Abstract
This study investigates the residue characteristics and potential ecological risks of glyphosate (GLY) and its primary metabolite, aminomethylphosphonic acid (AMPA), in the karst region, specifically focusing on the Yangmei River sub-basin. Water samples from the river were collected in April, June, August, and October of 2023, alongside 20 soil samples taken based on agricultural tillage practices. The residue characteristics of GLY and AMPA were analyzed, and their potential ecological risks were assessed using the Risk Quotient (RQ) method. The results indicated that the residues of GLY and AMPA in the soil of the Yangmei River basin exhibited spatial heterogeneity. The GLY content in the soil ranged from non-detectable (nd) to 888.85 μg/kg, with an average concentration of 262.53 μg/kg. The AMPA content varied from 47.90 to 2102.10 μg/kg, with an average of 465.52 μg/kg. Glyphosate pollution in the soil of the Yangmei River basin was determined to pose a moderate ecological risk. In the water of the Yangmei River basin, GLY concentrations ranged from 0 to 204.0 μg/L, with an average of 50.91 μg/L, while AMPA concentrations varied from 0 to 127.26 μg/L, averaging 26.51 μg/L. The highest GLY concentration was recorded in June, with detection rates for GLY being higher in April and June. The spatial distribution of GLY and AMPA was uneven. Glyphosate pollution in the water environment of the Yangmei River basin also presents a moderate ecological risk. Moreover, glyphosate has negatively impacted the aquatic environment, and its effects on water eutrophication should not be overlooked in efforts to prevent and control this phenomenon.
1. Introduction
Eutrophication of water bodies has emerged as a significant global ecological and environmental issue [], with agricultural non-point source pollution identified as a primary contributor []. Glyphosate (GLY), while playing a crucial role in agricultural production, is also a major factor in agricultural non-point source pollution. GLY is an efficient, broad-spectrum, non-selective organophosphorus herbicide widely employed for weed control across agriculture, forestry, aquatic environments, and urban areas []. Since its commercialization by Monsanto in 1974, GLY has become the most widely used herbicide in global agriculture, accounting for over 72% of total global pesticide usage []. In Europe, GLY holds a market share of 33% of all herbicide sales, positioning it as one of the most frequently utilized herbicides in European agriculture. Projections indicate that by 2024, global glyphosate usage will reach 2.5 million tons, with a continued upward trend []. China has emerged as the largest global producer of GLY, with production reaching 500,000 tons in 2018, while consumption was reported at 40,000 tons. By 2019, production further increased to 550,000 tons []. GLY exhibits high environmental persistence, with a half-life ranging from 222 to 835 days []. The widespread use of GLY has led to its significant accumulation in farmland and surrounding water bodies. GLY has been detected across various environmental media, including the atmosphere, precipitation, surface water, groundwater, and soil. Research indicates that the concentration of GLY in rainwater can reach up to 0.48 g/L []. In the United States, GLY concentrations in surface and groundwater range from 2 to 430 μg/L [], while in Europe, the concentrations vary from 0.59 to 165 μg/L []. Notably, these concentrations exceed the maximum contamination limits established by the United States (700 µg/L) and the European Union (0.1 µg/L) []. Furthermore, glyphosate has been detected in human urine at concentrations as high as 2.10 µg/L [], suggesting that GLY has infiltrated the human internal environment. Additionally, pollutants commonly found in glyphosate formulations, such as petroleum, heavy metals, and polyethylene oxide tallow amine, are frequently overlooked in risk assessments [,,].
The impacts of GLY are multifaceted. On one hand, it interferes with the metabolic pathways of plants and key microorganisms, potentially leading to physiological and behavioral dysfunctions in aquatic organisms and posing threats to human health [,]. On the other hand, GLY can regulate cyanobacterial phosphorus uptake, can influence bacterial community structures related to algal blooms, and may serve as the sole phosphorus source for the growth of certain phytoplankton [,]. For instance, phosphate produced from the degradation of GLY can significantly promote the growth of Microcystis aeruginosa, thereby increasing the risk of water eutrophication [].
Aquifers in karst regions supply drinking water to approximately 20–25% of the global population, and karst aquifers are therefore among the most important yet vulnerable freshwater resources. The highly developed conduit networks characteristic of karst systems permit rapid, often channelized transport of surface-derived solutes into groundwater; as a result, herbicides applied at the surface—including glyphosate and its primary metabolite AMPA—can reach groundwater with limited attenuation under certain hydrogeological conditions [,]. Guizhou Province, located on the Yungui Plateau at the headwaters/transition zone of the Yangtze and Pearl River basins, occupies a strategic hydrological position and therefore plays an important role in safeguarding drinking-water resources for downstream populations []. A survey on pesticide use in rural areas of Guizhou conducted by Yang et al. [] reports glyphosate application intensities in Guizhou that reflect intensive local usage; regional monitoring studies have also detected glyphosate/AMPA in surface water and drinking-water source areas in the province, underscoring the potential for contaminant transfer from agricultural land to water supplies. Consequently, research focused on the prevention, control, and remediation of glyphosate contamination in karst soils is vital for protecting basin-scale ecological security and drinking-water quality.
To design effective prevention and control measures, it is essential to obtain a systematic understanding of the contamination status and its spatiotemporal distribution in karst agricultural landscapes. Current investigations in karst settings tend to be spatially fragmented or localized, and long-term in situ monitoring data remain scarce, which limits regional or watershed-scale risk appraisal []. Therefore, this study quantifies glyphosate application in the study area and analyzes residual patterns of glyphosate and AMPA in both soils and waters. We apply the risk-quotient (RQ) approach to screen and map ecological risk (exposure concentration/PNEC) and identify priority risk areas, following established pesticide risk-assessment practice. Finally, geostatistical interpolation (ordinary Kriging) is used to resolve the spatial distribution patterns of glyphosate and AMPA across the study domain, providing the spatially explicit information necessary for zoning, classification, and graded management of herbicide pollution in karst regions.
2. Materials and Methods
2.1. Overview of the Study Area
Guizhou, one of the most representative regions globally with developed karst landforms, is characterized by exposed carbonate rocks on the surface, resulting in a highly fragile ecological environment. Calcareous soils, which have developed from the evolution of carbonate rocks, are primarily found in karst hilly areas, where the soil layer is thin, gravel content is high, and the erosion of the soil surface is severe due to the steep terrain []. Furthermore, the unique dual hydrological structure of karst regions exacerbates soil loss. The specific geological background and pedological environment contribute to the infertile, sparse, and scattered distribution of soil, leading to extremely limited agricultural land resources []. Soils in karst regions exhibit a low capacity to retain pollutants, allowing contaminants to migrate easily through surface and groundwater, resulting in widespread contamination with serious consequences and significant challenges for remediation. In recent years, the migration of a large number of middle-aged and young individuals for work has prompted farmers to extensively use GLY herbicides and other pesticides to reduce production costs. Consequently, farming on sloped land has emerged as a major source of agricultural non-point source pollution. The basin covers approximately 21.6 km2 and features convenient accessibility, limited industrial development, and an economy predominantly sustained by agriculture. Owing to its representative geomorphological setting and land use structure, the Yangmei River Basin serves as a typical small karst agricultural watershed, providing an ideal case study for investigating the interactions among rainfall, surface runoff, and non-point source pollution in karst environments [].
The region is primarily composed of carbonate rocks, including dolomite and limestone from the Huaxi and Longtou formations. These rocks have undergone dissolution and erosion, resulting in the formation of karst basins and peak-cluster landscapes. The soil types present in the area include yellow soils, calcareous soils, and paddy soils. The climate is characterized as subtropical monsoon, with an average annual temperature of 15.3 °C and annual precipitation ranging from 1100 to 1200 mm. The flood season extends from May to October, during which rainfall serves as the main source of groundwater recharge and surface runoff in the Yangmei River basin. As a representative small agricultural watershed, land use in the Yangmei River basin is primarily classified into dry land and paddy fields. Due to frequent agricultural activities and the extensive use of pesticides and fertilizers, rainfall-induced surface and subsurface flow processes significantly enhance the transport of agrochemicals, making dry sloping fields the principal contributors to non-point source pollution (Figure 1).
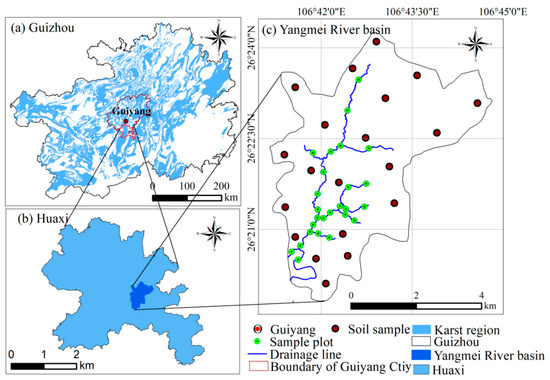
Figure 1.
The location of the study area.
2.2. Sample Collection and Preservation
In April, June, August, and October of 2023, soil and water samples were collected from the study area. Based on the confluence of tributaries and the main stream of the Yangmei River, 26 water sampling points were evenly distributed along the river in accordance with the principles of background, monitoring, and attenuation sections. Water samples were obtained using a dedicated 5 L acrylic water sampler, targeting surface water at a depth of 0–0.5 m. Immediately after collection, the water samples were placed in ice boxes and transported to the laboratory for cold storage, where they were subsequently analyzed for GLY, its metabolite AMPA, and other physicochemical parameters of the water. For soil sampling, six transects were established according to the spatial distribution and utilization of cultivated land within the watershed. A total of 20 soil sampling sites were designated, where surface soils (0–10 cm) were randomly collected to ensure representativeness of different land use types and topographic conditions. At each site, two soil samples were obtained: one was air-dried and sieved for the determination of basic physicochemical properties, and the other was freeze-dried, sieved, and stored for the analysis of GLY and AMPA concentrations.
2.3. Measurement Methods
- (1)
- Physicochemical Parameters of Water Samples
For water sample analysis, total phosphorus (TP) was determined using the potassium persulfate digestion-molybdenum blue colorimetric method. Chemical oxygen demand (CODMn) was measured by the acidic potassium permanganate index method. The total organic carbon (TOC) and total nitrogen (TN) in the water samples were measured using a Jinsima VCPH-type TOC analyzer (Shimadzu Corporation, Kyoto, Japan). Physicochemical parameters of the water, including water temperature, pH, dissolved oxygen, and electrical conductivity, were measured in real time on-site using the YSI 6600V2 multiparameter water quality analyzer (YSI Inc., Yellow Springs, OH, USA).
- (2)
- Measurement of Soil Basic Physicochemical Properties
Soil moisture content was determined by the drying to constant weight method, by drying the soil at 105 °C for 10 h. Electrical conductivity (EC) was measured using a conductivity meter, with a soil-to-water ratio of 5:1. In addition, the determination of soil pH, soil organic carbon (SOC), total nitrogen (TN), total phosphorus (TP), and other nutrient indicators, as well as the determination of soil enzyme activity, followed the methods outlined in “Soil Agricultural Chemical Analysis Methods” by Baosidan [] Specific measurement methods are detailed in Table 1.

Table 1.
Methods for determination of conventional soil indicators.
- (3)
- Determination Methods for GLY and AMPA
A 2 g soil sample was weighed and placed into a 50 mL plastic centrifuge tube, followed by the addition of 10 mL of 0.6 mol/L potassium hydroxide solution for extraction. The sample was subsequently shaken for 1 h on a shaker and then centrifuged at 3500 r/min for 15 min. After centrifugation, 1 mL of the supernatant was transferred into a 10 mL centrifuge tube, to which 80 µL of 6 mol/L hydrochloric acid was added to adjust the pH to approximately 9. Following this, 0.5 mL of 5% borate buffer and 0.5 mL of 6.5 mmol/L 9-fluorenylmethyl chloroformate (FMOC-Cl) were added and thoroughly mixed by shaking. The derivatization reaction was allowed to proceed at room temperature for 30 min. Upon completion, 50 µL of formic acid was added, and the solution was mixed once more. A 0.5 mL portion of the derivative was filtered through a 0.22 µm PTFE membrane and transferred into a vial for analysis. The standard curve concentrations were prepared as detailed in Table 2, following the same derivatization procedure as the samples []. For the collected earthworm samples, freeze-dried tissues were ground and sieved, and GLY and AMPA were quantified using the same procedure as that employed for the soil samples.

Table 2.
Gradient elution procedure.
To determine the concentrations of GLY and AMPA in water samples, begin by adding 0.5 mL of a 5% borate buffer and 0.5 mL of a 6.5 mmol/L solution of chloromethyl-9-fluorenylmethyl ester to the water sample. Shake the mixture thoroughly to ensure proper mixing. Allow the derivatization reaction to proceed for 30 min. Following this reaction period, introduce 50 µL of formic acid, mix the solution again, and then transfer 0.5 mL of the derivative into a test bottle that has been pre-filtered using a 0.22 µm PTFE membrane for analysis.
The samples were analyzed using a liquid chromatography-tandem mass spectrometry (LC-MS/MS) system (Agilent 1290 UHPLC coupled with an Agilent 6470A Triple Quadrupole Mass Spectrometer, Agilent Technologies, Santa Clara, CA, USA). Separation was achieved using a Hypersil GOLD™ C18 column (100 mm × 2.1 mm, 1.9 μm; Thermo Fisher Scientific, Waltham, MA, USA). The mobile phase comprised two components: (A) 0.1% formic acid and 10 mmol ammonium acetate in water, and (B) acetonitrile. Gradient elution was conducted according to the conditions detailed in Table 2. The flow rate was maintained at 0.3 mL/min, with a column temperature set at 35 °C and an injection volume of 2 µL.
Regarding the mass spectrometer settings, the instrument was equipped with an electrospray ionization (ESI) source operating in positive ion mode. The parameters for the instrument were as follows: drying gas temperature of 300 °C, drying gas flow rate of 9 L/min, nebulizer pressure of 45 psi, sheath gas temperature of 350 °C, and sheath gas flow rate of 10 L/min. Quantification was carried out using the external standard method. The retention times, characteristic ions, and quantification ions for the target analytes are presented in Table 3 [], while the standard curve equations for GLY and AMPA are provided in Table 4.

Table 3.
Retention time of pesticide compounds and monitoring ion pairs.

Table 4.
Linear equation and correlation coefficient of standard curve of GLY and AMPA.
Detection and Quantification Limits: Nine replicate analyses were conducted on low-concentration mixed standard solutions to determine the detection limit (LOD). The LOD was calculated by substituting the threefold standard deviation of the measurements and the mean concentration into the linear equation for GLY. Similarly, the quantification limit (LOQ) was derived using a tenfold standard deviation. Subsequently, a concentration close to the LOQ was prepared and analyzed (n = 9). Instrument-determined LODs for water samples were 0.0024 μg/L for GLY and 0.0022 μg/L for AMPA; for soil samples, LODs were 0.5 μg/kg (GLY) and 0.45 μg/kg (AMPA). All LODs and LOQs meet the requirements for LC-MS/MS analysis.
Precision and Accuracy: Samples at two different concentration levels were analyzed six times to compute the relative standard deviation (RSD) for precision evaluation. Accuracy was assessed through spiked recovery experiments, where the average measured concentration was divided by the actual spiked concentration (μg/L) and multiplied by 100%. The spike recoveries were found to be between 98.06% and 100.76% for GLY and 99.53% to 99.55% for AMPA. The precision (RSD) ranged from 1.20% to 2.05% for GLY and from 1.19% to 1.85% for AMPA. Both precision and accuracy met the criteria for LC-MS/MS analysis of GLY and AMPA.
2.4. GLY Risk Assessment Method
- (1)
- Water GLY Pollution Risk Diagnosis Method
The Risk Quotient (RQ) method is used to assess the potential risks of GLY in the aquatic environment, involving the calculation of both acute (short-term) and chronic (long-term) risk quotients []. The formulas for calculating the Acute Risk Quotient (ARQ) and Chronic Risk Quotient (CRQ) are as follows:
In the formulas, ARQ represents the Acute Risk Quotient, and CRQ represents the Chronic Risk Quotient; CMAX is the maximum concentration of GLY in the water; CAVG is the average concentration of GLY in the water; SWQC is the short-term water quality criterion; LWQC is the long-term water quality criterion. In this study, the short-term and long-term water quality criteria used are 0.14 mg/L and 0.007 mg/L, respectively [].
Based on the calculated RQ values, the water GLY pollution is evaluated according to the criteria in Table 5.

Table 5.
Risk judgment standard for RQ value in water.
- (2)
- Soil GLY Pollution Risk Diagnosis Method
The risk quotient (RQ) for GLY pollution in soil is calculated as follows:
In the formula, PNEC represents the predicted no-effect concentration of the target compound, in μg/g; AF is the assessment factor, which is set to 10 in this study; NOEC is the maximum no-observed-effect concentration for the target indicator organism. In this study, earthworms, known to be sensitive to GLY, are selected as the target indicator organism [,]. The NOEC value is obtained from the Pesticide Properties Database (PPDB), with specific values shown in Table 6. MEC is the measured concentration of the compound in the soil.

Table 6.
Toxicological data of chronic risk assessment of GLY and AMPA in soil.
Based on the RQ value, the soil GLY pollution risk zones are categorized according to the criteria in Table 7 [].

Table 7.
Risk judgment standard for RQ value in soil.
2.5. Data Processing and Analysis
Statistical analysis of the data was performed using Microsoft Excel 2019. Graphs were created using Origin 2021 software, and spatial interpolation of GLY and AMPA was conducted using Surfer 15 software. Correlation analysis (Pearson correlation analysis) was performed using SPSS 19.0, with significance levels set at p < 0.05 for general correlations and p < 0.01 to indicate highly significant correlations.
3. Results and Discussion
3.1. Characteristics of Changes in the Physicochemical Properties of Water
The physicochemical parameters of the water body in the study area for different months are presented in Table 8. The results indicate significant differences in the physicochemical parameters across different months (p < 0.05). The EC of the water body ranged from 370.56 to 608.26 μS/cm, with an average of 493.38 μS/cm, and showed significant differences among the months (p < 0.05). DO levels varied between 7.84 and 9.53 mg/L, with a mean value of 8.51 mg/L. The pH values ranged from 7.57 to 7.82, with an average of 7.73. Between April and October, the variations in DO and pH were not significant. Water temperature ranged from 22.35 to 27.00 °C, with an average of 25.07 °C, peaking during June to August. The CODMn varied between 3.88 and 5.82 mg/L, with a mean value of 4.71 mg/L. From April to October, both EC and CODMn showed significant decreases. This downward trend may be attributed to the rainy season. During the wet period, heavy rainfall contributes substantial runoff to the rivers, diluting the concentration of dissolved ions and pollutants in the water body, thereby reducing EC and CODMn levels. Carbon, nitrogen, and phosphorus are fundamental nutrients in wetland ecosystems, playing a vital role in maintaining primary productivity and constituting essential components of organismal structure and function. In the study area, the concentration of TP ranged from 0.09 to 0.19 mg/L, with an average of 0.14 mg/L. NH3-N concentration ranged from 0.37 to 1.69 mg/L, averaging 0.89 mg/L. TN concentrations ranged from 1.11 to 3.27 mg/L, with a mean of 2.17 mg/L. TOC concentrations ranged from 1.89 to 6.62 mg/L, with an average of 4.81 mg/L. The N/P and C/P ratios in the water body ranged from 33.20 to 73.30 and 10.21 to 20.33, with averages of 48.20 and 15.23, respectively. From April to August, concentrations of TP, TN, and TOC decreased continuously, while the N/P and C/P ratios increased. This suggests rapid phytoplankton growth during the summer, which leads to a significant increase in phosphorus demand and results in a phosphorus-limited state in the water body [].

Table 8.
Variation characteristics of basic physicochemical properties of water.
3.2. Basic Physicochemical Properties of Soil
The basic physicochemical properties of the soil in the study area are summarized in Table 9. The soil pH was 6.42 ± 0.74, and EC was 172.50 ± 66.38 μS/cm. The contents of TP, TN, and TK in the soil were 1.10 ± 0.74 g/kg, 4.37 ± 1.52 g/kg, and 1.13 ± 0.47 g/kg, respectively. As key indicators for assessing soil quality and guiding fertilization, the available nutrients in the soil were measured. The contents of AP, AK, and AN were 16.74 ± 11.22 mg/kg, 2.17 ± 0.48 mg/kg, and 149.09 ± 33.79 mg/kg, respectively. Additionally, SOM content was 39.34 ± 11.29 g/kg.

Table 9.
Basic physical and chemical properties of soil.
3.3. Glyphosate and AMPA Concentrations and Spatial Distribution in Surface Waters
3.3.1. Spatial Distribution Characteristics of Glyphosate in Surface Waters
In the study area, surface runoff primarily drains into the Yangmei River. Under rainfall conditions, GLY present in soils may be transported into the river via runoff. As illustrated in Figure 2, the spatial distribution of GLY concentrations in the Yangmei River varied across different months, ranging from 0 to 204.0 μg/L, with an average concentration of 50.91 μg/L. Detection frequencies of GLY differed significantly between months, ranging from 26.92% to 46.15%. The highest concentrations were observed in June, whereas relatively lower levels were detected in August. The detection frequencies were higher in April and June, followed by a decline in August and October, indicating a seasonal pattern in both concentration and detection rate.
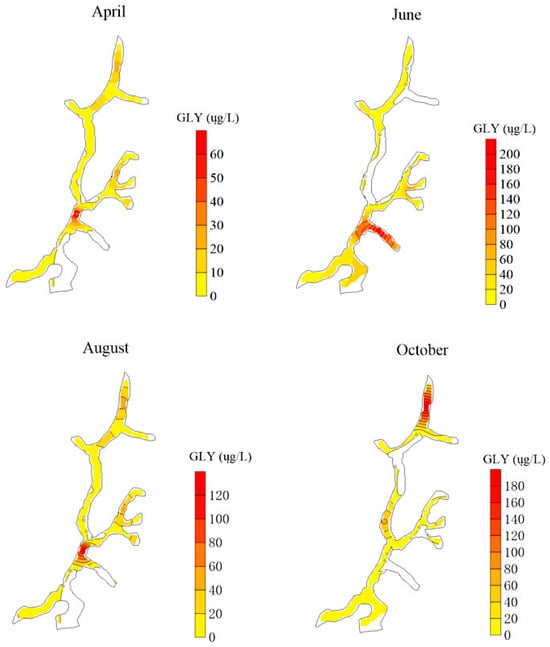
Figure 2.
Spatial distribution of GLY in Yangmei River.
The spatial and temporal distribution of GLY in surface waters exhibited pronounced heterogeneity. Overall, higher concentrations of GLY were predominantly observed in the downstream segments of the Yangmei River. According to the delineation of sub-watersheds by month, the primary sources of GLY in April were tributary catchments near Bagu Chong, Zhuangkeng, Xiaochong, and Baituo Village. In June, GLY was mainly concentrated in tributaries near Baituo Village and Badakuai. By August, elevated levels of GLY were detected in segments near the Yangmeibao Bridge, Baituo Village, and Badakuai tributaries. In October, major contributions originated from areas near the Yangmeibao Bridge and Jiguan region. Runoff during rainfall events likely mobilized soil-bound GLY into the watershed, resulting in higher concentrations near points of inflow from surrounding slopes, whereas lower concentrations were observed further from these entry points. Spatial distribution mapping indicates that GLY has been transported into the Lianjiang River, with a marked increase in GLY concentrations entering the river in June. These findings suggest that rainfall and surface runoff play critical roles in the mobilization and downstream transport of GLY within the watershed.
3.3.2. Spatial Distribution Characteristics of AMPA in Surface Waters
The spatial distribution of AMPA in surface waters within the study area is presented in Figure 3. AMPA concentrations ranged from 0 to 127.26 μg/L, with an average value of 26.51 μg/L. The detection frequency varied between 46.15% and 100% across different months, indicating a relatively high and consistent detection rate. This suggests that AMPA is widely distributed in the surface waters of the study area and may pose a long-term environmental risk due to its persistence.
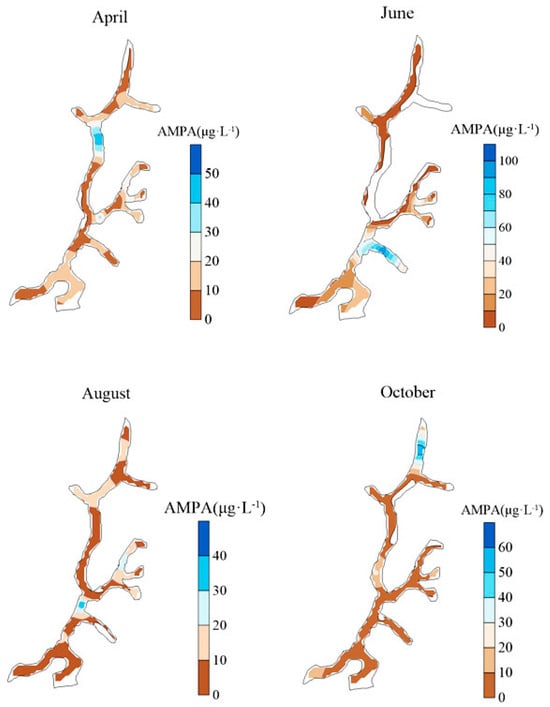
Figure 3.
Spatial Distribution of AMPA in Yangmei River.
The spatial and temporal distribution of AMPA also displayed significant heterogeneity. Based on watershed characteristics in different months, the primary sources of AMPA in April were tributaries located in the Zhuangkeng, Xiaochong, and Baituo Village regions. In June, elevated concentrations were primarily found in the Badakuai area. By August, the main distribution of AMPA had shifted upstream, with notable levels near the Yangmeibao Bridge and Baituo Village. In October, AMPA was predominantly concentrated in segments near the Yangmeibao Bridge and Jiguan region.
3.4. Concentrations and Spatial Distribution of Glyphosate and AMPA in Soils
The concentrations and detection rates of GLY and AMPA in soils from the study area are summarized in Table 10. GLY concentrations in soil samples ranged from 0 to 8.89 mg/kg, with an average of 2.63 mg/kg, and a detection frequency of 80%. Notably, 70% of the soil samples contained GLY at levels exceeding 1 mg/kg. AMPA concentrations ranged from 0.48 to 21.02 mg/kg, with an average value of 4.66 mg/kg, and a detection frequency of 100%. Among these, 90% of the samples had AMPA concentrations above 1 mg/kg. These results indicate relatively high levels of GLY and AMPA residues in soils across the study area. Furthermore, the high detection frequencies and concentration levels suggest a widespread distribution and a tendency for accumulation of both compounds in the soil environment.

Table 10.
Contents of GLY and AMPA in soil.
The spatial distribution of GLY and AMPA in soils is illustrated in Figure 4. Glyphosate exhibited pronounced spatial heterogeneity, characterized by a patchy distribution pattern. Higher concentrations of GLY were primarily concentrated in the middle to lower reaches of the watershed, while relatively lower concentrations were observed in the central region, displaying a decreasing trend from the center outward. Similarly, AMPA was predominantly distributed in the middle and lower portions of the watershed and exhibited a comparable spatial pattern, with concentrations gradually declining from central hotspots toward peripheral areas.
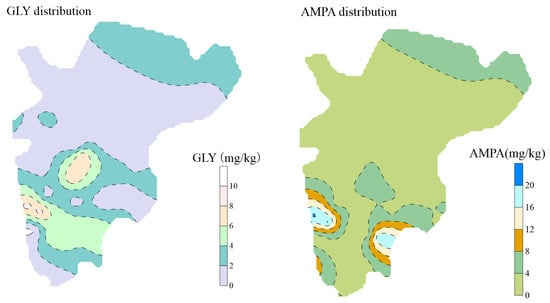
Figure 4.
Spatial distribution characteristics of soil GLY and AMPA.
3.5. Glyphosate Pollution Risk Assessment
3.5.1. Risk Assessment of Glyphosate Pollution in Surface Waters
The ARQ and CRQ of GLY in surface waters within the study area are presented in Figure 5. The results show that the ARQ values for GLY in April, June, August, and October were 1.09, 1.46, 0.95, and 1.40, respectively, while the corresponding CRQ values were 6.65, 8.43, 5.79, and 8.22. Except for August, the ARQ values in all other months exceeded 1, indicating a relatively high acute ecological risk of GLY in the aquatic environment. Furthermore, CRQ values in all months were substantially greater than 1, suggesting a considerable chronic risk posed by long-term exposure to GLY. Overall, the findings indicate that the pollution risk of GLY in surface waters of the study area is at a moderate level.
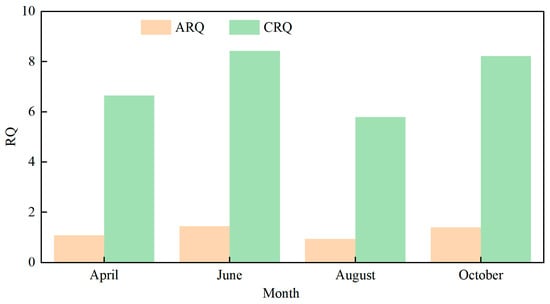
Figure 5.
Glyphosate Risk Quotient in Yangmei River Water in Different Months.
3.5.2. Zonation of Ecological Risk from Glyphosate Pollution in Soils
The spatial distribution of RQ for GLY and AMPA in soils is illustrated in Figure 6. The RQ values for GLY ranged from 0 to 4.17, with an average of 1.22 and a coefficient of variation of 95.96%. For AMPA, RQ values ranged from 0 to 7.48, with an average of 1.64 and a coefficient of variation of 114.66%. According to ecological risk assessment criteria (RQ < 0.1 indicates low risk; 0.1 ≤ RQ ≤ 1 indicates moderate risk; RQ > 1 indicates high risk), it can be concluded that the overall ecological risk from GLY and AMPA in the study area soils ranges from moderate to high.
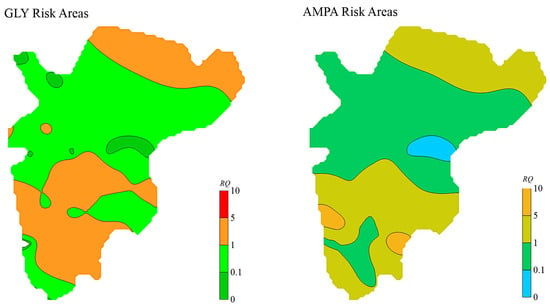
Figure 6.
Distribution Characteristics of GLY and AMPA Risk Quotients in Soil.
High-risk zones were mainly concentrated in the upstream (northern region near Yangmeibao Bridge) and downstream areas (Baituo Village and Badakuai regions) of the watershed. In contrast, the midstream regions—such as Yangmei Village, Bagu Chong, and Zhuangkeng—were classified as moderate-risk areas. Notably, the high-risk zones for GLY and AMPA were primarily located in areas of intensive and long-term agricultural cultivation, where crops such as maize, rice, vegetables, and strawberries are commonly grown. In comparison, low- to moderate-risk areas were mainly associated with residential zones, forests, and uncultivated lands. These findings suggest that land use type plays a critical role in influencing the application and environmental accumulation of GLY.
4. Discussion
4.1. Impacts of Glyphosate and AMPA on the Aquatic Environment
The effects of GLY and AMPA on water quality parameters are summarized in Table 11. The results indicate that GLY is significantly positively correlated with permanganate index CODMn, TN, and TP (p < 0.05), while AMPA shows a highly significant positive correlation with CODMn and TN (p < 0.01). These correlations may be attributed to the degradation pathways of GLY. Under the action of oxidoreductase enzymes, GLY degrades into AMPA and glycine, and AMPA can further be metabolized into phosphorus- and nitrogen-containing products []. In addition, as organophosphorus contaminants, GLY and AMPA contribute to the increase in organic matter content upon entering aquatic systems, thereby elevating CODMn levels []. A highly significant positive correlation was also observed between GLY and AMPA, which is consistent with the fact that AMPA is the primary degradation product of GLY. When applied to soil as a phosphorus source, GLY—due to its functional groups such as carboxyl and phosphonic acid—competes with phosphorus species bound to aluminum, iron, and calcium in the soil. This competition reduces the soil’s capacity to adsorb and immobilize phosphorus, thereby enhancing phosphorus mobility and promoting its transport into aquatic environments []. Residual GLY and AMPA in water bodies may consequently accelerate eutrophication and degrade water quality []. Furthermore, the amino, phosphonic acid, and carboxylic acid groups in GLY can form coordination complexes with metal ions, altering the toxicity and mobility of heavy metals in soils. For instance, GLY can chelate with heavy metals such as arsenic, cadmium, copper, lead, nickel, and zinc, exerting antagonistic effects and reducing their toxicity and bioavailability []. However, GLY may also enhance the release of heavy metals from sediments via complexation, promoting their transition from solid to aqueous phases and potentially intensifying heavy metal pollution in groundwater and surface waters []. In this study, the average AMPA concentration in soils (465.52 µg/kg) exceeded that of GLY (262.53 µg/kg), indicating that AMPA is more persistent in the environment and may act as a significant contributor to ecological risk. Therefore, the residual presence of GLY and AMPA poses a non-negligible threat to aquatic ecosystems and should be carefully considered in efforts aimed at controlling agricultural non-point source pollution and managing water quality.

Table 11.
Correlation analysis of GLY and AMPA with water environmental factors.
4.2. Spatiotemporal Distribution Characteristics of Glyphosate Pollution
In the study area, the distribution of GLY and AMPA in surface waters exhibited clear spatiotemporal heterogeneity. This pattern is influenced by multiple environmental and anthropogenic factors, including land use, slope gradient, agricultural practices, rainfall and runoff, microbial degradation, and the physical properties of the underlying surface. The frequency and amount of GLY application are closely related to local farming practices, particularly during the early stages of crop cultivation for weed control. Rainfall and surface runoff act as primary drivers of GLY transport, as higher summer precipitation can mobilize GLY adsorbed in soils and wash it into nearby water bodies. Future studies should quantitatively integrate these factors to better understand the mechanisms driving spatial variability of herbicide residues.
In karst terrains, the dynamics of subsurface karst (endokarst)—particularly the epikarst, vadose fractures and the conduit network—exert a primary control on the mobilization, storage and rapid transfer of surface-derived contaminants to groundwater. The epikarst operates both as a transient storage zone and as a preferential-flow interface: under low-intensity recharge it can attenuate and retain solutes, whereas under high-intensity or focused recharge events, it rapidly funnels water and sorbed contaminants into conduits with limited attenuation. Consequently, focused recharge, preferential flow along soil–rock interfaces and conduit-dominated flow can lead to rapid, channelized transport of pesticides such as glyphosate into springs and groundwater, often outpacing biodegradation or adsorption processes []. Recent work has further shown that short travel times from surface to spring in karst systems increase the vulnerability of aquifers to rapidly transported, short-lived pollutants [], and tracer and high-resolution monitoring studies have documented pesticide peaks in karst springs following rainfall-driven mobilization [].
Accordingly, while our present study emphasizes surface-water spatial patterns of GLY, interpretation of these patterns must account for potential subsurface contributions via endokarst processes. To quantify the relative importance of endokarst versus surface-derived sources in future work, we recommend targeted investigations including artificial tracer tests, high-frequency spring sampling, characterization of the epikarst and fracture connectivity, and modeling of conduit flow and solute transport. These approaches will allow for direct attribution of observed concentrations to focused subsurface recharge, diffuse surface inputs, or mixed pathways and thereby improve risk assessment and management strategies for glyphosate in karst basins.
A comparison of GLY and AMPA residues in water bodies from different regions (Table 12) revealed significant variations in their concentrations. In this study, GLY concentrations ranged from 0 to 204.0 µg/L and AMPA from 0 to 127.26 µg/L. Compared with the Bogue Phalia Basin in Mississippi, USA, where GLY ranged from 0.03 to 73 µg/L [], the upper limit of GLY in our study was higher but still lower than the extreme concentrations reported in global agricultural runoff (0.01–5153 µg/L) []. These differences may be attributed to variations in geographic and environmental conditions, GLY application rates, climate patterns, watershed characteristics, and analytical methods. Overall, our results are representative of GLY and AMPA contamination in karst agricultural watersheds while also highlighting the substantial spatial heterogeneity among different regions [,].

Table 12.
GLY and AMPA contents in surface water in different regions.
Similarly, a comparison of GLY and AMPA residues in soils from different regions (Table 13) reveals substantial variations in their concentrations across locations. The concentrations of both GLY and AMPA in soils within the study area are relatively high. Specifically, GLY levels exceed those reported in the Loess Plateau of Argentina, France, and Australia and are comparable to those observed in the Nandu River Basin of Hainan, China. Furthermore, the AMPA concentrations in soils from the study area are significantly higher than those reported in other regions. These elevated concentrations may be attributed to the intensive agricultural activities in the study area, where glyphosate is frequently and extensively applied. This high frequency of GLY usage likely contributes to the area being a hotspot for GLY contamination, resulting in significantly greater accumulation of GLY and its major metabolite AMPA in soils compared to other regions.

Table 13.
Residual amounts of GLY and AMPA in soils of different regions.
4.3. Limitations and Future Perspectives
This study provides valuable insights into the spatial and temporal distribution and ecological risks of GLY and its primary degradation product, AMPA, in both surface water and soil. However, several limitations should be considered in future research. First, water samples were collected only in April, June, August, and October, mostly during non-peak rainfall periods. This temporal sampling design may have missed pulse inputs during heavy rain events, potentially underestimating short-term peaks in GLY and AMPA concentrations. In addition, the study covered only four discrete months within a single year, which may not fully capture interannual variability or long-term trends in the distribution, degradation, and persistence of these compounds. Future studies should consider continuous, long-term monitoring across multiple years and seasons to better understand the dynamics of GLY and AMPA under varying climatic conditions and land use practices. Second, while the study assessed the ecological risks of GLY and AMPA using RQ models and correlation analyses, it did not integrate biological endpoints such as toxicity to aquatic organisms, microbial community changes, or impacts on soil health and crop productivity. To address this, future research should incorporate ecotoxicological studies, including in vitro and in vivo toxicity tests, as well as advanced molecular techniques like metagenomics and metabolomics, to gain a more comprehensive understanding of the biological and ecological consequences of GLY and AMPA exposure. Additionally, the study primarily relied on concentration measurements in environmental matrices without quantifying the contributions from specific sources of pollution, such as agricultural practices, crop types, and pesticide application rates. Therefore, future investigations should incorporate source apportionment models and detailed field-scale data on GLY application to better evaluate the contributions of different sources to the observed environmental levels. Lastly, it is important to acknowledge that the karst environment of the study area exhibits strong hydrological connectivity between surface and subsurface systems, characterized by the interaction between surface karst and subsurface karst. Due to the lack of available subsurface monitoring data, technical limitations, and potential risks associated with groundwater sampling in the karst terrain, this study did not directly investigate subsurface transport processes or the potential risk to groundwater. Nevertheless, future studies should focus on karst-specific hydrogeological modeling and targeted groundwater sampling to elucidate the mechanisms of vertical migration, preferential flow, and long-range transport of GLY and AMPA within karst aquifers.
5. Conclusions
- (1)
- In the study area, the concentration of GLY in surface water ranged from 0 to 204.0 μg/L, with a mean value of 50.91 μg/L, while the concentration of its primary degradation product AMPA ranged from 0 to 127.26 μg/L, with an average of 26.51 μg/L. The maximum GLY concentration was observed in June. The spatiotemporal distribution of GLY in the aquatic environment exhibited significant heterogeneity. During months with higher precipitation (April, June, and August), elevated GLY concentrations were predominantly found at the confluences of tributaries, whereas in October—a month with lower rainfall—concentration hotspots shifted to the upstream sections of the river. The spatial distribution patterns of GLY and AMPA were found to be consistent. Risk assessment based on the aquatic GLY concentrations indicated a moderate level of ecological risk in the study area.
- (2)
- In soils, GLY concentrations ranged from 0 to 8.89 mg/kg, with a mean of 2.63 mg/kg, while AMPA concentrations varied from 0.48 to 21.02 mg/kg, with an average of 4.66 mg/kg. High concentrations of GLY were primarily observed in the mid- and downstream regions of the watershed, showing a declining gradient from the center outward. Risk diagnostics revealed that both GLY and AMPA contamination in the soil posed moderate to high ecological risks and could have adverse impacts on adjacent aquatic ecosystems due to potential leaching and runoff.
- (3)
- Given that glyphosate contamination in soils of the study area has reached a moderate to high risk level and has adversely affected surrounding water quality, preventing its further diffusion into adjacent aquatic ecosystems has become an urgent priority. Effective control and mitigation measures should be tailored to regional characteristics, focusing on promoting the rational use of glyphosate through precision agriculture, establishing vegetative buffer zones to intercept surface runoff, and strengthening monitoring networks in karst regions to identify potential subsurface transport and groundwater contamination risks. The integrated implementation of these measures can effectively reduce the environmental persistence and ecological risks of glyphosate while promoting the sustainable management of agricultural ecosystems in karst watersheds.
Author Contributions
Y.Z.: Writing–conceptualization, designing methodology, software, formal analysis, review and editing. Y.Y.: Writing—study design, data interpretation, review and editing. Q.D.: Writing—study design, data analysis, review and editing. Z.S.: Writing—data collection, data analysis, original draft. H.Z.: Writing—data collection, figures, original draft. Z.H.: Writing—data collection, figures, original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Regional Fund of the National Natural Science Foundation of China (Grant No. 42167044), the National Natural Science Foundation of China (Grant No. 42007067), and the Tongren City Industry–Education Consortium Project for New Functional Materials (Project No. 2222165).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
Our acknowledgements are extended to the anonymous reviewers for their constructive review of this manuscript.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- de Raús, M.E.; Genki, T.; Joji, I.; Nicholas, C.; Michael, D. Globally consistent assessment of coastal eutrophication. Nat. Commun. 2021, 12, 6142. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zhang, R.; Liu, X.; Peng, Q.; Wang, L. Agricultural nonpoint source pollutant loads into water bodies in a typical basin in the middle reach of the yangtze river. Ecotoxicol. Environ. Saf. 2023, 268, 115728. [Google Scholar] [CrossRef] [PubMed]
- Delhomme, O.; Rodrigues, A.; Hernandez, A.; Chimjarn, S.; Bertrand, C.; Bourdat-Deschamps, M.; Fritsch, C.; Pelosi, C.; Nelieu, S.; Millet, M. A method to assess glyphosate, glufosinate and aminomethylphosphonic acid in soil and earthworms. J. Chromatogr. A 2021, 1651, 462339. [Google Scholar] [CrossRef] [PubMed]
- Benbrook, C.M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28, 3. [Google Scholar] [CrossRef]
- Yaah, V.B.K.; Ahmadi, S.; Quimbayo, J.M.; Morales-Torres, S.; Ojala, S. Recent technologies for glyphosate removal from aqueous environment: A critical review. Environ. Res. 2024, 240, 117477. [Google Scholar] [CrossRef]
- Xin, L.; Wu, W.; Xue, X.; Fu, G.; Chen, Y.; Wang, X.; Liu, D.; Yang, X. Effects of aggregate size on kinetics of glyphosate degradation in red soil. Chin. J. Eco-Agric. 2021, 29, 910–921. [Google Scholar] [CrossRef]
- García-Pérez, J.A.; Alarcón-Gutiérrez, E.; Perroni, Y.; Barois, I. Earthworm communities and soil properties in shaded coffee plantations with and without application of glyphosate. Appl. Soil Ecol. 2014, 83, 230–237. [Google Scholar] [CrossRef]
- Villamar-Ayala, C.A.; Carrera-Cevallos, J.V.; Vasquez-Medrano, R.; Espinoza-Montero, P.J. Fate, eco-toxicological characteristics, and treatment processes applied to water polluted with glyphosate: A critical review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1476–1514. [Google Scholar] [CrossRef]
- Mahler, B.J.; Metre, P.C.V.; Burley, T.E.; Loftin, K.A.; Meyer, M.T.; Nowell, L.H. Similarities and differences in occurrence and temporal fluctuations in glyphosate and atrazine in small midwestern streams (USA) during the 2013 growing season. Sci. Total Environ. 2017, 579, 149–158. [Google Scholar] [CrossRef]
- Villeneuve, A.; Larroudé, S.; Humbert, J.F. Herbicide Contamination of Freshwater Ecosystems: Impact on Microbial Communities//Herbicides: Properties, Synthesis and Control of Weeds; Intech Open: London, UK, 2011. [Google Scholar]
- Lyndsay, S.; Reza, P. Glyphosate in runoff waters and in the root-zone: A review. Toxics 2015, 3, 462–480. [Google Scholar] [CrossRef]
- Deng, X.; Li, Y. Effect of Glyphosate on Soil Microorganisms. Agrochemicals 2005, 44, 59–62. [Google Scholar] [CrossRef]
- Defarge, N.; Spiroux De Vendômois, J.; Séralini, G.E. Toxicity of formulants and heavy metals in glyphosate-based herbicides and other pesticides. Toxicol. Rep. 2018, 5, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Antoniou, M.N. Ignoring adjuvant toxicity falsifies the safety profile of commercial pesticides. Front. Public Health 2018, 5, 361. [Google Scholar] [CrossRef] [PubMed]
- Jungers, G.; Portet-Koltalo, F.; Cosme, J.; Séralini, G.E. Petroleum in pesticides: A need to change regulatory toxicology. Toxics 2022, 10, 670. [Google Scholar] [CrossRef]
- Ferrante, M.; Rapisarda, P.; Grasso, A.; Favara, C.; Oliveri Conti, G. Glyphosate and environmental toxicity with “one health” approach, a review. Environ. Res. 2023, 235, 116678. [Google Scholar] [CrossRef]
- Jaime, R.; Ricardo, D.C. Glyphosate residues in groundwater, drinking water and urine of subsistence farmers from intensive agriculture localities: A survey in hopelchén, campeche, mexico. Int. J. Environ. Res. Public Health 2017, 14, 595. [Google Scholar] [CrossRef]
- Ferreira, N.G.C.; da Silva, K.A.; Guimarães, A.T.B.; de Oliveira, C.M.R. Hotspots of soil pollution: Possible glyphosate and aminomethylphosphonic acid risks on terrestrial ecosystems and human health. Environ. Int. 2023, 179, 108135. [Google Scholar] [CrossRef]
- Wang, C.; Lin, X.; Li, L.; Lin, L.; Lin, S. Glyphosate shapes a dinoflagellate-associated bacterial community while supporting algal growth as sole phosphorus source. Front. Microbiol. 2017, 8, 2530. [Google Scholar] [CrossRef]
- An, J.; Jiang, Y.; Cao, H.; Yi, C.; Li, S.; Qu, M.; Liu, G. Photodegradation of glyphosate in water and stimulation of by-products on algae growth. Ecotoxicol. Environ. Saf. 2023, 263, 115211. [Google Scholar] [CrossRef]
- Hartmann, A.; Jasechko, S.; Gleeson, T.; Wada, Y.; Andreo, B.; Barberá, J.A.; Brielmann, H.; Bouchaou, L.; Charlier, J.; Darling, W.G.; et al. Risk of groundwater contamination widely underestimated because of fast flow into aquifers. Proc. Natl. Acad. Sci. USA 2021, 118, e2024492118. [Google Scholar] [CrossRef]
- Ford, D.; Williams, P. Karst Hydrogeology and Geomorphology; John Wiley & Sons Inc.: New York, NY, USA, 2007. [Google Scholar]
- Qiu, Y.; Yin, J.; Zhang, T.; Du, Y.; Zhang, B. Spatiotemporal dynamic analysis of a-level scenic spots in Guizhou Province, China. ISPRS Int. J. Geo Inf. 2021, 10, 568. [Google Scholar] [CrossRef]
- Yang, W.P.; Wei, C.; Lu, T.Y. Survey on pesticide use and the status of pesticide pollution in water sources in rural Guizhou. Adm. Tech. Environ. Monit. 2015, 27, 34–37. [Google Scholar]
- Bexfield, L.M.; Belitz, K.; Lindsey, B.D.; Toccalino, P.L.; Nowell, L.H. Pesticides and pesticide degradates in groundwater used for public supply across the United States: Occurrence and human-health context. Environ. Sci. Technol. 2020, 55, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.X.; Zhou, Y.C.; Zhou, X.W.; Zhang, C.L. ChunlaiDifferentiating Karst soil and soil in Karst region—A case study of Houzhai river watershed in puding county of Guizhou province. Soils 2020, 52, 414–420. [Google Scholar] [CrossRef]
- Ruan, Y.L.; Li, X.D.; Li, T.Y.; Chen, P.; Lian, B. Heavy metal pollution in agricultural aoils of the Karst areas and its harm to human health. Earth Environ. 2015, 43, 92–107. [Google Scholar] [CrossRef]
- Sun, Z.Y.; Zhou, Q.W.; Zhang, S.Q.; Wei, X.C.; Ma, L.S. Comparing and analyzing different spatial interpolation methods for soil-moisture estimation in karst areas. Trop. Geogr. 2019, 39, 770–779. [Google Scholar] [CrossRef]
- Bao, S. Soil Agrochemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Yang, X.; Wang, F.; Bento, C.P.M.; Meng, L.; van Dam, R.; Mol, H.; Liu, G.; Ritsema, C.J.; Geissen, V. Decay characteristics and erosion-related transport of glyphosate in chinese loess soil under field conditions. Sci. Total Environ. 2015, 530–531, 87–95. [Google Scholar] [CrossRef]
- Li, Z.M.; Kannan, K. A method for the analysis of glyphosate, aminomethylphosphonic acid, and glufosinate in human urine using liquid chromatography-tandem mass spectrometry. Int. J. Environ. Res. Public Health 2022, 19, 4966. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, X.; Zhang, B.; Bi, J.; Zhang, H.; Wang, J.; Fu, J.; Wang, Y. Screening of characteristic pollutants and risk pollutants in different media in the Yangtze River Basin based on comprehensive scoring and risk quotient methods. Environ. Sci. 2024, 45, 5214–5226. [Google Scholar] [CrossRef]
- Liu, S.; Wang, T.; Wang, Z.; Miao, F.; Wang, F.; Li, Z. Water quality criteria derivation and ecological risk assessment for glyphosate. Asian J. Ecotoxicol. 2023, 18, 335–350. [Google Scholar]
- Pérez, D.J.; Iturburu, F.G.; Calderon, G.; Oyesqui, L.A.E.; De Gerónimo, E.; Aparicio, V.C. Ecological risk assessment of current-use pesticides and biocides in soils, sediments and surface water of a mixed land-use basin of the pampas region, argentina. Chemosphere 2021, 263, 128061. [Google Scholar] [CrossRef]
- Vryzas, Z.; Alexoudis, C.; Vassiliou, G.; Galanis, K.; Papadopoulou-Mourkidou, E. Determination and aquatic risk assessment of pesticide residues in riparian drainage canals in northeastern greece. Ecotoxicol. Environ. Saf. 2011, 74, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.D.; He, X.Y.; Liang, Y.F.; Zhu, S.P.; Cui, Y.M.; Wu, C. Residual characteristics and ecological risk of glyphosate and aminomethylphosphonic acid residues in surface soils from Nandu River Basin, China. Agrochemicals 2022, 61, 894–899. [Google Scholar] [CrossRef]
- Artigas, J.; Batisson, I.; Carles, L. Dissolved organic matter does not promote glyphosate degradation in auto-heterotrophic aquatic microbial communities. Environ. Pollut. 2020, 259, 113951. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.Y.; Peng, Z.R.; Dai, Z. Advances in aquatic biotoxicity, environmental behavior and detection of glyphosate. Agrochemicals 2020, 59, 6–10. [Google Scholar] [CrossRef]
- Zhou, C.F.; Lin, J.W.; Li, Y.; Liu, A.Q. Effects of Glyphosate on Inorganic Phosphorus Transformation in Soil. J. Northwest For. Univ. 2016, 31, 71–77. [Google Scholar]
- Xu, Y.G.; Li, J.; Qin, J.H.; Li, Q.; Li, H.S. Joint toxicity of glyphosate and As(III) to Daphnia magna in aquatic environments. J. Agro-Environ. Sci. 2015, 34, 2076–2082. [Google Scholar]
- Wang, B.; Jiang, L.; Pan, B.; Lin, Y. Acute toxicity of Cu2+, Pb2+ and their combined contamination with two herbicides to earthworms. Agrochemicals 2020, 59, 425–429. [Google Scholar] [CrossRef]
- Paerl, H.W.; Hall, N.S.; Calandrino, E.S. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total Environ. 2011, 409, 1739–1745. [Google Scholar] [CrossRef]
- Sun, M.; Li, H.; Jaisi, D.P. Degradation of glyphosate and bioavailability of phosphorus derived from glyphosate in a soil-water system. Water 2019, 163, 114840. [Google Scholar] [CrossRef]
- Bauer, S.; Liedl, R.; Sauter, M. Modeling the influence of epikarst evolution on karst aquifer genesis: A time-variant recharge boundary condition for joint karst-epikarst development. Water Resour. Res. 2005, 41, W09409. [Google Scholar] [CrossRef]
- Rodríguez, A.G.P.; López, M.I.R.; Casillas, Á.D.; León, J.A.A.; Banik, S.D. Impact of pesticides in karst groundwater: Review of recent trends in Yucatan, Mexico. Groundw. Sustain. Dev. 2018, 7, 20–29. [Google Scholar] [CrossRef]
- U.S. Geological Survey (USGS). Concentrations of Glyphosate, Its Degradation Product (AMPA) and Other Herbicides in the Mississippi River Basin, 2001–2003. SIR 2007-5122. Available online: https://pubs.usgs.gov/sir/2007/5122/pdf/SIR2007-5122.pdf (accessed on 15 September 2025).
- Medalie, L.; Baker, N.T.; Shoda, M.E.; Stone, W.W.; Meyer, M.T.; Stets, E.G.; Wilson, M. Influence of land use and region on glyphosate and aminomethylphosphonic acid in streams in the USA. Sci. Total Environ. 2020, 707, 136008. [Google Scholar] [CrossRef]
- Qiao, C.; Wang, C.; Pang, R.; Tian, F.; Han, L.; Guo, L.; Luo, J.; Li, J.; Pang, T.; Xie, H.; et al. Environmental behavior and influencing factors of glyphosate in peach orchard ecosystem. Ecotoxicol. Environ. Saf. 2020, 206, 111209. [Google Scholar] [CrossRef]
- Lutri, V.F.; Matteoda, E.; Blarasin, M.; Aparicio, V.; Giacobone, D.; Maldonado, L.; Becher Quinodoz, F.; Cabrera, A.; Giuliano Albo, J. Hydrogeological features affecting spatial distribution of glyphosate and AMPA in groundwater and surface water in an agroecosystem. Córdoba, Argentina. Sci. Total Environ. 2020, 711, 134557. [Google Scholar] [CrossRef]
- Melendez-Pastor, I.; Hernández, E.I.; Navarro-Pedreño, J.; Almendro-Candel, M.B.; Gómez Lucas, I.; Jordán Vidal, M.M. Occurrence of Pesticides Associated with an Agricultural Drainage System in a Mediterranean Environment. Appl. Sci. 2021, 11, 10212. [Google Scholar] [CrossRef]
- Montiel-León, J.M.; Munoz, G.; Vo Duy, S.; Do, D.T.; Vaudreuil, M.; Goeury, K.; Guillemette, F.; Amyot, M.; Sauvé, S. Widespread Occurrence and Spatial Distribution of Glyphosate, Atrazine, and Neonicotinoids Pesticides in the St. Lawrence and Tributary Rivers. Environ. Pollut. 2019, 250, 29–39. [Google Scholar] [CrossRef]
- Morrás, H.; Behrends Kraemer, F.; Sainz, D.; Fernández, P.; Chagas, C. Soil Structure and Glyphosate Fate under No-Till Management in the Pampa Region. II. Glyphosate and AMPA Persistence and Spatial Distribution in the Long-Term: A Conceptual Model. Soil Tillage Res. 2022, 223, 105471. [Google Scholar] [CrossRef]
- Silva, V.; Montanarella, L.; Jones, A.; Fernandez-Ugalde, O.; Mol, H.; Ritsema, C.J.; Geissen, V. Distribution of Glyphosate and Aminomethylphosphonic Acid (AMPA) in Agricultural Topsoils of the European Union. Sci. Total Environ. 2018, 621, 1352–1359. [Google Scholar] [CrossRef]
- Wei, C.; Song, L.; Yang, W.; Zhao, Y. Research on Glyphosate Pesticide Residue in Surface Water in Guiyang. Environ. Sci. Technol. 2016, 39, 126–130. [Google Scholar]
- Fan, J.; Geng, J.; Wang, X. Determination of Glyphosate in Taihu Lake Water by Ion Chromatography. In Proceedings of the 6th National Conference on Environmental Chemistry and Exhibition on Environmental and Analytical Instruments, Shanghai, China, 21 September 2011; p. 2. [Google Scholar]
- Li, X.; Qi, J.; Chen, Y. Determination of Dichloroacetic Acid, Trichloroacetic Acid and Glyphosate in Environmental Water by Ion Chromatography with Large Volume Direct Injection. Chin. J. Appl. Chem. 2009, 26, 447–450. [Google Scholar]
- Bentoa, C.P.M.; van den Heuvel, A.S.; Yin, B.X.; Pérez, M.M.J.; Mol, H.G.J.; Ritsema, C.J.; Geissen, V. Dynamics of Glyphosate and AMPA in the Soil Surface Layer of Glyphosate-Resistant Crop Cultivations in the Loess Pampas of Argentina. Environ. Pollut. 2019, 244, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Froger, C.; Jolivet, C.; Budzinski, H.; Pierdet, M.; Caria, G.; Saby, N.P.A.; Arrouays, D.; Bispo, A. Pesticide Residues in French Soils: Occurrence, Risks, and Persistence. Environ. Sci. Technol. 2023, 57, 7818–7827. [Google Scholar] [CrossRef]
- Rose, M.T.; Zhang, P.; Rose, T.J.; Scanlan, C.A.; McGrath, G.; Van Zwieten, L. Herbicide Residues in Australian Grain Cropping Soils at Sowing and Their Relevance to Crop Growth. Sci. Total Environ. 2022, 833, 155105. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).