansion, later expressed as accelerated yellowing or loss of glossiness after harvest [100,104,105].

Table 3.
Effects of environmental factors during greenhouse cultivation on major quality traits and shelf life (SL) in cucumber. B, blue (spectra); Ca, calcium; CO2, carbon dioxide; DIF, day–night temperature difference; DM, dry matter; EC, electrical conductivity of the nutrient solution; IPM, integrated pest management; K, potassium; N, nitrogen; RH, relative air humidity; Si, silicon; T, temperature; UV-A, ultraviolet A radiation; VPD, vapor pressure deficit. Arrows indicate direction of projected change (↑ increase, ↓ decrease). Definitions of physiological disorders are provided in Supplementary Table S1.
Sweet pepper exhibits a slightly higher optimal temperature range than tomato, with 24–28 °C during the day and 18–20 °C at night promoting yield and pigment accumulation [95,141]. At moderate temperatures, ascorbic acid and carotenoid synthesis increase, enhancing nutritional quality and coloration (Table 4) [142,143]. However, prolonged temperatures above 32 °C cause flower abortion, sunscald, and irregular pigmentation, while sustained night temperatures above 22 °C promote softening and ascorbic acid loss [144,145,146,147]. When temperatures fall below 16 °C for extended periods, growth slows and fruit surface defects (e.g., microcracking) become more frequent [145,146]. A distinct diurnal rhythm, with sufficiently cool nights, improves tissue elasticity and supports thicker cuticle formation, contributing to extended shelf life [144,145,148].

Table 4.
Effects of environmental factors during greenhouse cultivation on major quality traits and shelf life (SL) in sweet pepper. ascorbic acid = vitamin C; B, blue (spectra); Ca, calcium; CO2, carbon dioxide; DIF, day–night temperature difference; DLI, daily light integral; EC, electrical conductivity of the nutrient solution; IPM, integrated pest management; K, potassium; N, nitrogen; R, red (spectra); RH, relative air humidity; Si, silicon; T, temperature; VPD, vapor pressure deficit. Arrows indicate direction of projected change (↑ increase, ↓ decrease). Definitions of physiological disorders are provided in Supplementary Table S1.
Across species, moderate day temperatures with cooler nights (i.e., positive DIF) support firmer, better-colored fruit, whereas excessive heat or warm nights accelerate deterioration.
3.2. Root-Zone Temperature
Root-zone temperature (RZT) also affects fruit quality by controlling nutrient uptake, water absorption, and root-to-shoot hormonal signaling [180,181]. In hydroponic and substrate-based systems, RZT can be decoupled from the aerial climate, enabling targeted manipulation to improve nutritional quality and stress tolerance.
In tomato, root zones maintained between 18 and 22 °C are generally considered optimal for root activity and nutrient absorption [182,183]. RZT below 15 °C limits water uptake and promotes Ca-related disorders (e.g., BER) [182,184]. Conversely, RZT above 25 °C favors vegetative over reproductive growth and reduces color and °Brix [185,186,187]. Seasonal RZT control (i.e., heating in cool periods and moderate cooling during heat) stabilizes nutrient flow, enhances lycopene and °Brix, and reduces blotchy ripening [188,189].
Cucumber roots are highly sensitive to thermal fluctuations because of their shallow distribution and high transpiration demand [190,191]. Optimal RZT is ~22–25 °C, supporting steady growth and fruit firmness [190,191,192]. Cooling below 18 °C reduces water uptake, causing dull fruit appearance and slower growth, whereas RZT above 28 °C increases respiration and promotes watery texture [191,192,193]. Warm root zones can ease chilling stress, but chronic overheating accelerates senescence. Studies using localized root-zone heating or cooling show that stable temperatures near 24 °C improve epidermal development and delay yellowing, highlighting the importance of thermal homogeneity [192,194].
Sweet pepper tolerates a slightly broader RZT range, with optimum values between 20 and 25 °C [195,196,197]. Cooler root zones delay growth and limit K and Ca uptake, reducing pericarp firmness, whereas warmer conditions promote rapid fruit enlargement but may lower dry matter (DM) and ascorbic acid content [195,197,198]. Root-zone heating in winter improves fruit set and enhances color uniformity, yet sustained high RZT in summer increases susceptibility to soft rot and shortens shelf life [195,196,197]. Balanced root-zone thermal management thus supports nutrient supply, pericarp thickness, and improved storage performance.
Across all three crops, stable moderate RZT complements optimal air temperature to maintain nutritional and structural quality [162,180], highlighting the root zone’s central role in regulating growth, nutrition, and fruit integrity.
3.3. Light Intensity, Photoperiod, and Spectral Quality
Light drives photosynthesis and regulates development, metabolism, and morphology. In greenhouses, its intensity and duration (together expressed as the daily light integral, DLI), along with spectral quality, shape both yield and fruit quality [65,85]. Modern glazing, light-emitting diodes (LEDs), and shading allow targeted control of these components to balance productivity and quality.
In tomato, moderate to high light integrals are associated with improved nutritional quality (Table 2) [59,64,199]. An increase in DLI enhances assimilate production, leading to higher soluble solids, sugars, and DM that support fruit flavor and firmness [61,63]. High irradiance also promotes lycopene and β-carotene accumulation through enhanced phytoene synthase activity and faster chloroplast-to-chromoplast conversion during ripening [55,64,86]. Conversely, light limitation caused by seasonal low radiation or excessive shading reduces pigment synthesis, lowers sugar-to-acid ratio, and produces pale, watery fruit [61,200]. Photoperiod effects are secondary, but extended daylength accelerates ripening and improves color uniformity [65,199]. Spectral composition further refines these responses: blue (B) light enhances ascorbic acid and phenolics, strengthens cuticle deposition, and improves firmness, whereas red (R) light boosts photosynthetic productivity and lycopene formation [64,201,202]. A balanced R–B ratio is widely regarded as optimal for both yield and quality. Far-red (FR) light influences plant morphology and assimilate partitioning by altering the R to FR ratio [203,204,205]. When FR is dominant, it can elongate stems and reduce fruit firmness, while moderate FR may enhance color development by facilitating chloroplast-to-chromoplast transition [202,204,205]. Ultraviolet-A (UV-A) exposure within safe limits may increase phenolic compounds and antioxidant capacity, although excessive UV causes surface damage and shortens shelf life [96].
Cucumber shows a distinct light response due to its thin pericarp and high WC [38]. Adequate light intensity during fruit development is essential to maintain firmness and avoid overly elongated or pale fruits (Table 3) [109]. Under low DLI conditions, fruits tend to be larger yet less dense, with reduced mechanical strength and shorter shelf life [206,207]. In contrast, high light, particularly with moderate B or UV-A enrichment, promotes epidermal chlorophyll retention and increases phenolics, enhancing green color and delaying yellowing during storage [108,109]. Spectral manipulation further modulates epidermal traits. B light generally promotes thicker cuticle and stronger cell adhesion, whereas R-dominant light accelerates elongation and can result in thinner pericarps [208,209,210]. FR supplementation increases fruit length and overall yield but may reduce firmness [211]. Moderate UV induces defensive secondary metabolites, improving resistance to postharvest decay, while excessive UV causes surface scalding and accelerated chlorophyll degradation, highlighting the narrow window between beneficial and damaging light regimes [108,193].
In sweet pepper, light intensity and spectrum strongly influence pigmentation and antioxidant status (Table 4) [143,149]. High DLI enhances photosynthetic activity and supports ascorbic acid and carotenoid accumulation, key determinants of nutritional quality and visual appeal [212]. Insufficient light, as in winter in low-tech greenhouses, delays ripening and leads to dull coloration and reduced sugar content [143,149,151]. B light enhances antioxidant enzyme activity and phenolic synthesis, contributing to higher radical-scavenging capacity, while R light favors carotenoid biosynthesis, particularly capsanthin and capsorubin during the red-ripening phase [212,213]. Supplemental R + B lighting improves color saturation and nutritional value compared with natural light alone [213,214]. FR radiation accelerates color transition but may cause softening when overapplied, whereas UV-A can boost phenolic and flavonoid concentrations without detrimental effects when carefully controlled [153,212]. The photoperiod effect is minor but can influence ascorbic acid retention by modulating the daily duration of photosynthetic activity [215].
Across all three crops, light intensity has a stronger influence on nutritional composition than on shelf life, though both are linked. High DLI improves DM and firmness via greater assimilate supply, while B and UV-A light enhance antioxidant metabolism and slow deterioration. By contrast, low or imbalanced light produces softer, poorly pigmented fruits with reduced storability. Integrated management of DLI, R−B balance, and limited FR or UV-A exposure yields produce with high nutritional value and improved postharvest stability.
3.4. CO2 Enrichment
CO2 enrichment is widely used in greenhouses to enhance photosynthesis and yield, but it also influences fruit composition, firmness, and postharvest behavior [114,216,217]. Its effects depend on CO2 concentration and exposure duration, as well as interacting factors such as light, temperature, RH, and species-specific physiology.
In tomato, CO2 enrichment from ~400 to 700–1000 µmol mol−1 enhances photosynthesis and increases carbohydrate availability for fruit growth [59,66]. Elevated CO2 generally raises soluble solids, sugars, and DM, improving flavor and density (Table 2) [59,62,66]. This effect reflects greater source strength and enhanced assimilate translocation to the fruits [59]. Several greenhouse studies report improved firmness and slower softening under moderate enrichment due to better cell wall integrity and reduced respiration rate at harvest [58,62,114]. At the biochemical level, CO2 enrichment may dilute organic acids, raising the sugar-to-acid ratio and altering flavor balance. Lycopene and β-carotene are typically maintained or modestly increased under adequate light, whereas excessive CO2 under high temperature or low radiation can limit pigment synthesis via feedback constraints on carbon (C) metabolism [59,62,66]. Ascorbic acid levels sometimes decline under high CO2, possibly due to lower oxidative stress and reduced need for antioxidant synthesis, although this response is inconsistent across cultivars [62,67]. Overall, elevated CO2 combined with sufficient light and balanced nutrition produces firmer, well-colored tomatoes with improved shelf life.
Cucumber shows a pronounced yield response to CO2 enrichment, reflecting its high photosynthetic demand and rapid fruit growth rate [218,219]. Concentrations of ~800–1000 µmol mol−1 increase fruit number and size, while maintaining or slightly improving firmness and DM (Table 3) [113]. Enhanced C assimilation accelerates cell expansion and cuticle formation, contributing to smoother, more uniform surfaces. However, when CO2 enrichment occurs under high RH or low light, the rapid enlargement may lead to watery tissue and thinner epidermis, reducing postharvest firmness and increasing decay susceptibility [112,113,220]. Studies indicate that moderate CO2 levels with adequate light and ventilation improve epidermal structure, lower transpiration rates, and reduce postharvest water loss. Effects on antioxidant compounds (e.g., ascorbic acid and phenolics) are variable, but can show mild increases when CO2 enrichment is paired with moderate light and balanced nutrition [112,221]. Under excessive enrichment or poor ventilation, internal CO2 buildup may alter pH and accelerate yellowing, emphasizing the need for tight control [218,222].
In sweet pepper, CO2 enrichment supports both productivity and nutritional quality [156,157]. Increased C availability boosts carbohydrate concentration and stimulates ascorbic acid and carotenoids, especially under strong light and moderate temperature (Table 4) [155,156]. Fruits grown under elevated CO2 typically exhibit thicker pericarp, higher DM, and improved firmness, traits associated with longer shelf life [151,155]. The mechanism involves more efficient photosynthesis and greater assimilate allocation to fruit tissues, enhancing cell wall deposition and turgor. However, as in tomato, excessive enrichment without sufficient light can cause carbohydrate dilution and lower pigment accumulation [59,223]. Postharvest responses are generally positive: lower respiration, delayed softening, and reduced water loss have been observed in CO2-enriched fruits [154,224]. Moreover, higher ascorbic acid levels increase antioxidant capacity, slowing oxidative degradation during storage [151,156].
Across all three crops, CO2 enrichment at 700–1000 µmol mol−1 under adequate light and ventilation improves nutritional and structural quality, increasing sugars, firmness, and sometimes antioxidants to extend shelf life. Responses, however, depend on conditions: under low light or high RH, yield gains may be accompanied by poorer tissue quality. Thus, effective CO2 management must be integrated with light and temperature control to ensure both high productivity and strong postharvest performance.
3.5. Relative Air Humidity, Vapor Pressure Deficit, and Air Movement
Greenhouse humidity, expressed as RH or its functional equivalent, the vapor pressure deficit (VPD), strongly regulates transpiration, nutrient transport, and fruit surface development, thereby shaping both growth and postharvest quality. Because RH control depends on ventilation and heating, it is difficult to manage and directly affects nutritional composition, firmness, and shelf life [225,226].
In tomato, moderate VPD levels (~0.5–1.0 kPa) promote balanced transpiration and adequate Ca transport to developing fruits (Table 2) [68,70,71]. Under such conditions, normal cuticle formation supports firm fruit with low susceptibility to cracking or BER. When RH is excessively high (above 85–90%), transpiration declines, Ca distribution becomes uneven, and the cuticle softens, increasing susceptibility to microcracks and pathogen infection [69,227]. High RH also suppresses the synthesis of structural epidermal waxes, producing a thinner and more permeable surface that accelerates postharvest water loss [116,227]. In contrast, very low RH (high VPD > 1.2–1.5 kPa) increases transpiration and mechanical stress, resulting in smaller fruits, tougher skin, and sometimes reduced sugar accumulation [70,71]. Moderate air circulation enhances boundary-layer exchange, stabilizes transpiration, and prevents condensation, which otherwise encourages fungal decay. Greenhouse studies consistently show that maintaining moderate VPD and gentle airflow results in thicker cuticles, firmer texture, and improved shelf life without compromising yield [69,71,227].
Cucumber responds more acutely to RH fluctuations because of its high transpiration rate and thin epidermis [115,228]. High RH during fruit expansion produces glossy, tender fruits with fragile epidermal layers prone to postharvest shrinkage and decay (Table 3) [117,118]. Under persistently humid conditions, Ca and boron transport to the fruit surface declines, weakening cell adhesion and promoting cuticle microfissures [228,229]. These defects subsequently manifest as accelerated yellowing and water loss during storage. Conversely, very low RH (<60%) increases transpiration beyond the plant’s hydraulic capacity, leading to localized wilting and roughened skin [220,230]. Greenhouse experiments demonstrate that maintaining VPD ~0.8–1.0 kPa ensures adequate water transport without compromising epidermal integrity [231,232]. Active airflow mitigates condensation on fruit surfaces, reduces microbial colonization, and improves uniform cooling at night, collectively contributing to extended shelf life. Some studies also link slightly higher VPD with elevated concentrations of soluble solids and phenolics, indicating that mild evaporative demand may stimulate secondary metabolism and improve nutritional quality when properly managed [120,233].
Sweet pepper shows greater tolerance to high RH compared with tomato and cucumber, yet it remains sensitive to prolonged saturated conditions [159,160]. When RH exceeds 90%, pollen viability declines, Ca transport becomes irregular, and the resulting fruits often exhibit softer pericarps and reduced firmness (Table 4) [159,160,161]. High RH also favors cuticle disorder, producing wrinkled or dull surfaces [160,234]. Conversely, very low RH increases transpiration and can cause microcracks at the fruit shoulder, leading to higher postharvest water loss. Maintaining intermediate VPD (~0.7–1.0 kPa) supports pericarp strength and uniform cuticular deposition, enhancing firmness and water-retention capacity [160,235]. Studies in greenhouse environments show that peppers grown under moderate VPD accumulate more soluble solids and ascorbic acid compared with those under extreme dryness or excessive RH [160,236]. Gentle but continuous air movement reduces local RH pockets in dense canopies, improves leaf cooling, and limits fungal contamination on fruit surfaces, thereby extending shelf life.
RH and VPD influence postharvest behavior mainly by regulating water relations and Ca distribution. High RH produces smooth but weak fruit, while moderate evaporative demand promotes denser tissues and thicker cuticles. With adequate air movement, moderate VPD and proper nutrition together support high nutritional quality and storage stability across all three crops.
3.6. Irrigation Regime and Water Status
Water management strongly influences crop physiology and the traits that determine postharvest longevity. Irrigation governs hydration, assimilate allocation, and nutrient transport, allowing precise control of yield and quality [237,238]. Regulated deficit irrigation (RDI), which applies mild, stage-specific water limitation, is increasingly used to enhance fruit nutritional density without causing lasting stress [238,239].
In tomato, greenhouse studies show that moderate irrigation reductions during fruit expansion or ripening increase soluble solids, sugars, organic acids, and DM, yielding fruit with improved flavor and a higher sugar-to-acid ratio (Table 2) [72,74]. The underlying mechanism reflects lower fruit WC and stimulated osmolyte and sugar synthesis under mild water deficit [74,238]. Phenolics and ascorbic acid often rise, indicating enhanced antioxidant metabolism [12]. However, when water deficit is too severe or imposed early, it restricts Ca transport and cell expansion, producing smaller fruit, softening, and higher BER susceptibility [240,241]. Thus, proper timing and intensity are critical: moderate RDI late in the cycle improves quality, whereas prolonged or erratic deficits compromise yield and shelf life. Overirrigation, in contrast, promotes vegetative growth, dilutes soluble solids, and leads to watery, fragile fruits that deteriorate rapidly after harvest [59,75]. Slight root-zone tension produces firmer fruit with thicker cuticle and reduced postharvest water loss [72,242].
Cucumber is particularly sensitive to fluctuations in water availability because of its high transpiration rate and limited osmotic buffering (Table 3) [38,95]. Consistent moisture is required to maintain steady fruit enlargement and epidermal turgor. Even short-term deficits result in curvature, reduced size, and roughened surface texture [120]. Severe water limitation causes pitting, dull coloration, and accelerated yellowing [243,244]. Nonetheless, mild deficit regimes (~10–20% irrigation reduction) can modestly increase DM and firmness without yield loss, provided that RH and nutrients remain favorable [119,120]. These slight restrictions enhance cuticle deposition and reduce internal voids, improving water retention [119]. Overirrigation, by contrast, produces fruits with high WC and low structural density, prone to rapid softening and decay [119,120,121]. Thus, while cucumber benefits less from RDI than tomato, maintaining near-saturated but uniform moisture remains essential for firm, durable fruits.
In sweet pepper, controlled irrigation deficits can enhance nutritional and sensory quality when properly timed (Table 4) [162,165]. Moderate reductions in irrigation during maturation elevate soluble solids, ascorbic acid, and phenolics, indicating activation of antioxidant pathways [162,164,165]. Mild water stress enhances assimilate partitioning to the fruits and can increase pericarp thickness, improving firmness and reducing postharvest water loss [162,165,245]. However, strong or prolonged deficits impair Ca transport, leading to soft pericarps and BER–like symptoms, particularly under high evaporative demand [151,164,165]. Overirrigation dilutes sugars and acids, producing bland-tasting fruits with thin walls and short shelf life [163,164]. Thus, optimal irrigation maintains near field-capacity moisture during flowering and fruit set, followed by moderate deficits during ripening.
Across the three species, balancing adequate water with mild stress is essential for maintaining yield and postharvest quality. Well-timed RDI enhances soluble solids, antioxidants, and firmness, while excessive deficits or overirrigation weaken structure and reduce storability. Sensor-based irrigation that maintains slight root-zone tension offers the most consistent quality and water-use efficiency [239,246].
3.7. Fertigation: Nitrogen, Potassium, Calcium, Electrical Conductivity, and Moderate Salinity
Nutrient management via fertigation strongly influences fruit composition and postharvest stability. The supply of nitrogen (N), K, Ca, and the electrical conductivity (EC) of the solution determine the balance between vegetative growth and quality [200,247]. Moderate salinity can enhance flavor, firmness, and antioxidants, whereas excessive or unbalanced fertilization leads to nutrient disorders and reduced storability.
In tomato, the interplay between N and K is central to fruit quality (Table 2) [80]. High N availability promotes vigorous vegetative growth and large fruit size but dilutes soluble solids and acids, producing fruit of inferior flavor and texture [248,249]. Conversely, moderate N reduction near ripening concentrates sugars and improves the sugar-to-acid ratio [249,250]. K enhances sweetness, color, and firmness by facilitating carbohydrate translocation and activating carotenoid and organic acid enzymes [76]. High K supply increases lycopene content and TA, while improving tissue strength through osmotic regulation [76,251]. Ca stabilizes cell walls and membranes [252], and adequate Ca transport during fruit development is essential to prevent BER and maintain firmness [253]. Ca-deficient fruits show localized tissue collapse and reduced shelf life due to weakened pectin cross-linking [18]. Optimal EC generally ranges from 2.5 to 4.0 dS m−1 [254], within which moderate salinity acts as a beneficial stress, reducing water uptake and increasing DM, sugars, acids, and phenolics [255,256]. When EC exceeds ~5 dS m−1, yield losses and physiological disorders outweigh quality gains [256,257]. Greenhouse trials show that controlled salinity and balanced K/Ca ratios produce tomatoes with higher °Brix, stronger pericarp, and longer shelf life [59,258].
Cucumber responds differently to nutrient concentration because of its high water demand and osmotic sensitivity. High N promotes rapid growth and large fruits but results in excessive WC and soft texture, with diluted sugars and reduced firmness [259]. Slight N reduction can increase DM and firmness without yield loss when K and Ca are sufficient [122,123]. K enhances color intensity and firmness, while maintaining turgor [260,261]. Ca supplementation strengthens the epidermis and cuticle, improving resistance to postharvest water loss [262]. Optimal EC for cucumber fertigation is ~2.0–2.5 dS m−1 [263,264]. Moderate salinity within this range can enhance structural density and reduce decay incidence, but higher salinity (>3.5 dS m−1) impairs water uptake, induces bitterness through cucurbitacin accumulation, and shortens shelf life [265,266]. Nitrate balance also affects fruit quality, as excess nitrate favors rapid elongation but produces pale, watery tissue [267,268]. Thus, fertigation regimes emphasizing K and Ca relative to N are essential for yield and postharvest resilience.
Sweet pepper responds strongly to nutrient balance, particularly to K and Ca availability. K enhances fruit firmness, color development, and ascorbic acid/carotenoid synthesis, while Ca ensures mechanical strength and limits softening [269,270]. High N during fruit development encourages vegetative growth and delays ripening, producing fruit with lower sugar and vitamin content [167,271]. Lowering N and increasing the K/N ratio near maturity often improves flavor and nutritional quality. Ca deficiency causes localized tissue collapse at the blossom end or pericarp, severely reducing marketability and storage life [159,272]. Maintaining adequate Ca in the nutrient solution and avoiding excessive RH that restricts transpiration are key to consistent uptake. The ideal EC for pepper fertigation is ~2.0–3.0 dS m−1 [273]. Moderate salinity enhances ascorbic acid and DM, whereas excessive salt stress reduces fruit size and firmness [274]. Balanced K– and Ca-rich fertigation under moderate EC produces fruit of higher nutritional density and improved keeping quality.
Optimal fertigation balances N, K, Ca, and moderate EC to impose mild, beneficial stress that boosts soluble solids, pigments, and antioxidants, producing firmer, denser fruit with better shelf life. Excessive N or low EC leads to dilute, soft fruit, while moderate salinity strengthens tissues and reduces postharvest water loss.
3.8. Canopy Management: Density, Pruning, Fruit Load, and Fruit Position
Canopy structure and management strongly influence fruit nutritional quality and postharvest performance. Plant density, pruning, and fruit load regulate light distribution, source–sink balance, and microclimate, thereby affecting assimilate supply and fruit maturation. Fruit position within the canopy further alters light and temperature exposure, contributing to variability in composition and shelf life.
In tomato, plant density and pruning strongly affect light interception and assimilate partitioning. High planting densities increase overall yield per unit area but reduce light penetration and elevate RH, producing softer fruits with lower soluble solids and greater decay susceptibility [275,276]. Reduced light also limits lycopene accumulation, leading to paler fruit and lower nutritional value [64,277]. Moderate plant density combined with systematic pruning maintains an open canopy, improves airflow, and supports uniform fruit development. Pruning to a single or double stem promotes efficient source–sink balance, yielding larger, sweeter, and firmer fruits [278,279]. Over-pruning can limit photosynthesis, leading to unbalanced ripening and lower antioxidant content [280,281]. Fruit load management is also critical. Excessive fruiting dilutes assimilates per fruit, lowering sugar and acid content, while moderate load improves compositional density and firmness [64,282]. The order of fruit within the truss affects quality. Basal fruits, which develop earlier and under stronger assimilate competition, often reach larger size but show lower DM and shorter shelf life than distal fruits [283,284]. Maintaining uniform cluster load and regular removal of side shoots helps standardize fruit quality across harvests.
Cucumber production relies on continuous fruiting along indeterminate vines, making canopy management essential for balancing vegetative vigor with fruit quality. High plant densities promote mutual shading and reduce airflow, leading to elongated, less firm fruits with thinner epidermis [24,285]. Lower densities improve light penetration and photosynthetic efficiency, producing firmer fruits with more uniform color [285,286]. Pruning mainly involves removing lateral shoots and old leaves to keep light reaching developing fruits. Excess foliage increases RH and disease incidence, thereby reducing shelf life [287,288]. Fruit load regulation is crucial because each fruit is a dominant sink. Allowing too many fruits reduces individual growth rate and firmness, whereas moderate load increases DM and structural integrity [289,290]. Fruit position also affects quality. Basal fruits often have thicker cuticle and higher firmness but may show curvature, whereas upper-node fruits, exposed to higher irradiance, retain better color and postharvest appearance [285,290]. Maintaining a steady harvest rhythm through consistent pruning and leaf renewal helps stabilize quality across harvests.
Sweet pepper, characterized by a more compact canopy, is sensitive to plant spacing and load management. Dense planting reduces airflow and light availability to lower leaves and fruits, resulting in thin-walled peppers with lower ascorbic acid and carotenoid contents [291,292]. Wider spacing improves light penetration and promotes thicker pericarp and higher pigment levels, though very low density can reduce yield [170,293]. Moderate pruning to remove shaded or senescent leaves improves microclimate and limits condensation-related disorders. Fruit load regulation strongly affects nutritional quality and shelf life. High load increases assimilate competition and delays ripening, producing fruits with lower sugars and vitamins, whereas moderate thinning enhances sweetness, firmness, and color uniformity [294,295]. Fruit position also creates compositional gradients. Upper-canopy fruits exposed to stronger irradiance accumulate more ascorbic acid and carotenoids but are more prone to sunscald, whereas inner-canopy fruits have paler color and softer texture but retain water better during storage [149,153]. A balanced canopy that provides partial shading under high light optimizes both quality and longevity.
Canopy management links greenhouse conditions to fruit physiology. Optimal plant density, pruning, and fruit load ensure uniform light and microclimate, enhancing sugars, acids, antioxidants, and firmness. Poorly structured canopies create uneven light and RH, causing variable ripening and inconsistent postharvest quality. Positional effects highlight the need for uniform exposure and balanced load. Thus, optimizing canopy structure is essential not only for yield but also for nutritional value and shelf life.
3.9. Seasonality: Temperature, Light Level, and Relative Air Humidity
Seasonal shifts in temperature, light, and RH strongly affect greenhouse vegetable quality. Cool, low-light periods slow growth and sugar/pigment formation but produce firmer, longer-lasting fruit, whereas summer heat and high radiation accelerate ripening, improving color and sweetness but reducing firmness and shelf life. RH further modulates these outcomes via transpiration and Ca mobility.
Tomato responds strongly to seasonal changes in temperature, light and RH. Under winter conditions (i.e., low radiation and moderate to high RH), ripening proceeds slowly, producing fruits with lower soluble solids, reduced lycopene, and higher acidity [64,282]. Such fruits retain greater firmness and storability due to slower respiration and cell-wall degradation. In contrast, summer conditions (i.e., high radiation and fruit temperatures > 30 °C) accelerate sugar and pigment accumulation but reduce firmness and shelf life [56,296]. RH fluctuations further modify these outcomes. High RH during cool periods impedes Ca transport and predisposes fruits to cracking, while low RH in hot months increases water loss and cuticular stress [242,297]. The magnitude of these seasonal contrasts depends on greenhouse technology level. Low-technology structures track ambient conditions, producing firm but pale fruits in winter and brightly colored yet short-lived fruits in summer [298,299,300]. Medium-technology greenhouses with partial heating and shading moderate these differences, while high-technology systems with full climate control largely eliminate them [298,301,302]. Across all systems, fruit temperature, driven by radiation load, remains the primary factor explaining seasonal differences in nutritional composition and shelf life.
Cucumber exhibits pronounced seasonal sensitivity because of its thin epidermis and high WC. During the cool season, low radiation and temperature slow growth, producing smaller but denser fruits with darker green color, higher firmness, and improved water retention [289,303]. These fruits store longer but contain lower soluble solids. In summer, abundant radiation and high air temperature accelerate fruit elongation, producing larger, lighter-colored fruits with higher initial sweetness but faster postharvest softening and yellowing [105,304]. Seasonal changes in RH strongly influence epidermal integrity. High RH during winter promotes smooth skin but favors decay after harvest, whereas the lower RH of hot periods enhances firmness but may cause surface roughness [104,305]. In low-technology greenhouses, these patterns are amplified, leading to greater variability in fruit firmness and shape [95,306]. Medium-technology systems reduce this variability through better ventilation and shading, while high-technology facilities maintain stable conditions that largely eliminate seasonal contrasts [307,308]. As with tomato, radiation-driven fruit temperature remains the dominant factor determining seasonal variation, controlling both firmness development and water-loss rate during storage.
In sweet pepper, seasonality governs both pigment biosynthesis and postharvest behavior. Winter fruits under low radiation accumulate less ascorbic acid and carotenoids but maintain high firmness and thick pericarps, supporting superior shelf life [142,146,309]. Summer fruits ripen rapidly under intense radiation and high temperature, achieving vivid coloration and elevated antioxidant content but undergo faster softening and higher water loss after harvest [142,310]. Seasonal RH differences compound these effects. High RH in cool months favors smooth surface texture but restricts Ca transport, whereas drier summer air increases transpirational stress and microcracking risk [116,160]. In low-technology greenhouses, summer fruits are visually attractive but short-lived, while winter fruits are mechanically strong yet less colorful [95,166]. Medium-technology systems with adjustable shading and ventilation improve microclimate stability and reduce heterogeneity in fruit size and maturity [95,311]. High-technology environments with automated temperature and RH regulation produce more uniform fruits throughout the year, minimizing seasonal variability [307,312]. For sweet pepper, as for tomato and cucumber, the primary determinant of seasonal quality differences is fruit-surface temperature, which integrates the effects of radiation intensity and RH on metabolic and structural processes.
Across all three crops, seasonal variation in quality is driven mainly by radiation load and the resulting fruit temperature, which regulate pigment and sugar formation as well as respiration, softening, and water loss. RH plays a secondary role. Seasonal contrasts are strongest in low-technology greenhouses, reduced in medium-technology systems, and minimal in high-technology facilities with stable climate control. Maintaining steady fruit temperature through shading, ventilation, and heating is key to achieving uniform year-round quality.
3.10. Cover Materials and Shading Strategies
Greenhouse cover properties control light transmission and internal temperature, strongly shaping fruit microclimate and quality. Shading reduces excess radiation in hot regions, while high transmission is essential in low-light periods to sustain pigment and sugar formation. The impact of these strategies varies with crop species, canopy structure, and greenhouse technology level.
Tomato benefits from cover materials that balance radiation transmission and heat control. Transparent polyethylene (PE) films with high light transmittance enhance photosynthesis in winter but can cause summer overheating [83,313]. Excessive radiation and fruit surface temperature above 30 °C reduce lycopene accumulation and increase softening, shortening shelf life [314,315]. The use of diffusive films, which scatter light without major loss of transmission, improves canopy light uniformity and reduces localized overheating [83,316]. Fruits under diffusive covers show higher lycopene and β-carotene, better color uniformity, and lower cracking incidence [317]. In high-radiation seasons, movable shading screens or reflective nets reduce fruit temperature and prevent sunscald while maintaining adequate photosynthetically active radiation (PAR) [300,318]. Studies show that moderate shading (~20–30%) preserves firmness and reduces postharvest decay without significantly decreasing soluble solids [87,300]. In low-radiation seasons, retracting shading materials or using high-transmission films enhances light interception and compensates for low DLI [83,319]. Thus, dynamic shading systems combining diffusive covers and adjustable screens provide the best balance of color development, firmness, and storability in greenhouse tomato.
Cucumber is particularly sensitive to high radiation and temperature due to its tender epidermis and rapid water loss. Cover materials that moderate radiation load and temperature are therefore critical to maintaining postharvest quality [127,320]. In low-tech structures, non-diffusive transparent films often permit excessive solar gain, resulting in elevated fruit temperature, curvature, and reduced firmness [304,321]. The adoption of diffusive PE films or white shading nets during summer lowers internal radiation by 20–40%, reducing heat stress and fruit yellowing [322,323]. Under diffusive light, canopy photosynthesis becomes more homogeneous, and fruits develop smoother surfaces with stronger cuticle integrity. However, excessive shading (>50%) reduces assimilate supply, leading to soft, watery fruits and diminished sugar content [322,324]. In winter, high-transmission films improve light penetration, maintaining green color retention [325,326]. The introduction of anti-drip coatings further enhances quality by preventing condensation, which otherwise promotes fungal infection and uneven epidermal development [327,328]. Overall, optimal cucumber management combines moderate diffusive shading in high-radiation periods with high-transmission films in low-radiation periods, ensuring balanced temperature control and consistent firmness.
Sweet pepper production benefits from controlled light diffusion and thermal moderation to prevent sunscald while maintaining pigment formation. In summer, direct radiation on exposed fruits can raise surface temperature above 40 °C, leading to bleaching and tissue collapse [329,330]. Diffusive films and aluminized shading nets reduce direct light, improve radiation distribution, and lower canopy temperature, preventing such injuries [149,170,331]. Fruits grown under diffusive light display improved color uniformity and higher carotenoid and ascorbic acid concentrations, likely due to reduced photooxidative stress and more uniform energy distribution within the canopy [149]. Moderate shading preserves firmness and limits water loss, extending shelf life, whereas heavy shading reduces sugar and pigment accumulation, producing dull-colored fruits with lower nutritional value. During winter, transparent or anti-reflective covers maximize radiation input, maintaining photosynthesis and promoting pigment synthesis [95,332]. Dynamic shading systems in mid- and high-technology greenhouses enable finer control of light intensity and reduce quality heterogeneity.
Across all three crops, effective optical management of covers and shading is essential for stabilizing microclimate, fruit composition, and shelf life. Diffusive films enhance light uniformity and lower fruit temperature, while moderate shading limits heat and oxidative stress during high-radiation periods. High-transmission covers in low-light seasons support assimilation and pigment formation, whereas excessive shading reduces sugars and carotenoids. Overall, the benefits of optimized optical materials stem from their ability to regulate fruit surface temperature, making adaptive shading and diffusion key components of climate-smart greenhouse design.
3.11. Biostimulants and Mineral Fortification
Beyond conventional fertilization, biostimulants and mineral fortifiers are increasingly used to improve fruit nutritional quality and postharvest resilience in greenhouse vegetables. Biostimulants (e.g., seaweed extracts, protein hydrolysates, humic substances, microbial inoculants, and chitosan) enhance nutrient uptake, modulate physiology and activate antioxidant defenses [333]. Mineral fortification with Ca or silicon (Si) strengthens cell walls and cuticular barriers, improving firmness and stress resistance. These inputs operate at the intersection of nutrition and stress physiology, complementing environmental control, although their effects vary among species.
In tomato, preharvest application of biostimulants and Ca fortification enhances nutritional and structural quality attributes [77,334]. Foliar or root applications of Ca salts increase membrane stability and reduce BER by improving Ca partitioning to the fruit [241,335]. Higher Ca concentrations in pericarp tissue correlate with slower softening and reduced postharvest decay [336,337]. Si supplementation strengthens epidermal tissues and improves tolerance to oxidative and thermal stress [338,339], resulting in firmer fruits and lower weight loss during storage. Biostimulants (e.g., chitosan, seaweed extracts, and amino-acid-based formulations) stimulate phenolic metabolism and elevate antioxidant capacity, increasing total phenolics, ascorbic acid, and lycopene [340]. Chitosan also forms a semi-permeable coating that reduces transpiration and respiration, thereby prolonging shelf life [341,342]. Studies show that biostimulants enhance antioxidant enzyme activity (superoxide dismutase, catalase, and peroxidase), delaying senescence and improving color stability during storage [343,344]. Thus, combining biostimulants with mineral fortification provides synergistic gains in nutritional density and mechanical resilience.
Cucumber responds markedly to Si and Ca supplementation, reflecting its high WC and thin cuticle [345,346]. Ca fortification during fruit development enhances epidermal strength and cohesion, reducing postharvest softening and surface collapse [299,347]. Adequate Ca supply also diminishes tipburn and localized pitting, disorders linked to uneven Ca distribution under fluctuating RH [348,349]. Si application improves resistance to biotic and abiotic stress by reinforcing the cuticular–cell wall complex and stimulating phenolic synthesis [135,350], producing firmer fruits with lower water loss and higher antioxidant capacity. Biostimulants (e.g., seaweed extracts and humic substances) promote root activity and improve mineral uptake, supporting turgor and chlorophyll maintenance [132,351]. Chitosan treatments delay postharvest yellowing by suppressing chlorophyll-degrading enzymes and enhancing antioxidant defense [352]. However, excessive or frequent biostimulant use can cause cuticular roughness or altered surface color, indicating the need for precise dosing. Overall, balanced Ca–Si nutrition combined with mild biostimulant use enhances both physical and biochemical stability of greenhouse-grown cucumber.
In sweet pepper, mineral fortification and biostimulant treatments strongly influence firmness, color development, and antioxidant status [353,354]. Ca supplementation, particularly during rapid fruit expansion, enhances pericarp thickness and firmness while preventing localized tissue collapse [269,272]. Adequate Ca also reduces physiological softening and postharvest decay. Si fortification increases cell-wall silicification and strengthens the antioxidant system, leading to higher ascorbic acid and carotenoid retention during storage [355]. Foliar Si sprays mitigate heat and light stress, resulting in brighter color and delayed senescence [355]. Biostimulants (e.g., seaweed extracts and protein hydrolysates) stimulate phenylpropanoid metabolism and ascorbic acid synthesis, enhancing nutritional quality [356,357]. Chitosan and microbial inoculants promote systemic resistance and reduce disease incidence, extending postharvest life [356,357,358]. Treated fruits typically display greater firmness, lower transpiration losses, and more uniform pigmentation [358]. These benefits are most evident in mid- and late-season crops under elevated radiation and temperature. Thus, integrating biostimulant and mineral treatments supports structural integrity and nutritional value under variable environmental conditions.
Across all three crops, biostimulants and mineral fortifiers improve fruit quality by enhancing physiological resilience rather than supplying nutrients. Ca strengthens membranes and cell walls, Si reinforces the epidermis and antioxidant systems, and biostimulants stimulate secondary metabolism. These actions increase firmness, antioxidant capacity, and shelf life, with best results under balanced fertigation and moderate stress. Combined Ca–Si fortification and periodic biostimulant use reliably improve structural integrity, nutritional value, and postharvest performance in greenhouse production.
3.12. Pest and Disease Pressure and Residue-Free Strategies Affecting Quality
Pest and disease management affects fruit quality both directly and indirectly. Pest pressure reduces photosynthesis, assimilate flow, and water balance, producing irregular fruits with weaker structure and altered composition. Conversely, excessive chemical control risks residue accumulation and may disrupt normal metabolism. Modern greenhouse production therefore relies on residue-free integrated pest management (IPM), combining biological control, natural elicitors, and cultural or physical measures to maintain plant health while supporting fruit quality and shelf life. The effectiveness of these strategies varies with species and their specific pest and pathogen sensitivities.
In tomato, pest and disease pressure affects fruit quality primarily through its influence on photosynthesis and vascular transport. Infestation by whiteflies, thrips, or spider mites reduces leaf assimilation area and induces stress responses that divert resources from fruit growth to defense [359,360]. Such stress often produces smaller fruits with elevated phenolic and flavonoid content [361]. While moderate biotic stress can enhance antioxidant capacity, severe or chronic infestation causes nutrient depletion and reduced firmness. Pathogenic infections (e.g., Botrytis cinerea or Cladosporium fulvum) reduce yield and accelerate postharvest decay by increasing fungal inoculum on the fruit surface [362,363]. Residue-free strategies based on biocontrol agents (e.g., Trichoderma harzianum, Bacillus subtilis) and elicitor compounds (e.g., chitosan, salicylic acid, seaweed extracts) strengthen plant defense and improve fruit biochemical profile [364,365]. Chitosan induces phenylpropanoid metabolism, enhancing phenolic and lycopene accumulation, while microbial biocontrol treatments stimulate systemic resistance [364,365]. These approaches reduce postharvest decay and maintain firmness by reinforcing cuticular and cell-wall structures. Thus, IPM-based, residue-free regimes often produce fruits with equal or superior nutritional quality compared with conventional chemical control while ensuring consumer safety.
Cucumber is highly susceptible to fungal and arthropod pests (e.g., powdery mildew, downy mildew, and whiteflies), which thrive under the humid greenhouse conditions [366,367]. Pest-induced stress decreases photosynthetic efficiency and disrupts water relations, producing irregular fruits with reduced firmness and shorter shelf life [368,369]. Infections by Pseudoperonospora cubensis or Sphaerotheca fuliginea also increase ethylene production, accelerating postharvest yellowing [370]. Residue-free management strategies using biological antagonists, essential oil formulations, and microbial inoculants help mitigate these effects [371,372]. Beneficial fungi and bacteria (e.g., Trichoderma spp. and Bacillus amyloliquefaciens) colonize roots or leaves, inducing systemic resistance and improving nutrient uptake [373,374]. Treated plants often show higher antioxidant and phenolic levels, indicating that mild induced defense enhances nutritional quality [374,375]. Physical measures (e.g., insect-proof screens and the introduction of predatory mites) further reduce pests without residues [376,377], though excessive shading from dense netting can limit light penetration. Cucumbers produced under residue-free regimes are typically firmer, less prone to decay, and retain green color longer during storage, reflecting enhanced physiological robustness and lower pathogen load.
In sweet pepper, pest pressure from aphids, thrips, and whiteflies, along with diseases (e.g., Botrytis, Alternaria, and bacterial leaf spot), can substantially reduce quality through direct damage and altered metabolism [378]. Infested plants divert assimilates to defense, resulting in smaller fruits with uneven coloration and thinner pericarps. Chemical pesticide overuse can leave residues and interfere with carotenoid accumulation [379,380]. Residue-free strategies centered on biological control (e.g., releasing Encarsia formosa, Amblyseius swirskii, and Orius laevigatus) maintain pest populations below thresholds while preserving conditions favorable to fruit development [176,381]. Elicitor treatments, including chitosan and microbial consortia, induce defense enzymes and enhance ascorbic acid and carotenoid synthesis, improving nutritional and antioxidant profiles [382,383]. These fruits also show delayed softening and reduced postharvest decay. The absence of residues facilitates colonization by beneficial epiphytic microflora, which may contribute to natural protection during storage [384]. Overall, residue-free regimes improve firmness and visual appeal without compromising flavor or safety, meeting consumer expectations for residue-free produce.
Across all three crops, pest and disease pressure strongly influence fruit quality. Severe infestation impairs photosynthesis and assimilate flow, weakening structure and reducing compositional value, while eliminating all stress can limit antioxidant formation. Residue-free biological control provides the best balance by maintaining plant health and inducing mild defenses that enhance phenolics and vitamins. Biocontrol agents and elicitors also strengthen epidermal and cell-wall integrity, reducing water loss and decay. Thus, residue-free IPM improves both food safety and the nutritional and postharvest performance of greenhouse vegetables.
3.13. Major Interactions Between Climate Factors
Greenhouse climate factors interact rather than act independently, and these interactions ultimately shape fruit nutritional quality and shelf life. Temperature, light, RH, CO2, and water or nutrient supply jointly influence photosynthesis, transpiration, and stress metabolism, producing either synergistic or antagonistic effects. Recognizing these interactions is essential for interpreting experimental variability and optimizing climate control for both yield and postharvest performance.
In tomato, temperature and light interact most strongly because they jointly regulate pigment synthesis and fruit metabolism. High light increases lycopene and β-carotene only when fruit temperature stays below ~30 °C [60,62]. Above this threshold, heat suppresses carotenoid biosynthesis and produces orange rather than red fruits [60,296]. Elevated CO2 enhances sugar accumulation only when light is sufficient to sustain the extra photosynthetic demand [58,62,71,241]. RH–Ca interactions are equally important. High RH limits transpiration and Ca transport, predisposing fruits to BER despite adequate soil Ca [71,241], especially when combined with high temperature and rapid fruit growth [184,241]. In contrast, moderate VPD and stable irrigation improve Ca allocation and firmness [71,74,241]. Mild water deficit under strong light can raise soluble solids and phenolics, but high temperature shifts this response towards faster softening [59,62,164,241]. Thus, the effect of any single environmental factor depends on its interaction with others, underscoring the need for integrated rather than isolated climate control.
In cucumber, strong interactions among light, RH, and temperature govern growth and epidermal development. High radiation at moderate temperature supports firm, well-colored fruits, whereas the same radiation under high temperature causes excessive elongation and soft texture [99,100]. High RH reduces transpiration and cuticle deposition, but this becomes critical mainly under high temperature, producing fruits with fragile skin and poor postharvest behavior [117,118]. The combination of low RH and high temperature induces excessive water stress and curvature [120,220,230]. Interactions between CO2 enrichment and RH are also important. Elevated CO2 improves growth and firmness under moderate RH but loses effectiveness when condensation or high RH limits gas diffusion [113,220]. Water status further modifies these outcomes. Under bright, dry conditions, even mild irrigation deficits reduce yield and firmness, while the same deficit under high RH increases DM and phenolics [119,120]. Thus, microclimate interactions determine whether stress enhances quality or creates structural weakness.
In sweet pepper, interactions among light, temperature, and RH control pigment accumulation, firmness, and water loss. Carotenoid synthesis is stimulated by high radiation but suppressed by excessive heat [253,329]. Thus, shading or diffusive films that moderate fruit temperature improve color and antioxidant levels [149,153,170]. High RH with elevated temperature delays Ca transport and weakens pericarp strength, whereas moderate RH at similar temperature supports uniform color development and firmness [159,160]. Water–nutrient interactions are equally important. Mild water deficit under moderate radiation increases ascorbic acid and phenolics, but when combined with high temperature it causes softening and irregular ripening [142,166]. Elevated CO2 enhances yield and ascorbic acid only when temperature and light are optimal. Otherwise, assimilate accumulation outpaces structural reinforcement, producing large but fragile fruits [151,156]. Overall, pepper quality reflects the combined effects of these factors on sink strength, pigment biosynthesis, and tissue integrity rather than any single variable.
Greenhouse climate factors influence fruit quality through tightly linked pathways. Temperature shapes how light drives pigment and antioxidant synthesis, while RH and VPD interact with Ca and water relations to determine firmness and disorder risk. CO2 and light regulate carbohydrate accumulation, and water or salinity stress modifies these responses. Across crops, fruit temperature is the main integrator of radiation, air temperature, and RH, predicting both compositional and structural outcomes. Synergistic combinations improve quality, whereas antagonistic ones accelerate softening. Thus, optimal management relies on coordinated control of radiation, ventilation, RH, and fertigation to maintain moderate fruit temperature and ensure consistent quality.
3.14. Climate Change Implications
Projected climate change will intensify heat, alter VPD dynamics, and increase radiation loads, challenging microclimate stability even in greenhouses. Despite their buffering capacity, greenhouses remain vulnerable to heat and moisture imbalances that alter physiological and biochemical processes [95], ultimately affecting fruit composition, firmness, and storability.
Tomato cultivation in a warmer climate will face greater risks of elevated fruit temperature. Temperatures above 30 °C suppress lycopene and favor β-carotene synthesis, producing paler, less antioxidant-rich fruits [55,314]. Warm nights accelerate respiration and reduce acidity, shortening shelf life. Although higher CO2 (up to 800–1000 µmol mol−1) can support yield and soluble solids, these gains decline once heat and VPD exceed optimal thresholds [221,223]. Higher VPD can also impair Ca transport, increasing susceptibility to BER and microcracking [7,184,241]. Future production will therefore require improved cooling strategies (shading, evaporative or hybrid systems) and heat-tolerant cultivars that preserve pigment stability and firmness [95].
Cucumber is highly sensitive to warming and RH fluctuations due to its high transpiration rate and delicate epidermis. Higher temperature and radiation accelerate fruit elongation and water uptake, producing larger but softer fruits with shorter postharvest life [99,100]. Extreme heat can impair chlorophyll retention and trigger premature yellowing, while high VPD or water deficits intensify turgor loss [104]. Elevated CO2 may enhance photosynthesis and yield, but under excessive RH or low light, it can lead to watery texture and thin cuticles [112]. Climate change will therefore increase within-crop variability and reduce storability unless mitigated by effective cooling, diffusive covers, and precise irrigation. Adaptive measures such as dynamic shading, RZT control, and sensor-based RH management will be essential to maintain epidermal integrity and firmness [95].
Sweet pepper is more heat-tolerant than tomato or cucumber, yet extreme thermal and radiative stress still disrupts pigment and vitamin synthesis. Day temperatures above 32 °C or intense radiation can cause sunscald and uneven coloration, while warm nights accelerate softening and ascorbic acid loss [149,253,329]. Moderate CO2 enrichment may enhance sugars, ascorbic acid, and carotenoids, but these gains diminish when heat or water stress restricts photosynthesis [155,156,157]. Under dry conditions, higher transpiration and reduced Ca mobility lead to thinner pericarps and reduced firmness [160,161,195], whereas excessive RH in closed systems promotes soft tissue and pathogen development [116,160,234]. Increasing climatic variability will therefore require integrated control of temperature, RH, and air movement to maintain pigment development and shelf life [95]. Breeding for stronger pericarps and improved oxidative-stress tolerance will also enhance resilience.
Across species, climate change will sharpen the trade-off between yield and postharvest quality [95]. Elevated CO2 may enhance photosynthesis, but rising temperature and VPD will speed metabolic decline and reduce storability, while shifting light regimes will alter pigment and antioxidant profiles. Greenhouse buffering capacity will determine the extent of quality loss. Adaptive measures in climate control and cultivar choice will be essential to maintain nutritional value and shelf life under the ongoing climate change conditions [95].
3.15. Cultivar Differences and Genetic Adaptation Potential
Beyond environmental regulation, cultivar choice defines the genetic axis of quality determination, shaping each species’ capacity to tolerate microclimatic stress and preserve postharvest integrity. Considerable genotypic variability exists in fruit quality and shelf life responses to environmental drivers. In tomato, cultivars differ markedly in maintaining pigment synthesis, firmness, and compositional stability under elevated temperature and fluctuating RH. Genotypes with thicker cuticles, higher Ca-use efficiency, and stronger cell wall metabolism show improved tolerance to microcracking, BER, and softening, resulting in more reliable postharvest performance [7,37]. Cucumber cultivars likewise vary in epidermal strength and water-retention capacity, with denser pericarps, higher cuticular wax deposition, and efficient stomatal regulation conferring reduced water loss and yellowing, and thus longer storage life [38,209,303]. In sweet pepper, key differences relate to carotenoid biosynthesis, pericarp thickness, and ascorbic acid retention [147,379]. Genotypes with stronger pigment formation and antioxidant metabolism better maintain nutritional value and firmness under variable temperature and light. These responses reflect both morpho-anatomical attributes and genetic regulation of stress-responsive pathways, including antioxidant defense, membrane stability, and heat- and RH- tolerance mechanisms. Advances in molecular breeding and phenotyping reveal broad intra-specific diversity that can be leveraged to enhance fruit integrity and storability [385]. Integrating cultivar selection with optimized environmental management offers a practical route to stable yield, high nutritional quality, and extended shelf life in greenhouse vegetable systems. The combined environmental and genetic influences shaping fruit storability are summarized in Figure 2.
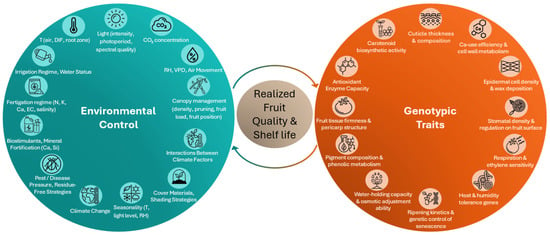
Figure 2.
Interplay between environmental control and cultivar-dependent genetic traits determining fruit quality and shelf life in greenhouse vegetables. Arrows indicate bidirectional interactions and feedbacks between environmental control factors and genotype-dependent traits, shaping realized fruit quality and shelf life. Ca, calcium; CO2, carbon dioxide; DIF, day–night temperature difference; EC, electrical conductivity; K, potassium; N, nitrogen; RH, relative air humidity; Si, silicon; T, temperature; VPD, vapor pressure deficit.
4. Comparison Across Environmental Factors: Relative Importance and Direction of Effects
The combined greenhouse evidence shows that preharvest environmental conditions interactively shape the nutritional composition and postharvest behavior of fruit and vegetables (Table 5). Although each factor (i.e., temperature, light, RH, CO2, irrigation, and nutrition) plays a distinct physiological role, their relative influence can be hierarchically interpreted according to their control of photosynthesis, assimilate partitioning, and structural stability. Among all variables examined, light intensity and fruit temperature emerge as the dominant determinants of overall quality and shelf life potential [83,200,300,314].

Table 5.
Relative importance and direction of major greenhouse environmental factors on nutritional quality and shelf life (SL) in tomato, cucumber, and sweet pepper. C, carbon; Ca, calcium; CO2, carbon dioxide; DM, dry matter; EC, electrical conductivity of the nutrient solution; IPM, integrated pest management; K, potassium; N, nitrogen; RH, relative air humidity; Si, silicon; T, temperature; VPD, vapor pressure deficit; +/−, positive/negative. Arrows indicate direction of projected change (↑ increase, ↓ decrease). Definitions of physiological disorders are provided in Supplementary Table S1.
Light availability governs the accumulation of carbohydrates, organic acids, pigments, and antioxidants, shaping the sensory and nutritional value of the produce. Adequate and spectrally balanced illumination enhances sweetness, coloration, and antioxidant activity, whereas light deficiency during winter or under excessive shading leads to pale fruits with low soluble-solids content. Conversely, excessive radiation, particularly when accompanied by high temperature, increases fruit surface heating and photooxidative stress, accelerating softening and reducing storage life. Thus, the light environment exerts a beneficial effect at moderate, well-distributed levels but a negative one under extremes.
Temperature, closely linked with radiation load, is the primary factor governing postharvest longevity. Moderate air and fruit temperatures sustain enzymatic activity conducive to sugar accumulation and pigment biosynthesis, while simultaneously preserving tissue firmness. When temperature exceeds species-specific optima, respiration and ethylene evolution rise sharply, hastening senescence and increasing susceptibility to decay. In this context, fruit temperature (rather than ambient air temperature per se) best explains seasonal and spatial variation in quality. Moderate warmth enhances flavor and coloration, whereas sustained heat stress shortens shelf life.
RH and its functional derivative, VPD, exert their influence primarily through water relations and Ca distribution. At moderate VPD (~0.8 kPa), transpiration proceeds in a controlled manner, ensuring uniform Ca transport to the fruits and adequate cuticle development. Under excessively high RH, transpiration and Ca flux decline, yielding thin, permeable cuticles and soft tissues prone to cracking and microbial decay. Conversely, very low RH accelerates water loss and induces mechanical stress in pericarp tissues. RH, therefore, exerts a biphasic effect, beneficial at moderate levels but detrimental at either extreme.
Water availability through irrigation management functions as both a nutritional and a stress signal. Mild water deficit stimulates osmotic adjustment and the accumulation of soluble solids, organic acids, and phenolic compounds, which enhance flavor and antioxidant capacity. When water limitation becomes severe, however, cell expansion and Ca mobility decline, resulting in smaller, softer fruits with reduced postharvest stability. Overirrigation has the opposite drawback. Excessive turgor produces large but watery fruits with low DM content. Hence, irrigation regime influences quality in a non-linear manner, positive under moderate deficit but negative when stress is either excessive or absent.
Nutrient balance, particularly the ratio of N, K, and Ca, and the overall EC of the fertigation solution, strongly affects compositional and structural attributes. High K and Ca concentrations coupled with moderate EC (2–3 dS m−1) promote the synthesis of sugars, organic acids, and carotenoids while reinforcing cell-wall integrity. Excessive N or salinity beyond 4–5 dS m−1, however, dilutes flavor compounds and impairs firmness. Proper nutrient management, thus, exerts a consistently positive effect on nutritional quality and shelf life, whereas imbalance or excess acts negatively.
CO2 enrichment generally exerts a positive influence when coupled with sufficient light and ventilation. It increases photosynthetic rate, carbohydrate accumulation, and fruit firmness while slightly reducing respiration at harvest. Under low light or excessive RH, however, the benefits diminish, as limited stomatal conductance restricts gas exchange and can lead to compositional dilution. Consequently, the effect of elevated CO2 is conditional rather than universally beneficial.
Seasonality reflects the combined fluctuation of these parameters rather than a single independent factor. Fruits harvested in cooler, low-radiation seasons often exhibit superior firmness and storability but lower sugar and pigment concentration, whereas those grown in warm, bright conditions show enhanced flavor at the cost of structural stability. This recurring trade-off is best explained by variations in fruit temperature rather than by calendar season itself. Similarly, cover materials and shading strategies act indirectly by moderating radiation and temperature. Diffusive films and moderate shading improve color uniformity and firmness, while over-shading suppresses assimilate accumulation.
Biostimulant and mineral fortification practices, notably those involving Ca and Si, consistently exert positive effects across species. By reinforcing cell-wall and membrane structures and activating antioxidant metabolism, these treatments enhance firmness, reduce postharvest water loss, and extend storage duration. Pest and disease pressure, in contrast, generally acts negatively by diminishing photosynthetic area and introducing decay pathogens, although integrated biological and elicitor-based management can partially offset this effect by inducing mild physiological resistance that enhances antioxidant content and surface protection.
When the cumulative influence of all factors is considered, a clear hierarchy emerges (Table 5). Light and fruit temperature exert the strongest influence, as they dictate both compositional and structural outcomes. RH, irrigation, and nutrient balance follow with high importance, primarily affecting firmness and disorder incidence. CO2 concentration, shading, and mineral or biostimulant treatments exert moderate influence, refining rather than determining quality outcomes. Pest and disease pressure generally has a low direct importance but notable indirect implications for safety and decay. The direction of each effect depends on its magnitude and the concurrent status of other factors. Moderate radiation, temperature, and VPD, combined with balanced irrigation and fertigation, synergistically enhance nutritional value and storability, whereas extremes reduce quality.
Overall, the interaction of these environmental drivers confirms that the highest fruit quality and shelf life are achieved not through maximizing individual parameters but through harmonizing multiple factors to maintain optimal fruit temperature, light interception, and water–nutrient balance. This integrative perspective underpins precision greenhouse management aimed at sustaining productivity, nutritional excellence, and postharvest durability.
5. Species-Specific Responses
5.1. Environmental Sensitivities and Quality Outcomes
Although each species shares many physiological responses to greenhouse microclimate, their differing ripening physiology, tissue structure, and metabolic profiles confer distinct sensitivities to environmental factors (Table 1). These differences lead to species-specific trade-offs between nutritional quality and shelf life under variable preharvest conditions (Table 6).

Table 6.
Comparative sensitivity of tomato, cucumber, and sweet pepper to major preharvest environmental factors affecting nutritional quality and shelf life (SL) in greenhouse cultivation. ascorbic acid = vitamin C; B, blue (spectra); Ca, calcium; CO2, carbon dioxide; DM, dry matter; EC, electrical conductivity of the nutrient solution; IPM, integrated pest management; K, potassium; N, nitrogen; R, red (spectra); RH, relative air humidity; Si, silicon; T, temperature; VPD, vapor pressure deficit. Arrows indicate direction of projected change (↑ increase, ↓ decrease). Definitions of physiological disorders are provided in Supplementary Table S1.
Tomato, being a climacteric fruit, continues metabolic activity after harvest and is therefore highly responsive to preharvest conditions that influence respiration and pigment biosynthesis. Moderate temperatures and balanced light environments promote sugar and lycopene accumulation, whereas excessive heat or RH accelerate softening and shorten shelf life. Tomato composition can be substantially modified through mild preharvest stress (e.g., RDI or moderate salinity), but these same treatments can reduce firmness if stress becomes excessive. Precise Ca nutrition and VPD management are essential to minimize Ca-related disorders (e.g., BER). Among the three species, tomato shows the most balanced response between flavor improvement and postharvest stability, yet also the highest susceptibility to overripe deterioration when preharvest stress is uncontrolled [59,95,282].
Cucumber, by contrast, is non-climacteric, with quality essentially fixed at harvest. Its postharvest fate is largely determined by the epidermal and cuticular characteristics formed during growth. Given its thin epidermis and high WC, cucumber is extremely sensitive to temperature, RH, and irrigation fluctuations. Even brief periods of high RH or water excess produce fruits with fragile cuticle and low firmness, while excessive radiation and temperature induce curvature, yellowing, and dehydration. The nutritional composition of cucumber is less responsive to preharvest modulation than tomato or pepper, though antioxidant capacity can be enhanced through moderate light and Si or Ca supplementation. Cucumber shelf life depends strongly on fruit surface integrity, meaning that uniform microclimatic control during fruit development is essential to maintain epidermal integrity and water retention.
Sweet pepper, another non-climacteric species, exhibits greater structural robustness than cucumber and a more stable composition at harvest. Its thick pericarp contributes to its natural firmness and moderate tolerance to RH and temperature fluctuations. However, pepper’s nutritional and aesthetic quality, particularly carotenoid and ascorbic acid content, is strongly dependent on radiation and temperature. High light promotes pigment formation and antioxidant activity, whereas excessive heat leads to color fading and softening. Ca nutrition remains crucial for preventing localized pericarp collapse, and mild water deficit can increase ascorbic acid and phenolic levels without impairing firmness. Pepper typically maintains firmness well but shows slower improvement from fine-tuned environmental adjustments [8,30,34].
When compared collectively, tomato displays the greatest sensitivity to temperature and RH, cucumber the highest susceptibility to water status and epidermal stress, and sweet pepper the strongest dependency on light for color and antioxidant traits. These distinctions imply that no single climatic optimization can be uniformly applied across species. Instead, management strategies must account for the primary physiological constraints governing each crop. In practical terms, this means emphasizing precise VPD and Ca control in tomato, stable irrigation and temperature in cucumber, and balanced radiation and shading in sweet pepper.
5.2. Underlying Physiological and Anatomical Mechanisms
Clear mechanistic differences among the three species underpin their contrasting responses to greenhouse microclimate. Tomato is characterized by high metabolic flux and a ripening program that remains closely coupled to environmental conditions, which makes its fruit physiology highly dynamic during development. This metabolic flexibility enhances responsiveness to changes in light, temperature, and water status, but also increases vulnerability when any of these drivers exceed optimal ranges. Cucumber, in contrast, invests proportionally more in rapid fruit enlargement than in epidermal and cuticular fortification. As a result, its sensitivity is directed toward processes that govern transpirational water fluxes, cuticular deposition, and epidermal cell cohesion. Sweet pepper occupies an intermediate position, combining substantial pericarp thickness and cell-wall robustness with pigment pathways that remain strongly regulated by the radiation environment.
These developmental architectures give rise to different environmental priorities. Tomato depends on conditions that stabilize its metabolic rate and support coordinated pigment formation because even moderate deviations can shift the balance between biosynthesis and degradation pathways and alter both color and firmness. In cucumber, the dominant constraint is the maintenance of surface barrier integrity. This requires minimal fluctuations in VPD, irrigation regime, and fruit surface temperature. Sweet pepper is distinct in its reliance on the light environment due to the regulatory features of its carotenoid and ascorbic acid pathways, which respond not only to total radiation but also to its temporal and spectral distribution.
The three crops also differ in how they integrate simultaneous environmental cues. Tomato exhibits strong interaction effects between temperature and light because multiple metabolic pathways converge during ripening. Cucumber tends to show additive responses, with independent contributions from RH, temperature, and water availability to epidermal strength, cuticular continuity, and water retention capacity. Sweet pepper displays threshold-type behavior: when radiation levels are sufficient, the crop tolerates a wider range of temperatures, but when light input falls below a certain level, pigment formation is constrained regardless of other conditions.
These mechanistic contrasts produce distinct risk profiles in greenhouse production. Tomato is most susceptible to combined heat and RH stress due to acceleration of respiration, enhanced softening enzyme activity, and destabilization of pigment pathways. Cucumber is vulnerable primarily to microclimatic instability, particularly abrupt changes in VPD or irrigation that impair cuticular formation and epidermal hydration balance. Sweet pepper is constrained mainly by insufficient or poorly distributed light, which limits its ability to complete chromoplast differentiation and carotenoid biosynthesis. Recognizing these species-specific vulnerabilities allows environmental control strategies to be tailored to the processes that govern quality and shelf life in each crop.
6. Methodological Notes: Experimental Design, Confounders, and Reporting Standards
Interpretation of greenhouse studies examining the impact of environmental conditions on fruit quality and shelf life is often constrained by methodological inconsistency. Variations in experimental design, control precision, and data reporting can obscure genuine physiological responses and limit cross-study comparability.
Many investigations manipulate single factors (e.g., temperature, light, or irrigation), while other co-varying parameters remain unmonitored. Yet, in greenhouse environments, variables like air temperature, radiation, RH, and CO2 are interdependent. Increasing temperature typically reduces RH and alters VPD. Supplemental lighting changes both canopy temperature and leaf transpiration. When these interactions are unmeasured, reported effects may be misattributed. True replication is another recurrent weakness. Plants within one climate compartment are often treated as independent replicates, inflating significance and masking spatial gradients. Reliable inference requires either independent greenhouse compartments or repeated experimental cycles.
Sampling heterogeneity further undermines consistency. Differences in harvest maturity, fruit position, and plant load often exceed the magnitude of treatment effects, while cultivar identity strongly modulates plant responsiveness to environmental stress. Tomato, cucumber, and sweet pepper varieties differ widely in cuticle thickness, pigment metabolism, and Ca sensitivity. Randomized sampling across canopy positions and strict maturity criteria are therefore essential to minimize bias, while the use of reference cultivars across experiments would further enhance comparability and reproducibility of quality responses.
Temporal and seasonal variations introduce additional confounding. Experiments performed at different times of year may attribute compositional changes to treatments rather than to ambient radiation or photoperiod differences. Replicating trials across seasons, or normalizing for DLI, is crucial for robust conclusions. Similar caution applies to postharvest assessments. Differences in storage temperature, RH, or handling can overshadow preharvest effects. Defining the “end of shelf life” consistently, based on standardized firmness, color, or decay thresholds, would greatly improve cross-study comparison, as has long been emphasized for vase life evaluations in ornamentals [386].
Accurate microclimate monitoring remains a core methodological gap. Many studies report only setpoints from climate controllers rather than measured conditions at fruit level. However, the actual microenvironment, especially fruit surface temperature, boundary-layer RH, and intercepted radiation, governs the physiological responses driving quality outcomes. Continuous monitoring of both ambient and fruit-level parameters is therefore indispensable [304].
Ultimately, methodological rigor is the foundation for meaningful synthesis. Incomplete climate characterization, pseudo-replication, or inconsistent sampling practices explain much of the contradictory evidence regarding how temperature, light, and RH influence fruit quality and shelf life. Future research should integrate multifactorial designs that reflect real greenhouse conditions, link preharvest monitoring with postharvest performance, and openly share raw environmental and compositional data. Only through transparent and standardized methodologies can the field progress from descriptive observation to predictive understanding of how controlled environments shape the nutritional and storage attributes of greenhouse vegetables.
7. Knowledge Gaps and Research Priorities
Despite significant progress in understanding how greenhouse environments affect vegetable quality, current knowledge remains fragmented and unevenly distributed among crops, factors, and analytical endpoints. Most available data describe isolated responses to single variables rather than integrated system behavior. As a result, the capacity to predict nutritional composition and shelf life under complex, dynamic greenhouse conditions remains limited. Addressing this limitation requires both methodological refinement and conceptual expansion.
A primary knowledge gap concerns the quantitative integration of environmental variables. Light, temperature, RH, CO2 concentration, and water or nutrient status act simultaneously, yet their interactions are rarely studied systematically. Even within advanced facilities, experiments often manipulate one factor at a time, assuming independence from others. Consequently, the true hierarchy of environmental drivers and their synergistic or antagonistic relationships remains uncertain. Multivariate and multifactorial experiments, supported by continuous climate monitoring and mechanistic modeling, are needed to disentangle these relationships and to predict combined effects on fruit composition and storability.
A second limitation is the uneven species coverage in the literature. Tomato has been extensively studied, particularly for light quality, CO2, and salinity effects, whereas cucumber and sweet pepper remain underrepresented in studies linking cultivation environment to postharvest physiology. Crop-specific sensitivities (e.g., cucumber’s epidermal fragility or pepper’s pigment dynamics) are poorly quantified. Comparative, cross-species studies using standardized analytical protocols would improve generalization across fruit types and enhance the transferability of findings to commercial production.
There is also a clear need to bridge the gap between preharvest physiology and postharvest performance. Many studies quantify compositional traits at harvest (e.g., sugars, acids, pigments) without evaluating how these translate into storage behavior. Conversely, shelf life tests are often detached from documented preharvest conditions. Integrated experimental designs that trace individual fruits from cultivation to storage, recording both microclimate exposure and postharvest evolution, are essential for establishing causal links between environmental control and storage stability. Non-destructive monitoring of firmness, color, and water status during storage would further strengthen this linkage [387].
Another unresolved question concerns the temporal dimension of environmental responses. Short-term exposure experiments dominate the literature, yet many quality-related processes are cumulative or developmental. Fruit responses to temperature or VPD may depend on the stage of growth at which stress occurs. Continuous, long-term measurements of fruit temperature, transpiration, and metabolite dynamics are therefore needed to define sensitive windows when environmental optimization has the greatest impact on final quality and shelf life.
Standardization of metrics is an additional priority. Definitions of nutritional quality and shelf life vary widely, with studies emphasizing sensory traits, sugars, antioxidants, or vitamins, while shelf life is variously defined by weight loss, firmness, or visual decay (Table 1). The absence of common benchmarks limits cross-study synthesis. Establishing harmonized analytical protocols and consensus indicators, potentially through international networks or open databases, would greatly enhance comparability and support meta-analytical modeling.
The integration of digital and modeling tools remains underexploited. Although greenhouse control systems routinely record temperature, RH, and radiation, these datasets are seldom linked to physiological measurements. Machine-learning (ML) approaches could identify key predictors of fruit quality and shelf life from large climatic datasets, provided standardized metadata exist. Likewise, mechanistic models coupling C allocation, water relations, and cuticular conductance could predict quality outcomes under different control scenarios. Future research should prioritize coupling of high-resolution climate data with fruit-level phenotyping and statistical modeling frameworks.
A further research frontier concerns genotype by environment interactions. Few studies explicitly compare cultivars differing in cuticle properties, fruit firmness, or metabolic pathways under controlled environmental variation. Such comparisons could reveal genotypic traits conferring resilience to high temperature, fluctuating RH, or low light, informing breeding targets for greenhouse adaptation. Integrating physiological and molecular analyses would help identify key regulatory nodes linking environmental stimuli to compositional and structural quality.
Greater attention is also needed to the sustainability and reproducibility of quality-oriented environmental strategies. Practices such as CO2 enrichment, supplemental lighting, or RDI must be evaluated not only for their effects on fruit quality but also for their energy, water, and C costs. Life-cycle and techno-economic assessments that include postharvest savings from extended shelf life would provide a holistic evaluation of sustainability.
Another underexplored area is the dynamic interaction between preharvest-conditioned quality and conventional postharvest technologies. Fruit with varying levels of structural integrity, cuticle strength, or metabolic activity may respond differently to cooling strategies, controlled atmosphere (CA) storage, or RH management. Systematic studies linking preharvest climate history with postharvest technology performance would help identify which environmental strategies enhance compatibility with commercial handling protocols and reduce losses along the supply chain.
In summary, future research should focus on five priorities: (1) multifactorial experiments integrating major climate variables, (2) standardized analytical and reporting protocols linking preharvest and postharvest phases, (3) continuous monitoring of fruit microclimate and development, (4) genotype-based investigations identifying resilience traits, and (5) data-driven modeling frameworks coupling environmental control with quality prediction. Addressing these gaps will enable a shift from descriptive observation to predictive design, optimizing environmental control not only for yield but also for nutritional quality and postharvest performance. The major methodological gaps and corresponding research priorities are summarized in Figure 3.
Figure 3.
Research roadmap outlining methodological gaps and future priorities for improving fruit quality and shelf life in greenhouse vegetables. Collectively, these directions aim to advance from descriptive understanding to predictive, sustainable greenhouse design optimizing both yield and postharvest excellence.
8. Conclusions
Greenhouse studies collectively show that preharvest climate strongly determines both nutritional value and postharvest longevity of fruit and vegetables. Across environmental drivers, fruit temperature and light intensity consistently emerge as the dominant regulators of assimilate availability, pigment formation, and metabolic stability. RH and VPD shape structural integrity by governing transpiration dynamics and Ca transport, while water and nutrient regimes modulate flavor concentration and firmness. These interactions highlight that the physical microclimate around the fruit, rather than mean air conditions, is the most accurate predictor of postharvest performance.
Clear species-specific patterns also emerge. Tomato is metabolically responsive and benefits from moderate radiation and temperature, yet its firmness and storability decline rapidly when heat or RH exceed optimal thresholds due to impaired lycopene synthesis, high respiration, and Ca-related disorders. Cucumber quality is dominated by epidermal and water-balance constraints, making stability of RH, VPD, and temperature the primary driver of storability. Sweet pepper is structurally more resilient, but its pigment and vitamin accumulation depend strongly on adequate radiation and moderate thermal regimes. These differences reflect underlying mechanisms including cuticle architecture, transpiration patterns, pigment-pathway sensitivity, and Ca-use efficiency.
From a practical standpoint, the most critical environmental levers differ among the three crops. In tomato, maintaining appropriate fruit temperature, controlling VPD, and providing moderate radiation are essential to preserve color development and firmness. In cucumber, stable RH and VPD, gentle cooling, and precise irrigation are particularly important to protect the delicate epidermis and limit water loss. In sweet pepper, adequate radiation combined with avoidance of direct heat is required to support complete pigment formation and nutritional quality. Integrating these species-specific requirements through improved shading, diffusive covers, targeted cooling, and informed cultivar selection can substantially enhance fruit quality and extend shelf life.
Although this review focuses on physiological and environmental determinants of fruit quality, the implementation of many optimized climate-control strategies in commercial greenhouses involves substantial energy use. Tighter control of temperature, VPD, and radiation often requires supplemental cooling, dehumidification, or lighting, all of which increase operational costs and C footprint. The economic feasibility and environmental sustainability of such interventions therefore depend on the balance between quality gains and the additional resource inputs required. Evaluating this “cost of quality” through techno-economic analyses and life-cycle assessments (LCA) represents an important future research direction, ensuring that physiological optimization aligns with both profitability and sustainability goals in greenhouse production.
Several research gaps remain. An important remaining gap concerns aroma-related VOCs, which strongly influence consumer acceptance but are rarely integrated into studies linking preharvest climate with postharvest behavior. Another missing dimension is the interaction between preharvest-conditioned fruit resilience and standard postharvest technologies, as fruits subjected to different environmental histories may respond differently to cooling strategies, controlled atmosphere (CA) storage, or modified atmospheres (MA). Most studies manipulate single factors, whereas greenhouse conditions arise from interacting drivers such as radiation–temperature–VPD combinations. Limited replication, inconsistent maturity indices, and incomplete reporting further constrain cross-study synthesis. Future work should adopt multifactorial designs, continuous climate and fruit-surface monitoring, and harmonized maturity criteria to link preharvest conditions to postharvest outcomes more reliably. Mechanistic insights combined with predictive modeling will enable precision greenhouse strategies that jointly optimize yield, nutritional quality, and storability.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy16010048/s1, Table S1. Definitions of physiological disorders and surface defects. BER, blossom-end rot; Ca, calcium; K, potassium; N, nitrogen; RH, relative air humidity; T, temperature; VPD, vapor pressure deficit.
Author Contributions
Conceptualization, D.F.; methodology, D.F.; validation, D.F., T.M., G.P.S., I.K., G.T. and G.N.; investigation, D.F., T.M., G.P.S., I.K., G.T. and G.N.; writing—original draft preparation, D.F., T.M., G.P.S., I.K., G.T. and G.N.; writing—review and editing, D.F., T.M., G.P.S., I.K., G.T. and G.N.; visualization, D.F., G.P.S. and I.K.; supervision, D.F.; project administration, D.F.; funding acquisition, G.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created in this study. Data sharing is not applicable to this review article.
Acknowledgments
Insightful discussions with Roland Pieruschka and Fabio Fiorani are greatly acknowledged. We also thank the Academic Editor and the four anonymous reviewers for their insightful and constructive comments, which substantially improved the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| a* | Green (–) → red (+) axis |
| b* | Blue (–) → yellow (+) axis |
| B | Blue (spectra) |
| β-carotene | Beta-carotene |
| BER | Blossom-end rot |
| C | Carbon |
| Ca | Calcium |
| CO2 | Carbon dioxide |
| DIF | Day–night temperature difference |
| DLI | Daily light integral |
| DM | Dry matter |
| EC | Electrical conductivity |
| FR | Far-red |
| FW | Fresh weight |
| IPM | Integrated pest management |
| K | Potassium |
| L* | Lightness |
| N | Nitrogen |
| PE | Polyethylene |
| PAR | Photosynthetically active radiation |
| R | Red (spectra) |
| RH | Relative air humidity |
| RDI | Regulated deficit irrigation |
| RZT | Root-zone temperature |
| Si | Silicon |
| T | Temperature |
| TA | Titratable acidity |
| TSS | Total soluble solids |
| UV | Ultraviolet |
| UV-A | Ultraviolet A radiation |
| VOCs | Volatile organic compounds |
| VPD | Vapor pressure deficit |
| WC | Water content |
References
- Zhao, X.; Peng, J.; Zhang, L.; Yang, X.; Qiu, Y.; Cai, C.; Hu, J.; Huang, T.; Liang, Y.; Li, Z.; et al. Optimizing the Quality of Horticultural Crop: Insights into Pre-Harvest Practices in Controlled Environment Agriculture. Front. Plant Sci. 2024, 15, 1427471. [Google Scholar] [CrossRef]
- Gruda, N.S.; Li, X.; Gallegos-Cedillo, V.M.; Samouline, G.; Dong, J.; Weiss, J.; Fernández, J.A. From Growth to Table: Exploring the Impact of Pre-Harvest Conditions on Greenhouse Vegetable Quality. Eur. J. Hortic. Sci. 2025, 90, 1. [Google Scholar] [CrossRef]
- Nitu, O.A.; Ivan, E.S.; Arshad, A. Optimizing Microclimatic Conditions for Lettuce, Tomatoes, Carrots, and Beets: Impacts on Growth, Physiology, and Biochemistry Across Greenhouse Types and Climatic Zones. Int. J. Plant Biol. 2025, 16, 100. [Google Scholar] [CrossRef]
- Semida, W.M.; Ammar, M.S.; El-Sawah, N.A. Effects of Shade Level and Microenvironment on Vegetative Growth, Physiological and Biochemical Characteristics of Transplanted Cucumber (Cucumis sativus). Arch. Agric. Environ. Sci. 2017, 2, 361–368. [Google Scholar] [CrossRef]
- Saltveit, M.E. Postharvest Physiology and Pathology of Vegetables, 2nd ed.; Bartz, J.A., Brecht, J.K., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 1–76. [Google Scholar]
- Osorio, S.; Alba, R.; Nikoloski, Z.; Kochevenko, A.; Fernie, A.R.; Giovannoni, J.J. Integrative Comparative Analyses of Transcript and Metabolite Profiles from Pepper and Tomato Ripening and Development Stages Uncovers Species-Specific Patterns of Network Regulatory Behavior. Plant Physiol. 2012, 159, 1713–1729. [Google Scholar] [CrossRef]
- Ho, L.C.; Adams, P. The Physiological Basis for High Fruit Yield and Susceptibility to Calcium Deficiency in Tomato and Cucumber. J. Hortic. Sci. 1994, 69, 367–376. [Google Scholar] [CrossRef]
- Martínez-Ispizua, E.; Calatayud, Á.; Marsal, J.I.; Mateos-Fernández, R.; Díez, M.J.; Soler, S.; Valcárcel, J.V.; Martínez-Cuenca, M.-R. Phenotypic Divergence among Sweet Pepper Landraces Assessed by Agro-Morphological Characterization as a Biodiversity Source. Agronomy 2022, 12, 632. [Google Scholar] [CrossRef]
- Thakur, N.; Sharma, D.; Kaur, J.; Sharma, V. Characterizing Tomato Genotypes in the Varied Climates of North-Western Himalayas and Implications for Environmental Resilience Using GGE Biplot Analyses. Sci. Rep. 2025, 15, 28524. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Kim, J.-G.; Kang, B.-C.; Song, K. Assessment of the Genetic Diversity of the Breeding Lines and a Genome Wide Association Study of Three Horticultural Traits Using Worldwide Cucumber (Cucumis spp.) Germplasm Collection. Agronomy 2020, 10, 1736. [Google Scholar] [CrossRef]
- Argento, S.; Garcia, G.; Treccarichi, S. Sustainable and Low-Input Techniques in Mediterranean Greenhouse Vegetable Production. Horticulturae 2024, 10, 997. [Google Scholar] [CrossRef]
- Hounsome, N.; Hounsome, B.; Tomos, D.; Edwards-Jones, G. Plant Metabolites and Nutritional Quality of Vegetables. J. Food Sci. 2008, 73, 4. [Google Scholar] [CrossRef]
- de la Rosa, L.A.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value, and Stability; Wiley: Hoboken, NJ, USA, 2009; ISBN 9780813803203. [Google Scholar]
- Yang, Y.; Luo, J.; Tang, Y.; Li, Z.; Yang, L.; Gao, J. Comparative Evaluation of Appearance and Nutritional Qualities of 57 Tomato (Solanum lycopersicum L.) Accessions. Horticulturae 2025, 11, 796. [Google Scholar] [CrossRef]
- Bénard, C.; Gautier, H.; Bourgaud, F.; Grasselly, D.; Navez, B.; Caris-Veyrat, C.; Weiss, M.; Génard, M. Effects of Low Nitrogen Supply on Tomato (Solanum lycopersicum) Fruit Yield and Quality with Special Emphasis on Sugars, Acids, Ascorbate, Carotenoids, and Phenolic Compounds. J. Agric. Food Chem. 2009, 57, 4112–4123. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Goodner, K.; Plotto, A. Interaction of Volatiles, Sugars, and Acids on Perception of Tomato Aroma and Flavor Descriptors. J. Food Sci. 2008, 73, S294–S307. [Google Scholar] [CrossRef]
- Gautier, H.; Diakou-Verdin, V.; Bénard, C.; Reich, M.; Buret, M.; Bourgaud, F.; Poëssel, J.L.; Caris-Veyrat, C.; Génard, M. How Does Tomato Quality (Sugar, Acid, and Nutritional Quality) Vary with Ripening Stage, Temperature, and Irradiance? J. Agric. Food Chem. 2008, 56, 1241–1250. [Google Scholar] [CrossRef]
- Ho, L.C. A Cellular Hypothesis for the Induction of Blossom-End Rot in Tomato Fruit. Ann. Bot. 2005, 95, 571–581. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Cho, M.-C.; Lee, J.G. Genotypic Variation in Carotenoid, Ascorbic Acid, Total Phenolic, and Flavonoid Contents, and Antioxidant Activity in Selected Tomato Breeding Lines. Hortic. Environ. Biotechnol. 2016, 57, 440–452. [Google Scholar] [CrossRef]
- Javid, H.; Fatima, U.; Rukhsar, A.; Hussain, S.; Bibi, S.; Bodlah, M.A.; Shahzad, H.H.; Dilshad, M.; Waqas, M.; Sharif, A. Phytochemical, Nutritional and Medicinal Profile of Cucumis sativus L. (Cucumber). Food Sci. Eng. 2024, 5, 358–377. [Google Scholar] [CrossRef]
- Manjunatha, M.; Anurag, R.K. Effect of Modified Atmosphere Packaging and Storage Conditions on Quality Characteristics of Cucumber. J. Food Sci. Technol. 2014, 51, 3470–3475. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Nema, N.K.; Maity, N.; Sarkar, B.K. Phytochemical and Therapeutic Potential of Cucumber. Fitoterapia 2013, 84, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Ghasemnezhad, M.; Sherafati, M.; Payvast, G.A. Variation in Phenolic Compounds, Ascorbic Acid and Antioxidant Activity of Five Coloured Bell Pepper (Capsicum annum) Fruits at Two Different Harvest Times. J. Funct. Foods 2011, 3, 44–49. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, S.; Yuan, J.; Wang, C.; Lu, T.; Wang, H.; Yu, C. The Formation of Fruit Quality in Cucumis sativus L. Front. Plant Sci. 2021, 12, 729448. [Google Scholar] [CrossRef]
- Deepa, N.; Kaur, C.; George, B.; Singh, B.; Kapoor, H.C. Antioxidant Constituents in Some Sweet Pepper (Capsicum annuum L.) Genotypes During Maturity. LWT-Food Sci. Technol. 2007, 40, 121–129. [Google Scholar] [CrossRef]
- Materska, M. Bioactive Phenolics of Fresh and Freeze-Dried Sweet and Semi-Spicy Pepper Fruits (Capsicum annuum L.). J. Funct. Foods 2014, 7, 269–277. [Google Scholar] [CrossRef]
- Trong, L.V.; Phuong, H.T.; Thinh, B.B. Changes in the Physiological and Biochemical Parameters of Cucumber (Cucumis sativus L.) During Fruit Development. Bull. Transilv. Univ. Brasov. 2023, 16, 143–154. [Google Scholar] [CrossRef]
- Klee, H.J.; Giovannoni, J.J. Genetics and Control of Tomato Fruit Ripening and Quality Attributes. Annu. Rev. Genet. 2011, 45, 41–59. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Ji, K.; Dai, S.; Hu, Y.; Sun, L.; Li, Q.; Chen, P.; Sun, Y.; Duan, C.; et al. The Role of Abscisic Acid in Regulating Cucumber Fruit Development and Ripening and Its Transcriptional Regulation. Plant Physiol. Biochem. 2013, 64, 70–79. [Google Scholar] [CrossRef]
- Guilherme, R.; Reboredo, F.; Guerra, M.; Ressurreição, S.; Alvarenga, N. Elemental Composition and Some Nutritional Parameters of Sweet Pepper from Organic and Conventional Agriculture. Plants 2020, 9, 863. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.V.R.; Gol, N.B.; Shah, K.K. Effect of Postharvest Treatments and Storage Temperatures on the Quality and Shelf Life of Sweet Pepper (Capsicum annum L.). Sci. Hortic. 2011, 132, 18–26. [Google Scholar] [CrossRef]
- Jahan, S.E.; Hassan, M.K.; Roy, S.; Ahmed, Q.M.; Hasan, G.N.; Muna, A.Y.; Sarkar, M.N. Effects of Different Postharvest Treatments on Nutritional Quality and Shelf Life of Cucumber. Asian J. Crop Soil Sci. Plant Nutr. 2020, 2, 51–61. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, N.; Upadhyay, A.; Sethi, S.; Singh, A. Edible Coating as Postharvest Management Strategy for Shelf-life Extension of Fresh Tomato (Solanum lycopersicum L.): An Overview. J. Food Sci. 2022, 87, 2256–2290. [Google Scholar] [CrossRef]
- Kalmykova, E.V.; Kalmykova, O.V. Changes in the Sweet Pepper Fruits Quality Indicators During Storage Taking into Account Agricultural Cultivation Technology. IOP Conf. Ser. Earth Environ. Sci. 2022, 1069, 012039. [Google Scholar] [CrossRef]
- Gebretsadik, K.; Qiu, X.; Dong, S.; Miao, H.; Bo, K. Molecular Research Progress and Improvement Approach of Fruit Quality Traits in Cucumber. Theor. Appl. Genet. 2021, 134, 3535–3552. [Google Scholar] [CrossRef]
- Jung, J.-M.; Shim, J.-Y.; Chung, S.-O.; Hwang, Y.-S.; Lee, W.-H.; Lee, H. Changes in Quality Parameters of Tomatoes During Storage: A Review. Korean J. Agric. Sci. 2019, 46, 239–256. [Google Scholar] [CrossRef]
- Bargel, H. Tomato (Lycopersicon esculentum Mill.) Fruit Growth and Ripening as Related to the Biomechanical Properties of Fruit Skin and Isolated Cuticle. J. Exp. Bot. 2005, 56, 1049–1060. [Google Scholar] [CrossRef]
- Grumet, R.; Lin, Y.-C.; Rett-Cadman, S.; Malik, A. Morphological and Genetic Diversity of Cucumber (Cucumis sativus L.) Fruit Development. Plants 2022, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Weryszko-Chmielewska, E.; Michałojć, Z. Anatomical Traits of Sweet Pepper (Capsicum annuum L.) Fruit. Acta Agrobot. 2012, 64, 181–188. [Google Scholar] [CrossRef]
- Shi, Y.; Vrebalov, J.; Zheng, H.; Xu, Y.; Yin, X.; Liu, W.; Liu, Z.; Sorensen, I.; Su, G.; Ma, Q.; et al. A Tomato LATERAL ORGAN BOUNDARIES Transcription Factor, SlLOB1, Predominantly Regulates Cell Wall and Softening Components of Ripening. Proc. Natl. Acad. Sci. USA 2021, 118, e2102486118. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Khan, A.S.; Azam, M.; Ayyub, S.; Kaleem, M.M.; Nawaz, S.; Abubakar, M.; Khalid, B.; Riaz, T.; Asim, M.; et al. Innovative Technologies in Postharvest Management of Fruits and Vegetables: A Review. Eur. Food Res. Technol. 2025, 251, 3445–3463. [Google Scholar] [CrossRef]
- Aguayo, E.; Escalona, V.; Artes, F. Quality of Fresh-Cut Tomato as Affected by Type of Cut, Packaging, Temperature and Storage Time. Eur. Food Res. Technol. 2004, 219, 492–499. [Google Scholar] [CrossRef]
- Tudor-Radu, M.; Vijan, L.E.; Tudor-Radu, C.M.; Tita, I.; Sima, R.; Mitrea, R. Assessment of Ascorbic Acid, Polyphenols, Flavonoids, Anthocyanins and Carotenoids Content in Tomato Fruits. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 477–483. [Google Scholar] [CrossRef]
- Deribe, H.; Beyene, B.; Beyene, B. Review on Pre and Post-Harvest Management on Quality Tomato (Lycopersicon esculentum Mill.) Production. Food Sci. Qual. Manag. 2016, 54, 72–79. [Google Scholar]
- Saha, S.; Hossain, M.; Rahman, M.; Kuo, C.; Abdullah, S. Effect of High Temperature Stress on the Performance of Twelve Sweet Pepper Genotypes. Bangladesh J. Agric. Res. 1970, 35, 525–534. [Google Scholar] [CrossRef]
- Yahia, E.M.; Carrillo-Lopez, A. Postharvest Physiology and Biochemistry of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128132784. [Google Scholar]
- Workneh, T.S. The Improvement of the Shelf Life of Vegetables Through Pre- and Post Harvest Treatments. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2002. [Google Scholar]
- Sousa Gallagher, M.J.; Mahajan, P.V. The Stability and Shelf Life of Fruit and Vegetables. In Food and Beverage Stability and Shelf Life; Elsevier: Amsterdam, The Netherlands, 2011; pp. 641–656. [Google Scholar]
- Arah, I.K.; Amaglo, H.; Kumah, E.K.; Ofori, H. Preharvest and Postharvest Factors Affecting the Quality and Shelf Life of Harvested Tomatoes: A Mini Review. Int. J. Agron. 2015, 2015, 78041. [Google Scholar] [CrossRef]
- Umeohia, U.; Olapade, A.A. Quality Attributes, Physiology, and Postharvest Technologies of Tomatoes (Lycopersicum esculentum)—A Review. Am. J. Food Sci. Technol. 2024, 12, 42–64. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Guzman-Puyol, S.; Benítez, J.J.; Athanassiou, A.; Heredia, A.; Domínguez, E. Plant Cuticle Under Global Change: Biophysical Implications. Glob. Change Biol. 2018, 24, 2749–2751. [Google Scholar] [CrossRef]
- Thole, V.; Vain, P.; Martin, C. Effect of Elevated Temperature on Tomato Post-Harvest Properties. Plants 2021, 10, 2359. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Rajametov, S.N.; Jeong, H.-B.; Cho, M.-C.; Lee, O.-J.; Kim, S.-G.; Yang, E.-Y.; Chae, W.-B. Comprehensive Understanding of Selecting Traits for Heat Tolerance During Vegetative and Reproductive Growth Stages in Tomato. Agronomy 2022, 12, 834. [Google Scholar] [CrossRef]
- Sato, S.; Peet, M.M.; Thomas, J.F. Physiological Factors Limit Fruit Set of Tomato (Lycopersicon esculentum Mill.) Under Chronic, Mild Heat Stress. Plant Cell Environ. 2000, 23, 719–726. [Google Scholar] [CrossRef]
- Dumas, Y.; Dadomo, M.; Di Lucca, G.; Grolier, P. Effects of Environmental Factors and Agricultural Techniques on Antioxidantcontent of Tomatoes. J. Sci. Food Agric. 2003, 83, 369–382. [Google Scholar] [CrossRef]
- Delgado-Vargas, V.A.; Ayala-Garay, O.J.; Arévalo-Galarza, M.d.L.; Gautier, H. Increased Temperature Affects Tomato Fruit Physicochemical Traits at Harvest Depending on Fruit Developmental Stage and Genotype. Horticulturae 2023, 9, 212. [Google Scholar] [CrossRef]
- Yuan, X.K. Effect of Day/Night Temperature Difference on Chlorophyll Content, Photosynthesis and Fluorescence Parameters of Tomato at Fruit Stage. Photosynthetica 2016, 54, 475–477. [Google Scholar] [CrossRef]
- Papadopoulos, A.P.; Hao, X. Effects of Day and Night Air Temperature in Early Season on Growth, Productivity and Energy Use of Spring Tomato. Can. J. Plant Sci. 2001, 81, 303–311. [Google Scholar] [CrossRef]
- Dorais, M.; Papadopoulos, A.P.; Gosselin, A. Greenhouse Tomato Fruit Quality. In Horticultural Reviews; Wiley: Hoboken, NJ, USA, 2000; pp. 239–319. [Google Scholar]
- Sawhney, V.K.; Polowick, P.L. Fruit Development in Tomato: The Role of Temperature. Can. J. Bot. 1985, 63, 1031–1034. [Google Scholar] [CrossRef]
- Kläring, H.-P.; Krumbein, A. The Effect of Constraining the Intensity of Solar Radiation on the Photosynthesis, Growth, Yield and Product Quality of Tomato. J. Agron. Crop Sci. 2013, 199, 351–359. [Google Scholar] [CrossRef]
- Pan, T.; Ding, J.; Qin, G.; Wang, Y.; Xi, L.; Yang, J.; Li, J.; Zhang, J.; Zou, Z. Interaction of Supplementary Light and CO2 Enrichment Improves Growth, Photosynthesis, Yield, and Quality of Tomato in Autumn Through Spring Greenhouse Production. HortScience 2019, 54, 246–252. [Google Scholar] [CrossRef]
- Lu, N.; Maruo, T.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Ito, Y.; Ichimura, T.; Shinohara, Y. Effects of Supplemental Lighting with Light-Emitting Diodes (LEDs) on Tomato Yield and Quality of Single-Truss Tomato Plants Grown at High Planting Density. Environ. Control Biol. 2012, 50, 63–74. [Google Scholar] [CrossRef]
- Alsina, I.; Erdberga, I.; Duma, M.; Alksnis, R.; Dubova, L. Changes in Greenhouse Grown Tomatoes Metabolite Content Depending on Supplemental Light Quality. Front. Nutr. 2022, 9, 830186. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Hao, X.; Wang, Q.; Khosla, S. Responses of a Long Greenhouse Tomato Crop to Summer CO2 Enrichment. Can. J. Plant Sci. 2006, 86, 1395–1400. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, H.; Liu, Y.; Li, J.; Zheng, J.; Sun, S.; Xing, G. Photosynthetic Responses of Tomato to Different Concentrations of CO2 Enrichment in Greenhouse. J. Plant Nutr. Fertil. 2018, 4, 1010–1018. [Google Scholar]
- Suzuki, M.; Umeda, H.; Matsuo, S.; Kawasaki, Y.; Ahn, D.; Hamamoto, H.; Iwasaki, Y. Effects of Relative Humidity and Nutrient Supply on Growth and Nutrient Uptake in Greenhouse Tomato Production. Sci. Hortic. 2015, 187, 44–49. [Google Scholar] [CrossRef]
- Shamshiri, R.R.; Jones, J.W.; Thorp, K.R.; Ahmad, D.; Man, H.C.; Taheri, S. Review of Optimum Temperature, Humidity, and Vapour Pressure Deficit for Microclimate Evaluation and Control in Greenhouse Cultivation of Tomato: A Review. Int. Agrophysics 2018, 32, 287–302. [Google Scholar] [CrossRef]
- Zhang, D.; Du, Q.; Zhang, Z.; Jiao, X.; Song, X.; Li, J. Vapour Pressure Deficit Control in Relation to Water Transport and Water Productivity in Greenhouse Tomato Production During Summer. Sci. Rep. 2017, 7, 43461. [Google Scholar] [CrossRef]
- Leonardi, C.; Guichard, S.; Bertin, N. High Vapour Pressure Deficit Influences Growth, Transpiration and Quality of Tomato Fruits. Sci. Hortic. 2000, 84, 285–296. [Google Scholar] [CrossRef]
- Ghannem, A.; Ben Aissa, I.; Majdoub, R. Effects of Regulated Deficit Irrigation Applied at Different Growth Stages of Greenhouse Grown Tomato on Substrate Moisture, Yield, Fruit Quality, and Physiological Traits. Environ. Sci. Pollut. Res. 2021, 28, 46553–46564. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Liu, H.; Wang, J.; Qiang, X. Response of Tomato Quality Parameters to Water Deficit Under Soil Salinity and Simulation Based on Stem Water Potential. Horticulturae 2025, 11, 114. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, W.; Du, T.; Kang, S.; Davies, W.J. Responses of Water Accumulation and Solute Metabolism in Tomato Fruit to Water Scarcity and Implications for Main Fruit Quality Variables. J. Exp. Bot. 2020, 71, 1249–1264. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, A.R.; Wahb-Allah, M.A.; Al-Omran, A.M. Effects of Salinity and Irrigation Management on Growth and Yield of Tomato Grown Under Greenhouse Conditions. Acta Hortic. 2009, 807, 201–206. [Google Scholar] [CrossRef]
- Hernández-Pérez, O.I.; Valdez-Aguilar, L.A.; Alia-Tejacal, I.; Cartmill, A.D.; Cartmill, D.L. Tomato Fruit Yield, Quality, and Nutrient Status in Response to Potassium: Calcium Balance and Electrical Conductivity in the Nutrient Solution. J. Soil Sci. Plant Nutr. 2020, 20, 484–492. [Google Scholar] [CrossRef]
- Dodgson, J.; Weston, A.; Marks, D. Tomato Firmness and Shelf-Life Increased by Application of Stimulated Calcium. Crops 2023, 3, 251–265. [Google Scholar] [CrossRef]
- Liu, S.; Qiang, X.; Liu, H.; Han, Q.; Yi, P.; Ning, H.; Li, H.; Wang, C.; Zhang, X. Effects of Nutrient Solution Application Rates on Yield, Quality, and Water–Fertilizer Use Efficiency on Greenhouse Tomatoes Using Grown-in Coir. Plants 2024, 13, 893. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Su, K.; Wang, L.; Zhou, J.; Sun, S.; Wang, J.; Xing, G. Modulation of Potassium-to-Calcium Ratio in Nutrient Solution Improves Quality Attributes and Mineral Composition of Solanum lycopersicum var. cerasiforme. Agronomy 2025, 15, 1380. [Google Scholar] [CrossRef]
- Qu, F.; Zhang, J.; Ma, X.; Wang, J.; Gao, Z.; Hu, X. Effects of Different N, P, K and Ca Levels on Tomato Yield, Quality and Fertiliser Use Efficiency. Plant Soil Environ. 2020, 66, 569–575. [Google Scholar] [CrossRef]
- Choi, H.-G.; Park, K.-S. Ripening Process of Tomato Fruits Postharvest: Impact of Environmental Conditions on Quality and Chlorophyll a Fluorescence Characteristics. Horticulturae 2023, 9, 812. [Google Scholar] [CrossRef]
- Arshad, A.; Sovorn, C.; Draghici, E.M.; Jerca, I.O.; Iqbal, N. Study on the Influence of Climatic Factors in Greenhouses on the Growth, Yield, and Biochemical Attributes of Tomatoes Grown in the Soilless System During the Summer Season. Mod. Concepts Dev. Agron. 2024, 14, 1350–1359. [Google Scholar] [CrossRef]
- Moreno-Teruel, M.d.L.Á.; Molina-Aiz, F.D.; Peña-Fernández, A.; López-Martínez, A.; Valera-Martínez, D.L. The Effect of Diffuse Film Covers on Microclimate and Growth and Production of Tomato (Solanum lycopersicum L.) in a Mediterranean Greenhouse. Agronomy 2021, 11, 860. [Google Scholar] [CrossRef]
- Moreno-Teruel, M.d.L.Á.; Valera, D.; Molina-Aiz, F.D.; López-Martínez, A.; Peña, A.; Marín, P.; Reyes-Rosas, A. Effects of Cover Whitening Concentrations on the Microclimate and on the Development and Yield of Tomato (Lycopersicon esculentum Mill.) Inside Mediterranean Greenhouses. Agronomy 2020, 10, 237. [Google Scholar] [CrossRef]
- He, X.; Maier, C.; Chavan, S.G.; Zhao, C.-C.; Alagoz, Y.; Cazzonelli, C.; Ghannoum, O.; Tissue, D.T.; Chen, Z.-H. Light-Altering Cover Materials and Sustainable Greenhouse Production of Vegetables: A Review. Plant Growth Regul. 2021, 95, 1–17. [Google Scholar] [CrossRef]
- Heuvelink, E.; Acevedo-Siaca, L.G.; Van de Poel, B.; Van der Jeucht, L.; Vialet-Chabrand, S.; Steppe, K.; Ji, Y.; Körner, O.; Kusuma, P.; Langer, S.; et al. Tomato in the Spotlight: Light Regulation of Whole-Plant Physiology. J. Exp. Bot. 2025, 76, 6289–6310. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, A.; Kornas, J.; Hanrieder, N.; González Rodríguez, S.; Hristov, L.; Fernández Solas, Á.; Wilbert, S.; Blanco, M.J.; Berzosa Álvarez, L.; Martínez Gallardo, A.; et al. Tomato Yield Under Different Shading Levels in an Agrivoltaic Greenhouse in Southern Spain. AgriEngineering 2025, 7, 178. [Google Scholar] [CrossRef]
- Dasgan, H.Y.; Aksu, K.S.; Zikaria, K.; Gruda, N.S. Biostimulants Enhance the Nutritional Quality of Soilless Greenhouse Tomatoes. Plants 2024, 13, 2587. [Google Scholar] [CrossRef] [PubMed]
- Distefano, M.; Steingass, C.B.; Leonardi, C.; Giuffrida, F.; Schweiggert, R.; Mauro, R.P. Effects of a Plant-Derived Biostimulant Application on Quality and Functional Traits of Greenhouse Cherry Tomato Cultivars. Food Res. Int. 2022, 157, 111218. [Google Scholar] [CrossRef]
- Islam, M.M.; Jahan, K.; Sen, A.; Urmi, T.A.; Haque, M.M.; Ali, H.M.; Siddiqui, M.H.; Murata, Y. Exogenous Application of Calcium Ameliorates Salinity Stress Tolerance of Tomato (Solanum lycopersicum L.) and Enhances Fruit Quality. Antioxidants 2023, 12, 558. [Google Scholar] [CrossRef]
- Terry, L. Elicitors of Induced Disease Resistance in Postharvest Horticultural Crops: A Brief Review. Postharvest Biol. Technol. 2004, 32, 1–13. [Google Scholar] [CrossRef]
- Dos, D.; Costa, S.; Moreno, D.S.A.; Alviano, C.S.; Jorge, A.; Da Silva, R. Extension of Solanaceae Food Crops Shelf Life by the Use of Elicitors and Sustainable Practices During Postharvest Phase. Food Bioprocess Technol. 2022, 15, 249–274. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Zhang, J.; He, T.; Huang, J.; Zhang, Z.; Wang, Y.; Hafeez, M.; Zhou, S.; Ren, X.; et al. Comprehensive Metabolome and Volatilome Analyses in Eggplant and Tomato Reveal Their Differential Responses to Tuta absoluta Infestation. Front. Plant Sci. 2021, 12, 757230. [Google Scholar] [CrossRef]
- Tortorici, S.; Biondi, A.; Pérez-Hedo, M.; Larbat, R.; Zappalà, L. Plant Defences for Enhanced Integrated Pest Management in Tomato. Ann. Appl. Biol. 2022, 180, 328–337. [Google Scholar] [CrossRef]
- Fanourakis, D.; Tsaniklidis, G.; Makraki, T.; Nikoloudakis, N.; Bartzanas, T.; Sabatino, L.; Fatnassi, H.; Ntatsi, G. Climate Change Impacts on Greenhouse Horticulture in the Mediterranean Basin: Challenges and Adaptation Strategies. Plants 2025, 14, 3390. [Google Scholar] [CrossRef]
- Bhattarai, S.; Jha, D.K.; Balyan, S.; Zhen, S.; Shivasankara, S.; Patil, B.S. UV-B and Blue Light Supplementation Improves Tomato Quality and Antioxidant Dynamics: A Novel Approach for Sustainable Greenhouse Production. J. Agric. Food Res. 2025, 22, 102054. [Google Scholar] [CrossRef]
- Petric, T.; Kiferle, C.; Perata, P.; Gonzali, S. Optimizing Shelf Life Conditions for Anthocyanin-Rich Tomatoes. PLoS ONE 2018, 13, e0205650. [Google Scholar] [CrossRef]
- Singh, M.C.; Singh, J.P.; Pandey, S.K.; Mahay, D.; Shrivastva, V. Factors Affecting the Performance of Greenhouse Cucumber Cultivation—A Review. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2304–2323. [Google Scholar] [CrossRef]
- Grimstad, S.O.; Frimanslund, E. Effect of Different Day and Night Temperature Regimes on Greenhouse Cucumber Young Plant Production, Flower Bud Formation and Early Yield. Sci. Hortic. 1993, 53, 191–204. [Google Scholar] [CrossRef]
- Kang, H.-M.; Park, K.-W.; Saltveit, M.E. Elevated Growing Temperatures During the Day Improve the Postharvest Chilling Tolerance of Greenhouse-Grown Cucumber (Cucumis sativus) Fruit. Postharvest Biol. Technol. 2002, 24, 49–57. [Google Scholar] [CrossRef]
- Slack, G.; Hand, D.W. The Effect of Day and Night Temperatures on the Growth, Development and Yield of Glasshouse Cucumbers. J. Hortic. Sci. 1983, 58, 567–573. [Google Scholar] [CrossRef]
- Xiong, J.; Patil, G.G.; Moe, R.; Torre, S. Effects of Diurnal Temperature Alternations and Light Quality on Growth, Morphogenesis and Carbohydrate Content of Cucumis sativus L. Sci. Hortic. 2011, 128, 54–60. [Google Scholar] [CrossRef]
- Xiong, J.; Patil, G.G.; Moe, R. Effect of DIF and End-of-Day Light Quality on Stem Elongation in Cucumis sativus. Sci. Hortic. 2002, 94, 219–229. [Google Scholar] [CrossRef]
- Ekman, J.H.; Pristijono, P.; Parks, S.; Jarvis, J. Growing Conditions Affect Postharvest Quality of Greenhouse Cucumbers. Acta Hortic. 2010, 877, 181–186. [Google Scholar] [CrossRef]
- Gómez-López, M.D.; Fernández-Trujillo, J.P.; Baille, A. Cucumber Fruit Quality at Harvest Affected by Soilless System, Crop Age and Preharvest Climatic Conditions During Two Consecutive Seasons. Sci. Hortic. 2006, 110, 68–78. [Google Scholar] [CrossRef]
- Pal, A. Cultivation of Cucumber in Greenhouse. In Protected Cultivation and Smart Agriculture; New Delhi Publishers: New Delhi, India, 2020. [Google Scholar]
- Christodoulou, P.; Ladika, G.; Tsiantas, K.; Kritsi, E.; Tsiaka, T.; Cavouras, D.; Zoumpoulakis, P.; Sinanoglou, V.J. Quality Assessment of Greenhouse-Cultivated Cucumbers (Cucumis sativus) During Storage Using Instrumental and Image Analyses. Appl. Sci. 2024, 14, 8676. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, C.; Wang, L.; Li, X.; Wang, G.; Yang, Y. The Combinations of White, Blue, and UV-A Light Provided by Supplementary Light-Emitting Diodes Promoted the Quality of Greenhouse-Grown Cucumber Seedlings. Agriculture 2022, 12, 1593. [Google Scholar] [CrossRef]
- Wang, S.; Fan, S.; Kong, Y.; Qingjun, C. Effect of Light Quality on the Growth and Photosynthetic Characteristics of Cucumber Cucumis sativus L. Under Solar Greenhouse. Acta Hortic. 2007, 731, 243–251. [Google Scholar] [CrossRef]
- Ahmed, S.J. Studying the Effect of Light Quality on Photosynthetic Efficiency and Yield in Greenhouse Grown Cucumber. Indus J. Anim. Plant Sci. 2025, 3, 1–14. [Google Scholar]
- Liu, X.; Liu, X.; Xu, Y.; Wang, Z.; Sun, Q.; Liu, S.; Liu, B.; Li, Q. Effects of Carbon Dioxide Enrichment and Environmental Factors on Photosynthesis, Growth and Yield and Their Interaction in Cucumber: A Meta-Analysis. arXiv 2025. [Google Scholar] [CrossRef]
- Dong, J.; Li, X.; Gruda, N.; Duan, Z. Interactive Effects of Elevated Carbon Dioxide and Nitrogen Availability on Fruit Quality of Cucumber (Cucumis sativus L.). J. Integr. Agric. 2018, 17, 2438–2446. [Google Scholar] [CrossRef]
- Koo, J.K.; Hwang, H.S.; Hwang, J.H.; Park, E.W.; Yu, J.; Yun, J.H.; Hwang, S.Y.; Choi, H.E.; Hwang, S.J. Supplemental Lighting and CO2 Enrichment on the Growth, Fruit Quality, and Yield of Cucumber. Hortic. Environ. Biotechnol. 2025, 66, 77–85. [Google Scholar] [CrossRef]
- Villagran, E.; Espitia, J.J.; Amado, G.; Rodriguez, J.; Gomez, L.; Velasquez, J.F.; Gil, R.; Baeza, E.; Aguilar, C.E.; Akrami, M.; et al. CO2 Enrichment in Protected Agriculture: A Systematic Review of Greenhouses, Controlled Environment Systems, and Vertical Farms—Part 2. Sustainability 2025, 17, 2809. [Google Scholar] [CrossRef]
- Rho, H.; Su, J.; Sim, H.S.; Moon, Y.H.; Woo, U.J.; Kim, S.K. Development of a Cucumber Transpiration Model Based on a Simplified Penman-Monteith Model in a Semi-Closed Greenhouse. HortScience 2023, 58, 1208–1216. [Google Scholar] [CrossRef]
- Trivedi, P.; Nguyen, N.; Hykkerud, A.L.; Häggman, H.; Martinussen, I.; Jaakola, L.; Karppinen, K. Developmental and Environmental Regulation of Cuticular Wax Biosynthesis in Fleshy Fruits. Front. Plant Sci. 2019, 10, 431. [Google Scholar] [CrossRef]
- Song, X.; Miao, L.; Jiao, X.; Ibrahim, M.; Li, J. Regulating Vapor Pressure Deficit and Soil Moisture Improves Tomato and Cucumber Plant Growth and Water Productivity in the Greenhouse. Horticulturae 2022, 8, 147. [Google Scholar] [CrossRef]
- Rodriguez Borbon, M.I.; Sohn, H.; Delgado, E.; Fuqua, D.O.; Rodríguez Medina, M.A.; Tlapa, D.; Baez-Lopez, Y. Shelf-Life Assessment on European Cucumber Based on Accelerated Temperature–Humidity Stresses. Appl. Sci. 2023, 13, 2663. [Google Scholar] [CrossRef]
- Zhang, H.; Chi, D.; Wang, Q.; Fang, J.; Fang, X. Yield and Quality Response of Cucumber to Irrigation and Nitrogen Fertilization Under Subsurface Drip Irrigation in Solar Greenhouse. Agric. Sci. China 2011, 10, 921–930. [Google Scholar] [CrossRef]
- Piri, H.; Naserin, A.; Albalasmeh, A.A. Interactive Effects of Deficit Irrigation and Vermicompost on Yield, Quality, and Irrigation Water Use Efficiency of Greenhouse Cucumber. J. Arid. Land 2022, 14, 1274–1292. [Google Scholar] [CrossRef]
- Rahil, M.H.; Qanadillo, A. Effects of Different Irrigation Regimes on Yield and Water Use Efficiency of Cucumber Crop. Agric. Water Manag. 2015, 148, 10–15. [Google Scholar] [CrossRef]
- Al-Jaloud, A.A.; Baig, M.S.; Errebhi, M.A.; AbdelGadir, A.H.; Sarhan, H.B. The Effect of Fertigating Different Levels of Nitrogen, Phosphorus and Potassium on Greenhouse Cucumber Yield. Acta Hortic. 2006, 710, 359–364. [Google Scholar] [CrossRef]
- Roosta, H.R.; Sharifi Azad, H.; Mirdehghan, S.H. Comparison of the Growth, Fruit Quality and Physiological Characteristics of Cucumber Fertigated by Three Different Nutrient Solutions in Soil Culture and Soilless Culture Systems. Sci. Rep. 2025, 15, 203. [Google Scholar] [CrossRef]
- Liang, X.; Gao, Y.; Zhang, X.; Tian, Y.; Zhang, Z.; Gao, L. Effect of Optimal Daily Fertigation on Migration of Water and Salt in Soil, Root Growth and Fruit Yield of Cucumber (Cucumis sativus L.) in Solar-Greenhouse. PLoS ONE 2014, 9, e86975. [Google Scholar] [CrossRef]
- Guo, R.; Li, X.; Christie, P.; Chen, Q.; Zhang, F. Seasonal Temperatures Have More Influence than Nitrogen Fertilizer Rates on Cucumber Yield and Nitrogen Uptake in a Double Cropping System. Environ. Pollut. 2008, 151, 443–451. [Google Scholar] [CrossRef]
- Gruda, N.S.; Gallegos-Cedillo, V.M.; Nájera, C.; Catalina, E.-G.; Ochoa, J.; Fernández, J.A. Advancing Protected Cultivation: A Pathway for Nutrient-Rich Vegetables. CRC Crit. Rev. Plant Sci. 2025, 44, 88–116. [Google Scholar] [CrossRef]
- Alsadon, A.; Al-Helal, I.; Ibrahim, A.; Abdel-Ghany, A.; Al-Zaharani, S.; Ashour, T. The Effects of Plastic Greenhouse Covering on Cucumber (Cucumis sativus L.) Growth. Ecol. Eng. 2016, 87, 305–312. [Google Scholar] [CrossRef]
- Abu-Zahra, T.R.; Ateyyat, M.A. Effect of Various Shading Methods on Cucumber (Cucumis sativus L.) Growth and Yield Production. Int. J. Environ. Sustain. 2016, 5, 10–17. [Google Scholar] [CrossRef]
- Hao, X.; Zheng, J.M.; Zhang, Y.; Little, C.; Khosla, S. Effects of Diffused Plastic Cover Materials on Greenhouse Microclimate, Plant Growth, Fruit Yield and Quality, and Energy Use in Greenhouse Fruit Vegetable Production. Acta Hortic. 2017, 1182, 73–78. [Google Scholar] [CrossRef]
- Moreno-Teruel, M.Á.; Molina-Aiz, F.D.; López-Martínez, A.; Valera-Martínez, D.L.; Peña-Fernández, A.; Baptista, F. Impact of a High-PAR Transmittance and High Light Diffusivity Plastic Cover on Photosynthetic Activity and Development of Cucumber (Cucumis sativus L.) Crops in a Mediterranean Greenhouse. arXiv 2024. [Google Scholar] [CrossRef]
- Li, R.; Gao, Y.; Cai, B.; Li, G.; Xue, Z.; Wang, X.-X.; Li, Q. RPO Film Effectively Promotes Fruit Quality and Yield of Cucumber Through Adjusting Greenhouse Environment and Hormone Contents. BMC Plant Biol. 2024, 24, 1250. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.M.; Ashour, M.; Sakai, N.; Zhang, L.; Hassanien, H.A.; Gaber, A.; Ammar, G. Impact of Seaweed Liquid Extract Biostimulant on Growth, Yield, and Chemical Composition of Cucumber (Cucumis sativus). Agriculture 2021, 11, 320. [Google Scholar] [CrossRef]
- Zamljen, T.; Šircelj, H.; Veberič, R.; Hudina, M.; Slatnar, A. Impact of Two Brown Seaweed (Ascophyllum nodosum L.) Biostimulants on the Quantity and Quality of Yield in Cucumber (Cucumis sativus L.). Foods 2024, 13, 401. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Xylia, P.; Stavrinides, M.; Tzortzakis, N. Evaluating a Natural-Based Solution for Its Stimulation in Cucumis sativus Plants and Fruits. Horticulturae 2025, 11, 499. [Google Scholar] [CrossRef]
- García, Y.G.; Flores-Robles, V.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; De la Fuente, M.C.; Sandoval Rangel, A.; Juárez-Maldonado, A. Application of Two Forms of Silicon and Their Impact on the Postharvest and the Content of Bioactive Compounds in Cucumber (Cucumis sativus L.) Fruits. BIOCELL 2022, 46, 2497–2506. [Google Scholar] [CrossRef]
- Kahramanoğlu, İ.; Usanmaz, S. Improving Postharvest Storage Quality of Cucumber Fruit by Modified Atmosphere Packaging and Biomaterials. HortScience 2019, 54, 2005–2014. [Google Scholar] [CrossRef]
- Al-Tamimi, A.A.H.; Jalalizand, A.; Salim, H.A.; Mahmoudi, E. Achievement of Induced Systemic Resistance in Greenhouse Cucumber against Bemisia tabaci Using Humic Acid, Salicylic Acid and Trichoderma harzianum. arXiv 2025. [Google Scholar] [CrossRef]
- He, J.; Bouwmeester, H.J.; Dicke, M.; Kappers, I.F. Transcriptional and Metabolite Analysis Reveal a Shift in Direct and Indirect Defences in Response to Spider-Mite Infestation in Cucumber (Cucumis sativus). Plant Mol. Biol. 2020, 103, 489–505. [Google Scholar] [CrossRef]
- Qi, X.; Chen, M.; Liang, D.; Xu, Q.; Zhou, F.; Chen, X. Jasmonic Acid, Ethylene and ROS Are Involved in the Response of Cucumber (Cucumis sativus L.) to Aphid Infestation. Sci. Hortic. 2020, 269, 109421. [Google Scholar] [CrossRef]
- Hong, I.; Yu, J.; Hwang, S.J.; Kwack, Y. Estimation of Cucumber Fruit Yield Cultivated Under Different Light Conditions in Greenhouses. Horticulturae 2024, 10, 1117. [Google Scholar] [CrossRef]
- Bakker, J.C.; van Uffelen, J.A.M. The Effects of Diurnal Temperature Regimes on Growth and Yield of Glasshouse Sweet Pepper. Neth. J. Agric. Sci. 1988, 36, 201–208. [Google Scholar] [CrossRef]
- Neocleous, D.; Nikolaou, G. Antioxidant Seasonal Changes in Soilless Greenhouse Sweet Peppers. Agronomy 2019, 9, 730. [Google Scholar] [CrossRef]
- Adibian, M.; Hamidoghli, Y.; Aliniaeifard, S. Effect of Supplemental Light Quality and Season on Growth and Photosynthesis of Two Cultivars of Greenhouse Sweet Pepper (Capsicum annuum L.). Int. J. Hortic. Sci. Technol. 2023, 10, 51–66. [Google Scholar]
- Si, Y.; Heins, R.D. Influence of Day and Night Temperatures on Sweet Pepper Seedling Development. J. Am. Soc. Hortic. Sci. 1996, 121, 699–704. [Google Scholar] [CrossRef]
- Aloni, B.; Karni, L.; Rylski, I.; Cohen, Y.; Lee, Y.; Fuchs, M.; Moreshet, S.; Yao, C. Cuticular Cracking in Pepper Fruit. I. Effects of Night Temperature and Humidity. J. Hortic. Sci. Biotechnol. 1998, 73, 743–749. [Google Scholar] [CrossRef]
- Thanopoulos, C.; Akoumianakis, K.A.; Passam, H.C. The Effect of Season on the Growth and Maturation of Bell Peppers. Int. J. Plant Prod. 2013, 7, 279–294. [Google Scholar]
- Stommel, J.R.; Griesbach, R.J. Differential Expression of Capsicum Anthocyanin Structural Genes in Response to Temperature Stress. HortScience 2004, 39, 867D–868D. [Google Scholar] [CrossRef]
- Kabir, M.Y.; Nambeesan, S.U.; Bautista, J.; Díaz-Pérez, J.C. Plant Water Status, Plant Growth, and Fruit Yield in Bell Pepper (Capsicum annum L.) Under Shade Nets. Sci. Hortic. 2022, 303, 111241. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Milenković, L.; Šunić, L.; Barać, S.; Mastilović, J.; Kevrešan, Ž.; Fallik, E. Effect of Shading by Coloured Nets on Yield and Fruit Quality of Sweet Pepper. Zemdirb.-Agric. 2017, 104, 53–62. [Google Scholar] [CrossRef]
- Rylski, I.; Spigelman, M. Effect of Shading on Plant Development, Yield and Fruit Quality of Sweet Pepper Grown Under Conditions of High Temperature and Radlation. Sci. Hortic. 1986, 29, 31–35. [Google Scholar] [CrossRef]
- O’Donoghue, E.M.; Brummell, D.A.; McKenzie, M.J.; Hunter, D.A.; Lill, R.E. Sweet Capsicum: Postharvest Physiology and Technologies. N. Z. J. Crop Hortic. Sci. 2018, 46, 269–297. [Google Scholar] [CrossRef]
- He, X. Light Altering Film Affects Capsicum Development and Quality in a Protected Cropping Facility. Ph.D. Thesis, The University of Sydney, Sydney, NSW, Australia, 2022. [Google Scholar]
- Jiménez-Viveros, Y.; Núñez-Palenius, H.G.; Fierros-Romero, G.; Valiente-Banuet, J.I. Modification of Light Characteristics Affect the Phytochemical Profile of Peppers. Horticulturae 2023, 9, 72. [Google Scholar] [CrossRef]
- Afolabi, A.S.; Choi, I.-L.; Lee, J.H.; Kwon, Y.B.; Yoon, H.S.; Kang, H.-M. Effect of Pre-Storage CO2 Treatment and Modified Atmosphere Packaging on Sweet Pepper Chilling Injury. Plants 2023, 12, 671. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kang, S.; Li, F.; Zhang, X.; Huo, Z.; Ding, R.; Tong, L.; Du, T.; Li, S. Light Supplement and Carbon Dioxide Enrichment Affect Yield and Quality of Off-Season Pepper. Agron. J. 2017, 109, 2107–2118. [Google Scholar] [CrossRef]
- Beny, A.; Leah, K. Effects of CO2 Enrichment on Yield, Carbohydrate Accumulation and Changes in the Activity of Antioxidative Enzymes in Bell Pepper (Capsicum annuum L.). J. Hortic. Sci. Biotechnol. 2002, 77, 534–540. [Google Scholar] [CrossRef]
- Alonso, F.J.; Lorenzo, P.; Medrano, E.; Sánchez-Guerrero, M.C. Greenhouse Sweet Pepper Productive Response to Carbon Dioxide Enrichment and Crop Pruning. Acta Hortic. 2012, 927, 345–351. [Google Scholar] [CrossRef]
- Porras, M.E.; Lorenzo, P.; Medrano, E.; Sánchez-González, M.J.; Otálora-Alcón, G.; Piñero, M.C.; del Amor, F.M.; Sánchez-Guerrero, M.C. Photosynthetic Acclimation to Elevated CO2 Concentration in a Sweet Pepper (Capsicum annuum) Crop Under Mediterranean Greenhouse Conditions: Influence of the Nitrogen Source and Salinity. Funct. Plant Biol. 2017, 44, 573–586. [Google Scholar] [CrossRef]
- Abdel-Mawg, A.M.R.; El-Abd, S.O.; Abou-Hadid, A.F.; Sassine, Y.N. Blossom End Rot of Sweet Pepper Fruits in Relation to Different Shoot and Root Conditions. Asian J. Plant Sci. 2005, 4, 619–624. [Google Scholar] [CrossRef]
- Abd-El-Baky, H.; Ali, S.; El Haddad, Z.; El Ansary, Z. Some Environmental Parameters Affecting Sweet Pepper Growth and Productivity Under Different Greenhouse Forms in Hot and Humid Climatic Conditions. J. Soil Sci. Agric. Eng. 2010, 1, 225–247. [Google Scholar] [CrossRef]
- Taia, W.; Basahi, J.; Hassan, I. Impact of Ambient Air on Physiology, Pollen Tube Growth, Pollen Germination and Yield in Pepper (Capsicum annuum L.). Pak. J. Bot. 2013, 45, 921–926. [Google Scholar]
- Gruda, N.S.; Samuolienė, G.; Dong, J.; Li, X. Environmental Conditions and Nutritional Quality of Vegetables in Protected Cultivation. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70139. [Google Scholar] [CrossRef] [PubMed]
- Rameshwaran, P.; Tepe, A.; Yazar, A.; Ragab, R. Effects of Drip-Irrigation Regimes with Saline Water on Pepper Productivity and Soil Salinity Under Greenhouse Conditions. Sci. Hortic. 2016, 199, 114–123. [Google Scholar] [CrossRef]
- Abdelkhalik, A.; Pascual, B.; Nájera, I.; Domene, M.A.; Baixauli, C.; Pascual-Seva, N. Effects of Deficit Irrigation on the Yield and Irrigation Water Use Efficiency of Drip-Irrigated Sweet Pepper (Capsicum annuum L.) Under Mediterranean Conditions. Irrig. Sci. 2020, 38, 89–104. [Google Scholar] [CrossRef]
- Haghighi, M.; Sharifani, M.J.; Parnianifard, F. Physiological Changes of Sweet Pepper Under Low Irrigation Regimes Applied in Three Phenological Stages of Vegetative Growth, Reproductive Growth, and Fruit Set. N. Z. J. Crop Hortic. Sci. 2025, 53, 419–441. [Google Scholar] [CrossRef]
- Byeon, S.-E.; Park, H.; Jeong, S.; Park, J.; Latt, T.T.; Lee, J.; Hwang, I.G.; Lee, J. Tissue Region-Dependent Differential Responses of Fruit Quality Attributes, Mineral Nutrients, and Targeted Major Metabolites in Blossom-End Rot of Paprika Fruit. Hortic. Environ. Biotechnol. 2025, 1–19. [Google Scholar] [CrossRef]
- Murashima, K.; Arakawa, R.; Maruyama, H.; Watanabe, T.; Shinano, T. Evaluation of Functional Components in Paprika Fruits (Capsicum annuum L.) Under Different Nitrogen Concentrations. Hortic. J. 2025, 94, 255–265. [Google Scholar] [CrossRef]
- Oliveira, F.A.; Duarte, S.N.; Medeiros, J.F.; Lima, C.J.; Oliveira, M.K.; Silva, R.C. Improving Sweet Pepper Yield and Quality by Means of Fertigation Management. Hortic. Bras. 2017, 35, 235–241. [Google Scholar] [CrossRef]
- Prieto, M.; Peñalosa, J.; José Sarro, M.; Zornoza, P.; Gárate, A. Seasonal Effect on Growth Parameters and Macronutrient Use of Sweet Pepper. J. Plant Nutr. 2007, 30, 1803–1820. [Google Scholar] [CrossRef]
- Ferreira, R.d.C.; Bezerra, R.d.S.; Rosa, J.Q.S. Effects of Light Intensity Modification by Reflective Aluminized Screenhouse on Sweet Pepper Growth and Yield. Eng. Agric. 2014, 34, 626–635. [Google Scholar] [CrossRef]
- Hamaiel, A.; Abdelhady, M.; Dawod, M. Enhancing Fruit Yield and Quality of Red Sweet Pepper Under Protected Conditions with Organic Acids and Bio-Stimulants. Damietta J. Agric. Sci. 2024, 3, 1–13. [Google Scholar] [CrossRef]
- Maraei, R.; Eliwa, N.; Aly, A. Use of Some Biostimulants to Improve the Growth and Chemical Constituents of Sweet Pepper. Potravin. Slovak J. Food Sci. 2019, 13, 553–561. [Google Scholar] [CrossRef]
- Baltazar, M.; Correia, S.; Guinan, K.J.; Sujeeth, N.; Bragança, R.; Gonçalves, B. Recent Advances in the Molecular Effects of Biostimulants in Plants: An Overview. Biomolecules 2021, 11, 1096. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Parvin, K.; Bardhan, K.; Nahar, K.; Anee, T.I.; Masud, A.A.C.; Fotopoulos, V. Biostimulants for the Regulation of Reactive Oxygen Species Metabolism in Plants Under Abiotic Stress. Cells 2021, 10, 2537. [Google Scholar] [CrossRef]
- Avasiloaiei, D.I.; Calara, M.; Brezeanu, P.M.; Bălăiță, C.; Brumă, I.S.; Brezeanu, C. Capsicum chinense Jacq. Response to Pyrolysis-Derived Amendments and Sustainable Fertilizers in Containerized Greenhouse Systems. Agronomy 2025, 15, 2125. [Google Scholar] [CrossRef]
- Attia, M.S.; Salem, M.S.; Abdelaziz, A.M. Endophytic Fungi Aspergillus spp. Reduce Fusarial Wilt Disease Severity, Enhance Growth, Metabolism and Stimulate the Plant Defense System in Pepper Plants. Biomass Convers. Biorefin. 2024, 14, 16603–16613. [Google Scholar] [CrossRef]
- Szarka, J.; Timár, Z.; Hári, R.; Palotás, G.; Péterfi, B. General Defense Response Under Biotic Stress and Its Genetics at Pepper (Capsicum annuum L.). Sustainability 2022, 14, 6458. [Google Scholar] [CrossRef]
- Ismail, M.S.M.; Hussien, M.-A.N.E. Potential of Plant Extracts and their Elicitor Effect on the Red Sweet Pepper (Capsicum annuum L.) Defence System against Tetranychus urticae (Acari: Tetranychidae) Infestation Under Greenhouse Conditions. Int. J. Acarol. 2022, 48, 130–138. [Google Scholar] [CrossRef]
- Martínez-Damián, M.T.; Ojeda-Barrios, D.L.; Cruz-Alvarez, O. Fungicides, Bio-Controllers and Resistance Inducers Affect Bioactive Compounds and Oxidative Metabolism in Bell Pepper Plants Inoculated with Phytophthora capsici. Folia Hortic. 2024, 36, 187–196. [Google Scholar] [CrossRef]
- He, J. Root Growth, Morphological and Physiological Characteristics of Subtropical and Temperate Vegetable Crops Grown in the Tropics Under Different Root-Zone Temperature. In Plant Growth; InTech: London, UK, 2016; Volume 7, pp. 1993–2005. [Google Scholar]
- Kawasaki, Y.; Matsuo, S.; Kanayama, Y.; Kanahama, K. Effect of Root-Zone Heating on Root Growth and Activity, Nutrient Uptake, and Fruit Yield of Tomato at Low Air Temperatures. J. Jpn. Soc. Hortic. Sci. 2014, 83, 295–301. [Google Scholar] [CrossRef]
- Tindall, J.A.; Mills, H.A.; Radcliffe, D.E. The Effect of Root Zone Temperature on Nutrient Uptake of Tomato. J. Plant Nutr. 1990, 13, 939–956. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.; Song, W. Physiological and Growth Characteristics of Tomato Seedlings in Response to Low Root-Zone Temperature. HortScience 2023, 58, 442–448. [Google Scholar] [CrossRef]
- Adams, P.; Ho, L.C. Effects of Environment on the Uptake and Distribution of Calcium in Tomato and on the Incidence of Blossom-End Rot. Plant Soil 1993, 154, 127–132. [Google Scholar] [CrossRef]
- Hurewitz, J.; Janes, H.W. Effect of Altering the Root-Zone Temperature on Growth, Translocation, Carbon Exchange Rate, and Leaf Starch Accumulation in the Tomato. Plant Physiol. 1983, 73, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, A.; Trudel, M.J. Interactions Between Root-Zone Temperature and Light Levels on Growth, Development and Photosynthesis of Lycopersicon esculentum Mill. Cultivar ‘Vendor’. Sci. Hortic. 1984, 23, 313–321. [Google Scholar] [CrossRef]
- Nkansah, G.O.; Ito, T. Comparative Studies on Growth and Development of Heat-Tolerant and Non Heat-Tolerant Tomato Plants Grown at Different Root-Zone Temperatures. J. Jpn. Soc. Hortic. Sci. 1994, 62, 775–780. [Google Scholar] [CrossRef]
- He, F.; Thiele, B.; Watt, M.; Kraska, T.; Ulbrich, A.; Kuhn, A.J. Effects of Root Cooling on Plant Growth and Fruit Quality of Cocktail Tomato During Two Consecutive Seasons. J. Food Qual. 2019, 2019, 3598172. [Google Scholar] [CrossRef]
- Anza, M.; Riga, P.; Epalza, B.; Arana, I.; Arazuri, S.; Jaren, C.; Arias, N. Effect of Root-Zone Heating on the Yield and Some Quality Parameters of Hydroponically Grown Tomato. Acta Hortic. 2012, 927, 897–903. [Google Scholar] [CrossRef]
- Yan, Q.; Duan, Z.; Mao, J.; Li, X.; Dong, F. Effects of Root-Zone Temperature and N, P, and K Supplies on Nutrient Uptake of Cucumber (Cucumis sativus L.) Seedlings in Hydroponics. Soil Sci. Plant Nutr. 2012, 58, 707–717. [Google Scholar] [CrossRef]
- Moon, J.-H.; Boo, H.-O.; Jang, I.-O. Effect of Root-Zone Temperature on Water Relations and Hormone Contents in Cucumber. Korean Soc. Hortic. Sci. 2007, 48, 257–264. [Google Scholar]
- Gosselin, A.; Trudel, M.-J. Influence of Root-Zone Temperature on Growth, Development and Yield of Cucumber Plants Cv. Toska. Plant Soil 1985, 85, 327–336. [Google Scholar] [CrossRef]
- Yan, Q.; Duan, Z.; Mao, J.; Li, X.; Dong, F. Low Root Zone Temperature Limits Nutrient Effects on Cucumber Seedling Growth and Induces Adversity Physiological Response. J. Integr. Agric. 2013, 12, 1450–1460. [Google Scholar] [CrossRef]
- Bi, X.; Wang, X.; Zhang, X. Effects of Different Root Zone Heating Methods on the Growth and Photosynthetic Characteristics of Cucumber. Horticulturae 2022, 8, 1137. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.; Sassine, Y.N.; El-Behairy, U.A.A.; Abou Hadid, A.F. Effect of Minimum Root-Zone Temperature on the Growth and Production of Greenhouse Sweet Pepper. J. Appl. Sci. Res. 2005, 1, 72–77. [Google Scholar]
- Choi, K.Y.; Ko, J.Y.; Yoo, H.J.; Choi, E.Y.; Lee, H.C.; Lee, Y.-B. Effect of Cooling Timing in the Root Zone on Substrate Temperature and Physiological Response of Sweet Pepper in Summer Cultivation. Hortic. Sci. Technol. 2014, 32, 53–59. [Google Scholar]
- Gosselin, A.; Trudel, M.-J. Root-Zone Temperature Effects on Pepper. J. Am. Soc. Hortic. Sci. 1986, 111, 220–224. [Google Scholar] [CrossRef]
- Sheibanirad, A.; Haghighi, M.; Pessarakli, M. The Effect of Root Zone Temperature at Low Nitrogen Level of Nutrient Solution on Sweet Pepper. J. Plant Nutr. 2023, 46, 3983–4000. [Google Scholar] [CrossRef]
- Hao, X.; Guo, X.; Lanoue, J.; Zhang, Y.; Cao, R.; Zheng, J.; Little, C.; Leonardos, D.; Kholsa, S.; Grodzinski, B.; et al. A Review on Smart Application of Supplemental Lighting in Greenhouse Fruiting Vegetable Production. Acta Hortic. 2018, 1227, 499–506. [Google Scholar] [CrossRef]
- Dorais, M.; Ehret, D.L.; Papadopoulos, A.P. Tomato (Solanum lycopersicum) Health Components: From the Seed to the Consumer. Phytochem. Rev. 2008, 7, 231–250. [Google Scholar] [CrossRef]
- Kaiser, E.; Ouzounis, T.; Giday, H.; Schipper, R.; Heuvelink, E.; Marcelis, L.F.M. Adding Blue to Red Supplemental Light Increases Biomass and Yield of Greenhouse-Grown Tomatoes, but Only to an Optimum. Front. Plant Sci. 2019, 9, 2002. [Google Scholar] [CrossRef]
- He, R.; Wei, J.; Zhang, J.; Tan, X.; Li, Y.; Gao, M.; Liu, H. Supplemental Blue Light Frequencies Improve Ripening and Nutritional Qualities of Tomato Fruits. Front. Plant Sci. 2022, 13, 888976. [Google Scholar] [CrossRef]
- Lanoue, J.; Zheng, J.; Little, C.; Thibodeau, A.; Grodzinski, B.; Hao, X. Alternating Red and Blue Light-Emitting Diodes Allows for Injury-Free Tomato Production with Continuous Lighting. Front. Plant Sci. 2019, 10, 1114. [Google Scholar] [CrossRef]
- Deram, P.; Lefsrud, M.G.; Orsat, V. Supplemental Lighting Orientation and Red-to-Blue Ratio of Light-Emitting Diodes for Greenhouse Tomato Production. HortScience 2014, 49, 448–452. [Google Scholar] [CrossRef]
- Kim, H.-J.; Yang, T.; Choi, S.; Wang, Y.-J.; Lin, M.-Y.; Liceaga, A.M. Supplemental Intracanopy Far-Red Radiation to Red LED Light Improves Fruit Quality Attributes of Greenhouse Tomatoes. Sci. Hortic. 2020, 261, 108985. [Google Scholar] [CrossRef]
- Hovi-Pekkanen, T.; Tahvonen, R. Effects of Interlighting on Yield and External Fruit Quality in Year-Round Cultivated Cucumber. Sci. Hortic. 2008, 116, 152–161. [Google Scholar] [CrossRef]
- de Freitas, I.S.; Roldán, G.Q.; Macedo, A.C.; Mello, S.d.C. The Responses of Photosynthesis, Fruit Yield and Quality of Mini-Cucumber to LED-Interlighting and Grafting. Hortic. Bras 2021, 39, 86–93. [Google Scholar] [CrossRef]
- Liang, Y.; Kang, C.; Kaiser, E.; Kuang, Y.; Yang, Q.; Li, T. Red/Blue Light Ratios Induce Morphology and Physiology Alterations Differently in Cucumber and Tomato. Sci. Hortic. 2021, 281, 109995. [Google Scholar] [CrossRef]
- Ménard, C.; Dorais, M.; Hovi, T.; Gosselin, A. Developmental and Physiological Responses of Tomato and Cucumber to Additional Blue Light. Acta Hortic. 2006, 711, 291–296. [Google Scholar] [CrossRef]
- Jin, D.; Su, X.; Li, Y.; Shi, M.; Yang, B.; Wan, W.; Wen, X.; Yang, S.; Ding, X.; Zou, J. Effect of Red and Blue Light on Cucumber Seedlings Grown in a Plant Factory. Horticulturae 2023, 9, 124. [Google Scholar] [CrossRef]
- López-Juez, E.; Buurmeijer, W.F.; Heeringa, G.H.; Kendrick, R.E.; Wesselius, J.C. Response of Light-Grown Wild-Type and Long Hypocotyl Mutant Cucumber Plants to End-of-Day Far-Red Light. Photochem. Photobiol. 1990, 52, 143–149. [Google Scholar] [CrossRef]
- Kim, D.; Son, J.E. Adding Far-Red to Red, Blue Supplemental Light-Emitting Diode Interlighting Improved Sweet Pepper Yield but Attenuated Carotenoid Content. Front. Plant Sci. 2022, 13, 938199. [Google Scholar] [CrossRef] [PubMed]
- Adibian, M.; Hamidoghli, Y.; Ghasemnezhad, M.; Aliniaeifard, S. Effects of Combined Red and Blue Light Spectra as Supplemental Light on Yield and Fruit Quality of Sweet Pepper. J. Hortic. Postharvest Res. 2023, 6, 317–330. [Google Scholar]
- López-Marin, J.; Profile, S.; Gálvez, A.; Manera, F.J. Photoselective Shade Netting in a Sweet Pepper Crop Accelerates Ripening Period and Enhances the Overall Fruits Quality and Yield. J. Agric. Sci. Technol. 2022, 24, 1171–1186. [Google Scholar]
- Demers, D.-A.; Gosselin, A.; Wien, H.C. Effects of Supplemental Light Duration on Greenhouse Sweet Pepper Plants and Fruit Yields. J. Am. Soc. Hortic. Sci. 1998, 123, 202–207. [Google Scholar] [CrossRef]
- Peet, M.M.; Willits, D.H. Greenhouse CO2 Enrichment Alternatives: Effects of Increasing Concentration or Duration of Enrichment on Cucumber Yields. J. Am. Soc. Hortic. Sci. 1987, 112, 236–241. [Google Scholar] [CrossRef]
- Peet, M.M.; Welles, G. Greenhouse Tomato Production. In Tomatoes; Heuvelink, E., Ed.; CABI Publishing: Wallingford, UK, 2005; pp. 257–304. [Google Scholar]
- Du, X.; Song, Y.; Pan, L.; Cui, S. Optimizing Cucumber (Cucumis sativus L.) Fruit Metabolomics Under Elevated CO2 and High-Temperature Stress in the Greenhouse. Horticulturae 2024, 11, 10. [Google Scholar] [CrossRef]
- Namizaki, H.; Iwasaki, Y.; Wang, R. Effects of Elevated CO2 Levels on the Growth and Yield of Summer-Grown Cucumbers Cultivated Under Different Day and Night Temperatures. Agronomy 2022, 12, 1872. [Google Scholar] [CrossRef]
- Granke, L.L.; Hausbeck, M.K. Effects of Temperature, Humidity, and Wounding on Development of Phytophthora Rot of Cucumber Fruit. Plant Dis. 2010, 94, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Shi, K. Carbon Dioxide (CO2) Enrichment in Protected Vegetable Production. In Growth Regulation and Quality Improvement of Vegetable Crops; Springer Nature: Singapore, 2025; pp. 333–347. [Google Scholar]
- van Berkel, N. Injurious Effects of High CO2 Concentrations on Cucumber, Tomato, Chrysanthemum and Gerbera. Acta Hortic. 1984, 162, 101–112. [Google Scholar] [CrossRef]
- Xu, H.-L.; Gauthier, L.; Desjardins, Y.; Gosselin, A. Photosynthesis in Leaves, Fruits, Stem and Petioles of Greenhouse-Grown Tomato Plants. Photosynthetica 1997, 33, 113–123. [Google Scholar] [CrossRef]
- Conesa, A.; Artés-Hernández, F.; Geysen, S.; Nicolaï, B.; Artés, F. High Oxygen Combined with High Carbon Dioxide Improvesmicrobial and Sensory Quality of Fresh-Cut Peppers. Postharvest Biol. Technol. 2007, 43, 230–237. [Google Scholar] [CrossRef]
- Gruda, N. Impact of Environmental Factors on Product Quality of Greenhouse Vegetables for Fresh Consumption. CRC Crit. Rev. Plant Sci. 2005, 24, 227–247. [Google Scholar] [CrossRef]
- Körner, O.; Challa, H. Process-Based Humidity Control Regime for Greenhouse Crops. Comput. Electron. Agric. 2003, 39, 173–192. [Google Scholar] [CrossRef]
- Domínguez, E.; Fernández, M.D.; Hernández, J.C.L.; Parra, J.P.; España, L.; Heredia, A.; Cuartero, J. Tomato Fruit Continues Growing While Ripening, Affecting Cuticle Properties and Cracking. Physiol. Plant 2012, 146, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Medrano, E.; Lorenzo, P.; Sánchez-Guerrero, M.C.; Montero, J.I. Evaluation and Modelling of Greenhouse Cucumber-Crop Transpiration Under High and Low Radiation Conditions. Sci. Hortic. 2005, 105, 163–175. [Google Scholar] [CrossRef]
- Ekinci, M.; Esringu, A.; Dursun, A.; Yildirim, E.; Turan, M.; Karaman, M.R.; Arjumend, T. Growth, Yield, and Calcium and Boron Uptake of Tomato (Lycopersicon esculentum L.) and Cucumber (Cucumis sativus L.) as Affected by Calcium and Boron Humate Application in Greenhouse Conditions. Turk. J. Agric. For. 2015, 39, 613–632. [Google Scholar] [CrossRef]
- Shibuya, T.; Terakura, R.; Kitaya, Y.; Kiyota, M. Effects of Low Relative Humidity and Illumination on Leaf Water Status of Cucumber Seedlings and Growth of Harvested Cuttings. HortScience 2006, 41, 410–413. [Google Scholar] [CrossRef]
- Shibuya, T.; Kano, K.; Endo, R.; Kitaya, Y. Photosynthetic Properties and Response to Drought in Cucumber Seedlings Acclimatized to Different Vapor-Pressure-Deficit Levels. Hortic. J. 2017, 86, 334–339. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Li, Y.; Qin, L.; Li, J.; Xu, F. Reducing the Excessive Evaporative Demand Improved the Water-Use Efficiency of Greenhouse Cucumber by Regulating the Trade-off Between Irrigation Demand and Plant Productivity. HortScience 2018, 53, 1784–1790. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, Y.; Zhao, X.; Li, J. Systemic Effects of the Vapor Pressure Deficit on the Physiology and Productivity of Protected Vegetables. Veg. Res. 2023, 3, 20. [Google Scholar] [CrossRef]
- Lara, I.; Belge, B.; Goulao, L.F. The Fruit Cuticle as a Modulator of Postharvest Quality. Postharvest Biol. Technol. 2014, 87, 103–112. [Google Scholar] [CrossRef]
- Parsons, E.P.; Popopvsky, S.; Lohrey, G.T.; Alkalai-Tuvia, S.; Perzelan, Y.; Bosland, P.; Bebeli, P.J.; Paran, I.; Fallik, E.; Jenks, M.A. Fruit Cuticle Lipid Composition and Water Loss in a Diverse Collection of Pepper (Capsicum). Physiol. Plant 2013, 149, 160–174. [Google Scholar] [CrossRef]
- Katsoulas, N.; Kittas, C.; Tsirogiannis, I.L.; Kitta, E.; Savvas, D. Greenhouse Microclimate and Soilless Pepper Crop Production and Quality as Affected by a Fog Evaporative Cooling System. Trans. ASABE 2007, 50, 1831–1840. [Google Scholar] [CrossRef]
- Costa, J.M.; Ortuño, M.F.; Chaves, M.M. Deficit Irrigation as a Strategy to Save Water: Physiology and Potential Application to Horticulture. J. Integr. Plant Biol. 2007, 49, 1421–1434. [Google Scholar] [CrossRef]
- Ripoll, J.; Urban, L.; Staudt, M.; Lopez-Lauri, F.; Bidel, L.P.R.; Bertin, N. Water Shortage and Quality of Fleshy Fruits—Making the Most of the Unavoidable. J. Exp. Bot. 2014, 65, 4097–4117. [Google Scholar] [CrossRef]
- Yang, H.; Du, T.; Qiu, R.; Chen, J.; Wang, F.; Li, Y.; Wang, C.; Gao, L.; Kang, S. Improved Water Use Efficiency and Fruit Quality of Greenhouse Crops Under Regulated Deficit Irrigation in Northwest China. Agric. Water Manag. 2017, 179, 193–204. [Google Scholar] [CrossRef]
- Sun, Y.; Feng, H.; Liu, F. Comparative Effect of Partial Root-Zone Drying and Deficit Irrigation on Incidence of Blossom-End Rot in Tomato Under Varied Calcium Rates. J. Exp. Bot. 2013, 64, 2107–2116. [Google Scholar] [CrossRef]
- Saure, M.C. Blossom-End Rot of Tomato (Lycopersicon esculentum Mill.)—A Calcium- or a Stress-Related Disorder? Sci. Hortic. 2001, 90, 193–208. [Google Scholar] [CrossRef]
- Romero, P.; Rose, J.K.C. A Relationship Between Tomato Fruit Softening, Cuticle Properties and Water Availability. Food Chem. 2019, 295, 300–310. [Google Scholar] [CrossRef]
- Navazio, J.P.; Staub, J.E. Effects of Soil Moisture, Cultivar, and Postharvest Handling on Pillowy Fruit Disorder in Cucumber. J. Am. Soc. Hortic. Sci. 1994, 119, 1234–1242. [Google Scholar] [CrossRef]
- Thomas, R.S.; Staub, J.E. Water Stress and Storage Environment Affect Pillowy Fruit Disorder in Cucumber. J. Am. Soc. Hortic. Sci. 1992, 117, 394–399. [Google Scholar] [CrossRef]
- Davies, W.J.; Zhang, J. Root Signals and the Regulation of Growth and Development of Plants in Drying Soil. Annu. Rev. Plant Biol. 1991, 42, 55–76. [Google Scholar] [CrossRef]
- Mao, X.; Liu, M.; Wang, X.; Liu, C.; Hou, Z.; Shi, J. Effects of Deficit Irrigation on Yield and Water Use of Greenhouse Grown Cucumber in the North China Plain. Agric. Water Manag. 2003, 61, 219–228. [Google Scholar] [CrossRef]
- Sonneveld, C.; Voogt, W. Plant Nutrition of Greenhouse Crops; Springer: Dordrecht, The Netherlands, 2009; pp. 393–403. ISBN 978-90-481-2531-9. [Google Scholar]
- Liu, R.; Zhu, P.F.; Wang, Y.S.; Chen, Z.; Zhu, J.R.; Shu, L.Z.; Zhang, W.J. Alternate Partial Root-Zone Drip Nitrogen Fertigation Reduces Residual Nitrate Loss While Improving the Water Use but Not Nitrogen Use Efficiency. Front. Plant Sci. 2021, 12, 722459. [Google Scholar] [CrossRef]
- Heeb, A.; Lundegårdh, B.; Ericsson, T.; Savage, G.P. Nitrogen Form Affects Yield and Taste of Tomatoes. J. Sci. Food Agric. 2005, 85, 1405–1414. [Google Scholar] [CrossRef]
- Liu, D.; Chen, J.; Hao, Y.; Yang, X.; Chen, R.; Zhang, Y. Effects of Extreme Root Restriction on the Nutritional and Flavor Quality, and Sucrose Metabolism of Tomato (Solanum lycopersicum L.). Horticulturae 2023, 9, 813. [Google Scholar] [CrossRef]
- Daoud, B.; Pawelzik, E.; Naumann, M. Different Potassium Fertilization Levels Influence Water-Use Efficiency, Yield, and Fruit Quality Attributes of Cocktail Tomato—A Comparative Study of Deficient-to-Excessive Supply. Sci. Hortic. 2020, 272, 109562. [Google Scholar] [CrossRef]
- Zhang, J.; Du, M.; Liu, G.; Ma, F.; Bao, Z. Lignin Sulfonate-Chelated Calcium Improves Tomato Plant Development and Fruit Quality by Promoting Ca2+ Uptake and Transport. Horticulturae 2024, 10, 1328. [Google Scholar] [CrossRef]
- Kabir, M.d.Y.; Díaz-Pérez, J.C. Calcium Route in the Plant and Blossom-End Rot Incidence. Horticulturae 2025, 11, 807. [Google Scholar] [CrossRef]
- Signore, A.; Serio, F.; Santamaria, P. A Targeted Management of the Nutrient Solution in a Soilless Tomato Crop According to Plant Needs. Front. Plant Sci. 2016, 7, 391. [Google Scholar] [CrossRef]
- Roșca, M.; Mihalache, G.; Stoleru, V. Tomato Responses to Salinity Stress: From Morphological Traits to Genetic Changes. Front. Plant Sci. 2023, 14, 1118383. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.L.; Contreras, J.I.; Salinas, R.; Lao, M.T. Influence of Salinity and Fertilization Level on Greenhouse Tomato Yield and Quality. Commun. Soil Sci. Plant Anal. 2009, 40, 485–497. [Google Scholar] [CrossRef]
- Tola, E.; Al-Gaadi, K.A.; Madugundu, R.; Patil, V.C.; Sygrimis, N. Impact of Water Salinity Levels on the Spectral Behavior and Yield of Tomatoes in Hydroponics. J. King Saud Univ.-Sci. 2023, 35, 102515. [Google Scholar] [CrossRef]
- Ortega-Amaro, M.A.; Loera-Alvarado, G.; López Martínez, L.A.; Martínez Paisano, G. Quality and Postharvest Life of Tomato Fruits (Solanum lycopersicum L.) Produced Under Saline Stress Conditions. Agro Product. 2025, 18, 121–126. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Cheng, M.; Zhang, F.; Wang, X.; Fan, J.; Wu, L.; Fang, D.; Zou, H.; Xiang, Y. Optimal Drip Fertigation Management Improves Yield, Quality, Water and Nitrogen Use Efficiency of Greenhouse Cucumber. Sci. Hortic. 2019, 243, 357–366. [Google Scholar] [CrossRef]
- Al-Moshileh, A.M.; Errebhi, M.A.; Obiadalla-Ali, H.A. Effect of Potassium Fertilization on Tomato and Cucumber Plants Under Greenhouse Conditions. Biosci. Res. 2017, 14, 68–74. [Google Scholar]
- Altunlu, H.; Gül, A. Effects of Different Amounts of Nitrogen and Potassium Nutrition on Post Harvest Quality of Cucumber. Acta Hortic. 1999, 491, 383–388. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Heydari, N.; Delshad, M.; Babalar, M.; Salehi, R. Growth and Yield of Greenhouse Cucumber as Influenced by Nutrient Solution EC and Number of Flowers per Node. Iran. J. Hortic. Sci. 2020, 51, 719–728. [Google Scholar]
- Sonali; He, J.; Wang, Y.; Liang, W.; Rasouli, F.; Li, L.; Bose, J.; Donovan-Mak, M.; Huda, S.; Jayasena, V.; et al. Optimized Fertigation Improves Yield and Quality of Cucumbers for Resource Efficiency and Economic Return in High-Tech Greenhouses. J. Agric. Food Res. 2024, 19, 101699. [Google Scholar]
- Al-Momany, B.; Abu-Romman, S. Cucumber and Salinity. Aust. J. Crop Sci. 2023, 17, 1835–2707. [Google Scholar] [CrossRef]
- Chen, T.-W.; Gomez Pineda, I.M.; Brand, A.M.; Stützel, H. Determining Ion Toxicity in Cucumber Under Salinity Stress. Agronomy 2020, 10, 677. [Google Scholar] [CrossRef]
- Ruiz, J.M.; Romero, L. Commercial Yield and Quality of Fruits of Cucumber Plants Cultivated Under Greenhouse Conditions: Response to Increases in Nitrogen Fertilization. J. Agric. Food Chem. 1998, 46, 4171–4173. [Google Scholar] [CrossRef]
- Jasso-Chaverria, C.; Hochmuth, G.J.; Hochmuth, R.C.; Sargent, S.A. Fruit Yield, Size, and Color Responses of Two Greenhouse Cucumber Types to Nitrogen Fertilization in Perlite Soilless Culture. HortTechnology 2005, 15, 565. [Google Scholar] [CrossRef]
- Rubio, J.S.; García-Sánchez, F.; Flores, P.; Navarro, J.M.; Martínez, V. Yield and Fruit Quality of Sweet Pepper in Response to Fertilisation with Ca2+ and K+. Span. J. Agric. Res. 2010, 8, 170–177. [Google Scholar] [CrossRef]
- Botella, M.Á.; Arévalo, L.; Mestre, T.C.; Rubio, F.; García-Sánchez, F.; Rivero, R.M.; Martínez, V. Potassium Fertilization Enhances Pepper Fruit Quality. J. Plant Nutr. 2017, 40, 145–155. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Wang, Y.; Ma, X.; Shen, Y.; Wang, X.; Yang, H.; Zhang, W.; Lakshmanan, P.; Hu, Y.; et al. Physiological and Metabolomic Analysis Reveals Maturity Stage-Dependent Nitrogen Regulation of Vitamin C Content in Pepper Fruit. Front. Plant Sci. 2023, 13, 1049785. [Google Scholar] [CrossRef] [PubMed]
- Mayorga-Gómez, A.; Nambeesan, S.U.; Coolong, T.; Díaz-Pérez, J.C. Temporal Relationship Between Calcium and Fruit Growth and Development in Bell Pepper (Capsicum annuum L.). HortScience 2020, 55, 906–913. [Google Scholar] [CrossRef]
- Shin, J.H.; Son, J.E. Development of a Real-Time Irrigation Control System Considering Transpiration, Substrate Electrical Conductivity, and Drainage Rate of Nutrient Solutions in Soilless Culture of Paprika (Capsicum annuum L.). Eur. J. Hortic. Sci. 2015, 80, 271–279. [Google Scholar] [CrossRef]
- Marín, A.; Rubio, J.S.; Martínez, V.; Gil, M.I. Antioxidant Compounds in Green and Red Peppers as Affected by Irrigation Frequency, Salinity and Nutrient Solution Composition. J. Sci. Food Agric. 2009, 89, 1352–1359. [Google Scholar] [CrossRef]
- Fandi, M.; Muhtaseb, J.; Hussein, M. Effect of Plant Density on Tomato Yield and Fruit Quality Growing in Tuff Culture. Acta Hortic. 2007, 741, 207–212. [Google Scholar] [CrossRef]
- Karpe, M.; Marcelis, L.F.M.; Heuvelink, E. Dynamic Plant Spacing in Tomato Results in High Yields While Mitigating the Reduction in Fruit Quality Associated with High Planting Densities. Front. Plant Sci. 2024, 15, 1386950. [Google Scholar] [CrossRef] [PubMed]
- Jarquín-Enríquez, L.; Mercado-Silva, E.M.; Maldonado, J.L.; Lopez-Baltazar, J. Lycopene Content and Color Index of Tomatoes Are Affected by the Greenhouse Cover. Sci. Hortic. 2013, 155, 43–48. [Google Scholar] [CrossRef]
- Maboko, M.M.; Du Plooy, C.P.; Chiloane, S. Effect of Plant Population, Fruit and Stem Pruning on Yield and Quality of Hydroponically Grown Tomato. Afr. J. Agric. Res. 2011, 6, 5144–5148. [Google Scholar]
- Lhamo, T.; Gyalmo, T.; Pem, T.; Bajgai, Y. Effect of Different Pruning Systems on Yield and Quality of Tomato Grown Under Greenhouse. Bhutanese J. Agric. 2022, 5, 71–82. [Google Scholar] [CrossRef]
- Appolloni, E.; Paucek, I.; Pennisi, G.; Manfrini, L.; Gabarrell, X.; Gianquinto, G.; Orsini, F. Winter Greenhouse Tomato Cultivation: Matching Leaf Pruning and Supplementary Lighting for Improved Yield and Precocity. Agronomy 2023, 13, 671. [Google Scholar] [CrossRef]
- Paponov, M.; Verheul, M.J.; Dobrev, P.I.; Paponov, I.A. Additive Effects of Light and Branching on Fruit Size and Chemical Fruit Quality of Greenhouse Tomatoes. Front. Plant Sci. 2023, 14, 1221163. [Google Scholar] [CrossRef]
- Kubota, C.; Kroggel, M.; Torabi, M.; Dietrich, K.A.; Kim, H.-J.; Fonseca, J.; Thomson, C.A. Changes in Selected Quality Attributes of Greenhouse Tomato Fruit as Affected by Pre- and Postharvest Environmental Conditions in Year-Round Production. HortScience 2012, 47, 1698–1704. [Google Scholar] [CrossRef]
- Bertin, N. Competition for Assimilates and Fruit Position Affect Fruit Set in Indeterminate Greenhouse Tomato. Ann. Bot. 1995, 75, 55–65. [Google Scholar] [CrossRef]
- Paponov, M.; Kechasov, D.; Lacek, J.; Verheul, M.J.; Paponov, I.A. Supplemental Light-Emitting Diode Inter-Lighting Increases Tomato Fruit Growth Through Enhanced Photosynthetic Light Use Efficiency and Modulated Root Activity. Front. Plant Sci. 2020, 10, 1656. [Google Scholar] [CrossRef]
- Ding, X.; Nie, W.; Qian, T.; He, L.; Zhang, H.; Jin, H.; Cui, J.; Wang, H.; Zhou, Q.; Yu, J. Low Plant Density Improves Fruit Quality without Affecting Yield of Cucumber in Different Cultivation Periods in Greenhouse. Agronomy 2022, 12, 1441. [Google Scholar] [CrossRef]
- Tafoya, F.A.; Orona, C.A.L.; Juárez, M.G.Y.; Valdez, T.D.; Alcaraz, T.d.J.V.; Delgado, J.M.P. Densidad de Plantas y Poda de Tallos En La Producción de Pepino En Invernadero. Rev. Mex. Cienc. Agric. 2019, 10, 79–90. [Google Scholar]
- Yadav, M.; Dhankhar, S.K. Factors Affecting Shelf Life of Vegetables: A Review. Agric. Rev. 2025, 46, 893–900. [Google Scholar] [CrossRef]
- Hettiarachchi, M.H.S.M.; Weerakkody, P.; Wijayasinghe, K.D.B.V. Chapter-7 Greenhouse Cucumber Production in the Humid Tropics Chapter-1 Greenhouse Cucumber Production in the Humid. In Advanced Research and Review in Horticultural Sciences; Bright Sky Publications: Delhi, India, 2024; p. 113. [Google Scholar]
- Marcelis, L.F.M. Fruit Growth and Biomass Allocation to the Fruits in Cucumber. 1. Effect of Fruit Load and Temperature. Sci. Hortic. 1993, 54, 107–121. [Google Scholar] [CrossRef]
- Yang, F.; Gu, Q.; He, W.; Hong, D.; Yu, M.; Yao, J. Case Study on the Application of Innovative Cultivation Techniques in Cucumber Production. Int. J. Hortic. 2025, 15, 29–40. [Google Scholar] [CrossRef]
- Topuz, A.; Ozdemir, F. Assessment of Carotenoids, Capsaicinoids and Ascorbic Acid Composition of Some Selected Pepper Cultivars (Capsicum annuum L.) Grown in Turkey. J. Food Compos. Anal. 2007, 20, 596–602. [Google Scholar] [CrossRef]
- Dasgan, Y.H.; Abak, K. Effects of Plant Density and Number of Shoots on Yield and Fruit Characteristics of Peppers Grown in Glasshouses. Turk. J. Agric. For. 2003, 27, 29–35. [Google Scholar]
- Lbhí, R.; Stadler, C. Effects of Lighting Time and Lighting Source on Growth, Yield and Quality of Greenhouse Sweet Pepper; Landbúnaðarháskóli Íslands: Borgarnes, Iceland, 2011; ISBN 9789979881117. [Google Scholar]
- González-Real, M.M.; Baille, A.; Liu, H.Q. Influence of Fruit Load on Dry Matter and N-Distribution in Sweet Pepper Plants. Sci. Hortic. 2008, 117, 307–315. [Google Scholar] [CrossRef]
- Ali, A.M.; Kelly, W.C. The Effects of Interfruit Competition on the Size of Sweet Pepper (Capsicum annuum L.) Fruits. Sci. Hortic. 1992, 52, 69–76. [Google Scholar] [CrossRef]
- Ruiz-Nieves, J.M.; Ayala-Garay, O.J.; Serra, V.; Dumont, D.; Vercambre, G.; Génard, M.; Gautier, H. The Effects of Diurnal Temperature Rise on Tomato Fruit Quality. Can the Management of the Greenhouse Climate Mitigate Such Effects? Sci. Hortic. 2021, 278, 109836. [Google Scholar] [CrossRef]
- Yu, X.; Niu, L.; Zhang, Y.; Xu, Z.; Zhang, J.; Zhang, S.; Li, J. Vapour Pressure Deficit Affects Crop Water Productivity, Yield, and Quality in Tomatoes. Agric. Water Manag. 2024, 299, 108879. [Google Scholar] [CrossRef]
- Zhou, D.; Meinke, H.; Wilson, M.; Marcelis, L.F.M.; Heuvelink, E. Towards Delivering on the Sustainable Development Goals in Greenhouse Production Systems. Resour. Conserv. Recycl. 2021, 169, 105379. [Google Scholar] [CrossRef]
- Sophie, P. Improving Greenhouse Systems and Production Practices (Greenhouse Technology Systems Component) (Parent-VG0796); Horticulture Australia Ltd.: Sydney, NSW, Australia, 2010; ISBN 0734125593. [Google Scholar]
- Gent, M.P.N. Effect of Degree and Duration of Shade on Quality of Greenhouse Tomato. HortScience 2007, 42, 514–520. [Google Scholar] [CrossRef]
- Serrano-Carreón, L.; Aranda-Ocampo, S.; Balderas-Ruíz, K.A.; Juárez, A.M.; Leyva, E.; Trujillo-Roldán, M.A.; Valdez-Cruz, N.A.; Galindo, E. A Case Study of a Profitable Mid-Tech Greenhouse for the Sustainable Production of Tomato, Using a Biofertilizer and a Biofungicide. Electron. J. Biotechnol. 2022, 59, 13–24. [Google Scholar] [CrossRef]
- Saraiva, R.; Dias, I.; Grego, J.; Oliveira, M. Greenhouse Tomato Technologies and Their Influence in Mediterranean Region. In Tomato Cultivation and Consumption—Innovation and Sustainability; IntechOpen: London, UK, 2024. [Google Scholar]
- Schouten, R.E.; Otma, E.C.; van Kooten, O.; Tijskens, L.M.M. Keeping Quality of Cucumber Fruits Predicted by Biological Age. Postharvest Biol. Technol. 1997, 12, 175–181. [Google Scholar] [CrossRef]
- Al-Mulla, Y.A.; Al-Balushi, M.I.; Al-Busaidi, H.A.; Al-Mahdouri, A.A.; Kittas, C.; Katsoulas, N. Analysis of Microclimate and Cucumber Fruit Yield in a Screenhouse and an Evaporatively Cooled Greenhouse in a Semi-Arid Location. Trans. ASABE 2018, 61, 619–629. [Google Scholar] [CrossRef]
- Amin, B.; Atif, M.J.; Kandegama, W.; Nasar, J.; Alam, P.; Fang, Z.; Cheng, Z. Low Temperature and High Humidity Affect Dynamics of Chlorophyll Biosynthesis and Secondary Metabolites in Cucumber. BMC Plant Biol. 2024, 24, 903. [Google Scholar] [CrossRef] [PubMed]
- Castilla, N.; Hernandez, J. Greenhouse Technological Packages for High-Quality Crop Production. Acta Hortic. 2007, 761, 285–297. [Google Scholar] [CrossRef]
- Allali, F.E.; Fatnassi, H.; Demrati, H.; Errais, R.; Wifaya, A.; Aharoune, A. Greenhouse Cooling Systems: A Systematic Review of Research Trends, Challenges, and Recommendations for Improving Sustainability. Clean. Eng. Technol. 2025, 26, 100973. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Al-Faraj, A.A.; Hegazy, M.A.; Abdel-Ghany, A.M. Effect of Cooling Strategies on the Uniformity of the Greenhouses Microclimate: A Review. Ciênc. Téc. Vitiviníc. 2016, 31, 249–288. [Google Scholar]
- Amalfitano, C.; Del Vacchio, L.; Somma, S.; Cuciniello, A.; Caruso, G. Effects of Cultural Cycle and Nutrient Solution Electrical Conductivity on Plant Growth, Yield and Fruit Quality of “Friariello” Pepper Grown in Hydroponics. Hortic. Sci. 2017, 44, 91–98. [Google Scholar] [CrossRef]
- Gidado, M.J.; Gunny, A.A.N.; Gopinath, S.C.B.; Ali, A.; Wongs-Aree, C.; Salleh, N.H.M. Challenges of Postharvest Water Loss in Fruits: Mechanisms, Influencing Factors, and Effective Control Strategies—A Comprehensive Review. J. Agric. Food Res. 2024, 17, 101249. [Google Scholar] [CrossRef]
- Brunner, C.; Gartner, H. Impact of Shading Techniques on the Growth and Yield of Greenhouse-Grown Pepper Plants. Int. J. Biosci. Biochem. 2022, 4, 38–40. [Google Scholar] [CrossRef]
- Chimankare, R.V.; Das, S.; Kaur, K.; Magare, D. A Review Study on the Design and Control of Optimised Greenhouse Environments. J. Trop. Ecol. 2023, 39, e26. [Google Scholar] [CrossRef]
- Papaioannou, C.; Katsoulas, N.; Maletsika, P.; Siomos, A.; Kittas, C. Effects of a UV-Absorbing Greenhouse Covering Film on Tomato Yield and Quality. Span. J. Agric. Res. 2012, 10, 959–966. [Google Scholar] [CrossRef]
- Helyes, L.; Lugasi, A.; Pék, Z. Effect of Natural Light on Surface Temperature and Lycopene Content of Vine Ripened Tomato Fruit. Can. J. Plant Sci. 2007, 87, 927–929. [Google Scholar] [CrossRef]
- Affandi, F.Y.; Pijnenburg, C.; Verdonk, J.C.; Woltering, E.J.; Schouten, R.E. Growth Temperature Influences Postharvest Quality and Cold Tolerance of Green Harvested Dwarf Tomatoes During Storage. Front. Sustain. Food Syst. 2022, 6, 876597. [Google Scholar] [CrossRef]
- Kittas, C.; Merkouris, O.; Arcadio, V.; Nardiello, C.; Katsoulas, N. Effect of a Diffuse Polyethylene Covering Film on Green-House and Crop Light Environment and on Tomato Crop Pro-Duction. In Proceedings of the International Conference of Agricultural Engineering, Zurich, Switzerland, 6–10 July 2014. [Google Scholar]
- Paradiso, R.; Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Pelosi, M.E.; Rippa, M.; Mormile, P.; Mori, M. Integrating Smart Greenhouse Cover, Reduced Nitrogen Dose and Biostimulant Application as a Strategy for Sustainable Cultivation of Cherry Tomato. Plants 2024, 13, 440. [Google Scholar] [CrossRef]
- Tezcan, N.Y.; Taspinar, H.; Korkmaz, C. Effects of Shade Nets on the Microclimate and Growth of Tomato. Tarim Bilim. Derg. 2022, 29, 443–454. [Google Scholar] [CrossRef]
- Montero, J.I.; Antón, A. Tomato Response to a High Light Transmission Film Used as Vovering Material for Greenhouses. Acta Hortic. 2003, 401–405. [Google Scholar] [CrossRef]
- Al-Madani, A.A.; Al-Helal, I.M.; Alsadon, A.A. Assessing the Effectiveness of Reflective and Diffusive Polyethylene Films as Greenhouse Covers in Arid Environments. Agronomy 2024, 14, 1082. [Google Scholar] [CrossRef]
- Lamnatou, C.; Chemisana, D. Solar Radiation Manipulations and Their Role in Greenhouse Claddings: Fluorescent Solar Concentrators, Photoselective and Other Materials. Renew. Sustain. Energy Rev. 2013, 27, 175–190. [Google Scholar] [CrossRef]
- Kitta, E.; Katsoulas, N.; Kittas, C. Effect of Shading on Photosynthesis in Greenhouse Hydroponic Cucumber Crops. Acta Hortic. 2021, 167–172. [Google Scholar] [CrossRef]
- Teitel, M. The Effect of Screens on the Microclimate of Greenhouses and Screenhouses—A Review. Acta Hortic. 2006, 719, 575–586. [Google Scholar] [CrossRef]
- Lin, W.C.; Jolliffe, P.A. Light Intensity and Spectral Quality Affect Fruit Growth and Shelf Life of Greenhouse-Grown Long English Cucumber. J. Am. Soc. Hortic. Sci. 1996, 121, 1168–1173. [Google Scholar] [CrossRef]
- Ávalos-Sánchez, E.; Moreno-Teruel, M.Á.; Molina-Aiz, F.D.; López-Martínez, A.; Peña-Fernández, A.; Baptista, F.; Valera-Martínez, D.L. Influence of the Diffusivity and Transmittance of a Plastic Greenhouse Cover on the Development of Fungal Diseases in a Cucumber Crop. Agronomy 2022, 12, 2743. [Google Scholar] [CrossRef]
- Kwon, J.K.; Khoshimkhujaev, B.; Lee, J.H.; Yu, I.H.; Park, K.S.; Choi, H.G. Growth and Yield of Tomato and Cucumber Plants in Polycarbonate or Glass Greenhouses. Hortic. Sci. Technol. 2017, 35, 79–87. [Google Scholar] [CrossRef]
- Katsoulas, N.; Bartzanas, T.; Kitta, E. Effects of Anti-Drip Polyethylene Covering Films on Microclimate and Crop Production. Acta Hortic. 2012, 952, 209–215. [Google Scholar] [CrossRef]
- Ziedan, E.S.H.; Khattab, A.E.-N.A.E.-H.; Sahab, A.F. New Fungi Causing Postharvest Spoilage of Cucumber Fruits and Their Molecular Characterization in Egypt. J. Plant Prot. Res. 2023, 58, 362–371. [Google Scholar] [CrossRef]
- López-Marín, J.; González, A.; Gálvez, A. Effect of Shade on Quality of Greenhouse Peppers. Acta Hortic. 2011, 893, 895–900. [Google Scholar] [CrossRef]
- Kabir, M.Y. Abiotic Factors Affecting Plant Physiology and Fruit Yield and Physiological Disorders in Bell Pepper and Tomato. Doctoral Dissertation, University of Georgia, Athens, GA, USA, 2024. [Google Scholar]
- Castronuovo, D.; Statuto, D.; Muro, N.; Picuno, P.; Candido, V. The Use of Shading Nets for the Greenhouse Cultivation of Sweet Pepper in the Mediterranean Area. Acta Hortic. 2017, 373–380. [Google Scholar] [CrossRef]
- Pandey, G.; Parks, S.; Thomas, R.G. Polymer and Photo-selective Covers on Plant and Fruit Development: A Review. Agron. J. 2023, 115, 3074–3091. [Google Scholar] [CrossRef]
- Kumar, A.; Sangwan, P.; Kumar, V.; Pandey, A.K.; Pooja; Kumar, A.; Chauhan, P.; Koubouris, G.; Fanourakis, D.; Parmar, K. Physio-Biochemical Insights of Endophytic Microbial Community for Crop Stress Resilience: An Updated Overview. J. Plant Growth Regul. 2025, 44, 2641–2664. [Google Scholar] [CrossRef]
- Al-Saif, A.M.; Ahmed, M.E.M.; Taha, M.A.; Sharma, A.; El-Sheshtawy, A.-N.A.; Abouelsaad, I.A.; El-Serafy, R.S.; Mahdy, R.M. Preharvest Applications Improve the Postharvest Storage and Quality of Tomato Fruits by Enhancing the Nutritional Value and Antioxidant System. Horticulturae 2024, 10, 1248. [Google Scholar] [CrossRef]
- Birgin, Ö.; Akhoundnejad, Y.; Dasgan, H.Y. The Effect of Foliar Calcium Application in Tomato (Solanum lycopersicum L.) Under Drought Stress in Greenhouse Conditions. Appl. Ecol. Environ. Res. 2021, 19, 2971–2982. [Google Scholar] [CrossRef]
- Souiden, Y.; Chéour, F. Calcium Delays the Postharvest Ripening and Related Membrane-Lipid Changes of Tomato. J. Nutr. Food Sci. 2015, 5, 1000393. [Google Scholar] [CrossRef]
- Haleema, B.; Rab, A. Calcium Sources and Concentrations Affecting Tomato Fruit Softening and Post-harvest Decay. Pak. J. Bot. 2014, 28, 145–152. [Google Scholar]
- He, B.; Wei, Y.; Wang, Y.; Zhong, Y.; Fan, M.; Gong, Q.; Lu, S.; Hassan, M.U.; Li, X. Silicon Application Improves Tomato Yield and Nutritional Quality. BMC Plant Biol. 2025, 25, 252. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, Y.; Zhang, Y.; Shi, Y.; Hou, L.; Khan, A.; Zhang, R.; Zhang, Y. Mechanism of Exogenous Silicon in Enhancing Cold Stress Tolerance in Solanum lycopersicum L. Seedlings: Insights from Resistance and Quality Indicators. Horticulturae 2024, 11, 4. [Google Scholar] [CrossRef]
- Francesca, S.; Arena, C.; Hay Mele, B.; Schettini, C.; Ambrosino, P.; Barone, A.; Rigano, M.M. The Use of a Plant-Based Biostimulant Improves Plant Performances and Fruit Quality in Tomato Plants Grown at Elevated Temperatures. Agronomy 2020, 10, 363. [Google Scholar] [CrossRef]
- Meena, M.; Pilania, S.; Meena, K.K.; Lakhawat, S.S.; Saharan, V. A Comprehensive Study of Chitosan Application for Extending Shelf Life of Tomato. J. Environ. Biol. 2021, 42, 1405–1414. [Google Scholar] [CrossRef]
- Paul, S.K.; Sarkar, S.; Sethi, L.N.; Ghosh, S.K. Development of Chitosan Based Optimized Edible Coating for Tomato (Solanum lycopersicum) and Its Characterization. J. Food Sci. Technol. 2018, 55, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Gil-Ortiz, R.; Naranjo, M.Á.; Atares, S.; Vicente, O. Antioxidant Responses of Water-Stressed Cherry Tomato Plants to Natural Biostimulants. Agronomy 2023, 13, 2314. [Google Scholar] [CrossRef]
- Gedeon, S.; Ioannou, A.; Balestrini, R.; Fotopoulos, V.; Antoniou, C. Application of Biostimulants in Tomato Plants (Solanum lycopersicum) to Enhance Plant Growth and Salt Stress Tolerance. Plants 2022, 11, 3082. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Zhang, N.; Li, F.D.; Bi, H.G.; Ai, X.Z. Optimization of Silicon and Calcium Fertilization in Grafted Cucumber in Solar-Greenhouse. J. Plant Nutr. Fertil. 2020, 26, 393–400. [Google Scholar]
- Emad, A.-A.; Yousry, B.; Elmahdy, M.; Mohamed, R. Silicon Supplements Affect Yield and Fruit Quality of Cucumber (Cucumis sativus L.) Grown in Net Houses. Afr. J. Agric. Res. 2017, 12, 2518–2523. [Google Scholar] [CrossRef]
- Casals, J.; Rull, A.; Segarra, J.; Schober, P.; Simó, J. Participatory Plant Breeding and the Evolution of Landraces: A Case Study in the Organic Farms of the Collserola Natural Park. Agronomy 2019, 9, 486. [Google Scholar] [CrossRef]
- Olle, M.; Bender, I. Causes and Control of Calcium Deficiency Disorders in Vegetables: A Review. J. Hortic. Sci. Biotechnol. 2009, 84, 577–584. [Google Scholar] [CrossRef]
- Bahloul, H.E.; Shafshak, S.A.; Nagar Mahran, E.M.; El-Said, M. Effect of Foliar Spray with Some Growth Stimulants on Growth, Productivity and Quality of Cucumber Plants Grown Under Greenhouse Conditions. Ann. Agric. Sci. 2021, 59, 975–986. [Google Scholar] [CrossRef]
- Wolff, S.A.; Karoliussen, I.; Rohloff, J.; Strimbeck, R. Foliar Applications of Silicon Fertilisers Inhibit Powdery Mildew Development in greenhouse Cucumber. Agric. Environ. 2005, 10, 355–359. [Google Scholar]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Lee, W. Influence of Chitosan Coating and Packaging Materials on the Quality Characteristics of Fresh-Cut Cucumber. Korean J. Food Preserv. 2019, 26, 371–380. [Google Scholar] [CrossRef]
- Moosavi-Nezhad, M.; Homayoonzadeh, M.; Tsaniklidis, G.; Roessner, U.; Woltering, E.J.; Fanourakis, D.; Aliniaeifard, S. Enhancing Shelf Life of Bell Peppers Through Preharvest Fertigation with Calcium and Potassium Thiosulfate: A Focus on Antioxidant and Cell Wall Degradation Enzymes. J. Agric. Food Res. 2024, 17, 101262. [Google Scholar] [CrossRef]
- Valero, D.; Zapata, P.; Martínez-Romero, D.; Guillén, F.; Castillo, S.; Serrano, M. Pre-Harvest Treatments of Pepper Plants with Nitrophenolates Increase Crop Yield and Enhance Nutritive and Bioactive Compounds in Fruits at Harvest and During Storage. Food Sci. Technol. Int. 2014, 20, 265–274. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.A.; Geeth, R.H. Effect of Foliar Spray With Some Silicon Sources and Paclobutrazol on Growth, Yield and Fruit Quality of Sweet Pepper (Capsicum annuum L.) Plants Under High Temperature Conditions. Egypt. J. Agric. Res. 2018, 96, 577–593. [Google Scholar] [CrossRef]
- Tahmasebi, A.; Mirzaalian Dastjerdi, A.; Jamali, B. Microbial-Based Biological Treatments Improved the Nutritional, Nutraceutical and Functional Properties of Greenhouse Sweet Pepper (Capsicum annuum L.). Front. Sustain. Food Syst. 2023, 7, 1145972. [Google Scholar] [CrossRef]
- Bonini, P.; Rouphael, Y.; Miras-Moreno, B.; Lee, B.; Cardarelli, M.; Erice, G.; Cirino, V.; Lucini, L.; Colla, G. A Microbial-Based Biostimulant Enhances Sweet Pepper Performance by Metabolic Reprogramming of Phytohormone Profile and Secondary Metabolism. Front. Plant Sci. 2020, 11, 567388. [Google Scholar] [CrossRef]
- Alpos, M.A.; Ruth, E.; Bayogan, V. Effects of Chitosan Coating on the Postharvest Quality and Antioxidant Properties of Sweet Pepper (Capsicum annuum L.). Philipp. J. Sci. 2023, 152, 919–929. [Google Scholar] [CrossRef]
- Reitz, S.R.; Funderburk, J. Management Strategies for Western Flower Thrips and the Role of Insecticides. Insectic.–Pest Eng. 2012, 1, 355–384. [Google Scholar]
- Johnson, M.W.; Caprio, L.C.; Coughlin, J.A.; Tabashnik, B.E.; Rosenheim, J.A.; Welter, S.C. Effect of Trialeurodes vaporariorum (Homoptera: Aleyrodidae) on Yield of Fresh Market Tomatoes. J. Econ. Entomol. 1992, 85, 2370–2376. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Dew, T.P.; Orfila, C.; Urwin, P.E. Influence of Combined Biotic and Abiotic Stress on Nutritional Quality Parameters in Tomato (Solanum lycopersicum). J. Agric. Food Chem. 2011, 59, 9673–9682. [Google Scholar] [CrossRef]
- Tzortzakis, N. Physiological and Proteomic Approaches to Address the Active Role of Botrytis Cinerea Inoculation in Tomato Postharvest Ripening. Microorganisms 2019, 7, 681. [Google Scholar] [CrossRef]
- Veloukas, T.; Bardas, G.A.; Karaoglanidis, G.S.; Tzavella-Klonari, K. Management of Tomato Leaf Mould Caused by Cladosporium fulvum with Trifloxystrobin. Crop Prot. 2007, 26, 845–851. [Google Scholar] [CrossRef]
- Qiu, C.; Bao, Y.; Lü, D.; Yan, M.; Li, G.; Liu, K.; Wei, S.; Wu, M.; Li, Z. The Synergistic Effects of Humic Acid, Chitosan and Bacillus Subtilis on Tomato Growth and against Plant Diseases. Front. Microbiol. 2025, 16, 1574765. [Google Scholar] [CrossRef]
- Vukelić, I.D.; Prokić, L.T.; Racić, G.M.; Pešić, M.B.; Bojović, M.M.; Sierka, E.M.; Kalaji, H.M.; Panković, D.M. Effects of Trichoderma harzianum on Photosynthetic Characteristics and Fruit Quality of Tomato Plants. Int. J. Mol. Sci. 2021, 22, 6961. [Google Scholar] [CrossRef]
- Daunde, A.T.; Baghele, R.D.; Khandare, V.S. Management of Prevalent Diseases of Cucumber (Cucumis sativus) Through Integrated Approach. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 3022–3028. [Google Scholar] [CrossRef]
- Weintraub, P.G.; Recht, E.; Mondaca, L.L.; Harari, A.R.; Diaz, B.M.; Bennison, J. Arthropod Pest Management in Organic Vegetable Greenhouses. J. Integr. Pest Manag. 2017, 8, 29. [Google Scholar] [CrossRef]
- Shi, Q.; You, L.; Wang, Y.; Du, X.; Chen, T. Effects of Downy Mildew Infection and Potassium on Growth and Physiological Traits of Greenhouse Cucumber. Agronomy 2025, 15, 1017. [Google Scholar] [CrossRef]
- Hao, X.; Shipp, J.L.; Wang, K.; Papadopoulos, A.P.; Binns, M.R. Impact of Western Flower Thrips on Growth, Photosynthesis and Productivity of Greenhouse Cucumber. Sci. Hortic. 2002, 92, 187–203. [Google Scholar] [CrossRef]
- Anarjan, M.B.; Zhang, C.; Begum, S.; Win, K.T.; Li, H.; Wang, C.; Wu, T.; Lee, S. Genetic Basis of Downy Mildew Resistance in Cucumber: Identification of Candidate Genes and Ethylene-Mediated Defense Mechanism. arXiv 2025. [Google Scholar] [CrossRef]
- Núñez-Palenius, H.G.; Orosco-Alcalá, B.E.; Espitia-Vázquez, I.; Olalde-Portugal, V.; Hoflack-Culebro, M.; Ramírez-Santoyo, L.F.; Ruiz-Aguilar, G.M.L.; Cruz-Huerta, N.; Valiente-Banuet, J.I. Biological Control of Downy Mildew and Yield Enhancement of Cucumber Plants by Trichoderma harzianum and Bacillus subtilis (Ehrenberg) Under Greenhouse Conditions. Horticulturae 2022, 8, 1133. [Google Scholar] [CrossRef]
- Sarhan, E.A.D.; Abd-Elsyed, M.H.F.; Ebrahiem, A.M.Y. Biological Control of Cucumber Powdery Mildew (Podosphaera xanthii) (Castagne) Under Greenhouse Conditions. Egypt. J. Biol. Pest Control 2020, 30, 65. [Google Scholar] [CrossRef]
- Li, Y.; Gu, Y.; Li, J.; Xu, M.; Wei, Q.; Wang, Y. Biocontrol Agent Bacillus amyloliquefaciens LJ02 Induces Systemic Resistance against Cucurbits Powdery Mildew. Front. Microbiol. 2015, 6, 883. [Google Scholar] [CrossRef]
- Yedidia, I.; Srivastva, A.K.; Kapulnik, Y.; Chet, I. Effect of Trichoderma harzianum on Microelement Concentrations and Increased Growth of Cucumber Plants. Plant Soil 2001, 235, 235–242. [Google Scholar] [CrossRef]
- Sabbagh, S.K.; Tanavar, L. Bio-Fertilizer Incidences on Cucumber Disease and Defense Reactions in Response to Phytophthora melonis. Biol. Control Pests Plant Dis. 2019, 8, 1–15. [Google Scholar]
- Abou-Haidar, A.; Tawidian, P.; Sobh, H.; Skinner, M.; Parker, B.; Abou-Jawdah, Y. Efficacy of Phytoseiulus persimilis and Amblyseius swirskii for Integrated Pest Management for Greenhouse Cucumbers Under Mediterranean Environmental Conditions. Can. Entomol. 2021, 153, 598–615. [Google Scholar] [CrossRef]
- Mahmood, A.; Hu, Y.; Tanny, J.; Asante, E.A. Effects of Shading and Insect-Proof Screens on Crop Microclimate and Production: A Review of Recent Advances. Sci. Hortic. 2018, 241, 241–251. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.-K.; Song, Y.-J.; Kang, S.C.; Kim, B.; Choi, I.-J.; Lee, D.-H. Evaluating Natural Compounds as Potential Insecticides against Three Economically Important Pests, Bemisia tabaci (Hemiptera: Aleyrodidae), Frankliniella occidentalis (Thysanoptera: Thripidae), and Myzus persicae (Hemiptera: Aphididae), on Greenhouse Sweet Peppers. Appl. Biol. Chem. 2018, 61, 313–323. [Google Scholar]
- Kim, E.-H.; Lee, K.M.; Lee, S.-Y.; Kil, M.; Kwon, O.-H.; Lee, S.-G.; Lee, S.-K.; Ryu, T.-H.; Oh, S.-W.; Park, S.-Y. Influence of Genetic and Environmental Factors on the Contents of Carotenoids and Phenolic Acids in Red Pepper Fruits (Capsicum annuum L.). Appl. Biol. Chem. 2021, 64, 85. [Google Scholar] [CrossRef]
- Klátyik, S.; Ciceoi, R.; Mladenova, G.; Orel, O.; Takács, E.; Mörtl, M.; Székács, A. Effect of Pesticides on the Nutritional Quality of Cultivated Spice Paprika. Sci. Papers. Ser. B Hortic. 2022, 66, 277–284. [Google Scholar]
- Weintraub, P.G. Integrated Control of Pests in Tropical and Subtropical Sweet Pepper Production. Pest Manag. Sci. 2007, 63, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Rana, V.; Kumari, P.; Singh, N.A.; Soni, R.; Devrani, M.; Kumar, B.; Singh, M. Microbial Induced Enhancement of Secondary Metabolites in Plants: A Comprehensive Review. J. Pure Appl. Microbiol. 2025, 19, 960–973. [Google Scholar] [CrossRef]
- Hafez, M.M.; Gad El-Rab, N.A. Effect of Edible Coatings on Quality Attributes and Storability of Sweet Pepper Fruits. Egypt. J. Agric. Res. 2023, 101, 1073–1085. [Google Scholar] [CrossRef]
- Mamphogoro, T.P.; Kamutando, C.N.; Maboko, M.M.; Aiyegoro, O.A.; Babalola, O.O. Epiphytic Bacteria from Sweet Pepper Antagonistic In Vitro to Ralstonia solanacearum BD 261, a Causative Agent of Bacterial Wilt. Microorganisms 2021, 9, 1947. [Google Scholar] [CrossRef] [PubMed]
- Fiorani, F.; Schurr, U. Future Scenarios for Plant Phenotyping. Annu. Rev. Plant Biol. 2013, 64, 267–291. [Google Scholar] [CrossRef]
- Fanourakis, D.; Pieruschka, R.; Savvides, A.; Macnish, A.J.; Sarlikioti, V.; Woltering, E.J. Sources of Vase Life Variation in Cut Roses: A Review. Postharvest Biol. Technol. 2013, 78, 1–15. [Google Scholar] [CrossRef]
- Makraki, T.; Tsaniklidis, G.; Papadimitriou, D.M.; Taheri-Garavand, A.; Fanourakis, D. Non-Destructive Monitoring of Postharvest Hydration in Cucumber Fruit Using Visible-Light Color Analysis and Machine-Learning Models. Horticulturae 2025, 11, 1283. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.