Abstract
Brazil is one of the largest producers of pulses globally, and soybean ranks highly in terms of production. However, pests increase crop production costs and affect oilseed production and quality. Pests are primarily controlled by chemicals, leading to changes in insect pest populations. For example, secondary pests can become primary pests because of the selection of resistant insects and the elimination of natural enemies. Farmers have widely accepted biological control because of its high control efficacy and low environmental contamination risk. Two successful biological control programs in soybean used Telenomus podisi Ashmead (Hymenoptera: Platygastridae) to manage the Neotropical brown stink bug, Euschistus heros (Fabricius) (Hemiptera: Pentatomidae), and Trissolcus basalis (Wollaston, 1858) (Hymenoptera: Scelionidae) to manage the southern green stink bug, Nezara viridula (Linnaeus) (Hemiptera: Pentatomidae), when these pests were the most abundant in soybean-producing regions. To release parasitoids, rearing protocols must be developed in order to optimize production. This study evaluated the effect of temperature on the biological characteristics of T. podisi and T. basalis when fresh or frozen E. heros eggs were provided. Fifty fresh or previously frozen eggs were placed with parasitoids for 24 h in a climate chamber (25 ± 1 °C, 70 ± 10% RH, and 14-h photophase). At the end of this period, the eggs were transferred to other chambers and maintained at different temperatures (19, 22, 25, 28, 31, and 34 ± 1 °C, 70 ± 10% RH, with a 14-h photoperiod). The number of emerging adults from eggs parasitized by T. basalis was lower than that from eggs parasitized by T. podisi. Differences in T. basalis and T. podisi parasitism were observed depending on whether the E. heros eggs were frozen or fresh, but neither development nor emergence were affected. The duration of egg–adult development and the longevity of the two parasitoids were inversely proportional to the temperature increase. The sex ratio of T. basalis and T. podisi progeny was not affected by different temperatures or by the use of frozen or fresh eggs. The estimated number of both parasitoids’ generations corresponded with temperature, reaching 14–15 generations/cycle of soybean for Rio Verde, GO, and 12–14 generations/cycle for Barreiras, BA. Given these characteristics, we conclude that a biological control program using T. podisi can benefit large areas of soybean production by controlling the eggs of phytophagous bugs in soybean crops.
1. Introduction
Pulse crops are grown in Brazil, with soybeans (Glycine max L.) being the most widely grown and important export product. In 2021/2022, Brazil produced 125.55 million tons of soybeans, leading global production [1]. If losses from soybean pests, caused mainly by Plusinae, Noctuidae, and the Pentatomidae complex, can be reduced, production would be even higher.
The most common management method used to reduce pests in soybean cultivation is chemical control, which can cause problems in the agro-ecosystem, including increased insecticide resistance, environmental contamination, and elimination of natural enemies. These changes can favor insects that were previously considered secondary pests, causing them to become primary pests [2].
Phytophagous stink bugs (Pentatomidae) include several species that are major pests of soybean crops in Brazil. They cause severe damage to both the yield and quality of the harvested product [3,4]. Among the phytophagous stink bugs that damage soybean crops, the brown stink bug, Euschistus heros (Fabricius, 1794) (Hemiptera: Pentatomidae), is the most abundant species in the Neotropical region, including Brazil [5], Argentina, Paraguay, Panama, Uruguay, and Bolivia [3,6,7].
Unfortunately, the management of E. heros populations is limited by the availability of effective insecticides. Only six active chemical ingredients registered for stink bug control are available in Brazil, most of which show low control efficacy because of insecticide resistance. Initially, the expansion of soybean plants with expression of protein crystals from Bacillus thuringiensis (Bt) was predicted to reduce damage from the stink bug complex. Because Bt technology requires fewer insecticide applications to control caterpillars during the vegetative period of soybean crop development, it was believed that fewer sprays would preserve the beneficial entomofauna while suppressing stink bug populations, which start to appear in the crop at the end of the vegetative stage. However, in the 2018/2019 crop, an increase in the stink bug population and crop damage was observed. This can likely be explained because, in the absence of the caterpillar complex, stink bugs benefited from reduced competition.
Ideally, modern agriculture utilizes a multidisciplinary approach to manage pests as part of an integrated pest management (IPM) program. The use of augmentative biological control, through the mass release of a pest’s natural enemies, can reduce the pest population to levels below those causing economic damage [8]. In this context, the use of biological control agents at the onset of stink bug occurrence is strategic because of the limited pesticide applications, especially in areas using Bt soybean.
The egg parasitoid Telenomus podisi (Ashmead, 1893) (Hymenoptera: Scelionidae) has shown promising results when released towards the end of the soybean growing season, with E. heros, the most abundant bug in soybean crops, being its preferred host [9]. The release of T. podisi reaches a control efficacy of approximately 80% if released at the end of the vegetative period when the first stink bugs migrate to the crop and begin laying eggs (22).
Despite the potential of T. podisi in managing the stink bug complex in soybeans, some factors should be considered to ensure the feasibility of parasitoid use. One crucial factor is the absence of hosts for T. podisi during autumn and winter because there is no stink bug oviposition in the off-season. Therefore, it is necessary to develop mass-production methods for confined systems so that biological control agents are available for release at the time of pest occurrence in the field.
One method for successfully producing egg parasitoids during the off-season is to store host eggs in liquid nitrogen until needed, as demonstrated for Nezara viridula (Linnaeus, 1758) (Hemiptera: Pentatomidae) and E. heros [10,11]. Thus, parasitoids can be reared in large quantities in the off-season, to be released when stink bug oviposition occurs in soybeans. In addition to being able to mass rear parasitoids as needed, it is also necessary to determine the abiotic conditions that induce and regulate the processes of quiescence, hibernation, or diapause in egg parasitoids because manipulation of these processes can affect the efficiency of large-scale parasitoid production for biological control purposes [12,13]. However, few studies have documented the effects of abiotic factors on biological control agents. Therefore, this study sought to determine the thermal requirements of T. podisi reared from frozen and fresh E. heros eggs to support the release of parasitoids in pulse production fields. The thermal requirements of T. basalis were also determined for comparison with those of T. podisi.
2. Materials and Methods
2.1. Stink Bug Colony and Eggs
The rearing of E. heros followed a methodology similar to that described in [11]. The insects were kept in climatized rooms at a temperature of 25 ± 2 °C, with relative humidity (RH) of 70 ± 10%, and a 14-h photophase. The first adult stink bugs were collected in the field and kept in a separate room for the observation of natural parasitism. Subsequently, the selected adults were kept in plastic containers with one male for each female, with a maximum capacity of 50 couples/cage. The stink bugs were fed soybean pods, groundnuts, and sunflower grains. The pods were glued onto strips of sulfite paper and suspended on the cage wall. Ligustrum fruits (Ligustrum lucidum) were provided in the cage as supplemental food [11]. Moistened cotton was placed on top of the cage to provide water and maintain environmental humidity. After the beginning of the oviposition period, eggs were collected daily. Some were returned to the breeding cage, while the rest were stored in liquid nitrogen for use in parasitoid multiplication.
2.2. Egg Parasitoid Colony
Rearing of the stink bug egg parasitoids was conducted according to the methodology described in [14]. Telenomus podisi was maintained on E. heros eggs [15], while T. basalis was maintained on N. viridula eggs, which are considered the best hosts for the development of these parasitoids.
The stink bug eggs for parasitism were provided in transparent plastic flasks of approximately 20 cm in length. One end of the flask was closed with cotton wool and the other with a fine mesh screen to allow air into the tube. A thin layer of honey was placed in the upper inner portion of the flask as a food source, and moistened cotton wool was placed in the central and upper portions of the flask.
Parasitoid adults were introduced into the flasks at the time of emergence, and subsequently, egg masses were provided, with approximately 7000 eggs per tube. Parasitism was allowed for 24 h, after which new parasitoids were introduced. After parasitism, the egg masses were placed in plastic tubes for adult emergence (brood maintenance).
2.3. Bioecological Parameters of Telenomus podisi and Trissolcus basalis on Frozen and Fresh Euschistus Heros Eggs
Fresh E. heros eggs with up to 24 h of embryonic development (from the broodstock and eggs previously stored in liquid nitrogen) were used to evaluate parasitoid biology. The E. heros eggs were glued onto white cards and then transferred to glass tubes (8 cm high × 2 cm diameter) closed with a PVC-type plastic film. Newly emerged females of T. podisi or T. basalis were introduced at a proportion of 1 parasitoid female to 50 eggs. Over a 24-h period, parasitism was allowed in a climatized chamber at 25 ± 1 °C, 70 ± 10% relative humidity, and a 14-h photophase. After parasitism, the eggs were manually removed and placed in plastic bags, and sets of 20 cards with eggs were transferred to climatized chambers, maintained at 19, 22, 25, 28, 31, or 34 ± 1 °C; 70 ± 10% relative humidity; and a 14-h photophase for observation of parasitoid development. The females remained in the tubes at the same temperatures as described above and were observed daily to determine longevity. The eggs placed in the plastic bags also remained at the same temperatures and were observed daily to determine the duration of development (egg to adult), emergence (viability), the sex ratio (given by the formula: ), and the number of individuals per egg mass. To determine the duration of development (egg to adult), daily observations of T. podisi emergence were made. The parasitoid emergence was evaluated under a stereoscopic microscope by counting host eggs that had an exit hole.
A completely randomized design was used in a 2 × 2 × 6 factorial arrangement (parasitoid species × fresh or previously frozen eggs × temperatures) with 20 replications. The variables parasitism (%), emergence (%), sex ratio, and duration of egg–adult development (days) were subjected to analysis of variance (ANOVA), and the experimental residuals were subjected to the Shapiro–Wilk and Bartlett’s tests to verify normality and homogeneity of variances. Subsequently, the means were compared using Tukey’s test (p < 0.05). The ExpDes. package of the R computer application (version 3.5.0) (R Core Team, 2018) was used to perform the analyses of variance and comparison of means.
2.4. Determination of the Thermal Requirements and Estimation of the Number of Generations of Stink Bug Egg Parasitoids
Calculations of the thermal requirements for parasitoid development were estimated using the base temperature (Tb) and the thermal constant (K), which were obtained from the duration of the development period (egg–adult) at the tested temperatures. The development rates of T. basalis and T. podisi as a function of temperature were analyzed using linear and nonlinear models (Table 1). The parameters included in each of the models were estimated by the Levenberg–Marquardt method, using the minpack.lm package [16] of the R computer application (version 3.5) [17]. The model with the best fit was selected based on the chi-square test of adherence (χ2), the adjusted coefficient of determination (adjR2), the logarithm of maximum likelihood (LogLik), the sum of squared residuals (RSS), and Akaike’s information criterion (AIC) and the Bayesian information criterion (BIC) and their respective weights (wAIC and wBIC, respectively).

Table 1.
Models used to relate the rate of development to room temperature.
The number of parasitoid generations per year in soybean-producing regions was estimated based on the average annual temperature of these locations using the equation , where K is the thermal constant, Tm is the average temperature for each locality studied, Tb is the base temperature, and T is the time in days.
3. Results
3.1. Bioecological Parameters of Telenomus podisi and Trissolcus basalis Reared from Frozen and Fresh Euschistus Heros Eggs
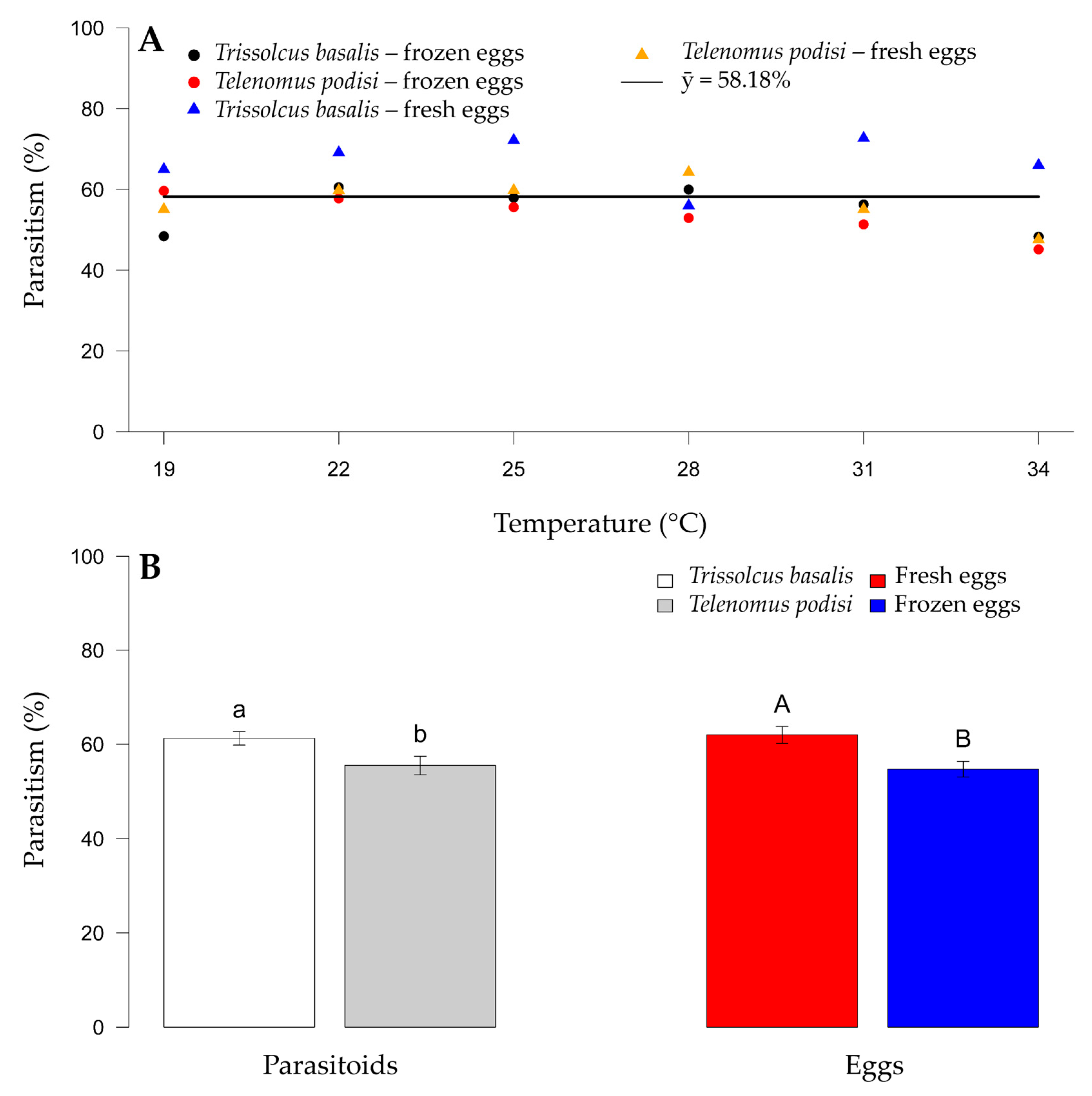
There was a mean parasitism rate of 58.18% for E. heros eggs, with no relationship between parasitoid species and temperature (Figure 1A). However, host egg type had an effect on the parasitism levels between species. Among the parasitoid species, T. basalis had a mean parasitism of 65.06%, and T. podisi had a significantly lower rate of 49.52%. A difference was also observed between fresh and frozen eggs, with parasitism being greater on fresh eggs for both species across temperatures (50.27 and 60.29% for T. basalis and T. podisi, respectively) (Figure 1B).
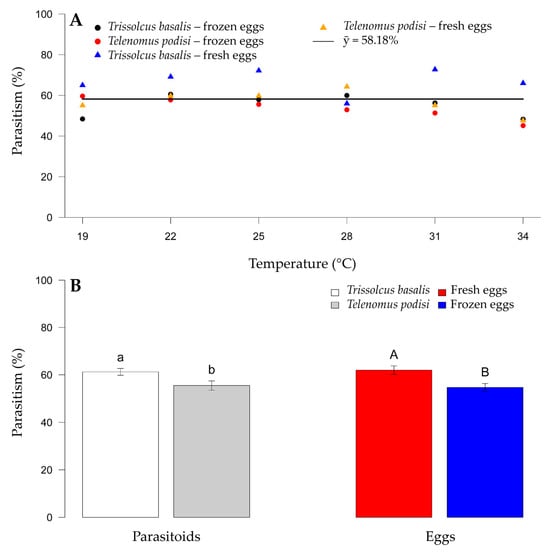
Figure 1.
Parasitism of Trissolcus basalis and Telenomus podisi on frozen or fresh eggs of Euschistus heros at different constant temperatures, under laboratory conditions. Average for each combination of parasitoid and type of egg (A) (continuous line). Comparison of parasitism between parasitoids and type of egg (B). Bars followed by the same letter, lowercase (comparison of parasitoid species) or capital letters (comparison of egg types), do not differ statistically according to the F test (p > 0.05).
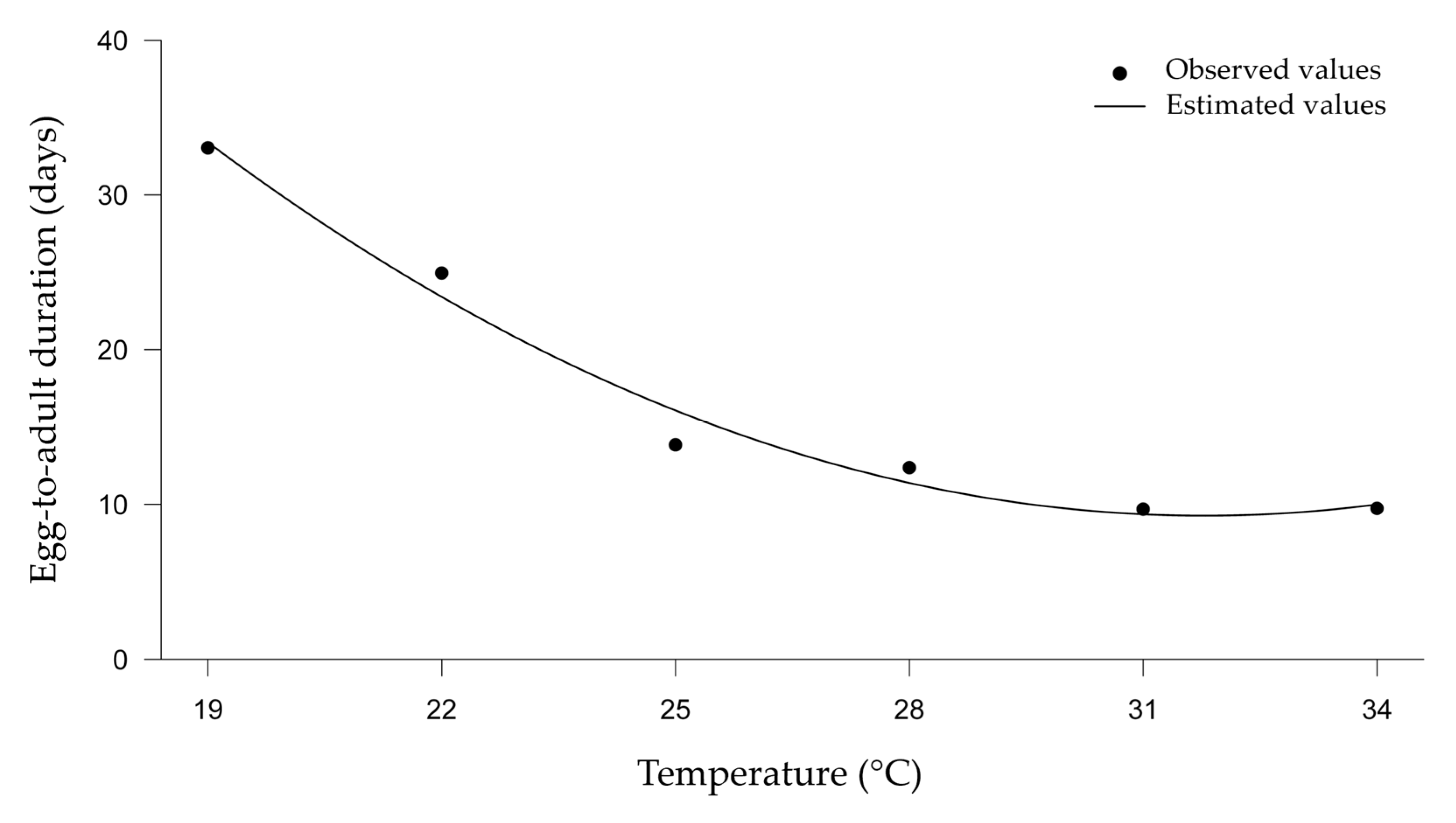
The duration of the egg-to-adult development of the two parasitoids was inversely proportional to the increase in temperature (Figure 2), and temperature was the only significant variable. The development of T. basalis and T. podisi on E. heros eggs showed no difference in any of the treatments compared at the same temperatures, showing that there was no difference in parasitoid development between fresh and frozen eggs (Figure 2).
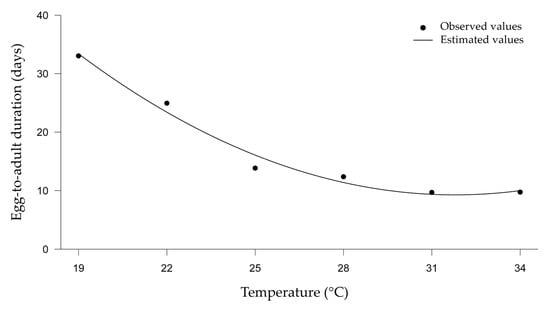
Figure 2.
Duration of egg–adult development (in days) of Trissolcus basalis and Telenomus podisi parasitizing frozen or fresh eggs of Euschistus heros at different constant temperatures, under laboratory conditions.
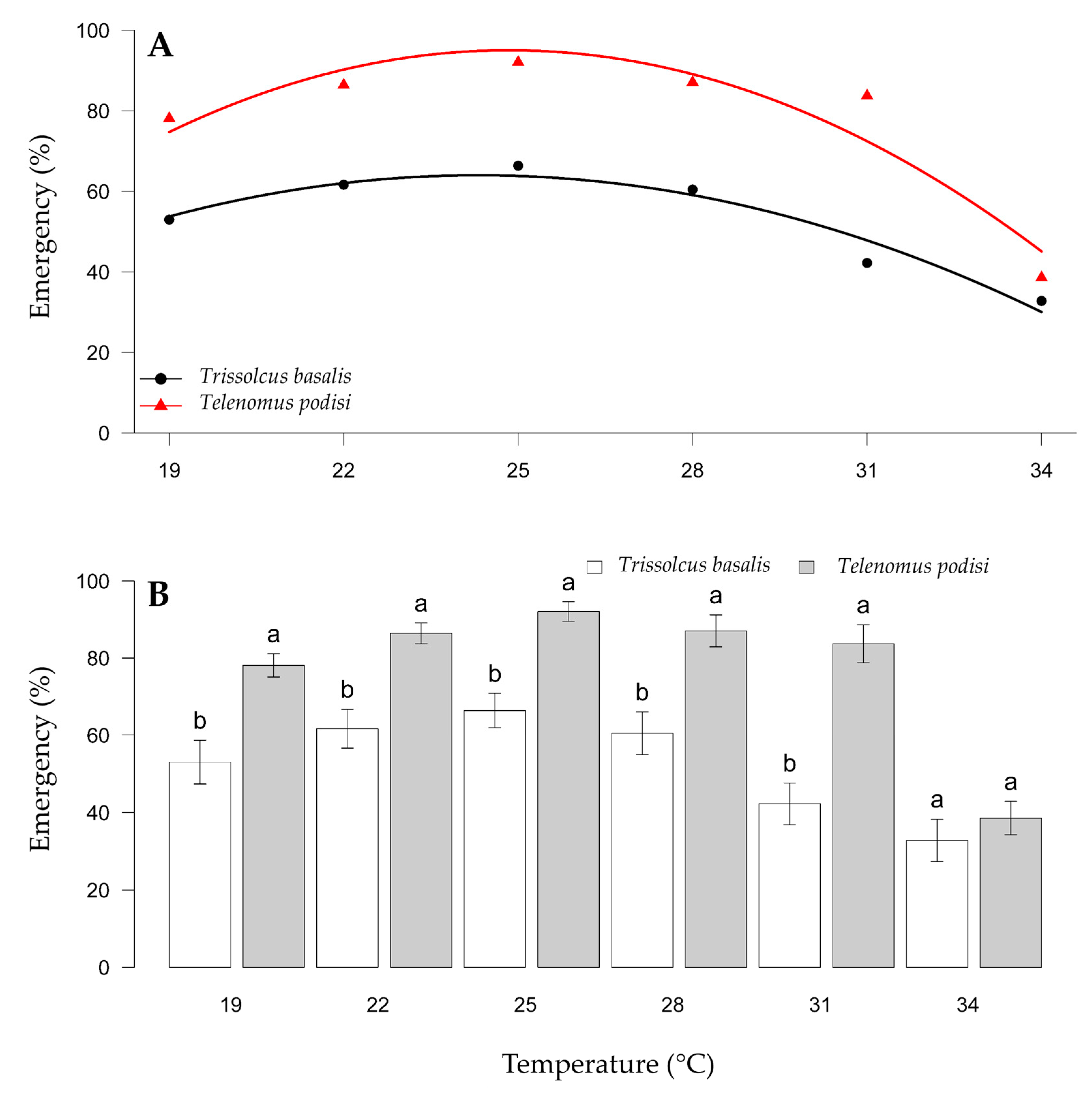
The viability of the parasitized eggs was affected by parasitoid species and temperature and was not influenced by whether the eggs were frozen or fresh. For biological control programs with the release of egg parasitoids, the objective is an emergence of >80% to achieve adequate control. The viability of eggs parasitized by T. basalis was lower than that of eggs parasitized by T. podisi (Figure 3A). The viability of T. basalis was higher between 22 and 28 °C, with adult emergence between 58.31% and 66.54%. The viability of eggs parasitized by T. podisi was >81% between 22 and 31 °C, with a variation of 81.79 (31 °C) to 97.5% (28 °C) between these temperatures (Figure 3B). Thus, the viability of T. podisi met the criteria to be an effective biological control agent, whereas the viability of T. basalis was lower.
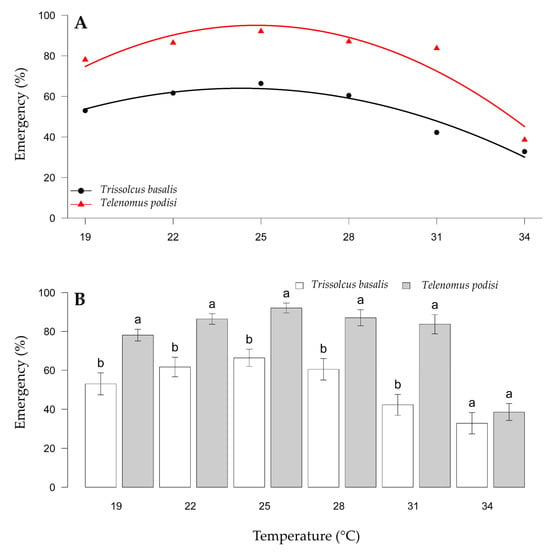
Figure 3.
Viability of Trissolcus basalis and Telenomus podisi in frozen or fresh eggs of Euschistus heros at different constant temperatures, under laboratory conditions. Regression analysis examining the temperature effect for each parasitoid (A). Comparison of emergence between parasitoids within each temperature (B). Averages with lowercase letters (comparison within each temperature) do not differ statistically according to the F test (p > 0.05).
There was no interaction among the factors evaluated (p > 0.05) for the sex ratio of the emerging adults. When the species were evaluated separately, they also did not differ significantly (p > 0.05), whereas the effect of temperature was observed for all biological parameters (p < 0.05). Because the sex ratio of parasitoids was not affected (average greater than 0.85) by any of the factors tested, there should be sufficient parasitoids of both sexes for reproduction following release.
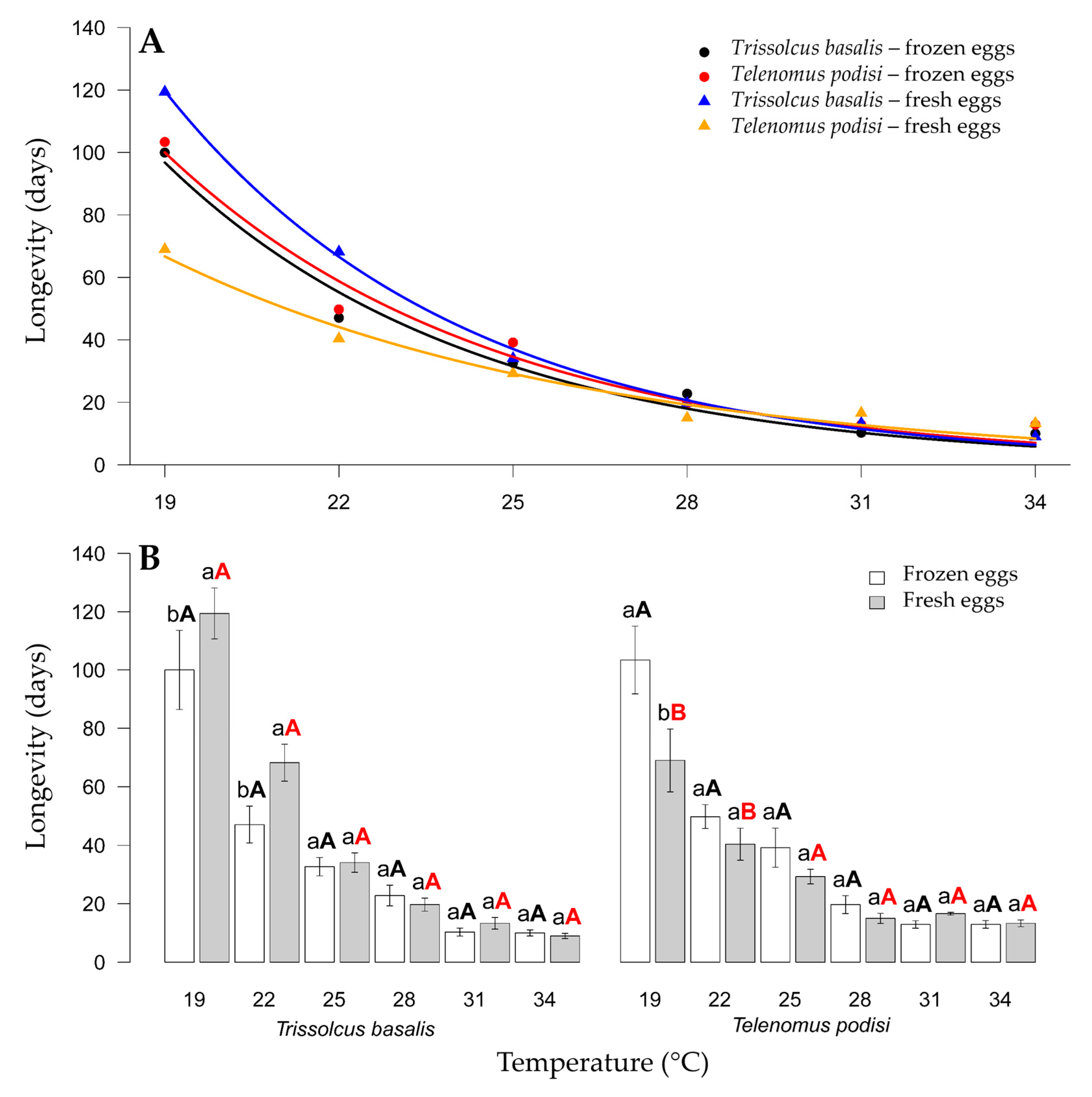
The longevity of T. basalis and T. podisi parasitizing frozen or fresh E. heros eggs at different constant temperatures was inversely proportional to the temperature increase. For T. basalis, only the treatments at 19 °C and 22 °C significantly differed, with longevities of 100 and 119.4 days, respectively, for frozen and fresh E. heros eggs. In contrast, T. podisi only had a difference in female longevity at 22 °C, with 116.0 and 55.00 days for frozen and fresh E. heros eggs, respectively (Figure 4).
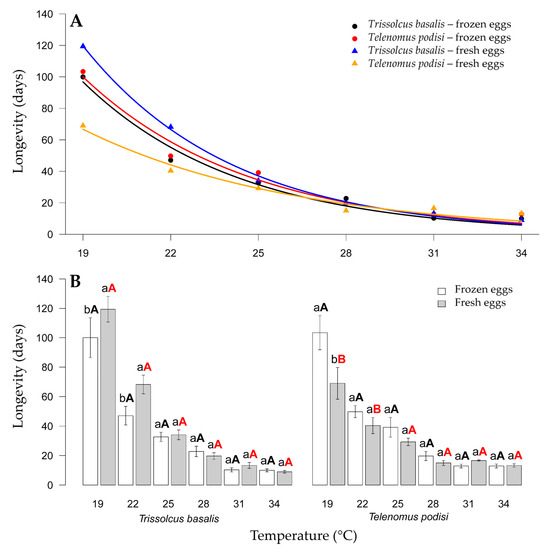
Figure 4.
Longevity (days) of Trissolcus basalis and Telenomus podisi parasitizing frozen or fresh eggs of Euschistus heros at different constant temperatures, under laboratory conditions. Regression analysis for each combination of parasitoids and egg types (A). Comparison of parasitoid longevity in each combination of temperature and type of egg (B). Averages with lowercase letters compare eggs within species, black uppercase letters compare species parasitizing frozen eggs, and red uppercase letters compare species parasitizing fresh eggs. In all cases, the F test was applied for statistical differences (p < 0.05).
3.2. Thermal Requirements Based on Temperature-Dependent Development Ratio and Estimated Number of Generations of Stink Bug Egg Parasitoids
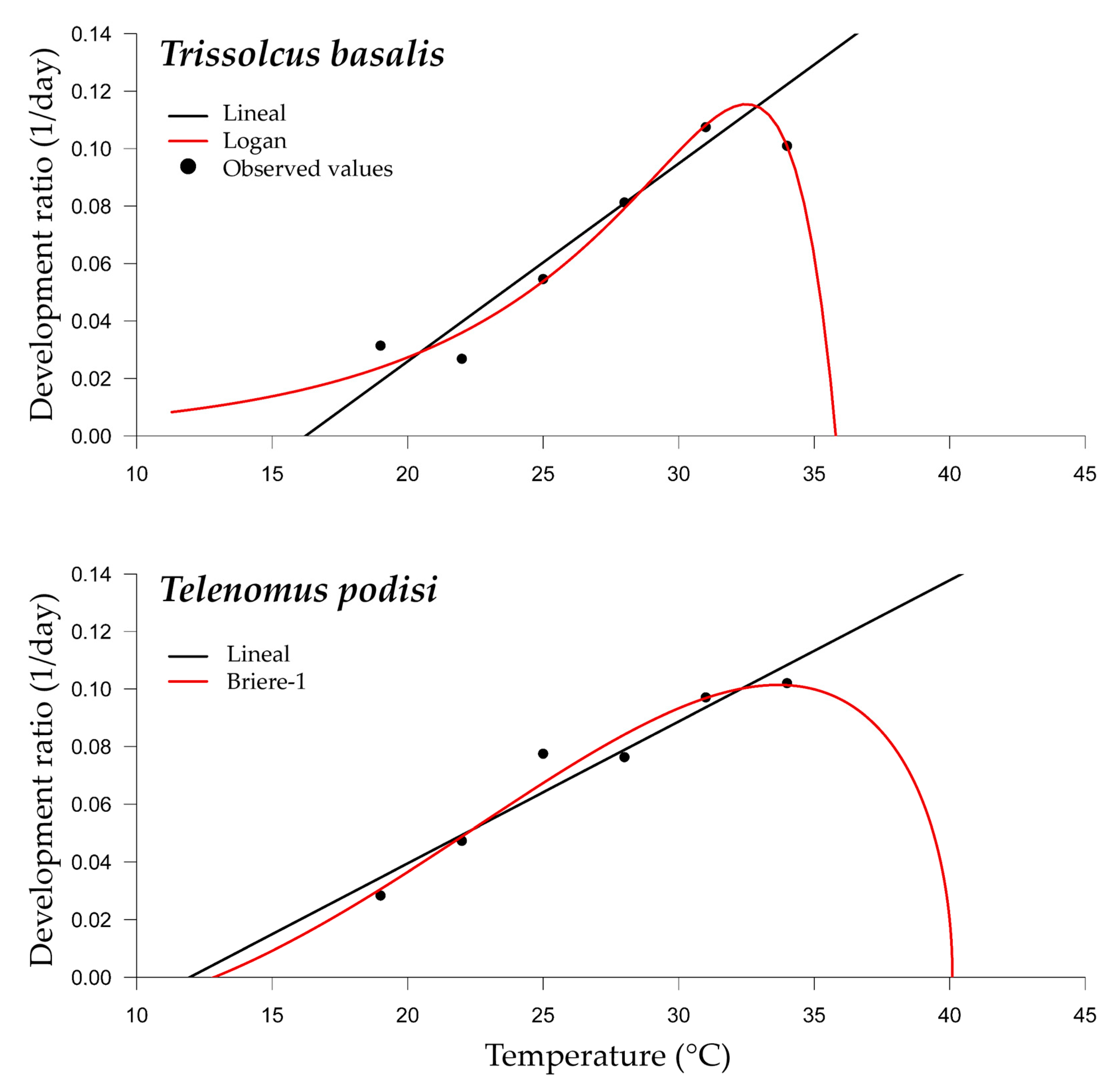
For the development rate from egg to adult for T. basalis and T. podisi, we determined the lower thermal threshold of development (Tb) and the thermal constant (K) for fresh and frozen E. heros eggs. The linear model had an adjusted R2 higher than 0.89 for T. basalis, with a lower temperature threshold (Tb) of 16.23 °C and a thermal constant (K) of 145.22 °days. For T. podisi, the adjusted R2 was 0.91, with a lower temperature threshold (Tb) of 11.96 °C and thermal constant (K) of 203.47 °days (Table 2 and Figure 5).

Table 2.
Parameters of the linear regression model and adjusted R2.
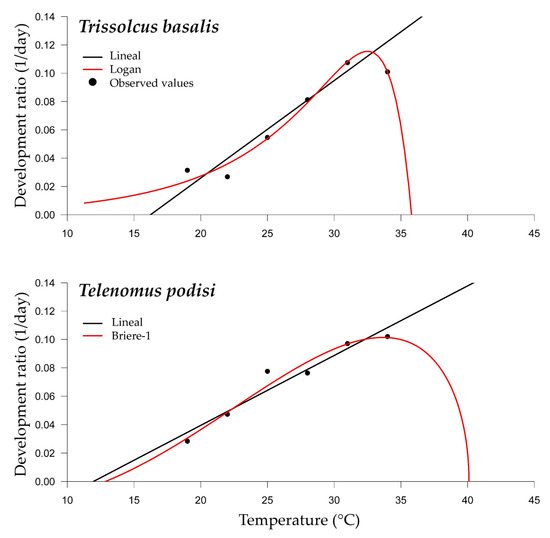
Figure 5.
Temperature-dependent nonlinear developmental regression models for Trissolcus basalis and Telenomus podisi in Euschistus heros eggs under laboratory conditions.
Among the nonlinear models tested, the Logan model, for T. basalis, and Briere-1 model, for T. podisi, presented the best adjustments for predicting the development data (Table 3 and Table 4 and Figure 5). The optimal (Topt) and upper (Tmax) thermal thresholds for development estimated for each parasitoid species were different (Table 4). For T. basalis, the Topt estimate was 32.5 °C, while for T. podisi, it was 40.0 °C. The Tmax for T. bassalis was 35.8 °C, lower than that of T. podisi, which was estimated at 40.1 °C.

Table 3.
Criteria for selection of nonlinear temperature-dependent regression models for Trissolcus basalis and Telenomus podisi in eggs of Euschistus heros.

Table 4.
Parameters of the nonlinear regression models of the temperature-dependent development ratio for Trissolcus basalis and Telenomus podisi in eggs of Euschistus heros, under laboratory conditions.
4. Discussion
The development of both T. basalis and T. podisi on E. heros eggs occurred at all tested temperatures and increased with warmer temperatures. Thus, it is suggested that these parasitoid species can be successfully used in biological control programs throughout the temperature ranges in which crops are grown in Brazil. Although the current assessment was conducted under laboratory conditions at constant temperatures, the authors of [22] found no differences in the biological characteristics of Trichogramma galloi (Zucchi) (Hymenoptera: Trichogrammatidae), an egg parasitoid, when reared at constant or fluctuating temperatures. These results indicate that laboratory studies can support field releases, because the insects showed performance similar to that in the laboratory, though caution should be applied.
It is still necessary to evaluate other biological parameters that depend on temperature to project parasitoid performance in the field [15,23]. Host quality is the main factor influencing the sex ratio, which can occur in two different ways. One involves the recognition of hosts of different qualities and then the laying of eggs (male or female) according to the host quality, and the other, which occurs after oviposition, allows the sex ratio to be determined by the developing progeny in response to host quality, with both male and female eggs laid, but one sex surviving competition [24,25].
Another critical factor for parasitoids is the presence of endosymbionts in the female reproductive organs, which may limit reproduction by inducing reproductive incompatibility, feminization, and parthenogenesis [26]. However, for the egg parasitoids, T. basalis and T. podisi, this needs to be documented before sex ratio verification because the presence of Proteobacteria can lead to erroneous and conflicting results [27]. After the biological control agents are released in plant production fields, they are subjected to different abiotic factors, which may interfere with the sex ratio of the parasitoids and affect the efficacy of pest control.
The estimated number of generations for both parasitoids corresponded to the temperature in warmer areas, reaching 14 to 15 generations/cycle for Rio Verde, GO, and 12 to 14 generations/cycle for Barreiras, BA. Despite using monthly averages for these locations and considering diurnal and nocturnal thermal variations, the modeled results were very close to those obtained under field conditions. Thus, it is likely that similar simulations can be performed for any soybean-growing region of interest, where parasitoids can be released. Temperatures in the regions of Rio Verde, GO, and Barreiras, BA seldom reach the lower threshold (Tb) for insects and are often above 24 °C. The amplitude of variations around the averages could influence the parasitoids’ biological parameters, resulting in higher development rates or lower viability than those obtained in the laboratory; therefore, additional research on the seasons of parasitoid release should be conducted.
The laboratory-based determination of biological characteristics and thermal requirements provides relevant information for implementing and maintaining a biological control program. The results suggest that released parasitoids can develop throughout the year under field conditions. Furthermore, the number of generations of these parasitoids indicates that they could significantly impact both stink bug populations, but other variables should be further considered. The reproductive potential should be determined, and the parasitoid’s ability to search for host eggs and its habitat preference should be documented because these factors may affect the functional response under field conditions. These effects may be enhanced because of the heterogeneity of areas under soybeans, especially in relation to plant architecture (number of leaves and plant height) compared with other agricultural ecosystems.
5. Conclusions
Our results for the thermal requirements were based on the lower thermal threshold of development (Tb) and the thermal constant (K) for fresh and frozen E. heros eggs for T. basalis, with a lower temperature threshold (Tb) of 16.23 °C and a thermal constant (K) of 145.22 °days. For T. podisi, the lower temperature threshold (Tb) of 11.96 °C and thermal constant (K) of 203.47 °days was determined. The estimated number of generations for T. basalis and T. podisi corresponded to the temperatures in warmer growing areas, reaching 14 and 15 generations/cycle for Rio Verde, GO, and 12 and 14 generations/cycle for Barreiras, BA, respectively.
Author Contributions
Conceptualization, R.C.d.O.; methodology, R.C.d.O.; P.H.P.I., D.P., J.R.d.C., W.W.H. and B.A.Z.S.; software, J.R.d.C.; validation, R.C.d.O., P.H.P.I., D.P., J.R.d.C., W.W.H. and B.A.Z.S.; formal analysis, R.C.d.O., P.H.P.I. and J.R.d.C. investigation, R.C.d.O.; resources, R.C.d.O., D.P., W.W.H. and B.A.Z.S.; data curation, R.C.d.O.; writing—original draft preparation, R.C.d.O., P.H.P.I. and J.R.d.C.; writing—review and editing, R.C.d.O., P.H.P.I., D.P., J.R.d.C., W.W.H. and B.A.Z.S.; visualization, R.C.d.O., P.H.P.I., D.P., J.R.d.C., W.W.H. and B.A.Z.S.; supervision, R.C.d.O.; project administration, R.C.d.O.; funding acquisition, R.C.d.O. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—finance code 001; Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP (processes number 2018/02317-5, 2019/10736-0 and 2018/19782-2); Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (304126/2019-5) Regiane C. de Oliveira hold a CNPq fellowship; and Bruno Zachrisson fellowship of the “Sistema Nacional de Investigación” (SNI)—(Secretaria Nacional de Ciencia, Tecnología e Innovación—SENACYT), for the support in the research in biological control of insect-pest programs in Panama by the grant number: SNI-19-2020-062-2023.
Data Availability Statement
All datasets used or analyzed during this study are included in this article.
Acknowledgments
The authors would like to acknowledge the Department of Entomology and Plant Pathology at Oklahoma State University for all the support given to this research and the financial support provided by the following agencies: Coordenação de Aperfeiçoamento de Pes- soal de Nível Superior—Brasil (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- CONAB. Companhia Nacional de Abastecimento. Acompanhamento de Safra Brasileira: Grãos. V. 12—Safra 2021/22–N.12–12° levantamento, Setembro 2022. Brasília: Conab. Available online: https://www.conab.gov.br/info-agro/safras/graos/boletim-da-safra-de-graos/item/download/44171_1d9f893d78f593b07d41887104acc43f (accessed on 21 November 2022).
- Bueno, A.F.; Paula-Moraes, S.V.; Gazzoni, D.L.; Pomari, A.F. Economic Thresholds in Soybean-Integrated Pest Management: Old Concepts, Current Adoption, and Adequacy. Neotrop. Entomol. 2013, 42, 439–447. [Google Scholar] [CrossRef]
- Panizzi, A.R. History and Contemporary Perspectives of the Integrated Pest Management of Soybean in Brazil. Neotrop. Entomol. 2013, 42, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Tuelher, E.S.; Silva, H.; Hirose, E.; Guedes, R.N.C.; Oliveira, E. Competition between the phytophagous stink bugs Euschistus heros and Piezodorus guildinii in soybeans. Pest Manag. Sci. 2016, 72, 1837–1843. [Google Scholar] [CrossRef]
- Krinski, D.; Favetti, B.M.; De Lima, A.G.; Brum, T.R. Oviposition preference of the neotropical brown stink bug Euschistus heros on artificial substrates of different colors. Ciência Rural 2013, 43, 2185–2190. [Google Scholar] [CrossRef]
- Bueno, A.d.F.; Bortolotto, O.C.; Pomari-Fernandes, A.; França-Neto, J.d.B. Assessment of a more conservative stink bug economic threshold for managing stink bugs in Brazilian soybean production. Crop. Prot. 2015, 71, 132–137. [Google Scholar] [CrossRef]
- Panizzi, A.R. Growing Problems with Stink Bugs (Hemiptera: Heteroptera: Pentatomidae): Species Invasive to the U.S. and Potential Neotropical Invaders. Am. Entomol. 2015, 61, 223–233. [Google Scholar] [CrossRef]
- Van Lenteren, J.C.; Bolckmans, K.; Köhl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. BioControl 2017, 62, 1–25. [Google Scholar] [CrossRef]
- Silva, G.V.; Bueno, A.D.F.; Neves, P.M.O.J.; Favetti, B.M. Biological Characteristics and Parasitism Capacity of Telenomus podisi (Hymenoptera: Platygastridae) on Eggs of Euschistus heros (Hemiptera: Pentatomidae). J. Agric. Sci. 2018, 10, 210–220. [Google Scholar] [CrossRef]
- Doetzer, A.K.; Foerster, L. Storage of Pentatomid Eggs in Liquid Nitrogen and Dormancy of Trissolcus basalis (Wollaston) and Telenomus podisi Ashmead (Hymenoptera: Platygastridae) Adults as a Method of Mass Production. Neotrop. Entomol. 2013, 42, 534–538. [Google Scholar] [CrossRef]
- Oliveira, R.C. Utilização de Telenomus podisi no manejo de Euschistus heros. In Controle Biológico com Parasitoides e Predadores na Agricultura Brasileira; Parra, J.R.P., Pinto, A.S., Nava, D.E., Oliveira, R.C., Diniz, A.J.F., Eds.; FEALQ: Piracicaba, Brazil, 2021; pp. 235–247. [Google Scholar]
- Pastori, P.L.; Zanuncio, J.C.; Pereira, F.F.; Pratissoli, D.; Cecon, P.R.; Serrão, J.E. Temperatura e tempo de refrigeração de pupas de Anticarsia gemmatalis (Lepidoptera: Noctuidae) afetam parâmetros biológicos de Trichospilus diatraeae (Hymenoptera: Eulophidae)? Semin. Ciências Agrárias 2013, 34, 1493–1508. [Google Scholar] [CrossRef]
- Ghosh, E.; Ballal, C.R. Effect of age dependent cold storage of factitious host Corcyra cephalonica (Stainton) (Lepidoptera: Pyralidae) for their continuous production and Trichogramma chilonis (Ishii) (Hymenoptera: Trichogrammatidae) rearing. J. Asia Pac. Entomol. 2017, 20, 928–934. [Google Scholar] [CrossRef]
- Corrêa-Ferreira, B.S. Criação Massal do Percevejo Verde Nezara viridula (L.); (EMBRAPA-CNPSo. Documentos, 11); EMBRAPA-CNPSo: Londrina, Brazil, 1985; 16p. [Google Scholar]
- Parra, L.M.; de Carvalho, J.R.; Hoback, W.W.; de Oliveira, R.C. Optimizing Mass Rearing of the Egg Parasitoid, Telenomus podisi, for Control of the Brown Stink Bug, Euschistus heros. Insects 2023, 14, 435. [Google Scholar] [CrossRef]
- Elzhov, T.V.; Mullen, K.M.; Spiess, A.-N.; Bolker, B. Package minpack.lm: R Interface to the Levenberg-Marquardt Nonlinear Least-Squares Algorithm Found in MINPACK, Plus Support for Bounds; R Package Version 1.2-4. 2023. Available online: https://cran.r-project.org/web/packages/minpack.lm/minpack.lm.pdf (accessed on 18 October 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 19 August 2019).
- Campbell, A.; Frazer, B.D.; Gilbert, N.; Gutierrez, A.P.; Mackauer, M. Temperature Requirements of Some Aphids and Their Parasites. J. Appl. Ecol. 1974, 11, 431–438. [Google Scholar] [CrossRef]
- Briere, J.-F.; Pracros, P.; Le Roux, A.-Y.; Pierre, J.-S. A Novel Rate Model of Temperature-Dependent Development for Arthropods. Environ. Entomol. 1999, 28, 22–29. [Google Scholar] [CrossRef]
- Logan, J.A.; Wollkind, D.J.; Hoyt, S.C.; Tanigoshi, L.K. An Analytic Model for Description of Temperature Dependent Rate Phenomena in Arthropods 1. Environ. Entomol. 1976, 5, 1133–1140. [Google Scholar] [CrossRef]
- Lactin, D.J.; Holliday, N.J.; Johnson, D.L.; Craigen, R. Improved Rate Model of Temperature-Dependent Development by Arthropods. Environ. Entomol. 1995, 24, 68–75. [Google Scholar] [CrossRef]
- Cônsoli, F.L.; Parra, J.R.P. Effects of constant and alternating temperatures on Trichogramma galloi Zucchi (Hym., Trichogrammatidae) biology II.—Parasitism capacity and longevity. J. Appl. Entomol. 1995, 119, 667–670. [Google Scholar] [CrossRef]
- Pratissoli, D.; Parra, J.R.P. Desenvolvimento e exigências térmicas de Trichogramma pretiosum Riley, criados em duas traças do tomateiro. Pesqui. Agropecuária Bras. 2000, 35, 1281–1288. [Google Scholar] [CrossRef][Green Version]
- Zago, H.B.; Pratissoli, D.; Barros, R.; Godim, M.G.C., Jr. Biologia e exigências térmicas de Trichogramma pratissolii Querino & Zucchi (Hymenoptera: Trichogrammatidae) em hospedeiros alternativos. Neotrop. Entomol. 2006, 35, 377–381. [Google Scholar] [CrossRef][Green Version]
- Vinson, S.B. Comportamento de seleção hospedeira de parasitóides de ovos, com ênfase na família Trichogrammatidae. In Trichogramma e o Controle Biológico; Parra, J.R.P., Zucchi, R.A., Eds.; FEALQ: Piracicaba, Brazil, 1997; pp. 67–119. [Google Scholar]
- Werren, J.H. Biology of Wolbachia. Annu. Rev. Entomol. 1997, 42, 587–609. [Google Scholar] [CrossRef]
- Stouthamer, R.; Breeuwer, J.A.J.; Luck, R.F.; Werren, J.H. Molecular identification of microorganisms associated with parthenogenesis. Nature 1993, 361, 66–68. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).