Effects and Mechanism of Auxin and Its Inhibitors on Root Growth and Mineral Nutrient Absorption in Citrus (Trifoliate Orange, Poncirus trifoliata) Seedlings via Its Synthesis and Transport Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Growth Conditions
2.2. Experiment Design
2.3. Study of Root Morphology
2.4. Mineral Nutrient Content Analysis
2.5. Root Auxin Content
2.6. Analysis of Gene Expression
2.7. Statistical Analysis
3. Results
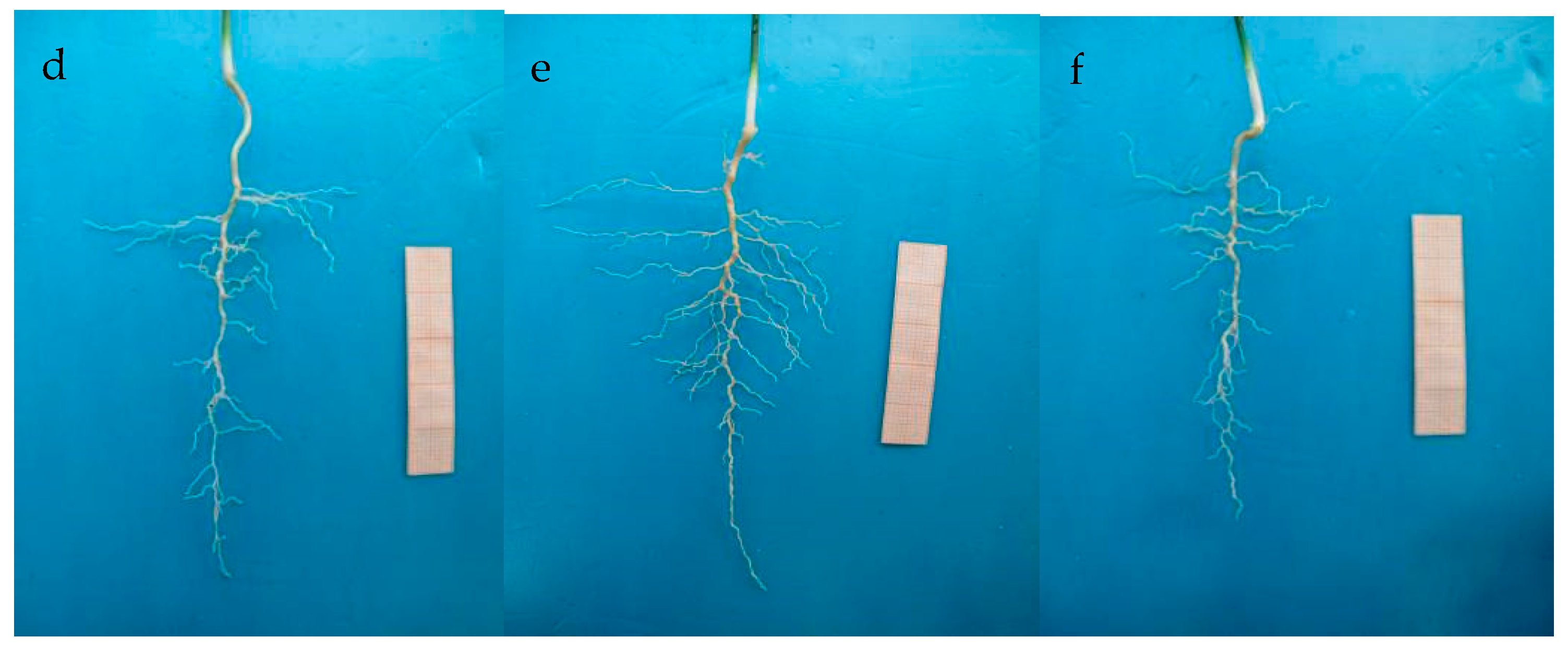
3.1. The Morphology of Trifoliate Orange Seedlings
3.2. The Growth of Main and Lateral Roots
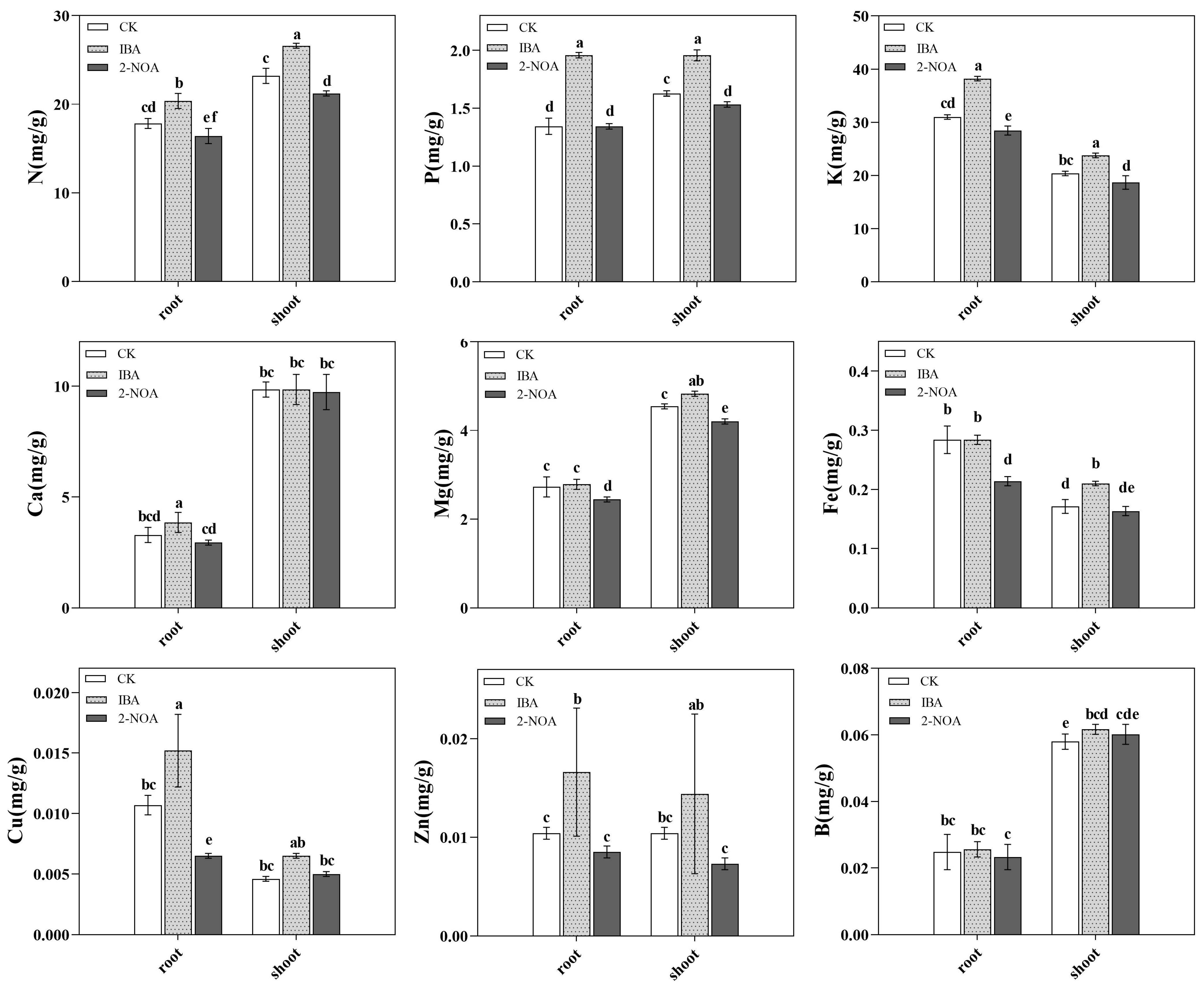
3.3. Variations in Plant Mineral Nutrient Composition
3.4. Measurement of Endogenous Auxin Content in Roots
3.5. The Expression Levels of Root Auxin Biosynthesis and Transport Genes
4. Discussion
4.1. Effect of Auxin on the Root Growth of Trifoliate Orange
4.2. Effect of Auxin on Mineral Nutrients of Trifoliate Orange
4.3. Expression of Auxin and Its Related Genes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.D. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef]
- Cavallari, N.; Artner, C.; Benkova, E. Auxin-regulated lateral root organogenesis. Csh Perspect. Biol. 2021, 13, a039941. [Google Scholar] [CrossRef] [PubMed]
- Lavy, M.; Estelle, M. Mechanisms of auxin signaling. Development 2016, 143, 3226–3229. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Barzee, T.J.; Zhang, R. Citrus: Integrated Processing Technologies for Food and Agricultural by-Products; Academic Press: Cambridge, MA, USA, 2019; pp. 217–242. [Google Scholar] [CrossRef]
- Eekhout, J.P.C.; de Vente, J. Global impact of climate change on soil erosion and potential for adaptation through soil conservation. Earth-Sci. Rev. 2022, 226, 103921. [Google Scholar] [CrossRef]
- Rivas, M.Á.; Friero, I.; Alarcón, M.V.; Salguero, J. Auxin-cytokinin balance shapes maize root architecture by controlling primary root elongation and lateral root development. Front. Plant Sci. 2022, 13, 836592. [Google Scholar] [CrossRef]
- Qin, H.; Huang, R. Auxin controlled by ethylene steers root development. Int. J. Mol. Sci. 2018, 19, 3656. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.W.; Kim, M.-J.; Pandey, S.K.; Oh, E.; Seo, P.J.; Kim, J. Recent advances in peptide signaling during Arabidopsis root development. J. Exp. Bot. 2021, 72, 2889–2902. [Google Scholar] [CrossRef]
- Thelander, M.; Landberg, K.; Muller, A.; Cloarec, G.; Cunniffe, N.; Huguet, S.; Soubigou-Taconnat, L.; Brunaud, V.; Coudert, Y. Apical dominance control by TAR-YUC-mediated auxin biosynthesis is a deep homology of land plants. Curr. Biol. 2022, 32, 3838–3846.e5. [Google Scholar] [CrossRef]
- Kim, H.; Jang, J.; Seomun, S.; Yoon, Y.; Jang, G. Division of cortical cells is regulated by auxin in Arabidopsis roots. Front. Plant Sci. 2022, 13, 953225. [Google Scholar] [CrossRef]
- Swarup, R.; Péret, B. AUX/LAX family of auxin influx carriers—An overview. Front. Plant Sci. 2012, 3, 32237. [Google Scholar] [CrossRef]
- Singh, G.; Retzer, K.; Vosolsobě, S.; Napier, R. Advances in understanding the mechanism of action of the auxin permease AUX1. Int. J. Mol. Sci. 2018, 19, 3391. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.J.; Luo, J. The PIN-FORMED auxin efflux carriers in plants. Int. J. Mol. Sci. 2018, 19, 2759. [Google Scholar] [CrossRef] [PubMed]
- Vosolsobě, S.; Skokan, R.; Petrášek, J. The evolutionary origins of auxin transport: What we know and what we need to know. J. Exp. Bot. 2020, 71, 3287–3295. [Google Scholar] [CrossRef]
- Fageria, N.K.; Moreira, A. The role of mineral nutrition on root growth of crop plants. Adv. Agron. 2011, 110, 251–331. [Google Scholar] [CrossRef]
- Pandey, R. Mineral nutrition of plants. Photosynth. Res. 2015, 46, 499–538. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Potassium control of plant functions: Ecological and agricultural implications. Plants. 2021, 10, 419. [Google Scholar] [CrossRef]
- Farhat, N.; Elkhouni, A.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Rabhi, M. Effects of magnesium deficiency on photosynthesis and carbohydrate partitioning. Acta Physiol. Plant. 2016, 38, 145. [Google Scholar] [CrossRef]
- Wang, J.; Yang, S. Dose-dependent responses of Arabidopsis thaliana to zinc are mediated by auxin homeostasis and transport. Environ. Exp. Bot. 2021, 189, 104554. [Google Scholar] [CrossRef]
- Borah, S.; Baruah, A.M.; Das, A.K.; Borah, J. Determination of mineral content in commonly consumed leafy vegetables. Food Anal. Method. 2009, 2, 226–230. [Google Scholar] [CrossRef]
- Kramberger, B.; Gselman, A.; Janzekovic, M.; Kaligaric, M.; Bracko, B. Effects of cover crops on soil mineral nitrogen and on the yield and nitrogen content of maize. Eur. J. Agron. 2009, 31, 103–109. [Google Scholar] [CrossRef]
- Ali, B.; Sabri, A.; Ljung, K.; Hasnain, S. Auxin production by plant associated bacteria: Impact on endogenous IAA content and growth of Triticum aestivum L. Lett. Appl. Microbiol. 2009, 48, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Chen, L.-L.; Ruan, X.; Chen, D.; Zhu, A.; Chen, C.; Bertrand, D.; Jiao, W.-B.; Hao, B.-H.; Lyon, M.P.; et al. The draft genome of sweet orange (Citrus sinensis). Nat. Genet. 2013, 45, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-H.; Yuan, S.-D.; Tong, C.-L.; Zhang, D.-J.; Huang, R.-H. Ethylene modulates root growth and mineral nutrients levels in trifoliate orange through the auxin-signaling pathway. Not. Bot. Horti Agrobo. 2023, 51, 13269. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhry, S.; Kepinski, S. Auxin in root development. CSH Perspect. Biol. 2022, 14, a039933. [Google Scholar] [CrossRef]
- Robert, H.S.; Friml, J. Auxin and other signals on the move in plants. Nat. Chem. Biol. 2009, 5, 325–332. [Google Scholar] [CrossRef]
- Saini, S.; Sharma, I.; Kaur, N.; Pati, P.K. Auxin: A master regulator in plant root development. Plant Cell Rep. 2013, 32, 741–757. [Google Scholar] [CrossRef]
- Bouain, N.; Krouk, G.; Lacombe, B.; Rouached, H. Getting to the root of plant mineral nutrition: Combinatorial nutrient stresses reveal emergent properties. Trends Plant Sci. 2019, 24, 542–552. [Google Scholar] [CrossRef]
- Chen, J.; Liu, L.; Wang, Z.; Zhang, Y.; Sun, H.; Song, S.; Bai, Z.; Lu, Z.; Li, C. Nitrogen fertilization increases root growth and coordinates the root–shoot relationship in cotton. Front. Plant Sci. 2020, 11, 880. [Google Scholar] [CrossRef]
- Xu, Q.; Fu, H.; Zhu, B.; Hussain, H.A.; Zhang, K.; Tian, X.; Duan, M.; Xie, X.; Wang, L. Potassium improves drought stress tolerance in plants by affecting root morphology, root exudates, and microbial diversity. Metabolites 2021, 11, 131. [Google Scholar] [CrossRef]
- Omondi, J.O.; Lazarovitch, N.; Rachmilevitch, S.; Yermiyahu, U. Phosphorus affects storage root yield of cassava through root numbers. J. Plant Nut. 2019, 42, 2070–2079. [Google Scholar] [CrossRef]
- Salas-González, I.; Reyt, G.; Flis, P.; Custódio, V.; Gopaulchan, D.; Bakhoum, N.; Dew, T.P.; Suresh, K.; Franke, R.B.; Dangl, J.L.; et al. Coordination between microbiota and root endodermis supports plant mineral nutrient homeostasis. Science 2021, 371, eabd0695. [Google Scholar] [CrossRef] [PubMed]
- Phuphong, P.; Cakmak, I.; Yazici, A.; Rerkasem, B.; Prom-U-Thai, C. Shoot and root growth of rice seedlings as affected by soil and foliar zinc applications. J. Plant Nut. 2020, 43, 1259–1267. [Google Scholar] [CrossRef]
- Laňková, M.; Smith, R.S.; Pešek, B.; Kubeš, M.; Zažímalová, E.; Petrášek, J.; Hoyerová, K. Auxin influx inhibitors 1-NOA, 2-NOA, and CHPAA interfere with membrane dynamics in tobacco cells. J. Exp. Bot. 2010, 61, 3589–3598. [Google Scholar] [CrossRef]
- Zhang, D.-J.; Yang, Y.-J.; Liu, C.-Y.; Zhang, F.; Hu, W.; Gong, S.-B.; Wu, Q.-S. Auxin modulates root-hair growth through its signaling pathway in citrus. Sci. Hortic. 2018, 236, 73–78. [Google Scholar] [CrossRef]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Chen, Y. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef]
- Ye, X.; Chen, X.-F.; Deng, C.-L.; Yang, L.-T.; Lai, N.-W.; Guo, J.-X.; Chen, L.-S. Magnesium-deficiency effects on pigments, photosynthesis and photosynthetic electron transport of leaves, and nutrients of leaf blades and veins in Citrus sinensis seedlings. Plants 2019, 8, 389. [Google Scholar] [CrossRef]
- Adil, M.; Bashir, S.; Bashir, S.; Aslam, Z.; Ahmad, N.; Younas, T.; Asghar, R.M.A.; Alkahtani, J.; Dwiningsih, Y.; Elshikh, M.S. Zinc oxide nanoparticles improved chlorophyll contents, physical parameters, and wheat yield under salt stress. Front. Plant Sci. 2022, 13, 932861. [Google Scholar] [CrossRef]
- Li, X.; Kang, H.; Chen, S.; Bai, M.; Li, F.; Liu, T.; Zhou, W.; Aladejana, J.T.; Li, J. Gln-Lys isopeptide bond and boroxine synergy to develop strong, anti-mildew and low-cost soy protein adhesives. J. Clean. Prod. 2023, 397, 136505. [Google Scholar] [CrossRef]
- Yang, X.M.; Ren, G.M.; Luo, K.J. Effects of Sepiolite and Mycorrhizals on Nutrient Element Absorptions of Maize in Heavy Metals Contaminated Soil. Adv. Mater. Res. 2013, 800, 153–158. [Google Scholar] [CrossRef]
- Jiang, H.-B.; Lu, X.-H.; Deng, B.; Liu, L.-M.; Qiu, B.-S. Adaptive mechanisms of the model photosynthetic organisms, cyanobacteria, to iron deficiency. In Microbial Photosynthesis; Wang, Q., Ed.; Springer: Singapore, 2020; pp. 197–244. [Google Scholar] [CrossRef]
- Rawat, J.; Pandey, N.; Saxena, J. Role of potassium in plant photosynthesis, transport, growth and yield. In Role of Potassium in Abiotic Stress; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–14. [Google Scholar] [CrossRef]
- Peaucelle, A.; Braybrook, S.; Höfte, H. Cell wall mechanics and growth control in plants: The role of pectins revisited. Front. Plant Sci. 2012, 3, 121. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Scheres, B. Lateral root formation and the multiple roles of auxin. J. Exp. Bot. 2018, 69, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Leftley, N.; Banda, J.; Pandey, B.; Bennett, M.; Voß, U. Uncovering how auxin optimizes root systems architecture in response to environmental stresses. Csh Perspect. Biol. 2021, 13, a040014. [Google Scholar] [CrossRef]
- Habets, M.E.J.; Offringa, R. PIN-driven polar auxin transport in plant developmental plasticity: A key target for environmental and endogenous signals. New Phytol. 2014, 203, 362–377. [Google Scholar] [CrossRef]
- Della Rovere, F.; Fattorini, L.; D’Angeli, S.; Veloccia, A.; Falasca, G.; Altamura, M.M. Auxin and cytokinin control formation of the quiescent centre in the adventitious root apex of Arabidopsis. Ann. Bot. 2013, 112, 1395–1407. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, L.; Qin, J.; Wan, J.; Wang, R.; Li, S.; Xu, J. cGMP is involved in Zn tolerance through the modulation of auxin redistribution in root tips. Environ. Exp. Bot. 2018, 147, 22–30. [Google Scholar] [CrossRef]
- Xi, D.; Chen, X.; Wang, Y.; Zhong, R.; He, J.; Shen, J.; Ming, F. Arabidopsis ANAC092 regulates auxin-mediated root development by binding to the ARF8 and PIN4 promoters. J. Integr. Plant Biol. 2019, 61, 1015–1031. [Google Scholar] [CrossRef]




| Gene | Accession No. | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Amplification Size (bp) |
|---|---|---|---|---|
| ABCB1 | Ciclev10010916m | GAGCCATTCACGCCACTTC | TCTTGTAACCGAGCCTTTGAGC | 186 |
| ABCB19 | Ciclev10010931m | GCATGAGTTTGGGTCAGTCTTT | CATCTTCCATTTGTTGGGTCTT | 127 |
| AUX1 | Ciclev10011596m | CTTGACTCTGCCCTATTCATTCTC | TGGACCCAGTAACCCATCAAGC | 205 |
| LAX1 | Ciclev10031413m | TTGGCGGACATGCAGTGAC | CAGCGGCAGCAGAAGGAAT | 123 |
| LAX2 | Ciclev10028271m | TGTGGGAAGATGGGTAGGGAC | TAGTCATGCTCGCCCACCC | 98 |
| LAX3 | Ciclev10001072m | ATCACTTTCGCTCCTGCTGC | CAAACCCAAATCCCACCACTA | 133 |
| PIN1 | Ciclev10007787m | GCTTTGGCAACAGAAGAGGATT | ATTACACTTGTCGGCGGCATA | 94 |
| PIN3 | orange1.1g006199m | CATGCCTCCAGCGAGTGTTAT | TGCCACCTGAAAGCGATTAGA | 126 |
| PIN4 | Ciclev10012938m | ATGGGGTTGAAAACGAAGGG | CCTGATAAGTTTCCTCCACACCA | 167 |
| TAR2 | Ciclev10020085m | CACACACGGCACACCCCTA | GCCTCCCACTCCCCAGATC | 137 |
| YUC3 | Ciclev10006828m | CCTTCAGGTTTAGCCGTTGC | GGAAGTTTGGAAGTTGGCAGA | 157 |
| YUC4 | Ciclev10008466m | GACCATCTGGGTTAGCCGTTT | GTATTTTGGGAAGTTTTCAGGGA | 185 |
| YUC6 | Ciclev10008473m | GTGGTTGCTAAAGTGGCTGC | GTTGAAGGGGACCCAAAAGA | 122 |
| YUC8 | Ciclev10020503m | GTGATAATGGTAGGGGCAGGA | GAATGGCAGGTGAGGGAGC | 183 |
| β-actin | Ciclev10025866m | CCGACCGTATGAGCAAGGAAA | TTCCTGTGGACAATGGATGGA | 190 |
| Treatment | Tap Root Length (cm) | Tap Root Diameter (cm) | Lateral Root Length (cm) | Lateral Root Number (#) |
|---|---|---|---|---|
| CK | 5.24 ± 1.48 b | 0.11 ± 0.01 b | 0.39 ± 0.08 b | 1.80 ± 0.44 b |
| 1.0 µmol·L−1 IBA | 6.16 ± 0.39 a | 0.13 ± 0.01 a | 0.87 ± 0.22 a | 3.40 ± 1.63 a |
| 50 µmol·L−1 2-NOA | 4.12 ± 0.14 c | 0.10 ± 0.01 b | 0.35 ± 0.03 c | 1.02 ± 0.10 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Shi, Y.; Tong, C.; Zhang, D. Effects and Mechanism of Auxin and Its Inhibitors on Root Growth and Mineral Nutrient Absorption in Citrus (Trifoliate Orange, Poncirus trifoliata) Seedlings via Its Synthesis and Transport Pathways. Agronomy 2025, 15, 719. https://doi.org/10.3390/agronomy15030719
Yang Y, Shi Y, Tong C, Zhang D. Effects and Mechanism of Auxin and Its Inhibitors on Root Growth and Mineral Nutrient Absorption in Citrus (Trifoliate Orange, Poncirus trifoliata) Seedlings via Its Synthesis and Transport Pathways. Agronomy. 2025; 15(3):719. https://doi.org/10.3390/agronomy15030719
Chicago/Turabian StyleYang, Yuwei, Yidong Shi, Cuiling Tong, and Dejian Zhang. 2025. "Effects and Mechanism of Auxin and Its Inhibitors on Root Growth and Mineral Nutrient Absorption in Citrus (Trifoliate Orange, Poncirus trifoliata) Seedlings via Its Synthesis and Transport Pathways" Agronomy 15, no. 3: 719. https://doi.org/10.3390/agronomy15030719
APA StyleYang, Y., Shi, Y., Tong, C., & Zhang, D. (2025). Effects and Mechanism of Auxin and Its Inhibitors on Root Growth and Mineral Nutrient Absorption in Citrus (Trifoliate Orange, Poncirus trifoliata) Seedlings via Its Synthesis and Transport Pathways. Agronomy, 15(3), 719. https://doi.org/10.3390/agronomy15030719





