Abstract
The growing world population is putting pressure on food and feed production. For many years, selenium deficiencies have been observed in the diets of the inhabitants of most European and other continental countries, both in the environment and in food and fodder. Therefore, it is becoming necessary to supplement these deficiencies. A 3-year field study was therefore carried out to determine the effect of sulphur (0, 15 and 30 kg S ha−1) and selenium (0, 10 and 20 g Se ha−1) on the yield and biometric traits of winter forms of spelt wheat and common wheat, as well as the timing of application (at the tillering stage, BBCH 22–24, and the stem-shooting stage, BBCH 31–34). Sulphur fertilisation had a slight but positive effect on both the grain and straw yields of both wheat species, especially spelt wheat. The highest increase in spelt wheat grain and straw yield and common wheat straw yield was obtained after applying sulphur at a dose of 15 kg S ha−1, by 3%, 5% and 5%, respectively. In the case of selenium, a higher dose (20 g Se ha−1) had the most beneficial effect on the grain yield of spelt wheat (5% increase) and common wheat (8% increase). In turn, a lower dose of this element (10 g Se ha−1) contributed to an increase in the straw yield of both wheat species, by 10% and 17%, respectively. The yield of spelt and common wheat was not dependent on the timing of Se application. The beneficial effect of S and Se fertilisation on the growth and development of the tested plants is also indicated by the high (exceeding 1) tolerance index for Se and the yield response for S. The effect of S and Se on the weight of a thousand grains was not clear-cut. The density of spelt and common wheat ears increased as a result of the impact of S and Se (S: by 6% and 5%; Se: by 10% and 15%, respectively). Delaying the application of Se contributed to an increase in the density of the tested plants.
1. Introduction
In order to obtain high-quality crops, it is necessary to provide them with adequate amounts of nutrients to meet their nutritional needs [1,2]. Selenium deficiency has been observed in the diets of most European countries for many years, resulting from processes taking place in the natural environment. The greatest impact on the selenium deficit is the reduction in emissions of this element from human activities, as well as the depletion of natural selenium resources [3].
Among the general public, knowledge of the role of this micronutrient in the proper development of humans and animals remains insufficient. This results in most European countries failing to introduce legal regulations to help prevent selenium deficiencies in humans and animals. One reason for the lack of interest in selenium as a plant nutrient is that, as a micronutrient, selenium is not essential for normal plant growth and development. It is also not considered to enhance yields. At higher concentrations, it is toxic and significantly increases cultivation costs [4]. Nevertheless, the health benefits for animals and humans resulting from selenium use far outweigh the disadvantages [5]. Therefore, it is crucial to disseminate knowledge about selenium among farmers and to study various approaches to eliminating its deficiency in humans [6]. As plant products are the main source of selenium for animals and humans, it is important to increase their selenium levels. This can be achieved through biofortification, genetic engineering and using materials that are naturally rich in selenium [7,8]. Finland is a good example. For many years, it has been required that mineral fertilisers contain added selenium. This has resulted in increased concentrations of selenium in the human body.
The selenium content of plant products largely depends on the selenium content of the soil, which is determined by various environmental factors, including the type of parent rock, the intensity of leaching and the transport of the element from rocks to water reservoirs, as well as the terrain and age of the soil. Additionally, the sorption of this element by iron oxides [9], the soil’s redox potential [10] and the clay content [11] also impact this. Selenium concentrations in soils ranging from 0.01 to 2 mg kg−1 are considered non-toxic [12,13]. Generally, however, the selenium content in soil is at a mg kg−1 level [14]. The highest concentrations of selenium are found in soils that are rich in iron compounds and organic matter, while lower concentrations are found in igneous rocks and acidic soils. The form in which selenium occurs in soil depends on pH, redox potential, free oxygen concentration and soil moisture [15].
The selenium content of soils has only been analysed since the early 1950s, when its importance to animals was recognised. The implementation of environmental policy, as well as the intensification of agriculture, has led to a significant loss of selenium in soils. The use of simple mineral fertilisers, mainly triple superphosphate, which has a lower content of this microelement (≤4 mg Se kg−1) than single superphosphate (≤55 mg Se kg−1), has also contributed to the reduction of selenium in the soil. Currently, the Se content in most Polish soils, with the exception of industrial areas, does not exceed 0.5 mg Se kg−1, classifying them as deficient [6]. The selenium content of plant products correlates with that of the soil in which they were grown [16].
Selenium is a microelement necessary for the proper functioning of the human body, due to its key role in the functioning of the immune system, antioxidant protection and the metabolism of thyroid hormones. Both selenium deficiency and excess can lead to health disorders, so it is important to follow the dietary recommendations regarding selenium intake [17]. The recommended daily intake of selenium according to the National Institutes of Health [17] is as follows (in µg day−1): infants, 15–20; children, 20–40; adolescents and adults, 55; pregnant women, 60; lactating women, 70. According to the EFSA [18], the recommended intake is as follows: infants, 15; children, 15–35; adolescents, 55; adults and pregnant women, 70; lactating women, 85. The upper limit of selenium ranges in children from in 90 µg day−1 (1–3 years) to 280 µg day−1 (9–13 years), and in adolescents and adults it is 400 µg day−1. Exceeding the upper limit of selenium may lead to selenosis (selenium poisoning), the symptoms of which include, among others, gastrointestinal disorders, brittle nails and hair loss [17].
Ionic antagonisms play an important role in the uptake and assimilation of selenium by plants. The similarities between sulphur and selenium as chemical elements should be emphasised, particularly with regard to selenium replacing sulphur in plants. The uptake of selenium and sulphur by plants is influenced by the antagonistic interaction between these elements, as selenium is a chemical analogue of sulphur. Therefore, both selenates (VI) and sulphates (VI) are taken up by plants via the same pathway. In plants, sulphur transporters with a high affinity for sulphur are used for this purpose [19]. Under conditions of sulphur deficiency, an increased expression of genes encoding sulphate permeases and enzymes involved in the metabolism of selenium and sulphur compounds is observed, resulting in the increased uptake of selenates (VI) [20]. Plants use sulphur transporters for membrane transport, which show a high affinity for Se [21,22]. Under conditions of sulphur deficiency, the expression of genes responsible for encoding sulphate permeases and the enzymes responsible for Se/S metabolism increases, resulting in an increased uptake of selenates (VI) [23]. After uptake, selenium enters the assimilation pathway and is then reduced to selenite (IV) via selenide. Selenide can be incorporated into selenocysteine, which can then be converted into selenomethionine in three enzymatic steps [21]. Selenates (IV) are likely absorbed by passive diffusion, and phosphate pathways are involved in their transport. Although Se (IV) uptake is not dependent on metabolism, it can be inhibited by the metabolic inhibitor CCCP (carbonyl cyanide m-chlorophenylhydrazone) and by phosphates present in the soil solution [24]. In summary, it can be said that, in the transport process, sulphates compete with selenate (VI) and phosphates with selenite (IV) [21].
Currently, sulphur fertilisation is one of the most important issues in modern European agriculture. The reduction in sulphur content in agroecosystems is observed, which is associated with a reduction in its emissions worldwide [25]. In 1990, SO2 emissions in OECD countries amounted to approximately 130 million tonnes. By 2023, they had fallen to around 50 million tonnes, a reduction of 62% [26]. This deficit is related to environmental constraints initiated in the 1980s to reduce the atmospheric emissions of sulphur compounds. Directives (2001/81/EC, 96/62/EC, 2004/42/EC and 99/32/EC) issued by the European Parliament and the Council on the protection of the atmosphere from pollution oblige EU Member States to implement and comply with them [27]. The European Parliament and the Council are preparing new national emission limits and further reduction commitments for the most significant atmospheric pollutants. For example, this means that sulphur dioxide emissions in Poland should be reduced by more than three times by 2030. This will involve further reductions in the atmospheric concentration of gaseous sulphur compounds, which may exacerbate the deficiency of this element in soils and, consequently, in plants in the coming years.
In soils, sulphur most often occurs in an organic form, such as in amino acids, proteins and oligopeptides. However, in order to be available to plants, it must undergo mineralisation [15]. The most accessible form of inorganic sulphur to plants is sulphate (SO42−) [28,29], but this only accounts for a few to several percent of the total sulphur content in soil, usually not exceeding 10%, and it varies little with soil depth [15]. The content of sulphates in some soils may increase with the soil depth in the event of significant precipitation (and intensive washing out to deeper soil layers) and the presence of compact soil layers (strongly binding sulphur), the properties of which make it difficult to move this element. The level of sulphate in the soil and its availability to plants are influenced by various factors, including the content of organic matter and the rate of its mineralisation, soil leaching or sorption processes, soil pH and temperature and soil type. Sulphate is highly susceptible to leaching, with losses amounting to as much as 300 kg S ha−1 year−1. Sulphates can be sorbed by iron (III) and aluminium hydroxides, clay substances and soil organic matter [27]. Sorption is primarily influenced by soil pH: an increase in pH is associated with an increase in sulphate content [15]. Conversely, a decrease in pH results in the increased sorption of sulphate ions onto soil solid-phase particles, reducing the availability of sulphur to plants [27]. In addition to sulphate, sulphur is present in soil in a free form (elemental sulphur, S0) and in the form of sulphides or thiosulphates [30].
To ensure food security, a balanced plant fertilisation that takes into account all the necessary macro- and micronutrients, including sulphur, is important. It is not only important to consider the yield of plants, but also their quality parameters, especially those that improve plant health. The aim of the research presented in this paper is therefore to assess the impact of sulphur and selenium on the yield and biometric parameters of winter spelt and common wheat. This study hypothesised that fertilisation with sulphur and selenium increases the grain and straw yields of winter spelt wheat and winter common wheat, and that the application of sulphur and selenium has a positive effect on their 1000 grain weight and ear density. Cereals are a good plant species for introducing Se into the human (and animal) organism due to their large share in the human and animal diet. Selenium applied in such amounts as in our experiment does not pose a risk of being absorbed by plants in excessive amounts.
2. Materials and Methods
2.1. Methodological Assumptions
The research was based on a three-year field experiment conducted at the Czesławice Experimental Station (51°18′23″ N 22°16′02″ E) of the University of Life Sciences in Lublin, Poland. The experiment was conducted on silt loam soil formed from loess [31] that was rich in phosphorus (105.5 mg P kg−1 d.m.) and magnesium (59.0 mg Mg kg−1 d.m.) and had an average sulphur (10.4 mg SO4 kg−1 d.m.) and potassium (134.5 mg K kg−1 d.m.) content. The wheat trial was planted after winter rapeseed. Two test plants were used in the experiment: spelt winter wheat (Triticum spelta L.) of the Rokosz variety and common winter wheat (Triticum aestivum L.) of the Astoria variety. It included three factors: sulphur dose at three levels (S0, SI and SII), selenium dose at three levels (Se0, SeI and SeII) and selenium application timing at two dates (at the tillering stage, BBCH 22–24, and at the stem elongation stage, BBCH 31–34). The experiment comprised 108 plots, each measuring 3 × 6 m (18 m2). Sowing was carried out in the first ten days of October each year of the field experiment, and harvesting in the second ten days of August. Sulphur was applied before sowing in the form of ammonium sulphate (NH4)2SO4 at the following doses: S0—0 kg S ha−1, SI—15 kg S ha−1 and SII—30 kg S ha−1. Selenium was applied in the form of sodium selenite (IV) (Na2Se2O3) in the following doses: Se0—0 g Se ha−1, SeI—10 g Se ha−1 and SeII—20 g Se ha−1. The selenium was applied in both tested doses at two stages: the tillering phase (BBCH 22–24) and the stem elongation phase (BBCH 31–34). Selenium was applied foliar by spraying plants with an aqueous solution.
In the field experiment, nitrogen was applied in the form of ammonium nitrate (NH4NO3) at a dose of 20 kg N ha−1 before sowing. The amount of N applied in the form of ammonium sulphate has been balanced with NH4NO3. Following the onset of spring vegetation, an additional 60 kg N ha−1 of nitrogen was applied in two instalments: 40 kg N ha−1 (including nitrogen applied with sulphur fertilisation) in the tillering phase (BBCH 22–24) and 20 kg N ha−1 in the stem elongation phase (BBCH 31–34). Phosphorus and potassium fertilisers were applied before sowing at the following doses: 60 kg P2O5 ha−1 and 80 kg K2O ha−1. Plant protection against weeds, diseases and pests was carried out in accordance with the recommendations for winter wheat cultivation. Field trials were conducted in three replicates. They were carried out in two separate blocks: one block was planted with spelt wheat and the other with common wheat. Within the blocks the plots were randomised.
The first year of research was the warmest (with an average annual temperature of 8.9 °C) and the third year was the coldest (with an average annual temperature of 8.5 °C). The coldest months were January and February, with average temperatures ranging from −1.1 to −5.9 °C. August was usually the warmest month (from 19.7 °C to 21.9 °C). Only in the second year of the study was July slightly warmer (18.7 °C). Unlike the following years, the first year of the study was characterised by lower monthly temperatures than the long-term average during the critical phases of wheat growth and development (March to June). Average precipitation (601.9 mm) was slightly lower than the long-term average (663.1 mm). The highest precipitation was recorded in the first year (752.3 mm), and the lowest in the final year of the study (520.4 mm). The months with the lowest precipitation totals were June (13.5 mm) in the first year, January (17.1 mm) in the second year and February (12.9 mm) in the third year. The highest monthly precipitation totals were recorded in May of the first year (169.6 mm), July of the second year (193.3 mm) and July of the third year (147.7 mm). Therefore, there was a significant variation in precipitation throughout the study.
Spelt wheat and common wheat were harvested at full maturity stage. Immediately after harvesting the test plants, grain and straw yields, plant density and 1000 grain weight (TGW) were determined.
2.2. Laboratory and Statistical Analysis Methods
At harvest, 0.25 m2 in the middle of each plot was cut 10 cm above surface, and the collected material was divided into straw and grain. The plant density was determined from the material collected in this way, and the grain and straw yield was determined using the weighing method. The thousand grain weight (TGW) was determined by taking three samples of 300 grains each from the cleaned yield of each plot. The grains were weighed to determine the thousand grain weight. Additionally, the tolerance index (Ti) for selenium and yield response (Yr) for sulphur were calculated as the ratio of the grain and straw yields obtained with the addition of the analysed elements to those obtained without them. This coefficient can have a value of less than 1, equal to 1 or greater than 1. If Ti (Yr) < 1, plant growth is inhibited; if Ti (Yr) = 1, the increased concentration of selenium (or sulphur) has no effect on yield; and, if Ti (Yr) > 1, this element has a positive effect on plant growth and development [32].
The results of the study were statistically analysed using a three-factor ANOVA from the Statistica package [33]. Tukey’s HSD test, at a significance level of p ≤ 0.05, was used to determine LSD values.
3. Results and Discussion
3.1. Wheat Grain and Straw Yield
Alongside rice and maize, wheat is one of the three most important cereals in the world. It provides the basis for the diet of around 30–40% of the global population. Therefore, the chemical composition of wheat is important, as low levels of micronutrients and macronutrients in wheat grains can cause deficiencies in people who consume large amounts of cereal products, including those made from wheat [34]. A major global issue is the lack of selenium, an essential nutrient for all animals and humans whose deficiency can cause serious health problems [16]. One of the best ways to provide them to humans and animals is to supplement wheat with selenium and other components (including sulphur) [34,35].
In this research, yields of spelt wheat grain ranged from 5.82 to 9.00 t ha−1. The average yield of common wheat was 6.94 t ha−1, ranging from 5.71 to 8.49 t ha−1. The average yield of common wheat was 6.94 t ha−1 and was slightly lower than the average yield of spelt wheat, −7.33 t ha−1 (Table 1, Figure 1). No significant changes in the grain yield of the two tested plant were observed in the three experimental years despite the differences in meteorological conditions. These values were similar in all three years of the experiment, with only the second year characterised by a slightly lower yield. However, the literature indicates that weather conditions have the greatest impact on the yield of both spelt and common wheat [36]. In both test plants, the lowest grain yield was obtained in the second year of the experiment, when there were extremely wet conditions in the month of sowing and in April. The yields of winter wheat depend largely on the distribution and amount of precipitation during the growing season, as well as on temperature, sunshine, snow cover thickness and weather conditions during the winter [37].

Table 1.
Grain yield (t ha−1) of spelt wheat (Triticum spelta L.) and common wheat (Triticum aestivum L.).
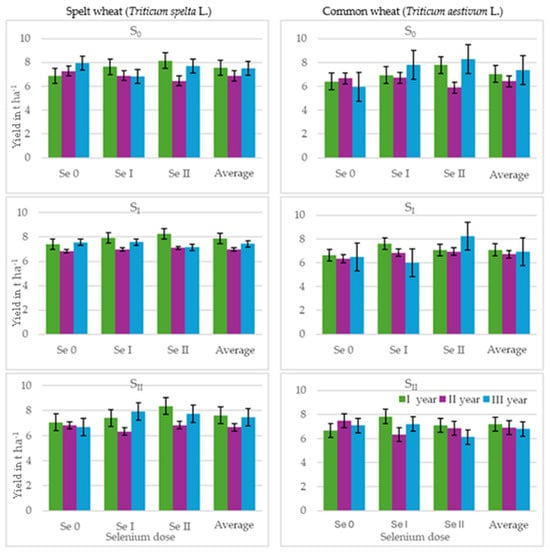
Figure 1.
Grain yield (t ha−1) of spelt wheat (Triticum spelta L.) and common wheat (Triticum aestivum L.) (average for the 1st and 2nd selenium application dates). Spelt wheat: LSD0.05 for A—n.s., B—0.33, C—n.s., A × B—n.s., A × C—n.s., B × C—n.s., A × B × C—n.s.; common wheat: LSD0.05 for A—n.s., B—0.33, C—n.s., A × B—0.75, A × C—n.s., B × C—n.s., A × B × C—n.s. LSD for A—sulphur dose, B—selenium dose, C—selenium application date; A × B, A × C, B × C, A × B × C—interactions; significant differences at p ≤ 0.05; n.s.—differences not significant.
In most cases, sulphur fertilisation resulted in a slight increase in the spelt wheat grain yield, with an increase in grain yield of only 3% observed when a lower dose was applied. Fertilisation with selenium at both doses increased the yield of spelt wheat. The highest yield increase recorded was 5%, following the application of a higher dose of selenium compared to the control plots. The timing of selenium application did not significantly affect the spelt wheat grain yield. Sulphur fertilisation did not result in a significant change in the common wheat grain yield. Adding selenium at both doses increased the grain yield of common wheat by 6% and 8%, respectively. The timing of selenium application did not significantly affect grain yield in any of the sulphur fertilisation variants.
The selenium tolerance index in the grain of both test plants fluctuated around 1 (Figure 2). In most cases, adding selenium to fertilisation was associated with a slight increase in the tolerance index above 1, which indicates a positive effect of selenium on wheat grain yield. In plots with a zero dose (control) or the first dose of sulphur, a yield response (Yr) was generally observed in common wheat grain. In contrast, in plots with the highest dose of sulphur, higher Yr values were usually recorded in spelt wheat grain (Figure 3). While it cannot be unequivocally stated that selenium fertilisation positively affected the yield of the test plants, it is important to emphasise that no negative relationship was observed. The yield response to sulphur fertilisation also fluctuated around 1 in most cases. Only in plots of common wheat fertilised with higher doses of sulphur and selenium in the second test period were values significantly below 1 recorded.
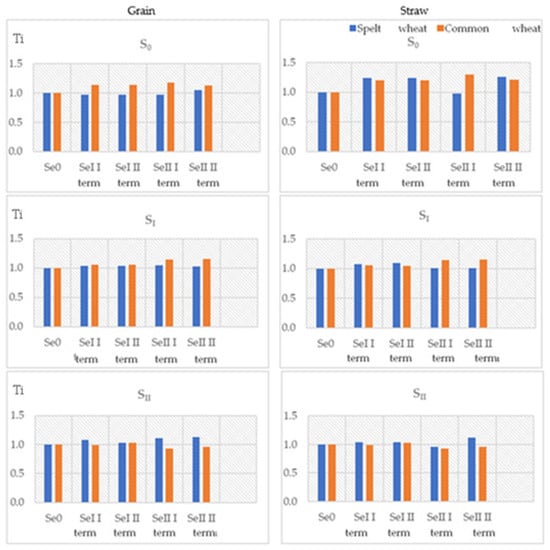
Figure 2.
Selenium tolerance index (Ti) for grain and straw of spelt wheat (Triticum spelta L.) and common wheat (Triticum aestivum L.).
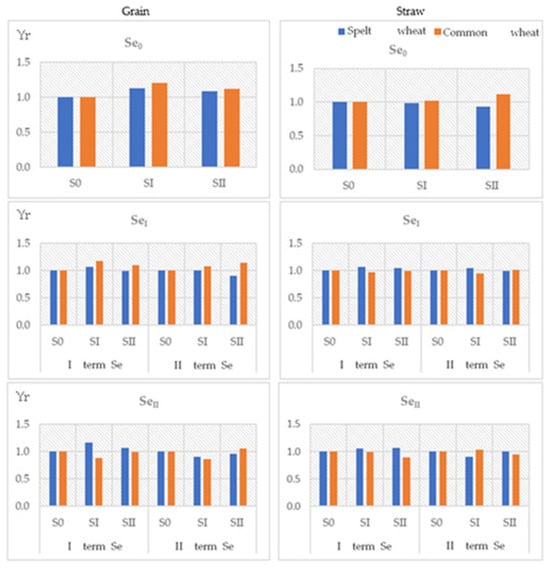
Figure 3.
Yield response (Yr) for grain and straw of spelt wheat (Triticum spelta L.) and common wheat (Triticum aestivum L.) on sulphur fertilisation.
The literature data indicate a positive effect of sulphur on wheat yield, associated with the more efficient use of organic nitrogen [38], as well as a lack of significant effects [39]. Zhao et al. [40] believe that sulphur fertilisation can increase winter wheat yield by between 5 and 50%, depending on soil conditions. They determined the optimal sulphur dose to be approximately 20 kg per hectare. However, the maximum increase in wheat yield obtained as a result of sulphur fertilisation in the study by Singh et al. [41] was 26%. According to Podleśna [27], sulphur fertilisation of winter wheat at a dose of 60 kg S ha−1 led to an 11% increase in grain yield. In a study by Potarzycki et al. [42], fertilising winter wheat with sulphur at a dose of 25 kg S ha−1 was associated with an increase in grain yield depending on the form of sulphur used. Sulphur deficiency can lead to chlorosis (yellowing of leaves), stunted growth and a reduction in the number of grains. It also affects the efficiency with which plants take up nitrogen and their overall shoot development.
Wheat has a significant ability to absorb large quantities of selenium without adversely affecting growth [43]. Numerous studies have demonstrated the positive impact of selenium fertilisation on crop yields [44,45], and it is widely accepted that selenium does not limit plant growth [46]. Our own research produced similar results to those of Wang et al. [47], who also reported a significant effect of selenium fertilisation on winter wheat grain yield. Other authors [48,49,50,51] have associated selenium fertilisation with increased yields of crops such as winter rapeseed, lettuce, potatoes and lentils. However, Radawiec et al. [52] did not observe any significant differences in wheat yield when fertilised with selenium. Nevertheless, it did contribute to an increase in the selenium content of spring wheat grain without causing the excessive accumulation of toxic elements such as iron, copper, zinc and manganese. Furthermore, De Vita et al. [53] also did not obtain a higher wheat yield, even after applying a high dose of selenium (120 g ha−1). As in our own studies, as well as in the experiments by Ducsay and Ložek [54] and Rodrigo et al. [55], the timing of selenium application did not significantly affect wheat grain yield. Applying selenium to winter wheat is particularly important in stressful conditions (e.g., heavy metal contamination), as it increases chlorophyll content, photosynthesis rate and efficiency, antioxidant capacity and enzymatic activity [56]. This positively affects plant growth, health and stress tolerance [57,58]. According to Alrashidi [59], selenium supplementation has beneficial effects on nitrogen metabolism, nitrate reductase activity and antioxidants in winter wheat under water-stressed conditions (i.e., drought), thereby limiting the negative impact of drought stress on photosynthesis, plant health and growth. The effect of selenium was analogous under salt stress conditions [60].
The straw yield of spelt wheat ranged from 4.83 to 9.11 tonnes per hectare, and that of common wheat ranged from 3.67 to 7.36 tonnes per hectare (Table 2, Figure 4). The respective average straw yields were 6.39 and 5.48 t ha−1. These values are consistent with those reported in previous studies [61,62]. On average, the highest straw yields for both crops were obtained in the second year of the experiment.

Table 2.
Straw yield (t ha−1) of spelt wheat (Triticum spelta L.) and common wheat (Triticum aestivum L.).
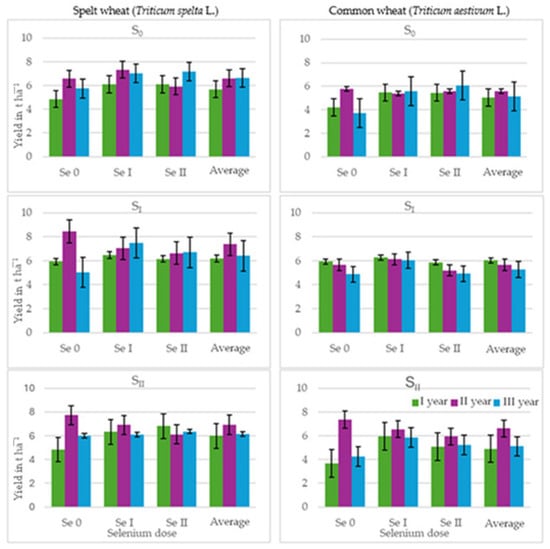
Figure 4.
Straw yield (t ha−1) of spelt wheat (Triticum spelta L.) and common wheat (Triticum aestivum L.) (average for the 1st and 2nd selenium application dates). Spelt wheat: LSD0.05 for A—n.s., B—n.s., C—n.s., A × B—n.s., A × C—n.s., B × C—n.s., A × Bx C—n.s. Common wheat: LSD0.05 for A—n.s., B—0.47, C—n.s. A × B—0.75, A × C—n.s., B × C—n.s., A × Bx C—n.s. LSD for A—sulphur dose, B—selenium dose, C—selenium application date; A × B, A × C, B × C, A × B × C—interactions; significant differences at p ≤ 0.05; n.s.—differences not significant.
The presence of a lower dose of sulphur in the soil was associated with a 5% increase in the spelt wheat straw yield, while a higher dose resulted in a decrease (Table 2, Figure 4). Fertilisation with a lower dose of selenium increased the straw yield by 10%, whereas a higher dose only increased it by 3%. A higher spelt wheat straw yield was also observed in plots fertilised with selenium at a later date.
Adding sulphur to fertilisers increased the straw yield of common wheat in most plots by an average of 8% and 6% (Table 2, Figure 4). Fertilisation with a lower dose of selenium resulted in a 17% increase in common wheat straw yield and a 9% increase with a higher dose. The timing of selenium application did not clearly affect the yield of common wheat straw.
Gondek and Gondek [61] also observed an increase in straw yield under the influence of NPK + S(S) fertilisation in spring wheat. In Gondek’s subsequent studies [62], spring wheat produced an even higher straw yield when a lower dose of sulphur was applied.
As with the grain, the selenium tolerance index in the straw of both test plants (Figure 2) was close to one. In most of the tested samples, adding selenium to the fertiliser dose was associated with a slight increase in the Ti value above 1. The sulphur tolerance index values were also around 1, and no clear influence of the experimental factors on the formation of this index was observed for either sulphur or selenium.
3.2. The 1000 Grain Weight (TGW) of Wheat
The weight of 1000 grains is one of the determinants of grain quality and indicates the extent to which it is filled with reserve substances, i.e., how plump it is. Various values for the weight of 1000 grains of spelt and common wheat can be found in the literature [63,64,65]. It typically falls within the range of 24 to 60 g, with the most common values being between 40 and 60 g [63]. Bernas et al. [64] give values ranging from 52 to 54 g, and Krawczyk et al. [65] give values ranging from 36.52 to 50.20 g. The results of our own research (Table 3, Figure 5) are similar to the above values. The weight of a thousand spelt grains ranged from 26.45 to 41.38 g, and the weight of a thousand common wheat grains ranged from 40.83 to 48.61 g. In both spelt and common wheat, the highest TGW was recorded in the third year of the experiment, but the differences were not significant.

Table 3.
The 1000 grain weight (g) of spelt wheat (Triticum spelta L.) and common wheat (Triticum aestivum L.).
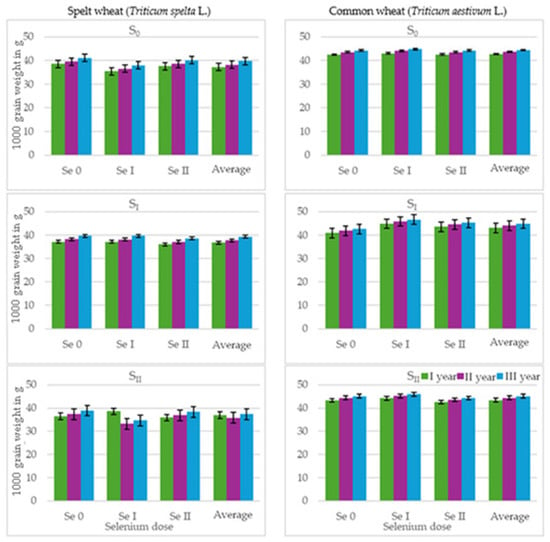
Figure 5.
The 1000 grain weight (g) of spelt wheat (Triticum spelta L.) and common wheat (Triticum aestivum L.) (average for the 1st and 2nd selenium application dates). Spelt wheat: LSD0.05 for A—n.s., B—n.s., C—n.s., A × B—n.s., A × C—3.38, B × C—n.s., A × B × C—7.15. Common wheat: LSD0.05 for A—n.s., B—0.95, C—0.65, A × B—2.18, A × C—n.s., B × C—1.63, A × B × C—n.s. LSD for A—sulphur dose, B—selenium dose, C—selenium application date; A × B, A × C, B × C, A × B × C—interactions; significant differences at p ≤ 0.05; n.s.—differences not significant.
The sulphur fertilisation of spelt wheat resulted in a decrease in its 1000 grain weight. A more noticeable effect (average 4% reduction) was observed in plots fertilised with a higher dose (30 kg S ha−1). The 1000 grain weight of spelt wheat decreased with the addition of selenium to the fertiliser dose, with a more noticeable effect after applying a lower dose. The weight of 1000 grains was then 4.5% lower than in the control plots. Plots fertilised with selenium in the second period had a higher TGW than those fertilised in the first period.
Sulphur fertilisation resulted in a slight increase in the weight of 1000 grains of common wheat. A similar effect was observed following selenium fertilisation, with a lower dose resulting in an increase in TGW. A higher weight of 1000 grains of common wheat was recorded in plots that had been fertilised with selenium at an earlier date.
Stankowski et al. [66] reported a positive effect of sulphur fertilisation on the weight of 1000 grains after applying sulphur at a dose of 40 kg ha−1. However, Łukasiewicz et al. [67] observed a decrease in the TGW of spring wheat after sulphur fertilisation, similarly to Järvan et al. [68] in winter wheat. Potarzycki et al. [42] observed an increase in the weight of 1000 grains under the influence of sulphur fertilisation and consider this indicator to be the most independent of mineral fertilisation. These results are consistent with our own research, in which the different effects of the experimental factors were observed in both test plants.
3.3. Wheat Ear Number
The number of ears in both common wheat and spelt wheat (Table 4, Figure 6) was consistent with the values most frequently reported in the literature [63]. The highest number of ears in spelt wheat was recorded in the second year of the experiment. No clear trend in changes to the number of ears of common wheat depending on the year of research was observed. Sulphur fertilisation increased the ear number of spelt wheat. Fertilisation with selenium at a lower dose resulted in a 10% increase in ear density and, at a higher dose, a 7% increase. A 6% higher ear density was observed in plots fertilised with selenium at a later date. The number of ears of common wheat increased by 5% in plots fertilised with a lower dose of sulphur. Fertilisation with a lower dose of selenium resulted in a 15% increase in the number of ears, while a higher dose resulted in a 9% increase. As with spelt wheat, a higher ear density was observed in plots fertilised with selenium at a later date. Our own research confirms the results obtained in the experiment by Järvan et al. [68], who also found that sulphur fertilisation increases ear density. Sulphur and selenium are chemically similar, meaning these elements are absorbed, metabolised and transported in the same way using similar mechanisms. Furthermore, sulphur deficiency leads to the increased expression of enzymes involved in selenium (Se)/sulphur (S) metabolism, which promotes an increased selenate uptake [69].

Table 4.
Ear density (pieces per m−2) of spelt wheat (Triticum spelta L.) and common wheat (Triticum aestivum L.).
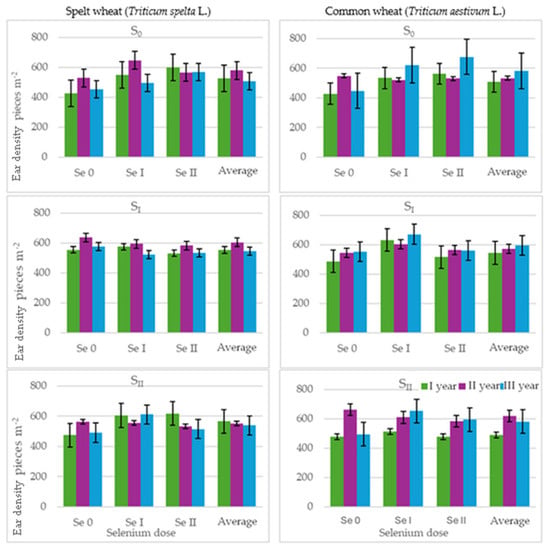
Figure 6.
Ear density (pieces per m−2) of spelt wheat (Triticum spelta L.) and common wheat (Triticum aestivum L.) (average for the 1st and 2nd selenium application dates). Spelt wheat: LSD0.05 for A—n.s., B—31.50, C—21.52, A × B—72.36., A × C—n.s., B × C—n.s., A × B × C—n.s. Common wheat: LSD0.05 for A—n.s., B—40.76, C—n.s., A × B—93.63, A × C—n.s., B × C—n.s., A × B × C—n.s.; LSD for A—sulphur dose, B—selenium dose, C—selenium application date; A × B, A × C, B × C, A × B × C—interactions; significant differences at p ≤ 0.05; n.s.—differences not significant.
The total S contents in the plant material clearly responded to the S fertilisation, indicating that the S status of the field is not sufficient to reach the full yield potential. The maximum Se content found in the grains accounted for below 0.40 mg kg−1 of dry matter (d.m.), which indicates that Se was not accumulated to contents which can become a problem in human nutrition (unpublished data).
4. Conclusions
Sulphur fertilisation resulted in an increase in the yield of the generative and vegetative organs of both spelt and common wheat. A lower dose of sulphur (15 kg S ha−1) in the soil was associated with a 3% (grain) and 5% (straw) increase in spelt wheat yield and a 5% (only straw) increase in common wheat yield. Selenium fertilisation, despite not being considered a yield-forming element, did not adversely affect plant yield and was associated with a slight increase in yields. A higher dose of selenium (20 g Se ha−1) increased the grain yield of spelt wheat by 5% and common wheat by 8%. A lower dose of selenium (10 g Se ha−1) resulted in a 10% increase in the straw yield of spelt wheat and 17% in common wheat. The positive effect of the experimental factors on the growth and development of the test plants is also evidenced by the tolerance index, which, in most cases, was greater than after application. However, the experimental factors did not clearly differentiate the weight of a thousand grains of spelt and common wheat. The number of ears in both test plants increased slightly under the influence of sulphur (by 6% in spelt wheat and 5% in common wheat) and selenium (by 10% and 15%, respectively) fertilisation. A later application of selenium favoured a higher number of test plants.
The studies conducted indicate a significant effect of sulphur and selenium fertilisers on plant yield and wheat quality.
Future Research Gap
These results constitute the basis for comprehensive field research aimed at fully verifying the effectiveness of the applied fertilisers containing selenium and sulphur and developing the possibility of cooperation between these fertilisers in shaping the amount and quality of crop yields, which is particularly important in the context of quite frequent selenium deficiencies in food. It is necessary to carry out research taking into account various soil conditions, with other plant species that are of great economic importance and, in particular, those intended for food production. The research should also include an analysis of the content of these elements in individual vegetation phases to identify the periods in which their deficiencies are most often observed, which will allow for the emergency use of sulphur and selenium.
Author Contributions
Conceptualization, M.S.B.; methodology, M.S.B. and M.K.-S.; investigation, M.S.B. and M.K.-S.; analysis, M.S.B. and M.K.-S.; visualisation, M.S.B., M.K.-S. and M.W.; writing—review and editing, M.S.B., M.K.-S. and M.W.; supervision, M.S.B. and M.W.; M.W., corresponding author. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded as a part of the projects realised by the University of Life Sciences in Lublin, Faculty of Agrobioengineering, Department of Agricultural and Environmental Chemistry (grant No. RKC/S/59).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bogusz, P.; Rusek, P.; Brodowska, M.S. Suspension fertilizers: How to reconcile sustainable fertilization and environmental protection. Agriculture 2021, 11, 1008. [Google Scholar] [CrossRef]
- Brodowska, M.S.; Wyszkowski, M.; Kordala, N. Use of organic materials to limit the potential negative effect of nitrogen on maize in different soils. Materials 2022, 15, 5755. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Sarkar, S.; Ahmad, Z.; Vemuri, H.; Garai, S.; Mondal, M.; Bhatt, R.; et al. Selenium biofortification: Roles, mechanisms, responses and prospects. Molecules 2021, 26, 881. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Nahar, K.; Fujita, M. Selenium toxicity in plants and environment: Biogeochemistry and remediation possibilities. Plants 2020, 9, 1711. [Google Scholar] [CrossRef] [PubMed]
- Avery, J.C.; Hoffmann, P.R. Selenium, selenoproteins, and immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef] [PubMed]
- Darecki, A.; Saeid, A.; Górecki, A. Perspective of selenium fortifications of plants with economic importance to Poland. Chem. News 2015, 69, 1067–1081. [Google Scholar]
- Brodowska, M.S.; Kurzyna-Szklarek, M.; Haliniarz, M. Selenium in the environment. J. Elem. 2016, 21, 1173–1185. [Google Scholar] [CrossRef]
- Danso, O.P.; Asante-Badu, B.; Zhang, Z.; Song, J.; Wang, Z.; Yin, X.; Zhu, R. Selenium biofortification: Strategies, progress and challenges. Agriculture 2023, 13, 416. [Google Scholar] [CrossRef]
- Martinez, M.; Giménez, J.; De Pablo, J.; Rovira, M.; Duro, L. Sorption of selenium (IV) an selenium (VI) onto magnetite. Appl. Surf. Sci. 2006, 252, 3767–3773. [Google Scholar] [CrossRef]
- Čuvardić, M. Selenium in soil. Proc. Nat. Sci. Matica Srp. Novi Sad 2003, 104, 23–37. [Google Scholar] [CrossRef]
- Borowska, K.; Koper, J. Dynamics of changes of selenium content in soil and red clover (Trifolium pretense L.) affected by long—Term organic fertilization on the background of selected soil oxidoreductases. Pol. J. Environ. Stud. 2011, 20, 1403–1410. [Google Scholar]
- Winkel, L.H.E.; Vriens, B.; Jones, G.D.; Schneider, L.S.; Pilon-Smits, E.; Bañuelos, G.S. Selenium cycling across soil-plant-atmosphere interfaces: A critical review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef]
- García Márquez, V.; Morelos Moreno, Á.; Benavides Mendoza, A.; Medrano Macías, J. Ionic selenium and nanoselenium as biofortifiers and stimulators of plant metabolism. Agronomy 2020, 10, 1399. [Google Scholar] [CrossRef]
- Yamada, H.; Kase, Y.; Usuki, M.; Kajiyama, S.; Yonebayashi, K. Selective determination and formation of elemental selenium in soils. Soil Sci. Plant Nutr. 1999, 45, 403–408. [Google Scholar] [CrossRef]
- Zenda, T.; Liu, S.; Dong, A.; Duan, H. Revisiting sulphur—The once neglected nutrient: It’s roles in plant growth, metabolism, stress tolerance and crop production. Agriculture 2021, 11, 626. [Google Scholar] [CrossRef]
- Fordyce, F.M. Selenium deficiency and toxicity in the environment. In Essentials of Medical Geology: Revised Edition; Selinus, O., Ed.; Springer: Cham, The Netherlands, 2013; pp. 375–416. [Google Scholar] [CrossRef]
- National Institutes of Health, Office of Dietary Supplements. Selenium—Fact Sheet for Health Professionals. NIH ODS 2022. Available online: https://ods.od.nih.gov/factsheets/Selenium-HealthProfessional/ (accessed on 30 October 2025).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on dietary reference values for selenium. EFSA J. 2014, 12, 3846. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.H.; Hwang, S.; Lytle, C.M.; Zhu, Y.; Tai, J.; Bravo, R.C.; Chen, Y.; Leustek, T.; Terry, N. Overexpression of ATP sulfurylase in Indian mustard leads to increased selenate uptake, reduction, and tolerance. Plant Physiol. 1999, 119, 123–132. [Google Scholar] [CrossRef]
- Schiavon, M.; Pilon-Smits, E.A.H. The fascinating facets of plant selenium accumulation—Biochemistry, physiology, evolution and ecology. New Phytol. 2017, 213, 1582–1596. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- Trippe, R.C.; Pilon-Smits, E.A.H. Selenium transport and metabolism in plants: Phytoremediation and biofortification implications. J. Hazard. Mater. 2020, 404, 124178. [Google Scholar] [CrossRef]
- El Mehdawi, A.F.; Jiang, Y.; Guignardi, Z.S.; Esmat, A.; Pilon, M.; Pilon-Smits, E.A.H.; Schiavon, M. Influence of sulfate supply on selenium uptake dynamics and expression of sulfate/selenate transporters in selenium hyperaccumulator and nonhyperaccumulator Brassicaceae. New Phytol. 2018, 217, 194–205. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Wang, Q.; Wan, Y.; Zhuang, Z.; Yu, Y.; Li, H. Selenite uptake and transformation in rice seedlings (Oryza sativa L.): Response to phosphorus nutrient status. Front. Plant Sci. 2020, 11, 874. [Google Scholar] [CrossRef]
- Srivastava, P.; Rana, N.; Kumar, R.; Ladohia, S.; Mehta, S. From emissions to agriculture: A comprehensive study of sulfur cycle alterations. Int. J. Res. Agron. 2024, 7, 371–376. [Google Scholar] [CrossRef]
- OECD. Air and GHG Emissions. Available online: https://www.oecd.org/en/data/indicators/air-and-ghg-emissions.html (accessed on 27 October 2025).
- Podleśna, A. Studies on role of sulfur at forming of mineral management and height and quality of chosen crops yield. In Monographs and Scientific Dissertations; Institute of Soil Science and Plant Cultivation—State Research Institute: Pulawy, Poland, 2013; Volume 37, p. 144. Available online: https://iung.pl/images/pdf/habilitacje/Podlesna–hab.pdf (accessed on 30 September 2025).
- Khan, N.A.; Khan, M.I.R.; Asgher, M.; Fatma, M.; Masood, A.; Syeed, S. Salinity tolerance in plants: Revisiting the role of sulfur metabolites. J. Plant Biochem. Physiol. 2014, 2, 120. [Google Scholar] [CrossRef]
- Anjum, N.A.; Gill, R.; Kaushik, M.; Hasanuzzaman, M.; Pereira, E.; Ahmad, I.; Tuteja, N.; Gill, S.S. ATP-sulfurylase, sulfurcompounds, and plant stress tolerance. Front. Plant Sci. 2015, 6, 210. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Lara, L.O.; Medrano-Macías, J.; Pérez-Labrada, F.; Rivas-Martínez, E.N.; García-Enciso, E.L.; González-Morales, S.; Juárez-Maldonado, A.; Rincón-Sánchez, F.; Benavides-Mendoza, A. From elemental sulfur to hydrogen sulfide in agricultural soils and plants. Molecules 2019, 24, 2282. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014; International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. In World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015; p. 182. Available online: https://www.fao.org/3/i3794en/I3794en.pdf (accessed on 20 May 2024).
- Kosiorek, M.; Wyszkowski, M. Remediation of cobalt-contaminated soil using manure, clay, charcoal, zeolite, calcium oxide, main crop (Hordeum vulgare L.), and after-crop (Synapis alba L.). Minerals 2020, 10, 429. [Google Scholar] [CrossRef]
- TIBCO Software Inc. Statistica (Data Analysis Software System), Version 13.3; TIBCO Software: Palo Alto, CA, USA, 2017.
- Sarwar, N.; Akhtar, M.; Kamran, M.A.; Imran, M.; Riaz, M.A.; Kamran, K.; Hussain, S. Selenium biofortification in food crops: Key mechanisms and future perspectives. J. Food Compos. Anal. 2020, 93, 103615. [Google Scholar] [CrossRef]
- Sunic, K.; Spanic, V. Genetic biofortification of winter wheat with selenium (Se). Plants 2024, 13, 1816. [Google Scholar] [CrossRef]
- Małecka-Jankowiak, I.; Blecharczyk, A.; Sawińska, Z.; Piechota, T.; Waniorek, B. Impact of crop sequence and tillage system on weed infestation of winter wheat. Fragm. Agron. 2015, 32, 54–63. [Google Scholar]
- Semenov, M.A.A.; Stratonovitch, P.; Alghabari, F.; Gooding, M.J.J. Adapting wheat in Europe for climate change. J. Cereal Sci. 2014, 59, 245–256. [Google Scholar] [CrossRef]
- Schnug, E.; Haneklaus, S.; Murphy, D. Impact of sulphur fertilization on fertilizer nitrogen efficiency. Sulphur Agric. 1993, 17, 8–12. [Google Scholar]
- Phillips, S.B.; Mullins, G.L. Foliar burn and wheat grain yield responses following topdress-applied nitrogen and sulfur fertilizers. J. Plant Nutr. 2004, 27, 921–930. [Google Scholar] [CrossRef]
- Zhao, F.J.; Hawkesford, M.J.; McGrath, S.P. Sulphur assimilation and effects on yield and quality of wheat. J. Cereal Sci. 1999, 30, 1–17. [Google Scholar] [CrossRef]
- Singh, P.S.; Singh, R.; Singh, M.P.; Singh, V.P. Impact of sulfur fertilization on different forms and balance of soil sulfur and the nutrition of wheat in wheat-soybean cropping sequence in Tarai soil. J. Plant Nutr. 2014, 37, 618–632. [Google Scholar] [CrossRef]
- Potarzycki, J.; Przygocka-Cyna, K.; Wendel, J.; Biniek, Ł.; Ridiger, B. The impact of sulphur fertilization on yield of winter wheat. Fragm. Agron. 2015, 32, 63–72. [Google Scholar]
- Lazo-Vélez, M.A.; Chávez-Santoscoy, A.; Serna-Saldivar, S.O. Selenium-enriched breads and their benefits in human nutrition and health as affected by agronomic, milling, and baking factors. Cereal Chem. 2015, 92, 134–144. [Google Scholar] [CrossRef]
- Nawaz, F.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A.; Shabbir, R.N.; Bukhari, M.A. Supplemental selenium improves wheat grain yield and quality through alterations in biochemical processes under normal and water deficit conditions. Food Chem. 2015, 175, 350–357. [Google Scholar] [CrossRef]
- Lara, T.S.; de Lima Lessa, J.H.; de Souza, K.R.D.; Corguinha, A.P.B.; Martins, F.A.D.; Lopes, G.; Guilherme, L.R.G. Selenium biofortification of wheat grain via foliar application and its effect on plant metabolism. J. Food Compos. Anal. 2019, 81, 10–18. [Google Scholar] [CrossRef]
- White, P.J.; Bowen, H.C.; Parmaguru, P.; Fritz, M.; Spracklen, W.P.; Spiby, R.E.; Meacham, M.C.; Mead, A.; Harriman, M.; Trueman, L.J.; et al. Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J. Exp. Bot. 2004, 55, 1927–1937. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, Y.; Li, J.; Wan, Y.; Huang, Q.; Guo, Y.; Li, H. Effects of different forms of selenium fertilizers on Se accumulation, distribution, and residual effect in winter wheat-summer maize rotation system. J. Agric. Food Chem. 2017, 65, 1116–1123. [Google Scholar] [CrossRef]
- Xue, T.; Hartikainen, H.; Piironen, V. Antioxidative and growth-promoting effect of selenium on senescing lettuce. Plant Soil 2001, 237, 55–61. [Google Scholar] [CrossRef]
- Turakainen, M.; Hartikainen, H.; Seppanen, M.M. Effects of selenium treatments on potato (Solanum tuberosum L.) growth and concentrations of soluble sugars and starch. J. Agric. Food Chem. 2004, 52, 5378–5382. [Google Scholar] [CrossRef]
- Lyons, G.H.; Genc, Y.; Soole, K.; Stangoulis, J.C.R.; Liu, F.; Graham, R.D. Selenium increases seed production in Brassica. Plant Soil 2009, 318, 73–80. [Google Scholar] [CrossRef]
- Ekanayake, L.J.; Thavarajah, D.; Vial, E.; Schatz, B.; McGee, R.; Thavarajah, P. Selenium fertilization on lentil (Lens culinaris Medikus) grain yield, seed selenium concentration, and antioxidant activity. Field Crops Res. 2015, 177, 9–14. [Google Scholar] [CrossRef]
- Radawiec, A.; Szulc, W.; Rutkowska, B. Effect of fertilization with selenium on the content of selected microelements in spring wheat grain. J. Elem. 2021, 26, 1025–1036. [Google Scholar] [CrossRef]
- De Vita, P.; Platani, C.; Fragasso, M.; Ficco, D.B.M.; Colecchia, S.A.; Del Nobile, M.A.; Padalino, L.; Di Gennaro, S.; Petrozza, A. Selenium-enriched durum wheat improves the nutritional profile of pasta without altering its organoleptic properties. Food Chem. 2017, 214, 374–382. [Google Scholar] [CrossRef]
- Ducsay, L.; Ložek, O.; Marček, M.; Varényiová, M.; Hozlár, P.; Lošák, T. Possibility of selenium biofortification of winter wheat grain. Plant Soil Environ. 2016, 62, 379–383. [Google Scholar] [CrossRef]
- Rodrigo, S.; Santamaria, O.; Poblaciones, M.J. Selenium application timing: Influence in wheat grain and flour selenium accumulation under mediterranean conditions. J. Agric. Sci. 2014, 6, 23–30. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Cheng, T.; Wang, H.; Zhou, Y.; Gong, Z.; Hu, T. Strategic selenium application methods and timing enhance grain yield, minimize cadmium bioaccumulation, and optimize selenium fortification in Triticum aestivum L. Agronomy 2025, 15, 199. Agronomy 2025, 15, 199. [Google Scholar] [CrossRef]
- Bayat, S.; Dalir, N.; Mokhtassi-Bidgoli, A.; Malakouti, M.J.; Shahbazi, K. Selenium alleviates cadmium-induced stress in durum wheat (Triticum durum) by enhancing the accumulation of cadmium in the roots and by modulating of photosynthesis parameters. J. Plant Nutr. 2022, 46, 1903–1919. [Google Scholar] [CrossRef]
- Wu, L.M.; Lu, N.H. Selenium improves wheat antioxidant capacity, photosynthetic capacity, and growth under cadmium stress. Photosynthetica 2024, 62, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Alrashidi, A.A. Selenium and potassium supplementation improve nitrogen metabolism, antioxidant activity, and osmolyte production, reducing the growth and photosynthetic inhibition caused by polyethylene glycol (PEG) in wheat. Not. Bot. Horti Agrobot. Cluj-Napoca 2024, 52, 13936. [Google Scholar] [CrossRef]
- Alrashidi, A.A. Alleviation of salinity triggered oxidative damage and photoinhibition in Vigna radiata by individual and combined treatments of selenium and jasmonic acid. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12704. [Google Scholar] [CrossRef]
- Gondek, K.; Gondek, A. The influence of mineral fertilization on the yield and content of selected macro and microelements in spring wheat. J. Res. Appl. Agric. Eng. 2010, 55, 30–36. [Google Scholar]
- Gondek, K. Assessment of the effect of sulphur supplied to the soil with mineral fertilizers and waste from magnesium sulphate production on its content in spring wheat (Triticum aestivum L.). Acta Agrophys. 2012, 15, 269–280. [Google Scholar]
- Rachoń, L.; Szumiło, G. Comparison of chemical composition of selected winter wheat species. J. Elem. 2009, 14, 135–146. [Google Scholar] [CrossRef]
- Bernas, J.; Konvalina, P.; Moudry, J., Jr.; Vlasek, O.; Jelinkova, Z. Technological quality of spelt wheat and environmental impact of spelt wheat growing. Int. J. Adv. Sci. Eng. Technol. 2016, 4, 128–131. [Google Scholar]
- Krawczyk, P.; Ceglińska, A.; Kardialik, J. Comparing the technological value of spelt grains to common wheat grains. Food Sci. Technol. Qual. 2008, 5, 43–51. [Google Scholar]
- Stankowski, S.; Hury, G.; Sobolewska, M.; Opatowicz, N.; Bashutska, U. The effect of sulphur fertilisation on field and grain quality of winter wheat. Sci. Bull. Nat. Forest. Univ. Ukr. Environ. Ecol. 2015, 2, 174–177. [Google Scholar]
- Łukaszewicz, S.; Politycka, B.; Smoleń, S. Accumulation of selected macronutrients and tolerance towards selenium of garden pea treated with selenite and selenate. J. Elem. 2019, 24, 245–256. [Google Scholar] [CrossRef]
- Järvan, M.; Edesi, L.; Adamson, A. Effect of sulphur fertilization on grain yield and yield components of winter wheat. Acta Agric. Scand. Sect. B Soil Plant Sci. 2012, 62, 401–409. [Google Scholar] [CrossRef]
- Goh, K.H.; Lim, T.T. Geochemistry of inorganic arsenic and selenium in a tropical soil: Effect of reaction time, pH, and competitive anions on arsenic and selenium adsorption. Chemosphere 2004, 55, 849–859. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).