Abstract
The red palm weevil (RPW), Rhynchophorus ferrugineus (Olivier) (Coleoptera: Dryophthoridae), is a destructive and voracious pest of palm species worldwide. Due to environmental and regulatory concerns, ecologically safe alternatives to synthetic chemical insecticides are needed to manage this cryptic insect species. Entomopathogenic fungi have the potential to manage this pest. The scope of management and effectiveness can be improved by direct control or horizontal transmission of entomopathogenic fungal isolates. We tested in the laboratory the virulence and pathogenicity of fifteen different entomopathogenic fungal isolates belonging to the following species: Beauveria bassiana, Metarhizium anisopliae, Beauveria brongniartii and Purpureocillium lilacinum. All fungal isolates were found virulent against larvae (14.9 ± 1.06 to 81.5 ± 1.48% mortality) and adults (5.6 ± 1.12 to 51.7 ± 1.51% mortality) at 12 d post-treatment. From a screening bioassay, five M. anisopliae (WG-08, WG-09) and B. bassiana (WG-23, WG-24, WG-25) isolates were tested for their concentration response mortality against larvae and adults after 7, 14 and 21 days (d) of treatment. Mortality was found positively correlated with concentration and time. At 21 d of treatment, WG-23 and WG-25 1 × 108 conidia/mL resulted in 100% mortality against larvae while only WG-25 1 × 109 conidia/mL caused 100% mortality of adults. Along with mortality, all the potential isolates have strong ovicidal effects that reduced 81.49% at 1 × 108 conidia/mL. The horizontal transmission bioassay indicated that the infected adults transmitted the disease to healthy individuals. Horizontal transmission of fungi from infected to non-infected adults not only caused significant mortality but also had a serious sublethal impact on insect development and fitness including reduced number of eggs/d fecundity, egg viability and neonate survival. Isolate WG-25 reduced oviposition (0.5 eggs/d), fecundity (11.7 eggs/female), egg viability (11.6%) along with larval survival 25.9% when infected male mated with normal female. In semi-field trials, all fungal isolates reduced survival of larvae found inside the palms and ultimately reduced infestations over a period of two months. The results of this study indicate that entomopathogenic fungi should be further tested for sustainable and efficient control of RPW in date palm production systems.
1. Introduction
Date palm, Phoenix dactylifera L. (Arecales: Arecaceae) is a diploid perennial plant known as one of the oldest and most common crops to Southwest Asia and North Africa. Over time, it spread to Australia, South America, southern Africa, Mexico, Pakistan and the United States [1] The red palm weevil (RPW) Rhynchophorus ferrugineus (Olivier) (Coleoptera: Dryophthoridae), is considered to be one of the most devastating insect pests of palm species [2]. It was first reported in India, but now it is widely distributed in Africa, America, Asia, Europe and Oceania [3,4].
In Pakistan, it is a serious pest of date palms [5,6]. The weevil has invaded a total of 69 countries over the last 30 years, and the host range has expanded from only 4 species in the mid-1950s to 40 species in 2020 [4,7]. It is an important insect pest against which quarantine and biosecurity measures should be taken by countries to try to limit its further spread, as it attacks 19 species from 15 genera [8]. It reproduces within the tree stem, damaging the vascular system which leads to the gradual death of the entire palm [9,10]. The female lays eggs in injured parts such as stems, leaf bases, offshoots, and tree crowns where larvae bore in and are usually invisible until infested palms die [11,12]. The concerns of farmers regarding economic and environmental issues of RPW are genuine and considerable. It severely affects the produce, as well as exerting negative impact on cultural and aesthetic values of palm trees [6,13].
Present management approaches against R. ferrugineus involve various control methods such as cultural techniques including burning of dead tree trunks to destroy immature stages inside, cutting down the infected palms, fertilization and irrigation, mass trapping, as well as monitoring, early detection, applications of acoustic devices, male sterile techniques, host plant resistance, entomopathogenic nematodes, insecticide applications, including fumigation, or application of natural substances [14,15,16,17,18,19,20]. Due to the high cost of pheromone traps and the prevalence of hot summers in date-producing areas, the trap strategy has not attained wide acceptance against R. ferrugineus [21]. Similarly, the sterile insect technique is also not an effective approach in the field as R. ferrugineus mates in a concealed environment, i.e., within the palm tree. Chemical insecticides have two major limitations: they harm the environment and have little or no effectiveness against the later larval stages of R. ferrugineus [21]. As curative and preventive treatments against R. ferrugineus, insecticides are usually applied on an irregular basis, which may result in environmental pollution, risks to human health and decreases in natural enemy fauna [22]. With these reservations associated with the use of traditional control approaches, there is an urgent need for novel techniques and methods for the control of invasive R. ferrugineus. In that vein, microbial control agents, particularly entomopathogenic fungi (EPF), may be considered as promising aids to existing control strategies against RPW [23].
Entomopathogenic fungi contribute to insect population regulation in natural habitats, and are usually harmless to non-target organisms, humans, and the environment [24,25]. EPFs are widely distributed in forest ecosystems and agricultural habitats; commercialized EPF products are used for the successful management of various insect pests [26,27,28]. Unlike other microbial agents (viruses, protozoa and bacteria) which need to be ingested by the host insect for the initiation of the infection process [29], fungal pathogens can directly penetrate the insect host’s cuticle [30]. Entomopathogenic fungi should be considered for inclusion in IPM programs for R. ferrugineus [20,31,32,33]. EPF can affect survival and reproduction of RPW [12,34]. However, there are certain limitations in the use of EPF such as issues during their production, interactions with fungicides, the feasibility of applying them in large-scale agricultural settings, the long-term sustainability of these biological control measures, and potential environmental impacts of widespread EPF use [20]
Horizontal transmission of a pathogen within a target population can extend the potential scope of microbial control for various insect species [35]. Auto-dissemination via the horizontal transmission of pathogens within a species has been used for biocontrol of insect pests belonging to different insect orders [36,37]. Horizontal transmission of EPFs offers advantages including reduction in area treated and volume of inoculums, diminishing harmful effects on non-target individuals [38,39] as well as providing another advantage over chemical insecticides as its impact on the pest population increases beyond the zone of direct contact [40]. Auto-dissemination in different target coleopteran hosts by Metarhizium brunneum Petch (Hypocreales: Clavicipitaceae) [37,41] has been previously documented.
Keeping in view the importance of EPF as potential candidates for biological control of RPW, this study was carried out to assess the efficacy of native isolates of B. bassiana, M. anisopliae, Purpureocillium lilacinum (Thom) Luangsa-ard, Houbraken, Hywel-Jones & Samson (Hypocereales: Ophiocordycipitaceae), recovered from soil and insect infected cadavers, to select the most virulent. This study also assessed, for the first time in RPW, the horizontal transmission of the most virulent fungal isolates and to determine the subsequent effects on oviposition, fecundity, egg hatching and larval survival among all four mating combinations of non-infected/infected males and females of R. ferrugineus. The efficacy of different EPF isolates was evaluated in semi-field trials, their persistence and impact on survival of R. ferrugineus was also assessed.
2. Materials and Methods
2.1. Insect Collection and Rearing RPW
The different developmental stages of RPW were manually collected from fallen and damaged trees of date palm, Jhang, Bahawalpur, Lodhran, Multan, Layyah and Bhakar districts of Punjab province, Pakistan. Each development stage was placed individually in boxes and transferred to the laboratory. Collected pupae were taken to the laboratory in clean perforated zip lock plastic bags.
In the laboratory, larvae were fed on sugarcane stalks depending upon the size of the larval instar. To induce larval feeding, a hole according to the size of larvae was made in the upper portion of sugarcane and larvae were released into these holes on each sugarcane stalks. The food was changed on average every three days, and larvae were allowed to feed on these pieces until they pupated. The pupae were placed in a plastic box (15 cm × 30 cm × 30 cm), which was kept moistened with distilled water using a hand sprayer; the pupae were examined daily to check adult emergence from pupae. The adults were placed in aerated plastic boxes (15 cm × 30 cm × 30 cm). Adults were fed on shredded sugarcane stems, which had been soaked in distilled water for one minute. In each box equal numbers of males and females were placed. The diet for adults was changed on alternate days and sugarcane stalks in which egg-laying occurred were moved to separate boxes for larval emergence. The sugarcane stalks were monitored daily and larvae hatching from eggs or loose in the box were transferred onto sugarcanes stalks with a camel hairbrush. The incubator was set for all the stages at 28 ± 2 °C and 65 ± 5% relative humidity (RH) and 16: 8 h (Light/Dark) photoperiod [42].
2.2. Culturing of Fungal Isolates
The virulence of fifteen isolates of different EPF including B. bassiana (WG-14, WG-21, WG-22, WG-23, WG-24, WG-25), M. anisopliae (WG-08, WG-09), Beauveria brongniartii (Sacc.) Petch (Hypocreales: Cordycipitaceae) (WG-26, WG-27) and P. lilacinum (WG-33, WG-34, WG-35, WG-36, WG-37) was evaluated against R. ferrugineus (Table S1). These isolates were obtained previously from insect cadavers and soil samples collected from various geographical areas (Table S1). The fungal isolates from soil samples were recovered using potato dextrose agar (PDA) (BD, Franklin Lakes, NJ, USA) with chloramphenicol (50 µg/mL), streptomycin sulphate (50 µg/mL) (Sigma, St Louis, MO, USA) and 0.5 g/L of dodine 65% w/w WP. All isolates were identified using taxonomic keys [43,44,45,46,47]). The cultures were stored at the Microbial Control Laboratory at Department of Entomology, University of Agriculture, Faisalabad (Pakistan) [48,49]. The isolates were inoculated in PDA Petri dishes (100 mm) and sealed with parafilm (Sigma Aldrich Chemie GmbH, Taufkirchen, Germany) at 25 °C with a 14: 10 h (Light/Dark) illumination period for 14 d. Conidia were harvested with sterile scalpel and suspended in a 50 mL conical tube with 30 mL of 0.05% Tween 80. The tubes were agitated with eight glass beads and vortexed for 5 min. The required conidial concentrations were ascertained using a hemocytometer under 400× magnification. Conidial germination was determined by spreading 0.1 mL (1 × 106 conidia/mL) suspensions on two sabouraud dextrose agar (SDA) (BD, Franklin Lakes, NJ, USA) in Petri dishes (100 mm) and incubated at 25 °C with a 14:10 h (Light/Dark) illumination period for 16–18 h. Germination was recorded by placing cover slips on plates and assessing 200 spores from each plate. We averaged two counts from two plates, and the final concentration was adjusted using serial dilutions, based on germinated conidia [50]. Germination of conidia of all the EPF was assessed under 400× magnification and found to be greater than 90%.
2.3. Screening of Fungal Isolates against RPW Larvae and Adults
Fifteen isolates were evaluated against 4th instars and adults (48–72 h old) of R. ferrugineus at two concentrations 1 × 107 and 1 × 108 conidia/mL. These rates were selected based on preliminary concentration assays conducted in the laboratory. Larvae were dipped into either concentration for 60 s while adults were immersed for 90 s [51]. The treated insects were then separately placed on sterile filter paper to dry. After 24 h, the insects were placed individually in plastic cups (150 mL) to avoid cannibalism. Larvae were provided with artificial diet [42,52], while adults were provided with shredded sugarcane (3 cm × 3 cm) for feeding. The plastic cups were covered with perforated lids for the purpose of aeration and were placed in an incubator (MIR-254, Sanyo, Tokyo, Japan) maintained at 25 °C and 75% RH with a 16: 8 h (Light/Dark) illumination period. For the control, individuals were dipped for same period in a 0.05% Tween 80 and otherwise handled the same as the treated insects. A total of 15 larvae and adults were used per replicate, and each treatment had 3 replicates (45 individuals per treatment). The entire experiment was repeated twice with 2880 individuals used in the experiment. The experiment end point was 12 d after treatment and mortality was observed on daily basis. After the last count, the remaining live individuals (larvae and adults) were disposed of. The dead larvae and adults were individually placed on sterile moistened filter paper and observed for mycosis on cadavers. The cadavers with no visible symptoms after 24 h were surface sterilized with sodium hypochlorite (0.1%) solution followed by three rinses with distilled water. Then, cadavers were placed on sterile moistened filter paper and incubated at 25 °C, 75 RH and 16: 8 h (Light/Dark) to stimulate the fungal growth [51].
2.4. Concentration Response Bioassay against Larvae and Adults of RPW
The isolates which caused the highest larval mortalities (M. anisopliae WG-08 and WG-09; B. bassiana WG-23, WG-24, and WG-25) during preliminary screening were selected for further study against both larvae and adults at different concentrations and exposure intervals. Each fungal isolate was applied at four concentrations (1 × 106, 1 × 107, 1 × 108 and 1 × 109 conidia/mL) against 6th instars and adults (48–72 h old) by dipping. Larvae were dipped into the four different concentrations for 60 s, while adults were dipped for 90 s [51]. For post-application, insects were handled as described in Section 2.3. Mortality counts were made at 7, 14 and 21 d post-treatment. For each replication, 15 insects were used making a total of 45 individuals per treatment in three replicates. The experiment was repeated twice.
2.5. Bioassay against Eggs
The ovicidal activity of the five previously selected fungal isolates (WG-08, WG-09, WG-23, WG-24 and WG-25) was evaluated using recently laid eggs. Before the bioassay, eggs (<24 h of age) were surface sterilized by immersing them in a 3% solution of sodium hypochlorite (NaClO) and then washing with distilled water three times [53]. Fifteen eggs per replicate were immersed into two fungal concentrations (1 × 106 and 1 × 108 conidia/mL) of each isolate for 1 min by placing them in a clean stainless steel mesh basket with dimensions of 16 × 17 mm [51]. A batch of eggs immersed in distilled water containing only 0.01% Tween 80 [54] served as the control. After dipping, eggs were moved to Petri dishes (2.5 cm diameter) lined with sterile moistened filter paper and incubated (MIR-254, Sanyo, Tokyo, Japan) at 25 °C with 75 RH and a 16: 8 h (Light/Dark) illumination period. Each treatment was replicated three times and the entire experiment was also repeated three times with new biotic and abiotic materials each time. Hatching data for eggs were recorded daily up to 5 d although most hatching occurred between 3 and 4 days; eggs not hatched by the 5th day were considered dead. After hatching, newly emerged neonates were transferred to another Petri dish and provided with clean sugarcane slices for feeding. Neonate survival times were recorded daily for 5 d.
2.6. Horizontal Transmission of Fungal Isolates among Adults and Effectiveness of Sublethal Concentrations on Reproductive Stages of RPW
The potential for horizontal transmission of five pre-selected EPF isolates (WG-08, WG-09, WG-23, WG-24, WG-25) was assessed in R. ferrugineus adults. EPF were applied at concentration 1 × 105 conidia/mL. Laboratory reared 48–72 h old male and female adults were immersed in a suspension of different isolates for 90 s and then allowed to mate. Four different mating pairs (10 adults with 1:1 ratio of both male and female) were arranged for each isolate: (1) non-infected females + non-infected males, (2) infected females + non-infected males, (3) non-infected females + infected males and (4) infected females + infected males. After treatment, each mating pair was transferred together into plastic boxes and provided with cotton wicks dipped into a 5% solution of sugar for feeding; the cotton wicks additionally served as a substrate for oviposition. Adult mortality and fecundity were recorded daily up to 28 d. Oviposition was recorded daily up to 7 d. Eggs laid by treated females from each combination were collected randomly and kept in Petri dishes lined with moistened filter paper. From each replication, 3 eggs/d were randomly selected up to 7 d post-treatment (21 eggs from each replicate) and hatching (%) was noted for the following 5 days (because most of hatching occurred between 3 and 4 days). The percent survival of the hatched neonates was then noted for up to 5 d. Each mating combination represented a single treatment with three replicates for individual isolates and the whole experiment was repeated three times [51].
2.7. Semi-Field Trials for RPW Survival
This experiment was conducted with 5 years old date palm plants that were purchased from a nursery with no infestation detected after careful visual observation. The plants were watered as needed and kept under a double mesh security enclosure. Each treatment consisted of six different treatments including five fungal isolates (WG-08, WG-09, WG-23, WG-24 and WG-25) and a control. Fungal isolates were suspended individually at concentration 1 × 109 conidia/mL in a 0.05% Tween 80 and 2000 mL of fungal solution. The fungal suspensions of each isolate were sprayed around the trunk with a knapsack sprayer while for the control, 2000 mL of 0.05% Tween 80 was sprayed without fungal conidia. Two hours post-application, a group of three female adults and two males were released on each plant and the trunk was wrapped with double mesh wire gauze to avoid the escape of weevils. Adults were removed 7 d post-release, and the palm plants were cut with a chainsaw at 30 d post-insect released. First, the palm plants were categorized as infested if a single larva was found inside or un-infested if no larva was found inside. The total numbers of dead and live individuals found inside were counted. The trial was arranged in a randomized complete block design (RCBD). Each treatment comprised five palm plants with four replications (total of 20 palms per treatment and total of 120 palms per trial).
2.8. Fungal Persistence over Time and Its Effectiveness against Larvae of RPW
This trial was aimed to assess the persistence of fungal isolates (WG-08, WG-09, WG-23, WG-24, WG-25) in palm plants over time. The treatment application and protocol were the same as in the previous trial. The treated and control plants were divided into five different groups that were infested with adults at 1, 15, 30, 45 and 60 d post-treatment application. Adults remained on the plants for 7 d and then were removed. Treatment efficacy was determined by dissecting palm plants at 30 d post-insect release and the total number of larvae present inside was counted as either live or dead [51].
2.9. Statistical Analysis
The normality of the distribution of the percentages of RPW mortality and mycosis was checked by Shapiro–Wilk test [55]). All data were not normally distributed and all percentages were log-transformed before further analysis. The mortality data from different treatments were corrected using Abbott’s [56]) formula and tested for comparison using Tukey HSD post hoc test [57]) at a 5% significance level. The statistical package Minitab [58] was used for analysis. Median lethal concentration (LC50) and Lethal time (LT50) values for fungal concentrations of isolates at different exposure intervals were calculated by Probit analysis. For semi-field trials, the mean number of live larvae per plant was analyzed (ANOVA) and significant differences were subjected to Duncan’s test at 5% level of significance for comparison. The efficacy of fungal isolates against larvae on palm trees was computed following Abbott’s [56] formula [51].
3. Results
3.1. Screening of Fungal Isolates against Larvae and Adults
The main effects and their possible associated interaction fungal isolates × concentration for mortality and mycosis were significant for both larvae and adults (Table S2). Larvae were more susceptible to fungal infection than adults (Figure 1 and Figure 2) at the two different concentrations. Mortality ranged from 14.9–52.9%, at 1 × 107 conidia/mL, to 18.3–81.5% at 1 × 108 conidia/mL for larvae and 5.6–31.8%, at 1 × 107 conidia/mL, to 9.1–51.7% at 1 × 108 conidia/mL in adults.
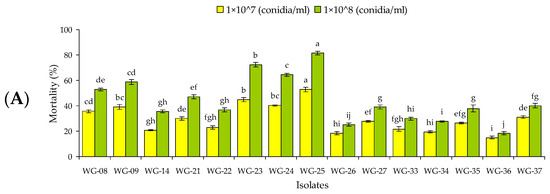
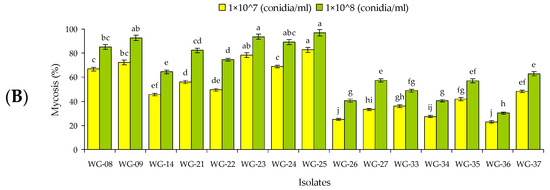
Figure 1.
Mean (A) mortality (% ± SE) and (B) mycosis (% ± SE) of 15 isolates of entomopathogenic fungi against Rhynchophorus ferrugineus larvae 12 d post-treatment. Means followed by the same lowercase letter each for mortality (F = 11.45, df = 14, 89, p < 0.01 for 1 × 107 conidia/mL and F = 18.23, df = 14, 89, p < 0.01 for 1 × 108 conidia/mL) and mycosis (F = 21.87, df = 14, 89, p < 0.01 for 1 × 107 conidia/mL and F = 29.34, df = 14, 89, p < 0.01 for 1 × 108 conidia/mL) are not significantly different, Tukey HSD test at p = 0.05.
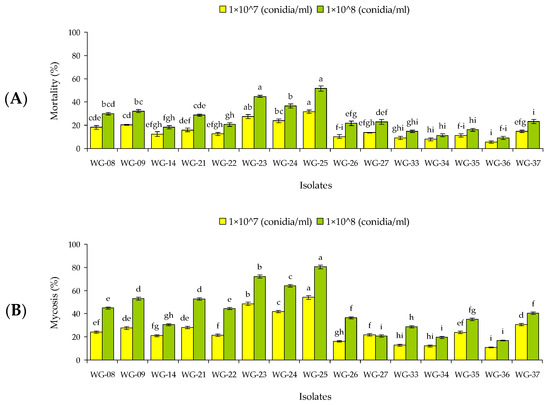
Figure 2.
Mean (A) mortality (% ± SE) and (B) mycosis (% ± SE) of 15 isolates of entomopathogenic fungi against Rhynchophorus ferrugineus adults 12 d post-treatment. Means followed by the same lowercase letter each for mortality (F = 14.56, df = 14, 89, p < 0.01 for 1 × 107 conidia/mL and F = 23.48, df = 14, 89, p < 0.01 for 1 × 108 conidia/mL) and mycosis, F = 13.71, df = 14, 89, p < 0.01 for 1 × 107 conidia/mL and F = 16.12, df = 14, 89, p < 0.01 for 1 × 108 conidia/mL) are not significantly different, Tukey HSD test at p = 0.05.
At 1 × 108 conidia/mL, only five isolates (WG-08, WG-09, WG-23, WG-24 and WG-25) against larvae and one isolate (WG-25) against adults caused >50% mortality at 12 d post-inoculation. At the same concentration, five isolates (WG-08, WG-09, WG-23, WG-24 and WG-25) against larvae and four isolates (WG-09, WG-23, WG-24 and WG-25) against the adults produced >50% mycosis (Figure 1 and Figure 2). Control mortality was lower than 5% against both developmental stages.
3.2. Concentration Response Bioassay against RPW Larvae and Adults
The main effects and their possible associated interactions isolate × concentration and isolate × interval, concentration × interval, and isolate × concentration × interval were significant for both larvae and adults (Table S3). Up to 7 d post-treatment, none of the isolates provided 100% mortality of larvae or adults. At 1 × 109 conidia/mL all isolates except WG-08 caused >50% mortality in larvae and only two isolates WG-23 and WG-25 caused >50% mortality in adults (Figure 3). Again, no isolate caused 100% mortality neither larval nor adult stage at 14 d post-inoculation. Maximum mortality of 98.5% and 79.3% were reported by isolate WG-25 in larvae and adults, respectively (1 × 109 conidia/mL) (Figure 3). After 21 d post-treatment, WG-23 and WG-25 caused 100% mortality of larvae at the two highest spore concentrations (1 × 108 and 1 × 109 conidia/mL) while WG-25 caused 100% mortality of adults at 1 × 109 conidia/mL (Figure 3).
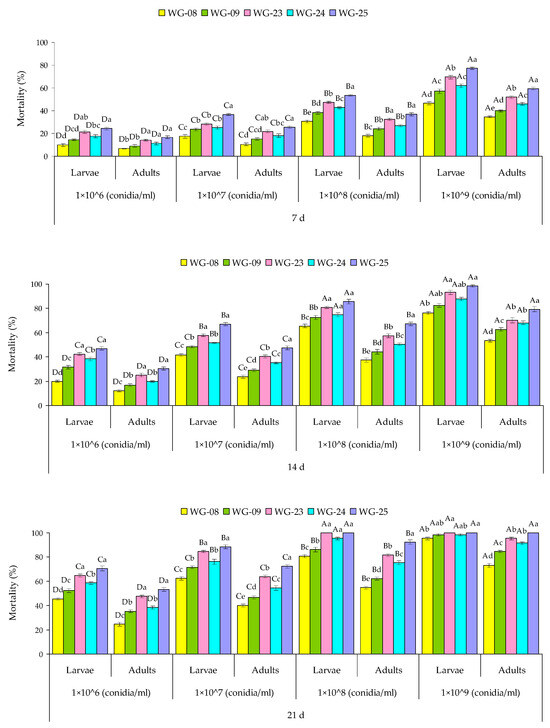
Figure 3.
Mean mortality (% ± SE) of larvae and adults of Rhynchophorus ferrugineus 7, 14 and 21 d post-treatment with selected isolates of entomopathogenic fungi. Per exposure and life stage, within each concentration (7 d, larvae F = 36.04; df = 4, 44; p < 0.01 for 1 × 106 conidia/mL, F = 39.70; df = 4, 44; p < 0.01 for 1 × 107 conidia/mL, F = 53.24; df = 4, 44; p < 0.01 for 1 × 108 conidia/mL, and F = 97.07; df = 4, 44; p < 0.01 for 1 × 1010 conidia/mL; adults F = 31.84; df = 4, 44; p < 0.01 for 1 × 106 conidia/mL, F = 37.28; df = 4, 44; p < 0.01 for 1 × 107 conidia/mL, F = 93.15; df = 4, 44; p < 0.01 for 1 × 108 conidia/mL, and F = 110.91; df = 4, 44; p < 0.01 for 1 × 1010 conidia/mL; 14 d, larvae F = 26.08; df = 4, 44; p < 0.01 for 1 × 106 conidia/mL, F = 77.45; df = 4, 44; p < 0.01 for 1 × 107 conidia/mL, F = 72.29; df = 4, 44; p < 0.01 for 1 × 108 conidia/mL, and F = 93.30; df = 4, 44; p < 0.01 for 1 × 1010 conidia/mL; adults F = 54.27; df = 4, 44; p < 0.01 for 1 × 106 conidia/mL, F = 66.05; df = 4, 44; p < 0.01 for 1 × 107 conidia/mL, F = 87.23; df = 4, 44; p < 0.01 for 1 × 108 conidia/mL, and F = 53.11; df = 4, 44; p < 0.01 for 1 × 1010 conidia/mL; 21 d, larvae F = 33.94; df = 4, 44; p < 0.01 for 1 × 106 conidia/mL, F = 48.26; df = 4, 44; p < 0.01 for 1 × 107 conidia/mL, F = 21.09; df = 4, 44; p < 0.01 for 1 × 108 conidia/mL, and F = 39.46; df = 4, 44; p < 0.01 for 1 × 1010 conidia/mL; adults F = 56.31; df = 4, 44; p < 0.01 for 1 × 106 conidia/mL, F = 31.09; df = 4, 44; p < 0.01 for 1 × 107 conidia/mL, F = 45.06; df = 4, 44; p < 0.01 for 1 × 108 conidia/mL, and F = 29.41; df = 4, 44; p < 0.01 for 1 × 1010 conidia/mL), means followed by the same lowercase letter are not significantly different, respectively, Tukey HSD test at p = 0.05. Per exposure and life stage, within each isolate (7 d, larvae F = 41.46; df = 3, 35; p < 0.01 for WG-08, F = 54.09; df = 3, 35; p < 0.01 for WG-09, F = 68.32; df = 3, 35; p < 0.01 for WG-23, F = 61.29; df = 3, 35; p < 0.01 for WG-24, F = 88.30; df = 3, 35; p < 0.01 for WG-25; adults F = 23.34; df = 3, 35; p < 0.01 for WG-08, F = 29.91; df = 3, 35; p < 0.01 for WG-09, F = 45.05; df = 3, 35; p < 0.01 for WG-23, F = 61.29; df = 3, 35; p < 0.01 for WG-24, F = 88.30; df = 3, 35; p < 0.01 for WG-25; 14 d larvae F = 39.29; df = 3, 35; p < 0.01 for WG-08, F = 78.90; df = 3, 35; p < 0.01 for WG-09, F = 28.42; df = 3, 35; p < 0.01 for WG-23, F = 30.06; df = 3, 35; p < 0.01 for WG-24, F = 27.13; df = 3, 35; p < 0.01 for WG-25; adults F = 33.14; df = 3, 35; p < 0.01 for WG-08, F = 67.36; df = 3, 35; p < 0.01 for WG-09, F = 58.19; df = 3, 35; p < 0.01 for WG-23, F = 36.65; df = 3, 35; p < 0.01 for WG-24, F = 20.08; df = 3, 35; p < 0.01 for WG-25; 21 d, larvae F = 21.14; df = 3, 35; p < 0.01 for WG-08, F = 23.46; df = 3, 35; p < 0.01 for WG-09, F = 47.27; df = 3, 35; p < 0.01 for WG-23, F = 30.41; df = 3, 35; p < 0.01 for WG-24, F = 19.95; df = 3, 35; p < 0.01 for WG-25; adults F = 100.02; df = 3, 35; p < 0.01 for WG-08, F = 90.27; df = 3, 35; p < 0.01 for WG-09, F = 43.15; df = 3, 35; p < 0.01 for WG-23, F = 79.21; df = 3, 35; p < 0.01 for WG-24, F = 42.09; df = 3, 35; p < 0.01 for WG-25), means followed by the same uppercase letter are not significantly different, respectively, Tukey HSD test at p = 0.05. Control mortality was <3% against both developmental stages and data were not included in analysis.
3.3. Lethal Concentration and Lethal Time
Lethal concentration (LC50) values for all tested isolates ranged from 2.6 × 105–1.7 × 106 conidia/mL for larvae and 8.9 × 105–3.1 × 107 conidia/mL for adults. The most virulent isolate WG-25 caused LC50 2.6 × 105 conidia/mL for larvae and 8.9 × 105 conidia/mL for larvae (Table 1).

Table 1.
Lethal concentration (LC50) estimates of five native entomopathogenic fungal isolates tested against Rhynchophorus ferrugineus larvae and adults.
Lethal time (LT50) values for all five isolates ranged from 7.5–22.3 d (WG-08), 5.5–19.5 d (WG-09), 3.6–14.2 d (WG-23), 4.4–17.4 (WG-24), and 3.2–14.2 (WG-25) for larvae. Similarly, LT50 values for the tested isolates ranged from 12.2–31.6 d (WG-08), 9.7–25.8 d (WG-09), 7.0–21.4 d (WG-23), 8.2–24.8 d (WG-24), and 5.7–19.5 d (WG-25) for adults, against four different concentrations (Table 2 and Table 3).

Table 2.
Lethal time (LT50) estimates of five native entomopathogenic fungal isolates tested against Rhynchophorus ferrugineus larvae.

Table 3.
Lethal time (LT50) estimates of five native entomopathogenic fungal isolates tested against Rhynchophorus ferrugineus adults.
3.4. Bioassay against Eggs
All tested isolates were virulent against R. ferrugineus eggs. Hatching (%) was significantly lower in treated eggs compared to non-treated eggs. Egg hatching was directly correlated with application rate as hatching (%) ranged from 49.6–75.5% and 18.5–46.6% at 1 × 106 and 1 × 108 conidia/mL, respectively (Table 4). Minimum hatching was reported with WG-25, 49.6% and 18.5% using 1 × 106 and 1 × 108 conidia/mL, respectively. The lowest AST was observed in WG-25 treated eggs, at 1.7 and 0.5 d at 1 × 106 and 1 × 108 conidia/mL, respectively, and compared to the controls. The controls normally had longer larval periods and control insects were not included in the interpretation of data (Table 4).

Table 4.
Egg hatching (% ± SE) and Average Survival Time (AST) (in days) for larvae which had been exposed to five selected entomopathogenic fungi isolates at two concentration rates. Means followed by the same lowercase letter in each column are not significantly different, Tukey HSD test at p = 0.05. Dashes (-) mean that all controls survived after five days and data were not included in analysis.
3.5. Horizontal Transmission of Fungal Isolates among Adults and Effectiveness of Sublethal Concentrations on Reproductive Stages of RPW
The results of horizontal transmission showed that mortality in non-treated pairs was significantly lower than in treated pairs. Isolate type and sex of insects significantly affected the results. For infected pairs, mortalities were 11.1–61.6% for females and 13.3–78.8% for males. Maximum mortality was observed with WG-25 against females (45.5–61.6%) and males (46.6–78.8%) (Table 5). Mortality was always observed to be less than 5% among all the non-infected pairs, not included in the analysis. Significant reductions in oviposition, fecundity, egg hatching and larval survival was observed when compared to the control (non-infected female) and were also dependent on the isolate and type of pair combination. The combination of infected females with infected males was highly significant when compared to other combinations. The results indicate that WG-25 was the most virulent isolate followed by WG-23, WG-24, WG-09 and WG-08 (Table 6).

Table 5.
Mortality (% ± SE) of Rhynchophorus ferrugineus pairs with five selected isolates of Beauveria bassiana and Metarhizium anisopliae. Means followed by same lowercase letter for each isolate in columns were not significantly different, df = 2, 26; Tukey HSD test at p = 0.05.

Table 6.
Oviposition rate (eggs per female per day up to 7 d), fecundity (eggs per female up to 28 d), egg hatching (% ± SE) up to 5 d and larval survival (% ± SE) 5 d after hatching of Rhynchophorus ferrugineus pairs infected in different combinations with five isolates of Beauveria bassiana and Metarhizium anisopliae 1 × 105 conidia/mL. Means followed by the same lowercase letter for each isolate in the columns were not significantly different, df = 3, 35; Tukey HSD test at p = 0.05.
3.6. Semi-Field Trials for RPW Survival
Significantly lower palm infestation was observed following treatment with different fungal isolates (F = 17.78; df = 5, 23; p < 0.01) compared to the controls. The lowest palm infestation was observed when palms treated with WG-25 (90.50% efficacy). From the non-treated palms, all of them were found to be infested. Statistically no difference was observed between WG-08 and control palms. For palm infestation, significantly lower numbers of larvae were observed from fungus-treated palms (F = 70.57; df = 5, 23; p < 0.01) when compared with control palms. Among the different fungal isolates, the lowest number of larvae per palm (4.15) was observed for WG-25-treated palms followed by WG-23 (13.30 larvae per palm) (Table 7).

Table 7.
Mean (% ± SE) number of infested palms, larvae of Rhynchophorus ferrugineus (number ± SE) and control efficacy when palms were treated with two isolates of Metarhizium anisopliae (WG-08 and WG-09) and three isolates of Beauveria bassiana (WG-23, WG-24 and WG-25) under semi-field conditions. Means followed by the same lowercase letter in the columns were not significantly different, Duncan test at p = 0.05. Dashes (-) mean that data for controls were not included in analysis and run after Abbott’s formula [56].
3.7. Fungal Persistence over Time and Its Effectiveness against RPW Larvae
Significant differences among all the fungal isolates (F = 70.57; df = 5, 23; p < 0.01) on the basis of the total number of larvae captured, with a maximum efficacy (82.21%) observed in WG-25 at 1 d post-insect release. At 15 d post-insect release, fungi persisted in a positive manner with lowest palm infestation (20.00%) and with maximum efficacy (85.77%) observed for the WG-25 treatment. From 30 to 60 d, efficacy exhibited a decreasing trend with a maximum treatment efficacy (57.65%) observed for WG-25 at 60 d. The lowest palm infestation for all the intervals was observed when using WG-23 and WG-25 (Table 8).

Table 8.
Mean (% ± SE) infested palms, larvae (number ± SE) and treatment efficacy when palms were treated with two isolates of Metarhizium anisopliae (WG-08 and WG-09) and three isolates of Beauveria bassiana (WG-23, WG-24 and WG-25). Adults were released over time intervals (1, 15, 30, 45 and 60 d) under semi-field setting. Within each interval, means followed by the same lowercase letter in the columns were not significantly different, Duncan test at p = 0.05. Dashes (-) mean that data for controls were not included in analysis and run after Abbott’s formula [56].
4. Discussion
Exotic fungal isolates previously deployed against a variety of insect pests in different countries have often been unsatisfactory due to factors such as differences between hosts, isolates and climatic conditions [59,60]. Noting this constraint in the present study, we used native 15 EPF isolates of different developmental stages of R. ferrugineus. Prior to use under field conditions, the most important aspect is to identify highly virulent isolates through laboratory screening bioassays [61]. Results of our screening assay showed that all isolates were pathogenic to R. ferrugineus and further supported the findings of Sun et al. and Yang et al. [61,62] who worked with different B. bassiana and M. anisopliae isolates. Although, R. ferrugineus was susceptible to all isolates, the isolates varied in their virulence. This is not an unusual phenomenon as Serna-Domínguez et al. Qayyum et al. and Ullah et al. [63,64,65] noted variation in virulence and attributed it to genetic variations among different isolates from specific geographical origins.
The current study did not support the hypothesis that virulent isolates must be isolated from the target or closely related organisms [66,67]. Five isolates (WG-08, WG-09, WG-23, WG-24 and WG-25) that we used in virulence assay were of B. bassiana and M. anisopliae, but none was originally isolated from R. ferrugineus and instead they originated from stored-grain insect pests and soil samples. Several studies [68,69,70] favored the concept that pathogens isolated from R. ferrugineus were more virulent to this invasive pest compared to non-host isolates. However, our result aligns with the findings of Liu et al. [71] who evaluated different fungal isolates against tarnished plant bug Lygus lineolaris (Palisot de Beauvois) (Hemiptera: Miridae) and found that the M. anisopliae isolate ARSEF 3540, originating from soil, was a suitable biological control agent for the mirid. Similar results were described by Qayyum et al. [72], who found that a virulent isolate of B. bassiana recovered from soil, caused >80% mortality. Although we did not use any isolates recovered from R. ferrugineus, our results indicate that strains obtained from a variety of media or non-hosts have potential to manage R. ferrugineus populations.
Mycosed cadavers play a key role in regulating the pest population by producing conidia, which may cause secondary infection and increase the persistence of the pathogen for a longer period [73]. We observed that some cadavers did not show any fungal outgrowth, which was similar to the studies by [74] in which no fungal outgrowth on the surface of Lycorma delicatula (White) (Hemiptera: Fulgoridae) living insects was observed and rarely from cadavers; however, the growth appeared when incubated under humid conditions in the laboratory. Due to their concealed nature, and suitable humid condition inside the palm tree, mycosed cadavers may play a significant role for secondary infection of R. ferrugineus.
Developmental stages of insects differ in their susceptibility towards different strains of EPF [75], and this was also noted in our study for R. ferrugineus larvae and adults. Both screening and virulence assays showed that larvae of R. ferrugineus were more susceptible than adults. Our results were similar to the findings of Dembilio et al., Francardi et al., Gindin et al., Güerri-Agulló et al., and Lo Verde et al. [22,51,53,68,76] in that adults were less susceptible than larvae to the fungal treatments. Güerri-Agulló et al. [76] claimed that the soft cuticle of larvae is more vulnerable to fungi than that of adults. During another investigation, Ansari and Butt [77] found that pine weevil Hylobius abietis (L.) (Coleoptera: Curculionidae) larvae and pupae were more susceptible than adults when treated with B. bassiana and M. anisoplaie. The reason behind the low susceptibility of adults compared to larvae is generally attributed to differences in biochemical composition of cuticle and the immune system of both stages [78], while it is suggested that during molting the shedding of the larva may get rid of infectious conidia [79]. Based on the current findings, to control R. ferrugineus the larval stage seems to be the best one to target, but keeping in mind their cryptic nature, it is essential to develop an effective method to infect the R. ferrugineus larvae with fungi.
In the screening assay, we identified five isolates with potential for development as biological control agents, three B. bassiana and two M. anisopliae. Beauveria bassiana performed well with higher mortality compared to M. anisopliae against different insect stages (larvae and adults) of R. ferrugineus irrespective of isolate origin (soil or insect cadaver). Lo Verde et al. [53] tested different isolates of EPF obtained from different stages of R. ferrugineus and found that B. bassiana was more effective than other fungi tested. El Kichaou et al. [80] found mortality in larvae of R. ferrugineus of 100% and 90% at six days post-inoculation with B. bassiana and M. anisopliae, respectively. Cherry et al. [81] tested 12 isolates of M. anisopliae and B. bassiana against cowpea weevil Callosobruchus maculatus (Fabricius) (Coleoptera: Chrysomelidae) and amongst the two highly effective isolates (B. bassiana 0362 and M. anisopliae 0351), B. bassiana 0362 was consistently more virulent against this insect. Contrary to our results Gindin et al. [68] and Francardi et al. [22] identified M. anisopliae as more virulent against R. ferrugineus compared with B. bassiana. We conclude that both species have considerable potential to control larvae and adults of R. ferrugineus with B. bassiana generally exhibiting higher virulence compared to M. anisopliae.
When eggs of R. ferrugineus were treated with fungi, the eggs showed a level of tolerance to infection. Previously, Gindin et al. [68] tested M. anisopliae against eggs of R. ferrugineus and obtained 43.5–80% egg mortality. Dembilio et al. [51] observed that B. bassiana caused a significant reduction in egg hatching compared with controls but concluded that eggs were less susceptible than 4th instar larvae, while more susceptible than adults [51]. In our study, neonates that emerged from eggs did not survive more than three days and this was in accordance with the findings of Dembilio et al. [82], Gindin et al. [68], and Lo Verde et al. [53]. The reason behind the low average survival rate of neonates could be the inability of fungi to infect the eggs due to the presence of chorion [33] and, consequently, they can infect the neonates. Under field conditions, damaged parts of the palm are where females are attracted for egg laying; therefore, treatment of these parts with fungi may increase the death of emerged neonates resulting in reduction of R. ferrugineus populations.
Several studies have confirmed the efficacy of various isolates of EPF against different developmental stages of R. ferrugineus [28,61,62,72,83,84]. In the field, larvae due to their cryptic nature [85], are difficult to infect with EPF. Only adults are mobile and able to disperse, remaining alive for a few days after becoming infected [76]. During infection, adults have conidia on their surface, and thus horizontal transmission between adults and its effect on subsequent progeny has been investigated [38,51,86]. Horizontal transmission of B. bassiana and M. anisopliae against different insect pests has been studied previously [36,86,87,88,89]. Mortality in the present study after 28 d post-infection confirmed the findings of Gindin et al. [68] and Dembilio et al. [51], which indicated that R. ferrugineus can transmit the pathogen from infected to normal adults. We found that the mortality was comparatively high in the case of mating with infected adult males which agrees with studies by Quesada-Moraga et al. [38] and Dembilio et al. [51]. According to Quesada-Moraga et al. [90], when adult males of the Mediterranean fruit fly, Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) were infected by inoculated adult females with dry conidia of the M. anisopliae isolate EAMa 01/58-Su, 100% mortality was obtained. Wai et al. [91] concluded that mated female R. ferrugineus consume much of their energy during their reproductive cycle. Consequently, they remain with less potential to survive in comparison to virgin females. The reason behind this observation may be because of the large number of conidia received by males from females and their mating behavior plays an important role. Contradictory to our results, Kaaya and Okech [92] reported that infected female tsetse flies, Glossina morsitans Wiedemann (Diptera: Glossinidae), caused higher mortality compared to males because the fly males are smaller in size and more susceptible compared to females. In another study, Quesada-Moraga et al. [88] found that mortality of the German cockroach Blattella germanica L. (Blattodea: Ectobiidae) was uniform irrespective of sex. The present study indicated that along with mortality against adults, fungal infection also reduced oviposition rate, fecundity and egg hatching. Dembilio et al. [51] found significant reduction in oviposition and fecundity against R. ferrugienus when treated with B. bassiana at a concentration rate of 1.5 × 109 conidia/mL. There was up to 70% reduction in fecundity when Western corn root worm Diabrotica virgifera virgifera LeConte (Coleoptera: Chrysomelidae) was infected with B. bassiana [93]. Castillo et al. [94] observed reductions in fecundity of C. capitata of up to 65% and 40–50% when treated with M. anisopliae and P. fumosoroseus, respectively. The reduction in oviposition we observed was in line with Meadow et al. [95], who showed that reduced oviposition was related to insect pathogens that weaken the female. Along with oviposition, infection also reduced fecundity. Fragues et al. [96] observed significant reductions in fecundity when Colorado potato beetle, Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae), was treated with B. bassiana. Sikura et al. [97] determined that B. bassiana induced histological and cytological types of injuries in the ovaries of L. decemlineata, which prevents follicle development and caused reductions in fecundity.
In semi-field survival and persistence trials, all of the tested EPF isolates significantly reduced R. ferrugineus larval survival with efficacy of WG-25 being as high as 90% compared to the control. In the persistence bioassay, although persistence decreased with the passage of time, significant levels of control were achieved even after 60 d post application. Similar findings were observed by Dembilio et al. [51], indicating that EPF persisted in palm plants over 45 d.
5. Conclusions
The present study showed that a range of EPF isolates have considerable potential against certain life stages of R. ferrugineus. The larval stage was found to be the most susceptible stage, due to its cryptic nature and the high humidity inside the trunk that favor the infection process. But a major constraint is how these microbial agents can be delivered to larvae within the palm trunk, and so further research is needed on application methods. Adults are the only freely mobile stage, able to horizontally transmit fungi to members of their colonies and are attracted to pheromone traps. Fungus treated traps could be developed to attract and infect adults which could in turn infect other adults during mating and also infect the immature stages. Eggs were also found to be susceptible to fungi, hence cut or injured parts of the palms suitable for egg laying could be treated with EPF to reduce the pest population. There is a need for field-oriented studies to assess the efficacy of fungal isolates in different climatic conditions. The impact of temperature, humidity and post-application persistence of these fungal isolates should be tested to identify the optimum conditions for infection of the target insect pests. These virulent fungal isolates may be helpful for developing a sustainable integrated management program of the red palm weevil in date palm production systems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14040642/s1, Table S1: Details of native isolates of entomopathogenic fungal isolates obtained from soil samples and insect cadavers in Pakistan, Table S2: Factorial analysis for screening of fifteen fungal isolates of entomopathogenic fungi and mycosis in larvae and adults of Rhynchophorus ferrugineus, Table S3: Factorial analysis for virulence of five selected isolates of entomopathogenic fungi against larvae and adults of Rhynchophorus ferrugineus.
Author Contributions
Conceptualization, W.W., W.S.A., N.G.K., K.G.R., M.H., A.S.A. and D.S.-I.; methodology, W.W., N.G.K. and D.S.-I.; investigation, W.W., M.A.Q. and M.T.; software, W.W., N.G.K., M.A.Q. and M.T.; formal analysis, W.W., M.A.Q., M.T., W.S.A., K.G.R., M.H., A.S.A. and D.S.-I.; validation, W.W., M.A.Q. and M.T.; resources, W.W.; data curation, W.W., N.G.K., M.A.Q. and M.T.; writing—original draft preparation, W.W., N.G.K., W.S.A., M.A.Q., M.T., K.G.R., M.H., A.S.A. and D.S.-I.; writing—review and editing, W.W., N.G.K., W.S.A., M.A.Q., M.T., K.G.R., M.H., A.S.A. and D.S.-I.; visualization, W.W., N.G.K., M.A.Q., M.T. and D.S.-I.; supervision, W.W. and N.G.K.; project administration, W.W., N.G.K. and D.S.-I.; funding acquisition, W.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partly supported by the grant from High Education Commission, Islamabad, Pakistan.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The King Saud University authors are thankful for the financial support from Researchers Supporting Project number (RSPD2024R721), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wakil, W.; Faleiro, J.R.; Miller, T.A.; Bedford, G.O.; Krueger, R.R. Date palm production and pest management challenges. In Sustainable Pest Management in Date Palm: Current Status and Emerging Challenges, Sustainability in Plant and Crop Protection; Wakil, W., Faleiro, J.R., Miller, T.A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–11. [Google Scholar]
- Tagliavia, M.; Messina, E.; Manachini, B.; Cappello, S.; Quatrini, P. The gut microbiota of larvae of Rhynchophorus ferrugineus Oliver (Coleoptera: Curculionidae). BMC Microbiol. 2014, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Fiaboe, K.K.M.; Peterson, A.T.; Kairo, M.T.K.; Roda, A.L. Predicting the potential worldwide distribution of the red palm weevil Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) using ecological niche modeling. Fla. Entomol. 2012, 95, 659–673. [Google Scholar] [CrossRef]
- EPPO (European and Mediterranean Plant Protection Organization). Rhynchophorus ferrugineus . EPPO Global Data Base. Available online: https://gd.eppo.int/taxon/RHYCFE/distribution (accessed on 25 February 2024).
- Manzoor, M.; Yang, L.; Wu, S.; El-Shafie, H.; Haider, M.S.; Ahmad, J.N. Feeding preference of Rhynchophorus ferrugineus (Oliver) (Coleoptera: Curculionidae) on different date palm cultivars and host biochemical responses to its infestation. Bull. Entomol. Res. 2022, 112, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.T. Red palm weevil, Rhynchophorus ferrugineus, a significant threat to date palm tree, global invasions, consequences, and management techniques. J. Plant Dis. Prot. 2024, 131, 9–26. [Google Scholar] [CrossRef]
- Abdel-Banat, B.M.A.; El-Shafie, H.A.F. Management of the red palm weevil in date palm plantations in Al-Ahsa oasis of Saudi Arabia. In Plant Health Cases; CABI Digital Library: Wallingford, UK, 2023; Volume 23, pp. 1–11. [Google Scholar]
- Dembilio, Ó.; Jacas, J.A.; Llácer, E. Are the palms Washingtonia filifera and Chamaerops humilis suitable hosts for the red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae). J. Appl. Entomol. 2009, 133, 565–567. [Google Scholar] [CrossRef]
- Fetoh, B.E. Latent effects of gamma radiation on certain biological aspects of the red palm weevil (Rhynchophorus ferrugineus Olivier) as a new control technology. J. Agric. Technol. 2011, 7, 1169–1175. [Google Scholar]
- Pu, Y.C.; Xiang, H.J.; Liang, X.Y.; Wang, Y.; Hou, Y.M.; Fu, L.; Wang, R. External immune inhibitory efficiency of external secretions and their metabolic profiling in red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Front. Physiol. 2020, 10, 1624. [Google Scholar] [CrossRef]
- Pinhas, J.; Soroker, V.; Hetzroni, A.; Mizrach, A.; Teicher, M.; Goldberger, J. Automatic acoustic detection of the red palm weevil. Comput. Electron. Agric. 2008, 63, 131–139. [Google Scholar] [CrossRef]
- El-Shafie, H.A.F.; Faleiro, J.R. Red palm weevil Rhynchophorus ferrugineus (Coleoptera: Curculionidae): Global invasion, current management options, challenges and future prospects. In Invasive Species-Introduction Pathways, Economic Impact, and Possible Management Options; El-Shafie, H.A.F., Ed.; IntechOpen: London, UK, 2020; pp. 1–30. [Google Scholar]
- Boulila, W.; Alzahem, A.; Koubaa, A.; Benjdira, B.; Ammar, A. Early detection of red palm weevil infestations using deep learning classification of acoustic signals. Comput. Electr. Agric. 2023, 212, 108154. [Google Scholar] [CrossRef]
- Faleiro, J.R. A review of the issues and management of the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Rhynchophoridae) in coconut and date palm during the last one hundred years. Int. J. Trop. Insect Sci. 2006, 26, 135–154. [Google Scholar]
- Al-Ballaa, S.R.; Faleiro, J.R. Studies on curative treatment of red palm weevil, Rhynchophorus ferrugineus Olivier infested date palms based on an innovative fumigation technique. Arab J. Plant Prot. 2019, 37, 119–123. [Google Scholar] [CrossRef]
- Jalinas, J.; Güerri Agulló, B.; Dosunmu, O.G.; Haseeb, M.; Lopez Llorca, L.V.; Mankin, R.W. Acoustic signal applications in detection and management of Rhynchophorus spp. In fruit-crops and ornamental palms. Fla. Entomol. 2019, 102, 475–479. [Google Scholar] [CrossRef]
- Al-Ballaa, S.R. Fumigant action of commonly used insecticides as a curative treatment of red palm weevil Rhynchophorus ferrugineus (Olivier) in infested date palms. Arab J. Plant Prot. 2020, 38, 333–338. [Google Scholar]
- Rehman, G.; Mammon-ur-Rashid, M. Evaluation of entomopathogenic nematodes against red palm weevil, Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae). Insects 2022, 13, 733. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Latif, A.O.; Genbi, Y.M.O.; Adel, M.M. Enzyme inhibitory potency of nano formulation of some plant oils on the red palm weevil Rhynchophorus ferrugineus Olivier. Nat. Prod. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Sabbahi, R.; Hock, V. Entomopathogenic fungi against the red palm weevil: Lab and field evidence. Crop. Prot. 2024, 177, 106566. [Google Scholar] [CrossRef]
- Zhu, H.; Qin, W.Q.; Huang, S.C.; Yan, W.; Sun, X.D. Isolation and identification of an entomopathogenic fungus strain of Rhynchophorus ferrugineus Oliver. Acta Phytophylacica Sin. 2010, 37, 336–340. [Google Scholar]
- Francardi, V.; Benvenuti, C.; Roversi, P.F.; Rumine, P.; Barzanti, G. Entomopathogenicity of Beauveria bassiana (Bals.) Vuill. and Metarhizium anisopliae (Metsch.) Sorokin isolated from different sources in the control of Rhynchophorus ferrugineus (Olivier) (Coleoptera Curculionidae). Redia 2012, 95, 49–55. [Google Scholar]
- Wakil, W.; Faleiro, J.R.; Miller, T.A. Sustainable Pest Management in Date Palm: Current Status and Emerging Challenges; Springer International Publishing: Cham, Switzerland, 2015; p. 429. [Google Scholar]
- Zimmermann, G. Review on safety of the entomopathogenic fungus Metarhizium anisopliae. Biocontrol. Sci. Technol. 2007, 17, 879–920. [Google Scholar] [CrossRef]
- Roy, H.E.; Vega, F.E.; Chandler, D.; Goettel, M.S.; Pell, J.K.; Wajnberg, E. The Ecology of Fungal Entomopathogens; Springer: Dordrecht, The Netherlands, 2010; p. 198. [Google Scholar]
- Tahir, M.; Wakil, W.; Ali, A.; Sahi, S.T. Pathogenicity of Beauveria bassiana and Metarhizium anisopliae isolates against larvae of the polyphagous pest Helicoverpa armigera. Entomol. Gen. 2019, 38, 225–242. [Google Scholar] [CrossRef]
- Yasin, M.; Wakil, W.; Ghazanfar, M.U.; Qayyum, M.A.; Tahir, M.; Bedford, G.O. Virulence of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae against red palm weevil, Rhynchophorus ferrugineus (Olivier). Entomol. Res. 2019, 49, 3–12. [Google Scholar] [CrossRef]
- Wakil, W.; Kavallieratos, N.G.; Ghazanfar, M.U.; Usman, M.; Habib, A.; El-Shafie, H.A.F. Efficacy of different entomopathogenic fungal isolates against four key stored-grain beetle species. J. Stored Prod. Res. 2021, 93, 101845. [Google Scholar] [CrossRef]
- Fan, Y.; Fang, W.; Guo, S.; Pei, X.; Zhang, Y.; Xiao, Y.; Li, D.; Jin, K.; Bidochka, M.J.; Pei, Y. Increased insect virulence in Beauveria bassiana strains overexpressing an engineered chitinase. Appl. Environ. Microbiol. 2007, 73, 295–302. [Google Scholar] [CrossRef]
- Vega, F.E.; Meyling, N.V.; Luangsa-Ard, J.J.; Blackwell, M. Fungal Entomopathogens’. In Insect Pathology; Vega, F.E., Kaya, H.K., Eds.; Elsevier: London, UK, 2012; pp. 171–220. [Google Scholar]
- Mazza, G.; Francardi, V.; Simoni, S.; Benvenuti, C.; Cervo, R.; Faleiro, J.R.; Llácer, E.; Longo, S.; Nannelli, R.; Tarasco, E.; et al. An overview on the natural enemies of Rhynchophorus palm weevils, with focus on R. ferrugineus. Biol. Control 2014, 77, 83–92. [Google Scholar] [CrossRef]
- Khun, K.K.; Wilson, B.A.; Stevens, M.M.; Huwer, R.K.; Ash, G.J. Integration of entomopathogenic fungi into IPM programs: Studies involving weevils (Coleoptera: Curculionoidea) affecting horticultural crops. Insects 2020, 11, 659. [Google Scholar] [CrossRef]
- Ment, D.; Levy, N.; Allouche, A.; Davidovitz, M.; Yaacobi, G. Efficacy of entomopathogenic fungi as prevention against early life stages of the red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae) in laboratory and greenhouse trials. Insects 2023, 14, 918. [Google Scholar] [CrossRef]
- Yasin, M.; Wakil, W.; El-Shafie, H.A.F.; Bedford, G.O.; Miller, T.A. Potential role of microbial pathogens in control of red palm weevil (Rhynchophorus ferrugineus)—A review. Entomol. Res. 2017, 47, 219–234. [Google Scholar] [CrossRef]
- Mkiga, A.M.; Mohamed, S.A.; Plessis, H.D.; Khamis, F.M.; Akutse, K.S.; Ekesi, S. Metarhizium anisopliae and Beauveria bassiana: Pathogenicity, horizontal transmission, and their effects on reproductive potential of Thaumatotibia leucotreta (Lepidoptera: Tortricidae). J. Econ. Entomol. 2020, 113, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Ekesi, S.; Dimbi, S.; Maniania, N.K. The role of entomopathogenic fungi in the integrated management of tephritid fruit flies (Diptera: Tephritidae) with emphasis on species occurring in Africa. In Use of Entomopathogenic Fungi in Biological Pest Management; Ekesi, S., Maniania, N.K., Eds.; Research SignPost: Kerala, India, 2007; pp. 239–274. [Google Scholar]
- Matveev, S.; Reingold, V.; Yossef, E.; Levy, N.; Kottakota, C.; Mechrez, G.; Ment, D. The dissemination of Metarhizium brunneum conidia by females of the red palm weevil, Rhynchophorus ferrugineus, suggests a new mechanism for prevention practices. J. Fungi 2023, 9, 458. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Martin-Carballo, I.; Garrido-Jurado, I.; Santiago-Álvarez, C. Horizontal transmission of Metarhizium anisopliae among laboratory populations of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). Biol. Control 2008, 47, 115–124. [Google Scholar] [CrossRef]
- Brandl, M.A.; Schumann, M.; Przyklenk, M.; Patel, A.; Vidal, S. Wireworm damage reduction in potatoes with an attract-and-kill strategy using Metarhizium brunneum. J. Pest Sci. 2017, 90, 479–493. [Google Scholar] [CrossRef]
- Yiğit, A.U. Auto-dissemination of Cordyceps fumosorosea amongst adult females of the two-spotted spider mite. Exp. Appl. Acarol. 2023, 91, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Gardescu, S.; Hajek, A.E. Transmission of Metarhizium brunneum conidia between male and female Anoplophora glabripennis adults. BioControl 2011, 56, 771–780. [Google Scholar] [CrossRef]
- Wakil, W.; Yasin, M.; Shapiro-Ilan, D. Effects of single and combined applications of entomopathogenic fungi and nematodes against Rhynchophorus ferrugineus (Olivier). Sci. Rep. 2017, 7, 5971. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A.; Evans, H.C.; Latge, J.P. Atlas of Entomopathogenic Fungi; Springer: Berlin\Heidelberg, Germany, 1988; p. 187. [Google Scholar]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi, 4th ed.; The American Phytopathological Society Press: St. Paul, MN, USA, 1998; p. 218. [Google Scholar]
- Domsch, K.H.; Gams, W.; Anderson, T.-H. Compendium of Soil Fungi, 2nd ed.; IHW-Verlag: Eching, Germany, 2007; p. 672. [Google Scholar]
- Rehner, S.A.; Minnis, A.M.; Sung, G.H.; Luangsa-ard, J.J.; Devotto, L.; Humber, R.A. Phylogeny and systematics of the anamorphic, entomopathogenic genus Beauveria. Mycologia 2011, 103, 1055–1073. [Google Scholar] [CrossRef] [PubMed]
- Humber, R.A. Identification of entomopathogenic fungi. In Manual of Techniques in Invertebrate Pathology; Lacey, L.A., Ed.; Academic Press: London, UK, 2012; pp. 151–187. [Google Scholar]
- Wakil, W.; Ghazanfar, M.U.; Riasat, T.; Kwon, Y.J.; Qayyum, M.A.; Yasin, M. Occurrence and diversity of entomopathogenic fungi in cultivated and uncultivated soils in Pak. Entomol. Res. 2013, 43, 70–78. [Google Scholar] [CrossRef]
- Wakil, W.; Ghazanfar, M.U.; Yasin, M. Naturally occurring entomopathogenic fungi infecting stored grain insect species in Punjab, Pakistan. J. Insect Sci. 2014, 14, 1–7. [Google Scholar] [CrossRef]
- Inglis, G.D.; Enkerli, J.; Goettel, M.S. Laboratory techniques used for entomopathogenic fungi: Hypocreales. In Manual of Techniques in Invertebrate Pathology; Lacey, L.A., Ed.; Academic Press: London, UK, 2012; pp. 189–253. [Google Scholar]
- Dembilio, Ó.; Quesada-Moraga, E.; Santiago-Álvarez, C.; Jacas, J.A. Potential of an indigenous strain of the entomopathogenic fungus Beauveria bassiana as a biological control agent against the red palm weevil, Rhynchophorus ferrugineus. J. Invertebr. Pathol. 2010, 104, 214–221. [Google Scholar] [CrossRef]
- Martín, M.M.; Cabello, T. Manejo de la cría del picudo rojo de la palmera, Rhynchophorus ferrugineus (Olivier, 1790) (Coleoptera, Dryophthoridae), en dieta artificial y efectos en su biometría y biología. Bol. Sanid. Veg. Plagas. 2006, 32, 631–641. [Google Scholar]
- Lo Verde, G.; Torta, L.; Mondello, V.; Caldarella, C.G. Pathogenicity bioassays of isolates of Beauveria bassiana on Rhynchophorus ferrugineus. Pest Manag. Sci. 2015, 71, 323–328. [Google Scholar] [CrossRef]
- Marannino, P.; Santiago-Álvarez, C.; de Lillo, E.; Quesada-Moraga, E. A new bioassay method reveals pathogenicity of Metarhizium anisopliae and Beauveria bassiana against early stages of Capnodis tenebrionis (Coleoptera: Buprestidae). J. Invertebr. Pathol. 2006, 93, 210–213. [Google Scholar] [CrossRef]
- Hanusz, Z.; Tarasińska, J. Normalization of the Kolmogorov–Smirnov and Shapiro–Wilk tests of normality. Biom. Lett. 2015, 52, 85–93. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry; Freeman: New York, NY, USA, 1995. [Google Scholar]
- Minitab, LLC. Getting Started with Minitab 18; Minitab Inc.: State College, PA, USA, 2017; p. 73. [Google Scholar]
- Bidochka, M.J.; Kasperski, J.E.; Wild, G.A.M. Occurrence of the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana in soils from temperate and near-northern habitats. Can. J. Bot. 1998, 76, 1198–1204. [Google Scholar]
- Qayyum, M.A.; Bilal, H.; Ali, H.; Raza, H.; Wajid, M. Factors affecting the epizootics of entomopathogenic fungi—A review. J. Bioresour. Manag. 2021, 8, 78–85. [Google Scholar] [CrossRef]
- Sun, X.; Yan, W.; Qin, W.; Zhang, J.; Niu, X.; Ma, G.; Li, F. Screening of tropical isolates of Metarhizium anisopliae for virulence to the red palm weevil Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae). SpringerPlus 2016, 5, 1100. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-H.; Wu, L.-H.; Liao, C.-T.; Li, D.; Shin, T.Y.; Kim, J.S.; Nai, Y.-S. Entomopathogenic fungi-mediated biological control of the red palm weevil Rhynchophorus ferrugineus. J. Asia-Pac. Entomol. 2023, 26, 102037. [Google Scholar] [CrossRef]
- Serna-Domínguez, M.G.; Andrade-Michel, G.Y.; Rosas-Valdez, R.; Castro-Félix, P.; Arredondo-Bernal, H.C.; Gallou, A. High genetic diversity of the entomopathogenic fungus Beauveria bassiana in Colima, Mexico. J. Invertebr. Pathol. 2019, 163, 67–74. [Google Scholar] [CrossRef]
- Qayyum, M.A.; Saeed, S.; Wakil, W.; Nawaz, A.; Iqbal, N.; Yasin, M.; Alamri, S. Diversity and correlation of entomopathogenic and associated fungi with soil factors. J. King Saud Univ.-Sci. 2021, 33, 101520. [Google Scholar] [CrossRef]
- Ullah, S.; Raza, M.; Alkafafy, M.; Sayed, S.; Hamid, M.I.; Majeed, M.Z.; Riaz, M.A.; Gaber, N.M.; Asim, M. Isolation, identification and virulence of indigenous entomopathogenic fungal strains against the peach-potato aphid, Myzus persicae Sulzer (Hemiptera: Aphididae), and the fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Egypt. J. Biol. Pest Control. 2022, 32, 1–11. [Google Scholar] [CrossRef]
- Soares, G.G.; Marchal, M.; Ferron, P. Susceptibility of Otiorhynchus sulcatus (Coleoptera: Curculionidae) larvae to Metarhizium anisopliae and Metarhizium flavoviridae (Deuteromycotina: Hyphomycetes) at two different temperatures. Environ. Entomol. 1983, 12, 1886–1890. [Google Scholar] [CrossRef]
- Poprawski, T.J.; Marchal, M.; Robert, P.-H. Comparative susceptibility of Otiorhynchus sulcatus and Sitona lineatus (Coleoptera: Curculionidae) early stages to five entomopathogenic hyphomycetes. Environ. Èntomol. 1985, 14, 247–253. [Google Scholar] [CrossRef]
- Gindin, G.; Levski, S.; Glazer, I.; Soroker, V. Evaluation of the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana against the red palm weevil Rhynchophorus ferrugineus. Phytoparasitica. 2006, 34, 370–379. [Google Scholar] [CrossRef]
- Güerri-Agulló, B.; López-Follana, R.; Asensio, L.; Barranco, P.; Lopez-Llorca, L.V. Use of a solid formulation of Beauveria bassiana for biocontrol of the red palm weevil (Rhynchophorus ferrugineus) (Coleoptera: Dryophthoridae) under field conditions in SE Spain. Fla. Entomol. 2011, 94, 737–747. [Google Scholar] [CrossRef]
- Francardi, V.; Benvenuti, C.; Barzanti, G.P.; Roversi, P.F. Autocontamination trap with entomopathogenic fungi: A possible strategy in the control of Rhynchophorus ferrugineus (Olivier, 1790) (Coleoptera, Curculionidae). Redia 2013, 96, 57–67. [Google Scholar]
- Liu, H.; Skinner, M.; Brownbridge, M.; Parker, B. Characterization of Beauveria bassiana and Metarhizium anisopliae isolates for management of tarnished plant bug, Lygus lineolaris (Hemiptera: Miridae). J. Invertebr. Pathol. 2003, 82, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, M.A.; Saleem, M.A.; Saeed, S.; Wakil, W.; Ishtiaq, M.; Ashraf, W.; Ahmed, N.; Ali, M.; Ikram, R.M.; Yasin, M.; et al. Integration of entomopathogenic fungi and eco-friendly insecticides for management of red palm weevil, Rhynchophorus ferrugineus (Olivier). Saudi J. Biol. Sci. 2020, 27, 1811–1817. [Google Scholar] [CrossRef] [PubMed]
- Riasat, T.; Wakil, W.; Ashfaq, M.; Sahi, S.T. Effect of Beauveria bassiana mixed with diatomaceous earth on mortality, mycosis and sporulation of Rhyzopertha dominica on stored wheat. Phytoparasitica 2011, 39, 325–331. [Google Scholar] [CrossRef]
- Hajek, A.E.; Everest, T.A.; Clifton, E.H. Accumulation of fungal pathogens infecting the invasive spotted lanternfly, Lycorma delicatula. Insects 2023, 14, 912. [Google Scholar] [CrossRef]
- Goettel, M.S.; Eilenberg, J.; Glare, T. Entomopathogenic fungi and their role in regulation of insect populations. In Insect Control: Biological and Synthetic Agents; Gilbert, L.I., Gill, S.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 361–405. [Google Scholar]
- Güerri-Agulló, B.; Gómez-Vidal, S.; Asensio, L.; Barranco, P.P.; Lopez-Llorca, L.V. Infection of the red palm weevil (Rhynchophorus ferrugineus) by the entomopathogenic fungus Beauveria bassiana: An SEM study. Microsc. Res. Techn. 2010, 73, 714–725. [Google Scholar] [CrossRef]
- Ansari, M.A.; Butt, T.M. Susceptibility of different developmental stages of large pine weevil Hylobius abietis (Coleoptera: Ccurculionidae) to entomopathogenic fungi and effect of fungal infection to adult weevils by formulation and application methods. J. Invertebr. Pathol. 2012, 111, 33–40. [Google Scholar] [CrossRef]
- Hajek, A.E.; Leger, R.J.S. Interactions between fungal pathogens and insect hosts. Annu. Rev. Entomol. 1994, 39, 293–322. [Google Scholar] [CrossRef]
- Inglis, G.D.; Goettel, M.S.; Butt, T.M.; Strasser, H. Use of hyphomycete fungi for managing insect pests. In Fungi as Biocontrol Agents. Progress, Problems and Potential; Butt, T.M., Jackson, C.W., Magan, N., Eds.; CABI Publishing: Wallingford, UK, 2001; pp. 23–69. [Google Scholar]
- El Kichaoui, A.Y.; Asaker, B.A.A.; El-Hindi, M.W. Isolation, molecular identification and under lab evaluation of the entomopathogenic fungi M. anisopliae and B. bassiana against the red palm weevil R. ferrugineus in Gaza strip. Adv. Microbiol. 2017, 7, 109–124. [Google Scholar] [CrossRef][Green Version]
- Cherry, A.J.; Abalob, P.; Hell, K. A laboratory assessment of the potential of different strains of the entomopathogenic fungi Beauveria bassiana (Balsamo) Vuillemin and Metarhizium anisopliae (Metschnikoff) to control Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) in stored cowpea. J. Stored Prod. Res. 2005, 41, 295–309. [Google Scholar] [CrossRef]
- Dembilio, Ó.; Tapia, G.V.; Téllez, M.M.; Jacas, J.A. Lower temperature thresholds for oviposition and egg hatching of the red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae), in a Mediterranean climate. Bull. Entomol. Res. 2012, 102, 97–102. [Google Scholar] [CrossRef]
- Hou, F.J.; Addis, K.; Azmi, W.A. Virulence evaluation of entomopathogenic fungi against the red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Dryopthoridae). Malays. Appl. Biol. 2018, 47, 25–30. [Google Scholar]
- Saleem, M.A.; Qayyum, M.A.; Ali, M.; Amin, M. Effect of sub-lethal doses of Beauveria bassiana and Nitenpyram on the development of red palm weevil, Rhynchophorus ferrugineus (Olivier). Pak. J. Zool. 2018, 51, 559–565. [Google Scholar] [CrossRef]
- Al-Manie, M.; Alkanhal, I. Acoustic detection of the red date palm weevil. Trans. Eng. Comput. Technol. 2004, 2, 209–212. [Google Scholar]
- Toledo, J.; Campos, S.E.; Flores, S.; Liedo, P.; Barrera, J.F.; Villaseñor, A.; Montoya, P. Horizontal transmission of Beauveria bassiana in Anastrepha ludens (Diptera: Tephritidae) under laboratory and field cage conditions. J. Econ. Entomol. 2007, 100, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Furlong, M.J.; Pell, J.K. Horizontal transmission of entomopathogenic fungi by the diamondback moth. Biol. Control 2001, 22, 288–299. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Santos-Quiros, R.; Valverde-Garcia, P.; Santiago-Álvarez, C. Virulence, horizontal transmission, and sublethal reproductive effects of Metarhizium anisopliae (anamorphic fungi) on the German cockroach (Blattodea: Blattellidae). J. Invertebr. Pathol. 2004, 87, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Scholte, E.J.; Knols, L.; Takken, W. Autodissemination of the entomopathogenic fungus Metarhizium anisopliae amongst adult of the malaria vector Anopheles gambiae. Malar. J. 2004, 3, 45. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Moraga, E.; Maranhão, E.A.; Valverde-Garcia, P.; Santiago-Álvarez, C. Selection of Beauveria bassiana isolates for control of the whiteflies Bemisia tabaci and Trialeurodes vaporarium on the basis of their virulence, thermal requirement and toxicogenic activity. Biol. Control 2006, 36, 274–287. [Google Scholar] [CrossRef]
- Wai, Y.K.; Bakar, A.A.; Azmi, W.A. Fecundity, fertility and survival of red palm weevil (Rhynchophorus ferrugineus) larvae reared on Sago palm. Sains Malays. 2015, 44, 1371–1375. [Google Scholar]
- Kaaya, G.P.; Okech, M.A. Horizontal transmission of mycotic infection in adult tsetse, Glossina morsitans morsitans. Entomophaga 1990, 35, 589–600. [Google Scholar] [CrossRef]
- Mulock, B.S.; Chandler, L.D. Effect of Beauveria bassiana on the fecundity of Western corn rootworm, Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae). Biol. Control. 2001, 22, 16–21. [Google Scholar] [CrossRef][Green Version]
- Castillo, M.-A.; Moya, P.; Hernández, E.; Primo-Yúfera, E. Susceptibility of Ceratitis capitata Wiedemann (Diptera: Tephritidae) to entomopathogenic fungi and their extracts. Biol. Control. 2000, 19, 274–282. [Google Scholar] [CrossRef]
- Meadow, R.; Vandenberg, J.D.; Shelton, A.M. Exchange of inoculum of Beauveria bassiana (Bals.) Vuill. (Hyphomycetes) between adult flies of the cabbage maggot Delia radicum L. (Diptera: Anthomyiidae). Biocontrol Sci. Technol. 2000, 10, 479–485. [Google Scholar] [CrossRef]
- Fragues, J.; Delmas, J.C.; Augé, J.; Lebrun, R.A. Fecundity and egg fertility in the adult Colorado beetle (Leptinostarsa decimilineata) surviving larval infection by the fungus Beauveria bassiana. Entomol. Exp. Appl. 1991, 61, 45–51. [Google Scholar] [CrossRef]
- Sikura, A.I.; Sikura, L.V.; Trebesava, R.M. Influence of white muscardine fungus (Beauveria bassiana Balsamo Vuillemin) on the reproductive system of the Colorado potato beetle. Zashch. Rast. Kichinev 1972, 2, 89–97. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).