Abstract
Reliable soil critical values (SCVs) for micronutrients are essential for accurate fertilizer recommendations. This study established crop-specific SCVs for boron (B), copper (Cu), iron (Fe), manganese (Mn), and zinc (Zn) extracted with Mehlich-3 under Polish soil and climatic conditions. Extensive paired soil–plant datasets were collected for wheat (n = 1921), oilseed rape (n = 1944), and maize (n = 916) across all provinces. Micronutrients were determined in all soil and plant samples, with soil extractions performed using the Mehlich-3 method. Two plant-based calibration approaches were applied: (i) regression models linking the bioaccumulation factor (plant-to-soil concentration ratio) to soil properties, and (ii) the highyield method, defining SCVs as the lower quintile of micronutrient levels in soils from high-yielding fields. Both approaches yielded comparable results. Soil pH, organic carbon, available phosphorus, and texture were the key variables influencing SCVs, which differed among crops and elements: B and Mn were pH-dependent, Cu correlated with organic carbon, while Fe and Zn were associated with phosphorus or texture. Final SCVs ranged from B 0.10–0.90, Cu 1.0–2.2, Fe 160–280, Mn 30–75, and Zn 2.5–7.0 mg kg−1, depending on crop and soil class. These empirically derived, crop-specific Mehlich-3 SCVs provide a robust basis for micronutrient diagnostics and fertilizer management in temperate agricultural soils.
Keywords:
sample collection; microelements; soil test; regression; thresholds; wheat; maize; oilseed rape 1. Introduction
Micronutrients such as boron, copper, iron, manganese, and zinc are essential for plant metabolism, acting as cofactors in numerous enzymatic processes and regulating physiological functions such as photosynthesis, respiration, and hormone synthesis [1,2]. Deficiencies of these elements in agricultural soils may cause physiological disorders, yield reductions, and deterioration of crop quality. Reliable soil testing and the establishment of well-calibrated critical values (SCVs) are therefore fundamental for accurate nutrient diagnostics and fertilizer recommendations.
A key concept in soil testing is the SCVs—the concentration of a nutrient in a specific extractant below which plant growth or nutrient concentration in tissue begins to decline [3,4,5]. Because extractants differ in chemical strength and selectivity, SCVs must be derived specifically for each extractant–crop–region combination. Their calibration links analytical data with biological response, allowing a distinction between deficient, marginal, and sufficient nutrient levels in soils.
The availability of micronutrients in soils depends on a complex set of physicochemical and biological factors. Soil pH exerts the most significant control: as pH increases, the solubility of cationic micronutrients such as Fe, Mn, Cu, and Zn decreases due to precipitation and sorption on Fe and Al oxides [6,7]. In acidic conditions, however, excessive solubility may cause toxicity or leaching losses. Organic matter plays a dual role, either chelating micronutrients and enhancing their bioavailability or immobilizing them within stable organo-metallic complexes, depending on the redox state and degree of humification [8].
Other soil properties—including texture, mineralogy, carbonate content, and available phosphorus—strongly affect micronutrient dynamics through sorption, competition, and complexation reactions [9,10,11]. These interactions determine the actual supply of bioavailable forms to plants, explaining why total soil concentrations often correlate poorly with plant uptake [12]. A holistic understanding of these processes is therefore essential when developing or interpreting soil test calibrations for micronutrients.
Various universal extractants are used worldwide to assess plant-available micronutrients, each differing in chemical strength and suitability across soil conditions. EDTA is a widely applied chelating extractant that performs consistently in diverse soils, although it may mobilize poorly labile metal fractions [13]. DTPA, proposed by Lindsay and Norvell [4], serves as the international standard for neutral to calcareous soils, providing selective extraction of Zn, Cu, Fe and Mn, but its efficiency declines under acidic conditions. 1 M HCl [14], used in Central and Eastern Europe, including Poland, offers simplicity and low analytical cost but sometimes can mobilize non-labile fractions, reducing its value for precise micronutrient diagnostics. Mehlich 1, used primarily in the southeastern United States [15], extracts not only micronutrients but also selected macronutrients; however, its applicability is largely restricted to strongly acidic soils, and its analytical range is narrower than that of Mehlich 3 (M3). The M3 extractant [16], with broader operational versatility, enables simultaneous extraction of micro- and macronutrients and is widely adopted as a multinutrient soil test, though regional calibration remains essential for reliable interpretation.
Numerous studies have compared the efficiency of the M3 extractant with Mehlich 1, DTPA, EDTA, HCl, and other multinutrient tests across different soil types [17,18,19,20,21,22,23,24]. However, despite these numerous comparisons, only a few studies have addressed the establishment or calibration of SCVs for these extractants [25,26]. Most research reports only correlations between these tests or correlations between extractable micronutrient concentrations and plant uptake, without defining thresholds that distinguish deficient from sufficient levels. Therefore, the absence of detailed calibration methodologies for extractants such as Mehlich 3, used to establish SCVs, represents a major research gap
Different crop species vary markedly in their sensitivity to micronutrient deficiencies because of differences in root morphology, exudation patterns, and nutrient-use efficiency. Oilseed rape (Brassica napus L.) is particularly sensitive to B deficiency, which impairs cell-wall formation, pollen viability, and seed development [27,28].
Maize (Zea mays L.) are especially prone to Zn deficiency, leading to chlorosis, stunted growth, and reduced grain yield [29,30]. Cu deficiency is characterized by poor tillering, weak stems, and impaired grain formation, particularly in wheat and other cereals grown on sandy or organic soils [31,32]. Fe and Mn deficiencies also occur frequently in high-pH soils and are particularly damaging in intensive cereal systems [6,10].
The study by Korzeniowska et al. [33] confirms that oilseed rape is highly susceptible to B deficiency, wheat is prone to Cu and Mn deficiencies, while maize is very sensitive to Zn deficiency under low soil reserves. These findings indicate that the three major crops commonly cultivated in Poland and Europe require close monitoring of B, Cu, and Zn levels to prevent hidden deficiencies that may limit yield and quality.
Such interspecific differences highlight the need for crop-specific SCVs that reflect variation in nutrient demand and uptake efficiency across species, providing a basis for targeted micronutrient management strategies.
In Poland, 1 M HCl has long been used as the standard advisory extractant, and SCVs for this method are well established [34]. However, it does not allow for simultaneous determination of macro- and micronutrients. In contrast, the Mehlich 3 extract can serve as a universal soil test for both nutrient groups, significantly reducing analytical costs. Transitioning to Mehlich 3, which provides multinutrient capability and lower analytical costs, requires verification of its suitability under Polish soil conditions and the development of corresponding SCVs calibrated for local soils and crops. The relationships between 1 M HCl and Mehlich 3 extractants were previously characterized [15], providing a basis for deriving SCVs for Mehlich 3 under Polish conditions.
Although Mehlich 3 is widely applied in soil testing, only limited information is available on how SCVs for this extractant were calibrated. In Poland, where 1 M HCl has been the standard method, establishing Mehlich 3 SCVs is an essential step before any methodological transition in advisory practice can be recommended. The aim of the present study was to determine soil critical values for B, Cu, Fe, Mn, and Zn extracted with Mehlich 3 for three major field crops—wheat, oilseed rape, and maize—under Polish soil and climatic conditions. The calibration will be based on plant critical concentrations and yield responses. By providing clearly defined, crop-specific thresholds, this study will enable the interpretation of Mehlich 3 soil test results and contribute to developing a consistent basis for micronutrient management and fertilizer advisory systems in temperate agricultural conditions.
2. Materials and Methods
2.1. Sample Collection
SCVs were established from three extensive sets of paired soil–plant samples. The datasets included 1921 pairs collected in 2016 from wheat fields, 1944 in 2017 from rapeseed fields, and 916 in 2018 from maize fields. Samples were collected by accredited personnel from agro-chemical laboratories across 16 provinces of Poland, generally one soil–plant pair per “gmina”, the smallest administration unit in Poland (Figure 1).
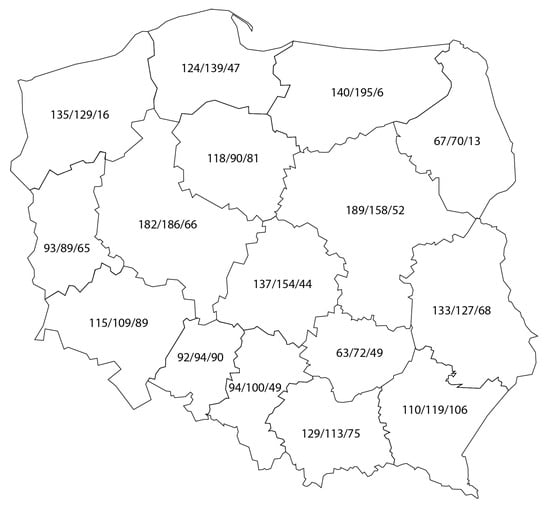
Figure 1.
Number of soil–plant samples collected in Polish provinces: wheat/maize/rape.
Samples from wheat fields were collected from 1 m2 plots, from rapeseed fields from 4 m2, and from maize fields from 8 m2. Each soil sample from wheat and rapeseed fields was composed of five subsamples, while maize soil samples combined ten subsamples. Soil was collected to a depth of 20 cm using a soil sampler, and corresponding plant samples were gathered simultaneously.
Whole wheat shoots were harvested 2 cm above the soil surface at the beginning of stem elongation (BBCH 30/31). Rapeseed shoots were cut 5 cm above the soil surface from the start of main stem elongation to the appearance of the first internode (BBCH 30/31). Maize shoots were similarly cut 5 cm above the soil surface, but only when the plants had reached 25–30 cm in height (BBCH 14–15). Each wheat sample included at least 80 shoots, while each rapeseed and maize sample included 20 shoots.
For each sampled field, farmers provided the harvested grain yield of wheat, rapeseed, and maize. Wheat yield data were available for only 1760 fields.
2.2. Chemical Analysis
Concentrations of B, Cu, Fe, Mn, and Zn were determined in all plant and soil samples. Plant micronutrients were analyzed by flame atomic absorption spectrometry (FAAS) following dry ashing in a muffle furnace and digestion with 20% nitric acid [35], except for B, which was measured using inductively coupled plasma atomic emission spectrometry (ICP-AES).
Soil micronutrients were extracted using the Mehlich 3 method [15,16] with a soil-to-solution ratio of 1:10. Samples were shaken on a rotary stirrer for 10 min at 35–40 rpm. Cu, Fe, Mn, and Zn were analyzed by FAAS, while B by ICP-AES.
Additional soil properties influencing micronutrient availability for plants—pH, organic carbon (Corg), available phosphorus (PM3), and texture—were also assessed. Soil pH was measured potentiometrically in 1 M KCl [36], Corg was determined using the Turin method with potassium dichromate [37], PM3 was analyzed following Mehlich 3 [38] and texture was measured by laser diffraction.
2.3. Database Construction
Three datasets were compiled corresponding to wheat, rapeseed, and maize. Each record represented one field from which samples were collected and included information on grain yield, micronutrient concentrations in plant tissue and soil, as well as basic soil properties (pH, Corg, PM3, and texture). At the initial stage of data processing, the datasets were checked for gross errors, that is, results that differed strongly from the rest of the data. Records showing excessively high plant micronutrient concentrations—defined as values exceeding twice the upper limit of the optimal range according to Bergmann [39]—were also excluded. Such values were considered inappropriate for studies focused on defining critical deficiency limits in soils. This process reduced skewness and improved normality. After data curation, the final datasets contained 1921 field records for wheat, 1944 for oilseed rape, and 916 for maize, which were used in further statistical evaluation.
2.4. Statistics and Calculations
The compiled datasets were characterized using descriptive statistics, including mean, range, and standard error. Differences in micronutrient concentrations among wheat, oilseed rape, and maize were evaluated using analysis of variance (ANOVA). Multiple comparisons between groups were performed with Tukey’s Honestly Significant Difference (HSD) test at a significance level of p < 0.05.
SCVs were calculated using two plant indicator-based methods: the first method based on the micronutrient plant critical value (PCV) and the second based on crop yield.
In the first method, referred to as the regression models method, simple correlation and linear regression analyses were applied. For all three crops and five micronutrients, the bioaccumulation factor was calculated as the ratio of the micronutrient concentration in the plant (Mip) to that in the soil (Mis). Pearson’s correlation coefficients were then computed between the bioaccumulation factor (Mip/Mis) and soil properties to identify the variables most strongly influencing micronutrient bioaccumulation. In the next step, several regression models were developed. The bioaccumulation factor was used as the dependent variable, while the selected soil properties that most strongly affected micronutrient bioaccumulation served as independent variables. The SCVs were then calculated from the best-fitting equations (with the highest r2 values) by substituting the plant critical value (PCV) for Mip.
In the second method, referred to as the high-yield method, the subset of fields with high yields—defined as at least 50% higher than the national average yield for each crop—was selected. Within this subset, soil micronutrient concentrations (Mis) were arranged in ascending order, and the lower quintile (QU1) was calculated. This quintile value was taken as the SCV, while observations below QU1 (representing 20% of the ordered dataset) were excluded as random data points.
All statistical analyses were performed using Statgraphics Plus 5.1 (StatPoint Technologies, Inc., Warrenton, VA, USA).
3. Results and Discussion
3.1. Soil and Plant Sample Characterization
Soil samples collected from wheat, rapeseed, and maize fields exhibited similar mean values of pH, organic carbon (Corg), and Mehlich 3-extractable phosphorus (PM3) (Table 1). Soils under rapeseed cultivation were characterized by slightly lighter texture compared with soils from wheat and maize fields.

Table 1.
Physicochemical properties of soil samples.
Mean concentrations of micronutrients in soils varied among the studied crops as shown in Table 2. B differs significantly among wheat, rapeseed, and maize. Fe in rapeseed soils is significantly higher than in wheat and maize soils. Cu, Mn, and Zn do not show significant differences among the three crops.

Table 2.
Mehlich 3-extractable micronutrient concentrations in soil samples (mg kg−1).
The mean concentrations of micronutrients in plant tissues differed significantly among species, however the ranges were very wide, particularly for Fe, reflecting the heterogeneity of soil conditions (Table 3). This variability corresponds to the wide ranges observed in the corresponding soil samples (Table 2).

Table 3.
Micronutrient concentrations in crop shoots (mg kg−1 dry matter).
The largest interspecific differences were observed for B. Boron concentrations in rapeseed shoots were more than ninefold, and in maize shoots more than twofold, higher than those in wheat. Maize generally accumulated the highest concentrations of Cu, Fe, and Mn, whereas rapeseed, in addition to its exceptionally elevated B levels, exhibited the highest Zn content. Considerable variability in micronutrient concentrations among the shoots of different crop species was also reported by Korzeniowska and Stanislawska-Glubiak [40]. This variability highlights the need to establish species-specific SCVs for micronutrients.
3.2. Yield Description
The mean grain yield of wheat was 6.0 t ha−1, while mean seed yield of rapeseed and grain yield of maize amounted to 3.6 and 9.0 t ha−1, respectively. Wheat grain yield most frequently ranged between 5.0 and 6.9 t ha−1, accounting for 45% of all analyzed fields, whereas yields of 7.0 t ha−1 and higher were recorded for 33% of the fields (Table 4).

Table 4.
Distribution of crop yields.
In the case of rapeseed, seed yields between 3.0 and 3.9 t·ha−1 predominated, representing 46% of all fields, while yields of at least 4.0 t ha−1 occurred on 39% of the fields. For maize, yields most commonly ranged from 6.0 to 9.9 t ha−1 (59% of the fields), whereas high-yielding sites with ≥10.0 t·ha−1 accounted for 27% of the total.
3.3. Influence of Soil Properties on Critical Values
The establishment of SCVs for soil micronutrient contents should consider soil parameters that govern their bioavailability to plants. The literature highlights the key roles of soil pH, texture, and organic carbon content [7]. Other soil parameters, such as redox potential, nutrient interactions, and the aeration–water regime, also influence micronutrient uptake. For practical applications in advisory systems, this study focused on relating SCV to soil parameters that are easily measurable and can be expressed numerically.
For this reason, our study considered soil pH, texture, organic carbon content (Corg), and Mehlich-3 extractable phosphorus (PM3), which may interact with micronutrients. To identify the soil parameter exerting the strongest control on micronutrient uptake, the bioaccumulation factor (Mip/Mis) was employed. This ratio, defined as the concentration of a micronutrient in plant tissue (Mip) relative to its concentration in soil (Mis), provides a measure of elemental phytoavailability. Pearson correlation analysis between Mip/Mis and soil parameters allowed the identification of the soil characteristics exerting the strongest influence on micronutrient availability, depending on both the plant species and the specific micronutrient (Table 5).

Table 5.
Pearson correlations between bioaccumulation factor (Mip/Mis) and soil properties for wheat (n = 1921), rapeseed (n = 1944) and maize (n = 916).
In wheat, the bioaccumulation of B and Mn showed the strongest negative correlations with soil pH, whereas Zn bioaccumulation was negatively correlated with both pH and soil P. Cu bioaccumulation was negatively related to the silt fraction and Corg. For Fe, the strongest correlation was positive with the fraction <0.02 mm, while a negative correlation was observed only with soil P.
In oilseed rape, B and Cu bioaccumulation was most strongly influenced by Corg, Mn bioaccumulation by pH, and Zn by soil P. All these correlations were negative. For Fe, the strongest correlations were positive and associated with soil pH and texture. Similarly to wheat, Fe bioaccumulation was negatively correlated only with soil P.
In maize, the strongest negative correlations were observed between B and Mn bioaccumulation and soil pH, as well as between Cu bioaccumulation and Corg. Zn showed a strong positive correlation with soil P, while Fe exhibited a strong positive correlation with soil texture. Additionally, Zn was weakly negatively correlated with the clay fraction, and Fe was negatively correlated with soil P.
The soil factors most strongly affecting Cu and Zn bioaccumulation varied among plant species. Differences in root system architecture, the intensity of root exudation, and the physiological demand for micronutrients likely explain these variations. Consequently, the same soil factor may influence the availability and uptake of a given element to different extents across crop species.
3.4. Calculation of SCVs Using Regression Models Method
SCVs were estimated using simple linear regression models. For each micronutrient–crop combination, the bioaccumulation factor (Mip/Mis) was used as the dependent variable, and the independent variable was the soil property (pH, Corg, PM3, or texture). Regression equations were developed using Statgraphics software. In most cases, the soil property showing the strongest negative correlation was included in the regression model (Table 5). However, in three cases—wheat–Cu, wheat–Zn, and maize–B—other soil properties were used when considered more appropriate; these exceptions are discussed in the following sections.
For each micronutrient, eight regression models were tested to identify the equation with the highest coefficient of determination (r2): linear, exponential, reciprocal, logarithmic, multiplicative, and square-root. The SCVs were calculated from the equations with the highest r2 values. Table 6 illustrates the equations describing the relationship between the boron bioaccumulation factor (Bp/Bs) and soil pH for wheat. In this case, the multiplicative model was selected for SCVs estimation.

Table 6.
Regression equations describing the relationship between boron bioaccumulation factor (Bp/Bs) and soil pH for wheat (n = 1921).
In the next step, the entire range of soil pH was divided into four class intervals. Class boundaries were defined based on the frequency distribution of pH values obtained from the histogram. For each interval, calculations were performed using the same multiplicative equation, with the mean pH of each interval used as the independent variable.
After appropriate transformation of the equation, the plant critical value (PCV) was substituted for Bp. Then, Bs was calculated for each pH interval. Bs represents the SCV for boron. The PCV for wheat was adopted from Korzeniowska et al. [33] (Table 7). For other crops and micronutrients, PCVs reported by Stanislawska-Glubiak et al. [41] and Korzeniowska et al. [34] were used.

A similar procedure was applied to the other soil properties (Corg, PM3, and the <0.02 mm fraction) used as independent variables in the regression equations for the remaining micronutrients. For each property, class intervals were defined based on the frequency distribution obtained from the histogram, and the mean value of each interval was used as the independent variable in the respective equations.
The equations with the highest coefficients of determination, which were subsequently used to calculate the SCV for each micronutrient, are presented in Table 8.

Table 8.
Selected regression equations for calculating SCVs.
A similar regression-based approach for determining SCVs of zinc for rice was applied by Slaton et al. [42]. Unlike our study, which used the bioaccumulation factor (Mip/Mis) as the dependent variable, these authors used relative grain yield at 90% of the maximum yield as the response variable. To examine the relationship between grain yield and the independent variables, which were soil ZnM3 and pH, they applied linear and quadratic regression models. The highest coefficient of determination obtained was r2 = 0.486. It should be noted, however, that their dataset was derived from fertilization experiments and included only 36 observations (n = 36).
In our study, the coefficients of determination (r2) obtained from the regression models were relatively low, although highly significant (p < 0.001), which is typical for large datasets. The value of r2 is strongly influenced by sample size: extensive datasets often yield lower r2 values yet provide more reliable and statistically robust estimates. In contrast, small datasets can produce artificially inflated r2 values that may lead to misleading interpretations [43,44]. High r2 values are achievable in tightly controlled field experiments conducted at a single site; however, such results have limited practical relevance because they reflect only local conditions. When SCVs are established at the national scale—where micronutrient bioaccumulation is affected by a wide range of interacting factors—low but statistically significant r2 values are unavoidable.
In large datasets, even models incorporating several measurable soil properties explain only part of the variance in the dependent variable. The remaining variation represents uncontrolled “noise,” driven by factors not included in the model and difficult to quantify, such as weather variability, interactions among soil constituents, and other site-specific processes.
However, the generally low r2 values in our models led us to adopt a complementary approach—the highyield method. We assumed that close agreement between SCVs de-rived from the highyield method and those from the PCV-based regression models would support the reliability of the regression-derived SCVs despite their low r2 values.
3.5. Calculation of SCVs Using the High-Yield Method
To validate the SCVs obtained by the regression models method, the highyield method was applied. This approach assumes that crops achieving high yields were grown under optimal soil nutrient conditions. Accordingly, the SCV was defined as the lowest soil concentration of a given micronutrient that still allowed high yield production. High yields were defined as at least 50% above the national average for each crop.
From each of the three datasets (n = 1921, n = 1944, and n = 916), cases with high yields were selected: ≥7.0 t ha−1 for wheat, ≥4.0 t ha−1 for rapeseed, and ≥10 t ha−1 for maize. The resulting high-yield subsets included 578 observations for wheat, 755 for rapeseed, and 416 for maize. Each subset was further divided according to the soil property used as the independent variable in the regression equation for the corresponding micronutrient (Table 9). Within each subset, soil micronutrient concentrations were ranked in ascending order, and the lower quintile (20 percentile) (QU1) was calculated for each micronutrient. This QU1 value was taken as the minimum soil concentration required to achieve high yield.

Table 9.
Comparison of the critical values of low BM3 concentration in soil for wheat calculated by various methods (mg kg−1).
For example, to determine the SCV for boron in wheat, the high-yield dataset was divided by soil pH, and QU1 was calculated for each pH interval. In this case, the SCVs obtained using the regression models method and the highyield method were very similar (Table 9). The final SCV for each interval was calculated as the mean of the two values. We also tested the 10th, 15th, and 20th percentiles, but the 20th percentile (QU1) produced the most similar SCVs to those obtained with the regression-model method and was therefore adopted as the SCV.
As noted earlier, the soil property showing the strongest correlation with the bioaccumulation factor (Mip/Mis) (Table 5) was usually selected for the regression models. In some cases, however, a different property was chosen. For example, for wheat and Cu, organic carbon (Corg) was used instead of silt, and for maize and B, the fine fraction (Fr < 0.02) was selected instead of soil pH. The choice of the independent variable was guided by general knowledge of micronutrient availability and by the agreement between the SCVs obtained from the regression models method and those calculated using the high- yield method. When larger discrepancies occurred, the soil property for which both methods produced the most similar results was selected.
The definitive SCVs for wheat, rapeseed, and maize are presented in Table 10, Table 11 and Table 12.

Table 10.
Critical values of low micronutrient concentrations in soil for wheat.

Table 11.
Critical values of low micronutrient concentrations in soil for rapeseed.

Table 12.
Critical values of low micronutrient concentrations in soil for maize.
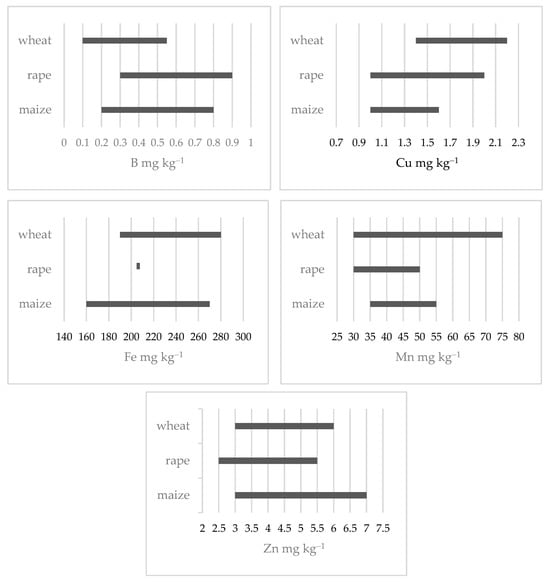
Figure 2 shows the comparative ranges of SCVs for micronutrients in three crops: wheat, rapeseed, and maize. The comparison includes SCVs related to specific soil properties, such as pH, Corg, PM3, and texture. These ranges indicate the micronutrient levels below which deficiency symptoms may occur. The comparison reveals clear species-specific differences in sensitivity to micronutrient deficiencies. Rapeseed shows the highest critical values for B, reflecting its known susceptibility to B deficiency. Wheat has relatively higher critical thresholds for Cu and Mn, indicating greater sensitivity to limited availability of these elements. Maize exhibits the highest SCVs for Zn.
3.6. Comparison of SCVs with Literature Values
The SCVs developed in this study are in some cases similar to those reported by other authors, but often differ considerably. These differences arise mainly because our SCVs were established specifically for individual crop species, whereas many previously published values are intended to be more general or universal. In addition, discrepancies may also result from variations in soil and climatic conditions among countries. Different studies account for distinct soil parameters in their calculations, and these parameters have a significant influence on the resulting SCVs.
The SCVs for B determined in this study differed from literature values in both their level and the soil properties influencing them (Table 13). In our study, SCVs for B depended on pH (wheat), Corg (rapeseed), and Fr < 0.02 (maize), ranging from 0.10 to 0.90 mg kg−1 depending on pH and crop species. A similar range (0.40–0.65 mg kg−1), dependent on soil pH, was reported for rice by Seth et al. [45]. Zbiral [26] proposed SCVs of 0.55–0.88 mg kg−1, depending on soil texture, while Haefele et al. [25] suggested a fixed SCV of 1.0 mg kg−1 for cereals, independent of soil properties.
SCVs for Cu in our study were dependent on Corg for all three crops, ranging from 1.0 to 2.2 mg kg−1. Zbiral [26] suggested similar values: 2.0 mg kg−1 for cereals and 1.6 mg kg−1 for other crops. Haefele et al. [25] proposed 0.5 mg kg−1 for cereals, whereas the Espinoza et al. [46] generally uses 1.0 mg kg−1 as the SCV for Cu.
SCVs for Fe showed substantial differences. In our study, they ranged from 190 to 280 mg kg−1 and were related to PM3 for wheat and maize, while for rapeseed no clear relation with soil properties was observed. By comparison, Zbiral [26] proposed a uniform Fe SCV of 60 mg·kg−1, which was calculated by converting DTPA-based SCVs from a dataset of only 95 soil samples. A similar value of 60 mg·kg−1 was also reported by Haefele et al. [25] for cereals.
Mn SCVs were linked to soil pH for all crops. For wheat, values ranged from 30 to 75 mg kg−1, depending on pH. Haefele et al. [25] suggested 60 mg kg−1 for cereals, similar to our results but without considering pH. Our SCVs for oilseed rape and maize ranged from 30 to 50 mg·kg−1, depending on soil pH. Similar SCV levels were reported by Zbíral [26] and Espinoza et al. [46], at 45 and 40 mg·kg−1, respectively, without considering soil properties.
The SCVs for Zn determined in our study depended on available soil P. For wheat, they ranged from 3 to 6 mg kg−1, for rapeseed 2.5 to 5.5 mg kg−1, and for maize 3 to 7 mg kg−1. In comparison, Murdock and Howe [47] reported Zn SCVs that varied not only with the level of P but also with soil pH. The lowest Zn SCVs reported by these authors ranged from 0.55 to 0.90 mg kg−1, while the highest reached 2.5 to 2.8 mg kg−1.
Other authors relate Zn SCVs only to soil pH. According to Slaton et al. [42], these values range from 1.3 to 4.2 mg·kg−1, while Seth et al. [45] reported values between 1.27 and 2.15 mg·kg−1. Zbiral [26] proposed a single Zn SCV of 2.2 mg·kg−1, independent of soil type. In turn, Cuesta et al. (2020) [48] adopted an SCV of 1.97 mg·kg−1 for maize, and Haefele et al. [25] proposed an SCV of 1.0 mg·kg−1 for cereals, irrespective of any soil properties.

Table 13.
SCVs for micronutrients in soil reported by other authors.
Table 13.
SCVs for micronutrients in soil reported by other authors.
| Element | Author | Crop | Soil Property | SCV (mg kg−1) |
|---|---|---|---|---|
| B | Haefele et al. 2024 [25] | cereals | x | <1.0 |
| Seth et al. 2018 [45] | x | pH 5.1 | 0.40 | |
| pH 6.2 | 0.65 | |||
| Zbiral 2016 [26] | x | light soils | <0.55 | |
| medium soils | <0.70 | |||
| heavy soils | <0.85 | |||
| Cu | Haefele et al. 2024 [25] | cereals | x | <0.5 |
| Zbiral 2016 [26] | cereals | x | <0.2 | |
| others | x | <1.6 | ||
| Espinoza et al. 2018 [46] | x | x | <1.0 | |
| Fe | Haefele et al. 2024 [25] | cereals | <60 | |
| Zbiral 2016 [26] | x | all soils | <60 | |
| Mn | Haefele et al. 2024 [25] | cereals | x | <60 |
| Zbiral 2016 [26] | x | all soils | <45.0 | |
| Espinoza et al. 2018 [46] | x | x | <40 | |
| Zn | Haefele et al. 2024 [25] | cereals | x | <1.0 |
| Cuesta et al. 2021 [48] | maize | x | <1.97 | |
| Murdock and Howe 2001 [47] | x | P-25 mg kg−1: | ||
| pH 6.0–6.4 | 0.55–0.90 | |||
| pH 7.3–7.6 | 1.60–2.00 | |||
| P-250 mg kg−1: | ||||
| pH 6.0–6.4 | 1.40–1.80 | |||
| pH 7.3–7.6 | 2.50–2.80 | |||
| Espinoza et al. 2018 [46] | x | x | <1.6 | |
| Seth et al. 2018 [45] | x | pH 5.1 | 2.15 | |
| pH 6.2 | 1.27 | |||
| Slaton et al. 2002 [42] | x | pH 6.2 | 1.3 | |
| pH 7.0 | 3.5 | |||
| pH 7.8 | 4.2 | |||
| Zbiral 2016 [26] | x | all soils | <2.2 |
4. Conclusions
The SCVs determined in this study differ from previously published values both in their levels and in the soil properties influencing them. Many literature values are generalized and do not account for differences between soils or crop species. In our study, we considered both crop sensitivity to micronutrient deficiencies and soil properties affecting micronutrient availability.
The SCVs calculated in this study from large, representative datasets and plant-based indicators show species-specific differences, highlighting the need for crop-specific calibration. These SCVs provide more accurate thresholds than generalized values, improving the assessment of soil micronutrient status and the effectiveness of fertilization recommendations in Poland.
In practice, assessing soil micronutrient status requires proper field sampling and laboratory analysis using the Mehlich-3 extract. In addition to measuring micronutrient concentrations, it is important to consider soil properties affecting SCVs, such as pH, Corg, texture, and PM3. If a micronutrient concentration is below the SCV, the soil is considered deficient, and fertilization is recommended. If the concentration is equal to or above the SCV, the nutrient level is sufficient, and additional fertilization is not necessary. Low micronutrient levels can be corrected with either soil-applied or foliar fertilization, following recommended doses.
Author Contributions
Conceptualization, J.K. and E.S.-G.; methodology, J.K. and E.S.-G.; formal analysis, J.K. and E.S.-G.; investigation, J.K. and E.S.-G.; resources, W.L.; data curation, J.K. and E.S.-G.; writing—original draft preparation, J.K. and E.S.-G.; writing—review & editing, J.K. and E.S.-G.; funding acquisition, W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Polish Ministry of Agriculture and Rural Development under 2.33 Scientific Research Program of the Institute of Soil Science and Plant Cultivation in Pulawy.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of Nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012; pp. 191–248. [Google Scholar]
- Cakmak, I. Enrichment of Cereal Grains with Zinc: Agronomic or Genetic Biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Filippi, D.; Gatiboni, L.; Crozier, C.; Osmond, D.; Hardy, D. Effect of Model Choice on Critical Soil Test Value of Phosphorus for Corn in Long-Term Trials in North Carolina. Soil Sci. Soc. Am. J. 2025, 89, e70104. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W. Development of a DTPA Soil Test for Zinc, Iron, Manganese, and Copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Slaton, N.A.; Correndo, A.A.; Pearce, A.W.; Bolster, C.H.; Osmond, D.L.; Spargo, J.T. Models and Sufficiency Interpretation for Estimating Critical Soil Test Values for the Fertilizer Recommendation Support Tool. Soil Sci. Soc. Am. J. 2024, 88, 1419–1437. [Google Scholar] [CrossRef]
- Alloway, B.J. (Ed.) Micronutrient Deficiencies in Global Crop Production; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Mandi, S.; Nayak, S.; Shivay, Y.S.; Singh, B.R. Soil Organic Matter: Bioavailability and Biofortification of Essential Micronutrients. In Soil Organic Matter and Feeding the Future; Springer: Singapore, 2021; pp. 203–234. [Google Scholar]
- Covelo, E.F.; Vega, F.A.; Andrade, M.L. Competitive Sorption and Desorption of Heavy Metals by Individual Soil Components. J. Hazard. Mater. 2007, 140, 308–315. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C.; Clark, R.B. Micronutrients in Crop Production. Adv. Agron. 2002, 77, 185–268. [Google Scholar]
- Wang, S.; Xu, L.; Hao, M. Impacts of Long-Term Micronutrient Fertilizer Application on Soil Properties and Micronutrient Availability. Int. J. Environ. Res. Public Health 2022, 19, 16358. [Google Scholar] [CrossRef]
- McLaughlin, M.J.; Zarcinas, B.A.; Stevens, D.P.; Cook, N. Soil Testing for Heavy Metals. Commun. Soil Sci. Plant Anal. 2000, 31, 1661–1700. [Google Scholar] [CrossRef]
- Trierweiler, J.F.; Lindsay, W.L. EDTA-ammonium carbonate soil test for zinc. Soil Sci. Soc. Am. J. 1969, 33, 49–54. [Google Scholar] [CrossRef]
- Korzeniowska, J.; Stanislawska-Glubiak, E. Comparison of 1 M HCl and Mehlich 3 for Assessment of the Micronutrient Status of Polish Soils in the Context of Winter Wheat Nutritional Demands. Commun. Soil Sci. Plant Anal. 2015, 46, 1263–1277. [Google Scholar] [CrossRef]
- Mehlich, A. Determination of P, Ca, Mg, K, Na, and NH4; North Carolina Soil Test Division (Mimeo 1953): Raleigh, NC, USA, 1953. [Google Scholar]
- Mehlich, A. Mehlich 3 Soil Test Extractant: A Modification of Mehlich 2 Extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Antonangelo, J.A.; Souza, J.L.B.; Whitaker, A.; Arnall, B.; Zhang, H. Evaluation of Mehlich-3 as a Multi-Element Extractant of Micronutrients and Sulfur in a Soil–Ryegrass System Amended with Varying Biochar Rates from Two Feedstocks. Land 2022, 11, 1979. [Google Scholar] [CrossRef]
- Bortolon, L.; Gianello, C. Multielement Extraction from Southern Brazilian Soils. Commun. Soil Sci. Plant Anal. 2012, 43, 1615–1624. [Google Scholar] [CrossRef]
- Brennan, D.; Coulter, B.; Mullen, G.; Courtney, R. Evaluation of Mehlich 3 for Extraction of Copper and Zinc from Irish Grassland Soils and for Prediction of Herbage Content. Commun. Soil Sci. Plant Anal. 2008, 39, 1943–1962. [Google Scholar] [CrossRef]
- Cancela, R.C.; de Abreu, C.A.; Paz-González, A. DTPA and Mehlich-3 Micronutrient Extractability in Natural Soils. Commun. Soil Sci. Plant Anal. 2002, 33, 2879–2893. [Google Scholar] [CrossRef]
- Liu, J.; Liao, Z.; Hu, C.; Qiu, W.; Sun, X.; Tan, Q. Relationship between Mehlich-3, ASI and Routine Methods of Soil Available Nutrients Analysis for Paddy Soils in China. J. Food Agric. Environ. 2011, 9, 516–520. [Google Scholar]
- Ostatek-Boczynski, Z.; Lee-Steere, P. Evaluation of Mehlich 3 as a Universal Nutrient Extractant for Australian Sugarcane Soils. Commun. Soil Sci. Plant Anal. 2012, 43, 623–630. [Google Scholar] [CrossRef]
- Rodriguez-Suarez, J.A.; Arias, M.; Lopez, E.; Soto, B. Comparison of Multi-Element to Single-Element Extractants for Macro- and Micronutrients in Acid Soils from Spain. Commun. Soil Sci. Plant Anal. 2008, 39, 231–240. [Google Scholar] [CrossRef]
- Sobral, L.F.; Smyth, J.T.; Fageria, N.K.; Stone, L.F. Comparison of Copper, Manganese, and Zinc Extraction with Mehlich 1, Mehlich 3, and DTPA Solutions for Soils of the Brazilian Coastal Tablelands. Commun. Soil Sci. Plant Anal. 2013, 44, 2507–2513. [Google Scholar] [CrossRef]
- Haefele, S.M.; Mossa, A.W.; Gashu, D.; Nalivata, P.C.; Broadley, M.R.; McGrath, S.P.; Thomas, C.L. Mehlich 3 as an Indicator of Grain Nutrient Concentration for Five Cereals in Sub-Saharan Africa. Field Crops Res. 2024, 307, 109243. [Google Scholar] [CrossRef]
- Zbíral, J. Determination of Plant-Available Micronutrients by the Mehlich 3 Soil Extractant—A Proposal of Critical Values. Plant Soil Environ. 2016, 62, 527–531. [Google Scholar] [CrossRef]
- Pommerrenig, B.; Junker, A.; Abreu, I.; Bieber, A.; Fuge, J.; Willner, E.; Bienert, M.D.; Altmann, T.; Bienert, G.P. Identification of Rapeseed (Brassica napus) Cultivars with a High Tolerance to Boron-Deficient Conditions. Front. Plant Sci. 2018, 9, 1142. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, H.; Shi, L.; Xu, F. Physiological and Genetic Responses to Boron Deficiency in Brassica napus: A Review. Soil Sci. Plant Nutr. 2014, 60, 304–313. [Google Scholar] [CrossRef]
- Sharma, P.N.; Chatterjee, C.; Sharma, C.P.; Agarwala, S.C. Zinc Deficiency and Anther Development in Maize. Plant Cell Physiol. 1987, 28, 11–18. [Google Scholar] [CrossRef]
- Suganya, A.; Saravanan, A.; Manivannan, N. Role of Zinc Nutrition for Increasing Zinc Availability, Uptake, Yield, and Quality of Maize (Zea mays L.) Grains: An Overview. Commun. Soil Sci. Plant Anal. 2020, 51, 2001–2021. [Google Scholar] [CrossRef]
- Karamanos, R.E.; Goh, T.B.; Harapiak, J.T. Determining Wheat Responses to Copper in Prairie Soils. Can. J. Soil Sci. 2003, 83, 213–221. [Google Scholar] [CrossRef]
- Brennan, R.F. Long-Term Residual Value of Copper Fertiliser for Production of Wheat Grain. Aust. J. Exp. Agric. 2006, 46, 77–83. [Google Scholar] [CrossRef]
- Korzeniowska, J.; Stanislawska-Glubiak, E.; Lipinski, W. Opracowanie liczb granicznych niedoboru mikroelementów w glebie oznaczanych przy użyciu ekstrahenta Mehlich 3 dla polskich warunków glebowych. Część I. Pszenica. Soil Sci. Annu. 2019, 70, 314–323. [Google Scholar] [CrossRef]
- Korzeniowska, J.; Stanislawska-Glubiak, E.; Lipinski, W. New Limit Values of Micronutrient Deficiency in Soil Determined Using 1 M HCl Extractant for Wheat and Rapeseed. Soil Sci. Annu. 2020, 71, 205–214. [Google Scholar] [CrossRef]
- PN-R 04014:1991; Agrochemical Plant Analyse. Methods of Mineralization of Plant Material for Determination of Macro- and Microelements. Polish Committee for Standardization: Warsaw, Poland, 1991.
- ISO 10390:2005; Soil Quality—Determination of pH. International Standardization Organization: Geneva, Switzerland, 2005.
- PN-ISO 14235:2003; Soil Quality: Determination of Organic Carbon in Soil by Sulfochromic Oxidation. Polish Committee for Standardization: Warsaw, Poland, 2003.
- Korzeniowska, J.; Stanislawska-Glubiak, E. Evaluation of the Egner–Riehm DL and Mehlich 3 Tests for the Determination of Phosphorus: The Influence of Soil Properties on Extraction Efficiency and Test Conversion. Agronomy 2024, 14, 2921. [Google Scholar] [CrossRef]
- Bergmann, W. Nutritional Disorders of Plants—Development, Visual and Analytical Diagnosis; Gustav Fischer Verlag: Jena, Germany; Stuttgart, Germany; New York, NY, USA, 1992. [Google Scholar]
- Korzeniowska, J.; Stanislawska-Glubiak, E. Differences in the Concentration of Micronutrients in Young Shoots of Numerous Cultivars of Wheat, Maize and Oilseed Rape. Agronomy 2022, 12, 2639. [Google Scholar] [CrossRef]
- Stanislawska-Glubiak, E.; Korzeniowska, J.; Lipinski, W. Opracowanie liczb granicznych niedoboru mikroelementów w glebie oznaczanych przy użyciu ekstrahenta Mehlich 3 dla polskich warunków glebowych. Czesc II. Rzepak. Soil Sci. Annu. 2019, 70, 324–330. [Google Scholar]
- Slaton, N.A.; Wilson, C.E.; Norman, R.J.; Gbur, E.E. Development of a Critical Mehlich 3 Soil Zinc Concentration for Rice in Arkansas. Commun. Soil Sci. Plant Anal. 2002, 33, 2759–2770. [Google Scholar] [CrossRef]
- Cramer, J.S. Mean and Variance of R2 in Small and Moderate Samples. J. Econom. 1987, 35, 253–266. [Google Scholar] [CrossRef]
- Zaarour, N.; Melachrinoudis, E. What’s in a Coefficient? The “Not So Simple” Interpretation of R2 for Relatively Small Sample Sizes. J. Educ. Train. Stud. 2019, 7, 27. [Google Scholar] [CrossRef]
- Seth, A.; Sarkar, D.; Masto, R.E.; Batabyal, K.; Saha, S.; Murmu, S.; Das, R.; Padhan, D.; Mandal, B. Critical Limits of Mehlich 3 Extractable Phosphorus, Potassium, Sulfur, Boron and Zinc in Soils for Nutrition of Rice (Oryza sativa L.). J. Soil Sci. Plant Nutr. 2018, 18, 512–523. [Google Scholar]
- Espinoza, L.; Slaton, N.; Mozaffari, M. Understanding the Numbers on Your Soil Test Report. Agric. Nat. Resour. 2018, FSA-2118, 1–4. Available online: https://www.uaex.uada.edu/publications/PDF/FSA-2118.pdf (accessed on 20 October 2025).
- Murdock, L.W.; Howe, P.L. Zinc Fertilizer Rates and Mehlich III Soil Test Levels for Corn. Agron. Notes 2001, 33, 1–7. Available online: http://uknowledge.uky.edu/pss_notes/8 (accessed on 20 October 2025).
- Cuesta, N.M.; Wyngaard, N.; Saínz-Rozas, H.; Reussi-Calvo, N.; Carciochi, W.; Eyherabide, M.; Colazo, J.C.; Barraco, M.; Guertal, E.A.; Barbieri, P. Determining Mehlich-3 and DTPA Extractable Soil Zinc Optimum Economic Threshold for Maize. Soil Use Manag. 2021, 37, 736–748. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).