Abstract
Drought limits tea yield and can also negatively impact its quality. In this study, constant humidity and dry–wet alternating modes were compared to determine their impacts on soil nitrogen transformation and ammonia-oxidizing microorganisms. Drought was found to reduce the soil NH4+-N concentration under the constant humidity mode, and the NO3−-N concentration was highest in 60% water-holding capacity (WHC) soil. Soil NO3−-N content increased rapidly after rewatering, and increasing the frequency of dry–wet watering resulted in a higher accumulation of NO3−-N. In the constant humidity mode, drought reduced the abundance of ammonia-oxidizing archaea (AOA), whereas that of ammonite-oxidizing bacteria (AOB) increased. Increases in drought duration and the frequency of dry–wet watering inhibited the activity of AOA under the dry–wet alternating mode, whereas the relative activity of AOB increased after rehydration. The water supply mode did not change the community structure of AOA or AOB at the genus level but affected their relative abundance. In the constant humidity mode, the contribution rate of AOA to nitrification potential (PNR) was 42.75–49.72%, whereas that of AOB was 50.28–57.25%. In the dry–wet alternating mode, the contribution rate of AOA to PNR increased, and the contribution rate of AOB decreased. Taken together, these findings indicate that ammonia oxidation might be primarily driven by AOA and AOB in weakly acidic and neutral soil. This study reveals the effects of different water supply modes on soil nitrogen transformation and ammonia-oxidizing micro-organisms and provides a scientific basis for improving nitrogen use efficiency.
1. Introduction
Tea plants (Camellia sinensis (L.) O. Kuntze) are grown for their leaves and require sufficient nitrogen to achieve high yields. Higher levels of soil nitrogen increase the photosynthetic rate, thereby improving both yield and quality [1]. The annual average precipitation in the Shandong tea growing area is less than 1000 mm, and rainfall often occurs intermittently. The dry–wet alternations presented by these pulses of rainfall could affect the soil nitrogen cycle and impact plant growth [2].
Soil moisture is one of the important factors affecting soil nitrogen mineralization rate. There was an approximately linear relationship between soil water content and nitrogen mineralization. With the increase in water content, soil nitrogen mineralization increased [3]. However, past a certain point, increasing soil moisture could result in a rapid decrease in nitrogen mineralization [4]. The soil nitrogen transformation varied greatly under different water supply modes. The humification processes of soil organic matter were also known to be affected in well-ventilated dry–wet alternating modes, resulting in differences in soil organic matter quality, and promoting the transformation of non-acidolysis nitrogen in complex soil structures to relatively simple acidolysis ammonia nitrogen and amino sugar nitrogen [1].
Microorganisms were the main drivers behind the biogeochemical cycle of soil elements. Soil microorganisms played a vital role in agricultural ecosystems, as they were involved in several soil functions and ecological services [5,6]. Soil microorganisms were known to be affected by changes in soil moisture, which could lead to alterations in nitrogen transformation. Additionally, drought had been shown to reduce the activity of soil microorganisms in tea gardens, thereby inhibiting the utilization of soil inorganic nutrients by microorganisms [7,8], and further affecting the release of nitrogen in soil. Although soil mineralization still occurs during drought, these conditions reduced the abundance of ammonia-oxidizing archaea (AOA) and ammonia-oxidizing bacteria (AOB) and slowed the transformation of ammonium nitrogen to nitrate nitrogen [9]. Unlike bacteria and fungi, archaea were known to have higher diversity in relatively arid regions. This phenomenon might be due to the unique niches created by more extreme environments, which archaea were able to fill. Additionally, Pett-Ridge et al. [10] found that their higher affinity for oxygen made ammonia-oxidizing archaea more competitive than ammonia-oxidizing bacteria in low-oxygen environments. Furthermore, ecological studies have shown that ammonia-oxidizing archaea were more adaptable to hypoxia than ammonia-oxidizing bacteria. In the soil ecosystem, the number of ammonia-oxidizing archaea was inversely proportional to the oxygen concentration in the soil, whereas the number of ammonia-oxidizing bacteria was positively correlated with oxygen concentration.
Tea garden soil is typically acidic, aluminum-rich, and high in polyphenols, forming a very unique chemical environment. The soil nitrogen transformation process and the microbial population driving nitrogen transformation were significantly different from other soil ecosystems. Although a large number of studies have been conducted on soil nitrogen transformation in tea gardens [1,3], the effects of water supply mode on soil nitrogen transformation and microbial communities in tea gardens had not been reported. In this study, the response of soil nitrogen transformation to different water supply modes was studied to better understand the mechanism of soil nitrogen transformation in Shandong tea gardens. We employed the ammoxidation inhibitors 1-octyne and acetylene, in addition to a quantitative real-time polymerase chain reaction (PCR) and high-throughput sequencing technology, to study the quantitative and structural characteristics of ammonia-oxidizing bacterial communities under different watering modes. We sought to understand the microbial driving mechanism of nitrogen transformation in tea garden soil under different water supply modes to reveal the interaction mechanism between soil nitrogen and microorganisms under different water supply modes. Our results provide new insights into the nitrogen transformation of tea garden soil, provide a scientific basis for improving nitrogen availability, and provide a scientific explanation of soil molecular ecology for the application of tea garden management.
2. Materials and Methods
2.1. Sample Collection
Soil samples were taken from a tea planting location at Shandong Agricultural University (N-36°28′, E-116°83’), which contained sandy loam soil. The surface vegetation of sampling points was removed, and the top 0–20 cm of soil was sampled with a soil drill at multiple random locations. Fresh soil samples were then passed through a 2-mm sieve. The soil water content was maintained at 20% of the maximum water holding capacity (WHC) by natural drying prior to bottling the samples.
2.2. Experimental Design
A total of 100 g dry weight of soil was placed into a culture bottle. Acetylene (Ace, 0.1% v/v) and 1-octyne (1-Oct, 5 μM aqueous solution) [11] were used as selective nitrification inhibitors for soil microcosm culturing. The culture temperature was maintained at 25 °C, and the relative humidity was maintained at 40%. The samples were placed in incubators set at 25 °C, with 40% relative humidity, in the dark. A summary of the experimental conditions is shown in Table 1. Samples were taken after 7 d, 14 d, and 21 d of culturing and utilized for nitrogen content measurement. After 21 d of incubation, soil DNA was extracted from six treatments of 20% WHC and 60% WHC in constant humidity mode and nine treatments in dry–wet alternating mode. The samples were then used to determine the abundance and diversity of soil ammonia-oxidizing microorganisms. Destructive sampling was used in all experiments, and each bottle of soil was used as a biological replicate, with three replicates per condition. During the incubation period, sterile deionized water was used to adjust the soil water content every 1–2 d.

Table 1.
Experimental design.
2.3. Determination of Ammonium Nitrogen and Nitrate Nitrogen in Soil
In brief, 10 g of cultivated soil sample was added to 50 mL of 2 mol/L KCl solution, followed by 1 h of shaking and then filtration. The contents of ammonium nitrogen and nitrate nitrogen were determined by a continuous flow analyzer (AA3, SEAL Analytic, Norderstedt, Germany), and the soil nitrification potential (PNR) was calculated.
2.4. Extraction of Total DNA, Real-Time Fluorescent Quantitative PCR
Soil total DNA was extracted with an “Omega Soil DNA Kit” (Omega Bio-tek, Norcross, Georgia, USA). The concentrations and purity of DNA were determined using a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Extracted DNA was stored at −80 °C prior to the determination of the abundance and diversity of soil ammonia-oxidizing microorganisms.
The copy numbers of AOA-amoA and AOB-amoA genes were determined by quantitative real-time PCR. The primer sequences of target genes are shown in Table 2. The reaction was performed with an ABI7500 quantitative real-time PCR instrument (Thermo Fisher Scientific, Waltham, MA, USA). The 20-μL reaction system contained 2 μL DNA template, 1 μL each forward and reverse primers, 6 μL double-distilled H2O, and 10 μL 2× SYBR® Green qPCR Master Mix (Applied Biosystems, Foster City, CA, USA). Each reaction was repeated three times, and the results were expressed as the copy number of genes per gram of dry soil weight.

Table 2.
The primer sequence of the target gene.
2.5. High-Throughput Sequencing of Soil Ammonia-Oxidizing Microorganisms
An ABI GeneAmp® 9700 PCR instrument (Thermo Scientific, USA) was used to amplify amoA genes of AOA and AOB. The primers were the same as those used for quantitative real-time PCR. The PCR products were detected by 2% agarose gel electrophoresis and recovered using an AxyPrepDNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA). The PCR products were quantitatively detected by a QuantiFluorTM-ST (Promega, Madison, WI, USA) blue fluorescence quantitative system, followed by the construction of a Miseq sequencing library. Sequencing was performed on the Illumina Novaseq 6000 sequencing platform at Biomarker Technologies Co., Ltd. (Beijing, China).
2.6. Data Processing
Acetylene was used to completely inhibit the ammonia oxidation of autotrophic nitrification, and the PNR of AOA + AOB was calculated by subtracting the PNR in the control from the PNR after acetylene inhibition. Additionally, 1-octyne was used to specifically inhibit AOB activity without affecting AOA activity. The PNR of AOA was determined by subtracting the PNR after 1-octyne inhibition from the PNR after acetylene inhibition. The PNR of AOB was determined by subtracting the PNR of AOA + AOB from the PNR of AOA.
Statistical analysis was conducted using SPSS v16.0 for Windows. One-way analysis of variance (ANOVA) and least significant difference (LSD) tests were used to compare the averages between treatments. The significance cutoff was set at p = 0.05. Origin 8.0 software was used to create graphs.
3. Results
3.1. Effects of Water Supply Model on Soil Mineralization in Tea Garden
Changes in the relative amounts of soil NH4+-N and NO3−-N during cultivation reflect nitrogen transformation. During the constant humidity mode, the NH4+-N concentration first increased and then decreased, whereas the NH4+-N content in T20 decreased. After 21 d of incubation, the NH4+-N level was highest in T40, followed by T80, T60, and then T20 (p < 0.05) (Figure 1a). Compared with the no-inhibitor culture, the relative levels of NH4+-N in samples treated with 1-octyne were T80-Oct > T60-Oc > T40-Oct > T20-Oct, with increases of 42.71%, 57.70%, 9.85%, and 5.11% respectively at the end of the culture period. However, T20-Ace, T40-Ace, T60-Ace and T80-Ace increased by 17.45%, 61.12%, 184.54% and 152.10%, respectively. At the end of the culture period, the relative amounts of NO3−-N in the no-inhibitor culture and inhibitor-treated culture were 60% WHC > 80% WHC > 40% WHC > 20% WHC, (Figure 1c), with the two inhibitors lower than that of the no-inhibitor culture. Compared with 1-octyne, T20-Ace, T40-Ace, T60-Ace and T80-Ace decreased by 5.22%, 14.61%, 9.38% and 7.59% respectively.
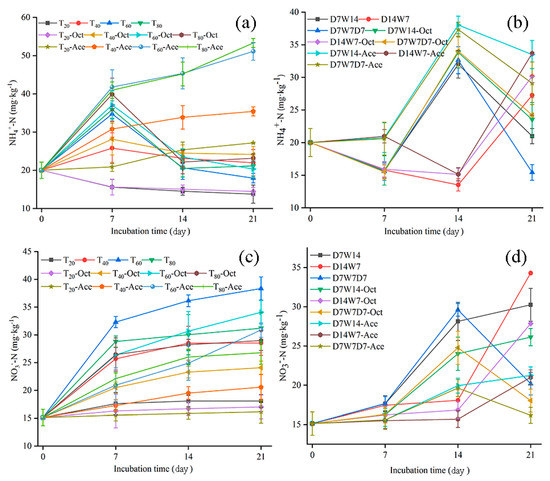
Figure 1.
The content of soil NH4+–N, NO3−–N under different water supply modes. (a,c) were constant humidity mode; (b,d) were dry–wet alternation mode. Values were the mean of three replicates ± S.D. T20: 20% WHC; T40: 40% WHC; T60: 60% WHC; T80: 80% WHC; D7W14: 20% WHC for 1–7 d, 60% WHC for 15–21 d; D14W7: 20% WHC for 1–14 d, 60% WHC for 15–21 d; D7W7D7: 20% WHC for 1–7 d, 60% WHC for 8–14 d, 20% WHC for 15–21 d. Treatments with added acetylene and 1-octane were labeled as Oct and Ace respectively.
In the dry–wet alternation mode, the content of NH4+-N and NO3−-N increased after rewatering and decreased after drought (Figure 1b,d). After 21 d of incubation, the NH4+-N content in D14W7 was the highest, followed by D7W14 and then D7W7D7, whereas the NO3−-N content was D14W7 > D7W14 > D7W7D7. The NH4+-N content in D7W14-Oct, D14W7-Oct, and D7W7D7-Oct increased by 12.14%, 10.68%, and 57.03%, whereas NH4+-N content in D7W14-Ace, D14W7-Ace, and D7W7D7-Ace increased by 59.64%, 23.55%, and 88.58% compared to the no-inhibitor culture, respectively. At the end of culture, the NO3−-N content in D7W14-Oct, D14W7-Oct, and D7W7D7-Oct decreased to 26.14, 27.86, and 18.03 mg/kg respectively, and those of D7W14-Ace, D14W7-Ace, and D7W7D7-Ace decreased to 21.24, 20.99 and 16.17 mg/kg, respectively.
3.2. Relative Contribution of Ammonia-Oxidizing Microorganisms to Soil Nitrate Nitrogen
In the constant humidity mode, the contribution rate of AOA to PNR was less than 50%, with the largest proportion detected in T80. The contribution rate of AOB was higher than that of AOA, which remained between 50.28% and 57.25%, whereas the contribution rate of AOB was the highest in T60 (Table 3). Compared with the constant humidity mode, the contribution rate of AOA to PNR increased under the dry–wet alternation mode, especially D7W14 (54.34%) and D14W7 (51.63%). The contribution rate of AOB decreased, with the highest proportion detected in D7W7D7 (53.8%).

Table 3.
The relative contribution rate of ammonia-oxidizing microorganisms to PNR. T20: 20% WHC; T40: 40% WHC; T60: 60% WHC; T80: 80% WHC; D7W14: 20% WHC for 1–7 d, 60% WHC for 15–21 d; D14W7: 20% WHC for 1–14 d, 60% WHC for 15–21 d; D7W7D7: 20% WHC for 1–7 d, 60% WHC for 8–14 d, 20% WHC for 15–21 d.
3.3. Effects of Different Water Supply Modes on Ammonia Oxidizing Microorganisms
3.3.1. Response of Gene Abundance of Ammonia Oxidizing Microorganisms to Different Water Supply Modes
The abundance of AOA-amoA steadily decreased in response to the constant humidity mode, whereas the level of AOB-amoA increased. The abundance of AOA-amoA and AOB-amoA in T60 (1.85 × 107–2.85 × 107 copies/g; 6.04 × 106–7.89 × 106 copies/g) were significantly higher than those in T20 (1.06 × 107–1.95 × 107 copies/g; 4.40 × 106–5.86 × 106 copies/g) (Figure 2a,c). And the addition of 1-octyne did not impact the abundance of AOA-amoA. At the end of the culture period, the AOB-amoA abundance in T20-Oct and T60-Oct decreased by 43.10% and 30.48%, whereas that in T20-Ace and T60-Ace decreased by 44.47% and 36.55%, respectively. In the dry–wet alternating mode, the abundance of AOA-amoA in D7W14 increased by 12.99%, whereas that in D14W7 and D7W7D7 decreased by 13.08% and 10.52% (Figure 2b); the abundance of AOB-amoA increased, and D14W7 increased the most (63.44%) (Figure 2d) at the end of culture, compared to after 7 d of culture.
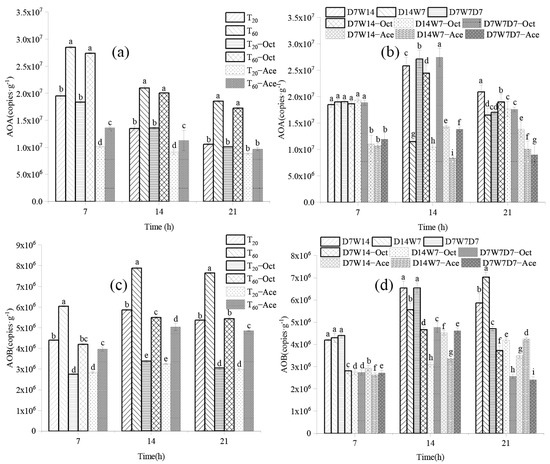
Figure 2.
The abundance of ammonia-oxidizing microorganisms under different water supply modes. (a,c) were constant humidity mode; (b,d) were dry–wet alternation mode. Data represent mean ± SE (n = 3) and statistically differences (p < 0.05), indicated by letters. T20: 20% WHC; T60: 60% WHC; D7W14: 20% WHC for 1–7 d, 60% WHC for 15–21 d; D14W7: 20% WHC for 1–14 d, 60% WHC for 15–21 d; D7W7D7: 20% WHC for 1–7 d, 60% WHC for 8–14 d, 20% WHC for 15–21 d. Treatments with added acetylene and 1-octane were labeled as Oct and Ace respectively.
3.3.2. The Community Composition of Ammonia Oxidizing Microorganisms
The AOA community was mainly composed of Candidatus Nitrosocaldus, Candidatus Nitrosocosmicus, Nitrososphaera, Nitrosoarchaeum, Nitrosopumilus, Candidatus Nitrosotalea, Candidatus Nitrosotenuis. Candidatus Nitrosocaldus had the highest abundance (constant humidity mode, 73–82%; dry–wet alternation mode, 75–80%) (Figure 3), but its abundance was unaffected by water content in the constant humidity mode. The relative abundance of Nitrososphaera, Nitrosoarchaeum, Nitrosopumilus, Candidatus Nitrosotalea, and Candidatus Nitrosotenuis in T60 was significantly higher than those in T20 (Table S1). At the genus level, the AOB community was mainly composed of Nitrosospira and Nitrosovibrio, which belong to β-Proteobacteria and γ-Proteobacteria, respectively. In the constant humidity mode, changes in water content had no significant effect on the relative abundance of AOB (Table S3).
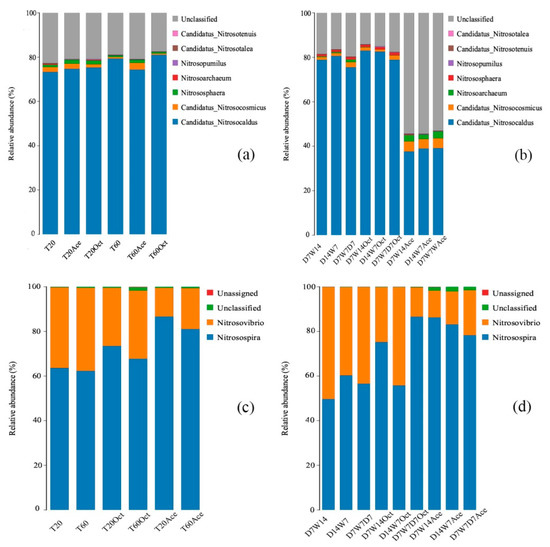
Figure 3.
Structure composition of ammonia-oxidizing microorganisms with different water supply modes at the genus level. (a,c) were the structure of AOA, AOB in constant humidity mode; (b,d) was the structure composition of AOA, AOB in dry–wet alternate mode. T20: 20% WHC; T60: 60% WHC; D7W14: 20% WHC for 1–7 d, 60% WHC for 15–21 d; D14W7: 20% WHC for 1–14 d, 60% WHC for 15–21 d; D7W7D7: 20% WHC for 1–7 d, 60% WHC for 8–14 d, 20% WHC for 15–21 d. Treatments with added acetylene and 1-octane were labeled as Oct and Ace respectively.
In the dry–wet alternative mode, the relative abundance of Candidatus Nitrosocaldus was significantly lower in D7W7D7 than that in D7W14 and D14W7 after incubation for 21 days, whereas the relative abundance of Candidatus Nitrosocosmicus, Nitrososphaera, Nitrosorchaeum, Nitrosopululus, Candidatus Nitrosotalea was significantly higher than those in D7W14 and D14W7 (p < 0.05). Except for Candidatus Nitrosocosmicus and Nitrosopumilus, the relative abundance of other genera in D14W7 was significantly higher than those in D7W14 (p < 0.05) (Table S2). Analysis of the AOB community structure indicated that there were significant differences between different drought-rewatering treatments (p < 0.05). The relative abundance of Nitrosospira was highest in D14W7, followed by D7W7D7 and then D7W14, whereas the relative abundance of Nitrosovibrio was highest in D14W7, followed by D7W7D7 and then D7W14 (Table S4).
3.4. The Correlation Analysis
3.4.1. Spearman Correlation Analysis
There was a significant positive correlation between PNR and NH4+, NO3− in both the constant humidity mode and dry–wet alternation mode (p < 0.01) (Table 4). In the constant humidity mode, the AOA abundance was positively correlated with NH4+ (p < 0.01), PNR (p < 0.05), and NO3− (p < 0.05). The AOB abundance was positively correlated with NH4+ (p < 0.05), PNR (p < 0.01), and NO3− (p < 0.01). In the dry–wet alternation mode, AOA and AOB were significantly positively correlated with NH4+, NO3−, and PNR (p < 0.01) (Table 4). To sum up, AOA and AOB might jointly lead the ammonia oxidation.

Table 4.
Correlation analysis between ammonia-oxidizing microbial abundance and nitrogen under constant humidity mode and dry–wet alternation mode. *, p < 0.05; ** p < 0.01.
3.4.2. Redundancy Analysis of Ammonia Oxidizing Microorganisms
Redundancy analysis (RDA) showed that NO3− and NH4+ had significant effects on soil microorganisms (p < 0.05), the contribution rate of NO3− and NH4+ was higher, reaching 44.90% and 43% respectively. It means that NO3− and NH4+ play a leading role in affecting soil microorganisms. Spearman correlation analysis found that NO3− was significantly positively correlated with Candidatus Nitrosocaldus and Nitrosovibrio (p < 0.01), and significantly negatively correlated with Candidatus Nitrosocosmicus, Nitrosopumilus, Candidatus Nitrosotalea and Nitrosospira. NH4+ was positively correlated with Nitrosospira (p < 0.05), and negatively correlated with Nitrososphaera, Candidatus Nitrosotalea, Nitrosovibrio (Figure 4).
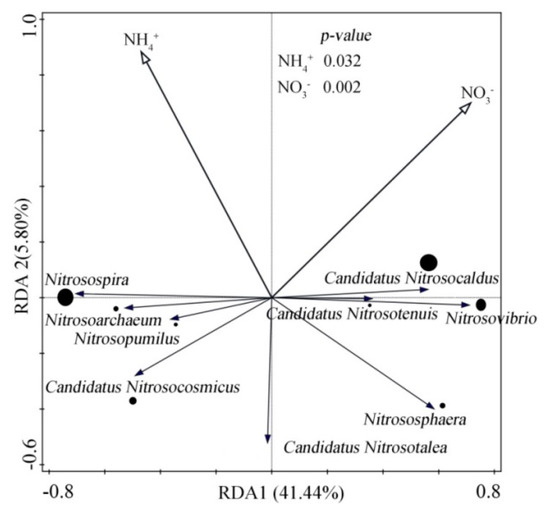
Figure 4.
Redundancy analysis (RDA) of soil bacteria (phylum lever) with soil environmental factors, viz. NH4+ and NO3−.
4. Discussion
4.1. Effects of Different Moisture Modes on Nitrogen Conversion
The inorganic nitrogen absorbed by tea plants was mainly NH4+ and NO3−. Changes in water content could have an impact on nitrogen conversion in soil. In this paper, it was found that the NH4+ content of soil increased with the increase of water content in the early stage of constant humidity culture, but the NH4+ content of all treated soils showed a decreasing trend in the later stage of culture (Figure 1a). The soil NO3− content maintained a trend of 60% WHC > 80% WHC > 40% WHC > 20% WHC throughout the culture period (Figure 1c). This might be because suitable water conditions were conducive to the release of soil nutrients due to the increased reproduction of microorganisms involved in mineralization and nitrification [12,13]. However, with the increase of soil water content, oxygen content, and soil permeability were reduced, resulting in the solidification of NH4+ stronger than nitrification [14], and the denitrification effect was enhanced, which in turn weakens the nitrogen mineralization amount and nitrogen mineralization rate [14]. This led to a gradual decrease in NH4+ content and an increase in ammonia volatilization [15,16].
Nitrification increased with higher soil moisture, increasing the conversion of NH4+ to NO3− [17,18], but very high moisture levels had the opposite effect [19]. Studies had shown that there was a significant positive correlation between soil moisture and soil nitrogen mineralization, with an optimum range of moisture [20]. When the soil moisture content was lower than field water holding capacity, the soil primary nitrogen mineralization rate and primary nitrogen fixation rate were mainly affected by substrate transport [21], and the diffusion of substrates to microorganisms was limited in low-water-content soils [22]. When the soil moisture content was in the range of 40–100% WHC, the effective carbon content of the soil increased, providing more energy and substrate for microorganisms [23,24]. Increases in the water level result in higher abundance and activity of microorganisms, up to a certain point, thereby increasing the primary nitrogen mineralization rate of soil [25]. In our experiments, 60% WHC was found to be optimal for Shandong tea plantation soil. Additionally, inhibition of nitrification by 1–octane and acetylene, the increase rate of NH4+ in 60% WHC soil was the largest. Changes in soil NO3− concentration were the result of the combined effects of nitrification, leaching loss, and denitrification [26,27]. These three factors affected the balance between NO3− production and consumption after 7 d of incubation, indicating that the NO3− content remained relatively stable across treatments (Figure 1c).
In the wet-dry alternating mode, the wet-dry conversion process destroyed soil aggregates, exposed more soil organic matter, provided a large number of nutrients for microorganisms [28], and increased the nitrogen conversion rate after soil rewatering [29]. Dijkstra et al. found that the total ammoniation rate and total nitrification rate increased rapidly 1–3 d after water input in dry grassland, and microbial activity was quickly activated after soil rewatering [30]. In this experiment, soil NH4+ and NO3− also showed “pulse” changes, whereas overall NH4+ decreased over time (Figure 1b). The alternation of dry and wet conditions also drove the conversion of NH4+ to NO3− in the soil, and the soil NO3− content increased rapidly after drought and rehydration (Figure 1d).
4.2. The Driving Effect of Ammonia-Oxidizing Microorganisms on Ammonia Oxidation under Different Moisture Modes
Ammonia-oxidizing microorganisms drove the process of soil ammonia oxidation, and both AOA and AOB were involved in this process. A previous work showed that autotrophic ammonia oxidation could be completely inhibited by the addition of low concentrations of acetylene [31]. In this study, the nitrification rate was significantly reduced after adding acetylene to moist soil, indicating that autotrophic ammonia oxidation was the dominant ammonia oxidation process under this condition. The soil PNR was higher after inhibition of AOA than after inhibition of AOB (Figure 2), and the contribution rate of AOB to PNR was higher than that of AOA (Table 3), indicating that AOB plays a key role in nitrification of weakly acidic and neutral soil in constant humidity mode.
The contribution of AOA and AOB to ammonia oxidation was affected by the external environment, with pH playing a major role. In our assays, the soil pH was weakly acidic to neutral (approximately 6.8), and AOA was more likely to survive in an acidic environment, making the abundance higher than AOB (Figure 2), which was similar to the results of most acidic soils [32,33]. As the neutral soil environment inhibits the ammonia oxidation of AOA [34,35], the contribution rate of AOA to PNR was slightly lower than that of AOB. Song et al. also found that ammonia-oxidizing bacteria were the primary drivers of soil nitrification in neutral and slightly acidic soils (pH 6.33), with nitrification contribution rates of 59.44% and 61.99%, respectively [36]. Our data showed that AOA and AOB were significantly positively correlated with NO3−, but the significance of AOB was higher than that of AOA in the constant humidity mode. However, the correlation between AOB and NO3− was not much different from that of AOA in the dry–wet alternation mode (Table 4). Although the abundance of AOA and AOB changed before and after incubation, the level of AOB was more highly correlated with the nitrification rate.
In addition, the soil nitrogen content affected the growth of ammonia-oxidizing microorganisms. Redundancy analysis showed that NO3− and NH4+ had significant effects on soil ammonia-oxidizing microorganisms (p < 0.05), with contribution rates of 44.90% and 43%, respectively (Figure 3), indicating that NO3− and NH4+ were major factors impacting soil microorganisms. In ammonia-oxidizing microorganisms, AOA tend to thrive in low nitrogen environments [32,37], whereas AOB prefer higher nitrogen settings [38,39]. In the constant humidity mode, the soil nitrogen content increased over the course of the culture, leading to the formation of the high nitrogen conditions favored by AOB. In the dry–wet alternating environment, soil osmotic potential increased during the drought period. These changes caused microorganisms to adapt to increasing osmotic potential by accumulating or producing osmotic substances [40], which leads to the fixation of a large amount of N [41]. After rewatering, the soil osmotic potential suddenly decreased, and microorganisms released the accumulated osmotic substances to avoid rupture. Recovery from low activity or dormancy resulted in enhanced nitrogen mineralization and nitrification [42,43], meaning that drought-rewatering cycles cause high NH4+ concentrations in soil. This adversely affected AOA, but had no significant effect on AOB abundance [44]. We also found that frequent dry–wet alternations caused AOB to contribute more to ammonia oxidation than AOA (Table 3). Overall, the relative contribution rate of AOA (constant humidity mode, 42.75–49.72%; dry–wet alternation mode, 46.17–54.34%) and AOB (constant humidity mode, 50.28–57.25%; dry–wet alternation mode, 45.66–53.83%) were not much different (Table 3), implying that ammonia oxidation may be dominated by AOA and AOB in weak acid and neutral brown soil.
5. Conclusions
Drought was found to reduce the soil NH4+-N concentration under the constant humidity mode, and the NO3−-N concentration was highest in 60% WHC soil. The increasing the frequency of dry–wet watering resulted in a higher accumulation of NO3−-N. The influence of dry–wet alternation mode on soil nitrogen transformation was greater than that of constant humidity mode. In the constant humidity mode, drought reduced the abundance of AOA, whereas that of AOB increased. Increases in drought duration and the frequency of dry–wet watering inhibited the activity of AOA under the dry–wet alternating mode, whereas the relative activity of AOB increased after rehydration. The water supply mode did not change the community structure of AOA or AOB at the genus level but affected their relative abundance. In weakly acidic and neutral soils, ammonia oxidation might be mainly driven by AOA and AOB. This study reveals the effects of different water supply modes on soil nitrogen transformation and ammonia-oxidizing micro-organisms and provides a scientific basis for improving nitrogen use efficiency. This study reveals the effects of different water supply modes on soil nitrogen transformation and ammonia-oxidizing micro-organisms and provides a scientific basis for improving nitrogen use efficiency.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/agronomy13051279/s1, Table S1: Relative abundance of ammonia-oxidizing archaea at the genus level in constant humidity mode; Table S2: Relative abundance of ammonia-oxidizing archaea at the genus level in dry–wet alternate mode; Table S3: Relative abundance of ammonia-oxidizing bacteria at the genus level in constant humidity mode; Table S4: Relative abundance of ammonia-oxidizing bacteria at the genus level in constant humidity mode.
Author Contributions
Conceptualization, X.H.; methodology, H.W.; formal analysis, J.H. and B.Z.; investigation, H.W. and J.H.; writing—original draft preparation, H.W.; writing—review and editing, X.H.; project administration, X.H.; funding acquisition, X.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation Committee of Shandong Province (the Natural Science Foundation of Shandong Province, Grant number: ZR2019BC062).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Han, W.; Ma, L.; Shi, Y.; Ruan, J.; Kemmitt, S. Nitrogen release dynamics and transformation of slow release fertiliser products and their effects on tea yield and quality. J. Sci. Food Agric. 2008, 88, 839–846. [Google Scholar] [CrossRef]
- Yahdjian, L.; Sala, O. Size of precipitation pulses controls nitrogen transformation and losses in an arid patagonian ecosystem. Ecosystems 2010, 13, 575–585. [Google Scholar] [CrossRef]
- Zhao, Z.; Yu, S.; Han, X.; Yang, S. Influence of drought and dry-wet alternation on nitrogen transformation and low abundance microorganisms in tea garden soil. J. Environ. Biol. 2022, 43, 231–238. [Google Scholar] [CrossRef]
- Saetre, P.; Stark, J. Microbial dynamics and carbon and nitrogen cycling following re-wetting of soils beneath two semi-arid plant species. Oecologia 2005, 142, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, L.; Dai, Y.; Di, H.; He, J. pH-dependent distribution of soil ammonia oxidizers across a large geographical scale as revealed by higher-throughput pyrosequencing. J. Soil Sediments 2013, 13, 1439–1449. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, W.; Hu, H.; Kim, Y.; Duan, J.; Luo, C.; Wang, S.; Guo, L.; Zheng, Y. Ammonia oxidizers and denitrifiers in response to reciprocal elevation translocation in an alpine meadow on the Tibetan Plateau. J. Soils Sediments 2014, 14, 1189–1199. [Google Scholar] [CrossRef]
- Muhr, J.; Franke, J.; Borken, W. Drying-rewetting events reduce C and N losses from a Norway pruce forest floor. Soil Boil. Biochem. 2010, 42, 1303–1312. [Google Scholar] [CrossRef]
- Chen, D.; Mi, J.; Chu, P.; Cheng, J.; Zhang, L.; Pan, Q.; Xie, Y.; Bai, Y. Patterns and drivers of soil microbial communities along a precipitation gradient on the Mongolian Plateau. Landsc. Ecol. 2015, 30, 1669–1682. [Google Scholar] [CrossRef]
- Wang, H.; Yang, J.; Yang, S.; Yang, Z.; Lv, Y. Effect of a 10 degrees C-elevated temperature under different water contents on the microbial community in a tea orchard soil. Eur. J. Soil Biol. 2014, 62, 113–120. [Google Scholar] [CrossRef]
- Pett-Ridge, J.; Petersen, D.; Nuccio, E.; Firestone, M. Influence of oxic/anoxic fluctuations on ammonia oxidizers and nitrification potential in a wet tropical soil. FEMS Microbol. Ecol. 2013, 85, 179–194. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, G.; Ju, X.; Liu, R. How nitrification-related N2O is associated with soil ammonia oxidizers in two contrasting soils in China? Sci. Total Environ. 2021, 770, 143212. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, L.; Li, Y.; Xu, J.; Brookes, P. Abiotic processes dominate soil organic matter mineralization: Investigating the regulatory gate hypothesis by inoculating a previously fumigated soil with increasing fresh soil inocula. Geoderma 2020, 373, 114400. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, S.; Zhu, Q.; Ding, W. Image grey value analysis for estimating the effect of microorganism inoculants on straws decomposition. Comput. Electron. Agric. 2016, 128, 120–126. [Google Scholar] [CrossRef]
- Bernal, S.; Sabater, F.; Butturini, A.; Nin, E.; Sabater, S. Factors limiting denitrification in a Mediterranean riparian forest. Soil Boil. Biochem. 2007, 39, 2685–2688. [Google Scholar] [CrossRef]
- Francisco, S.; Urrutia, O.; Martin, V.; Peristeropoulos, A.; Garcia-Mina, J. Efficiency of urease and nitrification inhibitors in reducing ammonia volatilization from diverse nitrogen fertilizers applied to different soil types and wheat straw mulching. J. Sci. Food Agr. 2011, 91, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Marcos, M.; Bertiller, M.; Cisneros, H.; Olivera, N. Nitrification and ammonia-oxidizing bacteria shift in response to soil moisture and plant litter quality in arid soils from the Patagonian Monte. Pedobiologia 2016, 59, 1–10. [Google Scholar] [CrossRef]
- Wang, X.; Park, S.; Lee, B.; Jeong, K. Changes in Nitrogen Mineralization as Affected by Soil Temperature and Moisture. J. Korean Soc. Grassl. Forage Sci. 2018, 38, 196–201. [Google Scholar] [CrossRef]
- Ma, L.; Guo, C.; Xin, X.; Yuan, S.; Wang, R. Effects of belowground litter addition, increased precipitation and clipping on soil carbon and nitrogen mineralization in a temperate steppe. Biogeosciences 2013, 10, 7361–7372. [Google Scholar] [CrossRef]
- Kiese, R.; Hewett, B.; Butterbach-Bahl, K. Seasonal dynamic of gross nitrification and N2O emission at two tropical rainforest sites in Queensland, Australia. Plant Soil 2008, 309, 105–117. [Google Scholar] [CrossRef]
- Guntiñas, M.; Leirós, M.; Trasar-Cepeda, C.; Gil-Sotres, F. Effects of moisture and temperature on net soil nitrogen mineralization: A laboratory study. Eur. J. Soil. Biol. 2012, 48, 73–80. [Google Scholar] [CrossRef]
- Butcher, K.; Nasto, M.; Norton, J.; Stark, J. Physical mechanisms for soil moisture effects on microbial carbon-use efficiency in a sandy loam soil in the western United States. Soil Biol. Biochem. 2020, 150, 107969. [Google Scholar] [CrossRef]
- Weerts, A.; Kandhai, D.; Bouten, W.; Sloot, P. Tortuosity of an unsaturated sandy soil estimated using gas diffusion and bulk soil electrical conductivity. Soil Sci. Soc. Am. J. 2001, 65, 1577–1584. [Google Scholar] [CrossRef]
- Rex, D.; Clough, T.; Lanigan, G.; Jansen-Willems, A.; Condron, L.; Richards, K.; Müller, C. Gross N transformations vary with soil moisture and time following urea deposition to a pasture soil. Geoderma 2021, 386, 114904. [Google Scholar] [CrossRef]
- Gleeson, D.; Müller, C.; Banerjee, S.; Ma, W.; Siciliano, S.; Murphy, D. Response of ammonia oxidizing archaea and bacteria to changing water filled pore space. Soil Biol. Biochem. 2010, 42, 1888–1891. [Google Scholar] [CrossRef]
- Sun, L.; Xia, Z.; Sang, C.; Wang, X.; Bai, E. Soil resource status affects the responses of nitrogen processes to changes in temperature and moisture. Biol. Fert. Soils 2019, 55, 629–641. [Google Scholar] [CrossRef]
- Klein, C.; Van Logtestijn, R. Denitrification in grassland soils in The Netherlands in relation to irrigation, N-application rate, soil water content and soil temperature. Soil Biol. Biochem. 1996, 28, 231–237. [Google Scholar] [CrossRef]
- Zhu, A.; Zhang, J.; Zhao, B.; Cheng, Z.; Li, L. Water balance and nitrate leaching losses under intensive crop production with Ochric Aquic Cambosols in North China Plain. Environ. Int. 2005, 31, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Funakawa, S.; Kosaki, T. Effect of repeated drying-rewetting cycles on microbial biomass carbon in soils with different climatic histories. Appl. Soil Ecol. 2017, 120, 1–7. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P. Effects of drying–rewetting frequency on soil carbon and nitrogen transformations. Soil Biol. Biochem. 2002, 34, 777–787. [Google Scholar] [CrossRef]
- Dijkstra, F.; Augustine, D.; Brewer, P.; von Fischer, C. Nitrogen cycling and water pulses in semiarid grasslands: Are microbial and plant processes temporally asynchronous? Oecologia 2012, 170, 799–808. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, L.; Bi, Q.; Dai, P.; Sun, D.; Sun, C.; Liu, W.; Lu, L.; Ni, W.; Lin, X. Comparative effects of 3,4-dimethylpyrazole phosphate (DMPP) and dicyandiamide (DCD) on ammonia-oxidizing bacteria and archaea in a vegetable soil. Appl. Microbiol. Biotechnol. 2015, 99, 477–487. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, H.; Shen, J.; He, J. Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J. 2012, 6, 1032–1045. [Google Scholar] [CrossRef]
- Lu, L.; Jia, Z. Urease gene-containing Archaea dominate autotrophic ammonia oxidation in two acid soils. Environ. Microbiol. 2013, 15, 1795–1809. [Google Scholar] [CrossRef]
- Pereira, E.; Poly, F.; Guillaumaud, N.; van Elsas, J.; Salles, J. Fluctuations in ammonia oxidizing communities across agricultural soils are driven by soil structure and pH. Front. Microbiol. 2012, 3, 77. [Google Scholar]
- Nicol, G.; Leininger, S.; Schlepe, C.; Prosser, J. The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ. Microbiol. 2008, 10, 2966–2978. [Google Scholar] [CrossRef]
- Song, H.; Che, Z.; Cao, W.; Huang, T.; Wang, J.; Dong, Z. Changing roles of ammonia-oxidizing bacteria and archaea in a continuously acidifying soil caused by over-fertilization with nitrogen. Environ. Sci. Pollut. Res. 2016, 23, 11964–11974. [Google Scholar] [CrossRef]
- He, J.; Hu, H.; Zhang, L. Current insights into the autotrophic thaumarchaeal ammonia oxidation in acidic soils. Soil Biol. Biochem. 2012, 55, 146–154. [Google Scholar] [CrossRef]
- Li, X.; Ying, J.; Ying, C.; Zhang, L.; Gao, Y.; Bai, Y. Effects of nitrogen addition on the abundance and composition of soil ammonia oxidizers in Inner Mongolia grassland. Acta Ecol. Sin. 2011, 31, 174–178. [Google Scholar] [CrossRef]
- Di, H.; Cameron, K.; Shen, J.; Winefield, C.; O’Callaghan, M.; Bowatte, S.; He, J. Ammonia oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions. Fems Microbiol. Ecol. 2010, 72, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Roesser, M.; Müller, V. Osmoadaptation in bacteria and archaea: Common principles and differences. Environ. Microbiol. 2001, 3, 743–754. [Google Scholar] [CrossRef]
- Schimel, J.; Balser, T.; Wallenstein, M. Microbial stress-response physiology and its implications for ecosystem function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef]
- Barnard, R.; Osborne, C.; Firestone, M. Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. ISME J. 2013, 7, 2229–2241. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.; Wallenstein, M. Soil microbial community response to drying and rewetting stress: Does historical precipitation regime matter? Biogeochemistry 2012, 109, 101–116. [Google Scholar] [CrossRef]
- Thion, C.; Prosser, J. Differential response of non-adapted ammonia oxidising archaea and bacteria to drying rewetting stress. FEMS Microbiol. Ecol. 2014, 90, 380–389. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).