Abstract
Bois noir (BN) is a grapevine yellows disease associated with ‘Candidatus Phytoplasma (Ca. P.) solani’ that is transmitted to grapevines by the planthopper Hyalesthes obsoletus Signoret which uses herbaceous plants such as Urtica dioica as a pathogen reservoir. Urtica dioica is often widespread along ditches bordering vineyards, and a gradient in decreasing BN symptomatic grapevines is observed from the vineyard edges facing these ditches. In two vineyards in north-eastern Italy, over eight or seven years, the ditch bordering one edge of each vineyard was divided into two sections; in one of these, U. dioica was chemically weeded in April, while the other one remained untreated. The impact of chemical weeding on the spatial distribution of both H. obsoletus captures and newly BN symptomatic grapevines was assessed. The reduction in H. obsoletus captures in the vineyard sector facing the section of the ditch subjected to weeding corresponded to a decrease in newly symptomatic grapevines. These findings demonstrated that nettle removal from areas surrounding vineyards can effectively control BN.
1. Introduction
Bois noir (BN) is the most widespread grapevine yellows disease (GY) in the Euro-Mediterranean region, where it causes both yield losses and grapevine death [1,2,3,4,5]. BN is associated with grapevine infections by ‘Candidatus Phytoplasma (Ca. P.) solani’ (subgroup 16SrXII-A) [5,6]. In the Euro-Mediterranean region, the main ‘Ca. P. solani’ insect vector is Hyalesthes obsoletus Signoret (Hemiptera: Cixiidae), a polyphagous planthopper living on herbaceous plants (e.g., stinging nettle Urtica dioica L., field bindweed Convolvulus arvensis L., stinking hawk’s-beard Crepis foetida L., Artemisia vulgaris L. and Artemisia verlotiorum Lamotte) and shrubby plants such as Vitex agnus-castus L. [7,8,9,10,11,12,13,14,15,16,17,18,19].
A sequence analysis of the tuf gene revealed the presence of two main ‘Ca. P. solani’ tuf-types on grapevines and alternative plant hosts in the Euro-Mediterranean region, which are associated with two diverse ecological pathosystems: (i) field bindweed—H. obsoletus—grapevine tuf-b (later called tuf-b1 genotype) and (ii) stinging nettle—H. obsoletus—grapevine tuf-a [9,20]. As a consequence, in Italy ‘Ca. P. solani’-positive H. obsoletus from C. arvensis was almost always infected by the tuf-b1 genotype, while the tuf-a genotype was prevalent in samples collected from U. dioica [12,20,21,22,23,24]. Recent studies in Austria and some Euro-Mediterranean countries have revealed that a genetic lineage, namely the tuf-b2 genotype, intermediate between the tuf-a and b1 genotypes, is associated with stinging nettle strains of ‘Ca. P. solani’ [20,25].
Convolvolus arvensis and U. dioica were not only associated with two different ‘Ca. P. solani’ tuf-types, but also with different phenologies of H. obsoletus. In particular, adult emergence starts earlier on C. arvensis than on U. dioica [23,26,27,28] as a consequence of a different overwintering nymphal instar (i.e., third on field bindweeds and second on stinging nettles) [13]. Hyalesthes obsoletus populations collected on field bindweeds or stinging nettles also showed better survival and adaptation to the origin host [23,27,29]. The genetic differences between H. obsoletus populations associated with field bindweeds and stinging nettles suggested the occurrence of host races [30].
After making sure of the health of the propagation material, strategies for reducing BN spread can theoretically be based on the removal of herbaceous plants, sources of inoculum and vector control before this inoculates healthy grapevines. The roguing of symptomatic grapevines is not considered to be a useful practice as grapevines are the end host of the phytoplasma [31]. Furthermore, both insecticides and push-and-pull strategies are ineffective [12,31] or impractical [16,32] to prevent grapevine infections.
Therefore, the only suitable strategy for the control of H. obsoletus and BN is the removal of herbaceous plants, which host both the phytoplasma and the vector nymphs [12,31,33]. Based on the different spatial distributions of C. arvensis and U. dioica in vineyard habitats [12], with the latter mostly growing outside vineyards (e.g., along ditches), different strategies were suggested. To avoid vineyard colonization by field bindweeds, selective grassing or cover crops were proposed; meanwhile, for its removal, inter-row tillage and selective herbicides along rows can be adopted [34]. However, inter-row tillage is an ambiguous practice because field bindweeds easily colonize soil after tillage [23] and H. obsoletus is attracted by soil without herbaceous vegetation [34]. To control U. dioica along hedgerows and ditches, localized chemical weeding and frequent cuts were suggested [35,36,37]. As an alternative to the removal of herbaceous hosts, the biological control of H. obsoletus nymphs by entomopathogenic nematodes and fungi gave good efficacy under laboratory and greenhouse conditions [38]. However, up to now, the efficacy of U. dioica removal on BN control has been inferred indirectly by relying on the observed positive correlation between the abundance of the stinging nettle and the incidence of ‘Ca. P. solani’ tuf-a type on grapevines [12,33,35]. A preliminary investigation in two north-eastern Italian vineyards affected by BN revealed a noticeable decreasing gradient in symptomatic grapevines from the edge facing a ditch with abundant stinging nettles. This observation led to the suspicion that this herbaceous plant might be a source of infectious H. obsoletus.
Building on this hypothesis, this study aimed to investigate, over several consecutive years, the impact of annual chemical weeding against stinging nettle on the spatial distribution of the vector and the evolution of BN, both in terms of incidence and spatial distribution.
2. Materials and Methods
2.1. Vineyards under Study
A multi-year study was carried out in two vineyards (vineyard A from 2007 to 2020 and vineyard B from 2012 to 2021) of cultivar Chardonnay heavily infected by BN located in north-eastern Italy (locality Cormons, Gorizia district, Friuli Venezia Giulia region).
Vineyard A (45°56′33.81″ N, 13°26′44.62″ E, 44 m a.s.l.) had 21 north–south-oriented rows approximately 400 m long; the grapevines were growing using the Guyot training system and with distances between and along rows of 2.8 and 1.0 m, respectively. Along the rows, there were support poles every four grapevines. On the north edge, the vineyard was bordered by a ditch, 20 m distant and perpendicular to the rows (Figure 1).
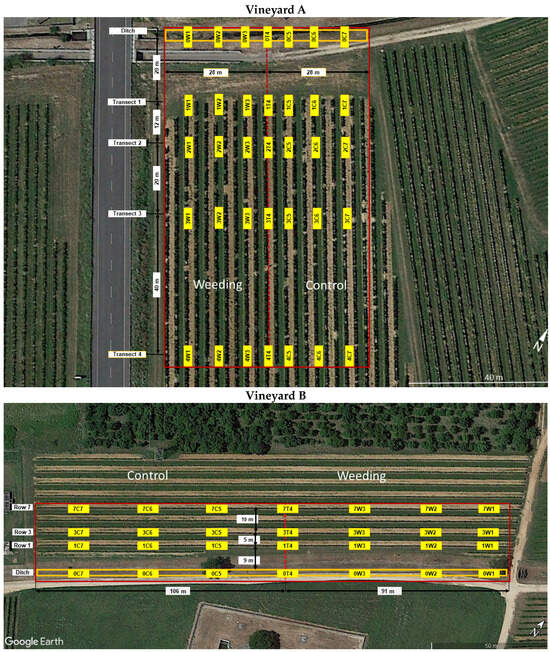
Figure 1.
Experimental design adopted in the study vineyards, with two treatments in comparison (modified from Google Earth). The red boxes delimit the ditch and the part of the vineyards being sampled. The location of the yellow sticky traps used for monitoring Hyalesthes obsoletus is also reported. The letters C, T and W in the acronym of the traps indicate control, transition and weeding, respectively.
Vineyard B (45°56′43.91″ N, 13°26′57.49″ E, 45 m a.s.l.) had seven east–west-oriented rows approximately 200 m long; the grapevines were growing using the Guyot training system with 2.6 and 0.8 m distances between and along rows, respectively. On the south edge, the vineyard was bordered by a ditch, 9 m distant and parallel to the rows (Figure 1). Along the rows, there were support poles every six grapevines.
In the area surrounding the two vineyards, other vineyards were present. Both vineyards were sprayed every late June–early July with neonicotinoids (vineyard A and B) and organophosphate (vineyard B) insecticides against Scaphoideus titanus Ball and a standard fungicide program was followed. Stinging nettle plants, potential sources of ‘Ca. P. solani’, were exclusively found along the ditch bordering the two vineyards, while field bindweeds predominantly proliferated within the vineyards. The presence of this last herbaceous plant was encouraged by the practice of alternating inter-row tilling every second year.
2.2. Treatments in Comparison
From 2012 to 2019 (vineyard A) and from 2014 to 2020 (vineyard B), the ditch bordering an edge of each vineyard was divided into two sections, in one of which the nettles were weeded every year in April, locating the glyphosate (Roundup® gold, Bayer Crop Science S.r.l., Milano, Italy, 34.4% of active ingredient, flow rate of 300 mL/hL) by a backpack sprayer.
For vineyard A, the section of the ditch subjected to nettle weeding bordered the sector of the vineyard from the 1st to the 11th row, while the control section of the ditch bordered the sector of the vineyard from the 11th to the 21st (Figure 1). For vineyard B, the section of the ditch subjected to nettle weeding bordered the sector of the vineyard from support poles 1 to 20, counted from the east side, while the control section of the ditch bordered the sector of the vineyard from support poles 20 to 43 (Figure 1). Hereafter, the vineyard sectors, facing the section of the ditch subjected or not to weeding were named “weeding sector” and “control sector”, respectively.
2.3. Molecular Identification and Characterization of ‘Ca. P. solani’ in Symptomatic Grapevines
From the second year after starting the annual chemical weeding, a number of the newly symptomatic grapevines, randomly chosen in both sectors, were submitted for molecular analyses (36% for vineyard A and 42% for vineyard B).
After total genomic DNA extraction following a CTAB method [39], the phytoplasma presence in symptomatic plants was determined by an EvaGreen qPCR protocol using phytoplasma universal primers 16S(RT)F1/16S(RT)R1 (138 bps) [40] and two microliters of diluted DNA (20 ng μL−1). qPCR was followed by a high-resolution melting (HRM) analysis as previously described [23,41], allowing a distinction between FD and BN phytoplasmas. ‘Ca. P. solani’ strains identified in symptomatic grapevines were typed by PCR/RFLP analyses based on tuf-type gene using primers TufAYf/TufAYr followed by digestion with HpaII [9]. PCR mixture and cycling conditions were performed as described in Mori et al. [23]. The thermal protocol underwent modifications in comparison to the original description by Langer & Maixner [9] and these modifications correspond specifically to the durations of the corresponding steps, while temperatures were the same as in the original protocol. To compare the proportions of plants infected with tuf-a and tuf-b1, the binomial test of significance was used. This and subsequent statistical analyses were performed using R software version 3.6.2 [42].
2.4. Monitoring of Hyalesthes obsoletus Populations
From 2014 to 2020, populations of H. obsoletus were monitored in two vineyards using yellow sticky traps that were replaced weekly, spanning from early June to late July. In each vineyard, seven traps were placed along the ditches—three in the weeding sector, three in the control sector and one at the transition point between the two sectors. This scheme was replicated in four transects for vineyard A and three rows for vineyard B (refer to Figure 1).
The yellow sticky traps were constructed using plastic yellow sheets (11 cm wide, 21 cm high and 0.2 cm thick, sourced from Plastibor s.r.l., Ponte San Nicolò, Padova, Italy). These sheets were coated on both sides with glue on four-fifths of their surface (Temo-O-Cid, Kollant Srl, Vigonovo, Italy). The traps were affixed vertically to stakes at the ground vegetation level.
The annual captures of H. obsoletus along the ditches and within the vineyards were quantified. Following the methodology of previous studies [23,28], the captures were divided into two equal-duration periods (1–25 June and 26 June–20 July). The accumulated captures, considering the entire sampling period and the two sub-periods (June and July), were analyzed using a Friedman test followed by Dunn’s Multiple Comparisons test. The replicates for each year comprised the accumulated captures recorded on each trap.
To assess the impact of nettle weeding on H. obsoletus captures, a Mann–Whitney U test was conducted to compare the accumulated captures over the years between weeding and control sections along ditches and the corresponding sectors within vineyards. In this analysis, the captures on transition point traps were not considered.
2.5. Map of BN-Symptomatic Grapevines
The two vineyards were mapped referring each grapevine to a row, a support pole and a position between two support poles. In early September, from 2007 to 2020 (vineyard A) or from 2012 to 2021 (vineyard B), BN-symptomatic grapevines were recorded. In vineyard A, only grapevines within the initial 110 m from the northern edge were considered, aligning with the area where yellow sticky traps monitored H. obsoletus. Conversely, in vineyard B, all grapevines were included as the H. obsoletus traps covered the entire surface. Symptomatic grapevines were identified based on the characteristic GY symptoms such as leaf rolling, sectorial discolorations of the blades and shoot lignification issues. For this purpose, each grapevine was examined simultaneously by two people, one on each side of the row. Newly symptomatic grapevines, starting from the second sampling year, were those showing BN symptoms despite being asymptomatic in the previous year. The annual mapping allowed us to track symptomatic grapevines before and after the start of chemical weeding of a section of the ditch (2007–2011 vs. 2012–2020 for vineyard A and 2012–2013 vs. 2014–2021 for vineyard B).
Symptomatic and newly symptomatic grapevine percentages were calculated annually, with the former relative to total plants and the latter relative to healthy grapevines in the previous year.
To describe the decreasing gradient in the percentage of symptomatic grapevines from the vineyard’s edge close to the ditch, in the first-year study (2007 for vineyard A and 2012 for vineyard B), regression analyses were performed, considering 10 transects for vineyard A and the seven rows for vineyard B. To compare the percentage of newly symptomatic grapevines in different years, Ryan’s test was performed. Then, for each year the percentages of both symptomatic and newly symptomatic grapevines were calculated separately for the ditch-weeded and non-weeded sectors using Fisher’s exact test for comparison.
For each vineyard and the two vineyards together, linear regressions between the cumulative annual captures of H. obsoletus within the vineyard and the percentage of grapevines that showed first symptoms in the same and the subsequent year were calculated.
A spatial analysis was performed to identify patterns in the accumulated symptomatic grapevines before and after the start of nettle weeding. To this purpose, the cumulative counts of symptomatic grapevines were tallied for five inter-pole groups along each row before the start of the weeding (2012 for vineyard A and 2014 for vineyard B). Similarly, counts of newly symptomatic grapevines after weeding initiation were calculated. SADIE red–blue analysis [43] was applied to determine spatial patterns in the distribution of symptomatic grapevines within the selected vineyards. This methodology identifies areas with relatively high-density counts (patches) or relatively small or zero counts (gaps) and calculates for each sampling point the indexes of clustering (vi; vj) that measure the local contribution to either patch or gap, respectively. For each variable (symptomatic or newly symptomatic grapevines), clustering significance (α = 0.05) was provided by comparing the vi and vj mean values with their corresponding values under the null hypothesis [43]. A two-dimensional map plotting the spatial distribution of local clustering indexes (vi; vj) for each variable was generated using linear kriging.
3. Results
3.1. First-Year Gradient in Symptomatic Grapevines
In the first-year study, a significant decreasing gradient in symptomatic grapevines from the ditch was recorded across 10 transects of equal width for vineyard A (power low regressions, Y = 16.52X − 0.918, p < 0.0001; R2 = 0.92) and across the seven rows for vineyard B (Y = 12.947X − 0.568, p = 0.0025; R2 = 0.86). All the grapevines with symptoms of grapevine yellows disease were affected by BN with a clear prevalence of ‘Ca. P. solani’ tuf-a (around 95% in both vineyards).
3.2. Molecular Analyses of Symptomatic Grapevines
In both vineyards, 100% of the grapevines sampled after the start of nettle weeding on a section of the ditch were infected by BN phytoplasmas based on results of real-time PCR/HRM analysis showing that grapevine DNA samples formed a cluster with ‘Ca. P. solani’ reference strains (P-TV and A-SLO, 16SrXII-A) maintained in periwinkles. This cluster was clearly different from the cluster comprising only the FD92 reference strain (16SrV-D), showing that no grapevines were infected by flavescence dorée phytoplamas.
RFLP analysis of HpaII allowed for the detection of tuf-a and tuf-b types; however, comprehensive molecular typing was performed on several ‘Ca. P. solani’ strains infecting tomatoes and acquired in 2020 through H. obsoletus individuals captured in the same two vineyards as those in the present study. In particular, tuf gene sequencing demonstrated that strains originating from stinging nettles and bindweeds belonged to the tuf-a and tuf-b1 genotypes, respectively [44]. This allowed us to assert that ‘tuf-b’ detected in the grapevines belongs to the tuf-b1 genotype [45].
In vineyard A, until 2016 only ‘Ca. P. solani’ tuf-a was recorded, whereas from 2017 to 2020 the ‘Ca. P. solani’ tuf-b1 genotype was also observed, with around 50% of the newly symptomatic grapevines belonging to this strain (Table 1).

Table 1.
BN tuf-type phytoplasmas identified on symptomatic grapevines in vineyard A.
In vineyard B, until 2020 ‘Ca. P. solani’ tuf-a was prevalent (87%), whereas in 2021, ‘Ca. P. solani’ tuf-b1 became prevalent (70%) (Table 2).

Table 2.
BN tuf-type phytoplasmas identified on symptomatic grapevines in vineyard B.
3.3. Hyalesthes obsoletus Populations and Newly Symptomatic Grapevines over the Sampling Years
In both vineyards, the total captures of H. obsoletus varied significantly over the years (Table S1 in the Supplementary Materials) both along the ditches (Figure 2A,G) and within the vineyards (Figure 2D,J). In both vineyards, the June captures were abundant within the vineyard (Figure 2E,K) and negligible along the ditch (Figure 2B,H).
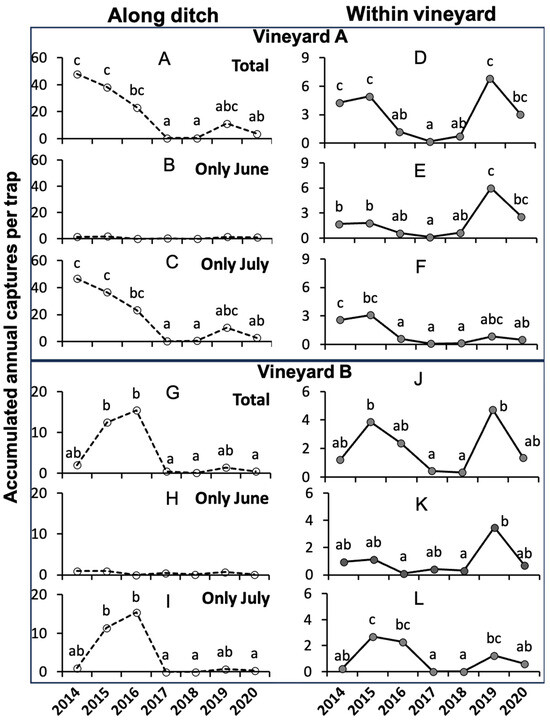
Figure 2.
Accumulated annual captures of Hyalesthes obsoletus recorded on yellow sticky traps in vineyards A (A–F) and B (G–L) over the sampling years along the ditch (A–C,G–I) and within the vineyard (D–F,J–L). Total captures and those referring to two distinct subperiods (i.e., 1–25 June and 26 June–20 July) are reported for each of the two positions. Different small letters above the lines indicate significant differences (p ≤ 0.050) between years using Dunn’s Multiple Comparisons test.
Along both ditches, the total captures were significantly high in the years 2014–2016, while in the years 2017–2018, they were close to zero (Figure 2A,G). Captures recovered in 2019 but then dropped again in 2020. The captures occurred almost exclusively in July (Figure 2B,C for vineyard A and Figure 2H,I for vineyard B).
Within both vineyards, captures were significantly higher during the three years from 2014 to 2016 compared to the subsequent two years from 2017 to 2018 (see Figure 2D,J). In 2019, captures experienced another peak, reaching levels similar to those observed in 2015, but they subsequently decreased again in 2020. Both June and July captures contributed to the two peaks, with a notable prevalence of June captures in the 2015 peak and July captures in the 2019 peak (Figure 2E,F for vineyard A and Figure 2K,L for vineyard B).
In both vineyards, the percentage of newly symptomatic grapevines varied greatly over the years (Figure 3), showing a peak in 2016, a clear decrease in the three years 2017–2019 and a new rise in 2020. In vineyard A, this last increase was particularly evident, reaching the same level as in 2016. The 2021 data relating to vineyard B alone showed a new decrease.
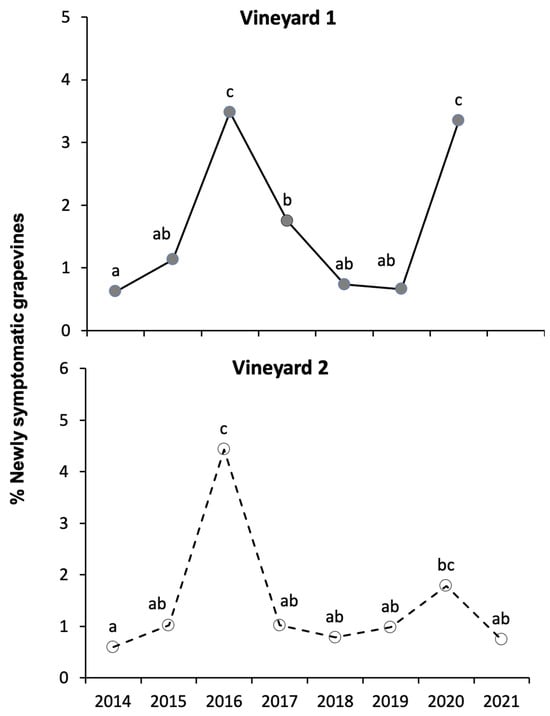
Figure 3.
Newly symptomatic grapevines recorded in the two vineyards over the sampling years. Different small letters above the lines indicate significant differences (p ≤ 0.050) with Ryan’s test.
In neither of the two vineyards did the percentage of newly symptomatic grapevines correlate with the captures of H. obsoletus recorded in the same year (Table 3). Conversely, the percentage of newly symptomatic grapevines showed a significant relationship with the vector captures recorded in the previous year in vineyards A; in vineyard B, the relationship, although positive, just missed the level of significance. Pooling together the two vineyards, the statistical significance level of this last relationship was improved.

Table 3.
Regressions between the cumulative annual captures of Hyalesthes obsoletus within the vineyard and the percentage of newly symptomatic grapevines in the same and the subsequent year. For each vineyard, the captures and the percentage of newly symptomatic grapevines from 2014 to 2020 were considered.
3.4. Influence of Stinging Nettle Weeding on Hyalesthes obsoletus Captures along the Ditch and within the Vineyards
Along both ditches, the accumulated captures of H. obsoletus were significantly lower in the weeding section than in the control (vineyard A: Mann–Whitney U test: n1 = 3, n2 = 3, U = 0, p = 0.05; vineyard B: Mann–Whitney U test: n1 = 3, n2 = 3, U = 0, p = 0.05; Figure 4). Within vineyard A, the total captures of the vector were not significantly different between the two sectors (Mann–Whitney U test: n1 = 12, n2 = 12, U = 68.5, p = 0.427). In contrast, in vineyard B they were significantly higher in the control sector than in the weeding sector (Mann–Whitney U test: n1 = 9, n2 = 9, U = 20.5, p = 0.04).
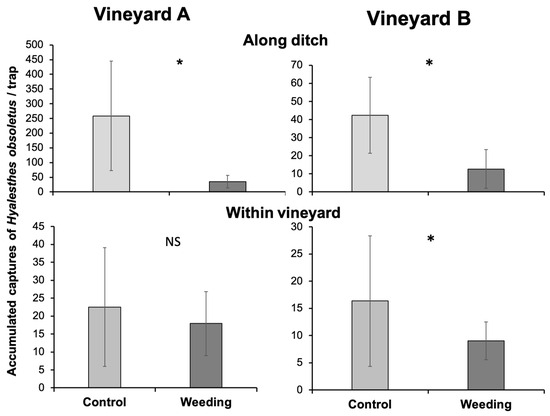
Figure 4.
Accumulated captures of Hyalesthes obsoletus per trap recorded on the yellow sticky traps placed in the two sections of the ditches and corresponding vineyard sectors (i.e., weeding and control). Asterisk indicates significant differences (p ≤ 0.05) between the two treatments at Mann–Whitney U test.
3.5. Influence of Stinging Nettle Weeding on the Spatial Distribution of Symptomatic Grapevines in the Vineyards
In vineyard A, the overall index of aggregation indicated an aggregated distribution of the accumulated symptomatic grapevines before the start of nettle weeding (Ia = 3.602, p < 0.001; Figure 5) as well as of the accumulated newly symptomatic grapevines that had manifested after the start of nettle weeding (Ia = 2.842, p = < 0.001). The average index of clustering indicated significant clustering into patches of the accumulated symptomatic grapevines (vi = 3.575; Pvi < 0.001), as well as the accumulated newly symptomatic grapevines (vi = 2.776; Pvi < 0.001). Before the start of nettle weeding, the patches of symptomatic grapevines were localized, respectively, in the first 60 m of both vineyard sectors facing the sections of the ditch that would or would not have been weeded. Considering the accumulated newly symptomatic grapevines after the start of nettle weeding, the patches were substantially in agreement with those recorded before the start of nettle weeding. The average index of clustering indicated significant clustering into gaps before the start of nettle weeding (vj = −3.609; Pvj < 0.001) and after (vj = −4.03; Pvj < 0.001), as well as of the accumulated newly symptomatic grapevines after the start of nettle weeding (vj = −2.884; Pvj < 0.001). Gaps were always localized along the southern edge, being more distant from the ditch.
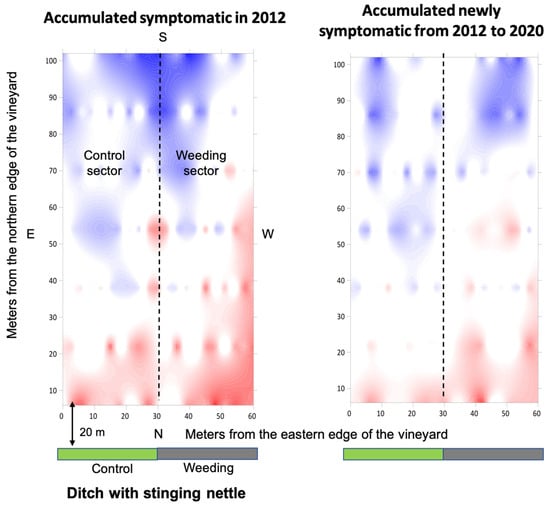
Figure 5.
Spatial distribution of the accumulated BN symptomatic grapevines in vineyard A before the start of annual nettle weeding (2012) and that of the accumulated newly symptomatic grapevines from 2012 to 2020 are reported. Red areas indicate aggregation (patches, vi ≥ 1.5), blue areas dispersion (gaps, vj ≤ 1.5) and white areas random distribution of symptomatic vines. The dotted lines separate the control and wedding sectors.
In vineyard B, the overall index of aggregation indicated an aggregated distribution of the accumulated symptomatic grapevines before the start of nettle weeding (Ia = 2.133, p = 0.015; Figure 6) as well as of the accumulated newly symptomatic grapevines that occurred after the start of nettle weeding (Ia = 2.643, p = 0.018). The average index of clustering indicated significant clustering into patches of the accumulated symptomatic grapevines (vi = 1.739; p = 0.047), as well as of the accumulated newly symptomatic grapevines (vi = 2.304; p = 0.007). Before the start of nettle weeding, two patches of symptomatic grapevines were present, with the first being on the eastern corner nearest to the ditch section that would have been weeded and the second being across the transition area part between two vineyard sectors facing the weeded and non-weeded sections of the ditch, respectively. Considering the accumulated newly symptomatic grapevines after the start of nettle weeding, the patches involved the southern edge of the control sector and only marginally the weeding sector.
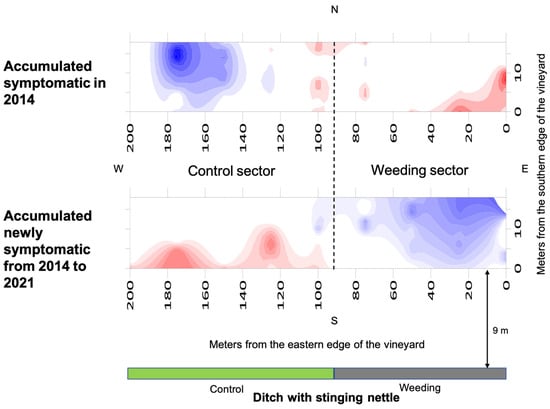
Figure 6.
Spatial distribution of the accumulated BN symptomatic grapevines in vineyard B before the start of annual nettle weeding (2014) and that of the accumulated newly symptomatic grapevines from 2014 to 2021 are reported. Red areas indicate aggregation (patches, vi ≥ 1.5), blue areas dispersion (gaps, vj ≤ 1.5) and white areas random distribution of symptomatic vines. The dotted lines separate the control and wedding sectors.
3.6. Evolution of BN Symptomatic and Newly Symptomatic Grapevine over the Sampling Years
In vineyard A, before the start of annual nettle weeding on a section of the ditch, there was no observable difference in the trend of symptomatic grapevines between the two sectors of the vineyard facing the weeding and control sections of the ditch, as depicted in Figure 7A. Even after the implementation of the nettle weeding in 2012, the disease trend in the two sectors of the vineyard was similar. This was because the incidence of newly symptomatic grapevines did exhibit significant differences between the two sectors of the vineyard, resulting in some years showing higher rates in the weeding sector and others in the control sector, with a significant difference only observed in 2014 (Figure 7B,C).
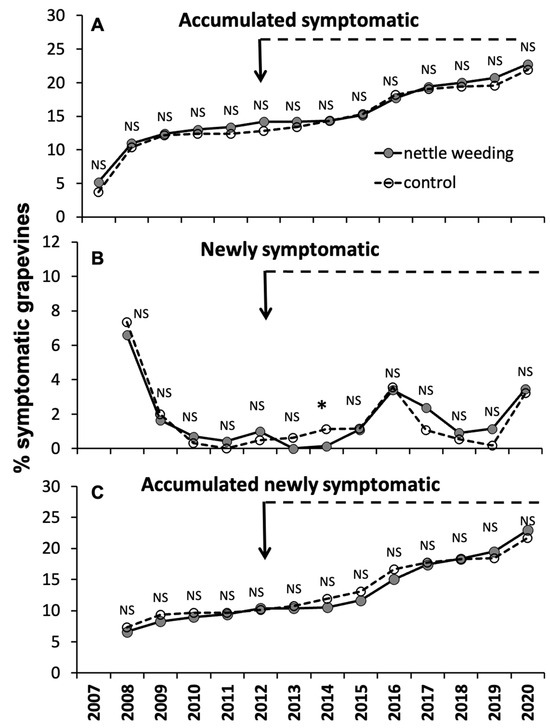
Figure 7.
Variations in the accumulated symptomatic grapevines (A), newly symptomatic grapevines (B) and accumulated newly symptomatic grapevines (C) recorded during the sampling years in the two treatments in comparison in Vineyard A. NS indicates non-significant differences, while * indicates significant differences at the 0.05 level, using Fisher’s exact test. The arrows indicate the year nettle weeding started.
In vineyard B, before the start of annual nettle weeding on a section of the ditch, the trend of symptomatic grapevines appeared with a significantly higher incidence in the sector of the vineyard facing the section of the ditch that would have been subjected to nettle weeding (Figure 8A). After the start of the nettle weeding, the trend showed first a gradual reduction in these differences, no longer significant in 2016. Following, there was subsequently an increase in these differences, albeit in favor of the control sector, which in 2020 and 2021 exhibited a significantly higher percentage of the accumulated symptomatic grapevines compared to the weeding sector. This shift occurred because, from 2016 onward, the incidence of newly symptomatic grapevines was constantly higher in the control sector (Figure 8B), showing often a statistically significant effect, resulting in a progressive increase in the differences accumulated (Figure 8C). After seven years of nettle weeding, the incidence of newly symptomatic grapevines in the sector of the vineyard bordering the section of the ditch subjected to nettle weeding was halved.
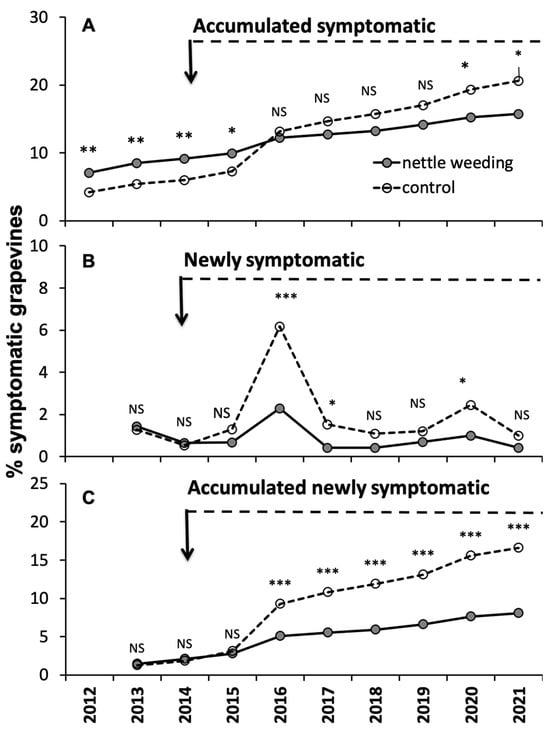
Figure 8.
Variations in the accumulated symptomatic grapevines (A), newly symptomatic grapevines (B) and accumulated newly symptomatic grapevines (C) recorded during the sampling years in the two treatments in comparison in Vineyard B. NS indicates non-significant differences, while *, **, *** indicate significant differences at the 0.05, 0.01 and 0.001 levels, respectively, using Fisher’s exact test. The arrows indicate the year nettle weeding started.
3.7. Influence of Stinging Nettle Weeding on ‘Ca. P. solani’ Tuf-Types
In both vineyards, the incidence of the two strains was not significantly different between the control and weeding sectors (Table 1 and Table 2; p ≥ 0.13 with Fisher’s exact test).
In vineyard A, the incidence of ‘Ca. P. solani’ tuf-a was significantly higher in the 10 inter-poles nearest to the ditch (96.6) than in the other 21 inter-poles (42.9%) (p = 0.0002). In these last inter-poles, the incidence of ‘Ca. P. solani’ tuf-a was on average higher in the weeding sector (50.0%) than in the control sector (33.3%).
In vineyard B, the incidence of ‘Ca. P. solani’ tuf-a was significantly higher in the four rows nearest to the ditch (88.2%) than in the other three rows (59%) (p = 0.0095). In these last rows, the incidence of ‘Ca. P. solani’ tuf-a was higher in the control sector (68.8%) than in the weeding sector (33.3%) but did not reach statistical significance (p = 0.17).
4. Discussion
This long-term study investigating the impact of nettle management on the vector H. obsoletus enabled us to evaluate the efficacy of nettle removal in controlling BN and to acquire new knowledge of the epidemiology of this GY. The results obtained are very clear and will be discussed in the following sections.
4.1. Annual and Seasonal Variation in Hyalesthes obsoletus Captures
The number of H. obsoletus captures showed high variability between years and the two sub-periods within each year (i.e., June and July).
The capture trend displayed elevated values in the three years 2014–2016, followed by a sharp drop in 2017–2018, and a subsequent recovery in the two years 2019–2020. This trend was consistent across both vineyard habitats and encompassed captures along ditches and within vineyards, suggesting common underlying factors. As reported in a previous study [23], the primary event likely responsible for the strong decline in the H. obsoletus populations was a late frost that occurred in the early morning of 21 April 2017. It is known that this planthopper finds shelter from winter rigors by burrowing into the soil, and in spring it migrates to shallower layers [27]. A frost during this period, unlike one occurring while the nymphs are still protected in the ground, can lead to significant nymph mortality. Following this decline, the recovery of H. obsoletus populations was gradual since the insect produces only one generation per year. This hypothesis is further supported by the subsequent decline in H. obsoletus populations observed in 2020, following a notable increase in adult captures in 2019 across both vineyards. Indeed, on 24 March 2020, the region where the two vineyards are located experienced a frost with minimum temperatures of −3 °C at two meters above ground and from −4 to −4.5 °C at ground level (https://www.arpa.fvg.it/temi/temi/meteo-e-clima/news/24-marzo-2020-gelata-tardiva-in-pianura/, accessed on 19 March 2024).
In both vineyard habitats, the recovery of H. obsoletus captures in 2019 was more pronounced within vineyards compared to the ditches containing stinging nettles. Comparing the captures recorded in the two sub-periods of the year considered (i.e., June and July), this difference was associated with higher captures in June within vineyards than along the ditches. According to the existing literature [23,28], captures recorded in June are mostly associated with adults that emerged from C. arvensis. This is consistent with the fact that U. dioica prevailed over C. arvensis along the diches, whereas only C. arvensis was present within the vineyards. Convolvolus arvensis was widespread in the vineyard inter-rows due to alternating tilling every second year. Furthermore, the fact that the recovery of captures observed in 2019 was greater within the vineyard, where only C. arvensis was found, and in June, when the adults emerged by C. arvensis fly, suggests that the late frost was more damaging to nymph populations living on U. dioica than on those living on C. arvensis. This could be the consequence of the different sizes of the overwintering nymphs (i.e., the third instar on C. convolvulus and the second one on U. dioica) [13] which in turn could be associated with a different susceptibility to frost.
4.2. Relationship between Hyalesthes obsoletus Captures and BN Newly Symptomatic Grapevines
It is known that the abundance of H. obsoletus and its herbaceous host plants influence a higher incidence of BN in vineyards [12,20,22,23,33,35,46]. However, the timing of BN symptom occurrence following grapevine inoculation by the vector remains less clear. In the case of the GY Flavescence dorée, symptoms typically occur in the year after inoculation [47]. Nevertheless, young grapevines affected by BN can exhibit symptoms in early autumn of the same year in which they were exposed to natural vectors [48]. In a previous study [12], which examined data from various vineyards (i.e., H. obsoletus density/newly symptomatic grapevines), newly symptomatic grapevines were not significantly associated with either the vector density within the vineyard in the current year or that of the previous year. However, in the present study, where the dataset (i.e., H. obsoletus captures/newly symptomatic grapevines) referred to different years within the same vineyards, newly symptomatic grapevines were significantly correlated with the previous year’s vector density in one of the two vineyards and even more by pulling the two vineyards together. Furthermore, the data indicated that the manifestation of symptoms in the same year as grapevine inoculation is not consistently observed for mature grapevines. The lack of a very close relationship between the population density of the vector and the newly symptomatic grapevines in the following year (coefficient of determination = 0.49) may be attributed to two factors: (i) symptoms may partially manifest in the year of inoculation or, vice versa, after more than a year and (ii) the efficiency of transmission by the vector may vary from year to year.
The discernible relationship between vector populations and the incidence of new diseased plants in the subsequent year underscores the significant influence of vector population abundance on disease progression over time. Therefore, phenomena such as late frosts can elucidate apparent declines in grapevine susceptibility, as observed in a previous study [49]. Indeed, both the drop in newly symptomatic grapevines that occurred in 1997 [49] and that in 2018 (as documented in this study) coincided with late frost [23], although only in this latter case were the H. obsoletus adults monitored, thereby revealing a collapse in the vector population coinciding with the frost event.
4.3. Effect of Nettle Weeding on the Spatial Distribution of Hyalesthes obsoletus
The effect of nettle weeding along the section of the ditches bordering the two vineyards on H. obsoletus population was evident along the ditches in both vineyards. This study confirms the efficacy of chemical weeding in H. obsoletus control in agreement with previous studies [27,35,36]. Unfortunately, repeated use of glyphosate, even if localized on the nettle alone, poses serious problems to the environment, such as herbicide drift and water contamination, with there also being possible effects on human health [50]. To avoid the undesirable side effects of chemical weeding, frequent cuts of ditch vegetation are a valid alternative, even if complete control of stinging nettles is expected only over a long year period [35,36].
The presence of a border effect in H. obsoletus captures associated with the presence of a ditch with stinging nettles confirms that the vector can colonize the vineyards. This occurred in a clear way both when the vineyard was 9 and 20 m distant from the ditch. The ability of H. obsoletus to colonize vineyards starting from a ditch with the presence of nettles is in agreement with other studies [11,12,37].
The effect of nettle weeding carried out along a section of the ditches on the bordering vineyards was significant only in one of the two vineyards. This discrepancy can be attributed to two key factors. Firstly, vineyard A is situated 20 m away from the ditch, whereas vineyard B is only 9 m distant. The distance from the ditch likely influences the dispersal range of adults, with a greater likelihood that an adult emerging from the control section of the ditch will migrate to the vineyard facing the weeded portion as the distance from the ditch increases. Secondly, the lengths of the ditch sections subjected or not to nettle weeding differ; they were 28 m in length in vineyard A and approximately 100 m in vineyard B. Consequently, the effect of nettle weeding in reducing colonization of the weeding sector by the vector is expected to be less pronounced in vineyard A due to its smaller width. We can therefore explain why vineyard A, with its greater distance from the ditch and narrower weeded section, experienced similar numbers of H. obsoletus adults colonizing the two sectors of the vineyard starting from the control section of the ditch.
4.4. Effect of Nettle Weeding on the Spatial Distribution and Dynamic of Symptomatic Grapevines
The effect of nettle weeding carried out along a section of the ditches on the spatial distribution of symptomatic grapevines was evident only in one of the two vineyards. Whitin vineyard A, aggregation patches were present not only in the sector facing the control section of the ditch but also in that facing the weeding section of the ditch. Within vineyard B, patches face the control section of the ditch whereas gaps face the weeding section of the ditch. Therefore, the spatial distribution of newly symptomatic grapevines was in agreement with that of H. obsoletus captures, indirectly confirming the positive association between the vector populations and BN incidence.
The different impact of nettle weeding along a section of the ditch on the vector spatial distribution within the two vineyards was evident when the dynamics of the accumulated newly symptomatic grapevines in the two vineyard sectors, facing the ditch section either submitted or not submitted to annual nettle weeding, were compared.
4.5. ‘Ca Phytoplasma solani’ Tuf-Types
In both vineyards, ‘Ca. P. solani’ tuf-a was predominantly present when H. obsoletus populations along the ditch were high, and the peaks of vector capture within vineyards coincided with those along the ditches. Conversely, when the captures of H. obsoletus along the ditches dropped, the ‘Ca. P. solani’ tuf-b1 became prevalent and the peaks of vector captures within vineyards occurred before those along the ditches, coinciding with the emergence of adults from field bindweeds.
This result indirectly confirms that adults of the vector emerging from stinging nettles along the ditch are associated with ‘Ca. P. solani’ tuf-a, whereas those emerging from field bindweeds, which are widespread within the two vineyards, are associated with ‘Ca. P. solani’ tuf-b1.
In both vineyards, the incidence of the ‘Ca. P. solani’ tuf-a was higher in vineyard parts nearest to the ditches but there were no differences in the incidence of the ‘Ca. P. solani’ tuf-types between the two vineyard sectors (i.e., control and weeding). However, in vineyard B, the expected greater incidence of the ‘Ca. P. solani’ tuf-a in the control sector was observed, although the scarce number of infected grapevines did not allow for reaching statistical significance.
In vineyard B, despite the presence of 20% of grapevines infected with the tuf-1b, which is associated with C. arvensis, the effectiveness of nettle weeding emerges.
5. Conclusions
This multi-year study confirmed that infectious H. obsoletus can colonize a vineyard from outside determining a border effect in the incidence of BN symptomatic grapevines. Furthermore, a significant relationship between the size of H. obsoletus populations and the occurrence of newly symptomatic grapevines was observed here for the first time. This suggests that the symptoms mostly appear in the year after infection.
Given the relationship between H. obsoletus populations and BN spread, it is evident that the disease can only be contained through vector control, but this cannot be achieved through insecticide spraying inside vineyards as a too large number of applications would be required due to the gradual emergence of adults from the ground, the scarce residual activity of currently available insecticides and the possibility that adults colonize vineyards from outside. The only possibility of controlling the vector is the elimination of the herbaceous host plants, the source of both the vector and the phytoplasma. This study is the first demonstration of the effectiveness of nettle removal on BN control obtained through the comparison between control and treated plots in the same vineyard. The possibility to control nettles not only with chemical weeding, as in this study, but also with frequent cuts of ditch vegetation can represent an example of vector control with an eco-friendly cultural control strategy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14040643/s1, Table S1: Statistical analyses on the influence of the sampling year on the accumulated captures recorded along the ditch and within the vineyard over the entire sampling period (total captures) and in the two monthly subperiods.
Author Contributions
Conceptualization, F.P. and E.C.; methodology, F.P. and E.C.; software, D.F.; formal analysis, D.F. and M.M.; investigation, F.P, E.C. and C.L.; data curation, D.F.; writing—original draft preparation, F.P. and E.C.; writing—review and editing, D.F. and M.M.; visualization, D.F.; supervision, F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
We thank the owner of the experimental vineyards, Giovanni Bigot of “Perleuve” S.r.l., for technical support. Finally, we thank Stefan Prazaru of University of Padova for SADIE analysis advice.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Garau, R.; Sechi, A.; Prota, V.A.; Moro, G. Productive Parameters in Chardonnay and Vermentino Grapevines Infected with “Bois Noir” and Recovered in Sardinia. Bull. Insectology 2007, 60, 233–234. [Google Scholar]
- Belli, G.; Bianco, P.A.; Conti, M. Grapevine Yellows in Italy: Past, Present and Future. J. Plant Pathol. 2010, 92, 303–326. [Google Scholar]
- Pavan, F.; Mori, N.; Bressan, S.; Mutton, P. Control Strategies for Grapevine Phytoplasma Diseases: Factors Influencing the Profitability of Replacing Symptomatic Plants. Phytopathol. Mediterr. 2012, 51, 11–22. [Google Scholar]
- Ember, I.; Bodor, P.; Zsófi, Z.; Pálfi, Z.; Ladányi, M.; Pásti, G.; Deák, T.; Nyitrainé, D.S.; Bálo, B.; Szekeres, A.; et al. Bois Noir Affects the Yield and Wine Quality of Vitis vinifera L. Cv. ‘Chardonnay’. Eur. J. Plant Pathol. 2018, 152, 185–197. [Google Scholar] [CrossRef]
- Quaglino, F.; Zhao, Y.; Casati, P.; Bulgari, D.; Bianco, P.A.; Wei, W.; Davis, R.E. ‘Candidatus Phytoplasma solani’, a Novel Taxon Associated with Stolbur- and Bois Noir-Related Diseases of Plants. Int. J. Syst. Evol. Microbiol. 2013, 63, 2879–2894. [Google Scholar] [CrossRef] [PubMed]
- Angelini, E.; Constable, F.; Duduk, B.; Fiore, N.; Quaglino, F.; Bertaccini, A. Grapevine phytoplasmas. In Phytoplasmas: Plant Pathogenic Bacteria—I: Characterisation and Epidemiology of Phytoplasma—Associated Diseases; Rao, G.P., Bertaccini, A., Fiore, N., Liefting, L.W., Eds.; Springer: Singapore, 2018; pp. 123–152. [Google Scholar]
- Alma, A.; Lessio, F.; Nickel, H. Insects as Phytoplasma Vectors: Ecological and Epidemiological Aspects. In Phytoplasmas: Plant Pathogenic Bacteria—II: Transmission and Management of Phytoplasma—Associated Diseases; Bertaccini, A., Weintraub, P.G., Rao, G.P., Mori, N., Eds.; Springer: Singapore, 2019; pp. 1–25. [Google Scholar]
- Sforza, R.; Clair, D.; Daire, X.; Larrue, J.; Boudon-Padieu, E. The Role of Hyalesthes obsoletus (Hemiptera: Cixiidae) in the Occurrence of Bois Noir of Grapevines in France. J. Phytopathol. 1998, 146, 549–556. [Google Scholar] [CrossRef]
- Langer, M.; Maixner, M. Molecular Characterisation of Grapevine Yellows Associated Phytoplasmas of the Stolbur-Group Based on RFLP-Analysis of Non-Ribosomal DNA. Vitis 2004, 43, 191. [Google Scholar]
- Sharon, R.; Soroker, V.; Wesley, S.D.; Zahavi, T.; Harari, A.; Weintraub, P.G. Vitex agnus-castus Is a Preferred Host Plant for Hyalesthes obsoletus. J. Chem. Ecol. 2005, 31, 1051–1063. [Google Scholar] [CrossRef]
- Bressan, A.; Turata, R.; Maixner, M.; Spiazzi, S.; Boudon-Padieu, E.; Girolami, V. Vector Activity of Hyalesthes obsoletus Living on Nettles and Transmitting a Stolbur Phytoplasma to Grapevines: A Case Study. Ann. Appl. Biol. 2007, 150, 331–339. [Google Scholar] [CrossRef]
- Mori, N.; Pavan, F.; Bondavalli, R.; Reggiani, N.; Paltrinieri, S.; Bertaccini, A. Factors Affecting the Spread of “Bois Noir” Disease in North Italy Vineyards. Vitis 2008, 47, 65–72. [Google Scholar]
- Cargnus, E.; Pavan, F.; Mori, N.; Martini, M. Identification and Phenology of Hyalesthes obsoletus (Hemiptera: Auchenorrhyncha: Cixiidae) Nymphal Instars. Bull. Entomol. Res. 2012, 102, 504–514. [Google Scholar] [CrossRef]
- Kosovac, A.; Radonjić, S.; Hrnčić, S.; Krstić, O.; Toševski, I.; Jović, J. Molecular Tracing of the Transmission Routes of Bois Noir in Mediterranean Vineyards of Montenegro and Experimental Evidence for the Epidemiological Role of Vitex agnus-castus (Lamiaceae) and Associated Hyalesthes obsoletus (Cixiidae). Plant Pathol. 2016, 65, 285–298. [Google Scholar] [CrossRef]
- Kosovac, A.; Jakovljević, M.; Krstić, O.; Cvrković, T.; Mitrović, M.; Toševski, I.; Jović, J. Role of Plant-Specialized Hyalesthes obsoletus Associated with Convolvulus arvensis and Crepis foetida in the Transmission of ‘Candidatus Phytoplasma solani’-Inflicted Bois Noir Disease of Grapevine in Serbia. Eur. J. Plant Pathol. 2019, 153, 183–195. [Google Scholar] [CrossRef]
- Moussa, A.; Mori, N.; Faccincani, M.; Pavan, F.; Bianco, P.A.; Quaglino, F. Vitex agnus-castus Cannot Be Used as Trap Plant for the Vector Hyalesthes obsoletus to Prevent Infections by ‘Candidatus Phytoplasma solani’ in Northern Italian Vineyards: Experimental Evidence. Ann. Appl. Biol. 2019, 175, 302–312. [Google Scholar] [CrossRef]
- Maixner, M.; Darimont, H.; Mohr, H.D. Studies on the Transmission of Bois Noir to Weeds and Potential Ground-Cover Plants by Hyalesthes obsoletus Signoret (Auchenorrhyncha: Cixiidae). IOBC/WPRS Bull. 2001, 24, 249–251. [Google Scholar]
- Weber, A.; Maixner, M. Habitat Requirements of Hyalesthes obsoletus Signoret (Auchenorrhyncha: Cixiidae) and Approaches to Control This Planthopper in Vineyards. IOBC/WPRS Bull. 1998, 21, 77–78. [Google Scholar]
- Mori, N.; Mitrović, J.; Smiljković, M.; Duduk, N.; Paltrinieri, S.; Bertaccini, B.; Duduk, B. Hyalesthes obsoletus in Serbia and Its Role in the Epidemiology of Corn Reddening. Bull. Insectology 2013, 66, 245–250. [Google Scholar]
- Aryan, A.; Brader, G.; Mörtel, J.; Pastar, M.; Riedle-Bauer, M. An Abundant ‘Candidatus Phytoplasma solani’ Tuf b Strain Is Associated with Grapevine, Stinging Nettle and Hyalesthes obsoletus. Eur. J. Plant Pathol. 2014, 140, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Riolo, P.; Landi, L.; Nardi, S.; Isidoro, N. Relationships among Hyalesthes obsoletus, Its Herbaceous Host Plants and “Bois Noir” Phytoplasma Strains in Vineyard Ecosystems in the Marche Region (Central-Eastern Italy). Bull. Insectology 2007, 60, 353–354. [Google Scholar]
- Mori, N.; Quaglino, F.; Tessari, F.; Pozzebon, A.; Bulgari, D.; Casati, P.; Bianco, P.A. Investigation on ‘Bois Noir’ Epidemiology in North-Eastern Italian Vineyards through a Multidisciplinary Approach. Ann. Appl. Biol. 2015, 166, 75–89. [Google Scholar] [CrossRef]
- Mori, N.; Cargnus, E.; Martini, M.; Pavan, F. Relationships between Hyalesthes obsoletus, its Herbaceous Hosts and Bois Noir Epidemiology in Northern Italian Vineyards. Insects 2020, 11, 606. [Google Scholar] [CrossRef]
- Landi, L.; Riolo, P.; Murolo, S.; Romanazzi, G.; Nardi, S.; Isidoro, N. Genetic Variability of Stolbur Phytoplasma in Hyalesthes obsoletus (Hemiptera: Cixiidae) and Its Main Host Plants in Vineyard Agroecosystems. J. Econom. Entomol. 2015, 108, 1506–1515. [Google Scholar] [CrossRef]
- Mehle, N.; Kavčič, S.; Mermal, S.; Vidmar, S.; Pompe Novak, M.; Riedle-Bauer, M.; Brader, G.; Kladnik, A.; Dermastia, M. Geographical and Temporal Diversity of ‘Candidatus Phytoplasma solani’ in Wine-Growing Regions in Slovenia and Austria. Front. Plant Sci. 2022, 13, 889675. [Google Scholar] [CrossRef]
- Lessio, F.; Tedeschi, R.; Alma, A. Population Dynamics, Host Plants and Infection Rate with Stolbur Phytoplasma of Hyalesthes obsoletus Signoret in North-Western Italy. J. Plant Pathol. 2007, 89, 97–102. [Google Scholar]
- Maixner, M. Biology of Hyalesthes obsoletus and Approaches to Control This Soilborne Vector of Bois Noir Disease. IOBC/WPRS Bull. 2007, 30, 3–9. [Google Scholar]
- Forte, V.; Angelini, E.; Maixner, M.; Borgo, M. Preliminary Results on Population Dynamics and Host Plants of Hyalesthes obsoletus in North-Eastern Italy. Vitis 2010, 49, 39. [Google Scholar]
- Kessler, S.; Schaerer, S.; Delabays, N.; Turlings, T.C.J.; Trivellone, V.; Kehrli, P. Host Plant Preferences of Hyalesthes obsoletus, the Vector of the Grapevine Yellows Disease ‘Bois Noir’, in Switzerland. Entomol. Exp. Appl. 2011, 139, 60–67. [Google Scholar] [CrossRef]
- Imo, M.; Maixner, M.; Johannesen, J. Sympatric Diversification vs. Immigration: Deciphering Host-Plant Specialization in a Polyphagous Insect, the Stolbur Phytoplasma Vector Hyalesthes obsoletus (Cixiidae). Mol. Ecol. 2013, 22, 2188–2203. [Google Scholar] [CrossRef] [PubMed]
- Maixner, M. Phytoplasma Epidemiological Systems with Multiple Plant Hosts. In Phytoplasmas: Genomes, Plant Hosts and Vectors; Weintraub, P.G., Jones, P., Eds.; CABI Publishing: Wallingford, UK, 2010; pp. 213–232. [Google Scholar]
- Zahavi, T.; Peles, S.; Harari, A.; Soroker, V.; Sharon, R. Push and Pull Strategy to Reduce Hyalesthes obsoletus Population in Vineyards by Vitex agnus castus as Trap Plant. Bull. Insectology 2007, 60, 297–298. [Google Scholar]
- Panassiti, B.; Hartig, F.; Fahrentrapp, J.; Breuer, M.; Biedermann, R. Identifying Local Drivers of a Vector-Pathogen-Disease System Using Bayesian Modeling. Basic Appl. Ecol. 2017, 18, 75–85. [Google Scholar] [CrossRef]
- Langer, M.; Darimont, H.; Maixner, M. Control of Phytoplasma Vectors in Organic Viticulture. IOBC/WPRS Bull. 2003, 26, 197–202. [Google Scholar]
- Mori, N.; Pavan, F.; Reggiani, N.; Bacchiavini, M.; Mazzon, L.; Paltrinieri, S.; Bertaccini, A. Correlation of Bois Noir Disease with Nettle and Vector Abundance in Northern Italy Vineyards. J. Pest Sci. 2012, 85, 23–28. [Google Scholar] [CrossRef]
- Mori, N.; Pavan, F.; Maixner, M. Control of Hyalesthes obsoletus Nymphs Based on Chemical Weeding and Insecticides Applied on Urtica dioica. Vitis 2014, 53, 103–109. [Google Scholar]
- Mori, N.; Pozzebon, A.; Duso, C.; Reggiani, N.; Pavan, F. Vineyard Colonization by Hyalesthes obsoletus (Hemiptera: Cixiidae) Induced by Stinging Nettle Cut Along Surrounding Ditches. J. Econ. Entomol. 2016, 109, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Moussa, A.; Maixner, M.; Stephan, D.; Santoiemma, G.; Passera, A.; Mori, N.; Quaglino, F. Entomopathogenic Nematodes and Fungi to Control Hyalesthes obsoletus (Hemiptera: Auchenorrhyncha: Cixiidae). BioControl 2021, 66, 523–534. [Google Scholar] [CrossRef]
- Martini, M.; Musetti, R.; Grisan, S.; Polizzotto, R.; Borselli, S.; Pavan, F.; Osler, R. DNA-Dependent Detection of the Grapevine Fungal Endophytes Aureobasidium pullulans and Epicoccum nigrum. Plant Dis. 2009, 93, 993–998. [Google Scholar] [CrossRef]
- Saccardo, F.; Martini, M.; Palmano, S.; Ermacora, P.; Scortichini, M.; Loi, N.; Firrao, G. Genome Drafts of Four Phytoplasma Strains of the Ribosomal Group 16SrIII. Microbiology 2012, 158, 2805–2814. [Google Scholar] [CrossRef]
- Martini, M.; Pavan, F.; Bianchi, G.L.; Loi, N.; Ermacora, P. Recent Spread of the “Flavescence Dorée” Disease in North-Eastern Italy. Phytopathol. Moll. 2019, 9, 207–208. [Google Scholar] [CrossRef]
- R Core Team. R-Version 3.6.2: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2019. Available online: https://www.R-project (accessed on 11 February 2024).
- Perry, J.N.; Winder, L.; Holland, J.M.; Alston, R.D. Red-Blue Plots for Detecting Clusters in Count Data. Eco. Lett. 1999, 2, 106–113. [Google Scholar] [CrossRef]
- Carminati, G.; Pavan, F.; Sommaro, D.; Sadallah, A.; Musetti, R.; Ermacora, P.; Martini, M. ‘Candidatus Phytoplasma solani’: From Insect Transmission to Biological and Molecular Characterization of Distinct Tuf-Type Strains in Tomato (Solanum lycopersicum L.) Plants. Department of Agricultural, Food, Environmental and Animal Sciences (DI4A), University of Udine, Via delle Scienze, Udine, Italy. 2024; manuscript in preparation. [Google Scholar]
- Carminati, G.; Firrao, G.; Ermacora, P.; Pavan, F.; Kube, M.; Martini, M. Abstracts of Presentations at the XXVII Congress of the Italian Phytopathological Society (SIPaV). J. Plant Pathol. 2022, 104, 1207–1280. [Google Scholar] [CrossRef]
- Panassiti, B.; Breuer, M.; Marquardt, S.; Biedermann, R. Influence of Environment and Climate on Occurrence of the Cixiid Planthopper Hyalesthes obsoletus, the Vector of the Grapevine Disease ‘Bois Noir’. Bull. Entomol. Res. 2013, 103, 621–633. [Google Scholar] [CrossRef]
- Caudwell, A. Epidemiology and Characterization of Flavescence Dorée (FD) and Other Grapevine Yellows. Agronomie 1990, 10, 655–663. [Google Scholar] [CrossRef]
- Osler, R. Symptom Expression and Disease Occurrence of a Yellows Disease of Grapevine in Northeastern Italy. Plant Dis. 1993, 77, 496. [Google Scholar] [CrossRef]
- Mutton, P.; Boccalon, W.; Bressan, S.; Coassin, C.; Colautti, M.; Del Cont Bernard, D.; Floreani, A.; Zucchiatti, D.; Pavan, F.; Mucignat, D.; et al. Legno nero della vite in vigneti di Chardonnay del Friuli-Venezia Giulia. Inf. Fitopatol. 2002, 52, 52–59. [Google Scholar]
- Dexter, A.G. Herbicide Spray Drift; North Dakota State University Extension Service, NDSU: Fargo, ND, USA, 1980. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).