Abstract
Avocado, a fruit consumed worldwide and essential for countries like Mexico and Chile, faces significant postharvest challenges, particularly during prolonged storage and transportation periods, where Botryosphaeriaceae and Glomerellaceae genera cause fruit rots that can generate substantial economic losses. This study investigated three Hass avocado orchards in the Valparaíso region of Chile to identify spore dispersion peaks, analyze the aerial dynamics of fungal inoculum, and evaluate the association with climatic conditions, as well as the incidence (I) and damage index (DI) of fruit rots. Spore traps were installed in symptomatic trees and monitored weekly over 13 months. Meteorological data were collected in parallel. Fruits from these orchards were sampled to evaluate postharvest rots, physiological maturity, and disease severity using molecular techniques, including DNA sequencing and phylogenetic analysis of isolated pathogens. The results revealed that spore peaks for both fungal families were closely associated with increased rainfall and high relative humidity, particularly from June to mid-September (winter season). The Santo Domingo orchard exhibited the highest disease pressure, with stem-end rot reaching an I of 44% and a DI of 17.25%, and anthracnose reaching an I of 23% and a DI of 12.25%. This study provides the first long-term, field-based evidence of airborne spore dynamics of Botryosphaeriaceae and Glomerellaceae in Chilean avocado orchards and their statistical relationship with environmental variables. These findings highlight the potential of incorporating climatic indicators—such as rainfall thresholds and humidity levels—into monitoring and early-warning systems to optimize fungicide application timing, reduce unnecessary chemical use, and improve postharvest disease management in avocado production.
1. Introduction
Persea americana Mill. (1768), commonly known as the avocado crop, belongs to the genus Persea, which includes approximately 85 species, whose first origin seems to be Mexico about 10,000 years ago [1]. In recent decades, avocado consumption has significantly increased worldwide. Among avocado cultivars, Hass avocado has become the most widely grown and exported due to its desirable sensory attributes and extended postharvest shelf life. Chile has become a key player in avocado production and export [2]. According to FAO data for 2022, Chile ranked 13th worldwide in avocado production, with an output of approximately 142,340 metric tons [3]. This level of production, along with high per capita consumption, underscores the national importance of avocado cultivation. This volume positions Chile among the top global producers. Furthermore, national per capita consumption averages 8.2 kg per person yearly, ranking Chile the second-highest consumer worldwide [4]. Historically, avocado cultivation was concentrated in Central America, where it has been grown for centuries. However, since the early 2000s, production has expanded into semi-arid regions of other countries, including central Chile, where environmental conditions have proven favorable for commercial avocado farming. Today, Chile ranks among the top 15 avocado-producing countries globally, with a planted area exceeding 30,000 hectares concentrated in central and semi-arid regions, from the Atacama Region (25°17′S to 29°30′ S) in the north to the O’Higgins Region (33°51′ S to 35°01′ S) in the south. Within this distribution, the Valparaíso Region (32°02′S to 33°57′ S) stands out as the most productive area, concentrating the highest density of avocado orchards in the country [5,6,7].
In Chile, avocado orchards are often established following significant soil modification, with deep ridges constructed using excavators to enhance root development and reduce compaction stress. Despite advances in agronomic practices and the implementation of quality standards, such as harvesting fruit with a minimum dry matter content of 23% to ensure ripeness upon arrival in export markets [8], postharvest quality loss remains a significant challenge. As a climacteric fruit, avocado is particularly vulnerable to physiological and pathological deterioration during storage and transport [9]. Postharvest fungal rots, especially stem-end rot caused by Botryosphaeriaceae species and anthracnose caused by Colletotrichum spp., represent the leading causes of economic losses, typically manifesting as circular black lesions and vascular browning in the pulp [7,10,11,12,13,14].
Environmental factors such as rainfall, relative humidity, and temperature are critical for spore dispersal. Botryosphaeriaceae spores are often dispersed via rain splash and wind [15]. Previous studies have shown that spore release is most significant during rainy periods, as observed in California [16,17] and Chile’s Casablanca Valley [18]. Similarly, Glomerellaceae spores are primarily dispersed during rainy seasons or periods of high humidity, as reported in studies on avocados in Mexico, where 72.5% of spores were released during the most humid months [19]. These findings highlight the importance of understanding meteorological influences on fungal spore dynamics for effective disease management.
Given the economic impact of fungal infections in postharvest avocados, spore quantification is essential for informed decision-making in disease control. Spore traps have been widely used to monitor Botryosphaeriaceae and Glomerellaceae spp. in various crops [16,18,20,21], including avocado in California [22]. A Chilean study used spore traps to monitor aerial pathogen dispersion in avocados. However, it lacked precipitation records and used a 15-day sampling interval [23]. These limitations underscore the need for more comprehensive, higher-resolution studies that integrate climatic variables with spore dynamics in a statistically robust framework.
Currently, postharvest disease management strategies in avocado rely heavily on broad-spectrum fungicide applications without predictive tools to assess inoculum pressure or environmental infection risk. There is a lack of long-term, field-based studies correlating airborne spore loads with weather patterns and disease severity in fruit, particularly in semi-arid regions such as central Chile. This gap hinders the development of precise forecasting systems for disease outbreaks and limits opportunities to implement targeted, cost-effective, and environmentally sound control measures.
In this context, the present study aims to determine the dispersion peaks of Glomerellaceae and Botryosphaeriaceae spores in three Hass avocado orchards with varying climatic and geographic conditions in central Chile (Santo Domingo, San Pedro, and La Palma). The study also evaluates the association between environmental factors, aerial inoculum, and postharvest disease expression by integrating high-frequency spore monitoring with weather data and molecular identification of pathogens. The results will contribute to the development of early warning systems and improve integrated disease management in avocado fruit production.
2. Materials and Methods
2.1. Study Area
Data were collected over a year (22 November 2017 to 27 December 2018) from three Hass avocado orchards in the Valparaíso Region, each with varying levels of postharvest fungal diseases. The orchards were located as follows: the first near Santo Domingo planted in 2008 (13 years old at the time of study), 6 km from the sea, at an altitude of 68 m.a.s.l., with an approximate contiguous planting area of 7 hectares; the second in San Pedro planted in 2002 (19 years old), 20.4 km from the sea, at 87 m.a.s.l., with ~12 hectares of contiguous avocado planting and the third in La Palma, Quillota planted in 1976 (45 years old), 28 km from the sea, at 158 m.a.s.l., with ~5 hectares of contiguous planting.
2.2. Measuring Spore Peaks
Five continuous rows were randomly selected in each orchard, with at least 20 plants per row, and spore traps were installed (five traps per orchard). Each trap consisted of a slide (25.4 × 76.2 mm) coated with a thin layer of petroleum jelly, secured with a paper clamp, and tied to tree branches at a height of 90 to 180 cm above the ground [16,22]. Traps were placed near plants exhibiting symptoms such as wood cankers, shoot blight, or necrotic vascular tissue. This methodology is consistent with previous studies conducted by Eskalen and Gubler [24], Eskalen et al. [22], and Valencia et al. [18].
The spore traps were collected and replaced weekly over a 13-month period. Before analysis, cotton blue in lactophenol was applied to the slides and then sealed with Entellan, as described in Valencia et al. [18]. Spores were identified at the Phytopathology Laboratory of the Pontifical Catholic University of Valparaíso using an optical microscope (analyzing 15 traps per week) based on their morphological characteristics and classified to the genus level using a taxonomic key [25].
The number of spores within each slide’s 18 × 18 mm cover area was quantified and recorded as the weekly total for each location. Data from the sampling period revealed peaks in spore presence for each species in the respective orchards.
2.3. Weather Data Monitoring
Weekly meteorological data collected included total precipitation, average relative humidity, and average temperature. For the Santo Domingo orchard, data were obtained from a meteorological station located at the Santo Domingo aerodrome (latitude −33.65, longitude −71.61), approximately 5.6 km from the sampling site.
In the San Pedro orchard, meteorological data were gathered using a weather station within the orchard. Sensors were positioned at latitude −32.94 and longitude −71.29, approximately 356.6 m from the sampling site.
For the La Palma orchard, data were collected via the HOBOS system from a meteorological station at latitude −32.89 and longitude −71.20, approximately 200 m from the sampling area.
2.4. Analysis of Avocado Fruit Dry Matter
The physiological maturity of harvested fruits was assessed by determining the percentage of dry matter. Fifteen fruits were collected from the different orchards during the second week of January 2019 for analysis. A longitudinal section of each fruit was cut, the peel was removed, and the pulp was diced into cubes. The cubes were weighed to obtain the initial sample weight and then dried in an oven at 65 °C for 24 h until a constant weight was achieved, representing the final sample weight [26].
The percentage of dry matter was calculated using the following formula [27]:
2.5. Incidence and Severity of Fruit Diseases
During the second week of January, fruit samples were collected from rows containing spore capture traps, with 100 fruits sampled per orchard. These fruits underwent traditional phytopathological analyses to determine postharvest disease incidence (I) and severity, expressed as a damage index (DI).
Sixty humid chambers (20 per orchard) were prepared for analysis. Each chamber was sterilized with chlorine and 95% alcohol, then fitted with a plastic grid over moistened sterile absorbent paper. Five avocado fruits, previously sterilized with 1% hypochlorite and rinsed with sterile distilled water, were placed in each chamber. The chambers were maintained at 25 ± 1 °C for one week.
2.5.1. Disease Incidence (I)
Incidence was calculated as the percentage of diseased fruits over the total number evaluated. Fruits were either healthy (0) or rotted (1).
2.5.2. Damage Index (DI)
Severity Was Assessed by Examining Internal Lesions on the Fruits:
For Glomerellaceae, severity was scored based on the number of lesions: 0 = healthy fruit; 1 = one or two lesions; 2 = three or four lesions; 3 = five or six lesions, and 4 = seven or more lesions.
For Botryosphaeriaceae, severity was scored based on the progression of streaks from the peduncle toward the other end of the fruit: 0 = healthy fruit; 1 = up to 25% injury progression; 2 = 26% to 50% injury progression; 3 = 51% to 75% injury progression and 4 = 76% to 100% injury progression.
The damage index (DI) was calculated using the following formula, adapted from McKinney [28]:
where: n = number of damaged fruits per score; v = severity score; V = maximum score level; N = total number of fruits evaluated.
2.6. Identification of Monosporic Cultures of Botryosphaeriaceae and Glomerellaceae
Preliminary identification of isolates was performed by comparing the morphological characteristics of the colony spores to those described in established taxonomic keys [25,29]. DNA was extracted from pure monosporic colonies using the DNeasy® Plant Mini Kit (Qiagen Hilden, Germany) for molecular identification. Ribosomal DNA was amplified through PCR following the methodology of Úrbez-Torres et al. [30]. The internal transcribed spacer (ITS) regions of ribosomal DNA were amplified using the following primers: ITS4: TCCTCCGCTTATTGATATGC and ITS5: GGAAGTAAAAGTCGTAACAAGG [31]. Additionally, elongation factor (EF) regions were amplified using EF1-728F: CATCGAGAAGTTCGAGAAGG and EF1-986R: TACTTGAAGGAACCCTTACC [32], and Macrogen Inc. (Seoul, Republic of Korea) sequenced the amplified DNA regions. The results were compared against the GenBank database using the BLAST (v1.4.0) (Basic Local Alignment Search Tool) program. Identification was confirmed for isolates showing greater than 97% similarity to reference sequences in the database.
2.7. Phylogenetic Tree
To confirm the taxonomic identity of the fungal isolates, two complementary phylogenetic analyses were performed: one based on maximum parsimony (MP) and another using Bayesian inference (BI) with concatenated gene regions.
First, MP analysis was conducted in MEGA (v5.2.2) software [33] using ITS sequences from isolates and GenBank reference strains of Botryosphaeriaceae and Glomerellaceae. The tree was inferred using the Tree Bisection and Reconnection (TBR) algorithm with 1000 bootstrap replicates to assess clade support. The analysis was rooted with Fusarium oxysporum (MT530052.1) as the outgroup. Tree quality metrics, including consistency index (CI) and retention index (RI), were calculated to evaluate the robustness of the resulting topology.
Additionally, a Bayesian phylogenetic analysis was performed using concatenated sequences of the internal transcribed spacer (ITS) region and elongation factor 1-alpha (EF1-α) gene. Sequences were aligned independently, concatenated in MEGA (v5.2.2), and exported in NEXUS format. The phylogeny was inferred using MrBayes (v3.2.7) under the GTR + gamma substitution model. The Markov Chain Monte Carlo (MCMC) simulation was run for 1,000,000 generations, sampling every 100 generations with four chains and a temperature of 0.2. The first 25% of trees (2500 generations) were discarded as burn-in. A 50% majority-rule consensus tree was constructed, and posterior probability values were reported for each node. Fusarium oxysporum was again used to root the tree.
2.8. Statistical Analysis and Correlation Visualization
To evaluate the relationship between weekly fungal spore dispersion and climatic variables (average temperature, relative humidity, and precipitation), Pearson correlation analyses were performed using R software (v4.0.0). Weekly total spore counts were calculated by summing Botryosphaeriaceae and Glomerellaceae spores per week and orchard. These data were then merged with meteorological records using the left_join function from the dplyr package (v1.1.4).
Pearson correlation coefficients (r) were calculated using the cor function and visualized through scatterplots generated with the ggpubr package (ggscatter function). Each plot included a fitted linear regression line with a 95% confidence interval, as well as the corresponding correlation coefficient (r) and p-value. These analyses were conducted independently for each orchard (Santo Domingo, San Pedro, and Quillota), covering timeframes of 48 to 54 weeks, depending on data availability. Data preprocessing, including removal of duplicated header rows and numeric column conversion, was performed using readxl and dplyr. Final figures were arranged using ggarrange to facilitate comparative visualization across sites.
Additionally, Pearson correlation analyses were conducted to assess the relationship between fruit dry matter content and two postharvest disease parameters: disease incidence and damage index. For each orchard, the correlation between dry matter percentage and disease outcomes was calculated based on individual fruit data (n = 18 per orchard). These correlations were visualized using scatterplots with regression lines and confidence intervals, following the same approach described above.
For statistical comparison of disease incidence and severity across orchards, one-way ANOVA followed by Tukey’s honest significant difference (HSD) test was applied (p < 0.05).
3. Results and Discussion
3.1. Spore Quantification and Meteorological Data Analysis
The study demonstrated that Botryosphaeriaceae and Glomerellaceae spores were dispersed throughout the year, with significant peaks during or near periods of rainfall and higher relative humidity (Figure 1, Figure 2 and Figure 3).
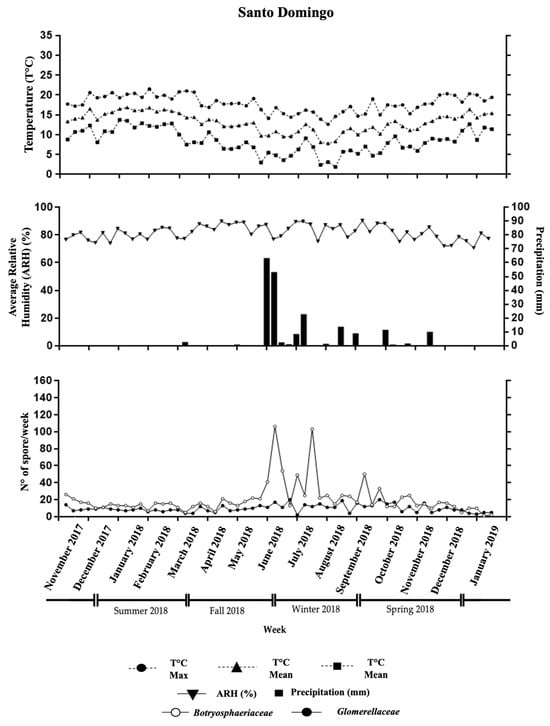
Figure 1.
Weekly data from the Santo Domingo orchard illustrating average temperature (°C), relative humidity (%), and total rainfall (mm). The lower section highlights the number of Glomerellaceae and Botryosphaeriaceae spores captured during the same period.
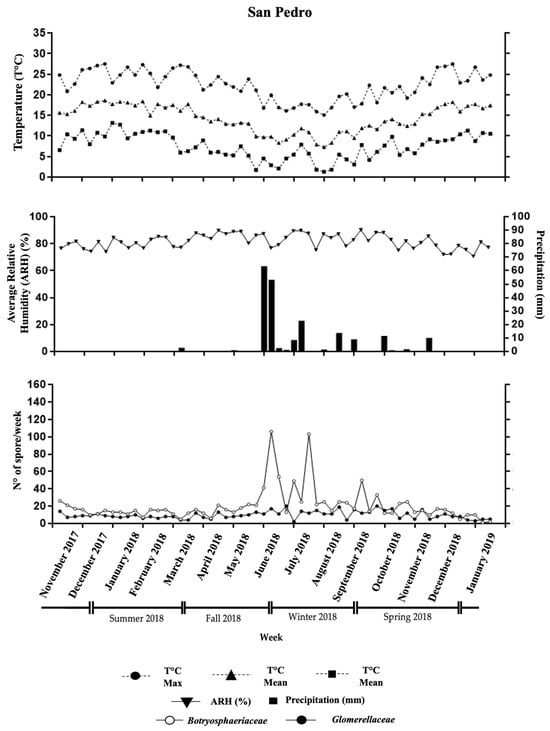
Figure 2.
Weekly data from the San Pedro orchard showing average temperature (°C), relative humidity (%), and total rainfall (mm). The lower section displays the number of Glomerellaceae and Botryosphaeriaceae spores captured each week.

Figure 3.
Weekly data from the Quillota orchard, presenting average temperature (°C), relative humidity (%), and total rainfall (mm). The lower section illustrates the number of Glomerellaceae and Botryosphaeriaceae spores captured weekly.
3.1.1. Santo Domingo Orchard
Spore dispersion was most pronounced between February and September, with Botryosphaeriaceae conidial peaks exceeding 30 spores per week during April, June, July, August, and September 2018 (Figure 1). These peaks coincided with weeks of notable rainfall (above 0.2 mm), with the highest weekly precipitation recorded (e.g., 14.6 mm in April, 32.4 mm in July, and up to 63.4 mm in June). Relative humidity ranged from 77% to 91.3%, and temperatures fluctuated between 7.8 °C and 16.8 °C. The most significant spore peak occurred during week 29 (June), with 106 spores per slide, closely following the wettest week (week 28, with 63.4 mm of rainfall) and one of the most humid weeks (week 28, with 89% relative humidity) (Figure 1).
Glomerellaceae spores remained above 10 spores per slide throughout the year, peaking at 20 spores during week 43 (September), when rainfall reached 0.4 mm, relative humidity averaged 89.9%, and the temperature was 10.2 °C (Figure 1).
3.1.2. San Pedro Orchard
Spore dispersal was highest from May to October (Figure 2). Botryosphaeriaceae exhibited peaks exceeding 30 spores per week during November 2017 in April, June, July, August, and September 2018. These peaks aligned with rainfall greater than 0.1 mm, with a maximum precipitation of 70.4 mm. Average temperatures during these months ranged from 9.7 °C to 15.6 °C. The most significant peak occurred in week 33 (July), with 109 spores per slide. This peak coincided with the wettest week (70.4 mm of rainfall) and the highest relative humidity (74.9%) recorded during the study period (Figure 2).
3.1.3. La Palma Orchard (Quillota)
Spore dispersion was primarily detected between April and November (Figure 3). Botryosphaeriaceae spores exceeded 30 per week in December 2017 and between May and December 2018. These peaks corresponded to periods of precipitation greater than 0.2 mm, with a maximum weekly rainfall of 52 mm. Relative humidity during these months ranged from 63.4% to 86.8%, with temperatures between 9 °C and 20.8 °C. The highest spore peak occurred during week 35 (July), with 140 spores per slide, near the wettest week (week 32, with 52 mm of rainfall) and one of the most humid weeks (week 36, with 86.6% relative humidity) (Figure 3).
Glomerellaceae, the highest peak (12 spores) also occurred in July, under conditions of 8.5 mm rainfall and 85.5% relative humidity (Figure 3)
Our findings on the conidial release dynamics of Botryosphaeriaceae and Glomerellaceae in avocado orchards under Mediterranean conditions are consistent with studies conducted in other crops and regions. For example, Mohankumar et al. [34] reported that in Australian macadamia orchards, the highest conidial dispersal of Lasiodiplodia pseudotheobromae and Neofusicoccum luteum occurred in summer and autumn, with a strong association with rainfall events and relative humidity levels exceeding 70%. Similarly, in our study, the most pronounced spore peaks coincided with periods of rainfall and high humidity, particularly between June and September. This alignment suggests that, regardless of crop type, moisture conditions play a critical role in the release and dissemination of fungal propagules. In parallel, Jiménez et al. [17] documented comparable dispersal patterns in table grape vineyards in central Mexico, where a strong positive correlation between rainfall and the number of trapped spores was also established. Unlike our findings, where temperature correlations were negative or non-significant, studies in tropical crops such as macadamia and grapes suggest that high temperature combined with humidity may further enhance sporulation. These comparisons emphasize the importance of incorporating local climatic variables into early-warning systems for postharvest diseases in avocado, highlighting the need to tailor disease management strategies to the seasonal dynamics of each agroclimatic zone.
3.2. Genera Identification on Slides
Using optical microscopy, Botryosphaeriaceae species were identified based on their spore morphology: Diplodia spores were generally hyaline, aseptate, and occasionally brown with one or two septa. They had smooth external walls, an ovoid shape, a wider middle, an obtuse apex, and a truncated or rounded base. Spore dimensions ranged from 19.26 to 27.94 µm in length and 10.56 to 14.9 µm in width, with an average of 23.12 × 12.51 µm. Dothiorella spores were hyaline to dark brown, featuring a septum in the middle, an ovoid shape, a broadly rounded apex, and a truncated base. Dimensions ranged from 16.69 to 26.93 µm in length and 6.02 to 14.83 µm in width, with an average of 22.14 × 10.25 µm. Neofusicoccum spores were predominantly hyaline, aseptate, smooth, ellipsoidal, and featured truncated bases. Dimensions ranged from 18.17 to 27.62 µm in length and 5.25 to 8.96 µm in width, with an average of 22.69 × 7.50 µm.
Figure 4 highlights the temporal distribution of spores by genus across the three orchards: Santo Domingo Orchard: The most pronounced peaks for Diplodia and Dothiorella occurred in week 29, with 46 and 37 spores, respectively. For Neofusicoccum, the peak was in week 34, with 25 spores. San Pedro Orchard: The highest spore counts were recorded in week 33, with 52 spores for Diplodia, 31 spores for Neofusicoccum, and 26 spores for Dothiorella. Quillota Orchard: The highest peaks for Dothiorella and Diplodia were observed in week 34, with 57 and 48 spores, respectively, while Neofusicoccum peaked in week 40, with 36 spores. All three orchards exhibited traces of Botryosphaeriaceae spores throughout the year, indicating continuous presence and potential spore dispersal across the seasons.
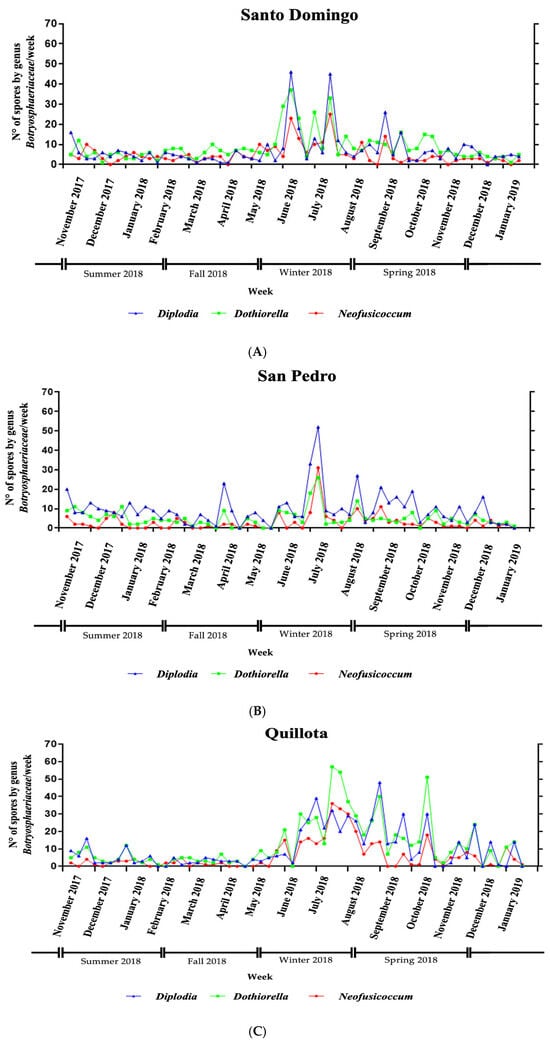
Figure 4.
Temporal distribution of Botryosphaeriaceae spores by genus in three avocado orchards—(A) Santo Domingo, (B) San Pedro, and (C) Quillota (La Palma)—in the Valparaíso Region, Chile, over a 13-month period (November 2017 to January 2019). Weekly conidial counts for the genera Diplodia (blue), Dothiorella (green), and Neofusicoccum (red) were quantified from spore traps and plotted to assess seasonal trends in aerial inoculum presence. Each panel represents data from one orchard, illustrating distinct temporal peaks and differences in dominant genera.
It is important to consider which genera are responsible for fruit damage and when they are most active. In this study, Diplodia, Dothiorella, and Neofusicoccum were consistently observed throughout the year, with peak dispersion aligning with wet and humid periods, especially between late autumn and winter. This suggests that these genera have a high potential for infection during cool, moist seasons, corresponding with stem-end rot symptoms observed in fruit.
For Colletotrichum (Glomerellaceae), spore detection was more limited, though peaks were evident during winter when relative humidity was consistently above 85% and temperatures were below 12 °C. These conditions align with previous reports associating Colletotrichum gloeosporioides infection with cool, wet environments conducive to appressorium formation and penetration [35]. Therefore, monitoring weather conditions may help predict periods of highest risk for anthracnose outbreaks.
Although Diplodia and Dothiorella spores were identified microscopically on spore traps, these genera were not recovered from infected fruit tissues during postharvest isolation. Several factors may explain this discrepancy. First, both genera have been reported as latent or weakly virulent pathogens in avocado, and their infection may be more dependent on host stress conditions or pruning wounds rather than direct fruit colonization. Second, Neofusicoccum spp., particularly N. parvum and N. australe, are considered more aggressive and may have competitively excluded Diplodia and Dothiorella during natural infection or incubation. Third, it is possible that infection by these genera occurred earlier in the season or in tissues not sampled (e.g., stems or peduncles), and thus remained undetected in the fruit-focused postharvest analysis. Similar patterns have been reported in previous studies in Chile and California, where Diplodia was frequently found in cankers and pruning wounds but less often in fruit [7,16].
3.3. Correlation Between Climatic Variables and Total Spore Dispersion
Pearson correlation analyses (Figure S1) revealed varying degrees of association between total weekly spore counts and climatic variables across the three avocado orchards evaluated.
In San Pedro, total spore counts exhibited a moderate to strong positive correlation with weekly rainfall (r = 0.66, p < 0.001) and a moderate positive correlation with relative humidity (r = 0.46, p = 0.002). In contrast, a weak negative correlation was observed with average temperature (r = −0.18, p = 0.24). These results suggest that in this orchard, rainfall events and higher humidity are key drivers of conidial release for both Botryosphaeriaceae and Glomerellaceae. In Santo Domingo, total spore counts were moderately and negatively correlated with temperature (r = −0.43, p = 0.002), while relative humidity showed no significant association (r = −0.03, p = 0.81). The negative association with temperature suggests that spore dispersal increased during cooler weeks, particularly in coastal conditions. In Quillota, no strong linear relationships were observed. Correlations were weak and non-significant for all three variables: temperature (r = −0.12, p = 0.40), relative humidity (r = 0.15, p = 0.28), and rainfall (r = 0.01, p = 0.92). These low correlations may be attributed to the use of estimated meteorological data in this location and a narrower range of climatic variation.
Together, these results highlight the site-specific role of climatic conditions in modulating airborne inoculum dynamics. While rainfall and humidity emerged as the most influential factors in San Pedro, lower temperatures were more closely associated with spore peaks in Santo Domingo. The findings support previous reports on the environmental drivers of conidial dispersal in avocado orchards.
These findings are consistent with previous research by Hernández-Lauzardo et al. [36] in Mexican avocado orchards, where over 72% of spore release occurred during the most humid months, and rainfall and high relative humidity were identified as key factors influencing Glomerellaceae dispersal. Similarly, Eskalen et al. [22] reported that Botryosphaeriaceae spores in Californian avocado orchards were released predominantly during rainy periods and cooler months, aligning with our observations in Santo Domingo and San Pedro. These results reinforce the notion that environmental triggers for spore dispersion are highly site-dependent and that localized monitoring systems integrating humidity and precipitation data may be essential to anticipate high-risk periods for fungal infection in avocado production systems.
3.4. Dry Matter Percentage in Sampled Fruits
The dry matter content of the sampled avocado fruits varied considerably among orchards: 19.12 ± 2.72 in Santo Domingo, 26.21 ± 1.90 in San Pedro, and 34.81 ± 1.83 in Quillota (Table S1). Notably, the orchard with the lowest dry matter content (Santo Domingo) also exhibited the highest incidence and damage index of fruit diseases. This observation suggests a potential relationship between fruit physiological maturity at harvest and increased susceptibility to postharvest pathogens, as less mature fruits may be more vulnerable during storage and ripening.
Spore dispersion of both Botryosphaeriaceae and Glomerellaceae was detected year-round; however, the most pronounced peaks occurred between April and September, corresponding to autumn and winter. These results are consistent with those of Eskalen et al. [22], who reported similar seasonal spore dynamics in California avocado orchards, with peaks extending from fall through mid-spring.
Interestingly, this study also recorded a Botryosphaeriaceae spore peak during spring that was not associated with rainfall. A plausible explanation is agrochemical applications with water volumes equal to or exceeding 2000 L/ha, which could physically disperse fungal propagules. In contrast, Valencia et al. [18] did not observe such dispersion during spring with applications using only 1000 L/ha, suggesting that the volume of liquid used may play a critical role in passive spore release. This hypothesis merits further investigation, especially in orchards that rely on high-volume foliar sprays.
Climatic variables also played a key role in inoculum dynamics. Valle [19] reported a positive correlation between high relative humidity and fungal spore dispersal in Mexican avocado orchards, where 72.5% of spores were released during the most humid months. Similarly, Crane et al. [37] found that Glomerellaceae spores dispersed predominantly during rainy periods or when relative humidity was high and temperatures were low. In Colletotrichum spp., which requires free water and specific thermal conditions to germinate, Prusky et al. [35] described that encystment and infection are enhanced under moderate temperatures (approximately 20 °C) and relative humidity above 80%. Thus, in this study, even though relative humidity levels were lower than those typically observed in Mexico, they were sufficient to promote fungal dispersal, especially during the Chilean winter.
Three Botryosphaeriaceae genera—Diplodia, Dothiorella, and Neofusicoccum—were visually identified on spore traps, consistent with the genera reported by Valencia et al. [7] for Chilean avocados. While their study also identified Lasiodiplodia theobromae, this species was not detected here. However, this difference likely reflects natural geographic variability rather than a methodological discrepancy, considering that Valencia et al. [7] sampled Alicahue, approximately 70 km northeast of the orchards studied here.
3.5. Incidence and Damage Index of Fruit Diseases
Following a modified method by Fischer et al. [38], avocado fruits were incubated in chambers at 25 °C with 80–85% relative humidity for seven days to assess the incidence (I) and damage index (DI) of stem-end rot and anthracnose. Santo Domingo Orchard: stem-end rot: I = 44%, DI = 17.25%, and anthracnose: I = 23%, DI = 12.25% (Figure 5). San Pedro Orchard: stem-end rot: I = 19%, DI = 5% and anthracnose: I = 19%, DI = 7.5% (Figure 5). Quillota Orchard: stem-end rot: I = 22%, DI = 7.25% and anthracnose: I = 12%, DI = 4% (Figure 5). These results highlight variations in disease prevalence and severity across the orchards, with Santo Domingo exhibiting the highest incidence and damage index values for both diseases.
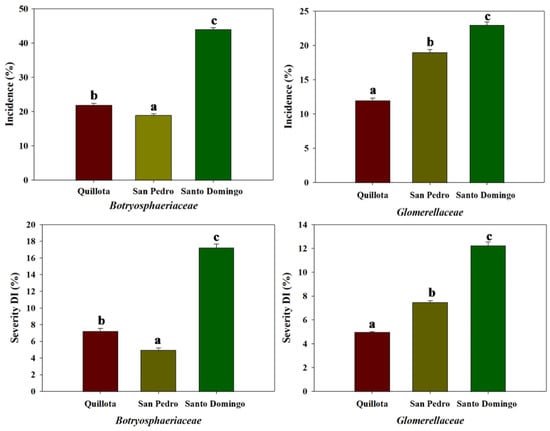
Figure 5.
Incidence (%) and severity index (DI, %) of postharvest fruit rot diseases caused by Botryosphaeriaceae (left panels) and Glomerellaceae (right panels) in three avocado orchards (Quillota, San Pedro, and Santo Domingo). Upper panels show disease incidence, while lower panels display disease severity (DI). Bars represent mean values ± standard error (SE). Different letters above bars indicate statistically significant differences between orchards (p < 0.05) based on Tukey’s honest significant difference (HSD) test.
Using humid chambers for incidence (I) and damage index (DI) measurements provided optimal conditions to enhance the expression of fungal pathogens, facilitating the identification of causative agents. However, it is important to note that these results reflect an upper bound of potential damage and may not directly correspond to postharvest conditions under commercial processing.
As shown in Figure 5, the highest I and DI values for diseases caused by both fungal families were observed in the Santo Domingo orchard, with Botryosphaeriaceae reaching an I = 44% and DI = 17.25%. These values were significantly higher than those recorded in San Pedro and Quillota. While Santo Domingo is the youngest orchard, this study highlights the substantial influence of coastal humidity and climatic conditions rather than orchard age. In particular, Santo Domingo’s proximity to the coast likely results in higher average humidity, which, as shown by Prusky et al. [34], favors the germination of fungal conidia and subsequent infection processes.
The high disease indices in this orchard may also reflect the greater presence of Colletotrichum spp., which are known to be highly responsive to elevated humidity and moderate temperatures (20–28 °C), conditions commonly recorded in Santo Domingo during summer. This coincides with Freeman et al. [39] and recent findings by Tapia et al. [40], who observed similar disease patterns in Chilean orchards under coastal influence.
3.6. Correlation Between Dry Matter and Disease Incidence and Severity Index
The correlation analysis between fruit dry matter content and postharvest disease parameters revealed site-specific patterns that may be influenced by microclimatic conditions (Table S1 and Figure S2). In the coastal orchard of Santo Domingo, a significant positive correlation was observed between dry matter and both disease incidence (r = 0.74, p = 0.00043) and damage index (r = 0.66, p = 0.0031), suggesting that more physiologically mature fruits may be more susceptible to pathogen colonization in humid environments. In contrast, the San Pedro orchard exhibited a strong negative correlation between dry matter and disease incidence (r = −0.75, p = 0.00034), as well as a moderate negative correlation with damage index (r = −0.59, p = 0.011), indicating that in more temperate conditions, less mature fruits may be at greater risk. Meanwhile, the Quillota orchard, characterized by lower precipitation and humidity, showed no significant correlations (r = −0.4 to ~0, p > 0.1), suggesting that fruit maturity may play a less influential role under drier conditions. These contrasting patterns align with previous reports that highlight the interaction between fruit physiology and environmental moisture in determining postharvest disease development [1,7].
3.7. Molecular Analysis of Fungal Species Isolated from Fruits
Monosporic isolates were analyzed using ITS. Only isolates with an identity percentage greater than 98% were selected for this study (Table 1). Santo Domingo Orchard: Eight isolates were identified, including two N. luteum and six C. gloeosporioides. San Pedro Orchard: Six isolates were identified, comprising three of N. parvum, one of N. australe, and two of C. gloeosporioides. Quillota Orchard: Five isolates were identified, including two of N. nonquaesitum, one of N. parvum, one of N. australe, and one of C. gloeosporioides. These findings provide a detailed molecular characterization of the fungal species in the sampled fruits across the three orchards.

Table 1.
Percentage (%) identity and GenBank matches of monosporic isolates obtained from fruits in the three orchards of the Valparaíso Region.
3.8. Phylogenetic Tree Analysis
Phylogenetic analysis was performed using 33 nucleotide sequences, including 15 reference strains and a final alignment of 513 positions. Among the 18 isolates recovered from avocado fruits, 10 belonged to the Botryosphaeriaceae family and 8 to Glomerellaceae. Maximum parsimony (MP) analysis grouped the isolates into five well-supported clades: Neofusicoccum luteum, N. australe, N. parvum, N. nonquaesitum, and Colletotrichum gloeosporioides (Figure 6). Of the aligned positions, 347 were constant and 118 were parsimony-informative. The MP method yielded the 10 most parsimonious trees, with a consistency index (CI) of 0.9161 and a retention index (RI) of 0.9892, confirming the robustness of the phylogenetic reconstruction.
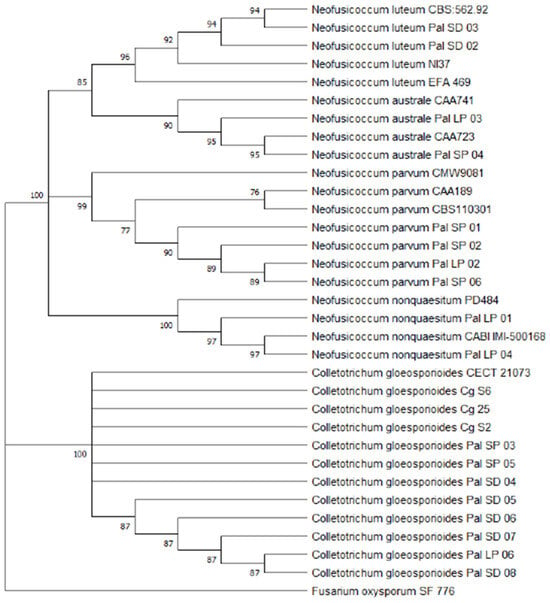
Figure 6.
Phylogenetic tree of identified species isolates based on Maximum Parsimony (MP) analysis of ITS loci. Isolates SP, SD, and LP, sequenced in this study, are included. MP bootstrap values > 50 are indicated on the branches. Fusarium oxysporum was used as the outgroup.
To complement and confirm these findings, a second phylogenetic tree was constructed using Bayesian inference (BI) based on concatenated ITS and EF1-α sequences (Figure S3). This analysis produced a 50% majority-rule consensus tree, revealing strong posterior probabilities (>0.95) for all major clades. The BI tree confirmed the presence of N. luteum, N. australe, N. parvum, and N. nonquaesitum among the Botryosphaeriaceae isolates, mirroring the results obtained through MP analysis and reinforcing the molecular identity of the isolates. Fusarium oxysporum was used as the outgroup in both analyses.
When comparing the orchards, La Palma (the oldest orchard, 45 years old) exhibited the highest cumulative number of Botryosphaeriaceae spores (1623 spores in 2018)—32% more than in Santo Domingo and 42% more than in San Pedro. In contrast, the youngest orchard (Santo Domingo, 13 years old) recorded the highest total of Glomerellaceae spores (517 spores), highlighting the likely influence of microclimatic factors such as relative humidity and temperature on spore dynamics. As previously reported, Colletotrichum spp. require free moisture and moderate temperatures for successful infection and sporulation, with optimal conditions between 20 °C and 28 °C and high relative humidity [35]. These conditions were frequently recorded in Santo Domingo during summer, which may explain the dominance of C. gloeosporioides in that orchard.
It is noteworthy that Lasiodiplodia theobromae, previously reported by Valencia et al. [7] in Alicahue avocado orchards, was not detected in this study. This absence likely reflects natural geographic and ecological differences, given that Alicahue is located approximately 70 km northeast of the orchards sampled here.
Overall, molecular identification of isolates was consistent with morphological observations. C. gloeosporioides was the predominant Glomerellaceae species in Santo Domingo, while Neofusicoccum spp. were dominant in San Pedro and La Palma. These distribution patterns align with previous findings showing that Colletotrichum spp. prefer warm and humid environments [35,39], and N. luteum is particularly common in coastal orchards in Chile [40]. The combination of MP and Bayesian approaches provides a robust framework for species-level identification and phylogenetic confirmation of avocado-associated pathogens.
Regarding agrochemical sprays, our observations suggest that spring spray events using water volumes ≥ 2000 L/ha may facilitate passive spore release or splash dispersion, particularly under humid conditions. While previous work by Valencia et al. [18] using 1000 L/ha found no effect, the role of spray volume and canopy wetness in spore mobilization warrants further investigation.
Compared to earlier studies focused mainly on short-term spore sampling or fruit-only analysis, our study provides a comprehensive 13-month dataset that integrates conidial dispersal, climatic data, and fruit disease assessments under field conditions. Recent research (e.g., Tapia et al. [40]; Fiorenza et al. [41]; Guarnaccia et al. [42]) has advanced fungal species identification in avocado but has not fully linked airborne inoculum dynamics to weather patterns and postharvest outcomes. Our findings thus contribute new insights toward early-warning strategies and more precise disease management in avocado production.
4. Conclusions
This study demonstrated a clear association between increased rainfall, relative humidity, and lower temperatures with peak aerial spore dispersal of Botryosphaeriaceae and Glomerellaceae in avocado orchards. Botryosphaeriaceae spores, particularly from Diplodia, Dothiorella, and Neofusicoccum, were dominant during wet and humid periods and correlated with stem-end rot in fruit. Colletotrichum gloeosporioides was the main species identified for Glomerellaceae, linked to anthracnose symptoms. Molecular identification confirmed the presence and diversity of pathogenic species across the orchards. These findings suggest that spore release and fruit infection are favored by periods of high humidity and precipitation, highlighting the need to monitor environmental conditions to predict disease risk. As a practical recommendation, growers should intensify disease surveillance and consider preventive fungicide applications during winter months or when humidity exceeds 80% and rainfall accumulates. The integration of weather-based monitoring with spore trap data may support early-warning systems and improve the timing of spray applications, contributing to more sustainable and targeted integrated disease management (IDM) strategies in avocado production.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15061453/s1, Figure S1: Pearson correlation analysis between total weekly spore counts (Botryosphaeriaceae + Glomerellaceae) and climatic variables (temperature, humidity, and precipitation) in three avocado orchards. Figure S2: Pearson correlation analysis between dry matter percentage and postharvest disease incidence and severity in fruits from three orchards. Figure S3: Bayesian phylogenetic tree based on concatenated ITS and EF1-α sequences of Botryosphaeriaceae isolates. Table S1: Average dry matter, incidence, and severity values of Botryosphaeriaceae and Glomerellaceae in avocado fruits from Santo Domingo, San Pedro, and Quillota.
Author Contributions
Conceptualization, L.T. and X.B.; methodology, L.T., D.C.-N., N.R., R.C., A.L. and X.B.; software, L.T., A.L.V., D.C.-N. and N.R.; validation, L.T. and X.B.; formal analysis, L.T., D.C.-N., N.R. and X.B.; investigation, X.B.; resources, X.B.; data curation, X.B.; writing—original draft preparation, L.T., D.C-N. and X.B.; writing—review and editing, D.C.-N. and X.B.; visualization, X.B.; supervision, X.B.; project administration, X.B.; funding acquisition, X.B. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank Laboratorio de Fitopatología, Escuela de Agronomía, PUCV, and ANID Millenium Nucleus of Bioproducts, Genomics, and Environmental Microbiology (BioGEM) NCN2023_054.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding authors.
Acknowledgments
We thank Iván Cortez and Katherine Vega for their invaluable technological support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Silva, T.A.; Ledesma, N. Avocado History, Biodiversity and Production. In Sustainable Horticultural Systems, Sustainable Development and Biodiversity; Nandwani, D., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 157–205. [Google Scholar]
- ODEPA. Boletín de Fruta, Diciembre 2021; Oficina de Estudios y Políticas Agrarias (ODEPA): San Diego, Chile, 2021; Available online: https://www.odepa.gob.cl/contenidos-rubro/boletines-del-rubro/boletin-de-fruta-diciembre-2021 (accessed on 12 January 2022).
- Food and Agriculture Organization of the United Nations (FAO) 2022. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 11 June 2025).
- Redagrícola. Producción de Palta a Nivel Mundial Creció 4% en 2023 y Chile se Ubicó Como el Cuarto Principal Consumidor. 2023. Available online: https://redagricola.com/produccion-de-palta-a-nivel-mundial-crecio-4-en-2023-y-chile-se-ubico-como-el-cuarto-principal-consumidor/ (accessed on 20 May 2025).
- Schaffer, B.N.; Wolstenholme, B.N.; Whiley, A.W. (Eds.) Introduction. In The Avocado: Botany, Production and Uses; CABI International Press: Wallingford, UK, 2013; pp. 1–9. [Google Scholar]
- ODEPA_CIREN. Catastro Frutícola, Región de Valparaíso; Principales Resultados: Santiago, Chile, 2017. [Google Scholar]
- Valencia, A.; Gil, P.; Latorre, B.; Rosales, I. Characterization and Pathogenicity of Botryosphaeriaceae Species Obtained from Avocado Trees with Branch Canker and Dieback and from Avocado Fruit with Stem End Rot in Chile. Plant Dis. 2019, 103, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Defilippi, B.; Ferreyra, R.; Rivera, S. Optimización de la calidad de palta “Hass”: Herramientas para enfrentar nuevos desafíos. In Boletín INIA- Instituto de Investigaciones Agropecuarias (INIA); INIA: La Cruz, Chile, 2015; Volume 307, p. 142. [Google Scholar]
- Woolf, A.; Clark, C.; Terander, E.; Phetsomphou, V.; Hofshi, R.; Arpaia, M.; Boreham, D.; Wong, M.; White, A. Measuring avocado maturity; ongoing developments. Orchadist 2003, 76, 40–45. [Google Scholar]
- Ferreyra, E.; Defilippi, B. Factores de precosecha que afectan la post-cosecha de palta Hass: Clima, suelo y manejo. In Boletín INIA- Instituto de Investigaciones Agropecuarias (INIA); INIA: La Cruz, Chile, 2012; Volume 248, p. 100. [Google Scholar]
- Molina, E.; Silva, H.; García, S. First report of black spots on avocado fruit caused by Neofusicoccum parvum in México. Plant Dis. 2012, 96, 287. [Google Scholar] [CrossRef]
- Montealegre, J.; Ramírez, M.; Riquelme, D. First report of Neofusicoccum australe in Chile causing avocado stem-end rot. Plant Dis. 2016, 100, 2532. [Google Scholar] [CrossRef]
- Bower, J.; Ferreyra, R.; Depilippi, B.; Arpaia, M. Factores de manejo agronómico que afectan la post-cosecha de la palta “Hass”: Herramientas para enfrentar nuevos desafíos. In Boletín INIA- Instituto de Investigaciones Agropecuarias (INIA); INIA: La Cruz, Chile, 2015; Volume 307, pp. 21–30. [Google Scholar]
- Kimaru, S.; Monda, E.; Heruiyot, R.; Mbaka, J.; Alakonya, A. Morphological and molecular identification of the causal agent of anthracnose disease of avocado in Kenya. Int. J. Microbiol. 2018, 4668420. [Google Scholar] [CrossRef]
- Pérez, G.; Pérez, C. Reconocimiento a campo de plagas y enfermedades forestales Cancro por Botryosphaeria. INIA: Tacuarembó, Uruguay, 2014; Available online: http://www.ainfo.inia.uy/digital/bitstream/item/3371/1/salida-inia-cartilla37-Botryosphaeria.pdf (accessed on 20 May 2025).
- Úrbez-Torres, J.; Battany, M.; Bettiga, L.; Gispert, C.; McGourty, G.; Roncoroni, J.; Smith, R.J.; Verdegaal, P.; Gubler, W. Botryosphaeriaceae species spore-trapping studies in California vineyards. Plant Dis. 2010, 94, 717–724. [Google Scholar] [CrossRef]
- Jiménez L., I.; Doll, D.; Ashworth, V.E.T.M.; Trouillas, F.P.; Rolshausen, P.E. Comparative profiling of wood canker pathogens from spore traps and symptomatic plant samples within california almond and walnut orchards. Plant Dis. 2022, 106, 2182–2190. [Google Scholar] [CrossRef]
- Valencia, D.; Torres, C.; Camps, R.; López, E.; Celis, J.; Besoain, X. Dissemination of Botryosphaeriaceae conidia in vineyards in the semiarid Mediterranean climate of the Valparaiso Region of Chile. Phytopathol. Mediterránea 2015, 54, 394–402. [Google Scholar]
- Valle, G. Dinámica de las Poblaciones Fúngicas Aéreas en un Agroecosistema de Aguacate. Master’s Thesis, Instituto Politécnico Nacional, Yautepec, México, 2015. [Google Scholar]
- Huerta, G.; Holguín, F.; Benítez, F.; Toledo, J. Anthracnose epidemiology (Colletotrichum gloeosporioides (Penz.) Penz. And Sacc.) on mango cv. Ataulfo (Mangifera indica L.) in the Soconusco, Chiapas, México. Rev. Mex. Fitopatol. 2009, 27, 93–105. [Google Scholar]
- Noriega, H.; Pereyda, J.; Garrido, R. Effects of climatological factors on fluctuation of spores at mango trees cv. Ataulfo, in Guerrero, México. Rev. Mex. Fitopatol. 2017, 35, 227–241. [Google Scholar]
- Eskalen, A.; Faber, B.; Bianchi, M. Spore trapping and pathogenicity of fungi in the Botryosphaeriaceae and Diaporthaceae associated with avocado branch canker in California. Plant Dis. 2013, 97, 329–332. [Google Scholar]
- Barcos, J.; Rebufel, P.; Soto, S. Hongos fitopatógenos causantes de pudrición peduncular en tres zonas productoras de paltos var. Hass. In Tierra Adentro (INIA); INIA: La Platina, Chile, 2020; Volume 113, pp. 62–64. [Google Scholar]
- Eskalen, A.; Gubler, W. Association of spores of Phaeomoniella chlamydospora, Phaeoacremoniu minflatipes, and Phaeoacremonium aleophilum with grapevine cordons in California. Phytopathol. Mediterr. 2001, 40, 429–432. [Google Scholar]
- Phillips, A.; Alves, A.; Abdollahzadeh, J.; Slippers, B.; Wingfield, M.; Groenewald, J.; Crous, P. The Botryosphaeriaceae: Genera and species known from culture. Mycology 2013, 76, 51–167. [Google Scholar] [CrossRef]
- INIA Perú. Técnica Adaptada Para Determinación de Momento Óptimo de Cosecha en Palta cv Hass. 2011. Available online: http://www.inia.gob.pe/wp-content/uploads/investigacion/programa/sistProductivo/tecnologia/palto/DetermOptimo-CosechaPaltaHass.pdf (accessed on 20 May 2025).
- Bergh, B.O.; Kumamoto, J.; Chen, P. Determining maturity in whole avocados. Calif. Avocado Soc. Yearb. 1989, 73, 173–176. [Google Scholar]
- Mc Kinney, H. Influence of soil temperature and moisture on infection of wheat seedlings by Helminthosporium sativum. J. Agric. Res. 1923, 26, 195–225. [Google Scholar]
- Weir, B.S.; Johnston, P.R.; Damm, U. The Colletotrichum gloeosporioides species complex. Stud. Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.; Leavitt, G.; Guerrero, J.; Guevara, J.; Gubler, W. Identification and pathogenicity of Lasiodiplodia theobromae and Diplodia seriata, the causal agents of bot canker disease of grapevines in México. Plant Dis. 2008, 92, 519–529. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. 1990, 18, 315–322. [Google Scholar]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1991, 91, 553–556. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Mohankumar, V.; Dann, E.K.; Akinsanmi, O.A. Seasonal dynamics of inoculum of Botryosphaeriaceae in macadamia orchards in Australia. Plant Pathol. 2023, 72, 1160–1170. [Google Scholar] [CrossRef]
- Prusky, D.; McEvoy, J.; Leverentz, R.; Conway, W. Local modulation of host pH by Colletotrichum species as a mechanism to increase virulence. Phytopathology 2001, 9, 1105–1113. [Google Scholar]
- Hernández-Lauzardo, A.; Campos-Martínez, A.; Valle, M.; Flores-Moctezuma, H.; Suárez, R.; Ramírez-Trujillo, J. First Report of Colletotrichum godetiae Causing Anthracnose on Avocado in Mexico. Plant Disease 2014, 99. [Google Scholar] [CrossRef]
- Crane, J.; Belerdi, C.; Maguire, I. Avocado Growing in the Florida Home Lands Cape. Institute of Food and Agricultural Sciences, University of Florida: Homestead, FL, USA, 2005; p. 16. [Google Scholar]
- Fischer, I.; Moraes, M.; Firmino, A.; Amorim, L. Detection and epidemiological progress of quiescent avocado diseases. Ciência Rural 2019, 49. [Google Scholar] [CrossRef]
- Freeman, S.; Katan, T.; Shabi, E. Characterization of Colletotrichum gloeosporioides isolates from avocado and almond fruits with molecular and pathogenicity test. Appl. Environ. Microbiol. 1995, 62, 1014–1020. [Google Scholar] [CrossRef]
- Tapia, L.; Larach, A.; Riquelme, N.; Guajardo, J.; Besoain, X. First Report of Neofusicoccum luteum Causing Stem-End Rot Disease on Avocado Fruits in Chile. Plant Dis. 2020, 104. [Google Scholar] [CrossRef]
- Fiorenza, A.; Gusella, G.; Vecchio, L.; Aiello, D.; Polizzi, G. Diversity of Botryosphaeriaceae species associated with canker and dieback of avocado (Persea americana) in Italy. Phytopathol. Mediterr. 2023, 62, 47–63. [Google Scholar] [CrossRef]
- Guarnaccia, V.; Vitale, A.; Cirvilleri, G.; Aiello, D.; Susca, A.; Epifani, F.; Perrone, G.; Polizzi, G. Characterisation and pathogenicity of fungal species associated with branch cankers and stemend rot of avocado in Italy. Eur. J. Plant Pathol. 2016, 146, 963–976. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).