Phenological Growth Stages of Abelmoschus manihot: Codification and Description According to the BBCH Scale
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Seed Germination and Seedling Establishment
2.3. Field Experiment and Phenological Observations
2.4. Statistical Analyses
3. Results
3.1. Principal Growth Stages
| Phenophase | Two-Digit Code | Three-Digit Code | Description | Period |
|---|---|---|---|---|
| 0 | 00 | 000 | Dry/inactive seed or seed dressing (Figure 2a) | Late February |
| 01 | 001 | Beginning of seed imbibition (Figure 2b) | ||
| 03 | 003 | Seed imbibition complete (Figure 2c) | ||
| 05 | 005 | Radicle emerged from the seed (Figure 2d) | ||
| 06a | 006a | Elongation of the radicle, no root hair (Figure 2e) | ||
| 06b | 006b | Formation of root hairs (Figure 2f) | ||
| 07 | 007 | Hypocotyl with cotyledons breaking through seed coat (Figure 2g) | ||
| 08 | 008 | Hypocotyl with cotyledons outside seed coat (Figure 2h) | ||
| 09a | 009a | Emergence: Cotyledons break through the soil surface, folded cotyledons (Figure 2i) | ||
| 09b | 009b | Emergence: elongation of hypocotyl, folded cotyledons (Figure 2j) | ||
| 09c | 009c | Emergence: cotyledons unfold slightly (Figure 2k) | ||
| 1 | 10 | 100 | Cotyledons completely unfolded (Figure 3a) | Early March |
| 11a | 101a | First leaf on main stem emergence (Figure 3b) | ||
| 11b | 101b | The first leaf starts to grow or elongate (Figure 3c) | ||
| 11c | 101c | The first leaf on the main stem unfolded (Figure 3d) | ||
| 12 | 102 | The second leaf on the main stem unfolded (Figure 3e) | ||
| 13 | 103 | The third leaf on the main stem unfolded (Figure 3f) | ||
| 14 | 104 | The fourth leaf on the main stem unfolded (Figure 3g) | ||
| 15 | 105 | The fifth leaf on the main stem unfolded (Figure 3h) | Late-March | |
| 19 | 109 | Ninth or more leaves on the main stem unfolded | ||
| 119 | 19th or more leaves on the main stem unfolded (Figure 3j) | Mid-April | ||
| 121 | The first leaf on the side shoots unfolded | |||
| 122 | The second leaf on the side shoots unfolded | |||
| 123 | The third leaf on the side shoots unfolded | |||
| 129 | Ninth or more leaves on side shoots unfolded (Figure 3j) | Late-April | ||
| 2 | 21 | 201 | The first side shoot is visible (Figure 3h) | Late-March |
| 22 | 202 | The second side shoots visible | ||
| 23 | 203 | The third side shoots visible (Figure 3i) | ||
| 25 | 205 | The fifth side shoots visible | ||
| 27 | 207 | The seventh side shoots visible (Figure 3j) | ||
| 29 | 209 | Ninth or more side shoots are visible (Figure 3m). | Late April | |
| 3 | 31 | 301 | Main stem up to 20 cm long (Figure 3i). | Late April |
| 32 | 302 | Main stem up to 50 cm long (Figure 3j). | ||
| 33 | 303 | Main stem up to 70 cm long (Figure 3k) | Mid-June | |
| 34 | 304 | Main stem up to 90 cm long (Figure 3m) | ||
| 35 | 305 | Main stem up to 120 cm long (Figure 3n). | Late June | |
| 37 | 307 | Main stem up to 200 cm long (Figure 3o). | Late August | |
| 39 | 309 | Maximum main stem length reached up to 240 cm long. | Late October | |
| 5 | 50 | 500 | The first floral buds on the main stem are visible (Figure 4a). | Mid-May |
| 51 | 501 | Flower bud swelling (Figure 4b). | ||
| 53 | 503 | The flower bud continues to swell, stigma visible inside the petals (Figure 4c). | ||
| 54 | 504 | Flower bud and flower pedicel elongate (Figure 4d). | ||
| 55 | 505 | First individual flowers visible until close (Figure 4e). | Late May | |
| 56 | 506 | The petals begin to turn yellow (Figure 4f). | ||
| 57 | 507 | The petals break through the sepals (Figure 4g). | ||
| 59 | 509 | The first flower petals visible, the petals elongated quickly, flower bud began to open. The first flower will blossom the next day (Figure 4h). | Early June | |
| 520 | First floral buds on side shoots visible | Late June | ||
| 529 | The first flower on side shoots will blossom in the next day. | |||
| 6 | 61 | 601 | First flower opening and fruit set on the main stem (Figure 5a). | Early June |
| 62 | 602 | Second flower opening and fruit set on the main stem. | ||
| 63 | 605 | Fifth flower opening and fruit set on the main stem. | ||
| 69 | 609 | Ninth or more flowers opening and fruit set on the main stem (Figure 5b). | ||
| 619 | 19th flower opening and fruit set on the main stem (Figure 5d). | |||
| 621 | First flower opening and fruit set on the side shoots. | |||
| 629 | Ninth or more flowers opening and fruit set on the side shoots (Figure 5e). | |||
| 7 | 70 | 700 | First flower on the main stem drops and fruit expose. | Early June |
| 71 | 701 | First fruit on the main stem reach the final size (Figure 5b). | Early July | |
| 72 | 702 | Second fruit on the main stem reach the final size. | ||
| 73 | 703 | Third fruit on the main stem reaches the final size. | ||
| 79 | 709 | Ninth or more fruits on the main stem reach the final size. | ||
| 719 | 19th fruit on the main stem reach the final size. | Mid-July | ||
| 721 | The first fruit on the side shoots reach the final size. | |||
| 722 | The second fruit on the side shoots reach the final size. | |||
| 723 | The third fruit on the side shoots reach the final size. | |||
| 729 | Ninth or more fruits on the side shoots reach the final size (Figure 5e) | |||
| 8 | 81 | 801 | 10% of fruits have a yellow pod. The seed coat becomes brown and hard. | Early August |
| 83 | 803 | 30% of fruits have a yellow pod. The seed coat becomes brown and hard. | ||
| 85 | 805 | 50% of fruits have a yellow pod. Seed coat becomes brown and hard (Figure 5g) | ||
| 87 | 807 | 90% of fruits have yellow pods. The seed coat becomes brown and hard. | ||
| 89 | 809 | All of the fruit pod seed coat becomes brown and hard. | ||
| 9 | 91 | 901 | All fruits open, the fruit pod becomes brown and hard, and the leaf turns yellow (Figure 5g). | Midder December |
| 95 | 905 | 50% of leaves fall. | Late December | |
| 96 | 906 | All leaves are brown. | ||
| 97 | 907 | All leaves fall, and aboveground parts dead (Figure 5h) | Late January |
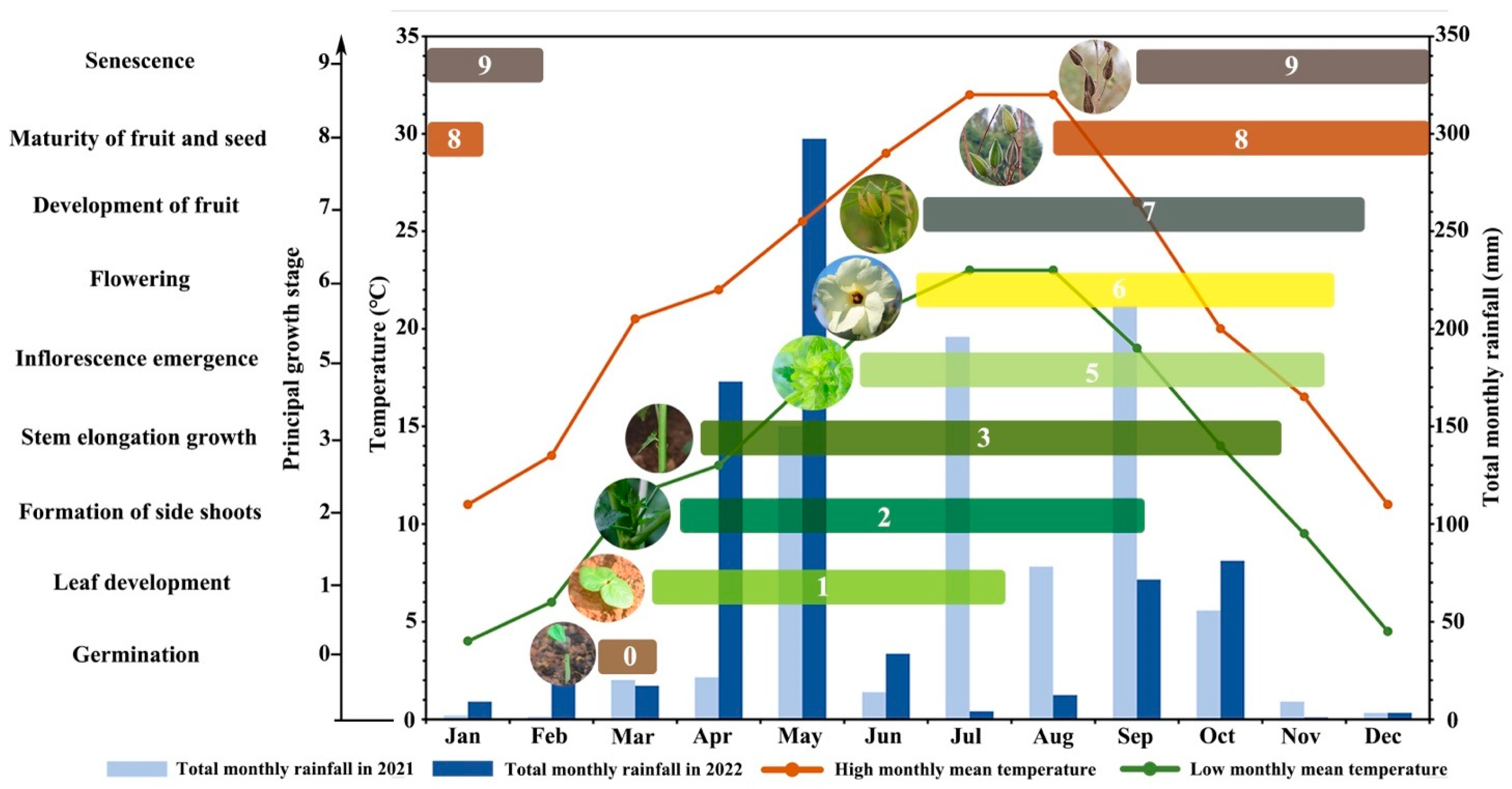




3.2. Specific Growth Stages
3.2.1. Principal Growth Stage 0: Germination
3.2.2. Principal Growth Stage 1: Leaf Development
3.2.3. Principal Growth Stage 2: Formation of Side Shoots
3.2.4. Principal Growth Stage 3: Shoot Development (Main Shoot)
3.2.5. Principal Growth Stage 5: Inflorescence Emergence
3.2.6. Principal Growth Stage 6: Flowering
3.2.7. Principal Growth Stage 7: Fruit and Seed Development
3.2.8. Principal Growth Stage 8: Maturity of Fruit and Seed
3.2.9. Principal Growth Stage 9: Senescence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDonough MacKenzie, C.; Gallinat, A.S.; Zipf, L. Low-cost Observations and Experiments Return a High Value in Plant Phenology Research. Appl. Plant Sci. 2020, 8, e11338. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, F.-M. Phenology in Agriculture and Horticulture. In Phenology: An Integrative Environmental Science; Schwartz, M.D., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 539–561. ISBN 978-94-007-6925-0. [Google Scholar]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A Decimal Code for the Growth Stages of Cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Meier, U.; Bleiholder, H.; Buhr, L.; Feller, C.; Hack, H.; Heß, M.; Lancashire, P.D.; Schnock, U.; Stauß, R.; van den Boom, T.; et al. The BBCH System to Coding the Phenological Growth Stages of Plants, History and Publications. J. Für Kult. 2009, 61, 41–52. [Google Scholar]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants; Blackwell Wissenschafts-Verlag: Berlin, Germany, 1997; ISBN 978-38-263-3152-7. [Google Scholar]
- Lancashire, P.D.; Bleiholder, H.; Boom, T.V.D.; Langelüddeke, P.; Stauss, R.; Weber, E.; Witzenberger, A. A Uniform Decimal Code for Growth Stages of Crops and Weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- Fadón, E.; Herrero, M.; Rodrigo, J. Flower Development in Sweet Cherry Framed in the BBCH Scale. Sci. Hortic. 2015, 192, 141–147. [Google Scholar] [CrossRef]
- Feldmann, F.; Rutikanga, A. Phenological Growth Stages and BBCH-Identification Keys of Chilli (Capsicum annuum L., Capsicum chinense JACQ., Capsicum baccatum L.). J. Plant Dis. Prot. 2021, 128, 549–555. [Google Scholar] [CrossRef]
- Kim, Y.S.; Park, C.S.; Lee, D.Y.; Lee, J.S.; Lee, S.H.; In, J.G.; Hong, T.K. Phenological Growth Stages of Korean Ginseng (Panax ginseng) According to the Extended BBCH Scale. J. Ginseng Res. 2021, 45, 527–534. [Google Scholar] [CrossRef]
- Vârban, R.; Ona, A.; Stoie, A.; Vârban, D.; Crișan, I. Phenological Assessment for Agronomic Suitability of Some Agastache Species Based on Standardized BBCH Scale. Agronomy 2021, 11, 2280. [Google Scholar] [CrossRef]
- Paradinas, A.; Ramade, L.; Mulot-Greffeuille, C.; Hamidi, R.; Thomas, M.; Toillon, J. Phenological Growth Stages of ‘Barcelona’ Hazelnut (Corylus avellana L.) Described Using an Extended BBCH Scale. Sci. Hortic. 2022, 296, 110902. [Google Scholar] [CrossRef]
- Niedbała, G.; Kurek, J.; Świderski, B.; Wojciechowski, T.; Antoniuk, I.; Bobran, K. Prediction of Blueberry (Vaccinium corymbosum L.) Yield Based on Artificial Intelligence Methods. Agriculture 2022, 12, 2089. [Google Scholar] [CrossRef]
- Luan, F.; Wu, Q.; Yang, Y.; Lv, H.; Liu, D.; Gan, Z.; Zeng, N. Traditional Uses, Chemical Constituents, Biological Properties, Clinical Settings, and Toxicities of Abelmoschus manihot L.: A Comprehensive Review. Front. Pharmacol. 2020, 11, 1068. [Google Scholar] [CrossRef] [PubMed]
- Prabawardani, S. Morphological Diversity and the Cultivation Practice of Abelmoschus manihot in West Papua, Indonesia. Biodiversitas J. Biol. Divers. 2016, 17, 894–899. [Google Scholar] [CrossRef]
- Preston, S.R.; Heller, J.; Engels, J. Aibika/Bele, Abelmoschus manihot (L.) Medik; IPK and IPGRI: Rome, Italy, 1998; ISBN 978-92-904-3381-1. [Google Scholar]
- Sutar, S.; Patil, P.; Aitawade, M.; John, J.; Malik, S.; Rao, S.; Yadav, S.; Bhat, K.V. A New Species of Abelmoschus Medik. (Malvaceae) from Chhattisgarh, India. Genet. Resour. Crop Evol. 2013, 60, 1953–1958. [Google Scholar] [CrossRef]
- Rubiang-Yalambing, L.; Arcot, J.; Greenfield, H.; Holford, P. Aibika (Abelmoschus manihot L.): Genetic Variation, Morphology and Relationships to Micronutrient Composition. Food Chem. 2016, 193, 62–68. [Google Scholar] [CrossRef]
- Hamon, S.; Koechlin, J. The Reproductive Biology of Okra. 1. Study of the Breeding System in Four Abelmoschus Species. Euphytica 1991, 53, 41–48. [Google Scholar] [CrossRef]
- Hamon, S.; Koechlin, J. The Reproductive Biology of Okra. 2. Self-Fertilization Kinetics in the Cultivated Okra (Abelmoschus esculentus), and Consequences for Breeding. Euphytica 1991, 53, 49–55. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Z.; Li, S.; Wang, L.; Lv, J.; Li, J.; Ma, X.; Fan, L.; Qian, F. Identification and Characterization of a Cytotoxic Polysaccharide from the Flower of Abelmoschus manihot. Int. J. Biol. Macromol. 2016, 82, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Lu, Y.; Shang, E.; Li, T.; Liu, Y.; Duan, J.; Qian, D.; Tang, Y. Metabolite Identification Strategy of Non-Targeted Metabolomics and Its Application for the Identification of Components in Chinese Multicomponent Medicine Abelmoschus manihot L. Phytomedicine 2015, 22, 579–587. [Google Scholar] [CrossRef]
- Tu, Y.; Sun, W.; Wan, Y.G.; Che, X.Y.; Pu, H.P.; Yin, X.; Chen, H.L.; Meng, X.J.; Huang, Y.R.; Shi, X.M. Huangkui Capsule, an Extract from Abelmoschus manihot (L.) Medic, Ameliorates Adriamycin-Induced Renal Inflammation and Glomerular Injury via Inhibiting P38MAPK Signaling Pathway Activity in Rats. J. Ethnopharmacol. 2013, 147, 311–320. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, C.; Han, Q.; Chen, Y.; Guo, J.; Wu, Q.; Zhu, B.; Shan, J.; Shi, L. Flos Abelmoschus Manihot Extract Attenuates DSS-Induced Colitis by Regulating Gut Microbiota and Th17/Treg Balance. Biomed. Pharmacother. 2019, 117, 109162. [Google Scholar] [CrossRef]
- National Meteorological Information Center. Available online: http://data.cma.cn/site/index.html (accessed on 15 January 2023).
- Boyes, D.C.; Zayed, A.M.; Ascenzi, R.; McCaskill, A.J.; Hoffman, N.E.; Davis, K.R.; Görlach, J. Growth Stage–Based Phenotypic Analysis of Arabidopsis: A Model for High Throughput Functional Genomics in Plants. Plant Cell 2001, 13, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Brandán, J.P. Phenological Growth Stages in Chia (Salvia hispanica L.) according to the BBCH Scale. Sci. Hortic. 2019, 255, 292–297. [Google Scholar] [CrossRef]
- Marchese, J.A.; Ferreira, J.F.S.; Moraes, R.M.; Dayan, F.E.; Rodrigues, M.F.F.; Jamhour, J.; Dallacorte, L.V. Crop Phenology and Floral Induction in Different Artemisia Annua L. Genotypes. Ind. Crops Prod. 2023, 192, 116118. [Google Scholar] [CrossRef]
- Ramírez, F.; Fischer, G.; Davenport, T.L.; Pinzón, J.C.A.; Ulrichs, C. Cape Gooseberry (Physalis peruviana L.) Phenology According to the BBCH Phenological Scale. Sci. Hortic. 2013, 162, 39–42. [Google Scholar] [CrossRef]
- Meier, U.; Bleiholder, H.; Brumme, H.; Bruns, E.; Mehring, B.; Proll, T.; Wiegand, J. Phenological Growth Stages of Roses (Rosa sp.): Codification and Description According to the BBCH Scale. Ann. Appl. Biol. 2008, 154, 231–238. [Google Scholar] [CrossRef]
- Mendez-Lopez, A.Y.; del CarmenLagunes-Espinoza, C.; González-Esquinca, A.R.; Hernández-Nataren, E.; Ortiz-García, C.F. Phenological Characterization of Chipilín (Crotalaria longirostrata Hook. & Arn.) and Relationship between the Phenological Stage and Chemical Composition of Leaves. S. Afr. J. Bot. 2023, 154, 140–148. [Google Scholar]
- Kishore, K. Phenological Growth Stages of Dragon Fruit (Hylocereus undatus) According to the Extended BBCH-Scale. Sci. Hortic. 2016, 213, 294–302. [Google Scholar] [CrossRef]
- Stoian, V.A.; Gâdea, Ș.; Vidican, R.; Vârban, D.; Balint, C.; Vâtcă, A.; Rotaru, A.; Stoian, V.; Vâtcă, S. Dynamics of the Ocimum Basilicum L. Germination under Seed Priming Assessed by an Updated BBCH Scale. Agronomy 2022, 12, 2694. [Google Scholar] [CrossRef]
- Gentallan, R.P.; Bartolome, M.C.B.; Cejalvo, R.D.; Timog, E.B.S.; Altoveros, N.C.; Borromeo, T.H.; Endonela, L.E. Seed Morphological Characteristics, Storage Behavior, and Germination Pattern of Combretum indicum (L.) DeFilipps. Genet. Resour. Crop Evol. 2021, 68, 2767–2773. [Google Scholar] [CrossRef]
- Archontoulis, S.V.; Struik, P.C.; Vos, J.; Danalatos, N.G. Phenological Growth Stages of Cynara cardunculus: Codification and Description According to the BBCH Scale. Ann. Appl. Biol. 2010, 156, 253–270. [Google Scholar] [CrossRef]
- Kishore, K. Phenological Growth Stages and Heat Unit Requirement of Indian Blackberry (Syzygium cumini L., Skeels). Sci. Hortic. 2019, 249, 455–460. [Google Scholar] [CrossRef]
- Piao, S.; Liu, Q.; Chen, A.; Janssens, I.A.; Fu, Y.; Dai, J.; Liu, L.; Lian, X.; Shen, M.; Zhu, X. Plant Phenology and Global Climate Change: Current Progresses and Challenges. Glob. Change Biol. 2019, 25, 1922–1940. [Google Scholar] [CrossRef] [PubMed]
- Cameron, W.; Petrie, P.R.; Barlow, E.W.R. The Effect of Temperature on Grapevine Phenological Intervals: Sensitivity of Budburst to Flowering. Agric. For. Meteorol. 2022, 315, 108841. [Google Scholar] [CrossRef]
- Xiao, D.; Zhao, J.J.; Hou, X.L.; Basnet, R.K.; Carpio, D.P.D.; Zhang, N.W.; Bucher, J.; Lin, K.; Cheng, F.; Wang, X.W.; et al. The Brassica Rapa FLC Homologue FLC2 Is a Key Regulator of Flowering Time, Identified through Transcriptional Co-Expression Networks. J. Exp. Bot. 2013, 64, 4503–4516. [Google Scholar] [CrossRef] [PubMed]
- Eeraerts, M. Increasing Wild Bee Richness and Abundance on Sequentially Flowering Cultivars of a Pollinator-Dependent Crop. Agric. Ecosyst. Environ. 2022, 325, 107745. [Google Scholar] [CrossRef]
- Krüger, E.; Woznicki, T.L.; Heide, O.M.; Kusnierek, K.; Rivero, R.; Masny, A.; Sowik, I.; Brauksiepe, B.; Eimert, K.; Mott, D.; et al. Flowering Phenology of Six Seasonal-Flowering Strawberry Cultivars in a Coordinated European Study. Horticulturae 2022, 8, 933. [Google Scholar] [CrossRef]
- Munger, P.; Bleiholder, H.; Hack, H.; Hess, M.; Stauß, R.; Boom, T.; Weber, E. Phenological Growth Stages of the Cotton Plant (Gossypium hirsutum L.): Codification and Description according to the BBCH Scale. J. Agron. Crop Sci. 1998, 180, 143–149. [Google Scholar] [CrossRef]
- Muengkaew, R.; Chaiprasart, P.; Warrington, I. Changing of Physiochemical Properties and Color Development of Mango Fruit Sprayed Methyl Jasmonate. Sci. Hortic. 2016, 198, 70–77. [Google Scholar] [CrossRef]
- Singh, A.K.; Bajpai, A.; Rajan, S.; Das, S.S.; Mishra, K.K. Modified BBCH Codification and Correlation of Phenological Characteristics with Climatic Variables in Jamun (Syzigium cuminii Skeels). Sci. Hortic. 2021, 283, 110081. [Google Scholar] [CrossRef]


| Stage | DAE | Bud Length (BUL, mm) | Bud Width (BUW, mm) | BUL/BUW | Pistil Length (PL, mm) | Stamen Length (SL, mm) | PIL/STL | Bracts Length (BRL, mm) | Pedicel Length (PEL, mm) |
|---|---|---|---|---|---|---|---|---|---|
| 500 | 1–2 | 4.45 ± 0.17 h | 3.38 ± 0.11 g | 1.32 ± 0.03 e | 1.56 ± 0.04 h | 2.65 ± 0.03 h | 0.59 ± 0.02 e | 6.63 ± 0.31 d | Not formed |
| 501 | 3–4 | 6.67 ± 0.28 g | 4.61 ± 0.18 f | 1.45 ± 0.10 d | 2.82 ± 0.19 g | 3.72 ± 0.39 g | 0.76 ± 0.05 d | 7.25 ± 0.42 c | 2.04 ± 0.19 f |
| 503 | 5–6 | 7.67 ± 0.13 f | 5.04 ± 0.10 e | 1.52 ± 0.01 d | 5.16 ± 0.15 f | 4.91 ± 0.04 f | 1.05 ± 0.02 c | 8.14 ± 0.66 c | 2.79 ± 0.19 e |
| 504 | 7–9 | 18.94 ± 0.13 e | 10.08 ± 0.65 d | 1.88 ± 0.08 c | 8.87 ± 0.11 e | 6.91 ± 0.05 e | 1.17 ± 0.02 b | 13.31 ± 0.88 b | 13.71 ± 1.26 d |
| 505 | 10–11 | 21.91 ± 0.92 d | 11.70 ± 0.57 c | 1.88 ± 0.11 c | 9.21 ± 0.39 d | 7.77 ± 0.41 d | 1.19 ± 0.02 a | 13.63 ± 1.01 b | 13.79 ± 0.46 d |
| 506 | 12–13 | 24.65 ± 0.48 c | 12.53 ± 0.25 b | 1.97 ± 0.04 c | 10.64 ± 0.60 c | 8.73 ± 0.41 c | 1.22 ± 0.03 a | 14.51 ± 0.89 b | 17.68 ± 0.61 c |
| 507 | 14–15 | 35.09 ± 1.95 b | 14.08 ± 0.88 a | 2.49 ± 0.07 b | 12.22 ± 0.14 b | 9.65 ± 0.43 b | 1.27 ± 0.04 a | 17.25 ± 0.52 a | 26.28 ± 1.50 b |
| 509 | 16 | 51.29 ± 3.84 a | 15.03 ± 0.89 a | 3.41 ± 0.08 a | 20.35 ± 0.31 a | 15.09 ± 1.07 a | 1.35 ± 0.09 a | 18.47 ± 1.20 a | 34.01 ± 2.89 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, W.; Hu, Y.; Lin, X.; Yu, D.; Jia, S.; Ye, Y.; Mao, Y.; Yi, L.; Gao, S. Phenological Growth Stages of Abelmoschus manihot: Codification and Description According to the BBCH Scale. Agronomy 2023, 13, 1328. https://doi.org/10.3390/agronomy13051328
Qian W, Hu Y, Lin X, Yu D, Jia S, Ye Y, Mao Y, Yi L, Gao S. Phenological Growth Stages of Abelmoschus manihot: Codification and Description According to the BBCH Scale. Agronomy. 2023; 13(5):1328. https://doi.org/10.3390/agronomy13051328
Chicago/Turabian StyleQian, Wenzhang, Yunyi Hu, Xi Lin, Deshui Yu, Shibing Jia, Yulin Ye, Yidong Mao, Lu Yi, and Shun Gao. 2023. "Phenological Growth Stages of Abelmoschus manihot: Codification and Description According to the BBCH Scale" Agronomy 13, no. 5: 1328. https://doi.org/10.3390/agronomy13051328
APA StyleQian, W., Hu, Y., Lin, X., Yu, D., Jia, S., Ye, Y., Mao, Y., Yi, L., & Gao, S. (2023). Phenological Growth Stages of Abelmoschus manihot: Codification and Description According to the BBCH Scale. Agronomy, 13(5), 1328. https://doi.org/10.3390/agronomy13051328







