Abstract
The massive and repetitive application of synthetic insecticides for the management of cotton pests results in the accumulation of resistance in Aphis gossypii Glover, a destructive pest worldwide. New chemistries are needed for pest management. Afidopyropen exhibits high efficacy against piercing-sucking pests and has been applied as a complementary alternative insecticide against aphids. This study was conducted to investigate the lethal and sublethal effects of afidopyropen on the life parameters and physiological responses of A. gossypii. Detoxifying enzyme activities and expression levels of P450 genes were compared after exposure to three generations of afidopyropen. Bioassay results indicate that afidopyropen possessed the highest toxicity, with a LC50 value of 0.30 mg/L. Sublethal concentrations (LC5 and LC10) caused adverse impacts on the F0 generation, reducing adult longevity and fecundity. A high concentration (LC10) also caused adverse effects on the F1 generation, while a low concentration (LC5) stimulated the fecundity. After continuous treatments with afidopyropen, the susceptibility decreased. GSTs and P450 were induced through sublethal concentrations; moreover, their activities in the F3 generation were higher than that in the F0 generation. Furthermore, the expression levels of 12 P450 genes in the F3 generation were higher than those in F0 generation. In conclusion, afidopyropen has excellent acute toxicity and continuous control effects on A. gossypii. GSTs and P450 may play important roles in the resistance of A. gossypii to afidopyropen.
1. Introduction
The cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae), also known as melon aphid, is a destructive pest worldwide that causes great harm to various plants [1]. In addition to sucking juice directly and causing wilt and death, A. gossypii transmits more than 60 plant pathogenic viruses [2]. In some cases, indirect harm caused by plant viruses transmitted by A. gossypii is more serious than direct harm. According to statistics, the losses caused by the direct and indirect damage of A. gossypii on various crops are more than 10% [1,3]. For a long time, the application of chemical pesticides has been the most effective way to treat A. gossypii [4,5,6]. However, the frequent application of insecticides leads to multiple environmental issues, accelerating the accumulation of insecticide resistance, killing natural enemies, aggravating environmental pollution, and threatening human health. To date, A. gossypii has been proven to show resistance to most commonly used insecticides in the field [7,8,9]. New chemistries and novel alternative management strategies are needed to overcome the resistance issues, such as toxicants from microbial metabolites, animal secretions and plant-derived extracts [10]. In addition to the invention of new compounds (cycloxaprid and flupyradifurone), some botanical and microbial insecticides have been proved to be applied for A. gossypii resistant management, such as cycloxaprid, azadirachtin, sophocarpidine and pyrethrin [10,11,12].
Afidopyropen (IUPAC: [(3S,4R,4aR,6S,6aS,12R,12aS,12bS)-3-(cyclopropylcarbonyloxy)-1,2,3,4,4a,5,6,6a,12a,12b-decahydro-6,12-dihydroxy-4,6a,12b-trimethyl-11-oxo-9-(3-pyridyl)-11H,12H-benzo(f)pyrano(4,3-b)chromen-4-yl]methyl cyclopropanecarboxylate), a relatively novel insecticide from microbial secondary extracts produced by Penicillium coprobium, is highly effective against sucking insects, such as Liviidae, Aleyrodidae and Aphididae [12,13,14,15]. By interfering with the insect stringer, afidopyropen causes the insect to lose coordinating ability and sense of direction, resulting in the cessation of feeding and consequently in starvation, desiccation and eventual death [13,15]. Previous reports have confirmed that afidopyropen is highly toxic to Aphis glycines Matsumura [14], Monelliopsis pecanis Bissell [16], Bemisia tabaci Gennadius [17], Diaphorina citri Kuwayama [18] and Stephanitis pyrioides Scott [19]. In view of the unique toxic mechanism, it is not easy to produce cross-resistance between afidopyropen and other insecticides [14,15], and therefore, afidopyropen provides alternative chemical control options to avoid the insecticide resistance. Moreover, afidopyropen is safe and nontoxic to nontarget organisms and beneficial to integrated biological and chemical pest management [13,14]. In summary, the application of afidopyropen is a good candidate for use in insecticide resistance management programs for aphid control.
After the insecticide is applied to the field, with biodegradation, soil adsorption, natural decomposition and rainwater leaching, it will be reduced to a series of low-dose or sublethal concentrations, which cannot kill the target organism, but the life behavior, development and reproduction of drug-contacting insects will show abnormal phenomena, which are known as sublethal effects [20,21,22]. Previous studies confirmed that sublethal doses have strong effects on physiological and behavioral responses and adverse impact the population fitness of target pests, such as extending development time and reducing fecundity [23]. Several common commercialized chemical insecticides, such as sulfoxaflor, acetamiprid, imidacloprid and dinotefuran, have been proved to induce noticeable sublethal effects on A. gossypii [23,24,25]. The LC25 of flupyradifurone extends the nymphal development and decreases the fecundity of the F1 generation [26], whereas sublethal concentrations of pesticides also sometimes exhibit positive influences (hormesis) on insects, accelerating development and stimulating fecundity, which has resulted in pest resurgence in the field [5,27,28]. Furthermore, studies on the sublethal effects of insecticides are considered to be an important part of exploring the accumulation of insecticide resistance in pests [29,30,31].
Continuous exposure to sublethal concentrations of pesticides will eliminate pesticide-sensitive individuals in the pest population and promote the population to mutate in the direction of resistance [29,32]. After continuous multigenerational accumulation, the resistance of the pest population gradually increases. For example, Shi et al. [23] confirmed that after being treated with LC25 of nitenpyram for more than one generation, the resistance level of Sogatella furcifera third-instar nymphs increased gradually, and the LC50 value increased from 1.23 mg/L (F0 generation) to 7.74 mg/L (F6 generation). The resistance levels of A. gossypii to acetamiprid increased 22.14-fold after 15 generations of sublethal concentration treatment, and the LC50 value increased from 0.55 mg/L (F0 generation) to 12.18 mg/L (F15 generation) [4]. Therefore, to better realize the potential value of afidopyropen as an alternative insecticide against aphid-resistant populations in the field, it is essential to determine the sublethal effects of afidopyropen on A. gossypii and assess the resistance risk, which is conducive to extending its service life in the field.
In addition to affecting the biological characteristics of insects, the sublethal concentration of insecticides also affects the physiological responses [17,33]. Sublethal concentrations of insecticides also affect various enzyme activities, gene expression and essential energy and substance metabolism in insects [34]. Long-term exposure to sublethal concentration stress will aggravate the enhancement of the insect’s detoxification metabolism ability, and the enhancement of detoxification metabolic enzyme activity is usually directly related to the enhancement of resistance to insecticides [35,36]. Mostafiz et al. [37] reported that the acetylcholinesterase (AChE) activity of A. gossypii decreased by more than 65% after treatment with the LC30 of methyl benzoate compared with untreated controls.
In this study, we detected the toxicity of afidopyropen against A. gossypii and studied the sublethal effects on the life parameters by building a life table, then the protective enzyme and metabolic enzyme activities were assayed. Furthermore, the effects of continuous treatments with LC10 on the susceptibility of A. gossypii to afidopyropen were studied. The objectives were to provide scientific bases for the chemical control of cotton aphids in the field and the rational application of afidopyropen.
2. Materials and Methods
2.1. Insect Materials
A laboratory A. gossypii colony was originally obtained from Hibiscus syriacus plants in Taian, China, in May 2020. Colonies were established on hydroponic cotton (Gossypium hirsutum L.) seedlings in cylinder molds and maintained in the laboratory at the College of Plant Protection Shandong Agricultural University for more than fifteen generations according to the method reported by Wang et al. [7]. The environmental conditions were 25 ± 1 °C, 60 ± 5% relative humidity and a 12:12 h light:dark cycle. During the breeding process, the A. gossypii colony was maintained without any exposure to insecticides since 2020.
2.2. Insecticides
Afidopyropen (92.5%) was provided by Badische Anilin-und-Soda-Fabrik (Shanghai, China); fluridine (95.9%) and trifluoropyrimidine (96%) were provided by Corteva Agriscience (Shanghai, China); fluridine (98.5%), acetamidine (99%) and clothianide (98%) were provided by Sino Agricultural Union Co., Ltd. (Jinan, China); and piraphidone (96%), imidacloprid (95%) and thiamethoxam (98%) were provided by Hailir Pesticides and Chemicals Group (Qingdao, China).
2.3. Bioassay
2.3.1. Toxicity Bioassay
The toxicity of 9 insecticides tested against A. gossypii was detected using a leaf-dip bioassay with minor modifications [32,37]. The stock solution of insecticides was prepared in analytical grade acetone and then diluted to the needed concentrations with distilled water containing 0.05% (v/v) Tween-80. The cotton leaves were dipped into insecticide solutions for 10 s and allowed to air dry on large filter paper. Then, the leaves were moved to a new disposable plastic petri dish (Φ = 4 cm) covered with 3 mm thick agar (15 g/L) at the bottom. Approximately 30 to 35 healthy and lively A. gossypii adults (within 24 h after molting) were transferred to the leaf in each dish. The tested dishes were sealed with plastic wrap, and the plastic wrap was pierced with an insect pin for air circulation. After 6 h, 30 aphids were retained in each petri dish. For the control, the leaves were dipped into distilled water containing 0.05% (v/v) Tween-80. The pre-experiment consisted of five concentration treatments (0.1, 1.0, 10, 100 and 1000 mg/L), and each treatment involved three replicates. According to the results of the pre-experiment, the formal experiment was designed as five concentration treatments (afidopyropen set at 0.04, 0.08, 0.16, 0.32 and 0.64 mg/L; pymetrozine set at 0.1, 0.2, 0.4, 0.8 and 1.6 mg/L; triflumezopyrim and flonicamid set at 0.5, 1.0, 2.0, 4.0 and 8.0 mg/L; sulfoxaflor and acetamiprid set at 0.625, 1.25, 2.5, 5.0 and 10 mg/L; thiamethoxam, clothianidin and imidacloprid set at 2.5, 5, 10, 20 and 40 mg/L). Aphid mortalities were recorded every 24 h, and the mortalities at 72 h were used to calculate the LC50 value. Aphids unable to move after being probed slightly with a soft brush were considered dead.
2.3.2. Sublethal Effect Bioassay
The sublethal effects of afidopyropen on A. gossypii were evaluated according to the method described by Shi et al. [23]. According to the toxicity bioassay results, the LC5 (0.0105 mg/L) and LC10 (0.0211 mg/L) of afidopyropen were prepared. A. gossypii adults (within 24 h after molting) were treated. After 72 h, individual surviving aphids were transferred to fresh cotton leaves placed in petri dishes covered with 3 mm thick agar at the bottom. All petri dishes were maintained in growth chambers. The survival rate, adult longevity and fecundity were recorded every day until all aphids died, and the leaf was replaced every two days. Every treatment contained 60 individuals and divided into three replicates, and the distilled water containing 0.05% (v/v) Tween-80 treatment was used as the control.
Continuing the above process, on the 2nd and 3rd days after the parental adults began to produce nymphs, the new nymphs born within 24 h were chosen as the F1 generation. One nymph was placed in one independent petri dish. All petri dishes were maintained in growth chambers according to the above description. The survival rate, nymphal developmental time, adult longevity and fecundity were recorded every day until all aphids died. The parental aphids treated with distilled water containing 0.05% (v/v) Tween-80 were regarded as the control.
2.3.3. Aphid Susceptibility Detection
Approximately 200 apterous A. gossypii were exposed to the LC10 of afidopyropen according to the toxicity methods. After 72 h, the surviving individuals were transferred to new cotton seedlings to breed F1 generation nymphs. Two days later, the parental aphids were removed. When F1 generation nymphs grew into adults, susceptibility to afidopyropen was detected, and the new LC10 was used to treat the F1 generation adults until the F3 generation. The toxicity ratio indicated the ratio of the value of LC50 for the FN generation to that for the F0 generation.
2.4. Enzyme Activity Assay
2.4.1. Sample Preparation
Approximately 800 A. gossypii adults of the F0 generation were exposed to the LC5 and LC10 of afidopyropen. At 24, 48 and 72 h, the surviving individuals were collected for the detection of all enzyme activity, while for the F3 generation, the surviving aphids were collected at 72 h for carboxylesterase (CarE), glutathione S-transferase (GST) and cytochrome P450 activity detection [32]. Each treatment contained 90 individuals and divided into three replicates. The aphids treated with distilled water containing 0.05% (v/v) Tween-80 treatment were used as the control. The test aphids were homogenized in a cold centrifuge tube with 0.05 M phosphate buffer (pH 7.8). Aphid homogenates were centrifuged at 10,000× g for 15 min at 4 °C. Supernatants were used as the enzyme samples. The protein content of every sample was determined according to the Bradford assay.
2.4.2. Enzyme Activity Assay
The catalase (CAT) enzyme activities were assayed by detecting the consumption of H2O2 at 240 nm for 2 min [38]. A yellow complex was formed between H2O2 and ammonium molybdate. The absorbance value was measured at 405 nm, and the consumption of H2O2 was calculated. One unit of CAT activity was defined as the amount (μmoL) of H2O2 decomposition per min per mg protein. The unit of enzyme activity was U mg−1 protein.
The peroxidase (POD) enzyme activities were assayed according to the guaiacol oxidation method [38]. The absorbance changes in the reaction mixture (2.9 mL of phosphate buffer, 0.5 mL of 0.05 M guaiacol solution, 0.5 mL of 2% H2O2 solution and 0.1 mL of enzyme sample) were detected at 470 nm (37 °C) within 1 min. One unit of POD activity was defined as the quantity of enzymes required to catalyze 1 μmol of substrate reaction per minute per mg protein.
The superoxide dismutase (SOD) enzyme activities were assayed based on the inhibition of the nitro blue tetrazolium photochemical reaction at 550 nm [38]. The absorbance value of the reaction solution (200 μL of substrate, 20 μL of enzyme sample and 20 μL of working solution) was detected after incubation at 37 °C for 20 min. One unit of SOD activity was defined as the amount of enzyme that caused a 50% inhibition of the nitro blue tetrazolium reduction.
α-naphthyl acetate was used as substrate to assay the CarE activity [33,39]. The reaction mixtures containing 50 μL of enzyme sample, 1.8 mL of 0.03 M substrate, and 0.45 mL of phosphate buffer (pH 7.0) were maintained at 37 °C for 10 min. Then, 0.9 mL (0.2 g of fast blue-B salt in 70 mL of 5% sodium dodecyl sulfate) were added to terminate the reactions. The absorbance changes at 450 nm per mg protein per min was used as the enzyme activity (∆/min/mg prot).
The activity of GST was determined using 1-chloro-2,4-dinitrobenzene (CDNB) as the substrate [33,39]. The reaction system contained 150 μL of 6 mM reduced glutathione (GSH), 75 μL of 0.6 mM CDNB and 50 μL of enzyme sample. The absorbance changes were determined at 340 nm and 37 °C for 5 min. The amount of CDNB binding with GSH catalyzed by enzyme per mg protein per min was used as the enzyme activity (nmol/min/mg prot).
P450 activities were determined using ethoxy coumarin as the substrate [39]. The reaction system contained 180 μL of ethoxy coumarin solution (100 μmol/L) and 20 μL of enzyme sample. The reaction systems were maintained at 37 °C for 30 min, at which point, 20 μL of 15% trichloroacetic acid (m/v) was added. Then, the fluorescence intensity of hydroxycoumarin produced by the enzymatic reaction was detected using a SpectraMax Gemini XPS (Thermo Fisher Scientific, Waltham, MA, USA). The amount of ethoxycoumarin converted to hydroxycoumarin per mg protein per min was used as the enzyme activity (nmol/min/mg prot).
2.5. Gene Expression Assay
The F0 and F3 generations of A. gossypii adults were exposed to the LC10 of afidopyropen, and the surviving aphids were chosen as the tested sample at 72 h. Total RNA was extracted from 40 aphids using an RNA Extraction and Purification Kit (ComWin Biotech, Beijing, China). cDNA was synthesized using the PrimeScript RT Kit (Takara Biotechnology, Dalian, China). The expression levels of 26 CYP450 genes were detected according to RT–qPCR. The threshold cycle (Ct) number was used for comparative quantitative analysis, and the 2−∆∆Ct method was used to evaluate the relative expression level compared with the elongation factor β-actin [40]. Each gene was analyzed in triplicate with three biologically independent treatments, and the aphids treated with distilled water containing 0.05% (v/v) Tween-80 were used as the control. All gene information is listed in Table S1.
2.6. Statistical Analyses
In the bioassays, the value of LC50 and the toxicity regression equation were performed through probit analyses in PASW Statistics 18.0.0 (SPSS Inc. Quarry Bay, Hong Kong).
The sublethal effects of afidopyropen on the F1 generation of A. gossypii were analyzed by forming a life table according to the age-stage two-sex life table theory [41]. The life table parameters were estimated using the bootstrap method included in the computer program TWOSEX-MS Chart [41].
The age-specific survival rate (lx) was calculated as follows:
where β = the number of stages.
The age-specific fecundity (mx) is calculated as follows:
The net reproductive rate (R0) is calculated as follows:
The intrinsic rate of increase (r) is calculated as follows:
The finite rate (λ) is calculated as follows:
The mean generation time (T) is calculated as follows:
The differences in other indices among different treatments were analyzed via one-way ANOVA followed by Tukey’s HSD multiple comparisons at the 0.05 level with PASW Statistics 18.0.0 (SPSS Inc. Quarry Bay, Hong Kong).
3. Results
3.1. Toxicity of Nine Insecticides to A. gossypii
We detected the toxicity of nine insecticides to A. gossypii, and the results indicate that afidopyropen showed the highest toxicity, with an LC50 value of 0.30 mg/L, followed by pymetrozine and triflumezopyrim (Table 1). For neonicotinoids (sulfoxaflor, acetamiprid, thiamethoxam, clothianidin and imidacloprid), the LC50 values were 2.61, 2.81, 4.42, 10.36 and 12.74 mg/L.

Table 1.
Toxicity of nine insecticides against A. gossypii adults.
3.2. Sublethal Effects of Afidopyropen on A. gossypii
3.2.1. F0 Generation
Sublethal concentrations (LC10 and LC5) of afidopyropen produced significant adverse effects on the F0 generation of A. gossypii (Figure 1). At 72 h after treatment, the survival rates of A. gossypii were 71.68% (LC10) and 83.43% (LC5), decreasing by 24.18% and 12.43% compared with the control (95.86%). The longevities with the LC10 and LC5 treatments were 3.03 d and 3.46 d, respectively, which were shortened by 3.81 d and 3.38 d compared with the control (6.84 d). The fecundities were 2.24 nymphs/adult (LC10) and 3.34 nymphs/adult (LC5), decreasing by 67.76% and 55.09%, respectively, compared with the control (7.17 nymphs/adult).
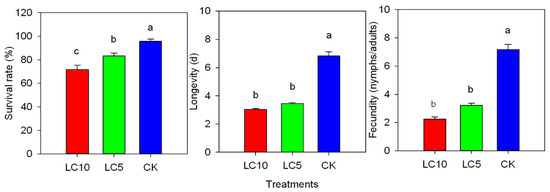
Figure 1.
Sublethal effects of afidopyropen on the survival, longevity and fecundity of the A. gossypii F0 generation. Each value is represented as mean ± SE. Different letters above the bars indicated significant differences among different treatments based on Tukey’s HSD (p < 0.05).
3.2.2. F1 Generation
The nymph developmental times of A. gossypii were shortened after sublethal concentrations of afidopyropen treatment (Table 2). The preadult development times of the LC10 and LC5 treatments were 5.52 and 5.25 d, respectively, shortened by 0.28 and 0.55 d compared with the control (5.80 d), and significant differences were discovered between the different treatments. Furthermore, high mortalities of nymph aphids were observed, and the values were 26.36% (LC10) and 22.44% (LC5), respectively, increasing by 11.82% and 7.90% compared with the control (14.54%).

Table 2.
Development, survival and fecundity of A. gossypii in the F1 generation after sublethal concentrations of afidopyropen treatment.
The adult longevities of A. gossypii treated with afidopyropen were shortened, and the values were 7.59 d (LC5) and 6.47 d (LC10), respectively, which were shorter than that of the control (8.32 d). It is interesting that the mean fecundity of the LC5 treatment was the highest (23.46 nymphs/female), higher than that of the control (19.75 nymphs/female), while the fecundity of the LC10 treatment was 16.90 nymphs/female, which was significantly lower than that of the control.
Additionally, the inhibitory effects of afidopyropen on A. gossypii were significantly different among the concentration treatments.
The results of the age-stage-specific survival rate (Sxj) of the A. gossypii F1 generation also indicated that the survival rate decreased, development slowed and longevity shortened (Figure 2). For the LC10 and LC5 treatments, the peak period of adult emergence was from the 7th to 8th d and from the 6th to 7th d, respectively, while it was at the 6th for the control. In addition, all aphids died on the 18th d for the control and on the 17th d and 16th d for the LC5 and LC10 treatments, respectively.
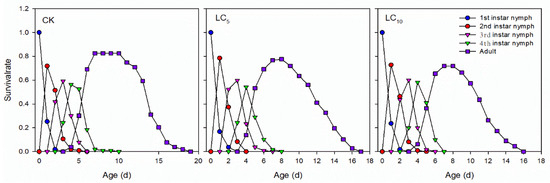
Figure 2.
Age-stage-specific survival rates (Sxj) of A. gossypii Glover F1 generations after sublethal concentrations of afidopyropen treatment.
The female age-specific fecundity (fxj), age-specific fecundity of the total population (mx) and age-specific maternity (lxmx) of the A. gossypii F1 generation after afidopyropen treatment also indicated that the highest reproductive potential of the LC5 treatment was observed on the 7th d, and the values of mx and lxmx were 5.04 and 3.91, respectively (Figure 3). The highest reproductive potential of LC10 was observed on the 8th d, and the values of mx and lxmx were 4.01 and 2.88, respectively, while on the 9th d, 3.93 and 3.28, respectively, for the control.
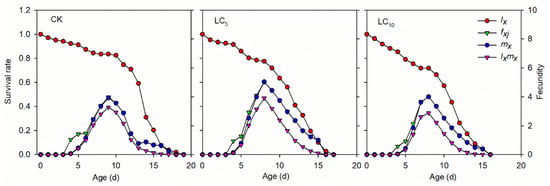
Figure 3.
Age-specific survival rate (lx), female age-specific fecundity (fxj), age-specific fecundity of the total population (mx) and age-specific maternity (lxmx) of the A. gossypii F1 generation after sublethal concentrations of afidopyropen treatment.
Differences in the population parameters of A. gossypii were observed across the different treatments (Table 3). The intrinsic rate of increase (r), finite rate of increase (λ), net reproductive rate (R0) and mean generation time (T) of A. gossypii treated with the LC5 concentration of afidopyropen were 0.0.327 d−1, 1.387 d−1, 18.97 nymphs and 8.99 d, respectively. Furthermore, the relative fitness Rf obtained for the LC5 treatment was 1.19, higher than that of the control and the LC10 treatment, with values of 1.00 and 0.86, respectively.

Table 3.
Population parameters of A. gossypii after sublethal concentrations of afidopyropen treatment in the F1 generation.
3.3. Sublethal Effects on Enzymatic Activities
The antioxidant enzymes of A. gossypii treated with sublethal concentrations of afidopyropen were induced (Figure 4). At 24, 48 and 72 h, the CAT activity of the LC5 treatment increased by 35.33%, 42.56% and 37.94%, respectively, compared with that of the control. However, the CAT activity of the LC10 treatment increased only at 48 h. The POD activity of A. gossypii increased, and with increasing concentration, the effect was more obvious. At 24, 48 and 72 h, the POD activity of A. gossypii treated with LC10 increased by 75.94%, 158.90% and 88.65%, respectively, compared with that of the control. SOD activity also increased, and the dosage effect was also obvious. At 24, 48 and 72 h, the SOD activity of A. gossypii treated with LC10 increased by 25.64%, 17.31% and 16.40%, respectively, compared with that of the control.
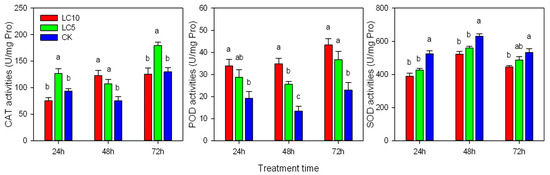
Figure 4.
CAT, POD and SOD activities of A. gossypii after sublethal concentrations of afidopyropen treatment. Each value was represented as mean ± SE. Different letters above the bars correspond to significant differences among different treatments based on Tukey’s HSD (p < 0.05).
The detoxification metabolism enzyme activities of A. gossypii (GST and P450) increased after treatment with sublethal concentrations of afidopyropen (Figure 5). The GST activity increased by 17.71% and 12.11% (LC5 treatment) and 27.60% and 26.75% (LC10 treatment) at 48 and 72 h, respectively, while it decreased by 22.28% (LC5 treatment) and 15.21% (LC10 treatment) at 24 h. CarE activity decreased at 24 h and 48 h and increased at 72 h. At 24 and 48 h, the CarE activity of the LC10 treatment decreased by 35.73% and 30.07% compared with the control and increased by 65.94% at 72 h. The P450 activity of aphids treated with afidopyropen increased significantly, and at 24, 48 and 72 h, the activity of aphids treated with the LC10 concentration increased by 35.16%, 102.71% and 49.28%, respectively.
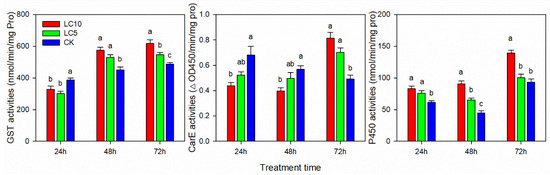
Figure 5.
GST, CarE and P450 activities of A. gossypii after sublethal concentrations of afidopyropen treatment. Each value is represented as mean ± SE. Different letters above the bars correspond to significant differences among different treatments based on Tukey’s HSD (p < 0.05).
3.4. Susceptibility of A. gossypii after Continuous Sublethal Treatment
After continuous treatment with afidopyropen, the susceptibility of the A. ossypii F1 to F3 generations decreased gradually (Table 4). The LC50 values of afidopyropen in the F0, F1, F2 and F3 generations were 0.30, 0.35, 0.59 and 0.78 mg/L, respectively, and the toxicity ratios (FN/F0) were 1.17 (F1/F0), 1.97 (F2/F0) and 2.60 (F3/F0).

Table 4.
Susceptibility of the F0 to F3 generations in A. gossypii after continuous sublethal treatment of afidopyropen.
3.5. Detoxification Metabolism Enzyme Activities of the F0 and F3 Generations
The P450 activity of the A. gossypii F3 generation was higher than that of the F0 generation and 183.54% higher than that of the F0 generation. Furthermore, after the LC5 and LC10 treatments, the P450 activity of the F3 generation was 113.16% and 48.97% higher than that of the F0 generation, respectively. The GST activity of F3 generation was higher than that of the F0 generation after the LC5 and LC10 treatments, and there was no significant difference between the F0 and F3 generations without afidopyropen treatment. The CarE activity of the F3 and F0 generations exhibited no significant differences whether they were treated with afidopyropen. Moreover, the activities of GST, CarE and P450 in the F0 generation increased with the increase of treatment concentration, while only P450 activity in the F3 generation was almost not affected by the treatment concentration (Figure 6).
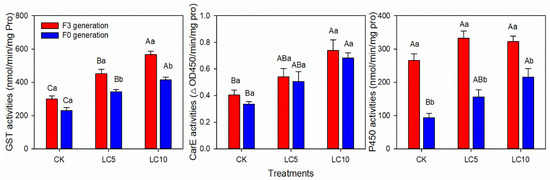
Figure 6.
GST, CarE and P450 activities of the F0 and F3 generations of A. gossypii after sublethal concentrations of afidopyropen treatment. Each value is represented as mean ± SE. Different lowercase letters above the bars correspond to significant differences at p < 0.05 between different generations under the same treatment, while the capital letters correspond to significant differences between different sublethal treatments for the F0 or F3 generation (Tukey’s HSD).
3.6. P450 Gene Expression in the F0 and F3 Generations
The expression levels of 26 cytochrome P450 genes were detected (Figure 7). The results indicate that treatment with afidopyropen induced high expression levels of four genes (CYP6G1, CYP6CY5, CYP380C6 and CYP6CY13) and low expression levels of five genes (CYP6CY19, CYP6CY22, CYP6CY18, CYP6CK1 and CYP6CY21) in the F0 generation. In the F3 generation, the expression levels of four genes (CYP4G15, CYP6CY18, CYP380C6, CYP306A1) increased significantly after LC10 treatment, and the expression levels of five genes (CYP4CH1, CYP6CY19, CYP6CY22, CYP6CY18, and CYP6CY21) decreased significantly. Compared with the F0 generation, the expression levels of 12 genes (CYP6DAI, CYP18AI, CYP6CY18, CYP6CY5, CYP4CH1, CYP6DA2, CYP380C6, CYP6CY13, CYP49A1, CYP6A14, CYP4CK1 and CYP380C12) in the F3 generation increased significantly, and the expression levels of 5 genes (CYP6DB1, CYP6CY19, CYP6CY7, CYP6CY18, and CYP6CY21 and CYP380C12) in the F3 generation decreased significantly. However, after treatment with the LC10 afidopyropen, the expression levels of CYP18AI, CYP6CY18, CYP380C6, CYP6A14 and CYP306A1 were still higher than those in the F0 generation, and the expression levels of CYP305E1, CYP6DB1, CYP6CY7 and CYP6CY21 were lower. In all these genes, CYP380C6 was induced in both the F0 and F3 generations.
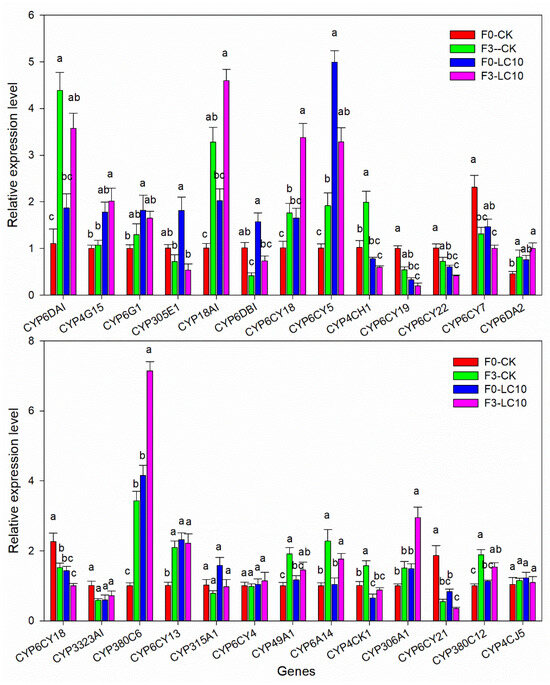
Figure 7.
Expression levels of cytochrome P450 genes in the F0 and F3 generations of A. gossypii after sublethal concentrations of afidopyropen treatment. Each value is represented as mean ± SE. Different letters above the bars correspond to significant differences among different treatments based on Tukey’s HSD (p < 0.05).
4. Discussion
Screening new insecticides with high efficiency, low toxicity and environmental friendliness is an important means to achieve effective control of cotton aphids [12,13,14]. As a newly developed insecticide, afidopyropen has no toxic effect on natural enemies or nontarget organisms [13,14]. It has become a new favorite for the prevention and control of piercing-sucking pests. Here, we found that afidopyropen possessed the highest toxicity to A. gossypii among nine insecticides, and the LC50 value was 0.30 mg/L, which was significantly higher than that of sulfoxaflor, which is considered to be the current special pesticide for aphid control [25,42]. Similarly, previous researchers have also confirmed that afidopyropen has good toxic effects on other aphids, such as A. glycines [14] and Myzus persicae [43]. In addition, Tang et al. [15] reported that the LC50 value of afidopyropen to A. gossypii was 1.062 mg/L, which was higher than our results. We speculate that the difference in toxicity values may be attributed to regional population and host plant differences. As previously reported, the sensitivity of A. gossypii collected from different regions or reared on different host plants to the same pesticide varies greatly [9,44,45].
After application, afidopyropen degrades gradually over time in the environment, and the half-life period in plants and soil are 1.65 and 1.21 d, respectively [46]. Therefore, during the natural degradation process in the field, aphids will be stressed by low concentrations of afidopyropen. In our study, significant decreases were shown in the longevity and fecundity of A. gossypii adults directly exposed to the LC10 and LC5 of afidopyropen, similar to Liu et al. [43] and Tang et al. [15], who also reported that the LC5, LC15 and LC25 of afidopyropen resulted in longevity shortening and fecundity decreases. In addition, afidopyropen was confirmed to cause adverse effects on the F0 generation of D. citri, such as adult longevity shortening [18]. In summary, in addition to its rapid lethal effect, afidopyropen also has excellent continuous control effects on surviving pests, playing a continuous role in controlling aphids.
Sublethal concentrations of pesticides will not only affect the exposed parental generation (F0) of target pests but also have transgenerational effects on the progeny (F1) [30,43]. The transgenerational effects on progeny are usually divided into two types. One is adverse effects on the progeny, and the other is stimulation of reproduction [30,47]. The latter easily causes an increase in pest population and more mutations beneficial to resistance, which is an important factor causing the resurgence of pests [48,49]. In this study, sublethal concentrations of afidopyropen showed only two effects on the A. gossypii F1 population. First, the LC10 and LC5 treatments caused a decrease in the survival rate, prolonged development and shortened longevity, and significant dose effects were observed. With increasing concentration, the negative effects became more significant. Similar to most insecticides, sublethal concentrations have negative effects on progeny [17,26,37]. In addition, the fecundity of the A. gossypii F1 population pretreated with a high concentration (LC10) was lower than that of the control. However, the fecundity of the F1 population pretreated with a low concentration (LC5) increased by 18.78% compared with that of the control, indicating that the low concentration had a stimulatory effect on the progeny reproduction of A. gossypii. Similarly, LC20 sulfoxaflor can significantly stimulate the reproduction of the A. gossypii F1 generation [49], and LC10 thiacloprid resulted in an increase in the fecundity of the M. azadirachtin F1 generation [47]. In contrast to this study, Tang et al. [15] and Liu et al. [43] did not report the reproductive stimulation of afidopyropen on A. gossypii and M. persicae. We speculate that the reason may be that the concentrations of afidopyropen involved in the above two reports were high (LC10, LC15 and LC25), and the minimum concentration required to stimulate reproduction has not been determined.
Calculating the population parameters of insects can better reflect fitness, which is conducive to the prediction of environmental adaptability, proliferation and attenuation [41]. Our results indicate that the life parameters of the A. gossypii F1 population pretreated with a low concentration (LC5) were better than those pretreated with a high concentration (LC10), and r, R0 and GRR were considerably higher than those of the control. Therefore, from the perspective of population parameters, it was found that low-concentration afidopyropen is beneficial to the expansion of aphid progeny populations. Combined with other reports on the effects of afidopyropen on A. gossypii [15] and M. persicae [43], high concentrations can significantly inhibit the propagation of the progeny population, while low concentrations stimulate reproduction.
Continuous multigeneration treatments using insecticides in the laboratory are conducive to evaluating the risk of pest resistance to insecticides [6,49,50]. A. gossypii, as an important agricultural pest, has a prominent problem of resistance accumulation to various insecticides [8,9]. In the present study, after three consecutive generations of treatment, the LC50 value of afidopyropen to A. gossypii increased from 0.3008 to 0.7084 mg/L, and the resistance ratio increased by 2.36 times compared with the F0 generation. After 10 and 20 generations of continuous treatment, the resistance of A. gossypii to sulfoxaflor increased by 1.63 and 4.78 times, respectively, compared with the susceptible strain [51]. After 9, 18 and 27 continuous generations of screening, the resistance ratios of A. gossypii to imidacloprid increased by 6.4, 22.7 and 26.1 times, respectively [52]. Although resistance screening was performed only for three generations in this study, the results also indicate that the resistance level of A. gossypii to afidopyropen increased gradually.
When pests ingest insecticides, dramatic physiological changes occur to eliminate toxicity [22,39,41]. Protective enzymes can help adapt to the oxidative stress caused by various stress factors (including insecticides), remove oxyradicals and maintain the homeostasis of the reactive oxygen metabolism, and detoxifying metabolic enzymes can transform and degrade pesticide molecules to reduce toxicity or protect insect target sites through blocking actions [53]. In this study, sublethal concentrations (LC10 and LC5) of afidopyropen caused POD activity to increase and SOD activity to decrease, while CAT activity was induced by LC5 and inhibited by LC10. It has been found that acetamiprid treatment has significant inhibitory effects on SOD, CAT and POD activities in A. gossypii [54]. Furthermore, the activities of GST and P450 in A. gossypii were induced by sublethal concentrations of afidopyropen, and the enzyme activity increased with increasing concentration. The increase in P450 activity was significantly higher than that of GSTs. Therefore, we speculate that the detoxification metabolic enzymes (GSTs and P450) play an important role in the resistance of A. gossypii to afidopyropen. Similarly, the activities of CarE, GSTs and P450s in acetamiprid-resistant strains of A. gossypii were significantly higher than those in acetamiprid-susceptible strains [55]. Furthermore, the activities of GSTs and P450 in the F3 generation of A. gossypii were significantly higher than that in the F0 generation after continuous sublethal concentration treatment, and the increase in P450 activity was significantly higher than that of GSTs. After sublethal treatment, the activities of GSTs and P450 of the F3 generation of A. gossypii were still higher than those without treatment. Therefore, we concluded that P450 plays the most critical role in the resistance of A. gossypii to afidopyropen, followed by GSTs.
Insect cytochrome P450 can usually be divided into four families, namely, the CYP2, CYP3, CYP4 and mitochondrial CYP families [56]. It is confirmed that the replication or amplification of P450 genes has an enormous impact on the evolution of insect resistance to insecticides and enables insects to adapt to insecticide stress [57,58,59]. In this study, the expression levels of eight genes (CYP6DAI, CYP6CY18, CYP6CY5, CYP6DA2, CYP380C6, CYP6CY13, CYP6A14, and CYP380C12) in the CYP3 family of the F3 generation were significantly higher than those of the F0 generation, and the expression levels of five genes (CYP6DB1, CYP6CY19, CYP6CY7, CYP6CY18, and CYP6CY21) in the F3 generation decreased. By comprehensively analyzing the expression levels of P450 genes in the F3 and F0 generations of A. gossypii after sublethal concentration treatment, six genes (CYP18AI, CYP6DA2, CYP6CY18, CYP380C6, CYP6A14 and CYP306A1I) were still higher, which is closely related to the resistance of Aphis craccivora to imidacloprid [39]. In verifying the relationship between P450 genes and insecticide resistance, researchers have confirmed that various P450 genes are related to insecticide resistance through heterologous overexpression in Drosophila [60]. For example, the overexpression of CYP380C6, CYP6CY7, CYP6CY21, CYP4CJ1, UGT341A4, UGT344B4 and UGT344M2 in Drosophila resulted in cross-resistance to cyantraniliprole and α-cypermethrin [6]. Ma et al. [42] reported that P450s activities were higher in a sulfoxaffor-resistant strain of A. gossypii than in a sulfoxaffor-susceptible strain, that nine P450 genes increased significantly, and that the suppression of the expressions of CYP6CY13 and CYP6CY19 by RNAi significantly enhanced the susceptibility of the resistant strain. Wang et al. [61] and Zhou et al. [17] also reported that P450s and GSTs could contribute to the field-evolved afidopyropen resistance in Bemisia tabaci. To clarify the P450 genes closely related to the resistance of A. gossypii to afidopyropen, molecular biology experiments involving gene function need to be carried out.
5. Conclusions
Our study confirmed that afidopyropen had an excellent toxic effect on A. gossypii. In addition to causing rapid death, it also had a continuous adverse effect on surviving aphids and caused transgenerational effects on the progeny, in which a high concentration (LC10) inhibited the life parameters, and a low concentration (LC5) stimulated reproduction. However, after continuous resistance screening, the resistance of A. gossypii to afidopyropen was enhanced, and the activities of GSTs and cytochrome P450s were enhanced significantly after insecticide exposure. The latter plays a significant role in the resistance process, as confirmed via gene detection. Therefore, afidopyropen can be used as a new pesticide to control cotton aphids in view of its extremely high toxicity, unique toxic mechanism and environmental safety; however, the risk of resistance during application cannot be ignored, and adequate cross-application with other insecticides is necessary. Therefore, exploring the cross-resistance of afidopyropen with other insecticides and monitoring the resistance levels of A. gossypii populations in the field will be our future research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14020258/s1, Table S1: Primers used in RT–qPCR.
Author Contributions
Conceptualization, W.D., L.G. and Y.X.; Data curation, M.W., C.L., R.Z. and S.Z.; Formal analysis, W.D. and Y.X.; Funding acquisition, X.X.; Investigation, W.D., L.G., C.L., R.Z. and S.Z.; Methodology, W.D. and L.G.; Project administration, X.X.; Resources, L.G., M.W. and S.Z.; Software, L.G. and Y.X.; Supervision, M.W. and X.X.; Visualization, X.X.; Writing—original draft, W.D. and X.X.; Writing—review and editing, W.D. and X.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 32072459).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank Guodong Zhu (Shandong Agricultural University, Taian, China) for his assistance in life table analysis during the preparation of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ebert, T.A.; Cartwright, B. Biology and ecology of Aphis gossypii Glover (Homoptera: Aphididae). Southwest. Entomol. 1997, 22, 116–153. [Google Scholar]
- Sudhakar, N.; Thajuddin, N.; Murugesan, K. Plant growth-promoting rhizobacterial mediated protection of tomato in the field against cucumber mosaic virus and its vector Aphis gossypii. Biocontrol Sci. Technol. 2011, 21, 367–386. [Google Scholar] [CrossRef]
- Heilsnis, B.; Mahas, J.B.; Conner, K.; Pandey, S.; Clark, W.; Koebernick, J.; Jacobson, A.L. Characterizing the vector competence of Aphis gossypii, Myzus persicae and Aphis craccivora (Hemiptera: Aphididae) to transmit cotton leafroll dwarf virus to cotton in the United States. J. Econ. Entomol. 2023, 116, 719–725. [Google Scholar] [CrossRef]
- Mokbel, E.S. Resistance risk assessment: Realized heritability, cross resistance and resistance stability of acetamiprid in the cotton aphid, Aphis gossypii Glover (Homoptera: Aphididae). J. Plant Prot. Res. 2018, 58, 328–334. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Tariq, K.; Desneux, N.; Gao, X.; Song, D. Acetamiprid resistance and fitness costs of melon aphid, Aphis gossypii: An age-stage, two-sex life table study. Pestic. Biochem. Phys. 2021, 171, 104729. [Google Scholar] [CrossRef]
- Zeng, X.; Pan, Y.; Song, J.; Li, J.; Lv, Y.; Gao, X.; Shang, Q. Resistance risk assessment of the ryanoid anthranilic diamide insecticide cyantraniliprole in Aphis gossypii Glover. J. Agric. Food Chem. 2021, 69, 5849–5857. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.Y.; Guo, Q.L.; Xia, X.M.; Wang, H.Y.; Liu, T.X. Resistance of Aphis gossypii (Homoptera: Aphididae) to selected insecticides on cotton from five cotton production regions in Shandong, China. J. Pestic. Sci. 2007, 32, 372–378. [Google Scholar] [CrossRef]
- Shi, D.; Liang, P.; Zhang, L.; Lv, H.; Gao, X.; You, H.; Ma, K. Susceptibility baseline of Aphis gossypii Glover (Hemiptera: Aphididae) to the novel insecticide afidopyropen in China. Crop Prot. 2022, 151, 105834. [Google Scholar] [CrossRef]
- Cheng, S.; Li, R.; Chen, Z.; Ni, J.; Lv, N.; Liang, P.; Gao, X. Comparative susceptibility of Aphis gossypii Glover (Hemiptera: Aphididae) on cotton crops to imidacloprid and a novel insecticide cyproflanilide in China. Ind. Crop Prod. 2023, 192, 116053. [Google Scholar] [CrossRef]
- Rizvi, S.A.H.; Liu, Z.H.; Ozuzu, S.A.; Arafat, Y.; Han, C.X.; Maggi, F.; Shao, H. Toxicity of Delphinium brunonianum Royle alkaloids against the adults of Diaphorina citri and its mechanism study in insect SF9 cell line. Ind. Crop Prod. 2024, 208, 117826. [Google Scholar] [CrossRef]
- Cui, L.; Qi, H.L.; Yang, D.; Yuan, H.Z.; Rui, C.H. Cycloxaprid: A novel cis-nitromethylene neonicotinoid insecticide to control imidacloprid-resistant cotton aphid (Aphis gossypii). Pestic. Biochem. Phys. 2016, 132, 96–101. [Google Scholar] [CrossRef]
- Leichter, C.A.; Thompson, N.; Johnson, B.R.; Scott, J.G. The high potency of ME-5343 to aphids is due to a unique mechanism of action. Pestic. Biochem. Phys. 2013, 107, 169–176. [Google Scholar] [CrossRef]
- Kandasamy, R.; London, D.; Stam, L.; von-Deyn, W.; Zhao, X.; Salgado, V.L.; Nesterov, A. Afidopyropen: New and potent modulator of insect transient receptor potential channels. Insect Biochem. Mol. 2017, 84, 32–39. [Google Scholar] [CrossRef]
- Koch, R.L.; Queiroz, O.; Aita, R.C.; Hodgson, E.W.; Potter, B.D.; Nyoike, T.; Ellers-Kirk, C.D. Efficacy of afidopyropen against soybean aphid (Hemiptera: Aphididae) and toxicity to natural enemies. Pest Manag. Sci. 2020, 76, 375–383. [Google Scholar] [CrossRef]
- Tang, Q.L.; Liang, P.Z.; Li, J.H.; Gao, X.W. A sublethal concentration of afidopyropen suppresses the population growth of the cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae). J. Integr. Agric. 2022, 21, 2055–2064. [Google Scholar] [CrossRef]
- Slusher, E.K.; Cottrell, T.; Acebes-Doria, A.L. Effects of aphicides on pecan aphids and their parasitoids in pecan orchards. Insects 2021, 12, 241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, Z.; Zheng, H.; Zhang, Q.; Gong, J.; Li, C.; Wang, R. Physiological and biochemical responses to sublethal concentrations of the novel pyropene insecticide, afidopyropen, in whitefly Bemisia tabaci MED (Q Biotype). Agronomy 2021, 11, 2260. [Google Scholar] [CrossRef]
- Chen, X.D.; Ashfaq, M.; Stelinski, L.L. Susceptibility of Asian citrus psyllid, Diaphorina citri (Hemiptera: Liviidae), to the insecticide afidopyropen: A new and potent modulator of insect transient receptor potential channels. Appl. Entomol. Zool. 2018, 53, 453–461. [Google Scholar] [CrossRef]
- Joseph, S.V. Repellent effects of insecticides on Stephanitis pyrioides Scott (Hemiptera: Tingidae) under laboratory conditions. Crop Prot. 2020, 127, 104985. [Google Scholar] [CrossRef]
- Deng, D.; Duan, W.; Wang, H.; Zhang, K.; Guo, J.; Yuan, L.; Wu, S. Assessment of the effects of lethal and sublethal exposure to dinotefuran on the wheat aphid Rhopalosiphum padi (Linnaeus). Ecotoxicology 2019, 28, 825–833. [Google Scholar] [CrossRef]
- Stapel, J.O.; Cortesero, A.M.; Lewis, W.J. Disruptive sublethal effects of insecticides on biological control: Altered foraging ability and life span of a parasitoid after feeding on extrafloral nectar of cotton treated with systemic insecticides. Biol. Control 2000, 17, 243–249. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.B.; Jiang, L.L.; Wang, H.Y.; Qiao, K.; Wang, D.; Wang, K. Toxicities and sublethal effects of seven neonicotinoid insecticides on survival, growth and reproduction of imidacloprid-resistant cotton aphid, Aphis gossypii. Pest Manag. Sci. 2011, 67, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Yuan, H.; Wang, Q.; Wang, Q.; Rui, C. Sublethal effects of the novel cis-nitromethylene neonicotinoid cycloxaprid on the cotton aphid Aphis gossypii Glover (Hemiptera: Aphididae). Sci. Rep. 2018, 8, 8915. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, Q.; Liu, X.; Liang, G. Differences in the sublethal effects of sulfoxaflor and acetamiprid on the Aphis gossypii Glover (Homoptera: Aphididae) are related to its basic sensitivity level. Insects 2022, 13, 498. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.Z.; Ma, K.S.; Chen, X.W.; Tang, C.Y.; Xia, J.; Chi, H.; Gao, X.W. Toxicity and sublethal effects of flupyradifurone, a novel butenolide insecticide, on the development and fecundity of Aphis gossypii (Hemiptera: Aphididae). J. Econ. Entomol. 2019, 112, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, E.M.G.; De-Moura, I.L.T.; Fadini, M.A.M.; Guedes, R.N.C. Beyond selectivity: Are behavioral avoidance and hormesis likely causes of pyrethroid-induced outbreaks of the southern red mite Oligonychus ilicis? Chemosphere 2013, 93, 1111–1116. [Google Scholar] [CrossRef]
- Qu, Y.; Xiao, D.; Li, J.; Chen, Z.; Biondi, A.; Desneux, N.; Song, D. Sublethal and hormesis effects of imidacloprid on the soybean aphid Aphis glycines. Ecotoxicology 2015, 24, 479–487. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Walse, S.S.; Throne, J.E. Sublethal exposure, insecticide resistance, and community stress. Curr. Opin. Insect Sci. 2017, 21, 47–53. [Google Scholar] [CrossRef]
- Wang, S.; Qi, Y.; Desneux, N.; Shi, X.; Biondi, A.; Gao, X. Sublethal and transgenerational effects of short-term and chronic exposures to the neonicotinoid nitenpyram on the cotton aphid Aphis gossypii. J. Pest Sci. 2017, 90, 389–396. [Google Scholar] [CrossRef]
- Shang, J.; Yao, Y.; Chen, L. Sublethal exposure to deltamethrin stimulates reproduction and alters symbiotic bacteria in Aphis gossypii. J. Agric. Food Chem. 2020, 69, 15097–15107. [Google Scholar] [CrossRef]
- Wang, K.Y.; Liu, T.X.; Yu, C.H.; Jiang, X.Y.; Yi, M.Q. Resistance of Aphis gossypii (Homoptera: Aphididae) to fenvalerate and imidacloprid and activities of detoxification enzymes on cotton and cucumber. J. Econ. Entomol. 2002, 95, 407–413. [Google Scholar] [CrossRef]
- Rizvi, S.A.H.; Ling, S.; Tian, F.; Xie, F.; Zeng, X.N. Toxicity and enzyme inhibition activities of the essential oil and dominant constituents derived from Artemisia absinthium L. against adult Asian citrus psyllid Diaphorina citri Kuwayama (Hemiptera: Psyllidae). Ind. Crop Prod. 2018, 121, 468–475. [Google Scholar] [CrossRef]
- Leelaja, B.C.; Rajini, P.S. Biochemical and physiological responses in Caenorhabditis elegans exposed to sublethal concentrations of the organophosphorus insecticide, monocrotophos. Ecotox. Environ. Saf. 2013, 94, 8–13. [Google Scholar] [CrossRef]
- Wen, S.; Xue, Y.; Du, R.; Liu, C.; Wang, X.; Wang, Y.; Xia, X. Toxicity and sublethal effects of triflumezopyrim on the development and detoxification enzymatic activities in the small brown planthopper (SBPH), Laodelphax striatellus (Fallen). Crop Prot. 2021, 150, 105813. [Google Scholar] [CrossRef]
- Voudouris, C.C.; Kati, A.N.; Sadikoglou, E.; Williamson, M.; Skouras, P.J.; Dimotsiou, O.; Margaritopoulos, J.T. Insecticide resistance status of Myzus persicae in Greece: Long-term surveys and new diagnostics for resistance mechanisms. Pest Manag. Sci. 2016, 72, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Mostafiz, M.M.; Alam, M.B.; Chi, H.; Hassan, E.; Shim, J.K.; Lee, K.Y. Effects of sublethal doses of methyl benzoate on the life history traits and acetylcholinesterase (AChE) activity of Aphis gossypii. Agronomy 2020, 10, 1313. [Google Scholar] [CrossRef]
- Lin, Y.; He, S.Q.; Lu, Z.H.; Gao, Y.L.; Duan, Y.R.; Li, Z.Y.; Chen, B.; Gui, F.R. Influence of Aphis gossypii feeding on defense strategy of native and introduced populations of Ageratina adenophora. Arthropod-Plant Interact. 2020, 14, 345–356. [Google Scholar] [CrossRef]
- Yang, Y.X.; Lin, R.H.; Li, Z.; Wang, A.Y.; Xue, C.; Duan, A.L.; Zhang, J.H. Function analysis of P450 and GST genes to imidacloprid in Aphis craccivora (Koch). Front. Physiol. 2021, 11, 624287. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, H.; Pan, Y.; Gao, X.; Xi, J.; Zhang, J.; Shang, Q. Expression profile changes of cytochrome P450 genes between thiamethoxam susceptible and resistant strains of Aphis gossypii Glover. Pestic. Biochem. Phys. 2018, 149, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Su, H.Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Ma, K.; Tang, Q.; Zhang, B.; Liang, P.; Wang, B.; Gao, X. Overexpression of multiple cytochrome P450 genes associated with sulfoxaflor resistance in Aphis gossypii Glover. Pestic. Biochem. Phys. 2019, 157, 204–210. [Google Scholar] [CrossRef]
- Liu, X.; Fu, Z.; Zhu, Y.; Gao, X.; Liu, T.X.; Liang, P. Sublethal and transgenerational effects of afidopyropen on biological traits of the green peach aphid Myzus persicae (Sluzer). Pestic. Biochem. Phys. 2022, 180, 104981. [Google Scholar] [CrossRef] [PubMed]
- Carletto, J.; Martin, T.; Vanlerberghe-Masutti, F.; Brévault, T. Insecticide resistance traits differ among and within host races in Aphis gossypii. Pest Manag. Sci. 2010, 66, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.N.; An, J.J.; Park, S.E.; Kim, J.I.; Kim, G.H. Regional susceptibilities to 12 insecticides of melon and cotton aphid, Aphis gossypii (Hemiptera: Aphididae) and a point mutation associated with imidacloprid resistance. Crop Prot. 2014, 55, 91–97. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, M.; Liu, X.; Xu, J.; Dong, F.; Wu, X.; Zheng, Y. Determination and dissipation of afidopyropen and its metabolite in wheat and soil using QuEChERS–UHPLC–MS/MS. J. Sep. Sci. 2018, 41, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Christopher, C.G.; Ramanaidu, K.; Astatkie, T.; Isman, M.B. Green peach aphid, Myzus persicae (Hemiptera: Aphididae), reproduction during exposure to sublethal concentrations of imidacloprid and azadirachtin. Pest Manag. Sci. 2009, 65, 205–209. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Magalhaes, L.C.; Cosme, L.V. Stimulatory sublethal response of a generalist predator to permethrin: Hormesis, hormoligosis, or homeostatic regulation? J. Econ. Entomol. 2009, 102, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Yao, Y.S.; Zhu, X.Z.; Wang, L.; Li, D.Y.; Zhang, K.X.; Cui, J.J. Evaluation of sublethal and transgenerational effects of sulfoxaflor on Aphis gossypii via life table parameters and 16S rRNA sequencing. Pest Manag. Sci. 2021, 77, 3406–3418. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Y.; Liu, Y.; Wang, X.; Zhu, Z. The effects of sublethal levels of insecticide on the wing dimorphism, development and reproduction of two aphid species. Chin. J. Appl. Entomol. 2018, 55, 896–903. [Google Scholar]
- An, J.J.; Gao, Z.L.; Dang, Z.H.; Yan, X.; Pan, W.L.; Li, Y.F. Development and risk assessment of resistance to sulfoxaflor in cotton aphid (Aphis gossypii). J. Hebei Agric. Univ. 2020, 43, 76–81. [Google Scholar] [CrossRef]
- Guo, T.; Ma, Y.; Ding, R.; Zhou, J.; Li, G.; Cai, X.; Gao, X. Selection and realized heritability analysis of resistance to imidacloprid in cotton aphid (Aphis gossypii). Acta Entomol. Sin. 2014, 57, 330–334. [Google Scholar] [CrossRef]
- Karuppaiah, V.; Srivastava, C.; Subramanian, S. Effect of host plants on insecticide susceptibility and detoxiffcation enzymes activity in Spodoptera litura Fabricius (Noctuidae: Lepidoptera). Proc. Natl. Acad. Sci. USA 2015, 86, 715–721. [Google Scholar] [CrossRef]
- Chen, X.; Xia, X.; Wang, H.; Qiao, K.; Wang, K. Cross-resistance to clothianidin and acetamiprid in the imidacloprid-resistant strain of Aphis gossypii (Hemiptera: Aphididae) and the related enzyme mechanisms. Acta Entomol. Sin. 2013, 56, 1143–1151. [Google Scholar]
- Guo, T.F.; Zhou, J.; Ye, Y.X. Study on the activity of the detoxification enzymes and synergist synergism of cotton Aphis gossypii on acetamiprid of different strains. Xinjiang Agric. Sci. 2015, 52, 1334–1339. [Google Scholar]
- Feyereisen, R. Insect P450 enzymes. Annu. Rev. Entomol. 1999, 44, 507–533. [Google Scholar] [CrossRef] [PubMed]
- Weedall, G.D.; Mugenzi, L.M.J.; Menze, B.D.; Tchouakui, M.; Ibrahim, S.S.; Amvongo-Adjia, N.; Wondji, C.S. A cytochrome P450 allele confers pyrethroid resistance on a major African malaria vector, reducing insecticide-treated bednet efficacy. Sci. Transl. Med. 2019, 11, eaat7386. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, C.T.; Garrood, W.T.; Singh, K.S.; Randall, E.; Lueke, B.; Gutbrod, O.; Bass, C. Neofunctionalization of duplicated P450 genes drives the evolution of insecticide resistance in the brown planthopper. Curr. Biol. 2018, 28, 268–274.e5. [Google Scholar] [CrossRef] [PubMed]
- Nauen, R.; Bass, C.; Feyereisen, R.; Vontas, J. The role of cytochrome P450s in insect toxicology and resistance. Annu. Rev. Entomol. 2022, 67, 105–124. [Google Scholar] [CrossRef]
- Jones, R.T.; Bakker, S.E.; Stone, D.; Shuttleworth, S.N.; Boundy, S.; McCart, C.; Elsen, J.M. Homology modelling of Drosophila cytochrome P450 enzymes associated with insecticide resistance. Pest Manag. Sci. 2010, 66, 1106–1115. [Google Scholar] [CrossRef]
- Wang, R.; Gao, B.; Che, W.; Qu, C.; Zhou, X.; Luo, C. First report of field resistance to afidopyropen, the novel Pyropene insecticide, on Bemisia tabaci Mediterranean (Q Biotype) from China. Agronomy 2022, 12, 724. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).