Abstract
This study investigates the impact of light conditions on the growth, rooting, and photosynthetic performance of in vitro cultivated Cistus creticus L. explants. Initially, different plant growth regulators were tested for multiple shoot growth [5 and 10 μM 6-Benzylaminopurine (BA), 5 and 10 μM meta-Topolin (mT), and 0.5 and 1 μM melatonin (Mel)] and rooting [5 and 10 μM mT, 0.5 and 1 μM Mel, and 0.5 and 1 μM Indole-3-butyric acid (IBA)]. The media with the best results were Murashige and Skoog (MS) with 5 μM BA or 5 μM mT for shoot formation and 0.5 μM Mel or 1 μM IBA for rooting. Also, the explants were cultured under red (R), blue (B), or white (W) LED lights or fluorescent (FL) lamps. After four weeks, the photosynthetic rate, chlorophyll concentration, fluorescence (Fv/Fm), and shoot/root measurements were recorded. The optimal plant regulator for shoot generation was 5 μM mT under a W LED. For rooting, 1 μM IBA combined with a R LED resulted in 100% rooting, 3.53 roots/explant, and a 1.25 cm root length. The B LED led to the highest photosynthetic rate, while the chlorophyll concentration was highest with 5 μM BA under a FL lamp (CCI = 3.03). During acclimatization, a R LED and 1 μM IBA yielded the highest survival rate (70%). The current findings could reinforce the in vitro cultivation of the species for use in the floriculture industry, as well as for medicinal and other industrial purposes. Growth rooms equipped with automated LED lighting systems could optimize the micro-environment and create suitable climatic conditions to enhance in vitro plant growth.
1. Introduction
Plants of the genus Cistus (f. Cistaceae), also known as “Rockroses” or “Ladania”, consist of almost 200 species. They are dicotyledonous, perennial, flowering, herbaceous plants, native plants of the Mediterranean and Western Africa. They can grow even in difficult climatic and soil conditions, such as arid and rocky soils [1,2]. Plants of the genus Cistus can be used as ornamental plants and medicinal plants. The aerial parts of most Cistus plants (especially Cistus creticus L.) are rich in labdane-type diterpenes and polyphenols, and their extracts have strong antimicrobial, antiviral, anticancer, and antioxidant properties [2,3,4], while its members can be used in xerophytic habitats and for the establishment of urban green roofs [5].
The Cistus genus is propagated either by seed or by asexual, vegetative means. The seeds exhibit physical dormancy due to a hard coat which can last for a long period of time and are interrupted by the effect of very high temperatures (>100 °C). This dormancy renders the seeds more resistant to adverse conditions, thus enabling the plant to regenerate more easily after a disaster, being a typical Mediterranean species adaptation [6,7]. The term “in vitro propagation” or “micropropagation” refers to the cultivation of organs or small sections of plant tissue in aseptic and controlled conditions [8]. This method of propagation renders the production of large numbers of healthy plants easier and also helps produce highly uniform plants in a shorter period of time, in comparison to conventional propagation techniques [9].
Typically, the in vitro cultivation of Cistus involves the use of cytokinin 6-benzyloaminopurine (BA or 6-BAP) for shoot proliferation [10]; the auxin indole-3-butyric acid (IBA) is employed for in vitro rooting [10]. In the recent past, meta-Topolin (mT) and melatonin (Mel), two naturally occurring hormones, have been proven very effective for the in vitro plant growth of various woody-plant species [11,12].
Light is a critical environmental factor for plant growth and development; it serves as the primary energy source for the photosynthetic process. It is a key regulator of various physiological processes, acting as an elicitor affecting various biochemical parameters, such as secondary metabolites [13,14,15]. Nowadays, light-emitting diodes (LEDs) have become widely popular. They have a beneficial effect on plant growth, providing a better alternative to artificial lighting compared to the conventional ones, decreasing the wide range of wavelengths of fluorescent lamps. LEDs reduce costs, being long-life and providing the ability to manipulate the light spectra that the plants will be exposed to depending on the desired results [16,17,18]. Furthermore, they give off less heat and cultures do not have to be placed close to their source. Munoz and et al. found that an average of 46% of the fluorescent electrical energy in a fluorescent tube transformed into heat [19]. It has also been proven that red, blue, and white LEDs significantly influence plant growth, morphology, and rooting, and they also affect the photosynthetic rhythm by activating different photoreceptors [20,21,22,23,24,25]. Additionally, LEDs offer a more sustainable lighting option, consuming less electricity and having a longer lifespan compared to fluorescent lamps [26]. The LEDs have high potential for in vitro propagation technology, being essential for the plant production [27,28]. It is crucial for each plant species to be cultivated in vitro under the appropriate blue/red ratio to achieve a high plant growth rate. According to previous studies, the growth process and morphogenesis of ornamental species, such as Lilium, Chrysanthemum, Myrtus communis, and Dendrobium officinale, were affected by light spectra, while different light spectra in conjunction with various concentrations of cytokinin’s affected both the morphological traits and phytochemical properties of M. communis [29,30,31,32]. Such lighting systems can be established in growth rooms with automated systems that offer the option of complete micro-environmental control tailored for each species [33]. This is particularly important during in vitro propagation, where pest and pathogen control is crucial, while each climatic parameter is a sensitive factor affecting every aspect of plant growth, such as cell formation and organogenesis. Regarding the lighting source used in in vitro culture systems of C. creticus L., to the best of our knowledge, only fluorescent lamps have been employed. In the present study, firstly, different plant growth regulators were examined in in vitro MS culture systems of C. creticus. In addition, the combined effects of four different light spectra with the best plant growth regulators were tested for the purpose of optimizing the in vitro culture protocol. The evaluation was conducted in terms of the shoot proliferation, rooting induction, photosynthetic rhythm, chlorophyll concentration, and fluorescence. Previous studies demonstrated the effect of lighting and PGRs on the in vitro propagation but few focused on the combined effect of both factors on the micropropagation of C. creticus, a species with significant ecological and pharmaceutical value in the Mediterranean. The objective of this study was to develop appropriate energy-efficient and effective in vitro cultivation practices using the best combination of plant growth regulators and LED light, which could be applied in the floriculture and pharmaceutical industries throughout the various stages of species production.
2. Materials and Methods
2.1. Plant Material
Seeds of C. creticus were collected on 28 July 2022 from native plants of Crete Island (Greece) (35°24′31.3″ N, 24°52′19.8″ E), when the seeds had reached full maturity. The collection was performed from 10 randomly selected individuals. The seeds were transferred to the laboratory and manually separated from the pericarp. They were then placed in paper bags and stored for 4 months under constant conditions in a growth chamber (25 °C; 30% RH; complete darkness). Ιn vitro germination was conducted on half-strength Murashige and Skoog media [34] after boiling the seeds for 5 min in water and placing the tubes in a growth chamber (23 ± 1 °C; 16/8 h light/dark by fluorescent light 50 ± 5 μmol m−2 s−1).
2.2. Effect of Plant Growth Regulators on Shoot Proliferation and Root Initiation
The first part of this study aimed to assess the appropriate plant growth regulator for the formation of multiple micro-shoots. A total of seven different MS media were tested: hormone-free (Hf, with no growth regulator); 5 μM BA; 10 μM BA; 5 μM mT; 10 μM mT; 0.5 μM Mel; and 1 μM Mel. After two cycles subculturing on the same medium, the rooting stage was conducted on the seven MS media as follows: Hf; 5 μM mT; 10 μM mT, 0.5 μM Mel; 1 μM Mel, 0.5 μM IBA; and 1 μM IBA.
2.3. Light Spectra
Inside the growth chamber, each light treatment was separated with cardboard to avoid light contamination among the different light treatments. The properties of light treatments are described in Table 1, while the spectral distributions are presented in Figure 1. Briefly, the control treatment was fluorescent (FL; cool white) lamps versus a monochromatic blue (B) LED, a monochromatic red (R) LED, and a broad-spectrum white (W; cool white) LED. The FL lamp was denoted as the control treatment due to its traditional use in in vitro culture until today. White spectra are popular nowadays because they facilitate plant scouting and general operations in growth chambers where humans work. Monochromatic red or blue lights were selected to examine and isolate the effects of these specific wavelengths to better understand plant responses. The photosynthetic photon flux density (PPFD) was maintained at 50 ± 5 μmol m−2 s−1, while the photoperiod was 16 h. This PPFD was selected upon the literature review, as well as after unpublished preliminary experiments conducted by our group.

Table 1.
The spectra of the light treatments used (these results were obtained with the use of an HD 30.1 spectroradiometer, DeltaOhm Srl, Padova, Italy). The peak wavelengths of the UV (380–399 nm), blue (B) (400–499 nm), green (G) (500–599 nm), red (R) (600–699 nm), far red (FR) (700–780 nm), and red-to-far red (R/FR) ratio, color rendering index (CRI), and correlated color temperature (CCT) of the tested light treatments.
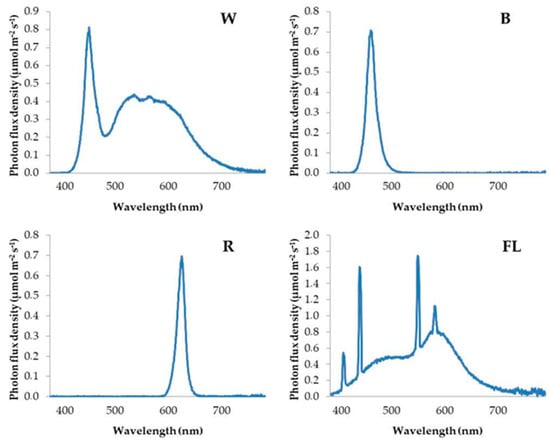
Figure 1.
Spectral distribution of white (W), blue (B), red (R), and fluorescent (FL) light treatments.
2.4. Treatments of Different Light Spectra
This stage was conducted on MS media containing the best growth regulators. The explants during the multiplication stage were incubated under fluorescent lights or R, B, or W LED lights on MS media that were Hf or supplemented with 5 μM of BA or mT. Additionally, the effects of the different light spectra and growth regulator treatments on the in vitro micro-shoot rooting were also studied. A total of 12 treatments were used (30 explants each). The rooted plantlets were cultured on MS media that were Hf or supplemented with 0.5 μM Mel or 1 μM IBA under four different light spectra, as before mentioned.
2.5. Photosynthetic Rate, Chlorophyll Concentration, and Fluorescence Measurement
In weekly intervals during the experiments mentioned above, and for a period of four weeks, the photosynthetic rate (A, μmol m−2 s−1) was measured. The measurements were carried out using the LCi (compact portable photosynthesis system, ADC BioScientific Ltd., Hoddesdon, UK), with the small chamber attachment (2.15 cm2) being used for measuring small leaves. The photosynthetic rate was recorded in situ, inside the growth chamber where the explants were located.
Also, the chlorophyll concentration was measured using the CCM-200 plus (Chlorophyll Content Meter; Opti-Sciences, Hudson, NH, USA). Finally, the chlorophyll fluorescence parameter (the ratio of the variable to the maximum fluorescence after dark adaptation) of the explants (Fv/Fm ratio) was also measured, as described by Martins et al. [35], using the instrument OS30p+ (Opti-Sciences, USA). For the measurements, in all the cases, ten explants per treatment were removed from the protective glass test tube, and their base was placed in deionized water. For the photosynthetic rate and for each treatment, 10 measurements (1 measurement per explant) were recorded, and for the chlorophyll concentration, 15 measurements (1 or 2 measurements per explant) for each treatment were recorded. For the chlorophyll fluorescence, the explants remained for 30 min in a dark place before the measurement, and after that, for each treatment, 20 measurements (2 measurements per explant) were recorded. Young leaves that had reached their full size were used (the 3rd or 4th leaf from the top of the explant).
2.6. Acclimatization
Well-rooted plantlets were transplanted into 1 L pots containing peat (a pH of 5.5–6.5, Klasmann-Delimann Gmbh, Geeste, Germany) and perlite (particle diameter 1–5 mm, Perloflor, ISOCON S.A., Athens, Greece) substrate (2:1, v:v). The plantlets were covered with a transparent membrane (SANITAS; Sarantis S.A., Marousi, Greece) and placed in a plant growth chamber. The temperature for the first week was maintained at 20 °C, and the second week it increased to 25 °C, and the membrane was removed. In the third week, the plantlets were transferred from the growth chamber to a bench in the glass, heated greenhouse of the Floriculture Laboratory of the Aristotle University of Thessaloniki. For the acclimatization, 10 micro-shoots were used per treatment, except in the Hf group under blue LED, where 5 micro-shoots were used due to reduced rooting. The light intensity gradually increased from 65 to 170 μmol−2 s−1 and finally to 340 μmol−2 s−1 over a period of 3 weeks. The relative humidity was gradually reduced from 95% to 80% and finally to 65% over the same period. After 3 weeks, the total number of plantlets from each treatment that were successfully acclimatized was recorded.
2.7. In Vitro Culture Condition
All the culturing of the in vitro seedlings, subcultures, and rooting experiments took place in glass tubes (1 explant/tube) containing 10 mL of solid (7 g L−1 agar) Murashige and Skoog media (MS) [34], supplemented with 20 g L−1 sucrose and covered with a plastic film (SANITAS; Sarantis S.A., Greece). The pH of the medium was adjusted to 5.8. All the cultures were incubated in the growth chamber at 23 ± 1 °C, and a 16/8 h light/dark photoperiod was provided for the different light spectra.
2.8. Statistical Analysis
The preliminary experiment investigated only one factor, the plant growth regulators, with 7 levels for multiple micro-shoot production (Hf (no PGR), 5 μM BA,10 μM BA, 5 μM mT, 10 μM mT, 0.5 μM MEL, and 1 μM MEL), as well as 7 levels for rooting (Hf, 0.5 μM MEL, 1 μM MEL, 0.5 μM IBA, 1 μM IBA, 5 μM mT, and 10 μM mT). For each part (multiple micro-shoot production and rooting), 30 replicates were done in each treatment.
The main experiment consisted of 2 factors.
Factor 1: lighting, with 4 levels.
- (1)
- Fluorescent light (FL) (this light spectrum was considered as the “control” for factor 1).
- (2)
- Red LED (R).
- (3)
- Blue LED (B).
- (4)
- White LED (W).
Factor 2: the type of PGR.
Multiple micro-shoot production (3 levels):
- (1)
- Hf (no PGR).
- (2)
- 5 µM BA.
- (3)
- 5 μM mT.
Rooting (3 levels):
- (1)
- Hf (no PGR).
- (2)
- 0.5 µM melatonin.
- (3)
- 1 μM IBA.
The total number of treatments, when the different nutrient mediums were combined with the light spectra, was 12 (3 × 4) for both multiple micro-shoot production and rooting, and each had 60 replicates. The data collected during the multiplication stage were recorded after 4 weeks, including the total reaction (shoot formation %), the number and length of the developed micro-shoots, and callus formation. The rooting data included the percentage of the micro-shoots that had rooted in each treatment, as well as the number and length of the roots that had developed.
All the parts of the experiments are considered as a completely randomized factorial design (CRD). The data were checked for normality and the homogeneity of variance and was then analyzed using an ANOVA within the GLM (General Linear Model) [36].
The preliminary experiment was analyzed using a one-way ANOVA since there was only 1 factor (PGR’s with 7 levels), and the main experiment was analyzed using a two-way ANOVA since there were 2 factors (PGRs and light). The reaction and rooting percentage data were transformed to arc-sine square values prior to the analysis, in order to achieve a normal distribution. For the statistical analysis of the photosynthetic rate, a two-way ANOVA was carried out for each week of measurements separately, with the photosynthetic rate being the dependent variable. The comparisons were made using a protected LSD test at a significance level of p ≤ 0.05, with the use of Levene’s test to ensure homogeneity. All the statistical analyses were performed using SPSS 29.0 (SPSS, Inc., Chicago, IL, USA).
3. Results
3.1. Effect of Growth Regulators on In Vitro Multiple Micro-Shoot Production
The highest overall reaction and micro-shoot production were achieved with 5 µM mT (90% response, 2.78 micro-shoots/explant) and 5 µM BA (80% response, 2.26 micro-shoots/explant) (Figure 2a,b). The longest micro-shoots were observed with 5 µM BA (2.54 cm) and 5 µM mT (2.19 cm) (Figure 2c). It is also interesting to note that higher concentrations of mT (10 µM) resulted in 100% callus formation, while lower concentrations (5 µM) and BA treatments produced minimal calluses (Figure 2d). These findings indicate that 5 µM mT and 5 μM BA are optimal for micro-shoot production, providing the best balance of reaction, shoot number, and length.
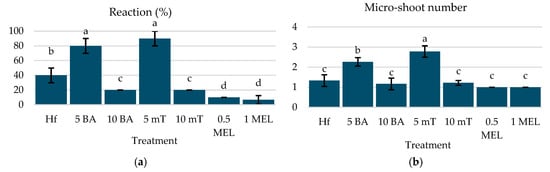
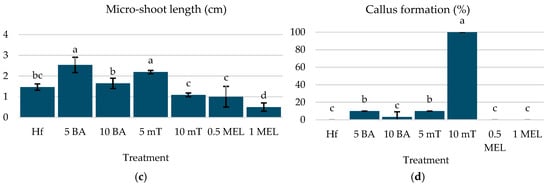
Figure 2.
(a) Reaction (%), (b) micro-shoot number, (c) micro-shoot length (cm), and (d) callus formation of C. creticus explants, four weeks after in vitro establishment under FL lamps on MS media without plant growth regulator (Hf) or with 5 μM BA, 10 μM BA, 5 μM mT, 10 μM mT, 0.5 μM Mel, or 1 μM Mel. The bars represent the standard deviation. The same letters per figure indicate a statistical similarity among the treatments (LSD test, p ≤ 0.05).
3.2. Effect of Growth Regulators on In Vitro Rooting
For the rooting stage of the C. creticus micro-shoots, the optimal treatments were found to be 0.5 µM Mel and 1 µM IBA, which achieved rooting rates of 90% and 80%, respectively, with no significant difference (Figure 3a). These treatments significantly outperformed the Hf treatment (60%) and other treatments, which exhibited minimal or no rooting (Figure 3a). The highest root numbers were observed with 1 µM IBA (3.66 roots per shoot), followed by 0.5 µM Mel (2.33 roots per shoot) (Figure 3b). The root length was also higher with 1 µM IBA (0.75 cm) and 0.5 µM melatonin (0.65 cm), both surpassing the Hf treatment (0.43 cm) (Figure 3c). Higher concentrations of mT (10 µM) entirely inhibited rooting, while lower concentrations showed limited effectiveness.
Figure 3.
(a) Rooting (%), (b) root number, (c) and root length of C. creticus explants four weeks after in vitro establishment under FL lamps on MS nutrient media without plant growth regulator (Hf) or with 0.5 μM Mel, 1 μM Mel, 0.5 μM IBA, 1 μM IBA, 5 μM mT, or 10 μM mT. The bars represent the standard deviation. The same letters per figure indicate a statistical similarity among the treatments (LSD test, p ≤ 0.05).
3.3. Treatments of Growth Regulators Combined with Different Light Treatments
3.3.1. Effect on In Vitro Multiple Micro-Shoot Production
The plantlets of C. creticus cultivated under a R LED showed an improved reaction when 5 µM BA or 5 µM mT was added to the MS medium, significantly outperforming the Hf treatment (63.33%, 66.67%, and 23.33%, respectively) (Figure 4a). Similar results were observed under a B LED, with the BA and mT treatments yielding better results (60% and 53.33%, respectively) compared to the Hf group (16.67%) (Figure 4a). The best response occurred under a W LED, with 5 µM BA and mT obtaining 83.33% and 90% reactions, respectively; both were significantly higher than the Hf group (26.67%) (Figure 4a). FL lighting also supported higher reaction rates (66.67% and 73.33% for BA and mT, respectively) compared to the Hf group (50%) (Figure 4a).
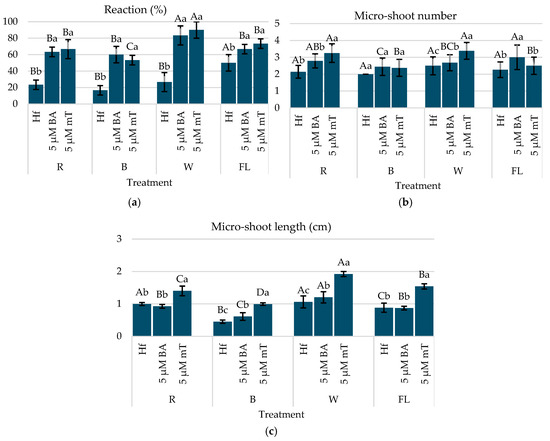
Figure 4.
(a) Reaction (%), (b) micro-shoot number, (c) and micro-shoot length of C. creticus explants four weeks after in vitro establishment under R, B, or W LED or FL lamps on MS nutrient media without plant regulator (Hf) or with 5 μM BA or 5 μM mT. The same capital letters for the factor “lighting” and the same lowercase letters for the factor “growth regulator” indicate statistical similarity among the treatments (LSD test, p ≤ 0.05).
Regarding the micro-shoot development, the highest number of shoots per explant was observed under a R LED with 5 µM mT (3.25 shoots per explant), followed by BA (2.79) and the Hf group (2.14) (Figure 4b). Similar patterns were noted under a W LED, with 5 µM mT producing the most shoots (3.38), significantly surpassing BA (2.68) and the Hf (2.50) group (Figure 4b). The best overall combination for promoting micro-shoot production was 5 µM mT under a W or R LED, achieving the more uniform growth of the micro-shoots and a bushy plantlet morphology.
Shoot length was also enhanced by 5 µM mT, with the longest micro-shoots observed under a W LED (1.92 cm) and a R LED light (1.40 cm), with there being a significant difference from the Hf group and BA treatment (Figure 4c). The B LED had a generally negative effect on micro-shoot growth, and browning was observed in the explants from the Hf group under this light after four weeks. The addition of mT gave a uniform growth and more leaf development, while the BA treatments favored the elongation of the central micro-shoot, with less growth in lateral micro-shoots (Figure 5).

Figure 5.
C. creticus explants in MS nutrient media with (1) no plant growth regulator (Hf), (2) 5 μM mT, or (3) 5 μM BA after four weeks of in vitro culture under a (A) FL lamp, (B) W LED, (C) R LED, or (D) B LED. The yellow bars in the images indicate the size of 1 cm.
The optimal combination for enhancing both in vitro growth and the development of C. creticus micro-shoots was 5 µM mT under a W LED light, as it consistently supported superior micro-shoot production and gave overall better plantlet growth quality.
3.3.2. Effect on In Vitro Rooting
Treatments under a R LED with 0.5 µM Mel (90%) or 1 µM IBA (100%) resulted in the highest rooting percentages, surpassing the Hf group (70%) significantly. A B LED, when combined with 1 µM IBA, also led to significant rooting (83.3%), while a W LED or a FL lamp with 1 µM IBA produced moderate results. The addition of 0.5 µM melatonin under B or W LEDs reduced rooting compared to a R LED or a FL lamp (Figure 6a).
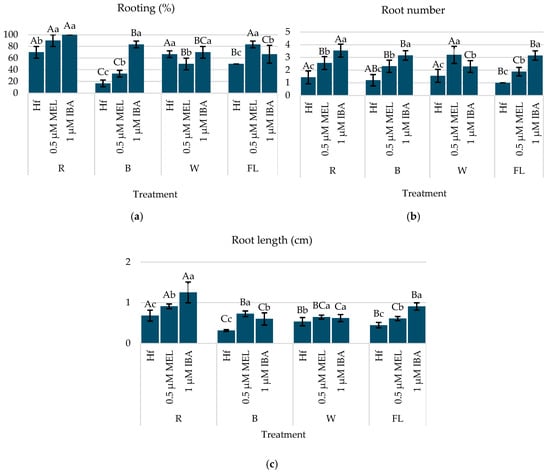
Figure 6.
(a) Rooting (%), (b) root number, and (c) root length of C. creticus explants four weeks after in vitro establishment under R, B, or W LEDs or FL lamps on MS media without plant regulator (Hf) or with 0.5 μM Mel or 1 μM IBA. The bars represent the standard deviation. The same capital letters for the factor “lighting” and the same lowercase letters for the factor “growth regulator” indicate a statistical similarity among the treatments (LSD test, p ≤ 0.05).
Micro-shoots under a R LED with 1 µM IBA exhibited the highest root number (3.53 roots/explant) and the longest root length (1.25 cm), making this the most effective combination for rooting (Figure 6b,c). A B LED with 1 µM IBA also resulted in higher root numbers (3.16 roots/explant) and a longer root length (0.73 cm), while a W LED with 0.5 µM Mel produced the highest root number (3.20 roots/explant) but with moderate a root length (0.64 cm) (Figure 6b,c).
This part of the study highlighted that a R LED with 1 µM IBA gave the most favorable rooting response in terms of both the root number and elongation, supporting its use for the efficient in vitro rooting of C. creticus (Figure 7).

Figure 7.
C. creticus micro-shoots, rooted in MS media with (A) 1μM IBA under R LED, (B) 0.5 μM Mel under R LED, and (C) 0.5 μM Mel under B LED, after four weeks of in vitro culture. The yellow bar in the image indicates the size of 1 cm.
3.3.3. Effect on In Vitro Photosynthetic Rhythm
Multiplication Stage
The effects of different light spectra and plant growth regulators (PGRs) on the photosynthesis rate (A, μmol m−2 s−1) of C. creticus explants showed significant variations in photosynthetic activity across four weeks of culture. The first week showed no significant differences in the A values between the explants grown under the R LED. However, under the B LED, the mT treatment had the highest A values (2.32 μmol m−2 s−1), significantly differing from the explants grown in the MS media with BA (1.93 μmol m−2 s−1) and the Hf group (1.70 μmol m−2 s−1). The explants under the W LED did not show significant differences in A values (Figure 8).
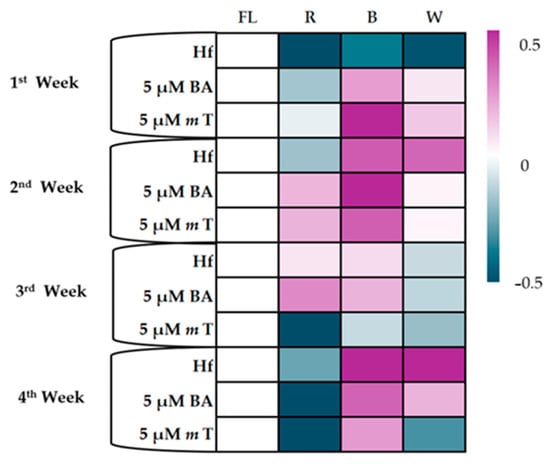
Figure 8.
Heatmap for the photosynthetic rate (A μmol m−2 s−1) of C. creticus explants in in vitro cultures at 1, 2, 3, and 4 weeks in MS nutrient media under lights of different spectra (R, B, and W LEDs and FL lamps): (a) without plant growth regulator (Hf); (b) with 5 μM BA; (c) with 5 μM mT. On the scale, 0 represents the Hf treatment (FL), and values up to 0.5 represent a positive effect of the treatment, while negative values to −0.5 represent the negative effect of the treatment.
In the second week, the R LED increased the A values across all the MS media treatments compared to the Hf F. Under the B LED, 5 μM BA led to the highest A values (3.24 μmol m−2 s−1), significantly differing from the mT (1.89 μmol m−2 s−1) and the Hf groups (1.50 μmol m−2 s−1). The B and W LEDs, without PGRs, gave higher A values (1.50 και 1.48 μmol m−2 s−1) than the FL and R LED (1.15 and 1.02 μmol m−2 s−1, respectively). The mT treatment showed improved photosynthesis rates under B LED but did not significantly differ from the FL and R LED (Figure 8).
By the third week, significant differences in A values were mainly observed under R LED, where explants in MS media with 5 μM BA had significantly higher A values than those with mT (1.88 and 0.54 μmol m−2 s−1, respectively). The explants grown without PGRs or with BA showed no significant differences across the light treatments, while the mT-treated explants under the R LED showed a marked decrease in photosynthesis rates (Figure 8).
In the fourth week, there were no significant differences between each treatment under the R and B LEDs. Under the W LED, the explants with mT showed lower A values than the Hf treatment. The B and W LEDs gave higher A values compared to the R LED, and the explants treated with mT showed improved photosynthesis under the B LED compared to the R or W LEDs (Figure 8).
Overall, the B LED was most effective in enhancing photosynthesis, and there were significant reductions in the A values under the R LED, particularly in the later weeks of the experiment.
Rooting Stage
During the first week of the in vitro rooting of C. creticus micro-shoots on MS media supplemented with 0.5 µM Mel or 1 µM IBA, no significant differences in the photosynthesis rate (A) were observed under the R or W LEDs compared to the Hf group. However, under the B LED, 1 µM of IBA enhanced photosynthesis (2.00 µmol m2 s−1) in comparison to the Hf treatment (1.72 µmol m2 s−1) (Figure 9).
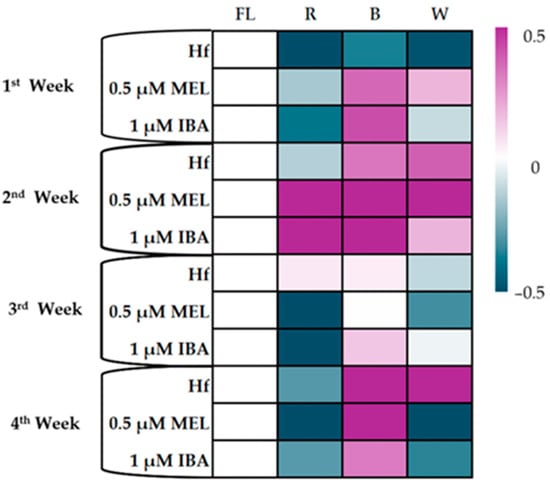
Figure 9.
Heatmap for the photosynthetic rate (A μmol m−2 s−1) of C. creticus explants in in vitro cultures at 1, 2, 3, and 4 weeks in MS nutrient media under lights of different spectra (R, B, or W LEDs or FL lamps): (a) without rooting regulator (Hf); (b) with 0,5 μM melatonin; (c) with 1 μM IBA. On the scale, 0 represents the Hf treatment (FL), and values up to 0.5 represent a positive effect of the treatment, while negative values to −0.5 represent the negative effect of the treatment.
In the second week, under the R LED, all the treatments exhibited higher A values compared to the Hf treatment, with the highest value recorded for the micro-shoots rooted in the MS media with 1 µM IBA (2.93 µmol m2 s−1). Similarly, under the B LED, higher A values were observed in the treatments with 1 µM IBA (2.11 µmol m2 s−1), while under the W LED, the best result was found in micro-shoots with 0.5 µM Mel (2.47 µmol m2 s−1) (Figure 8). In contrast, no statistical differences were noted under the FL lamps (Figure 9).
In the third week, significant differences in the photosynthesis rates were only recorded under the R LED, where all the treatments showed a negative effect on the A values (Figure 7). The lowest A values were recorded in the treatments with 0.5 µM Mel or 1 µM IBA under the R LED (Figure 9).
By the fourth week, a decrease in the photosynthesis rate was observed across all the treatments under the R LED (Figure 7). The B LED maintained higher A values compared to the other treatments, with the best results in the 0.5 µM Mel treatment (1.86 µmol m2 s−1) (Figure 9).
Overall, the B LED consistently supported higher photosynthesis rates, especially in the treatments with 1 µM IBA, while the R LED negatively impacted the photosynthesis rate, particularly in the later weeks of rooting.
3.3.4. Effect on In Vitro Chlorophyll Concentration
Multiplication Stage
The chlorophyll concentration in the C. creticus explants was influenced by both the light spectra and the plant growth regulators during the four-week in vitro culture. Under the R LED, the addition of 5 μM mT to the MS medium negatively affected chlorophyll levels, with lower concentrations observed compared to the Hf group (CCI = 1.39 και 1.77, respectively). In contrast, the B LED promoted higher chlorophyll contents in the explants supplemented with 5 µM BA or 5 µM mT. The W LED had a generally negative impact on the chlorophyll concentration across all the treatments (Table 2).

Table 2.
Chlorophyll concentration (CCI) of C. creticus micro-shoots in MS media with 5 μM BA, 5 μM mT, or without plant growth regulator (Hf) for multiple micro-shoot production and with 0.5 μM Mel, 1 μM IBA, or Hf for rooting, after 4 weeks of in vitro culture under R, B, or W LEDs or FL lamps. The same capital letters for the factor “lighting” and the same lowercase letters for the factor “growth regulator” indicate a statistical similarity among the treatments (LSD test, p ≤ 0.05).
Among the explants in the Hf group, the highest chlorophyll concentration was recorded under the FL lamps (CCI = 2.72), and similar results were observed with 5 µM BA (CCI = 3.03) and 5 µM mT (CCI = 1.61), where the FL lamp resulted in superior chlorophyll accumulation (Table 2).
Rooting Stage
Under the R LED, the addition of melatonin to the MS medium negatively affected chlorophyll levels, which were significantly lower compared to the Hf, while the micro-shoots with 1 µM IBA did not show significant differences compared to the Hf group. Under the B LED, no significant differences were observed across the treatments. However, under the W LED, both melatonin and IBA negatively impacted chlorophyll concentration compared to the Hf group (CCI = 1.72, 1.78 και 2.32, respectively) (Table 2).
The micro-shoots cultivated under the FL lamps showed the highest chlorophyll concentrations, with melatonin negatively affecting the chlorophyll content again and resulting in statistically lower values compared to both the Hf and 1 µM IBA treatments (Table 2). In the absence of PGRs, the micro-shoots exposed to R, B, or W LEDs had lower chlorophyll concentrations (CCI = 1.72, 1.15 και 2.32, respectively) compared to the Hf under the FL lamps (CCI = 2.69) (Table 2).
3.3.5. Effect on In Vitro Chlorophyll Fluorescence
Multipication Stage
In the R LED, 5 μM BA significantly reduced the Fv/Fm (0.567) compared to the Hf group (0.682) (Table 3). Similarly, under the B LED, the addition of 5 μM mT led to statistically lower Fv/Fm values (0.458) than the Hf treatment (0.614) (Table 3). The C. creticus explants grown under the W LED exhibited no significant differences from the Hf group (Table 3). The FL lamp induced the highest Fv/Fm when no plant growth regulators were added (0.703); however, the addition of 5 μM BA to the MS nutrient medium resulted in a significantly reduced Fv/Fm (0.275). In contrast, the treatments with 5 μM BA across all the light spectra improved the Fv/Fm compared to the reduced values observed under the FL lamp (Table 3).

Table 3.
Chlorophyll fluorescence (Fv/Fm) of C. creticus micro-shoots in MS media with 5 μM BA, 5 μM mT, or without plant growth regulator (Hf) for multiple micro-shoot production and with 0.5 μM Mel, 1 μM IBA, or Hf for rooting, after 4 weeks of in vitro culture under R, B, or W LEDs or FL lamps.
Rooting Stage
Under the R LED, no significant differences were observed between the treatments with the plant growth regulators and the Hf treatment. Under the B LED, 1 μM IBA significantly reduced the Fv/Fm (0.321) compared to the Hf treatment (0.625). Under the W LED, melatonin supplementation also reduced Fv/Fm values (0.525) compared to the Hf treatment (0.673) (Table 3).
In contrast, under the FL lamp, the MS media with melatonin and IBA demonstrated a positive interaction with light, resulting in higher Fv/Fm values compared to the Hf treatment (0.733, 0.724, and 0.632, respectively). The micro-shoots without plant growth regulators exhibited no significant differences in the Fv/Fm across lighting conditions. However, the MS nutrient media with 0.5 μM melatonin under the R or W LEDs and 1 μM IBA under the B LED resulted in reduced Fv/Fm values compared to the FL lamps (Table 3).
3.4. Acclimatization Stage
Three weeks after the transplantation of the rooted plantlets, the highest survival rate (70%) was observed in the plantlets that rooted in the previous stage in vitro and were under the R LED in the MS medium with 1 μM IBA; or 0.5 μM Mel under the R LED (60%); or 1 μM IBA and the B LED (60%) (Figure 10 and Figure 11).
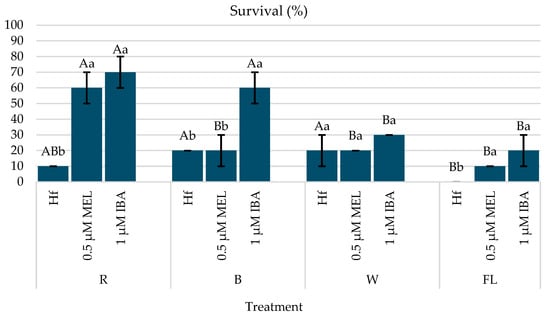
Figure 10.
Survival (%) of C. creticus plantlets after 3 weeks of greenhouse acclimatization on peat and perlite substrate (2:1, v:v) (figure based on in vitro rooting under different light spectra). The bars represent the standard deviation. The same capital letters for the factor “lighting” and the same lowercase letters for the factor “growth regulator” indicate a statistical similarity among the treatments (LSD test, p ≤ 0.05).

Figure 11.
C. creticus plantlet rooted in vitro on MS nutrient medium with 1 μM IBA and under R LED during acclimatization. The bar in the image shows the size of 1 cm.
4. Discussion
In vitro propagation is crucial for the exploitation of native species and the production of certified plant material. New high-value clones can be produced for employment in the floriculture and pharmaceutical industry, offering uniform and high-quality plants with special traits. In terms of sustainability, the use of suitable LED technology could have synergistic effects for the optimization of environmental conditions during the production process [37]. This study aligns with prior research, demonstrating the significant combined influence of specific plant growth regulators (PGRs) and LED lighting on the in vitro cultivation of plants. The use of LEDs not only provided customizable light spectra but also exhibited notable advantages over traditional fluorescent lighting, enhancing plant growth, rooting, and photosynthetic performance under specific conditions.
Meta-Topolin is known to stimulate latent meristematic cells, as noted by Ahmad and Strnad [11]. Furthermore, it has been reported that mT-treated plantlets have more tolerance to abiotic stress, reducing their morpho-physiological disorders [38,39,40]. On the other hand, low concentrations of BA have been used successfully for the in vitro establishment of Cistus crispus [41]. In the present study, the positive effects of BA and mT on shoot proliferation were consistent with previous findings for producing multiple shoots and improving the shoot length. In a recent study, Ioannidis and Koropouli [42] recorded a high proliferation rate for in vitro cultures of C. creticus in terms of shoot number using MS media without plant regulators, in contrast with our findings. Moreover, in the previous study, the initial plant material was excised from actively grown shoots of plants in the wild. This finding highlights the importance of genotype-specific optimization in developing tissue culture protocols for different plant species [42]. In the present study, mT and a W LED enhanced shoot proliferation, in accordance with Badhepuri et al. [43], who established an effective in vitro propagation protocol for Coleus forskohlii under white LEDs. The white spectrum contains wavelengths in the red and blue spectral zones, both of which are essential to drive photosynthesis and trigger photomorphogenic responses. In addition, a CRI of over 50 units (such as our W and FL treatments) enhances tasks within the growth room, such as plant scouting and care, while also creating a more suitable light environment for staff.
The role of IBA in promoting rooting was evident, as supported by earlier studies on many woody species [44]. In this study, a R LED combined with 1 μM IBA yielded the highest rooting success, in accordance with Bertazza et al. [45], who observed an increased root number and length under red light with IBA supplementation. This suggests a synergy between red light and auxin-like PGRs, facilitating root initiation as well as elongation. The auxin distribution is largely mediated through the phytochrome photoreceptors, which are activated by red and far red wavelengths. This increased auxin transport within the plant expectedly contributed to the enhanced root growth. The present findings are in line with previous research, indicating that red light can significantly stimulate root formation in various plant species, enhancing the signaling pathways associated with root induction and leading to improved rooting rates [46]. Moreover, Picea abies seedlings showed greater adventitious root initiation when treated with red light, possibly due to suppressing the jasmonic acid signaling pathway and cytokinin accumulation, two plant regulators known to inhibit root formation [47].
Regarding melatonin’s role as a PGR, it was particularly noteworthy. Arnao and Hernandez-Ruiz [48] highlighted its ability to enhance rooting through mechanisms similar to auxins, a finding that was also observed in this study. However, the concentration-dependent effects of melatonin were evident, aligning with the research on Prunus cerasus [49] and Arabidopsis thaliana [50], which reported optimal rooting at lower concentrations but adverse effects at higher levels.
The light spectrum played a crucial role in the growth and photosynthetic efficiency of C. creticus. The R LED promoted shoot elongation and multiple shoot formation, consistent with findings on Rubus idaeus [23] and Rehmannia glutinosa [51], where red light was shown to stimulate gibberellin activity, facilitating cell elongation. Also, the B LED enhanced photosynthetic rates, as found in studies on Lactuca sativa [52] and Cucumis sativus [53]. This improvement in photosynthetic performance under the B LED is attributed to better-developed chloroplast structures, as reported by Chen et al. [54]. Moreover, blue light, acting through the cryptochrome and phototropin photoreceptors, is known to induce stomatal opening, subsequently leading to increased intercellular CO2 concentration and enhanced photosynthetic rates [55]. In accordance with the present findings, it has been mentioned that a combination of these two special spectra has been used in various ratios for the production of many plant species with promising results [56,57]. FL lamps, while less energy-efficient, demonstrated higher chlorophyll concentrations in certain conditions, as noted in studies on Myrtus communis [32]. However, LEDs, particularly those with B and W spectra, showed promise in optimizing the chlorophyll content, as observed in Vaccinium corymbosum [58] and Rehmannia glutinosa [51]. The absorption peaks of chlorophylls lie within the blue and red wavelengths. In our case, the two treatments that combined blue and red wavelengths, FL lamps and W LEDs, showed high chlorophyll concentrations. In addition, the FL spectrum is considered sub-optimal for photosynthesis and photomorphogenesis due to the high green light emission, which is inefficient in driving the aforementioned processes. It is possible that plantlets grown under the FL light partitioned more energy and nutrients towards chlorophyll biosynthesis in order to increase light capture through the increased number of pigment molecules.
Finally, the acclimatization results indicated a survival rate comparable to previous studies on Cistus species [10]. The plantlets rooted under the R LED exhibited superior survival, aligning with reports by Pela et al. [59] and Zygomala et al. [60] on the successful transition of C. creticus from in vitro to ex vitro conditions.
These findings emphasize the critical interaction between LED lighting and PGRs in optimizing in vitro micropropagation protocols, offering a pathway to more energy-efficient and effective cultivation practices.
Another important note about the implementation of LEDs is the economic aspect for commercial micropropagation. LEDs are known for their energetic efficiency and longer lifespan compared to traditionally employed light sources such as FL lamps. Recent studies involving the production of various ornamental plants (Tillandsia ionantha and Pachyphytum species) demonstrated that despite the greater capital costs of LEDs, the saved energy and enhanced plant production leads to a greater return on investment [14,15].
5. Conclusions
This study highlighted the potential use of different light spectra during different stages of the in vitro micropropagation of C. creticus. The findings revealed that the most effective treatment for shoot proliferation and micro-shoot multiplication was the use of 5 μM mT in combination with a W LED. For rooting induction, the treatment with 1 μM IBA under a R LED resulted in a higher in vitro rooting percentage, as well as an increased root number and length. Still, the last treatment resulted in the highest survival rates for rooted micro-shoots under greenhouse conditions. To our knowledge, the present study demonstrated for the first time that the combined use of mT for shoot proliferation and IBA for rooting with targeted LED lighting is an effective strategy for the in vitro propagation of C. creticus. Further research examining the response surface design could reveal the exact blue-to-red light ratio for optimal plant growth and physiological outcomes, contributing to the optimization of in vitro protocols for the exploitation of the species.
Author Contributions
Conceptualization, S.H. and S.K.; methodology, S.H., F.B., K.B. and S.K.; software, K.B. and C.E.K.; validation, C.E.K.; formal analysis F.B. and C.E.K.; investigation, C.E.K.; resources, C.E.K.; data curation, C.E.K.; writing—original draft preparation, C.E.K., F.B., K.B., S.H. and S.K.; writing—review and editing, C.E.K., F.B., K.B., S.H. and S.K.; visualization, F.B., S.H. and S.K.; supervision, S.H. and S.K.; project administration, S.H. and S.K.; funding acquisition, S.H. and S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Catoni, R.; Gratani, L.; Varone, L. Physiological, Morphological and Anatomical Trait Variations between Winter and Summer Leaves of Cistus Species. Flora-Morphol. Distrib. Funct. Ecol. Plants 2012, 207, 442–449. [Google Scholar] [CrossRef]
- Papaefthimiou, D.; Papanikolaou, A.; Falara, V.; Givanoudi, S.; Kostas, S.; Kanellis, A.K. Genus Cistus: A Model for Exploring Labdane-Type Diterpenes’ Biosynthesis and a Natural Source of High Value Products with Biological, Aromatic, and Pharmacological Properties. Front. Chem. 2014, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Atsalakis, E.; Chinou, I.; Makropoulou, M.; Karabournioti, S.; Graikou, K. Evaluation of Phenolic Compounds in Cistus creticus Bee Pollen from Greece. Antioxidant and Antimicrobial Properties. Nat. Prod. Commun. 2017, 12, 1813–1816. [Google Scholar] [CrossRef]
- Abu-Orabi, S.T.; Al-Qudah, M.A.; Saleh, N.R.; Bataineh, T.T.; Obeidat, S.M.; Al-Sheraideh, M.S.; Al-Jaber, H.I.; Tashtoush, H.I.; Lahham, J.N. Antioxidant Activity of Crude Extracts and Essential Oils from Flower Buds and Leaves of Cistus creticus and Cistus salviifolius. Arab. J. Chem. 2020, 13, 6256–6266. [Google Scholar] [CrossRef]
- Varela-Stasinopoulou, D.S.; Nektarios, P.A.; Ntoulas, N.; Trigas, P.; Roukounakis, G.I. Sustainable Growth of Medicinal and Aromatic Mediterranean Plants Growing as Communities in Shallow Substrate Urban Green Roof Systems. Sustainability 2023, 15, 5940. [Google Scholar] [CrossRef]
- Valbuena, L.; Tarrega, R.; Luis, E. Influence of Heat on Seed Germination of Cistus laurifolius and Cistus ladanifer. Int. J. Wildland Fire 1992, 2, 15–20. [Google Scholar] [CrossRef]
- Raimundo, J.R.; Frazão, D.F.; Domingues, J.L.; Quintela-Sabarís, C.; Dentinho, T.P.; Anjos, O.; Alves, M.; Delgado, F. Neglected Mediterranean Plant Species Are Valuable Resources: The Example of Cistus ladanifer. Planta 2018, 248, 1351–1364. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.C.; Sheng Gerald, L.T.; Teixeira da Silva, J.A. Micropropagation in the Twenty-First Century. Methods Mol. Biol. 2018, 1815, 17–46. [Google Scholar] [CrossRef]
- Madesis, P.; Konstantinidou, E.; Tsaftaris, A.; Nianiou-Obeidat, I. Micropropagation and Shoot Regeneration of Cistus creticus ssp. Creticus. J. Appl. Pharm. Sci. 2011, 1, 54–58. [Google Scholar]
- Iriondo, J.M.; Moreno, C.; Pérez, C. Micropropagation of Six Rockrose (Cistus) Species. HortScience 1995, 30, 1080–1081. [Google Scholar] [CrossRef]
- Ahmad, N.; Strnad, M. (Eds.) Meta-Topolin: A Growth Regulator for Plant Biotechnology and Agriculture; Springer: Singapore, 2021; ISBN 9789811590450. [Google Scholar]
- Iqbal, R.; Khan, T. Application of Exogenous Melatonin in Vitro and in Planta: A Review of Its Effects and Mechanisms of Action. Biotechnol. Lett. 2022, 44, 933–950. [Google Scholar] [CrossRef]
- Soltani, F.; Hasanpour, H.; Hekmati, M. Study of Light Spectrums Effects on Some Growth Parameters and Antioxidant Capacity of Anthemis gilanica. J. Space Sci. Technol. 2021, 14, 15–22. [Google Scholar] [CrossRef]
- Lee, J.H.; Nam, S.Y. Vegetative Propagation of Six Pachyphytum Species as Influenced by Different LED Light Qualities. Hortic. Sci. Technol. 2023, 41, 237–249. [Google Scholar] [CrossRef]
- Lee, J.H.; Jang, I.T.; Kim, E.A.; Shin, E.J.; Lee, S.; Lee, M.; Nam, S.Y. Evaluating the Influence of Various Light Spectra on the Growth and Morphological Responses of Air Plant (Tillandsia ionantha Planch.) Grown under Non-Substrate and Restricted Irrigation Conditions in a Controlled Environment Facility. J. Agric. Life Environ. Sci. 2024, 36, 546–561. [Google Scholar] [CrossRef]
- Brown, C.S.; Schuerger, A.C.; Sager, J.C. Growth and Photomorphogenesis of Pepper Plants under Red Light-Emitting Diodes with Supplemental Blue or Far-Red Lighting. J. Am. Soc. Hortic. Sci. 1995, 120, 808–813. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Z.; Yuan, X.; Chen, X.; Lu, C. A Review on the Effects of Light-Emitting Diode (LED) Light on the Nutrients of Sprouts and Microgreens. Trends Food Sci. Technol. 2020, 99, 203–216. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Barceló-Muñoz, A.; Barceló-Muñoz, M.; Gago-Calderon, A. Effect of LED Lighting on Physical Environment and Microenvironment on in Vitro Plant Growth and Morphogenesis: The Need to Standardize Lighting Conditions and Their Description. Plants 2021, 11, 60. [Google Scholar] [CrossRef]
- Wu, H.-C.; Lin, C.-C. Red Light-Emitting Diode Light Irradiation Improves Root and Leaf Formation in Difficult-to-Propagate Protea cynaroides L. Plantlets in Vitro. HortScience 2012, 47, 1490–1494. [Google Scholar] [CrossRef]
- Lee, M.-J.; Son, K.-H.; Oh, M.-M. Increase in Biomass and Bioactive Compounds in Lettuce under Various Ratios of Red to Far-Red LED Light Supplemented with Blue LED Light. Hortic. Environ. Biotechnol. 2016, 57, 139–147. [Google Scholar] [CrossRef]
- Kozai, T. (Ed.) Smart Plant Factory: The Next Generation Indoor Vertical Farms; Springer: Singapore, 2018; ISBN 9789811310645. [Google Scholar]
- Nacheva, L.; Dimitrova, N.; Koleva-Valkova, L.; Tarakanov, I.; Vassilev, A. Effect of Led Lighting on the Growth of Raspberry (Rubus idaeus L.) Plants in Vitro. Agric. Sci. 2021, 13, 126–140. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Cho, K.M.; Lee, H.Y.; Cho, D.Y.; Lee, G.O.; Jang, S.N.; Lee, Y.; Kim, D.; Son, K.-H. Effects of White LED Lighting with Specific Shorter Blue and/or Green Wavelength on the Growth and Quality of Two Lettuce Cultivars in a Vertical Farming System. Agronomy 2021, 11, 2111. [Google Scholar] [CrossRef]
- Kochetova, G.V.; Avercheva, O.V.; Bassarskaya, E.M.; Kushunina, M.A.; Zhigalova, T.V. Effects of Red and Blue LED Light on the Growth and Photosynthesis of Barley (Hordeum vulgare L.) Seedlings. J. Plant Growth Regul. 2023, 42, 1804–1820. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwon, Y.B.; Roh, Y.H.; Choi, I.-L.; Kim, J.; Kim, Y.; Yoon, H.S.; Kang, H.-M. Effect of Various LED Light Qualities, Including Wide Red Spectrum-LED, on the Growth and Quality of Mini Red Romaine Lettuce (Cv. Breen). Plants 2023, 12, 2056. [Google Scholar] [CrossRef]
- Nhut, D.T.; Takamura, T.; Watanabe, H.; Okamoto, K.; Tanaka, M. Responses of Strawberry Plantlets Cultured in Vitro under Superbright Red and Blue Light-Emitting Diodes (LEDs). Plant Cell Tissue Organ Cult. 2003, 73, 43–52. [Google Scholar]
- Dutta Gupta, S.; Jatothu, B. Fundamentals and Applications of Light-Emitting Diodes (LEDs) in in Vitro Plant Growth and Morphogenesis. Plant Biotechnol. Rep. 2013, 7, 211–220. [Google Scholar] [CrossRef]
- Lian, M.-L.; Murthy, H.N.; Paek, K.-Y. Effects of Light Emitting Diodes (LEDs) on the in Vitro Induction and Growth of Bulblets of Lilium Oriental Hybrid ‘Pesaro’. Sci. Hortic. 2002, 94, 365–370. [Google Scholar] [CrossRef]
- Kurilčik, A.; Miklušytė-Čanova, R.; Dapkūnienė, S.; Žilinskaitė, S.; Kurilčik, G.; Tamulaitis, G.; Duchovskis, P.; Žukauskas, A. In Vitro Culture of Chrysanthemum Plantlets Using Light-Emitting Diodes. Open Life Sci. 2008, 3, 161–167. [Google Scholar] [CrossRef]
- Lin, Y.; Li, J.; Li, B.; He, T.; Chun, Z. Effects of Light Quality on Growth and Development of Protocorm-like Bodies of Dendrobium officinale in Vitro. Plant Cell Tissue Organ Cult. 2011, 105, 329–335. [Google Scholar] [CrossRef]
- Cioć, M.; Szewczyk, A.; Żupnik, M.; Kalisz, A.; Pawłowska, B. LED Lighting Affects Plant Growth, Morphogenesis and Phytochemical Contents of Myrtus communis L. in Vitro. Plant Cell Tissue Organ Cult. 2018, 132, 433–447. [Google Scholar] [CrossRef]
- Bantis, F.; Chatzigeorgiou, I.; Sismanis, M.; Ntinas, G.K.; Koukounaras, A. Vegetable Production in PFALs: Control of Micro-Environmental Factors, Principal Components and Automated Systems. Agriculture 2024, 14, 642. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Martins, J.P.R.; Rodrigues, L.C.A.; Santos, E.R.; Batista, B.G.; Gontijo, A.B.P.L.; Falqueto, A.R. Anatomy and Photosystem II Activity of in Vitro Grown Aechmea blanchetiana as Affected by 1-Naphthaleneacetic Acid. Biol. Plant. 2018, 62, 211–221. [Google Scholar] [CrossRef]
- Gomez, K.; Gomez, A. Statistical Procedures for Agricultural Research; John Wiley & Sons: New York, NY, USA, 1984. [Google Scholar]
- Van Delden, S.H.; SharathKumar, M.; Butturini, M.; Graamans, L.J.A.; Heuvelink, E.; Kacira, M.; Kaiser, E.; Klamer, R.S.; Klerkx, L.; Koostra, G.; et al. Current Status and Future Challenges in Implementing and Upscaling Vertical Farming Systems. Nat. Food 2021, 2, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Aremu, A.O.; Bairu, M.W.; Doležal, K.; Finnie, J.F.; Van Staden, J. Topolins: A Panacea to Plant Tissue Culture Challenges? Plant Cell Tissue Organ Cult. 2012, 108, 1–16. [Google Scholar] [CrossRef]
- Naaz, A.; Hussain, S.A.; Anis, M.; Alatar, A.A. Meta-Topolin Improved Micropropagation in Syzygium cumini and Acclimatization to Ex Vitro Conditions. Biol. Plant. 2019, 63, 174–182. [Google Scholar] [CrossRef]
- Jayaprakash, K.; Manokari, M.; Badhepuri, M.K.; Raj, M.C.; Dey, A.; Shekhawat, M.S. Influence of Meta-Topolin on in Vitro Propagation and Foliar Micro-Morpho-Anatomical Developments of Oxystelma esculentum (L.f.) Sm. Plant Cell Tissue Organ Cult. 2021, 147, 325–337. [Google Scholar] [CrossRef]
- Saia, S.; Giovino, A. An Efficient Protocol for Cistus crispus L. (Cistaceae) Micropropagation. Folia Hortic. 2020, 32, 1–9. [Google Scholar] [CrossRef]
- Ioannidis, K.; Koropouli, P. Effects of Different Media and Their Strengths in in Vitro Culture of Three Different Cistus creticus L. Populations and Their Genetic Assessment Using Simple Sequence Repeat Molecular Markers. Horticulturae 2024, 10, 104. [Google Scholar] [CrossRef]
- Badhepuri, M.K.; Manokari, M.; Cokul Raj, M.; Jogam, P.; Dey, A.; Faisal, M.; Alatar, A.A.; Joshee, N.; Singisala, N.R.; Shekhawat, M.S. Meta-Topolin Enhanced Direct Shoot Organogenesis and Regeneration from Leaf Explants of Coleus Forskohlii (Willd.) Briq. Ind. Crops Prod. 2023, 197, 116584. [Google Scholar] [CrossRef]
- Abdalla, N.; El-Ramady, H.; Seliem, M.K.; El-Mahrouk, M.E.; Taha, N.; Bayoumi, Y.; Shalaby, T.A.; Dobránszki, J. An Academic and Technical Overview on Plant Micropropagation Challenges. Horticulturae 2022, 8, 677. [Google Scholar] [CrossRef]
- Bertazza, G.; Baraldi, R.; Predieri, S. Light Effects on in Vitro Rooting of Pear Cultivars of Different Rhizogenic Ability. Plant Cell Tissue Organ Cult. 1995, 41, 139–143. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Zeng, B.; Jiang, Q.; Wang, S.; Li, X. Growth and Transcriptomics Analysis of Michelia Macclurei Dandy Plantlets with Different LED Quality Treatments. Phyton 2023, 92, 2891–2906. [Google Scholar] [CrossRef]
- Alallaq, S.; Ranjan, A.; Brunoni, F.; Novák, O.; Lakehal, A.; Bellini, C. Red Light Controls Adventitious Root Regeneration by Modulating Hormone Homeostasis in Picea abies Seedlings. Front. Plant Sci. 2020, 11, 586140. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Growth Activity, Rooting Capacity, and Tropism: Three Auxinic Precepts Fulfilled by Melatonin. Acta Physiol. Plant. 2017, 39, 127. [Google Scholar] [CrossRef]
- Sarropoulou, V.N.; Therios, I.N.; Dimassi-Theriou, K.N. Melatonin Promotes Adventitious Root Regeneration in in Vitro Shoot Tip Explants of the Commercial Sweet Cherry Rootstocks CAB-6P (Prunus cerasus L.), Gisela 6 (P. cerasus × P. canescens), and MxM 60 (P. avium × P. mahaleb). J. Pineal Res. 2012, 52, 38–46. [Google Scholar] [CrossRef]
- Hernández, I.G.; Gomez, F.J.V.; Cerutti, S.; Arana, M.V.; Silva, M.F. Melatonin in Arabidopsis thaliana Acts as Plant Growth Regulator at Low Concentrations and Preserves Seed Viability at High Concentrations. Plant Physiol. Biochem. 2015, 94, 191–196. [Google Scholar] [CrossRef]
- Manivannan, A.; Soundararajan, P.; Halimah, N.; Ko, C.H.; Jeong, B.R. Blue LED Light Enhances Growth, Phytochemical Contents, and Antioxidant Enzyme Activities of Rehmannia Glutinosa Cultured in Vitro. Hortic. Environ. Biotechnol. 2015, 56, 105–113. [Google Scholar] [CrossRef]
- Kang, W.H.; Park, J.S.; Park, K.S.; Son, J.E. Leaf Photosynthetic Rate, Growth, and Morphology of Lettuce under Different Fractions of Red, Blue, and Green Light from Light-Emitting Diodes (LEDs). Hortic. Environ. Biotechnol. 2016, 57, 573–579. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; Van Ieperen, W.; Harbinson, J. Blue Light Dose-Responses of Leaf Photosynthesis, Morphology, and Chemical Composition of Cucumis sativus Grown under Different Combinations of Red and Blue Light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Chen, L.; Wang, H.; Gong, X.; Zeng, Z.; Xue, X.; Hu, Y. Transcriptome Analysis Reveals Effects of Red and Blue Light-Emitting Diodes (LEDs) on the Growth, Chlorophyll Fluorescence and Endogenous Plant Hormones of Potato (Solanum tuberosum L.) Plantlets Cultured in Vitro. J. Integr. Agric. 2021, 20, 2914–2931. [Google Scholar] [CrossRef]
- Lim, M.-J.; Murthy, H.N.; Song, H.-Y.; Lee, S.-Y.; Park, S.-Y. Influence of White, Red, Blue, and Combination of LED Lights on In Vitro Multiplication of Shoots, Rooting, and Acclimatization of Gerbera jamesonii Cv. ‘Shy Pink’ Plants. Agronomy 2023, 13, 2216. [Google Scholar] [CrossRef]
- Meng, X.; Wang, Z.; He, S.; Shi, L.; Song, Y.; Lou, X.; He, D. LED-Supplied Red and Blue Light Alters the Growth, Antioxidant Status, and Photochemical Potential of in Vitro-Grown Gerbera jamesonii Plantlets. Hortic. Sci. Technol. 2019, 37, 473–489. [Google Scholar] [CrossRef]
- Silva, M.M.A.; De Oliveira, A.L.B.; Oliveira-Filho, R.A.; Gouveia-Neto, A.S.; Camara, T.J.R.; Willadino, L.G. Effect of Blue/Red LED Light Combination on Growth and Morphogenesis of Saccharum officinarum Plantlets in Vitro. In Proceedings of the Proceedings Volume 8947, Imaging, Manipulation, and Analysis of Biomolecules, Cells, and Tissues XII, SPIE BiOS, San Francisco, CA, USA, 3–6 February 2014; Farkas, D.L., Nicolau, D.V., Leif, R.C., Eds.; p. 89471. [Google Scholar]
- Hung, C.D.; Hong, C.-H.; Kim, S.-K.; Lee, K.-H.; Park, J.-Y.; Nam, M.-W.; Choi, D.-H.; Lee, H.-I. LED Light for in Vitro and Ex Vitro Efficient Growth of Economically Important Highbush Blueberry (Vaccinium corymbosum L.). Acta Physiol. Plant. 2016, 38, 152. [Google Scholar] [CrossRef]
- Pela, Z.; Pentcheva, M.; Gerasopoulos, D.; Maloupa, E. In Vitro Induction of Adventitious Roots and Proliferation of Cistus creticus Creticus L. Plants. Acta Hortic. 2000, 541, 317–322. [Google Scholar] [CrossRef]
- Zygomala, A.M.; Ioannidis, C.; Koropouli, X. In Vitro Propagation of Cistus creticus L. Acta Hortic. 2003, 616, 391–396. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).