Abstract
Elevated CO2 (eCO2) influences crop nutrition, but the impact of its interaction with soil amendments on selenium (Se) bioavailability is unclear. This study investigated how eCO2 (+200 ppm), biochar (BC, 1% w/w), and phosphate fertilizer (PF, 1 g kg−1) affect Se uptake in garlic—a model crop chosen for its efficiency in accumulating and transforming Se into bioactive forms. The results showed that eCO2 significantly enhanced garlic biomass by 19.1–34.2% and decreased soil pH by 0.05–0.13 units. Concurrently, eCO2 increased Se concentration in garlic tissues by 2.9–13.3% compared to ambient CO2 (aCO2). Biochar amendment reduced soil Se bioavailability, leading to a 15.2–22.8% decrease in garlic Se concentration under eCO2. In contrast, phosphate fertilizer enhanced Se bioavailability via competitive ligand exchange, increasing Se uptake by 18.7–31.4%. These findings demonstrate that PF can be strategically co-managed with eCO2 to optimize Se biofortification in garlic, providing a practical strategy to safeguard nutritional security under future climate scenarios.
1. Introduction
Selenium (Se) is an essential micronutrient for human health, and its dietary deficiency is linked to several pathologies [1,2,3]. The security of the soil–plant–human Se pathway is a critical public health concern [4,5]. Understanding how this pathway is altered under future climate scenarios is therefore paramount. The pathway of Se from soil to consumer is complex, governed by soil properties, microbial activity, and agricultural management [6,7]. Garlic (Allium sativum L.), was selected as the model plant for this study because it is an efficient secondary accumulator of Se and has a unique capacity to transform inorganic soil Se into bioactive organoselenium compounds, which enhances its nutritional value and makes it an ideal system for investigating Se bioavailability [8,9,10].
Anthropogenic elevation of atmospheric CO2 (eCO2) is a major environmental factor that can modulate nutrient dynamics in this pathway, presenting a multifaceted challenge to global agriculture [11,12,13]. While eCO2 can enhance the biomass of C3 plants like garlic, it often concurrently leads to a dilution of essential micronutrients in edible tissues [14,15]. However, this quantitative growth often conceals a critical qualitative decline, as it frequently leads to the dilution of essential micronutrients like zinc and iron in edible tissues [16]. This unintended consequence threatens to exacerbate global “hidden hunger”, yet the impact of eCO2 on the bioavailability of selenium (Se), a micronutrient of profound importance to human health, remains particularly elusive and poorly understood.
Current literature on eCO2 and Se bioaccumulation is sparse and contradictory, with reports ranging from increased uptake to significant dilution, highlighting a significant knowledge gap [17,18]. This inconsistency suggests that the effect of eCO2 is likely modulated by interactions with key agronomic factors. In contemporary agriculture, soil amendments are increasingly deployed to enhance sustainability [19,20]. Biochar (BC), a carbon–rich porous material, is celebrated for improving soil health, water retention, and nutrient availability [21,22,23,24]. BC can immobilize Se via its strong adsorption capacity, whereas phosphate fertilizer can mobilize Se through competitive anion exchange with selenate [25,26,27,28,29]. This creates a critical, and largely unexplored, antagonism in how common agricultural practices might influence food nutritional quality under future climate conditions.
To address this critical knowledge gap, this study employed a soil–garlic system in open-top chambers to investigate the individual and interactive effects of eCO2, BC, and PF. We hypothesize that eCO2 will increase garlic Se concentration by enhancing soil Se bioavailability. And BC amendment will suppress, whereas PF will enhance, the uptake of Se under both CO2 levels. Therefore, our specific objectives were to: (1) quantify the responses of garlic growth and Se accumulation; (2) decipher the associated changes in soil physicochemical properties and Se fractionation; (3) characterize the shifts in soil microbial community structure; and (4) integrate these findings using structural equation modeling (SEM) to elucidate the dominant mechanistic pathways. This comprehensive approach provides novel, systems-level insights essential for developing climate-resilient biofortification strategies to ensure the nutritional quality and safety of agricultural products in a high–CO2 future.
2. Materials and Methods
2.1. Experimental Site, Soil, and Amendments
The experiment was conducted in open-top chambers (OTCs) located at Yangzhou University, Jiangsu Province, China (119°41′ E, 32°35′ N). The experimental soil was collected from the surface layer (0–20 cm) of a farmland in Taihua Town, Yixing City. This site is recognized as a natural selenium-rich area by the Chinese Geological Society. The total selenium concentration was 0.72 ± 0.05 mg kg−1 for the selenium-enriched soil (Se) and 0.15 ± 0.02 mg kg−1 for the control soil (CK), which are within the typical range for natural Se-rich areas in China. The soil, classified as an Alfisol according to the Chinese Soil Taxonomy [30], was air-dried, homogenized, and sieved through a 4 mm mesh.
The biochar (BC) used in this study was produced from rice straw via slow pyrolysis at 500 °C. It was characterized by a high specific surface area and a pH of approximately 9.8. The biochar contained total N 0.12 g kg−1, total P 0.05 g kg−1, and total K 0.8 g kg−1. The Se content in the biochar was not determined as it was produced from biomass grown in non-Se-enriched areas and is known to contain negligible Se concentrations, thus having a minimal impact on the soil Se balance [31]. The phosphate fertilizer applied was triple superphosphate (TSP, Ca(H2PO4)2·H2O), with an N:P:K formulation of 0:46:0, which contained 43% water-soluble P2O5.
2.2. Experimental Design and Plant Cultivation
A complete factorial design was employed with the following factors: two CO2 levels [ambient (aCO2, ~415 ppm) and elevated (eCO2, ~615 ppm; simulating RCP 6.0 by 2100, IPCC 2021)] [32], two biochar levels (0% and 1% w/w), two phosphate levels (0 and 2.29 g P2O5 kg−1 soil, equivalent to 1 g P kg−1 soil), and two soil types (Control soil, CK, and Selenium-enriched soil, Se). The study employed a full factorial of CO2, BC, and PF on the selenium–enriched soil (2 × 2 × 2 = 8 treatments), while the control soil (CK) only received the CO2 treatments (ambient and elevated) to serve as a baseline (2 treatments), resulting in 10 distinct treatment, each with three replicates, totaling 30 experimental units (Figure S1).
The prepared soils were potted, and the amendments (BC and TSP) were thoroughly mixed into the respective pots before planting. Each pot was filled with 2.5 kg of prepared soil. Uniform, disease-free garlic cloves (Allium sativum L.) were germinated and then transplanted into the pots in early March 2024. The plants were grown until the end of May 2024 (86 days after transplanting) under controlled CO2 conditions in the OTCs. A flow meter-controlled pure CO2 release system maintains eCO2 levels in OTC at approximately 615 ppm. The system monitors concentrations in real time and adjusts CO2 flow rates as needed to ensure concentrations reach predetermined values. Throughout the experiment, the average daily temperature and relative humidity inside the OTCs were maintained at 25 ± 3 °C and 70 ± 10%, respectively, with natural light conditions.
2.3. Sample Collection and Analysis
At harvest, the entire above-ground shoot and bulb from each pot were collected as one plant sample. Soil samples were collected by pooling five cores from each pot. Approximately 200 g of fresh soil and the entire plant biomass per replicate were used for subsequent analysis. The tissues were rinsed with deionized water, freeze-dried, weighed for biomass determination, and ground into a fine powder for subsequent analysis [33]. Soil samples were collected from each pot. One subset was air–dried for the analysis of physicochemical properties and selenium fractionation [34], while another subset was stored at −80 °C for molecular microbial analysis [35].
Soil pH was measured potentiometrically in a 1:2.5 (w/v) soil-to-deionized water suspension using a pH meter (PHS-3E, INESA Instrument Co., Ltd., Shanghai, China) after 30 min of equilibration [33]. Soil redox potential (Eh) was measured using an ORP electrode. Soil organic matter (SOM) was determined by the potassium dichromate oxidation method (external heating method) using a titration apparatus (TAS-990, PERSEE, Beijing, China) [33]. The total Se concentration in plant tissues and soil was determined using hydride generation-atomic fluorescence spectrometry (HG-AFS, AFS-930, Beijing Jitian Instrument Co., Ltd., Beijing, China) after acid digestion. Specifically, plant samples (0.2 g) were digested with a 5:1 (v/v) mixture of HNO3 (65%) and HClO4 (70%) at 180 °C until a clear digest was obtained [33]. Soil samples (0.5 g) were digested with a 4:1 (v/v) mixture of HNO3 (65%) and HF (40%) in a closed-vessel microwave digestion system (MDS-6G, Since Microwave Chemical Technology Co., Ltd., Shanghai, China). The digests were then analyzed for total Se using an HG-AFS system (AFS-930, Beijing Jitian Instrument Co., Ltd., Beijing, China). For the determination of Se species (Se(IV) and Se(VI)) in garlic tissues, the samples were extracted with deionized water in a water bath at 95 °C for 30 min [36]. The extracts were then centrifuged and filtered through a 0.45 μm membrane. The Se species were separated and quantified using high-performance liquid chromatography coupled with hydride generation-atomic fluorescence spectrometry (HPLC-HG-AFS, Jitian, Beijing, China). The chromatographic separation was performed on an anion-exchange column (Dionex IonPac AS7, 4 × 250 mm, Thermo Fisher Scientific, Waltham, MA, USA) with a mobile phase of 30 mM (NH4)2HPO4 (pH 6.0) at a flow rate of 1.0 mL min−1. Soil Se was sequentially extracted into five operationally defined fractions: soluble (SOL-Se), exchangeable (EXE-Se), iron-manganese oxide bound (FMO-Se), organically bound (ORG-Se), and residual (RES-Se), following the procedure outlined by [36] with modifications. The specific extraction reagents and procedures were as follows: SOL-Se was extracted with 0.25 M KCl; EXE-Se with 0.5 M K2HPO4 (pH 7.0); FMO-Se with 0.25 M NH2OH·HCl (pH 2.0); and ORG-Se with 0.1 M Na4P2O7 (pH 10). The final RES-Se fraction was digested with a mixture of HNO3-HClO4 (4:1, v/v). The Se concentration in all extracts and digests was determined using hydride generation-atomic fluorescence spectrometry (HG-AFS, AFS-930, Beijing Jitian Instrument Co., Ltd., Beijing, China).
2.4. Analysis of Additional Soil Physicochemical Properties
Soil total carbon (TC) and total nitrogen (TN) were determined by dry combustion using an elemental analyzer (vario EL cube, Elementar, Langenselbold, Germany). Dissolved organic carbon (DOC) was extracted from fresh soil with deionized water (1:5 w/v) by shaking for 30 min, followed by centrifugation and filtration through a 0.45 μm membrane. The DOC concentration in the filtrate was measured using a TOC analyzer (TOC-L CPH, Shimadzu, Kyoto, Japan). Ammonium nitrogen (NH4+-N) and nitrate nitrogen (NO3−-N) were extracted from fresh soil with 2 M KCl (1:10 w/v) and determined by a continuous flow analyzer (AA3, Seal Analytical Ltd., Mequon, WI, USA). The C/N ratio was calculated as the ratio of TC to TN. Given that the experimental soil is acidic (initial pH < 6.5) and located in a humid subtropical region, carbonate content was considered negligible; thus, the measured TC represents total organic carbon [33].
2.5. Soil DNA Extraction and High-Throughput Sequencing
Microbial genomic DNA was extracted from 0.5 g of fresh soil using the D5625 soil DNA kit (Omega Bio-Tek, Norcross, GA, USA) following the instructions provided by the manufacturer. The V3–V4 hypervariable region of the bacterial 16S rRNA gene was amplified with the universal primers 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTA AT-3) [37]. The PCR products were purified, quantified, and paired-end sequenced (2 × 300 bp) on an Illumina MiSeq PE300 platform (Majorbio Bio-Pharm Technology Co., Ltd., Shanghai, China). The thermal cycling conditions for PCR were as follows: initial denaturation at 95 °C for 3 min; 30 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 45 s; with a final extension at 72 °C for 10 min. The raw sequencing data were processed using QIIME2 [38] and MOTHUR [39], clustered into operational taxonomic units (OTUs) at a 97% similarity threshold, and taxonomically classified against the SILVA database (v138) [40]. Alpha-diversity indices (chao1, Shannon) were calculated. The raw sequences were deposited in the NCBI Sequence Read Archive under the accession number SRP224943.
2.6. Statistical Analyses
All data are presented as the mean ± standard deviation (n = 3). One-way analysis of variance (ANOVA) followed by Fisher’s Least Significant Difference (LSD) test at a significance level of p < 0.05 was performed using SPSS 22.0 (IBM, Armonk, NY, USA) to identify significant differences among treatments. Spearman’s rank correlation analysis was used to assess relationships between variables. Principal component analysis (PCA) and redundancy analysis (RDA) were conducted using the R software (v3.5.2) with the vegan package [41]. Structural equation modeling (SEM) was performed in R using the lavaan package [42] to quantify the direct and indirect pathways influencing garlic Se concentration.
3. Results and Discussion
3.1. Garlic Biomass and Soil Physicochemical Properties
The data presented in Figure 1 unequivocally demonstrates that elevated CO2 (E-CO2) served as a primary driver for enhanced garlic growth, with soil amendments further modulating this response. Under ambient CO2 (A-CO2), the total biomass in the control (CK) treatment was 25.6 ± 2.2 g per pot. The application of phosphate fertilizer (PF) significantly increased biomass to 30.1 ± 2.5 g, while biochar (BC) alone resulted in 28.3 ± 2.3 g. The synergistic effect of Se and PF was evident, yielding the highest A-CO2 biomass of 32.7 ± 2.7 g. Under E-CO2, these values were substantially amplified across all treatments. The control biomass under E-CO2 (33.5 ± 2.8 g) was 31% higher than its A-CO2 counterpart. This CO2 fertilization effect was further enhanced by amendments, with the E-CO2 + Se-PF combination achieving a peak biomass of 41.8 ± 3.3 g. This supra-additive effect suggests that E-CO2 not only enhances photosynthetic carbon fixation but also potentiates the efficacy of soil amendments, likely by stimulating root development and exudation, which improves the utilization of the improved nutrient and water environment created by BC and PF [43,44].
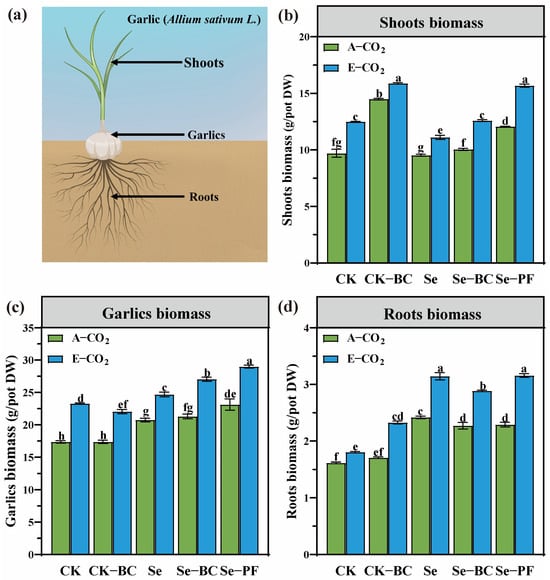
Figure 1.
Total garlic biomass under ambient CO2 (A-CO2) and elevated CO2 (E-CO2, A-CO2 + 200 ppm) across different amendment treatments. (a) Diagram of different parts of garlic plant; (b) Shoots biomass; (c) Garlics biomass; (d) Roots biomass. Different lowercase letters above bars indicate statistically significant differences among treatments (p < 0.05). (CK: Control soil; Se: Selenium–enriched soil; BC: Biochar; PF: Phosphate fertilizer).
Concurrent with the biomass response, significant alterations in soil physicochemical properties were observed (Figure 2). E-CO2 induced a consistent acidification, reducing soil pH by 0.05 to 0.13 units. The most substantial drop was noted in the Se-PF treatment. It is acknowledged that the magnitude of this pH change falls within the repeatability range (up to 0.14 units) of the standard measurement method. However, the consistent direction of change across all treatments and its correlation with other pronounced biogeochemical shifts (e.g., a substantial decrease in redox potential) suggest that the observed acidification trend, albeit small, may still hold biological significance in the context of this integrated soil–plant system. This acidification is mechanistically driven by the primary pathway of the increased dissolution of CO2 forming carbonic acid [45]. More profoundly, E-CO2 led to a substantial reduction in soil redox potential (Eh), with values decreasing by 13.7% to 29.6%. This shift towards a more reducing environment is a direct consequence of heightened microbial respiration, fueled by the increased input of labile root-derived carbon, which consumes dissolved oxygen [46].
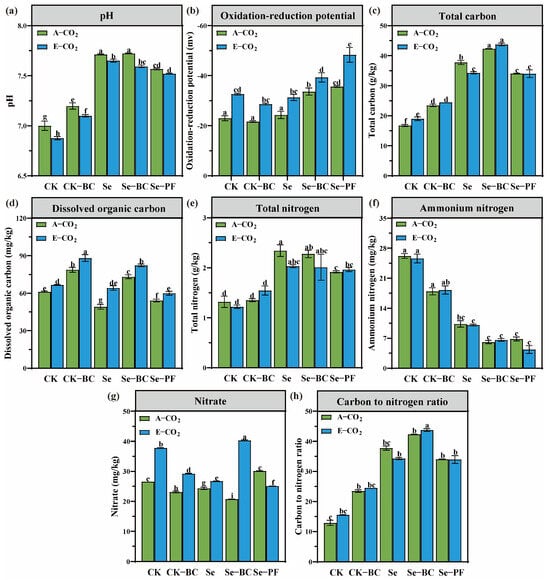
Figure 2.
Soil physicochemical properties under different treatments. (a): pH value; (b): Oxidation-reduction potential; (c): Total carbon content; (d): Dissolved organic carbon content; (e): Total nitrogen content; (f): Ammonium nitrogen content; (g): Nitrate content; (h): Carbon to nitrogen ratio. Different lowercase letters above bars indicate statistically significant differences among treatments (p < 0.05). (CK: Control soil; Se: Selenium–enriched soil; BC: Biochar; PF: Phosphate fertilizer).
The interplay between the observed acidification and the development of reducing conditions has profound implications for nutrient biogeochemistry. The decrease in pH can increase the solubility of metal oxides and phosphates. Concurrently, the lowered Eh fundamentally alters the speciation of redox-sensitive elements like selenium. The shift from oxidizing to reducing conditions favors the reduction of selenate (SeO42−, Se(VI)) to selenite (SeO32−, Se(IV)) [47,48]. While selenite is generally more strongly adsorbed, its increased presence in the soil solution under these specific conditions (e.g., competitive anion displacement by phosphate, complexation with dissolved organic matter) could enhance its phytoavailability [49,50,51]. Furthermore, the reductive dissolution of iron and manganese oxides, a process accelerated under low Eh, can release copious amounts of adsorbed selenite into solution, thereby priming the system for increased plant uptake [52]. This E-CO2–induced shift in soil chemistry created an environment fundamentally different from ambient conditions, setting the stage for the observed changes in Se dynamics.
3.2. Selenium Accumulation in Garlic and Soil Selenium Speciation
The selenium concentration in different garlic tissues, detailed in Figure 3, reveals a complex interaction between atmospheric CO2 and soil management. E-CO2 consistently enhanced Se bioaccumulation, increasing bulb Se concentration by 2.9% to 13.3% across various treatments. This enhancement is attributed to a synergy of plant and soil factors. Physiologically, E-CO2 often leads to increased transpiration and root biomass, enhancing nutrient uptake capacity. From a soil chemistry perspective, the E-CO2–induced reduction in soil Eh is the dominant driver. The reduction of selenate to selenite is crucial, as selenite is the preferred form for uptake by many plant root transporters [53]. The amendments, however, exerted powerful and opposing influences. Biochar (BC) acted as a strong sink, reducing garlic Se by approximately 41% under both CO2 levels, due to immobilization via adsorption onto its vast surface area and functional groups [54,55]. In stark contrast, phosphate fertilizer (PF) acted as a potent mobilizer, increasing garlics Se by 18.7–31.4% through competitive anion exchange, displacing selenate from soil adsorption sites [56].
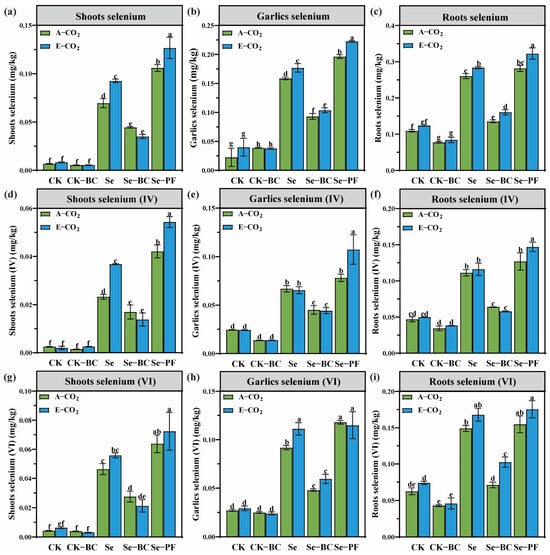
Figure 3.
Selenium accumulation in different garlic tissues under different treatments. (a): Shoots selenium concentration; (b): Garlics selenium concentration; (c): Roots selenium concentration; (d): Shoots selenium(IV) concentration; (e): Garlics selenium(IV) concentration; (f): Roots selenium(IV) concentration; (g): Shoots selenium(VI) concentration; (h): Garlics selenium(VI) concentration; (i): Roots selenium(VI) concentration. Different lowercase letters above bars indicate statistically significant differences among treatments (p < 0.05). (CK: Control soil; Se: Selenium–enriched soil; BC: Biochar; PF: Phosphate fertilizer).
The sequential extraction data in Figure 4 provides the mechanistic key to these uptake patterns, showing dynamic transformations between Se fractions. The most striking effect of E-CO2 was the significant redistribution of Se from the stable residual fraction (RES-Se) to more bioavailable forms. The RES-Se pool decreased by 9.3% to 17.3% under E-CO2, quantitatively mirrored by a dramatic surge in the iron-manganese oxide bound fraction (FMO-Se), which increased by 26.2% to 121.3%. This transformation is driven by the E-CO2-induced reducing environment. The reductive dissolution of Fe/Mn oxides liberates associated selenite into the soil solution, where it can be re-adsorbed into the FMO pool or become directly available for plant uptake [57].
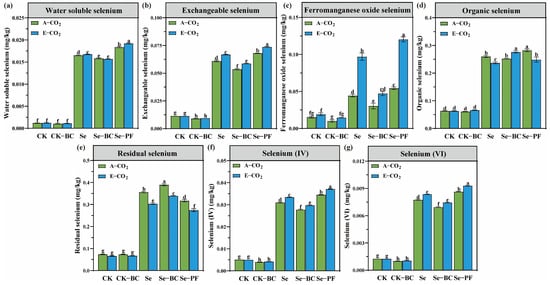
Figure 4.
Concentrations of different selenium fractions in the soil under different treatments. (a): Water-soluble selenium concentration; (b): Exchangeable selenium concentration; (c): Ferromanganese oxide selenium concentration; (d): Organic selenium concentration; (e): Residual selenium concentration; (f): Selenium(IV) concentration; (g): Selenium(VI) concentration. Different lowercase letters above bars indicate statistically significant differences among treatments (p < 0.05). (CK: Control soil; Se: Selenium–enriched soil; BC: Biochar; PF: Phosphate fertilizer).
The amendments powerfully engineered this speciation. Biochar consistently promoted Se stabilization, increasing RES-Se by 9.4–11.8% while suppressing mobile fractions (SOL-Se, EXE-Se, FMO-Se). This indicates biochar facilitates long-term sequestration, possibly through physical encapsulation or the formation of stable organo-selenium complexes [58]. Conversely, phosphate fertilizer promoted Se lability, decreasing RES-Se by ~10% and expanding the bioavailable pools (AVE-Se, FMO-Se) by 22.6–42.1%. This confirms that P fertilization fundamentally re-equilibrates Se among its solid-phase pools via competitive desorption [59]. The antagonism between BC and P treatment is thus a “tug-of-war”: phosphate mobilizes Se into solution, while biochar simultaneously re-immobilizes a significant portion of it. This has critical implications for precision biofortification, suggesting that the timing and sequence of amendment application are crucial to achieve desired Se outcomes in a high–CO2 future.
3.3. Soil Microbial Community Dynamics and Its Regulatory Role
The soil microbial community, as analyzed in Figure 5 and Figure S2, exhibited significant shifts in diversity and structure, revealing its role as a key mediator of the observed biogeochemical changes. Alpha–diversity indices (Figure 5a,b) showed that microbial richness (Chao1) and evenness (Shannon) were generally higher in the native selenium-enriched soil (Se) compared to the control (CK), suggesting that elevated Se selects for a specialized and more diverse microbial consortium capable of tolerating or metabolizing selenium [60]. The response to E-CO2 was treatment-dependent, in the Se treatment, the combination of E-CO2 and biochar (Se-BC) increased species richness, likely because biochar provided a protective habitat, buffering microbes from stress while allowing them to capitalize on increased root exudates [61]. Conversely, in the CK and P-fertilized treatments, E-CO2 reduced richness, indicating a potential homogenizing pressure favoring fewer, more adaptable taxa.
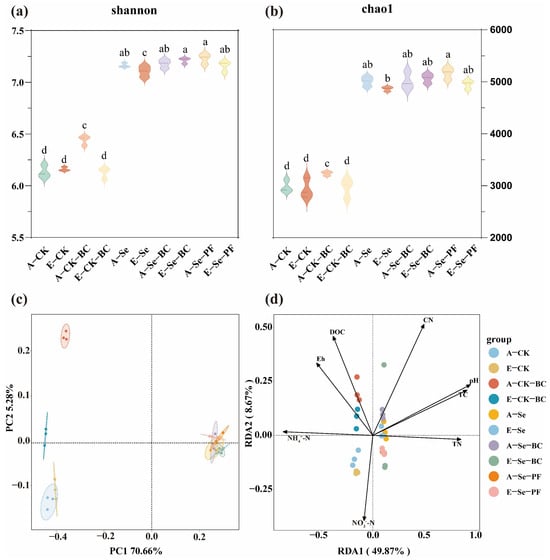
Figure 5.
Soil microbial community responses to eCO2, BC and P amendment. (a) Alpha diversity indices: Shannon; (b) Alpha diversity indices: chao1; (c) Principal Component Analysis (PCA) of bacterial community structure based on Bray–Curtis distance; (d) Redundancy analysis (RDA) ordination plots to analyze the relationship between soil properties and soil microbial community structure. Different lowercase letters indicate significant differences among treatments (p < 0.05). Error bars represent standard deviation (n = 3).
The Principal Component Analysis (PCA) in Figure 5c clearly separated the microbial communities based on soil type (Se vs. CK) and, within the Se soils, by amendment and CO2 level. This indicates deterministic selection where the native Se level sets the initial template, and the treatments act as secondary filters. The Redundancy Analysis (RDA) in Figure 5d revealed strong correlations between soil properties, Se fractions, and microbial community structure. The first axis, strongly associated with pH and Eh, shows that the E-CO2–induced acidification and reduction potential were major forces shaping the microbiome. Figure S2 further illustrates the shifts in microbial composition at the phylum level, showing, for instance, that E-CO2 and amendments altered the relative abundances of key phyla such as Proteobacteria, Acidobacteria, and Bacteroidetes.
Further elucidating these relationships, the Mantel test network in Figure 6 visualizes the robust correlations between soil properties, Se fractions, microbial phyla, and garlic Se accumulation. The network likely shows strong positive links between soil Eh, pH, and the RES-Se fraction, while SOL-Se and EXE-Se fractions are negatively correlated with Eh and positively linked to specific microbial phyla (e.g., Proteobacteria, Actinobacteria, and Firmicutes) and ultimately to garlic Se. This analysis positions the microbial community not as a passive responder but as an active participant in the Se cycle. The shifts in the microbial community were associated with changes in Se bioavailability, particularly the increase in copiotrophic bacteria under E-CO2, point to accelerated carbon cycling. More importantly, the correlation between key taxa and bioavailable Se pools, making it more accessible for plant uptake [62]. This intricate bio-geochemical coupling is a critical mechanism through which E-CO2 enhances Se phytoavailability.
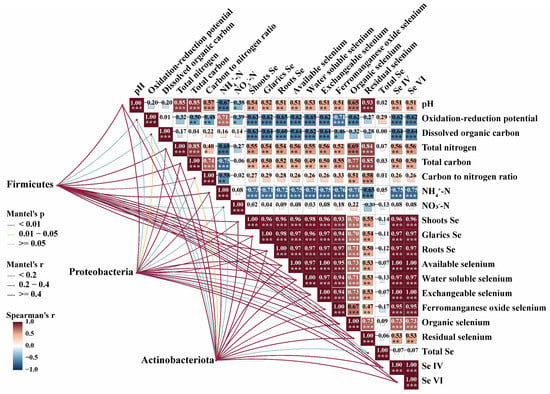
Figure 6.
Mantel test network correlating soil properties, soil Se fractions, Se accumulation in garlic, and the dominant phyla with Se dynamics. Line widths indicate correlation strength (p < 0.05). Significance levels are indicated: * p < 0.05, ** p < 0.01, and *** p < 0.001.
3.4. Integrated Mechanisms Elucidated by Structural Equation Modeling
The Structural Equation Model (SEM) in Figure 7 provides a powerful quantitative synthesis of the complex pathways influencing garlic Se concentration [Total garlic Se, garlic Se(IV), and garlic Se(VI)]. The model’s exceptional fit indices (χ2 test p = 0.85, GFI = 0.991, RMSEA = 0.000) offer strong confidence that the hypothesized structure accurately represents the causal relationships within the system. The model successfully disentangles the direct and indirect effects of the experimental drivers, moving from qualitative observation to quantitative attribution.
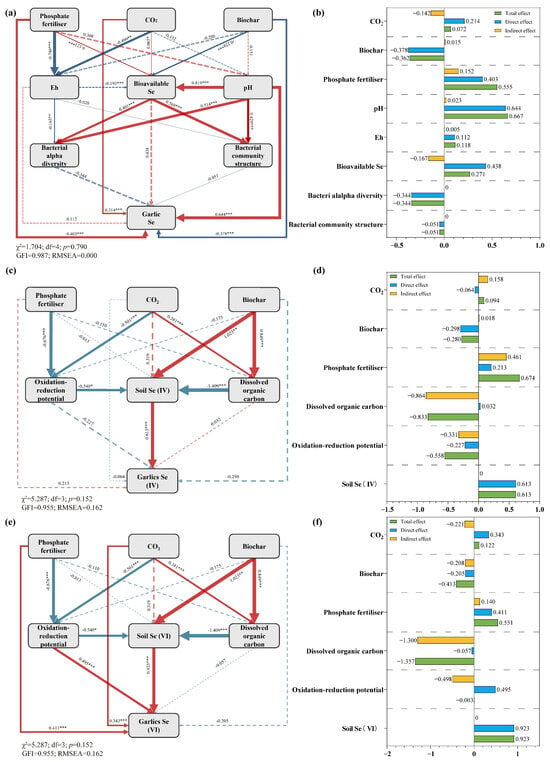
Figure 7.
Structural Equation Model (SEM) illustrating the direct and indirect effects of elevated CO2, biochar, and phosphate fertilizer on garlic selenium concentration. Standardized path coefficients are shown. Solid blue lines indicate significant positive paths, and solid red lines indicate significant negative paths. Dashed lines represent non-significant paths. (*** p < 0.001, ** p < 0.01, and * p < 0.05). The R2 value represents the proportion of variance explained for the endogenous variable. Model fit indices are provided (X2 p-value, GFI, RMSEA).
Path analysis reveals the nuanced strategies of each driver. Elevated CO2 (E-CO2) exerted a significant positive total effect on garlic Se, but this was primarily mediated through a powerful indirect pathway involving a decrease in soil pH. The direct path from E-CO2 to garlic Se was weaker, indicating that the CO2 fertilization effect on plant physiology alone is insufficient; the crucial step is the alteration of soil chemistry. The lowered pH subsequently had a strong positive effect on the pool of bioavailable soil Se. Phosphate fertilizer (PF) displayed a different strategy, characterized by an overwhelmingly strong direct positive effect on soil available Se, quantitatively capturing the competitive ligand exchange mechanism [56]. In stark contrast, biochar (BC) exhibited a significant negative direct effect on garlic Se, a clear numerical confirmation of its role as a Se immobilizing agent [63].
The SEM’s true power lies in its holistic integration, explaining the interactions and collective outcome. The model successfully contextualizes the observed antagonism between BC and P. The strong positive path from P to available Se and the negative path from BC to garlic Se visually represent the "tug-of-war" described earlier; their opposing forces are simultaneously quantified. The high explanatory power (R2 value) for garlic Se concentration indicates that the model has captured the dominant drivers. Furthermore, the model implies that the E-CO2 effect is critically contingent on soil chemistry–without the pH–mediated mobilization of Se, the benefit of E-CO2 would be markedly diminished. This integrated view underscores that predicting Se bioaccumulation under future climate scenarios requires a systems-level understanding that incorporates the cascading effects of CO2 on soil abiotic properties and the consequent shifts in microbial community function, all of which are then modulated by human management practices. The SEM provides this integrated, quantitative framework, highlighting that optimizing Se biofortification in a high–CO2 world will require careful consideration of these interacting pathways.
4. Conclusions
This study provides a systematic mechanistic understanding of how elevated CO2 (eCO2), biochar, and phosphate fertilizer interact to regulate selenium bioavailability in a soil–garlic system. The principal novel contribution lies in quantifying the antagonism between BC and PF under eCO2 and in identifying the pivotal role of eCO2–induced soil acidification in shifting Se speciation, as validated by structural equation modeling. These findings highlight that future Se biofortification strategies must be co-designed with climate and nutrient management policies. These results underscore that future CO2 levels will fundamentally reshape Se dynamics in soil–plant systems, and effective biofortification strategies must integrate synergistic, rather than opposing, soil management practices to optimize crop nutritional quality under changing climates. While this study provides valuable insights into the interactive effects of eCO2, biochar, and phosphate fertilizer on Se dynamics in garlic, it is important to note that the experiment was conducted over a single growing season. Future multi–year studies are recommended to validate these findings under varying climatic and soil conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15112579/s1, Figure S1: Schematic illustration of the experimental design; Figure S2: Soil microbial community composition at the phylum level responses to eCO2, BC and PF amendment.
Author Contributions
Y.W.: Writing—original draft, Visualization, Investigation, Data curation, Writing—review & editing. W.L.: Investigation, Software, Data curation, Formal analysis, Writing—review & editing. Y.S.: Methodology, Visualization, Investigation, Software, Formal analysis, Writing—review & editing. Z.Z.: Investigation, Software, Formal analysis. M.X.: Writing—review & editing, Funding acquisition, Data curation. F.X.: Investigation, Validation, Writing—review & editing. W.Y.: Resources, Writing—review & editing. S.W.: Validation, Project administration, Conceptualization, Writing—review & editing. X.W.: Writing—review & editing, Funding acquisition, Supervision, Resources, Conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Jiangsu Funding Program for Excellent Postdoctoral Talent (2024ZB041), the Natural Science Foundation of Jiangsu Province (BK20250922), the National Natural Science Foundation of China (Grant No. 42577031), the National Key Research and Development Program of China (2021YFD1700804), and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX24_3775 and SJCX24_2258).
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, J.R.; Feng, T.; Wang, B.; He, R.H.; Xu, Y.L.; Gao, P.P.; Zhang, Z.H.; Zhang, L.; Fu, J.Y.; Liu, Z.; et al. Enhancing organic selenium content and antioxidant activities of soy sauce using nano-selenium during soybean soaking. Front. Nutr. 2022, 9, 17. [Google Scholar] [CrossRef]
- Schomburg, L. Selenium, selenoproteins and the thyroid gland: Interactions in health and disease. Nat. Rev. Endocrinol. 2012, 8, 160–171. [Google Scholar] [CrossRef]
- Zhang, F.; Li, X.L.; Wei, Y.M. Selenium and Selenoproteins in Health. Biomolecules 2023, 13, 799. [Google Scholar] [CrossRef]
- Thiry, C.; Ruttens, A.; De Temmerman, L.; Schneider, Y.J.; Pussemier, L. Current knowledge in species-related bioavailability of selenium in food. Food Chem. 2012, 130, 767–784. [Google Scholar] [CrossRef]
- Tan, L.C.; Nancharaiah, Y.V.; van Hullebusch, E.D.; Lens, P.N.L. Selenium: Environmental significance, pollution, and biological treatment technologies. Biotechnol. Adv. 2016, 34, 886–907. [Google Scholar] [CrossRef]
- Nie, L.L.; Zhou, B.Q.; Hong, B.; Wang, X.D.; Chang, T.; Guan, C.Y.; Guan, M. Application of selenium can alleviate the stress of cadmium on rapeseed at different growth stages in soil. Agronomy 2023, 13, 2228. [Google Scholar] [CrossRef]
- Wang, D.; Rensing, C.; Zheng, S.X. Microbial reduction and resistance to selenium: Mechanisms, applications and prospects. J. Hazard. Mater. 2022, 421, 11. [Google Scholar] [CrossRef]
- Larsen, E.H.; Lobinski, R.; Burger-Meyer, K.; Hansen, M.; Ruzik, R.; Mazurowska, L.; Rasmussen, P.H.; Sloth, J.J.; Scholten, O.; Kik, C. Uptake and speciation of selenium in garlic cultivated in soil amended with symbiotic fungi (mycorrhiza) and selenate. Anal. Bioanal. Chem. 2006, 385, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.Y.; Luo, D.Y.; Ma, H.F.; Wang, L.Y.; Yang, C.; Tian, X.K.; Nie, Y.L. Different effects of selenium speciation on selenium absorption, selenium transformation and cadmium antagonism in garlic. Food Chem. 2024, 443, 8. [Google Scholar] [CrossRef]
- Yuan, L.; Yuan, J.F.; Gao, C.; Zhao, H.M.; Wu, C.Y.; Yang, Z.H. Lactiplantibacillus plantarum S1 as a Novel Dual-Functional Probiotic Strain for High-Efficiency Organoselenium Biotransformation in Functional Food Development. Foods 2025, 14, 1851. [Google Scholar] [CrossRef]
- Grimm, N.B.; Chapin, F.S.; Bierwagen, B.; Gonzalez, P.; Groffman, P.M.; Luo, Y.Q.; Melton, F.; Nadelhoffer, K.; Pairis, A.; Raymond, P.A.; et al. The impacts of climate change on ecosystem structure and function. Front. Ecol. Environ. 2013, 11, 474–482. [Google Scholar] [CrossRef]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.B.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef]
- Toreti, A.; Deryng, D.; Tubiello, F.N.; Müller, C.; Kimball, B.A.; Moser, G.; Boote, K.; Asseng, S.; Pugh, T.A.M.; Vanuytrecht, E.; et al. Narrowing uncertainties in the effects of elevated CO2 on crops. Nat. Food 2020, 1, 775–782. [Google Scholar] [CrossRef]
- Beringer, T.; Müller, C.; Chatterton, J.; Kulak, M.; Schaphoff, S.; Jans, Y. CO2 fertilization effect may balance climate change impacts on oil palm cultivation. Environ. Res. Lett. 2023, 18, 10. [Google Scholar] [CrossRef]
- McGrath, J.M.; Lobell, D.B. Regional disparities in the CO2 fertilization effect and implications for crop yields. Environ. Res. Lett. 2013, 8, 9. [Google Scholar] [CrossRef]
- Ogden, L.E. Elevated CO2 reduces crop yield and nutrition. Front. Ecol. Environ. 2019, 17, 367. [Google Scholar]
- Zhang, Z.H.; Kau, M.; Zang, H.W.; Wang, Y.D.; Duan, Y.H.; Zhang, L.; Liu, Y.X.; Yuan, L.X. Selenium regulated the responses of soil bacterial communities to short-term elevated atmospheric CO2 stress. Environ. Res. 2025, 285, 12. [Google Scholar] [CrossRef]
- Zang, H.W.; Shi, W.Y.; Kau, M.; Li, J.Y.; Li, J.X.; Zhang, W.Y.; Zhou, Z.M.; Sun, B.W.; Yuan, L.X.; Zhu, R.B. Effects of elevated CO2 concentration on Se accumulation and associated rhizobacterial community in Cardamine hupingshanensis. Plant Soil 2025, 511, 1553–1573. [Google Scholar] [CrossRef]
- Kamali, M.; Sweygers, N.; Al-Salem, S.; Appels, L.; Aminabhavi, T.M.; Dewil, R. Biochar for soil applications-sustainability aspects, challenges and future prospects. Chem. Eng. J. 2022, 428, 131189. [Google Scholar] [CrossRef]
- Garbowski, T.; Bar-Michalczyk, D.; Charazinska, S.; Grabowska-Polanowska, B.; Kowalczyk, A.; Lochynski, P. An overview of natural soil amendments in agriculture. Soil Tillage Res. 2023, 225, 20. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Liu, Q.; Zheng, H.; Li, M.; Liu, Y.F.; Wang, X.; Peng, Y.; Luo, X.X.; Li, F.M.; Li, X.Y.; et al. Biochar as a sustainable tool for improving the health of salt-affected soils. Soil Environ. Health 2023, 1, 21. [Google Scholar] [CrossRef]
- Carvalho, M.L.; de Moraes, M.T.; Cerri, C.E.P.; Cherubin, M.R. Biochar Amendment Enhances Water Retention in a Tropical Sandy Soil. Agriculture 2020, 10, 62. [Google Scholar] [CrossRef]
- Neththasinghe, N.; Dissanayaka, D.; Karunarathna, A.K. Rhizosphere nutrient availability and nutrient uptake of soybean in response to biochar application. J. Plant Nutr. 2023, 46, 4085–4095. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Mo, T.D.; He, J.Y.; Li, C.X.; Jiang, D.H. The Combined Application of Biological Nanoselenium and Biochar Promotes Selenium Enrichment and Cadmium Content Reduction in Rice. Agronomy 2025, 15, 1398. [Google Scholar] [CrossRef]
- Li, Y.Y.; Yu, S.H.; Zhou, X.B. Effects of phosphorus on absorption and transport of selenium in rice seedlings. Environ. Sci. Pollut. Res. 2019, 26, 13755–13761. [Google Scholar] [CrossRef]
- Nakamaru, Y.; Tagami, K.; Uchida, S. Effect of phosphate addition on the sorption-desorption reaction of selenium in Japanese agricultural soils. Chemosphere 2006, 63, 109–115. [Google Scholar] [CrossRef]
- Li, B.Y.; Zhou, D.M.; Cang, L.; Zhang, H.L.; Fan, X.H.; Qin, S.W. Soil micronutrient availability to crops as affected by long-term inorganic and organic fertilizer applications. Soil Tillage Res. 2007, 96, 166–173. [Google Scholar] [CrossRef]
- Hartley, W.; Riby, P.; Waterson, J. Effects of three different biochars on aggregate stability, organic carbon mobility and micronutrient bioavailability. J. Environ. Manag. 2016, 181, 770–778. [Google Scholar] [CrossRef]
- Gong, Z.T.; Zhang, G.L.; Chen, Z.C. Pedogenesis and Soil Taxonomy; Science Press: Beijing, China, 2007. (In Chinese) [Google Scholar]
- Li, Z.; Liang, D.; Peng, Q.; Cui, Z.; Huang, J.; Lin, Z. Interaction between selenium and soil organic matter and its impact on soil selenium bioavailability: A review. Geoderma 2017, 295, 69–79. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Goldfarb, L., Gomis, M.I., Huang, M., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Kurkova, T.; Skrypnik, L.; Zalieckiene, E. Features of plant material pre-treatment for selenium determination by atomic absorption and fluorimetric methods. Chemija 2008, 19, 40–43. [Google Scholar]
- Omega Bio-tek. Soil DNA Kit D5625 Manual; Omega Bio-tek: Norcross, GA, USA, 2023. [Google Scholar]
- Winkel, L.H.E.; Johnson, C.A.; Lenz, M.; Grundl, T.; Leupin, O.X.; Amini, M.; Charlet, L. Environmental Selenium Research: From Microscopic Processes to Global Understanding. Environ. Sci. Technol. 2012, 46, 571–579. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Rosseel, Y. lavaan: An Rpackage for structural equation modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef]
- Ahmad, S.; Sehrish, A.K.; Tabassam, R.; Ai, F.X.; Naeem, M.K.; Jamil, A.; Ali, S.; Guo, H.Y. Nutrient strengthening and stress alleviation in rice (Oryza sativa L.) via foliar ceria nanoparticles and biochar amendment under elevated CO2-mediated warming. Plant Physiol. Biochem. 2025, 229, 19. [Google Scholar] [CrossRef]
- Pei, J.M.; Li, J.Q.; Fang, C.M.; Zhao, J.Y.; Nie, M.; Wu, J.H. Different responses of root exudates to biochar application under elevated CO2. Agric. Ecosyst. Environ. 2020, 301, 7. [Google Scholar] [CrossRef]
- Oh, N.H.; Richter, D.D., Jr. Soil acidification induced by elevated atmospheric CO2. Glob. Change Biol. 2004, 10, 1936–1946. [Google Scholar] [CrossRef]
- Noyce, G.L.; Smith, A.J.; Kirwan, M.L.; Rich, R.L.; Megonigal, J.P. Oxygen priming induced by elevated CO2 reduces carbon accumulation and methane emissions in coastal wetlands. Nat. Geosci. 2023, 16, 63–68. [Google Scholar] [CrossRef]
- Navarro, R.R.; Aoyagi, T.; Kimura, M.; Koh, H.; Sato, Y.; Kikuchi, Y.; Ogata, A.; Hori, T. High-resolution dynamics of microbial communities during dissimilatory selenate reduction in anoxic soil. Environ. Sci. Technol. 2015, 49, 7684–7691. [Google Scholar] [CrossRef] [PubMed]
- Bao, P.; Huang, H.; Hu, Z.Y.; Häggblom, M.M.; Zhu, Y.G. Impact of temperature, CO2 fixation and nitrate reduction on selenium reduction, by a paddy soil Clostridium strain. J. Appl. Microbiol. 2013, 114, 703–712. [Google Scholar] [CrossRef]
- Zhai, H.; Liu, Y.M.; Pan, L.; Wang, Y.J.; Gong, H.T.; Ren, M.Q.; Wu, J.C. Selenite adsorption and desorption characteristics in soils: Effects of soil amendments and underlying mechanisms. Eur. J. Soil Sci. 2025, 76, 13. [Google Scholar] [CrossRef]
- Eich-Greatorex, S.; Krogstad, T.; Sogn, T.A. Effect of phosphorus status of the soil on selenium availability. J. Plant Nutr. Soil Sci. 2010, 173, 337–344. [Google Scholar] [CrossRef]
- Weng, L.P.; Vega, F.A.; Supriatin, S.; Bussink, W.; Van Riemsdijk, W.H. Speciation of Se and DOC in soil solution and their relation to Se bioavailability. Environ. Sci. Technol. 2011, 45, 262–267. [Google Scholar] [CrossRef]
- Zafeiriou, I.; Gasparatos, D.; Ioannou, D.; Kalderis, D.; Massas, I. Selenium biofortification of lettuce plants (Lactuca sativa L.) as affected by Se species, Se rate, and a biochar co-application in a calcareous soil. Agronomy 2022, 12, 131. [Google Scholar] [CrossRef]
- White, P.J. Selenium accumulation by plants. Ann. Bot. 2016, 117, 217–235. [Google Scholar] [CrossRef]
- Tang, Z.Y.; Feng, X.; Li, R.J.; Fan, F.L.; Miao, Z. Mechanisms of biochar in modulating soil organic selenium transformation and enhancing soil selenium availability. Agronomy 2025, 15, 701. [Google Scholar] [CrossRef]
- An, L.J.; Zhao, L.P.; Wei, A.; Shi, K.X.; Li, M.S.; Dawwam, G.E.; Zheng, S.X. Balancing application of plant growth-promoting bacteria and biochar in promoting selenium biofortification and remediating combined heavy metal pollution in paddy soil. Environ. Geochem. Health 2025, 47, 17. [Google Scholar] [CrossRef]
- Nakamaru, Y.M.; Altansuvd, J. Speciation and bioavailability of selenium and antimony in non-flooded and wetland soils: A review. Chemosphere 2014, 111, 366–371. [Google Scholar] [CrossRef]
- Wang, M.K.; Cui, Z.W.; Xue, M.Y.; Peng, Q.; Zhou, F.; Wang, D.; Dinh, Q.T.; Liu, Y.X.; Liang, D.L. Assessing the uptake of selenium from naturally enriched soils by maize (Zea mays L.) using diffusive gradients in thin-films technique (DGT) and traditional extractions. Sci. Total Environ. 2019, 689, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.J.; Zhu, G.W.; Parhat, R.; Jin, Y.Y.; Wang, X.S.; Wu, W.P.; Xu, W.L.; Wang, Y.L.; Chen, W.F. Exogenous selenium and biochar application modulate the growth and selenium uptake of medicinal legume Astragalus species. Plants 2023, 12, 1957. [Google Scholar] [CrossRef]
- Lee, S.; Woodard, H.J.; Doolittle, J.J. Effect of phosphate and sulfate fertilizers on selenium uptake by wheat (Triticum aestivum). Soil Sci. Plant Nutr. 2011, 57, 696–704. [Google Scholar] [CrossRef]
- Luo, L.; Hou, X.; Yi, D.D.; Deng, G.G.; Wang, Z.Y.; Peng, M. Selenium-enriched microorganisms: Metabolism, production, and applications. Microorganisms 2025, 13, 1849. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Lindblom, S.D.; Valdez-Barillas, J.R.; Fakra, S.C.; Marcus, M.A.; Wangeline, A.L.; Pilon-Smits, E.A.H. Influence of microbial associations on selenium localization and speciation in roots of Astragalus and Stanleya hyperaccumulators. Environ. Exp. Bot. 2013, 88, 33–42. [Google Scholar] [CrossRef]
- Sami, H.; Ashraf, K.; Sultan, K.; Alamri, S.; Abbas, M.; Javied, S.; Zaman, Q.U. Remediation potential of biochar and selenium for mitigating chromium-induced stress in spinach to minimize human health risk. S. Afr. J. Bot. 2023, 163, 237–249. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).