Phosphorus Availability and Uptake following a Maize-Pigeon Pea Rotation under Conservation Agriculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of Experimental Site, Design, and Treatments
2.2. Soil Sampling and Analysis
2.2.1. Field Trials
2.2.2. Greenhouse Study
2.3. Statistical Analysis
3. Results
3.1. Effect of Rotation with Pigeon Pea on Plant-Available Phosphorus under Field Conditions
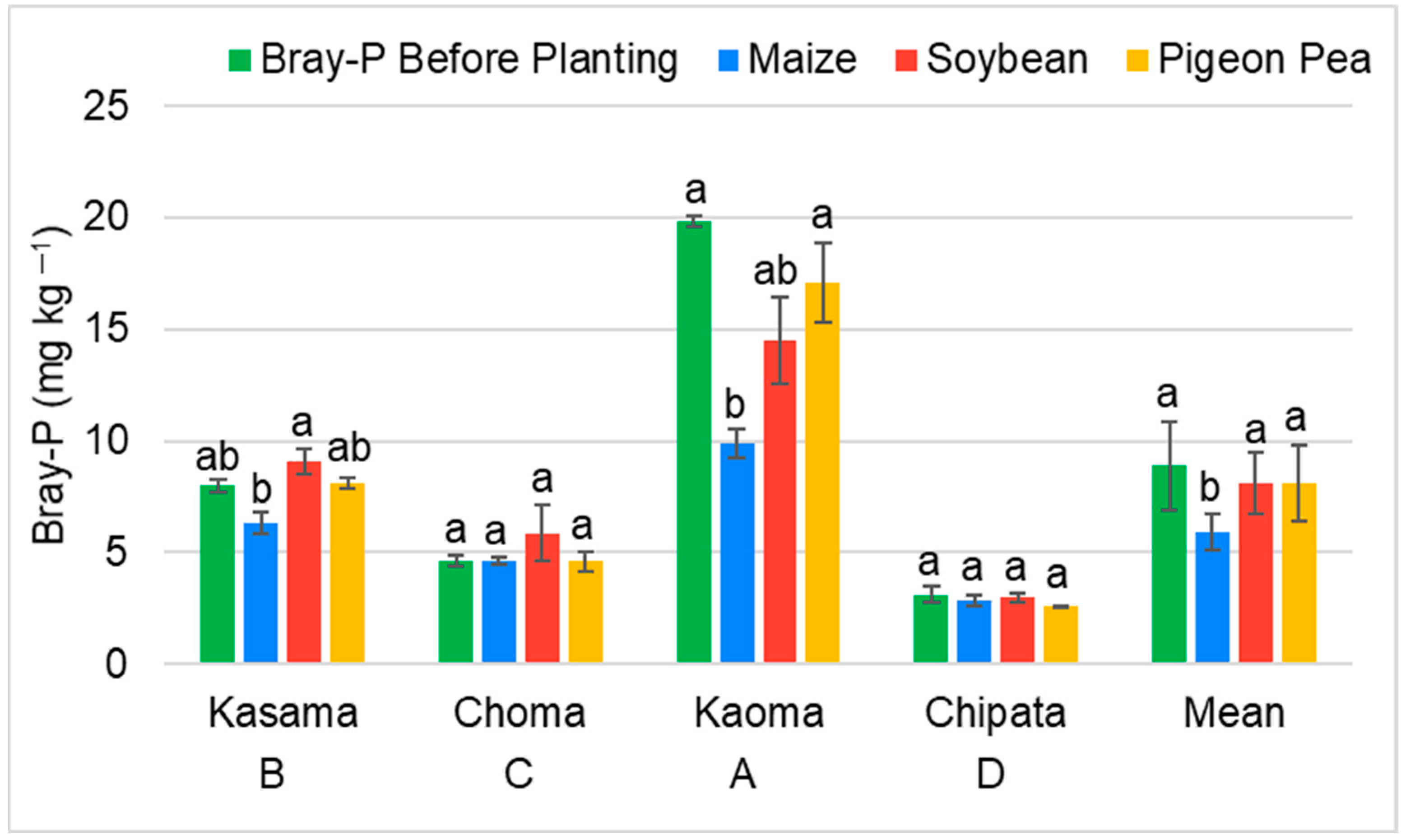
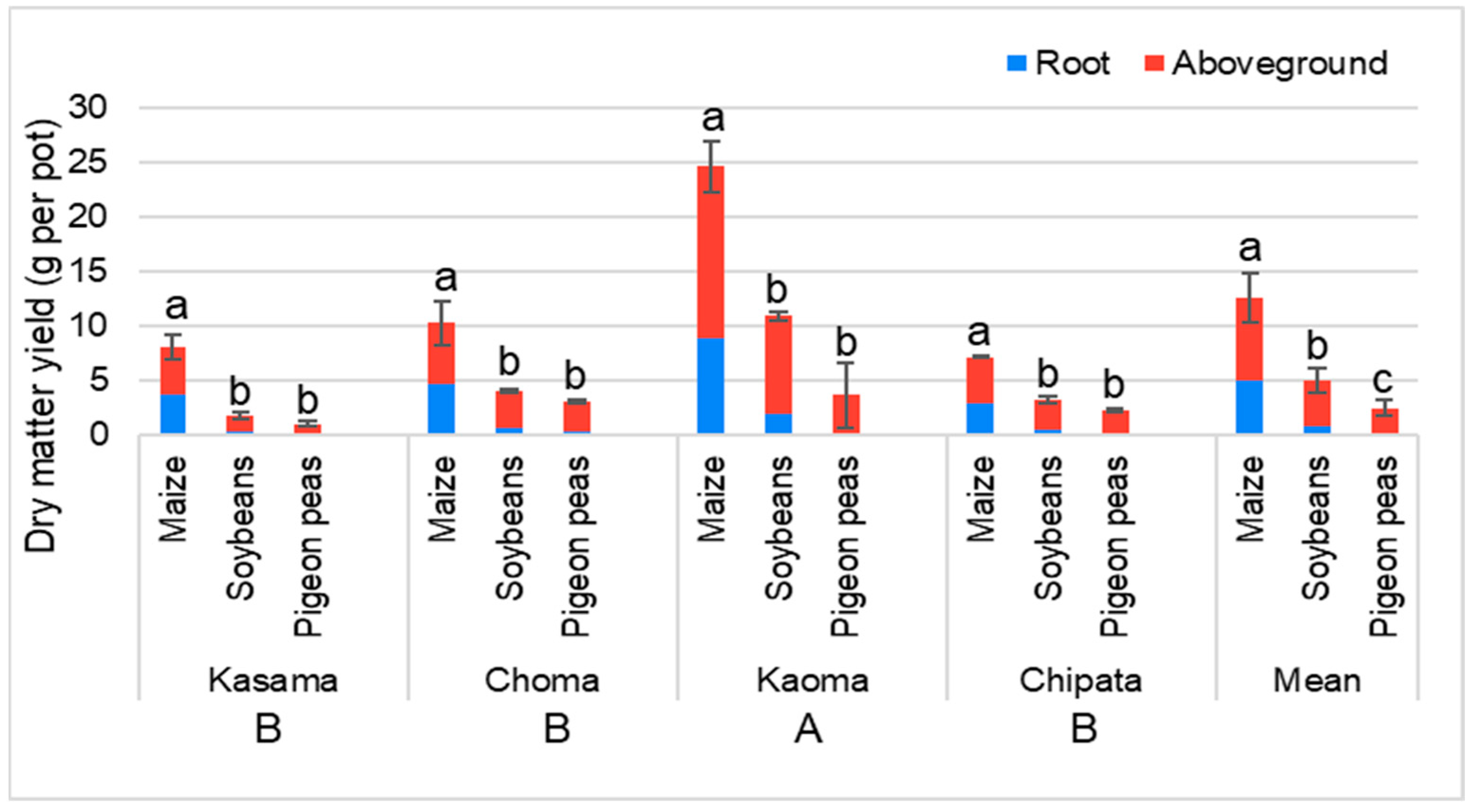
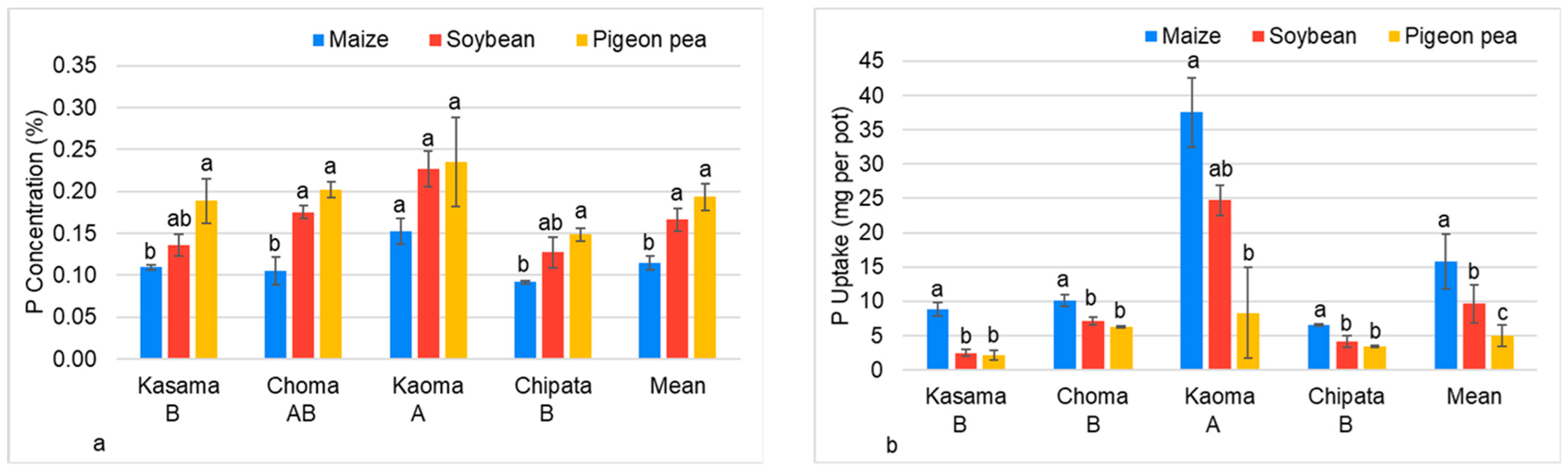
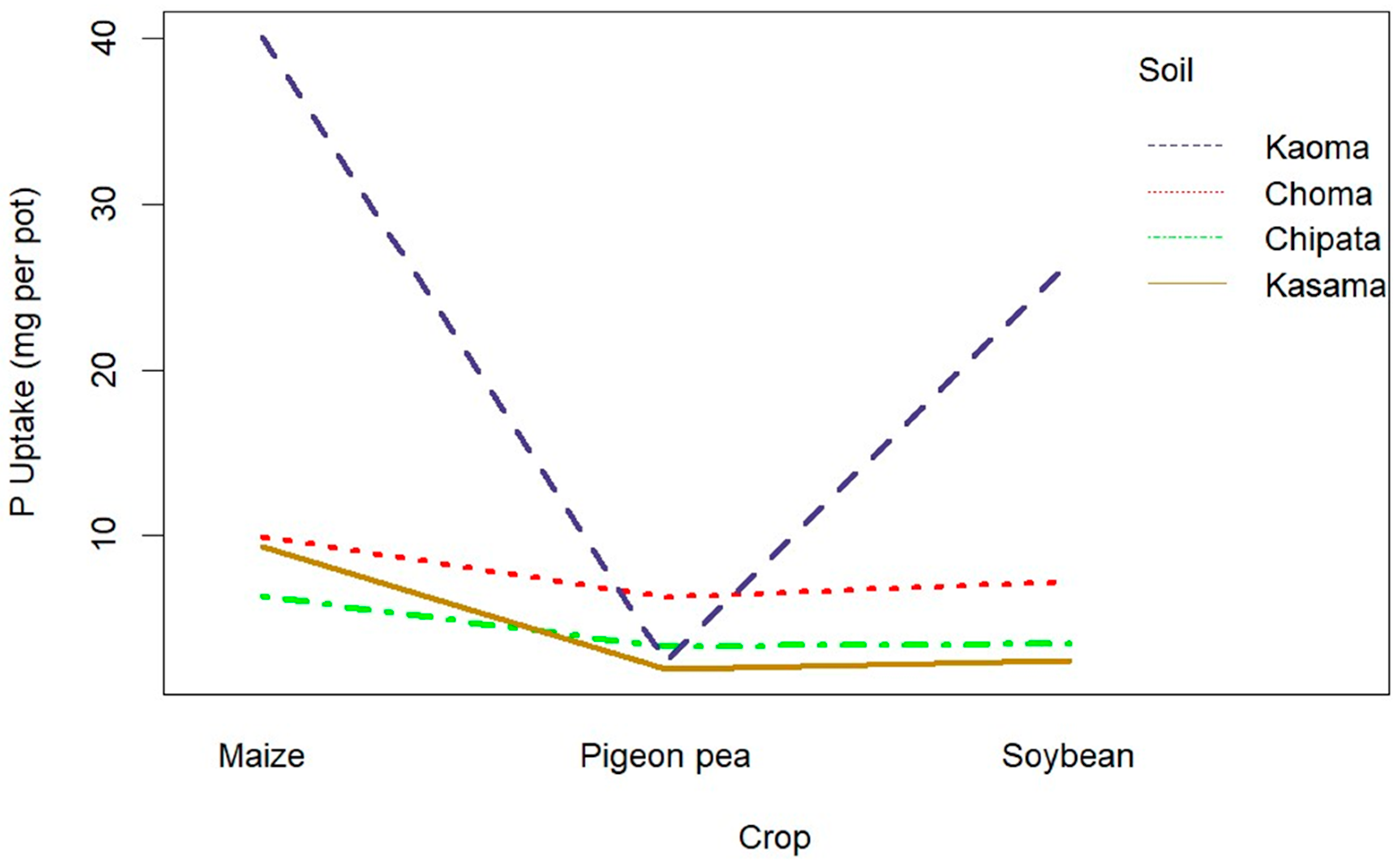
3.2. Effect of Pigeon Pea on Plant-Available Phosphorus under Greenhouse Study
3.2.1. Effect of Maize, Soybean, and Pigeon Pea on Plant-Available Phosphorus
3.2.2. Dry Matter Yield of Maize, Soybean, and Pigeon Pea
3.2.3. Phosphorus Concentration and Uptake by Maize, Soybean, and Pigeon Pea
3.2.4. Enrichment of Plant-Available P after Maize, Soybean, and Pigeon Pea Cropping
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Yerokun, O.A. Chemical Characteristics of Phosphorus in Some Representative Benchmark Soils of Zambia. Geoderma 2008, 147, 63–68. [Google Scholar] [CrossRef]
- Høgh-Jensen; Myaka, F.A.; Sakala, W.D.; Kamalongo, D.; Ngwira, A.; Odgaard, R. Adu-Gyamfi Yields and Qualities of Pigeonpea Varieties Grown under Smallholder Farmers’ Conditions in Eastern and Southern Africa. Afr. J. Agric. Res. 2007, 2, 269–278. [Google Scholar]
- Bahl, G.S.; Pasricha, N.S. Efficiency of P Utilization by Pigeonpea and Wheat Grown in a Rotation. Nutr. Cycl. Agroecosyst. 1998, 51, 225–229. [Google Scholar] [CrossRef]
- Pahalvi, H.N.; Rafiya, L.; Rashid, S.; Nisar, B.; Kamili, A.N. Chemical Fertilizers and Their Impact on Soil Health. In Microbiota and Biofertilizers, Volume 2: Ecofriendly Tools for Reclamation of Degraded Soil Environs; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 1–20. ISBN 9783030610104. [Google Scholar]
- Kiwia, A.; Kimani, D.; Harawa, R.; Jama, B.; Sileshi, G.W. Sustainable Intensification with Cereal-Legume Intercropping in Eastern and Southern Africa. Sustainability 2019, 11, 2891. [Google Scholar] [CrossRef]
- Thierfelder, C.; Rusinamhodzi, L.; Ngwira, A.R.; Mupangwa, W.; Nyagumbo, I.; Kassie, G.T.; Cairns, J.E. Conservation Agriculture in Southern Africa: Advances in Knowledge. Renew. Agric. Food Syst. 2015, 30, 328–348. [Google Scholar] [CrossRef]
- Bogužas, V.; Skinulienė, L.; Butkevičienė, L.M.; Steponavičienė, V.; Petrauskas, E.; Maršalkienė, N. The Effect of Monoculture, Crop Rotation Combinations, and Continuous Bare Fallow on Soil CO2 Emissions, Earthworms, and Productivity of Winter Rye after a 50-Year Period. Plants 2022, 11, 431. [Google Scholar] [CrossRef] [PubMed]
- Nuruzzaman, M.; Lambers, H.; Bolland, M.D.A.; Veneklaas, E.J. Phosphorus Uptake by Grain Legumes and Subsequently Grown Wheat at Different Levels of Residual Phosphorus Fertiliser. Aust. J. Agric. Res. 2005, 56, 1041–1047. [Google Scholar] [CrossRef]
- Mangaba, D.A. The Impact of the Farmer Input Support Program on Crop Diversification among Small-Scale Farmers in Zambia: A Case Study of Nega-Nega Agricultural Camp. Int. J. Multi-Displinary Res. 2017, 1–34. Available online: https://www.multiresearch.net/cms/publications/CFP4482017.pdf (accessed on 13 December 2023).
- Farooq, M.; Siddique, K.H.M. Conservation Agriculture; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; ISBN 9783319116204. [Google Scholar]
- Kassam, A.; Friedrich, T.; Derpsch, R. Global Spread of Conservation Agriculture. Int. J. Environ. Stud. 2019, 76, 29–51. [Google Scholar] [CrossRef]
- Muchabi, J.; Lungu, O.I.; Mweetwa, A.M. Conservation Agriculture in Zambia: Effects on Selected Soil Properties and Biological Nitrogen Fixation in Soya Beans (Glycine max (L.) Merr). Sustain. Agric. Res. 2014, 3, 28–36. [Google Scholar] [CrossRef]
- Sommer, R.; Paul, B.K.; Mukalama, J.; Kihara, J. Reducing Losses but Failing to Sequester Carbon in Soils—The Case of Conservation Agriculture and Integrated Soil Fertility Management in the Humid Tropical Agro-Ecosystem of Western Kenya. Agric. Ecosyst. Env. 2018, 254, 82–91. [Google Scholar] [CrossRef]
- Liu, X.; Herbert, A.M.; Hashemi, X.; Zhang, X.; Ding, G. Effects of Agricultural Management on Soil Organic Matter and Carbon Transformation—A Review. Plant Soil Env. 2006, 52, 531–543. [Google Scholar] [CrossRef]
- Venter, Z.S.; Jacobs, K.; Hawkins, H.J. The Impact of Crop Rotation on Soil Microbial Diversity: A Meta-Analysis. Pedobiologia 2016, 59, 215–223. [Google Scholar] [CrossRef]
- Mwila, M.; Mhlanga, B.; Thierfelder, C. Intensifying Cropping Systems through Doubled-up Legumes in Eastern Zambia. Sci. Rep. 2021, 11, 8101. [Google Scholar] [CrossRef] [PubMed]
- Vesterager, J.M.; Nielsen, N.E.; Høgh-Jensen, H. Variation in Phosphorus Uptake and Use Efficiencies between Pigeonpea Genotypes and Cowpea. J. Plant Nutr. 2006, 29, 1869–1888. [Google Scholar] [CrossRef]
- Abebe, Z.; Tola, M.; Tadesse, S. Multiple Advantages of Pigeon Pea (Cajanas cajan) in Maize Based Cropping Systems: Used as Live Stake for Climbing Bean with Phosphorus Rates and Maize Productivity Enhancement in Mono Cropping Areas. J. Nat. Sci. Res. 2016, 6, 58–69. [Google Scholar]
- Odeny, D.A. The Potential of Pigeonpea (Cajanus cajan (L.) Millsp.) in Africa. Nat. Resour. Forum. 2007, 31, 297–305. [Google Scholar] [CrossRef]
- Ae, N.; Arihara, J.; Okada, K.; Yoshihara, T.; Johansen, C. Phosphorus Uptake by Pigeon Pea and Its Role in Cropping Systems of the Indian Subcontinent. Science 1990, 248, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Garland, G.; Bünemann, E.K.; Oberson, A.; Frossard, E.; Snapp, S.; Chikowo, R.; Six, J. Phosphorus Cycling within Soil Aggregate Fractions of a Highly Weathered Tropical Soil: A Conceptual Model. Soil Biol. Biochem. 2018, 116, 91–98. [Google Scholar] [CrossRef]
- Saidia, P.S.; Asch, F.; Kimaro, A.A.; Germer, J.; Kahimba, F.C.; Graef, F.; Semoka, J.M.R.; Rweyemamu, C.L. Soil Moisture Management and Fertilizer Micro-Dosing on Yield and Land Utilization Efficiency of Inter-Cropping Maize-Pigeon-Pea in Sub Humid Tanzania. Agric. Water Manag. 2019, 223, 105712. [Google Scholar] [CrossRef]
- Kaoneka, S.R.; Saxena, R.K.; Silim, S.N.; Odeny, D.A.; Ganga Rao, N.V.P.R.; Shimelis, H.A.; Siambi, M.; Varshney, R.K. Pigeonpea Breeding in Eastern and Southern Africa: Challenges and Opportunities. Plant Breed. 2016, 135, 148–154. [Google Scholar] [CrossRef]
- Adu-Gyamfi, J.J.; Myaka, F.A.; Sakala, W.D.; Odgaard, R.; Vesterager, J.M.; Høgh-Jensen, H. Biological Nitrogen Fixation and Nitrogen and Phosphorus Budgets in Farmer-Managed Intercrops of Maize-Pigeonpea in Semi-Arid Southern and Eastern Africa. Plant Soil 2007, 295, 127–136. [Google Scholar] [CrossRef]
- Murata, M. Crop Varieties Released under the Agricultural Productivity Programme for Southern Africa (APPSA)—Zambia 2019. Available online: https://www.ccardesa.org/knowledge-products/crop-varieties-released-under-agricultural-productivity-programme-southern-0 (accessed on 18 May 2023).
- Nordic Climate Facility Completion Report Testing Biochar-Pigeon Pea Agroforestry Businesses in Zambia (ClimChar Zambia), Zambia, NCF7, NCF-C7-091. 2023. Available online: https://smartme.adalia.fi/NCF/fact_sheet/document/2318/field_XWJFUubB:9SoVPVQ8:KPHkG1W0/en (accessed on 29 October 2023).
- Ministry of Agriculture Exploratory Soil Map of Zambia. 1991. Available online: https://edepot.wur.nl/487478 (accessed on 23 April 2023).
- Egner, H.; Riehm, H.; Domigo, W.R. Investigations on the Chemical Soil Analysis as a Basis for Assesing the Soil Nutrient Status II: Chemical Extraction Methods for Phosphorus and Potassium Determination. Ann. R. Swed. Agric. Coll. 1960, 26, 199–215. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Organic Carbon. In Methods of Soil Analysis, Part 2: Chemical and Biological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madson, WI, USA, 1982. [Google Scholar]
- ISO 11272; ISO Soil Quality-Determination of Dry Bulk Density. ISO: Geneva, Switzerland, 2017.
- McLean, E.O. Soil PH and Lime Requirement. In Methods of Soil Analysis, Part 2: Chemical and Biological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madson, WI, USA, 1982. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen—Total. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of Total, Organic and Inorganic Phosphorus in Soil. Soil Sci 1945, 59, 39–45. [Google Scholar] [CrossRef]
- Thomas, G.W. Exchangeable Cations. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Day, P.R. Particle Fractionation and Particle Size Analysis. In Methods of Soil Analysis. Part 1, Agronomy 9; Clark, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 545–567. [Google Scholar]
- R Core Team R. A Language and Environment for Statistical Computing, R Foundation for Statistical Computing: Vienna, Austria, 2023.
- Posit Team RStudio. Integrated Development Environment for R, Posit Software; PBC: Boston, MA, USA, 2023.
- FAO. Food and Agriculture Organization of the United Nations World Reference Base for Soil Resources 2014: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2014; ISBN 9789251083697. [Google Scholar]
- Hocking, P.J.; Keerthisinghe, G.; Smith, F.W.; Randaul, P.J. Comparison of the Ability of Different Crop Species to Access Poorly-Available Soil Phosphorus. Plant Nutr. Sustain. Food Prod. Environ. 1997, 305–308. [Google Scholar]
- Bortolon, L.; Bortolon, E.S.O.; de Camargo, F.P.; Seraglio, N.A.; Lima, A.d.O.; Rocha, P.H.F.; de Souza, J.P.; Sousa, W.C.; Tomazzi, M.; Lago, B.C.; et al. Yield and Nutrient Uptake of Soybean Cultivars under Intensive Cropping Systems. J. Agric. Sci. 2018, 10, 344–357. [Google Scholar] [CrossRef]
- Amissah, S.; Ankomah, G.; Agyei, B.K.; Lee, R.D.; Harris, G.H.; Cabrera, M.; Franklin, D.H.; Diaz-Perez, J.C.; Habteselassie, M.Y.; Sintim, H.Y. Nutrient Sufficiency Ranges for Corn at the Early Growth Stage: Implications for Nutrient Management. Plants 2023, 12, 713. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.R.; Haegele, J.W.; Ruffo, M.L.; Below, F.E. Modern Corn Hybrids’ Nutrient Uptake Patterns. Better Crops 2013, 97, 7–10. [Google Scholar]
- Klamer, F.; Vogel, F.; Li, X.; Bremer, H.; Neumann, G.; Neuhäuser, B.; Hochholdinger, F.; Ludewig, U. Estimating the Importance of Maize Root Hairs in Low Phosphorus Conditions and under Drought. Ann. Bot. 2019, 124, 961–968. [Google Scholar] [CrossRef]
- Morris, R.A.; Garrity, D.P. Resource Capture and Utilization in Intercropping: Non-Nitrogen Nutrients. Field Crops Res 1993, 34, 319–334. [Google Scholar] [CrossRef]
- Tiziani, R.; Mimmo, T.; Valentinuzzi, F.; Pii, Y.; Celletti, S.; Cesco, S. Root Handling Affects Carboxylates Exudation and Phosphate Uptake of White Lupin Roots. Front. Plant Sci. 2020, 11, 584568. [Google Scholar] [CrossRef] [PubMed]
- Balemi, T.; Negisho, K. Management of Soil Phosphorus and Plant Adaptation Mechanisms to Phosphorus Stress for Sustainable Crop Production: A Review. J. Soil Sci. Plant Nutr. 2012, 12, 547–561. [Google Scholar] [CrossRef]
- Lyu, Y.; Tang, H.; Li, H.; Zhang, F.; Rengel, Z.; Whalley, W.R.; Shen, J. Major Crop Species Show Differential Balance between Root Morphological and Physiological Responses to Variable Phosphorus Supply. Front. Plant Sci. 2016, 7, 1939. [Google Scholar] [CrossRef]
- Adu-Gyamfi, J.J.; Fujita, K.; Ogata, S. Phosphorus Fractions in Relation to Growth in Pigeon Pea (Cajanus cajan (L.) Millsp.) at Varions Levels of P Supply. Soil Sci. Plant Nutr. 1990, 36, 531–543. [Google Scholar] [CrossRef]
- Mahanta, D.; Rai, R.K.; Mishra, S.D.; Raja, A.; Purakayastha, T.J.; Varghese, E. Influence of Phosphorus and Biofertilizers on Soybean and Wheat Root Growth and Properties. Field Crops Res 2014, 166, 1–9. [Google Scholar] [CrossRef]
- Subramanian, K.S.; Bharathi, C.; Jegan, A. Response of Maize to Mycorrhizal Colonization at Varying Levels of Zinc and Phosphorus. Biol. Fertil. Soils 2008, 45, 133–144. [Google Scholar] [CrossRef]
- Phiri, M.; Yerokun, O.A.; Chilombo, G. Phosphorus Fractions and Uptake in Semi-Arid Soils of Zambia. Proc. Afr. Crop Sci. Conf. Proc. 2013, 11, 831–834. [Google Scholar]


| Site | Baseline | After Pigeon Pea | Difference | |
|---|---|---|---|---|
| P kg ha−1 | P kg ha−1 | P kg ha−1 | % Decrease | |
| Choma | 17.7 ± 3.2 | 13.7 ± 1.2 | −4.0 | 22.6 |
| Kaoma | 41.8 ± 4.6 | 32.2 ± 3.4 | −9.6 | 23.0 |
| Mkushi | 23.6 ± 3.4 | 12.4 ± 3.4 | −11.2 | 47.5 * |
| Chipata | 25.3 ± 5.3 | 17.3 ± 2.8 | −8.0 | 31.6 |
| Soil ID | pH | SOC | N | FeAl-P | P | K | Sand | Clay | Silt | Texture | Soil Type |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.01 MCaCl2 | W & Black | Kjeldahl | NaOH-NaCl | Bray 1 | NH4OAc | Hydrometer Method | USDA | * WRB | |||
| % | % | mg kg−1 | mg kg−1 | cmol (+) kg−1 | % | % | % | ||||
| Kasama | 3.93 ± 0.02 | 0.83 ± 0.05 | 0.05 ± 0.01 | 11.21 ± 0.61 | 8.00 ± 0.26 | 0.09 ± 0.00 | 81.2 ± 0.0 | 6.4 ± 0.0 | 12.4 ± 0.0 | Loamy Sand | Acrisols |
| Choma | 4.71 ± 0.06 | 0.48 ± 0.02 | 0.05 ± 0.00 | 1.52 ± 1.29 | 4.62 ± 0.23 | 0.08 ± 0.00 | 83.9 ± 0.7 | 3.7 ± 0.7 | 12.4 ± 1.2 | Loamy Sand | Lixisols |
| Kaoma | 4.85 ± 0.07 | 0.71 ± 0.05 | 0.05 ± 0.00 | 7.12 ± 0.72 | 19.84 ± 0.27 | 0.14 ± 0.01 | 91.2 ± 0.0 | 2.4 ± 0.0 | 6.4 ± 0.0 | Sand | Arenosols |
| Chipata | 4.55 ±0.03 | 1.71 ± 0.05 | 0.19 ± 0.01 | 3.86 ± 1.17 | 3.12 ± 0.36 | 0.29 ± 0.00 | 68.5 ±0.7 | 9.1 ± 0.7 | 22.4 ± 1.2 | Sandy Loam | Acrisols |
| Site | Plant-Available P | P before Planting | Maize | Soybean | Pigeon Pea |
|---|---|---|---|---|---|
| mg per pot | mg per pot | mg per pot | mg per pot | ||
| Kasama | Bray-P | 20.01 ± 0.66 | 15.78 ± 1.25 | 22.70 ± 1.49 | 20.27 ± 0.61 |
| Plant P | - | 8.84 ± 0.98 | 2.49 ± 0.49 | 2.18 ± 0.71 | |
| Bray-P + Plant P | 20.01 ± 0.66 b | 24.62 ± 0.79 a | 25.19 ± 1.39 a | 22.45 ± 0.53 ab | |
| Mobilized P | - | 4.61 ± 0.79 | 5.18 ± 1.39 | 2.44 ± 0.53 | |
| Choma | Bray-P | 11.56 ± 0.57 | 11.58 ± 0.41 | 14.71 ± 3.18 | 11.53 ± 1.09 |
| Plant P | - | 10.13 ± 0.88 | 7.17 ± 0.55 | 6.28 ± 0.19 | |
| Bray-P + Plant P | 11.56 ± 0.57 b | 21.71 ± 0.52 a | 21.88 ± 2.75 a | 17.81 ± 0.90 ab | |
| Mobilized P | - | 10.15 ± 0.52 | 10.32 ± 2.75 | 6.25 ± 0.90 | |
| Kaoma | Bray-P | 49.59 ± 0.68 | 24.80 ± 1.63 | 36.25 ± 4.89 | 42.76 ± 4.46 |
| Plant P | - | 37.54 ± 5.02 | 24.76 ± 2.20 | 8.34 ± 6.58 | |
| Bray-P + Plant P | 49.59 ± 0.68 a | 62.34 ± 4.29 a | 61.01 ± 2.85 a | 51.10 ± 2.36 a | |
| Mobilized P | - | 12.75 ± 4.29 | 11.42 ± 2.85 | 1.51 ± 2.36 | |
| Chipata | Bray-P | 7.79 ± 0.90 | 7.17 ± 0.57 | 7.46 ± 0.42 | 6.47 ± 0.15 |
| Plant P | - | 6.60 ± 0.19 | 4.15 ± 0.80 | 3.40 ± 0.13 | |
| Bray-P + Plant P | 7.79 ± 0.90 c | 13.77 ± 0.74 a | 11.61 ± 0.72 ab | 9.87 ± 0.08 bc | |
| Mobilized P | - | 5.98 ± 0.74 | 3.82 ± 0.72 | 2.08 ± 0.08 | |
| Mean | Bray-P | 22.24 ± 4.95 | 14.83 ± 2.02 | 20.28 ± 3.47 | 20.26 ± 4.31 |
| Plant P | - | 15.78 ± 3.97 | 9.65 ± 2.73 | 5.05 ± 1.59 | |
| Bray-P + Plant P | 22.24 ± 4.95 b | 30.61 ± 5.73 a | 29.93 ± 5.69 a | 25.31 ± 4.72 b | |
| Mobilized P | - | 8.37 ± 1.36 a | 7.69 ± 1.33 a | 3.07 ± 0.79 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phiri, M.; Mulder, J.; Chishala, B.H.; Chabala, L.M.; Martinsen, V. Phosphorus Availability and Uptake following a Maize-Pigeon Pea Rotation under Conservation Agriculture. Agronomy 2024, 14, 169. https://doi.org/10.3390/agronomy14010169
Phiri M, Mulder J, Chishala BH, Chabala LM, Martinsen V. Phosphorus Availability and Uptake following a Maize-Pigeon Pea Rotation under Conservation Agriculture. Agronomy. 2024; 14(1):169. https://doi.org/10.3390/agronomy14010169
Chicago/Turabian StylePhiri, Mirriam, Jan Mulder, Benson H. Chishala, Lydia M. Chabala, and Vegard Martinsen. 2024. "Phosphorus Availability and Uptake following a Maize-Pigeon Pea Rotation under Conservation Agriculture" Agronomy 14, no. 1: 169. https://doi.org/10.3390/agronomy14010169
APA StylePhiri, M., Mulder, J., Chishala, B. H., Chabala, L. M., & Martinsen, V. (2024). Phosphorus Availability and Uptake following a Maize-Pigeon Pea Rotation under Conservation Agriculture. Agronomy, 14(1), 169. https://doi.org/10.3390/agronomy14010169




