Abstract
The high application rate and low utilization efficiency of inorganic phosphorus (Pi) fertilizer could lead to significant P accumulation in soil. Soil P cycling is greatly affected by the planting time in perennial fruit yards. However, the mechanism by which soil Pi fractions and pqqC-harboring bacterial communities, and their relationships, are affected by the planting time of fruit vines, remains unclear. Here, the soil Pi fractions, the pqqC-harboring bacterial communities, and their relationships in the grape yards with 0.5, 4, 16 and 22 growth years, designated as Y0.5, Y4, Y16 and Y22, were examined. The results showed that with the increasing growth years, soil organic carbon (SOC) contents and pH values, respectively, increased and decreased. In addition, the contents and percentages of soil labile Pi and moderately labile Pi increased, whereas those of soil stable Pi decreased. In the soils of Y4, Y16 and Y22, the abundance and α-diversity of pqqC decreased compared to the soils of Y0.5. In the soils of Y16, the composition of pqqC-harboring bacterial communities was altered significantly, showing a great difference compared to the soils of Y0.5, Y4 and Y22. At genus level, the relative abundance of pqqC-harboring bacteria was highly correlated with soil P fractions. Further structural equation modeling revealed that the relationships between the abundance and community richness of the pqqC gene and soil Pi transformation were regulated by soil pH. These findings suggest that changes in soil Pi fractions are closely associated with soil pH, pqqC gene abundance, pqqC-harboring bacterial community richness and SOC content in grape orchards with different planting years.
1. Introduction
Phosphorus (P) plays a vital role in grape growth, soil fertility and agricultural production. However, P is easily fixed by metal cations, or sorbed onto mineral surfaces, or immobilized by microorganisms within soil, consequently resulting in low P availability [,,,,]. Thus, ample mineral P fertilizer is continually applied to soil to maintain the maximum level of grape yields and economic benefits. The added inorganic P (Pi) fertilizer will first increase soil labile Pi and then, part of it is rapidly transformed into moderately labile Pi and stable Pi [,]. These scenarios lead to a significant accumulation of P in perennial grape yard soil, which further triggers the exhaustion of finite rock phosphate and environmental problems such as water eutrophication [,,]. A meta-analysis on the global fate of Pi fertilizer added to terrestrial ecosystems showed that the global annual Pi fertilizer consumption rose from 5 million tons (Mt) in 1961 to about 25 Mt in 2020, and it is expected to hit 27 Mt in 2050 []. Meanwhile, the meta-analysis also revealed that 67.2% of Pi fertilizer was stored in soils and 4.4% was lost via runoff and leaching. China was the country with the largest Pi fertilizer consumption in 2022, with nearly 11 Mt, followed by India and Brazil, with 7.92 and 5.81 Mt, respectively []. Accordingly, elucidating the dynamics and transformation of various Pi fractions is essential and crucial for predicting soil P availability and supply potential in grape yards.
The changes in soil Pi fractions are highly affected by a wide range of factors such as fertilization strategies, soil pH, soil organic carbon (SOC), soil microorganisms and grape planting years [,,,,]. Specifically, long-term (over 10 years) application of P fertilizer resulted in an accumulation of proportions of easily soluble P, aluminum-P and iron-P, which enhanced P bioavailability and environmental risk in the acidic soil of pomelo orchards []. Similar results indicated that the microbial turnover of bioavailable Pi pools (H2O-Pi, NaHCO3-Pi and NaOH-Pi) was facilitated after 27-year P fertilization due to the higher microbial activities []. Moreover, nitrogen (N)-induced soil acidification could increase the concentrations of labile Pi and moderately occluded Pi and accelerate the conversion of recalcitrant Pi into labile and moderately occluded Pi- [,]. A decrease in soil pH could reduce the adsorption of mineral ions to phosphates, consequently promoting the solubilization of inorganic phosphates [,,]. Additionally, SOC also has a profound impact on the dynamics and transformation of soil Pi fractions [,]. For instance, Zhang et al. [] found a significant and positive correlation between soil Pi (especially labile Pi) fractions and SOC, which was likely related to the competitive sorption and complexations between SOC and P, as well as the microbial mineralization of soil organic P (Po).
Microorganisms are integral drivers in soil P cycling because they can improve soil P availability through Po mineralization and Pi solubilization [,,,,]. Therefore, soil Pi solubilization is an important process to enhance soil available P. In this process, the bacterial gene pqqC, encoding a pyrroloquinoline quinone synthase, plays an important role and has been widely used as a bioindicator for mediating soil Pi dissolution [,,,]. The pqqC-harboring bacterial communities have been shown to be affected by soil pH, SOC and fertilizer regimes, which will subsequently influence the transformation and availability of soil Pi [,,]. Besides, grape planting years can also change soil P forms and soil microorganisms. In the 8-year-old vineyard soil, the structure of bacterial communities altered significantly, and the highest concentration of available P (AP) was also found, while soil total P (TP) content gradually increased after 3, 8 and 20 growth years []. In addition, soil TP and AP contents decreased after 12 years of cultivation with grapes compared to 7 years, and long-term continuous cropping reduced the diversity and richness of soil microbial communities []. Moreover, the α-diversity of bacterial communities in the topsoil was significantly and positively correlated with AP and TP in four vineyards with different reclamation years of 5, 10, 15 and 20 years []. Hence, the dynamics and conversion of soil Pi fractions substantially rely on soil physicochemical properties along with the microbial community in grape yards with different planting years.
China’s grape-planting area was about 780,000 hectares in 2021, ranking third globally []. Yantai has been identified as one of the most appropriate areas in the world for wine grape growing and high-end wine making. However, most grape yards are undergoing certain phenomena such as long-term excessive fertilizer addition, soil acidification, continuous cropping and the input of vine leftovers and leaf litter to soil as grape planting years progress, which will alter soil physicochemical properties and microbial communities and further impact soil Pi fractions [,,,]. Nevertheless, it remains poorly understood how soil physicochemical properties and pqqC-harboring bacterial communities regulate soil Pi fractions in grape orchards with different planting years in the test area. Therefore, the objectives of this study were to investigate (1) the changes in soil properties, different soil Pi fractions and pqqC-harboring bacterial communities under different planting years, and (2) the relationships between soil properties, pqqC-harboring bacterial communities and soil Pi fractions. We hypothesized that (1) SOC content and pH value, respectively, increased and decreased with increasing planting years; (2) soil labile Pi and moderately labile Pi increased, accompanied by decreases in soil stable Pi with increasing planting years; and (3) soil Pi fractions would be significantly influenced by soil physicochemical properties and pqqC-harboring bacterial communities in grape orchards with different planting years.
2. Materials and Methods
2.1. Site Description and Soil Sampling
This study was conducted in Penglai District (37°45′ N, 120°50′ E), Yantai City, Shandong Province, China. This area is characterized by a typical warm temperate continental monsoon climate, with a mean annual temperature of 12.9 °C and mean annual precipitation of 661 mm. The soil is classified as brown soil (Udic Luvisols, WRB) with sandy loam texture.
Soil sampling was carried out in September 2022. Four different grape-planting years were selected for this study: 0.5 years (Y0.5, newly established), 4 years (Y4), 16 years (Y16) and 22 years (Y22). Each grape-planting year involves four grape yards, representing four repetitions. Some potential confounding effects in soil spatial variations might still exist, even though all grape yards were subject to the same fertilization regime, cultivation and other agronomic strategies. Therefore, we tried to minimize such effects by selecting the grape yards located on similar geomorphologic units. Each grape yard was at least 1 km away from the others. Within each grape yard, five soil cores (5 cm in diameter) were sampled randomly at depth of 0–20 cm using an auger. Then, these five soil cores were blended into one homogenized composite soil sample, which was passed through a 2-mm sieve. A portion of each soil sample was stored at −80 °C for DNA extraction and high-throughput sequencing, while another portion of soil was preserved at 4 °C for measuring soil microbial biomass. The remaining samples were airdried for chemical analysis, such as soil pH, SOC and P fractions.
2.2. Soil Properties Analysis
Soil pH was determined by a digital pH meter (soil: water = 1:2.5). The SOC content was determined using a K2Cr2O7 oxidation method []. Soil P fractions were analyzed using a modified sequential extraction method [,]. Briefly, 0.5 g air-dried soil was successively extracted by an anion exchange resin (Resin-IP), 0.5 M NaHCO3 (NaHCO3-IP and NaHCO3-OP), 0.1 M NaOH (NaOH1-IP and NaOH1-OP), 1 M HCl (HCl-IP) and 0.1 M NaOH (NaOH2-IP and NaOH2-OP). Subsequently, the residual soil was digested with H2SO4-H2O2 to analyze Residue-P []. Concentrations of soil P fractions were measured using molybdenum blue colorimetric method []. Based on the availability of P to plants and microbes [], Pi fractions were further classified as labile Pi (Resin-IP and NaHCO3-IP), moderately labile Pi (NaOH1-IP) and stable Pi (HCl-IP and NaOH2-IP), while NaHCO3-OP, NaOH1-OP and NaOH2-OP represent labile Po, moderately labile Po and stable Po, respectively.
2.3. Soil DNA Extraction and pqqC Gene Quantification
The extraction of soil total DNA from 0.50 g of each sample was carried out by using the FastDNA® Spin Kit for Soil (MP Biomedicals, Solon, OH, USA). The analysis of the concentration and quality of the extracted DNA was performed with a NanoDrop2000 device (Thermo Fisher Scientific, Wilmington, DE, USA) and via 1% agarose gel electrophoresis. The absolute abundance of pqqC gene was determined by qPCR with the primer Fw (5′-AACCGCTTCTACTACCAG-3′) and Rv (5′-GCGAACAGCTCGGTCAG-3′) []. The reaction system included 5 μL of 2× ChamQ SYBR Color qPCR Master Mix, 0.4 μL of each primer, 1 μL of DNA template, 0.2 μL of 50× ROX Reference Dye 1 and 3 μL of ddH2O. The amplification protocol involved an initial denaturation phase at 95 °C for 3 min, followed by 40 cycles of 95 °C for 5 s, 58 °C for 30 s and 72 °C for 1 min. The standard curve was made by performing serial dilutions (10-fold) of cloned plasmids, and the copy number of the pqqC gene was expressed as per gram of dry soil according to the standard curve. The amplification efficiency was 100.71% with an R2 value equaling 0.999.
2.4. High-Throughput Sequencing and Sequences Analysis
Partial fragments of the bacterial pqqC gene were amplified for all samples using the primer in the qPCR experiment. The amplification procedure comprised an initial denaturation for 3 min at 95 °C, followed by 35 cycles (30 s at 95 °C, 30 s at 55 °C and 45 s at 72 °C), as well as a final extension of 10 min at 72 °C. The sequencing was carried out using the Illumina MiSeq platform (Majorbio, Shanghai, China). The raw sequences were merged with FLASH 1.2.11 (https://ccb.jhu.edu/software/FLASH/index.shtml 28 September 2023), and subsequently, the merged sequences underwent filtering within QIIME 1.9.1 [] (http://qiime.org/install/index.html 12 October 2023). Clean sequences were clustered at 97% similarity [] to generate representative operational taxonomic units (OTUs) sequences using UPARSE 11.0 [] (http://www.drive5.com/uparse/ 8 March 2024). Upon normalizing the sample that had the lowest number of sequences, the α-diversity estimated by Chao1 and Shannon was computed by QIIME 1.9.1 (http://qiime.org/install/index.html 20 March 2024). Principal coordinate analysis (PCoA) based on the Bray–Curtis distance was performed to assess the dissimilarities in the composition of pqqC-harboring bacterial communities across grape orchards with different planting years using the “vegan” package in R 4.4.1 (http://www.r-project.org 30 May 2024), and the significance of the results was tested by permutational multivariate analysis (PERMANOVA). The scores of the first axis in PCoA for each sample served as the indicators representing the community structure of the pqqC gene in subsequent analysis. The biomarkers corresponding to different planting years were detected by the linear discriminant analysis (LDA) effect size (LEfSe) algorithm with the Kruskal–Wallis test showing logarithmic LDA > 4.0 and p < 0.05. The correlation network between pqqC-harboring genera (with relative abundance in the top 50) and soil P fractions was constructed based on Spearman’s correlations; then, correlations with p < 0.05 and |coefficient| > 0.6 were visualized in the network using Networkx 1.11 (https://networkx.org/documentation/networkx-1.11/install.html 7 June 2024). The calculation of betweenness centrality was carried out to reflect the positions and roles of the actors in the network [,,] (Table A1).
2.5. Statistical Analysis
One-way ANOVA with a Duncan test (p < 0.05) was performed to analyze the effects of planting years on soil parameters (soil pH, SOC and soil P fractions, as well as the abundance, Chao1 and Shannon indices of pqqC gene). The aforesaid statistical analyses were executed with SPSS 26.0 (SPSS, Chicago, IL, USA). To test the normality of data, we fitted the data to a normal distribution and evaluated the goodness of fit via the Shapiro–Wilks test. Structural equation modeling (SEM) was established based on a priori model to explore the relationship between soil properties, pqqC-harboring bacterial communities and soil Pi fractions. The scores of the first axis in PCoA for each sample served as the indicators of soil Pi fractions (Figure A1). The model fit was assessed using the maximum likelihood χ2 test, comparative fit index (CFI), goodness of fit (GFI) and root square mean error of approximation (RMSEA). A nonsignificant χ2 test (p > 0.05) indicated adequate model fit. As the calculated standardized coefficients, the path coefficients were determined using the correlation matrices analysis. All the SEM analyses were conducted by Amos 21.0 (IBM, SPSS, New York, NY, USA).
3. Results
3.1. Soil Properties and P Fractions
Soil properties exhibited changes among grape orchards with different planting years (Figure 1). Compared with Y0.5 and Y4, Y16 and Y22 significantly increased SOC concentration, but decreased soil pH (Figure 1A,B, p < 0.05). The effects of planting years on the concentrations and proportions of soil P fractions were significantly different (Table 1, Figure 2, p < 0.05). The concentrations and proportions of soil resin-IP, NaHCO3-IP, labile-IP, NaOH1-IP (moderately labile-IP) and NaOH2-IP were significantly higher in Y16 than that in other orchards (Table 1, Figure 2, p < 0.05). Significantly higher concentrations and proportions of HCl-IP and stable-IP were found in Y0.5 than that in other orchards (Table 1, Figure 2, p < 0.05). Compared with Y0.5 and Y4, Y16 and Y22 significantly increased the concentration and proportion of soil NaHCO3-OP (Table 1, Figure 2A, p < 0.05). There was no significant difference in NaOH1-OP concentration across the four planting years (Table 1, p < 0.05), while significantly higher proportions of NaOH1-OP and NaOH2-OP were observed in Y22 than in Y0.5 (Figure 2A, p < 0.05). Soil NaOH2-OP concentration in Y22 was significantly higher than that in Y4, but it was not significantly different from that in Y0.5 and Y16 (Table 1, p < 0.05). Soil residual P showed the highest proportion and lowest concentration in Y4, with no significant difference in concentration between Y4 and Y22 (Table 1, Figure 2A, p < 0.05).

Figure 1.
Soil organic carbon (A) and pH (B) of grape orchards with different planting years. Values are means and error bars represent standard errors (n = 4). Different lowercase letters indicate significant (p < 0.05) differences between grape orchards with different planting years. SOC: soil organic carbon. Y0.5, Y4, Y16 and Y22 represent grape orchards planted for 0.5, 4, 16 and 22 years, respectively.

Table 1.
Concentrations (mg kg−1) of soil P fractions in grape orchards with different planting years.
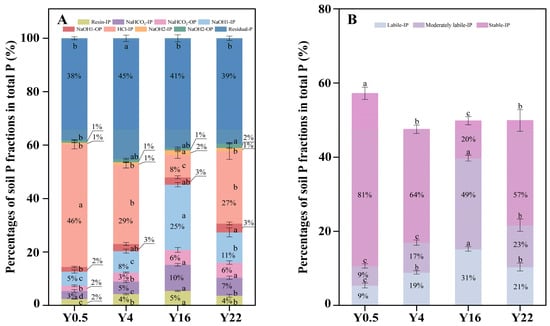
Figure 2.
Distribution (%) of each soil P fraction (A) and P fractions grouped by lability (B) in grape orchards with different planting years. Values are means and error bars represent standard errors (n = 4). Different lowercase letters indicate significant (p < 0.05) differences between grape orchards with different planting years. IP: inorganic P; OP: organic P; total P: sum of soil P fractions. Y0.5, Y4, Y16 and Y22 represent grape orchards planted for 0.5, 4, 16 and 22 years, respectively.
3.2. Abundance and Community Diversity of pqqC Gene
The abundance of the pqqC gene was significantly affected by planting years, with significantly higher values found in Y0.5 compared with other treatments (Figure 3A, p < 0.05). After the normalization of trimmed reads from 16 soil samples, a total of 781586 high-quality sequences were obtained, which were clustered into 5062 OTUs. The α-diversity of pqqC gene communities was estimated by Chao1 and Shannon indices. Compared with Y0.5, the Chao1 index was significantly decreased in Y4, Y16 and Y22, with the lowest values in Y16 (Figure 3B, p < 0.05). Similarly, the Shannon index showed a significantly lower value in Y16 than in other orchards (Figure 3C, p < 0.05). The PCoA and further analysis using PERMANOVA showed that different planting years significantly influenced the β-diversity of pqqC gene communities (Figure 3D, R2 = 0.564, p < 0.01). In the PCoA plot, the pqqC gene communities were significantly different between Y16 and other treatments (Figure 3D, p < 0.01).

Figure 3.
Comparisons of the abundance (A), α-diversity (B,C) and β-diversity (D) of pqqC-harboring bacterial communities between grape orchards with different planting years. α-diversity estimated by Chao1 and Shannon. Values are means and error bars represent standard errors (n = 4). Different lowercase letters indicate significant (p < 0.05) differences between grape orchards with different planting years. Comparisons of β-diversity across grape orchards with different planting years in principle coordinate analysis (PCoA) plot based on the Bray–Curtis distance matrix. Ellipses were drawn for each group with a confidence limit of 0.95. The differences of communities were examined by PERMANOVA analysis. Y0.5, Y4, Y16 and Y22 represent grape orchards planted for 0.5, 4, 16 and 22 years, respectively.
3.3. Composition and Biomarkers of pqqC-Harboring Bacterial Communities
All pqqC gene sequences in our soil samples were affiliated with 5 phyla and 100 genera. The dominant phyla across all samples were Actinobacteria (29.12%) and Proteobacteria (17.56%) (Figure 4A). The relative abundance of Actinobacteria significantly decreased in Y4 and Y16 as compared with Y0.5 and Y22, with the lowest value in Y16 (Figure 4B, p < 0.05). No significant difference was detected in the relative abundance of Proteobacteria across the four planting years (Figure 4B, p < 0.05). At the genus level, the dominant pqqC-harboring genera with relative abundance in the top six contained Rubrobacter (18.62%), Pseudomonas (4.69%), Variovorax (2.94%), Streptomyces (2.58%), Mycobacterium (2.45%) and Geodermatophilus (1.76%) (Figure 4C). The relative abundance of Rubrobacter significantly increased in Y22 but decreased in Y16 relative to Y0.5 and Y4 (Figure 4D, p < 0.05). The relative abundance of Variovorax in Y4 was significantly higher than that in Y16 but was not significantly different from that in Y0.5 and Y22 (Figure 4D, p < 0.05). Moreover, the Y0.5 treatment exhibited significantly higher relative abundances of Streptomyces and Mycobacterium than other treatments (Figure 4D, p < 0.05). A significant reduction in the relative abundance of Geodermatophilus was observed in Y16, compared to other orchards (Figure 4D, p < 0.05). Additionally, the relative abundance of Pseudomonas showed no significant differences among all orchards (Figure 4D, p < 0.05).
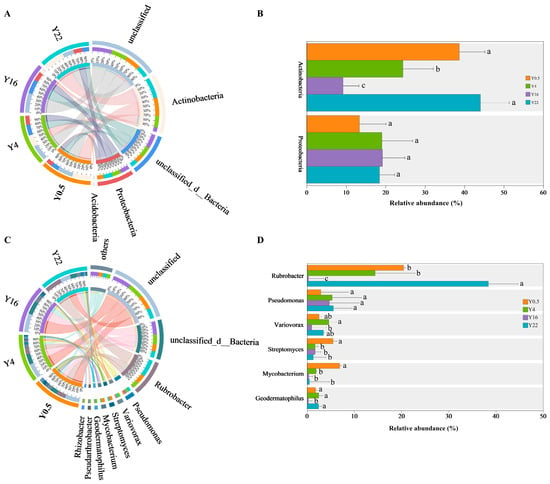
Figure 4.
Relative abundances of pqqC-harboring bacterial communities at the phylum (A,B) and genus (C,D) level in grape orchards with different planting years. Values are means and error bars represent standard errors (n = 4). Different lowercase letters indicate significant (p < 0.05) differences between grape orchards with different planting years. Y0.5, Y4, Y16 and Y22 represent grape orchards planted for 0.5, 4, 16 and 22 years, respectively.
The LEfSe analysis revealed biomarkers that indicated significant differences in pqqC-harboring bacterial communities in grape orchards with different planting years (Figure 5A,B, Kruskal–Wallis test with p < 0.05 and log LDA > 4.0). The results showed that there were 35 bacterial taxa with significant differences (Figure 5A,B, p < 0.05). Among them, there were 8 taxa for Y0.5, 11 taxa for Y4, 10 taxa for Y16 and 6 taxa for Y22 (Figure 5A,B). The biomarkers in Y0.5, Y4 and Y22 mainly derived from Actinobacteria and Proteobacteria, while those biomarkers in Y16 mainly derived from Acidobacteria, Proteobacteria and Actinobacteria (Figure 5A).

Figure 5.
Biomarkers of pqqC-harboring bacterial communities revealed by the linear discriminant analysis (LDA) effect size in grape orchards with different planting years. Circles in the cladogram (A) from inside to outside represent phylum, class, order, family and genus, respectively. The color-coded taxa within the cladogram (A) and histogram (B) indicate significantly enriched taxa in a treatment by Kruskal–Wallis test with p < 0.05 and logarithmic LDA > 4.0. Y0.5, Y4, Y16 and Y22 represent grape orchards planted for 0.5, 4, 16 and 22 years, respectively.
3.4. Relationships Between Soil Properties, pqqC-Harboring Bacterial Communities and Soil P Fractions
A total of 55 significant Spearman’s correlations were detected between pqqC-harboring microbial taxa (genera with relative abundance in the top 50) and soil P fractions, but only the strongly significant correlations were shown in the network (Figure 6, r > 0.6 and p < 0.05). Concentrations of soil Pi fractions (resin-IP, NaHCO3-IP, NaOH1-IP, HCl-IP and NaOH2-IP) and residual P were significantly correlated with pqqC-harboring genera (Figure 6, r > 0.6 and p < 0.05). With respect to soil organic P (Po) fractions (NaHCO3-OP and NaOH2-OP), significant correlations with pqqC-harboring genera were also found (Figure 6, r > 0.6 and p < 0.05). Based on the betweenness centrality, Azotobacter played a vital role in the network, followed by Rhizobacter, Caballeronia, Mycobacterium and Rhodoplanes (Table A1).
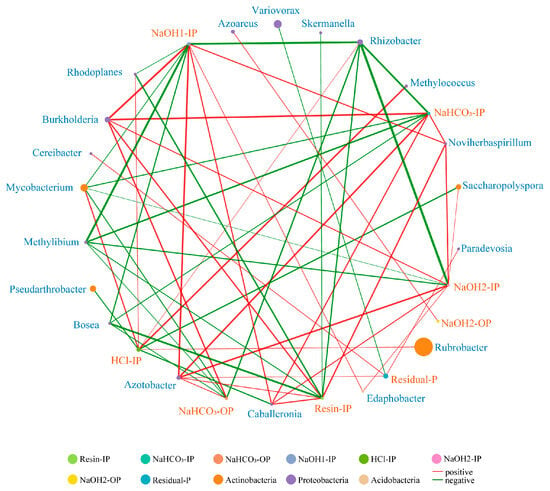
Figure 6.
The correlation network between pqqC-harboring genera (with relative abundance in the top 50) and soil P fractions based on Spearman’s correlations. Only strongly significant correlations (r > 0.6, p < 0.05) were shown in the network. Red and green lines indicate positive and negative correlations, respectively. The color of each genus indicates the phylum affiliation. The node size represents the relative abundance of pqqC-harboring genera and P fractions. The thickness of the line indicates the correlation coefficient. IP: inorganic P; OP: organic P.
The SEM was conducted further to investigate the relationships soil properties, pqqC-harboring bacterial communities and soil Pi fractions (Figure 7). The most parsimonious model explained 95% of the variations in soil Pi fractions. Notably, planting years had significant negative and positive effects on soil pH and SOC, respectively (p < 0.01). Subsequently, soil pH showed significant and positive direct effects on the abundance and α-diversity indices of pqqC gene and negative direct effects on the composition of pqqC-harboring bacterial communities and soil Pi fractions (p < 0.01). Meanwhile, the abundance and Chao 1 index of pqqC gene directly and negatively influenced soil Pi fractions (p < 0.05). In addition, direct negative and positive effects of SOC on the composition of pqqC-harboring bacterial communities and soil Pi fractions were observed, respectively (p < 0.05).
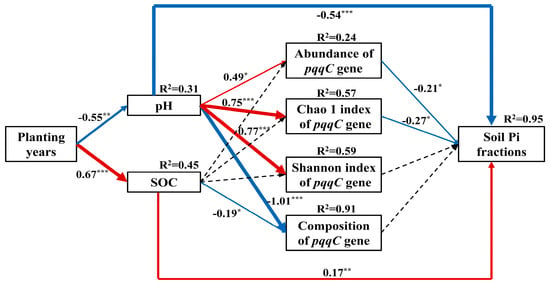
Figure 7.
Results of structural equation modeling for relationships between soil properties, pqqC-harboring bacterial communities and soil inorganic P fractions. Numbers in bold indicate the variance explained by the model (R2). Numbers on arrows are standardized path coefficients. The thickness of the arrows represents the extent of influence. Red and blue arrows indicate positive and negative effects, respectively. Dashed arrows indicate nonsignificant paths, which are removed in the final model. Significance levels are as follows: ⁎ p < 0.05; ⁎⁎ p < 0.01 and ⁎⁎⁎ p < 0.001. χ2 = 22.632, df = 15, p = 0.092, CFI = 0.947, GFI = 0.777 and RMSEA = 0.184. SOC: soil organic carbon.
4. Discussion
The decreased soil pH in the 16- and 22-year orchards shown here was ascribed to soil acidification resulting from the long-term excessive application of fertilizer, which was consistent with many previous studies [,,]. In contrast to soil pH, SOC accumulated more in 16- and 22-year orchards than in the newly established (Y0.5) and 4-year orchards, which was due to the continuous input of organic matter such as plant and litter debris to the soil of long-term orchard plantation. This result agreed with that of Zheng et al. [], who reported that the SOC storage in topsoil (0–20 cm) was promoted with the increased planting years.
Our findings suggested that the 16- and 22-year orchards increased the proportions of labile Pi and moderately labile Pi but reduced stable Pi, indicating that long-term orchard plantation could stimulate the transformation of recalcitrant Pi fractions into more readily available forms. These observations were mainly related to the drop in soil pH caused by long-term fertilizer input. It was well established that soil pH had a profound influence on soil P chemical reactions and was confirmed to be a key factor in regulating the turnover of soil Pi fractions []. The lower soil pH in 16- and 22-year orchards could reduce the adsorption of phosphates by weakening cation–oxygen bonds and facilitating the release of cations, which would consequently promote the solubilization of inorganic phosphates and transformation of stable Pi to both labile Pi and moderately labile Pi [,]. This explanation was supported by the fact that the concentration and proportion of stable Pi were significantly and negatively correlated with labile Pi and moderately labile Pi (Table A2). Moreover, it was also observed that there were significant and positive correlations of SOC content with labile Pi and moderately labile Pi in our study (Table A2). The result was consistent with that of Zhang et al. [] showing that the enhanced SOC content could increase soil Pi concentration, especially for soil labile Pi. This was likely because negatively charged organic matter could compete with phosphates for adsorption sites on the surfaces of positively charged clays and oxide minerals, thus mobilizing the adsorbed P into the soil solution [,]. In addition, some organic molecules released from organic matter decomposition blocked the complexation reactions of phosphates with cations in soil, which led to an increase in soil Pi concentration [,,]. Lastly, the mineralization of soil Po to Pi could be driven by the microbial need for C []. This might be another explanation for the increase in proportions of labile Pi and moderately labile Pi in 16- and 22-year orchards with high SOC contents. It should be noted that the accumulation in labile Pi and moderately labile Pi fractions might increase the risk of soil P loss to water bodies [,].
Likewise, the increase in concentrations and proportions of soil Po fractions (particularly NaHCO3-OP) in 16- and 22-year orchards might also be attributed to the significant accumulation of SOC and the continuous input of fertilizer in these orchards with long-term plantations. It has been found that soil organic matter (SOM) was considered as the primary source of soil Po and the main form of Po in soil was bound to SOM by phosphoester bonds [,,,]. Actually, significant and positive correlations between the content and proportion of NaHCO3-OP and SOC content were found in the present study (Table A2). Additionally, Chen et al. [] reported that long-term excessive input of P fertilizer significantly increased the soil Po concentration but reduced the proportion in pomelo orchards due to plant- and soil-related biological processes. However, Cade-Menun et al. [] and Tian et al. [] proved that fertilizer P application did not significantly affect soil Po content under grazed pasture because of the absence of any obvious accumulation of SOC. These inconsistent findings may be due to the differences in soil and plant types, regional climate and soil management.
Numerous studies have demonstrated that long-term application of chemical fertilizer could cause reductions in the abundances of P cycling genes involved in P dissolution (including pqqC gene), uptake and transportation, and alter the diversity and community composition of soil P-acquiring microbes because of soil acidification [,,,]. Moreover, long-term continuous cropping of the same crop decreased microbial abundance and diversity and altered the community composition of microbes in the soil [,,]. In concordance with these observations, our results revealed that the abundance and α-diversity estimated by Chao1 and Shannon indices of pqqC gene showed decreasing trends in orchards of 4, 16 and 22 years compared to those in the newly established orchard (0.5 years). In contrast, the pqqC gene was found in a higher abundance in the long-term (28-year field experiments) mineral fertilizer application treatment than that in the no-fertilizer treatment, because the gene expression of inorganic phosphate solubilizing bacteria was inhibited due to the lower P content in the no-fertilizer treatment, thereby leading to a lower abundance of the pqqC gene []. Notably, the 16-year orchard resulted in a higher level of the reduction in pqqC gene abundance and α-diversity. In addition to the lower soil pH mentioned above, the phenomenon might also be related to the highest labile Pi content of this treatment. As described by Qin et al. [], the production of the pqqC gene could be limited by the rapid increase in soil inorganic orthophosphate concentration.
With regard to β-diversity, the composition of the pqqC-harboring bacterial community was significantly different between the 16-year orchard and other orchards. In agreement with previous studies, the dominant pqqC-harboring bacteria were Actinobacteria and Proteobacteria at the phylum level, and at the genus level, they were Rubrobacter and Pseudomonas, with all of them being capable of P solubilization in this study [,,,]. Our results suggested that Proteobacteria and Pseudomonas played dominant roles in 16-year orchard, whereas Actinobacteria and Rubrobacter were the most prevalent phylum and genus in other orchards, respectively. Furthermore, according to the LEfSe analysis, it was found that five of the 10 biomarkers in the 16-year-old orchard were derived from Acidobacteria. It had been widely accepted that the Actinobacteria phylum with unique cell wall compositions exhibited an oligotrophic life strategy and had a strong ability to hydrolyze complex carbon substrates, such as pectin, cellulose, xylan and starch, which could enable them to cope with nutrient-deficient conditions in soil [,,]. Conversely, Proteobacteria were regarded as eutrophic bacteria and preferred a nutrient-enriched soil environment [,]. As previously described [,,,], Acidobacteria containing the pqqC gene were also involved in the solubilization of inorganic phosphate and were subject to regulation by soil pH. In addition, Rubrobacter, an obligate aerobic microorganism, exhibited a propensity to colonize microaggregates containing higher recalcitrant SOC []. Therefore, these trends in the present study likely occurred due to the substantial increases in the contents of labile Pi and SOC, the enhanced availability of SOM (as indicated by the ratio of microbial biomass carbon to SOC, Figure A2) and the lower soil pH in the 16-year orchard.
The correlation network clearly revealed an intimate connection between pqqC-harboring bacteria and soil P fractions. Abbasi et al. [] proposed that the member with a higher betweenness centrality had the power of controlling the communication and information flow in the network. It was found that the pqqC-harboring genus, Azotobacter, harbored the highest value of betweenness centrality in this study, indicating that Azotobacter had the most power and influence in linking soil P fractions in response to different planting years. The key genus Azotobacter had the ability to not only mobilize insoluble P but also fix nitrogen (N), implying the coupling of soil P transformation with N cycling [,]. Similarly, Yang et al. [] demonstrated that the keystone taxa within pqqC gene networks possessed diverse ecological functions, revealing that soil P turnover was coupled with the nutrient cycles of C and, particularly, N. In addition, Jiang et al. [] confirmed that soil stable Po could affect the pqqC-harboring bacterial community mainly via the interrelation with Pi forms, and they supposed that the two microbial processes (Pi solubilization and Po mineralization) were likely interdependent. Actually, our results observed that all soil Pi fractions, residual P and Po fractions (labile and stable Po) showed highly significant correlations with pqqC-harboring genera, which indicated the crucial role of these bacteria in Pi transformation and that the solubilization of Pi might be accompanied by Po mineralization. Therefore, the coupling process between P (including Pi and Po) and N cycling should be the aim of future study.
Consistent with the prevailing notion that soil pH was the primary regulator of the abundance, diversity and composition of inorganic P-solubilizing bacterial communities [,], the SEM results in the present study emphasized the importance of soil pH in soil Pi transformation and pqqC-harboring bacterial communities. In the model, the direct and significant effects of soil pH on the abundance, diversity and composition of pqqC-harboring bacterial communities were likely related to the nutrient availability, microbial respiration rates and the sum of whole microbial activities under abundant fertilizer input []. Moreover, soil pH and SOC showed significantly negative and positive effects on soil Pi fractions, respectively, which could be ascribed to the mechanisms of desorption and competition as discussed above. Additionally, the significant and negative effects of the abundance and community richness (Chao 1) of the pqqC gene on soil Pi fractions not only were related to the ability of pqqC-harboring bacterial communities to dissolve Pi, but also depended on the fact that the proliferation and variation of inorganic P-mobilizing bacteria could be stimulated by soil P deficiency [,]. Overall, our results suggest that changes in soil Pi fractions are closely associated with soil pH, the abundance and community richness of the pqqC gene and SOC.
Herein, our findings provide valuable insights for predicting soil P cycling under long-term orchard plantation, having implications regarding the development of sustainable agriculture. Nevertheless, this study may be subject to the following limitations. First, our study concentrated solely on the topsoil layer (0–20 cm). This was because the topsoil was the site where most soil microbial activities occurred, and it also served as a key zone for soil distribution affected by anthropogenic actions []. However, in the process of increasing cropping years, soil available P migrated to the deeper soil layers and moderately labile Pi forms (Al-P and Fe-P) gradually emerged as the dominant P pools []. Second, given that wine grapes have a considerably longer lifespan than other crops, this research might be restricted by the time scale, and moreover, it may also be limited by specific geographic areas and climatic conditions []. Third, the 16-year orchard resulted in a higher level of reduction in soil pH, which might be related to the initial soil pH. Unfortunately, we failed to obtain the initial soil samples to verify this conjecture. Fourth, we used a specific molecular marker (pqqC gene) instead of a standard molecular marker such as 16S rRNA. The advantages of using the pqqC gene include its function orientation, enabling the precise identification of P-cycling microbes and providing unique insights into P-related biogeochemical processes, and niche-centricity for exploring the unique ecology of relevant microbes, while the limitations are its lower taxonomic resolution compared to 16S rRNA and scarce database resources that hamper accurate sequence annotation and analysis. Hence, future studies should focus on the soil P cycling in deep soil under long-term orchard plantation over a broader geographic area.
5. Conclusions
Our results demonstrated that continuous grape cultivation accelerated the transformation of soil stable Pi to both labile Pi and moderately labile Pi, which might increase the risk of P loss. Moreover, the abundance and α-diversity of the pqqC gene showed decreasing trends as planting years increased due to soil acidification and continuous cropping. The composition of pqqC-harboring bacterial community changed greatly in the 16-year orchard likely because of the lower soil pH, the substantial increases in the contents of labile Pi and SOC and the enhanced availability of SOM. The correlation network revealed an intimate connection between pqqC-harboring bacteria and soil P fractions, which is bridged specifically by an important bacterial genus (Azotobacter) involved in N cycling. In addition, the SEM results further confirmed that the relationships between the pqqC-harboring bacterial communities and soil Pi fractions were mainly governed by soil pH. Remarkably, the abundance and community richness of the pqqC gene play a greater role in regulating soil Pi transformation than the diversity and composition of pqqC gene communities. Collectively, these findings suggested that changes in soil Pi fractions are closely associated with soil pH, the abundance and community richness of pqqC gene and SOC in grape orchards with different planting years in the test area. Nevertheless, future studies are also needed to explore the coupling process between P (including Pi and Po) and N cycling in grape orchards with different planting years.
Author Contributions
Conceptualization, X.G. and G.W.; Data curation, X.W. and Z.S.; Formal analysis, J.C. and J.Z.; Funding acquisition, G.W.; Investigation, Z.S. and S.F.; Methodology, J.C. and J.Z.; Project administration, H.Z.; Resources, J.C., X.G. and J.Z.; Software, C.Y. and T.M.; Supervision, C.Y. and T.M.; Validation, C.Y. and T.M.; Visualization, S.F.; Writing—original draft, X.W.; Writing—review and editing, G.W. and H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Shandong Province, China (ZR2022QD114) and the National Natural Science Foundation of China (42207387).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| P | Phosphorus |
| Pi | Inorganic P |
| Po | Organic P |
| SOC | Soil organic carbon |
| N | Nitrogen |
| Y0.5 | 0.5 years |
| Y4 | 4 years |
| Y16 | 16 years |
| Y22 | 22 years |
| IP | Inorganic P |
| OP | Organic P |
| OTUs | Operational taxonomic units |
| PCoA | Principal coordinate analysis |
| PERMANOVA | Permutational multivariate analysis |
| LDA | Linear discriminant analysis |
| LEfSe | linear discriminant analysis effect size |
| SEM | Structural equation modeling |
| SOM | Soil organic matter |
| Mt | Million tons |
| AP | Available P |
| TP | Total P |
| CFI | Comparative fit index |
| GFI | Goodness of fit |
| RMSEA | Root square mean error of approximation |
Appendix A

Table A1.
Betweenness centrality of members in the correlation network.
Table A1.
Betweenness centrality of members in the correlation network.
| Node Name | Betweenness Centrality |
|---|---|
| g_Azotobacter | 0.2603 |
| HCl-IP | 0.2248 |
| Residual-P | 0.2031 |
| Resin-IP | 0.1469 |
| NaOH1-IP | 0.1331 |
| NaOH2-IP | 0.1329 |
| g_Rhizobacter | 0.0793 |
| NaHCO3-IP | 0.0676 |
| g_Caballeronia | 0.0601 |
| g_Mycobacterium | 0.0555 |
| NaHCO3-OP | 0.0393 |
| g_Rhodoplanes | 0.0301 |
| g_Burkholderia | 0.0142 |
| g_Methylibium | 0.0142 |
| g_Saccharopolyspora | 0.0111 |
| g_Noviherbaspirillum | 0.0083 |
| g_Bosea | 0.0075 |
| g_Edaphobacter | 0.001 |
| g_Rubrobacter | 0 |
| g_Variovorax | 0 |
| g_Pseudarthrobacter | 0 |
| g_Azoarcus | 0 |
| NaOH2-OP | 0 |
| g_Methylococcus | 0 |
| g_Paradevosia | 0 |
| g_Skermanella | 0 |
| g_Cereibacter | 0 |
IP: inorganic P; OP: organic P.

Table A2.
Correlations between soil properties and P fractions (n = 16).
Table A2.
Correlations between soil properties and P fractions (n = 16).
| pH | SOC | cLPi | cMLPi | cSPi | pLPi | pMLPi | pSPi | cNaHCO3-OP | cNaOH1-OP | cNaOH2-OP | pNaHCO3-OP | pNaOH1-OP | pNaOH2-OP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | −0.676 ** | −0.876 ** | −0.929 ** | 0.45 | −0.706 ** | −0.715 ** | 0.679 ** | −0.785 ** | −0.656 ** | −0.265 | −0.562 * | −0.262 | 0.124 |
| SOC | −0.676 ** | 1 | 0.721 ** | 0.773 ** | −0.238 | 0.509 * | 0.641 ** | −0.524 * | 0.668 ** | 0.35 | 0.550 * | 0.524 * | 0.138 | 0.265 |
| cLPi | −0.876 ** | 0.721 ** | 1 | 0.948 ** | −0.556* | 0.874 ** | 0.850 ** | −0.818 ** | 0.897 ** | 0.644 ** | 0.321 | 0.697 ** | 0.359 | 0.044 |
| cMLPi | −0.929 ** | 0.773 ** | 0.948 ** | 1 | −0.444 | 0.781 ** | 0.804 ** | −0.715 ** | 0.840 ** | 0.590 * | 0.256 | 0.618 * | 0.197 | −0.104 |
| cSPi | 0.45 | −0.238 | −0.556 * | −0.444 | 1 | −0.844 ** | −0.832 ** | 0.900 ** | −0.476 | −0.165 | 0.235 | −0.641 ** | −0.468 | −0.032 |
| pLPi | −0.706 ** | 0.509* | 0.874 ** | 0.781 ** | −0.844 ** | 1 | 0.950 ** | −0.965 ** | 0.774 ** | 0.447 | 0.047 | 0.782 ** | 0.491 | 0.076 |
| pMLPi | −0.715 ** | 0.641 ** | 0.850 ** | 0.804 ** | −0.832 ** | 0.950 ** | 1 | −0.947 ** | 0.750 ** | 0.353 | 0.065 | 0.776 ** | 0.412 | 0.088 |
| pSPi | 0.679 ** | −0.524 * | −0.818 ** | −0.715 ** | 0.900 ** | −0.965 ** | −0.947 ** | 1 | −0.738 ** | −0.435 | −0.038 | −0.782 ** | −0.550* | −0.085 |
| cNaHCO3-OP | −0.785 ** | 0.668 ** | 0.897 ** | 0.840 ** | −0.476 | 0.774 ** | 0.750 ** | −0.738 ** | 1 | 0.744 ** | 0.341 | 0.871 ** | 0.506 * | 0.068 |
| cNaOH1-OP | −0.656 ** | 0.35 | 0.644 ** | 0.590 * | −0.165 | 0.447 | 0.353 | −0.435 | 0.744 ** | 1 | 0.294 | 0.568 * | 0.671 ** | −0.079 |
| cNaOH2-OP | −0.265 | 0.550 * | 0.321 | 0.256 | 0.235 | 0.047 | 0.065 | −0.038 | 0.341 | 0.294 | 1 | 0.144 | 0.129 | 0.753 ** |
| pNaHCO3-OP | −0.562 * | 0.524 * | 0.697 ** | 0.618* | −0.641 ** | 0.782 ** | 0.776 ** | −0.782 ** | 0.871 ** | 0.568 * | 0.144 | 1 | 0.679 ** | 0.171 |
| pNaOH1-OP | −0.262 | 0.138 | 0.359 | 0.197 | −0.468 | 0.491 | 0.412 | −0.550 * | 0.506 * | 0.671 ** | 0.129 | 0.679 ** | 1 | 0.271 |
| pNaOH2-OP | 0.124 | 0.265 | 0.044 | −0.104 | −0.032 | 0.076 | 0.088 | −0.085 | 0.068 | −0.079 | 0.753 ** | 0.171 | 0.271 | 1 |
SOC: soil organic carbon; cLPi: concentration of labile inorganic P; cMLPi: concentration of moderately labile inorganic P; cSPi: concentration of stable inorganic P; pLPi: proportion of labile inorganic P; pMLPi: proportion of moderately labile inorganic P; pSPi: proportion of stable inorganic P; cNaHCO3-OP: concentration of NaHCO3-OP; cNaOH1-OP: concentration of NaOH1-OP; cNaOH2-OP: concentration of NaOH2-OP; pNaHCO3-OP: proportion of NaHCO3-OP; pNaOH1-OP: proportion of NaOH1-OP; pNaOH2-OP: proportion of NaOH2-OP; OP: organic P. ** Correlation is significant at the 0.01 level. * Correlation is significant at the 0.05 level.
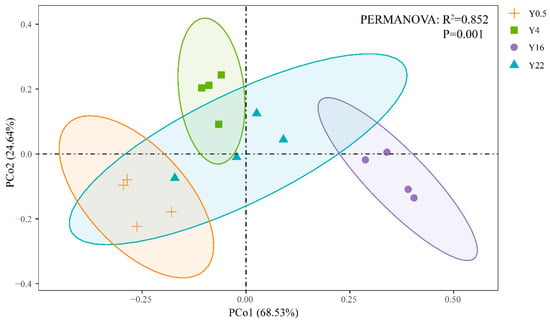
Figure A1.
Principal coordinate analysis depicts the soil inorganic P fractions in grape orchards with different planting years based on the Bray–Curtis distance. Ellipses were drawn for each group with a confidence limit of 0.95. The differences of soil inorganic P fractions were examined by PERMANOVA analysis. Y0.5, Y4, Y16 and Y22 represent grape orchards planted for 0.5, 4, 16 and 22 years, respectively.
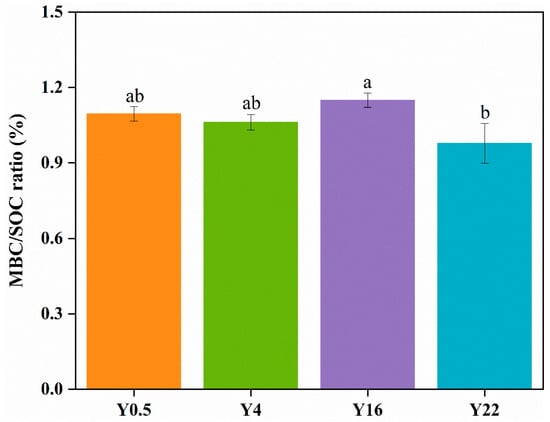
Figure A2.
The ratio (%) of microbial biomass carbon to soil organic carbon in grape orchards with different planting years. Different lowercase letters indicate significant (p < 0.05) differences between grape orchards with different planting years. MBC: microbial biomass carbon; SOC: soil organic carbon. Y0.5, Y4, Y16 and Y22 represent grape orchards planted for 0.5, 4, 16 and 22 years, respectively.
References
- Bi, Q.F.; Zheng, B.X.; Lin, X.Y.; Li, K.J.; Liu, X.P.; Hao, X.L.; Zhang, H.; Zhang, J.B.; Jaisi, D.P.; Zhu, Y.G. The microbial cycling of phosphorus on long-term fertilized soil: Insights from phosphate oxygen isotope ratios. Chem. Geol. 2018, 483, 56–64. [Google Scholar] [CrossRef]
- Mutwale, N.M.; Jorge, F.; Chabala, L.M.; Shepande, C.; Chishala, B.H.; Cambule, A.; Nhantumbo, A.; Matangue, M.; Braun, M.; Sandhage-Hofmann, A.; et al. Climatic effects on soil phosphorus pools and availability in sub-Saharan Africa. Eur. J. Soil Sci. 2024, 75, e13448. [Google Scholar] [CrossRef]
- Martinengo, S.; Schiavon, M.; Santoro, V.; Said-Pullicino, D.; Romani, M.; Miniotti, E.F.; Celi, L.; Martin, M. Assessing phosphorus availability in paddy soils: The importance of integrating soil tests and plant responses. Biol. Fertil. Soils 2023, 59, 391–405. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Lin, H.Y.; Chang, Z.F.; Li, Z.M.; Riaz, A.; Hou, E.Q. Magnesium-doped biochars increase soil phosphorus availability by regulating phosphorus retention, microbial solubilization and mineralization. Biochar 2024, 6, 68. [Google Scholar] [CrossRef]
- Xiao, D.; Tang, X.; Chen, S.; Chu, G.; Liu, Y.; Wang, D.; Xu, C. Aeration treatment promotes transformation of soil phosphorus fractions to plant-available phosphorus by modulating rice rhizosphere microbiota. Soil Tillage Res. 2025, 245, 106318. [Google Scholar] [CrossRef]
- Luo, X.; Elrys, A.S.; Zhang, L.; Ibrahim, M.M.; Liu, Y.; Fu, S.; Yan, J.; Ye, Q.; Wen, D.; Hou, E. The global fate of inorganic phosphorus fertilizers added to terrestrial ecosystems. One Earth 2024, 7, 1402–1413. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Xu, C.; Zhong, Y.; Xu, X.; Yuan, J.; Wang, J.; Zhang, Y. Ten years of urea fertilization alter the pqqC-harbouring community and increase soil inorganic phosphorus mobilization. Eur. J. Soil Sci. 2024, 75, e13563. [Google Scholar] [CrossRef]
- Gilbert, N. Environment: The disappearing nutrient. Nature 2009, 461, 716–718. [Google Scholar] [CrossRef]
- EL-Sharkawy, M.; Sleem, M.; Du, D.L.; El Baroudy, A.; Li, J.; Mahmoud, E.; Ali, N. Nano-water treatment residuals: Enhancing phosphorus kinetics and optimization in saline soils. Land Degrad. Dev. 2024, 35, 3314–3329. [Google Scholar] [CrossRef]
- Ishida, T.; Tamura, M.; Kimbi, S.B.; Tomozawa, Y.; Saito, M.; Hirayama, Y.; Nagasaka, I.; Onodera, S.I. Evaluation of Phosphorus Enrichment in Groundwater by Legacy Phosphorus in Orchard Soils with High Phosphorus Adsorption Capacity Using Phosphate Oxygen Isotope Analysis. Environ. Sci. Technol. 2024, 58, 5372–5382. [Google Scholar] [CrossRef]
- Global Consumption of Phosphate Fertilizer 2022, by Country. Available online: https://www.statista.com/statistics/1252669/phosphate-fertilizer-consumption-worldwide-by-country/ (accessed on 2 December 2024).
- Chen, X.; Wang, Y.; Wang, J.; Condron, L.M.; Guo, B.; Liu, J.; Qiu, G.; Li, H. Impact of ryegrass cover crop inclusion on soil phosphorus and pqqC- and phoD-harboring bacterial communities. Soil Tillage Res. 2023, 234, 105823. [Google Scholar] [CrossRef]
- Chen, X.; Yan, X.; Wang, M.; Cai, Y.; Weng, X.; Su, D.; Guo, J.; Wang, W.; Hou, Y.; Ye, D.; et al. Long-term excessive phosphorus fertilization alters soil phosphorus fractions in the acidic soil of pomelo orchards. Soil Tillage Res. 2022, 215, 105214. [Google Scholar] [CrossRef]
- Jiang, N.; Wei, K.; Pu, J.; Huang, W.; Bao, H.; Chen, L. A balanced reduction in mineral fertilizers benefits P reserve and inorganic P-solubilizing bacterial communities under residue input. Appl. Soil Ecol. 2021, 159, 103833. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, R.; Ghimire, R.; Zhang, N.; Zhou, S.; Zhao, F.; Wang, J. Linking soil phosphorus fractions to associated microbial functional profiles under crop rotation on the Loess Plateau of China. Soil Tillage Res. 2023, 233, 105809. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Zhang, J.; Ji, H.; Liao, Y.; Ma, X.; Li, Q.; Zhang, Y.; Jiang, L.; Wang, R.; et al. Differential responses of soil phosphorus fractions to varied nitrogen compound additions in a meadow steppe. J. Environ. Manag. 2024, 369, 122337. [Google Scholar] [CrossRef]
- Wang, R.Z.; Yang, J.J.; Liu, H.Y.; Sardans, J.; Zhang, Y.H.; Wang, X.B.; Wei, C.Z.; Lu, X.T.; Dijkstra, F.A.; Jiang, Y.; et al. Nitrogen enrichment buffers phosphorus limitation by mobilizing mineral-bound soil phosphorus in grasslands. Ecology 2022, 103, e3616. [Google Scholar] [CrossRef] [PubMed]
- Penn, C.J.; Camberato, J.J. A Critical Review on Soil Chemical Processes that Control How Soil pH Affects Phosphorus Availability to Plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, R.; Liu, Q.; Qiang, W.; Liang, J.; Hou, E.; Zhao, C.; Pang, X. Linking soil phosphorus fractions to abiotic factors and the microbial community during subalpine secondary succession: Implications for soil phosphorus availability. Catena 2023, 233, 107501. [Google Scholar] [CrossRef]
- Jindo, K.; Audette, Y.; Olivares, F.L.; Canellas, L.P.; Smith, D.S.; Paul Voroney, R. Biotic and abiotic effects of soil organic matter on the phytoavailable phosphorus in soils: A review. Chem. Biol. Technol. Agric. 2023, 10, 29. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Yuan, J.; Tang, Z.; Wang, J.; Zhang, Y. Long-Term Organic Fertilization Strengthens the Soil Phosphorus Cycle and Phosphorus Availability by Regulating the pqqC- and phoD-Harboring Bacterial Communities. Microb. Ecol. 2023, 86, 2716–2732. [Google Scholar] [CrossRef]
- Deng, P.; Zhou, Y.; Chen, W.; Tang, F.; Wang, Y. Microbial mechanisms for improved soil phosphorus mobilization in monoculture conifer plantations by mixing with broadleaved trees. J. Environ. Manag. 2024, 359, 120955. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Z.; Yang, Y.; Gao, Y.; Mahmood, M.; Jiao, H.; Wang, Z.; Liu, J. Long-term high-P fertilizer input shifts soil P cycle genes and microorganism communities in dryland wheat production systems. Agric. Ecosyst. Environ. 2023, 342, 108226. [Google Scholar] [CrossRef]
- Pu, Y.; Lang, S.; Li, Y.; Li, T.; Zhang, S.; Xu, X.; Yuan, D.; Jia, Y.; Wang, G.; Li, B. Regulation of soil phosphorus availability in alpine meadows: Insights from phosphate-mobilising bacteria. Appl. Soil Ecol. 2024, 204, 105730. [Google Scholar] [CrossRef]
- Shi, Q.; Song, Q.; Shan, X.; Li, X.; Wang, S.; Fu, H.; Sun, Z.; Liu, Y.; Li, T. Microorganisms regulate soil phosphorus fractions in response to low nocturnal temperature by altering the abundance and composition of the pqqC gene rather than that of the phoD gene. Biol. Fertil. Soils 2023, 59, 973–987. [Google Scholar] [CrossRef]
- Yang, L.; Du, L.; Li, W.; Wang, R.; Guo, S. Divergent responses of phoD- and pqqC-harbouring bacterial communities across soil aggregates to long fertilization practices. Soil Tillage Res. 2023, 228, 105634. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Y.; Xiangmin, R.; Fei, J.; Peng, J.; Luo, G. Coupling amendment of biochar and organic fertilizers increases maize yield and phosphorus uptake by regulating soil phosphatase activity and phosphorus-acquiring microbiota. Agric. Ecosyst. Environ. 2023, 355, 108582. [Google Scholar] [CrossRef]
- Qin, X.; Guo, S.; Zhai, L.; Pan, J.; Khoshnevisan, B.; Wu, S.; Wang, H.; Yang, B.; Ji, J.; Liu, H. How long-term excessive manure application affects soil phosphorous species and risk of phosphorous loss in fluvo-aquic soil. Environ. Pollut. 2020, 266, 115304. [Google Scholar] [CrossRef]
- Siles, J.A.; Starke, R.; Martinovic, T.; Parente Fernandes, M.L.; Orgiazzi, A.; Bastida, F. Distribution of phosphorus cycling genes across land uses and microbial taxonomic groups based on metagenome and genome mining. Soil Biol. Biochem. 2022, 174, 108826. [Google Scholar] [CrossRef]
- Shi, W.; Xing, Y.; Zhu, Y.; Gao, N.; Ying, Y. Diverse responses of pqqC- and phoD-harbouring bacterial communities to variation in soil properties of Moso bamboo forests. Microb. Biotechnol. 2022, 15, 2097–2111. [Google Scholar] [CrossRef]
- Wei, C.; Liu, S.; Li, Q.; He, J.; Sun, Z.; Pan, X. Diversity analysis of vineyards soil bacterial community in different planting years at eastern foot of Helan Mountain, Ningxia. Rhizosphere 2023, 25, 100650. [Google Scholar] [CrossRef]
- Li, Q.; Andom, O.; Li, Y.; Cheng, C.; Deng, H.; Sun, L.; Li, Z. Responses of grape yield and quality, soil physicochemical and microbial properties to different planting years. Eur. J. Soil Biol. 2024, 120, 103587. [Google Scholar] [CrossRef]
- Song, R.; Li, Y.; Zhu, Z.; Zhang, L.; Wang, H.; Li, H. Vineyard reclamation alters soil properties and microbial community in desertified land. Catena 2024, 246, 108399. [Google Scholar] [CrossRef]
- Song, J.; Zhang, A.; Gao, F.; Li, M.; Zhao, X.; Zhang, J.; Wang, G.; Hou, Y.; Cheng, S.; Qu, H.; et al. Reduced nitrogen fertilization from pre-flowering to pre-veraison alters phenolic profiles of Vitis vinifera L. Cv. Cabernet Gernischt wine of Yantai, China. Food Res. Int. 2023, 173, 113339. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.W.; Sommers, L.E. Chemical and microbiological properties. In Methods of Soil Analysis, 2nd ed.; Page, A.L., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Condron, L.M.; Goh, K.M. Effects of long-term phosphatic fertilizer applications on amounts and forms of phosphorus in soils under irrigated pasture in New Zealand. J. Soil Sci. 1989, 40, 383–395. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in Inorganic and Organic Soil Phosphorus Fractions Induced by Cultivation Practices and by Laboratory Incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Kuo, S. Phosphorus. In Methods of Soil Analysis, 3rd ed.; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 869–919. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Hu, M.; Le, Y.; Sardans, J.; Yan, R.; Zhong, Y.; Sun, D.; Tong, C.; Peñuelas, J. Moderate salinity improves the availability of soil P by regulating P-cycling microbial communities in coastal wetlands. Glob. Chang. Biol. 2023, 29, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Abbasi, A.; Hossain, L.; Leydesdorff, L. Betweenness centrality as a driver of preferential attachment in the evolution of research collaboration networks. J. Informetr. 2012, 6, 403–412. [Google Scholar] [CrossRef]
- Assenov, Y.; Ramírez, F.; Schelhorn, S.E.; Lengauer, T.; Albrecht, M. Computing topological parameters of biological networks. Bioinformatics 2008, 24, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Miao, N.; Wang, P.; Ju, X.; Chen, Z.; Zhou, J.; Kuzyakov, Y. Dramatic loss of inorganic carbon by nitrogen-induced soil acidification in Chinese croplands. Glob. Chang. Biol. 2020, 26, 3738–3751. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Wang, L.; Zhao, J.S.; Niu, Y.H.; Xiao, H.B.; Wang, Z.; Yu, S.X.; Shi, Z.H. Forty-year-old orchards promote carbon storage by changing aggregate-associated enzyme activities and microbial communities. Catena 2022, 213, 106195. [Google Scholar] [CrossRef]
- Guppy, C.N.; Menzies, N.W.; Moody, P.W.; Blamey, F.P.C. Competitive sorption reactions between phosphorus and organic matter in soil: A review. Soil Res. 2005, 43, 189–202. [Google Scholar] [CrossRef]
- Cao, N.; Zhi, M.; Zhao, W.; Pang, J.; Hu, W.; Zhou, Z.; Meng, Y. Straw retention combined with phosphorus fertilizer promotes soil phosphorus availability by enhancing soil P-related enzymes and the abundance of phoC and phoD genes. Soil Tillage Res. 2022, 220, 105390. [Google Scholar] [CrossRef]
- George, T.S.; Gregory, P.J.; Wood, M.; Read, D.; Buresh, R.J. Phosphatase activity and organic acids in the rhizosphere of potential agroforestry species and maize. Soil Biol. Biochem. 2002, 34, 1487–1494. [Google Scholar] [CrossRef]
- Malik, M.A.; Marschner, P.; Khan, K.S. Addition of organic and inorganic P sources to soil—Effects on P pools and microorganisms. Soil Biol. Biochem. 2012, 49, 106–113. [Google Scholar] [CrossRef]
- Joshi, S.R.; Tfaily, M.M.; Young, R.P.; McNear, D.H., Jr. Root exudates induced coupled carbon and phosphorus cycling in a soil with low phosphorus availability. Plant Soil 2024, 498, 371–390. [Google Scholar] [CrossRef]
- Cavalcante, H.; Araújo, F.; Noyma, N.P.; Becker, V. Phosphorus fractionation in sediments of tropical semiarid reservoirs. Sci. Total Environ. 2018, 619–620, 1022–1029. [Google Scholar] [CrossRef]
- Garland, G.; Bünemann, E.K.; Oberson, A.; Frossard, E.; Snapp, S.; Chikowo, R.; Six, J. Phosphorus cycling within soil aggregate fractions of a highly weathered tropical soil: A conceptual model. Soil Biol. Biochem. 2018, 116, 91–98. [Google Scholar] [CrossRef]
- Tiessen, H.; Moir, J.O. Characterization of available P by sequential extraction. In Soil Sampling and Methods of Analysis, 2nd ed.; Cartar, M.R., Ed.; Lewis Publishers: Boca Raton, FL, USA, 2007; pp. 75–86. [Google Scholar]
- Cade-Menun, B.J.; Doody, D.G.; Liu, C.W.; Watson, C.J. Long-term Changes in Grassland Soil Phosphorus with Fertilizer Application and Withdrawal. J. Environ. Qual. 2017, 46, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Boitt, G.; Black, A.; Wakelin, S.; Condron, L.M.; Chen, L. Accumulation and distribution of phosphorus in the soil profile under fertilized grazed pasture. Agric. Ecosyst. Environ. 2017, 239, 228–235. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, G.; Chen, H.; Chen, C.; Wang, J.; Ai, S.; Wei, D.; Li, D.; Ma, B.; Tang, C.; et al. Long-term nutrient inputs shift soil microbial functional profiles of phosphorus cycling in diverse agroecosystems. ISME J. 2020, 14, 757–770. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. The Influence of Soil Fertilization on the Distribution and Diversity of Phosphorus Cycling Genes and Microbes Community of Maize Rhizosphere Using Shotgun Metagenomics. Genes 2021, 12, 1022. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Xue, C.; Jiang, Q.; Xiao, Y.; Zhang, F.; Guo, S.; Shen, Q.; Ling, N. Soil Carbon, Nitrogen, and Phosphorus Cycling Microbial Populations and Their Resistance to Global Change Depend on Soil C:N:P Stoichiometry. mSystems 2020, 5, e00162-20. [Google Scholar] [CrossRef]
- Ghani, M.I.; Ali, A.; Atif, M.J.; Pathan, S.I.; Pietramellara, G.; Ali, M.; Amin, B.; Cheng, Z.H. Diversified crop rotation improves continuous monocropping eggplant production by altering the soil microbial community and biochemical properties. Plant Soil 2022, 480, 603–624. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Li, K.; Qiao, J.; Guo, Y.; Liu, Z.; Guo, X. Responses of soil bacterial and fungal communities to the long-term monoculture of grapevine. Appl. Microbiol. Biot. 2021, 105, 7035–7050. [Google Scholar] [CrossRef] [PubMed]
- Favet, J.; Lapanje, A.; Giongo, A.; Kennedy, S.; Aung, Y.Y.; Cattaneo, A.; Davis-Richardson, A.G.; Brown, C.T.; Kort, R.; Brumsack, H.J.; et al. Microbial hitchhikers on intercontinental dust: Catching a lift in Chad. ISME J. 2013, 7, 850–867. [Google Scholar] [CrossRef]
- Manzoni, S.; Schimel, J.P.; Porporato, A. Responses of soil microbial communities to water stress: Results from a meta-analysis. Ecology 2012, 93, 930–938. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, R.; Song, R.; An, X.; Chu, G.; Jia, H. Soil pqqC-harboring bacterial community response to increasing aridity in semi-arid grassland ecosystems: Diversity, co-occurrence network, and assembly process. Front. Microbiol. 2022, 13, 1019023. [Google Scholar] [CrossRef]
- Dai, Y.; Chen, D.; Zang, L.; Zhang, G.; Liu, Q.; He, Y.; Ding, F.; Wang, S.; Zhou, C.; Yang, Y.; et al. Natural restoration of degraded karst vegetation shifts soil microbial phosphorus acquisition strategies. Plant Soil 2023, 490, 201–215. [Google Scholar] [CrossRef]
- Davinic, M.; Fultz, L.M.; Acosta-Martinez, V.; Calderón, F.J.; Cox, S.B.; Dowd, S.E.; Allen, V.G.; Zak, J.C.; Moore-Kucera, J. Pyrosequencing and mid-infrared spectroscopy reveal distinct aggregate stratification of soil bacterial communities and organic matter composition. Soil Biol. Biochem. 2012, 46, 63–72. [Google Scholar] [CrossRef]
- El-Sorady, G.A.; El-Banna, A.A.A.; Abdelghany, A.M.; Salama, E.A.A.; Ali, H.M.; Siddiqui, M.H.; Hayatu, N.G.; Paszt, L.S.; Lamlom, S.F. Response of Bread Wheat Cultivars Inoculated with Azotobacter Species under Different Nitrogen Application Rates. Sustainability 2022, 14, 8394. [Google Scholar] [CrossRef]
- Azene, B.; Zhu, R.; Pan, K.; Sun, X.; Nigussie, Y.; Gruba, P.; Raza, A.; Guadie, A.; Wu, X.; Zhang, L. Land use change alters phosphatase enzyme activity and phosphatase-harboring microbial abundance in the subalpine ecosystem of southeastern Qinghai-Tibet Plateau, China. Ecol. Indic. 2023, 153, 110416. [Google Scholar] [CrossRef]
- Lagos, L.M.; Acuña, J.J.; Maruyama, F.; Ogram, A.; de la Luz Mora, M.; Jorquera, M.A. Effect of phosphorus addition on total and alkaline phosphomonoesterase-harboring bacterial populations in ryegrass rhizosphere microsites. Biol. Fertil. Soils 2016, 52, 1007–1019. [Google Scholar] [CrossRef]
- Bargaz, A.; Noyce, G.L.; Fulthorpe, R.; Carlsson, G.; Furze, J.R.; Jensen, E.S.; Dhiba, D.; Isaac, M.E. Species interactions enhance root allocation, microbial diversity and P acquisition in intercropped wheat and soybean under P deficiency. Appl. Soil Ecol. 2017, 120, 179–188. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).