Local Beneficial Microorganisms Impact Carbon and Nitrogen Mineralization in a Lixisol Incubated with Organic Waste Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil
2.2. Organic Waste Products
2.3. Local Beneficial Microorganisms
2.4. Incubation Experiment
2.5. Carbon Mineralization Kinetics
2.6. Dynamics of Organic Nitrogen Transformation into Its Main Mineral Forms
2.7. Statistical Analysis
3. Results
3.1. BM Microbial Composition
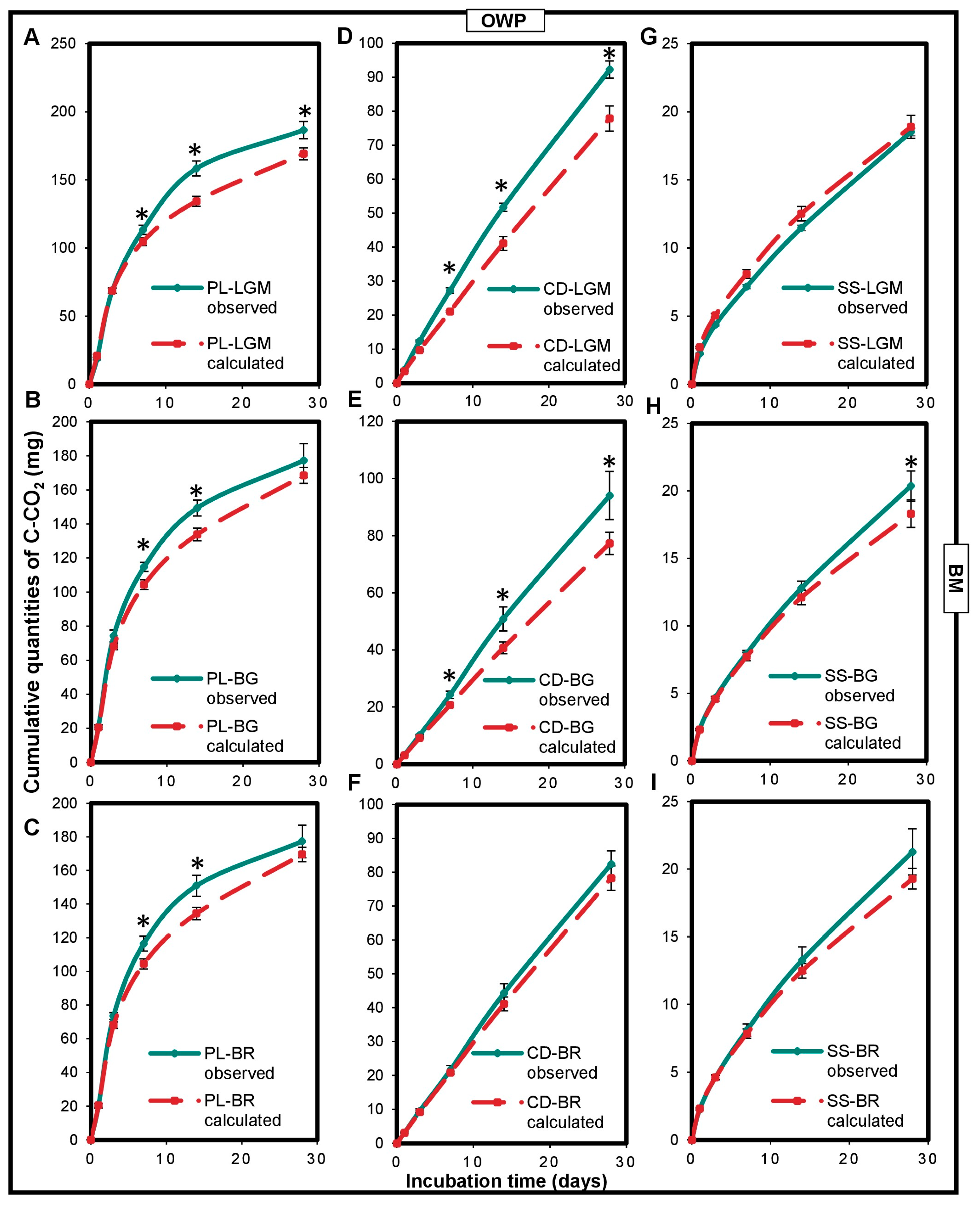
3.2. Impact of OWP or BM on Carbon Mineralization in Microcosms
3.3. Impact of the Interaction between OWP and BM on Microcosm Carbon Mineralization
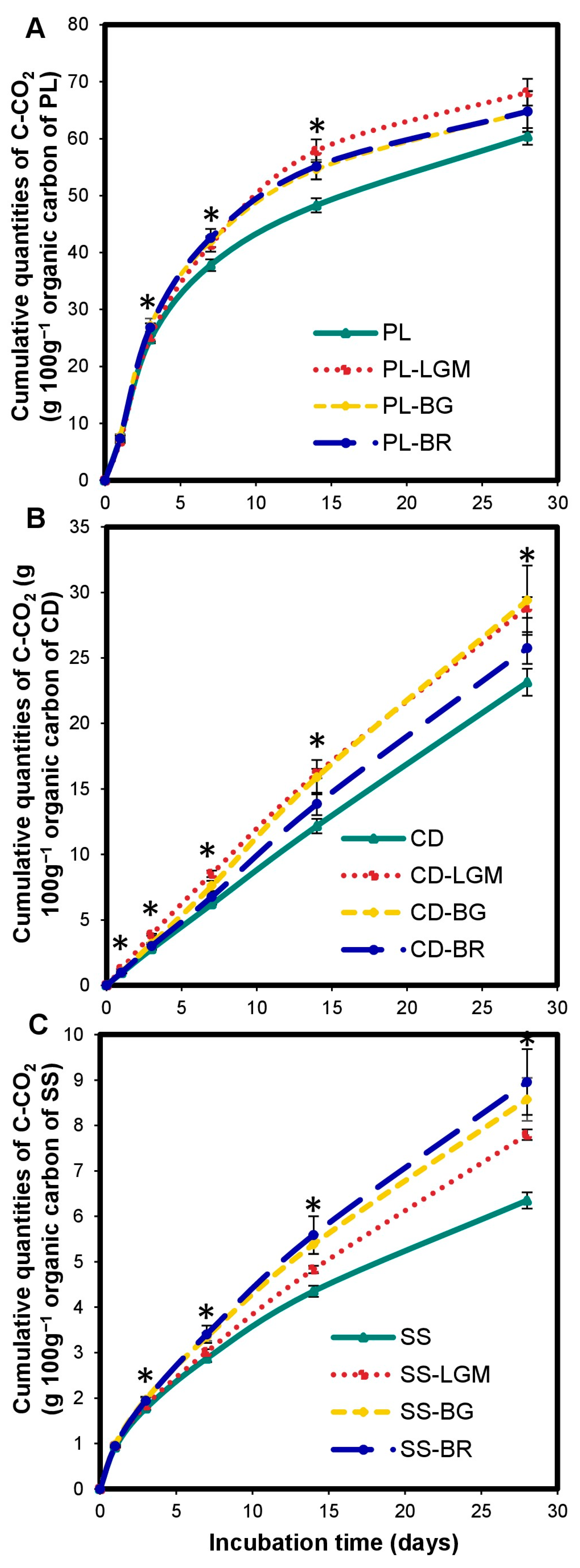
3.4. Impact of BM on OWP Carbon Mineralization
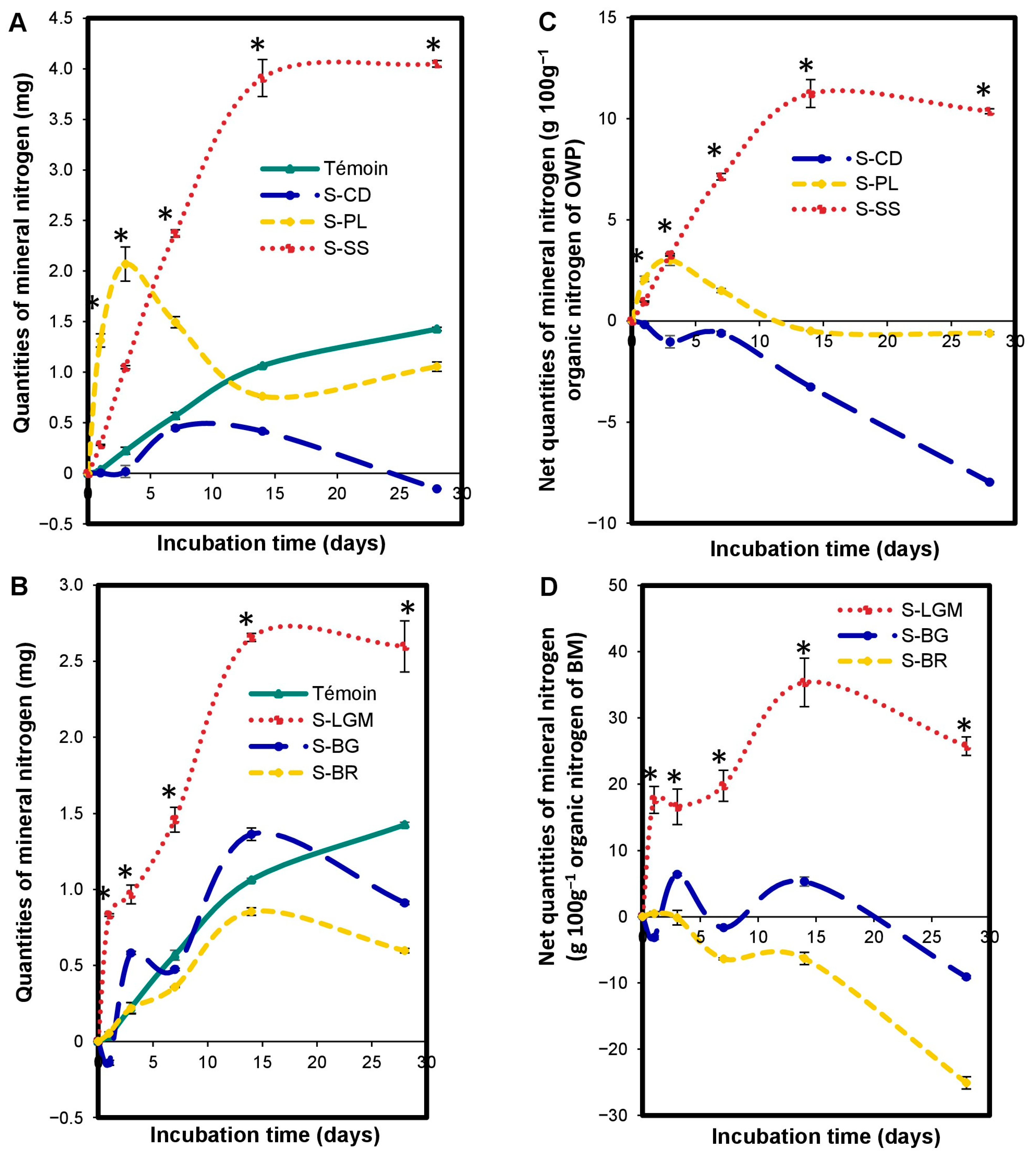
3.5. Impact of OWP or BM on Microcosm Nitrogen Mineralization
3.6. Impact of the Interaction between OWP and BM on Microcosm Nitrogen Mineralization
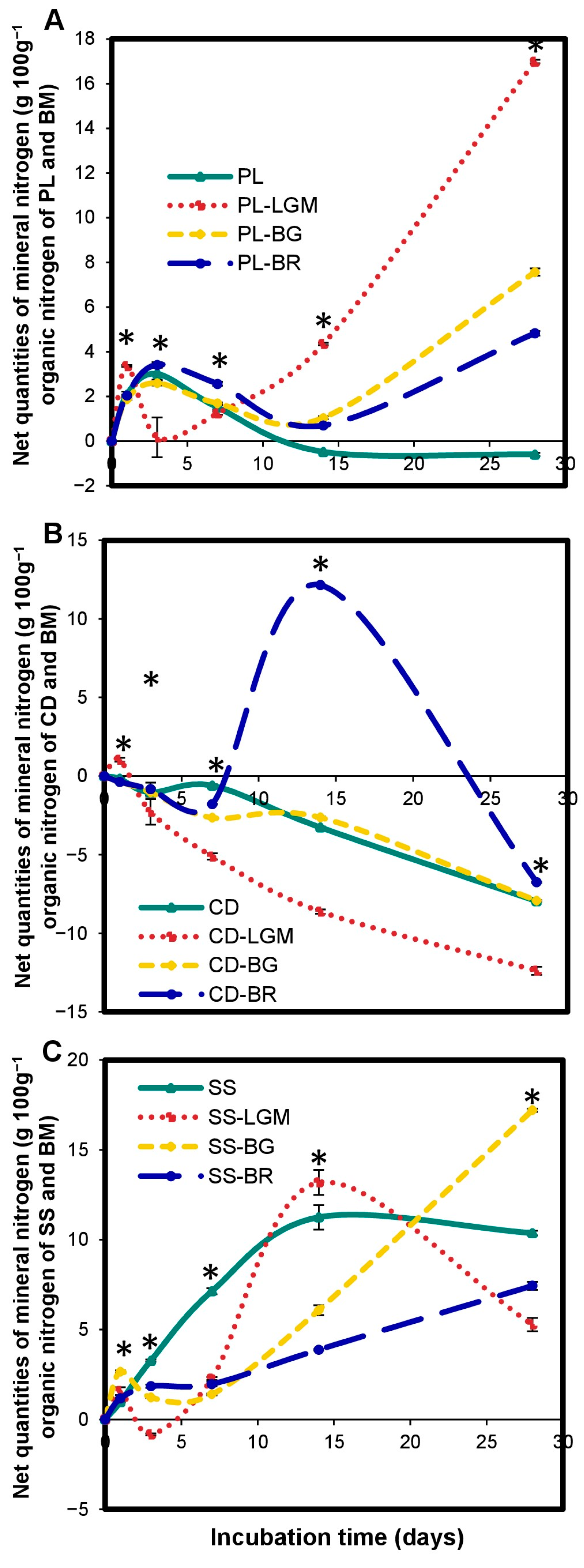
3.7. Impact of BM on OWP Nitrogen Mineralization
4. Discussion
4.1. Impact of OWP or BM on Carbon Mineralization in Microcosms
4.2. Impact of the Interaction between OWP and BM on Microcosm Carbon Mineralization
4.3. Impact of BM on OWP Carbon Mineralization
4.4. Impact of OWP or BM on Microcosm Nitrogen Mineralization
4.5. Impact of the Interaction between OWP and BM on Microcosm Nitrogen Mineralization
4.6. Impact of BM on OWP Nitrogen Mineralization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| OWP | organic waste products |
| BM | local beneficial microorganisms |
| PL | poultry litter |
| CD | cow dung |
| SS | sewage sludge |
| LGM | BM made using a forest litter collected in the Saint-Louis region and a carbon source of millet husks and groundnut shells |
| BG | BM made using a forest litter collected in the south of the Groundnut Basin and a carbon source of groundnut shells |
| BR | BM made using a forest litter collected in the south of the Groundnut Basin and a carbon source of rice bran |
| N-NO3 | nitrate–nitrogen |
| N-NH4 | ammonium–nitrogen |
| Nmin | net mineral nitrogen |
| C-CO2 | carbon of carbon dioxide |
| OC | organic carbon |
| ON | organic nitrogen |
| TOC | total organic carbon |
| TON | total organic nitrogen |
| C: N | carbon-to-nitrogen ratio |
| DOC | dissolved organic carbon |
| OTU | operational taxonomic units |
| Assim. P | assimilable phosphorus |
| WC | water content |
| CEC | cation exchange capacity |
| DM | dry matter |
References
- Marmo, L. EU strategies and policies on soil and waste management to offset greenhouse gas emissions. Waste Manag. 2008, 28, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Poore, J.; Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Francaviglia, R.; Almagro, M.; Vicente-Vicente, J.L. Conservation agriculture and soil organic carbon: Principles, processes, practices and policy options. Soil Syst. 2023, 7, 17. [Google Scholar] [CrossRef]
- Matson, P.A.; Naylor, R.; Ortiz-Monasterio, I. Integration of environmental, agronomic, and economic aspects of fertilizer management. Science 1998, 280, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.P.; Vitousek, P.M. Nitrogen in agriculture: Balancing the cost of an essential resource. annu. Rev. Environ. Resour. 2009, 34, 97–125. [Google Scholar] [CrossRef]
- Battye, W.; Aneja, V.P.; Schlesinger, W.H. Is nitrogen the next carbon? Earth’s Future 2017, 5, 894–904. [Google Scholar] [CrossRef]
- Pahalvi, H.N.; Rafiya, L.; Rashid, S.; Nisar, B.; Kamili, A.N. Chemical Fertilizers and Their Impact on Soil Health. In Microbiota and Biofertilizers, Vol 2: Ecofriendly Tools for Reclamation of Degraded Soil Environs; Dar, G.H., Bhat, R.A., Mehmood, M.A., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–20. ISBN 978-3-030-61010-4. [Google Scholar]
- Hoornweg, D.; Bhada-Tata, P.; Kennedy, C. Environment: Waste production must peak this century. Nature 2013, 502, 615–617. [Google Scholar] [CrossRef]
- Lehmann, J. Recycle waste for nourishing soils. Nature 2013, 504, 33. [Google Scholar] [CrossRef]
- Schiermeier, Q. Farmers dig into soil quality. Nature 2013, 502, 607. [Google Scholar] [CrossRef]
- Balasubramani, R.; Awasthi, M.K.; Varjani, S.; Karmegam, N. Aerobic and anaerobic digestion of agro-industrial and livestock wastes: A green and sustainable way toward the future. Agronomy 2023, 13, 2607. [Google Scholar] [CrossRef]
- Sharma, V.K.; Canditelli, M.; Fortuna, F.; Cornacchia, G. Processing of urban and agro-industrial residues by aerobic composting: Review. Energy Convers. Manag. 1997, 38, 453–478. [Google Scholar] [CrossRef]
- Schievano, A.; D’Imporzano, G.; Adani, F. Substituting energy crops with organic wastes and agro-industrial residues for biogas production. J. Environ. Manag. 2009, 90, 2537–2541. [Google Scholar] [CrossRef] [PubMed]
- Carpio, M.J.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S.; Marín-Benito, J.M. Effect of organic residues on pesticide behavior in soils: A review of laboratory research. Environments 2021, 8, 32. [Google Scholar] [CrossRef]
- Giusquiani, P.L.; Pagliai, M.; Gigliotti, G.; Businelli, D.; Benetti, A. Urban waste compost: Effects on physical, chemical, and biochemical soil properties. J. Environ. Qual. 1995, 24, 175–182. [Google Scholar] [CrossRef]
- Odlare, M.; Arthurson, V.; Pell, M.; Svensson, K.; Nehrenheim, E.; Abubaker, J. Land application of organic waste—effects on the soil ecosystem. Appl. Energy 2011, 88, 2210–2218. [Google Scholar] [CrossRef]
- Minasny, B.; Malone, B.P.; McBratney, A.B.; Angers, D.A.; Arrouays, D.; Chambers, A.; Chaplot, V.; Chen, Z.-S.; Cheng, K.; Das, B.S.; et al. Soil carbon 4 per mille. Geoderma 2017, 292, 59–86. [Google Scholar] [CrossRef]
- Lashermes, G.; Nicolardot, B.; Parnaudeau, V.; Thuriès, L.; Chaussod, R.; Guillotin, M.L.; Linères, M.; Mary, B.; Metzger, L.; Morvan, T.; et al. Typology of exogenous organic matters based on chemical and biochemical composition to predict potential nitrogen mineralization. Bioresour. Technol. 2010, 101, 157–164. [Google Scholar] [CrossRef]
- Bouajila, K.; Chibani, R.; Mechri, M.; Moussa, M.; Jeddi, F.B. Carbon and nitrogen mineralization dynamics in tow amended soils collected from the semi-arid and arid regions of Tunisia. Arab. J. Geosci. 2021, 14, 1005. [Google Scholar] [CrossRef]
- Pansu, M.; Thuriès, L. Kinetics of C and N mineralization, N immobilization and N volatilization of organic inputs in soil. Soil Biol. Biochem. 2003, 35, 37–48. [Google Scholar] [CrossRef]
- Birouste, M.; Kazakou, E.; Blanchard, A.; Roumet, C. Plant traits and decomposition: Are the relationships for roots comparable to those for leaves? Ann. Bot. 2012, 109, 463–472. [Google Scholar] [CrossRef]
- Kyulavski, V.; Recous, S.; Thuries, L.; Paillat, J.-M.; Garnier, P. Investigating interactions between sugarcane straw and organic fertilizers recycled together in a soil using modelling of C and N mineralization. Eur. J. Soil Sci. 2019, 70, 1234–1248. [Google Scholar] [CrossRef]
- Zhang, B.-Y.; Dou, S.; Guo, D.; Guan, S. Straw inputs improve soil hydrophobicity and enhance organic carbon mineralization. Agronomy 2023, 13, 2618. [Google Scholar] [CrossRef]
- Santos, C.; Fonseca, J.; Coutinho, J.; Trindade, H.; Jensen, L.S. Chemical properties of agro-waste compost affect greenhouse gas emission from soils through changed C and N mineralisation. Biol. Fertil. Soils 2021, 57, 781–792. [Google Scholar] [CrossRef]
- Kumar, K.; Goh, K.M. Crop Residues and Management Practices: Effects on Soil Quality, Soil Nitrogen Dynamics, Crop Yield, and Nitrogen Recovery. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: New York NY, USA, 1999; Volume 68, pp. 197–319. ISBN 0065-2113. [Google Scholar]
- Walker, D.J.; Clemente, R.; Bernal, M.P. Contrasting effects of manure and compost on soil pH, heavy metal availability and growth of Chenopodium album L. in a soil contaminated by pyritic mine waste. Chemosphere 2004, 57, 215–224. [Google Scholar] [CrossRef]
- Bolan, N.; Adriano, D.; Mahimairaja, S. Distribution and bioavailability of trace elements in livestock and poultry manure by-products. Crit. Rev. Environ. Sci. Technol. 2004, 34, 291–338. [Google Scholar] [CrossRef]
- Paul, E.A. The nature and dynamics of soil organic matter: Plant inputs, microbial transformations, and organic matter stabilization. Soil Biol. Biochem. 2016, 98, 109–126. [Google Scholar] [CrossRef]
- Hargreaves, J.C.; Adl, M.S.; Warman, P.R. A review of the use of composted municipal solid waste in agriculture. Agric. Ecosyst. Environ. 2008, 123, 1–14. [Google Scholar] [CrossRef]
- Iqbal, A.; Garnier, P.; Lashermes, G.; Recous, S. A new equation to simulate the contact between soil and maize residues of different sizes during their decomposition. Biol. Fertil. Soils 2014, 50, 645–655. [Google Scholar] [CrossRef]
- Lazicki, P.; Geisseler, D.; Lloyd, M. Nitrogen Mineralization from Organic Amendments Is Variable but Predictable; Wiley Online Library: New York, NY, USA, 2020; Volume 49, pp. 483–495. [Google Scholar]
- Khalil, M.I.; Hossain, M.B.; Schmidhalter, U. Carbon and nitrogen mineralization in different upland soils of the subtropics treated with organic materials. Soil Biol. Biochem. 2005, 37, 1507–1518. [Google Scholar] [CrossRef]
- Khalil, M.I.; Boeckx, P.; Rosenani, A.B.; Van Cleemput, O. Nitrogen transformations and emission of greenhouse gases from three acid soils of humid tropics amended with N sources and moisture regime. II. nitrous oxide and methane fluxes. Commun. Soil Sci. Plant Anal. 2001, 32, 2909–2924. [Google Scholar] [CrossRef]
- Pascual, J.A.; García, C.; Hernandez, T. Lasting microbiological and biochemical effects of the addition of municipal solid waste to an arid soil. Biol. Fertil. Soils 1999, 30, 1–6. [Google Scholar] [CrossRef]
- Hamarashid, N.H.; Othman, M.A.; Hussain, M.-A.H. Effects of soil texture on chemical compositions, microbial populations and carbon mineralization in soil. Egypt. J. Exp. Biol. 2010, 6, 59–64. [Google Scholar]
- Olle, M.; Williams, I.H. Effective microorganisms and their influence on vegetable production—A review. J. Hortic. Sci. Biotechnol. 2013, 88, 380–386. [Google Scholar] [CrossRef]
- Ney, L.; Franklin, D.; Mahmud, K.; Cabrera, M.; Hancock, D.; Habteselassie, M.; Newcomer, Q.; Dahal, S. Impact of inoculation with local effective microorganisms on soil nitrogen cycling and legume productivity using composted broiler litter. Appl. Soil Ecol. 2020, 154, 103567. [Google Scholar] [CrossRef]
- Mahmud, K.; Franklin, D.; Ney, L.; Cabrera, M.; Habteselassie, M.; Hancock, D.; Newcomer, Q.; Subedi, A.; Dahal, S. Improving inorganic nitrogen in soil and nutrient density of edamame bean in three consecutive summers by utilizing a locally sourced bio-inocula. Org. Agric. 2021, 11, 133–143. [Google Scholar] [CrossRef]
- Daly, M.J.; Stewart, D.P.C. Influence of “Effective Microorganisms” (EM) on vegetable production and carbon mineralization–a preliminary investigation. J. Sustain. Agric. 1999, 14, 15–25. [Google Scholar] [CrossRef]
- Ortiz, A.; Sansinenea, E. The role of beneficial microorganisms in soil quality and plant health. Sustainability 2022, 14, 5358. [Google Scholar] [CrossRef]
- Sousa, W.D.S.; de Pontes, J.R.V.; Melo, O.F.P. Effect of biofertilizer on soil fertility and lettuce nutrition. Rev. Agrogeoambient. 2020, 12, 26–39. [Google Scholar] [CrossRef]
- Joshi, H.; duttand, S.; Choudhary, P.; Mundra, S.L. Role of Effective Microorganisms (EM) in sustainable agriculture. Int. J. Curr. Microbiol. App. Sci. 2019, 8, 172–181. [Google Scholar] [CrossRef]
- Manresa, C.F.; Chaviano, P.F.F.; Calderón, D.M. Uso de microorganismos e cientes autóctonos, en el manejo de Meloidogyne incognita en el cultivo del tomate. Cent. Agrícola 2019, 46, 38–43. [Google Scholar]
- dos Santos, L.F.; Silva, M.D.C.S.; Lana, R.D.P.; Diogo, N.V.; Kasuya, M.C.M.; Ribeiro, K.G. Effective microorganisms: Microbial diversity and its effect on the growth of palisade grass. Trop. Grasslands-Forrajes Trop. 2020, 8, 177–186. [Google Scholar] [CrossRef]
- Hidalgo, D.; Corona, F.; Martín-Marroquín, J.M. Manure biostabilization by effective microorganisms as a way to improve its agronomic value. Biomass Convers. Biorefinery 2022, 12, 4649–4664. [Google Scholar] [CrossRef]
- Lambert, T.; Santiesteban, R.; Ceiro, W.G.; Fernández, M.E.; López, G.D.L.M.; Corrales, W.C. Efecto de bioproductos en la producción de Phaseolus vulgaris L. y Arachis hipogea L. Rev. Cienc. Agrícolas 2019, 36, 59–66. [Google Scholar] [CrossRef]
- Noda-Leyva, Y.; Martin-Martin, G.J. Effect of fertilization on the yield of Jatropha curcas (Linn.) and associated crops. Pastos y Forrajes 2020, 43, 79–87. [Google Scholar]
- Henriksen, T.; Breland, T. Carbon mineralization, fungal and bacterial growth, and enzyme activities as affected by contact between crop residues and soil. Biol. Fertil. Soils 2002, 35, 41–48. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, W.; Liu, Y.; Jia, Z.; Li, H.; Yang, Y.; Wang, D.; He, H.; Zhang, X. Identification of microbial strategies for labile substrate utilization at phylogenetic classification using a microcosm approach. Soil Biol. Biochem. 2021, 153, 107970. [Google Scholar] [CrossRef]
- Chen, R.; Senbayram, M.; Blagodatsky, S.; Myachina, O.; Dittert, K.; Lin, X.; Blagodatskaya, E.; Kuzyakov, Y. Soil C and N availability determine the priming effect: Microbial N mining and stoichiometric decomposition theories. Glob. Chang. Biol. 2014, 20, 2356–2367. [Google Scholar] [CrossRef]
- Lladó, S.; Žifčáková, L.; Větrovský, T.; Eichlerová, I.; Baldrian, P. Functional screening of abundant bacteria from acidic forest soil indicates the metabolic potential of acidobacteria subdivision 1 for polysaccharide decomposition. Biol. Fertil. Soils 2016, 52, 251–260. [Google Scholar] [CrossRef]
- Ling, L.; Luo, Y.; Jiang, B.; Lv, J.; Meng, C.; Liao, Y.; Reid, B.J.; Ding, F.; Lu, Z.; Kuzyakov, Y.; et al. Biochar induces mineralization of soil recalcitrant components by activation of biochar responsive bacteria groups. Soil Biol. Biochem. 2022, 172, 108778. [Google Scholar] [CrossRef]
- Ding, X.; Lu, X.; Ding, X.; Wu, B.; Wang, D.; Huang, L.; Wang, X.; Tan, Y.; Zhang, X.-X.; Liu, B. The total and functional bacterial community of nitrogen removal in the SND ditches. Int. Biodeterior. Biodegrad. 2017, 118, 102–109. [Google Scholar] [CrossRef]
- Shu, D.; He, Y.; Yue, H.; Wang, Q. Metagenomic and quantitative insights into microbial communities and functional genes of nitrogen and iron cycling in twelve wastewater treatment systems. Chem. Eng. J. 2016, 290, 21–30. [Google Scholar] [CrossRef]
- Zhu, X.; Mao, L.; Chen, B. Driving forces linking microbial community structure and functions to enhanced carbon stability in biochar-amended soil. Environ. Int. 2019, 133, 105211. [Google Scholar] [CrossRef] [PubMed]
- WRB, I.; Schád, P.; van Huyssteen, C.; Micheli, E. World Reference Base for Soil. Resources 2014, Update 2015; FAO: Rome, Italy, 2015; ISBN 978-92-5-108369-7, E-ISBN 978-92-5-108370-3. [Google Scholar]
- Sall, S.N.; Masse, D.; Hélène Diallo, N.; Sow, T.M.B.; Hien, E.; Guisse, A. Effects of residue quality and soil mineral n on microbial activities and soil aggregation in a tropical sandy soil in senegal. Eur. J. Soil Biol. 2016, 75, 62–69. [Google Scholar] [CrossRef]
- Tournier, E.; Amenc, L.; Pablo, A.-L.; Legname, E.; Blanchart, E.; Plassard, C.; Robin, A.; Bernard, L. Modification of a commercial DNA extraction kit for safe and rapid recovery of DNA and RNA simultaneously from soil, without the use of harmful solvents. MethodsX 2015, 2, 182–191. [Google Scholar] [CrossRef]
- Edgar, R.C. Uparse: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16s rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- AFNOR-XPU44-163; Soil Improvers and Growing Media—Characterization of Organic Matter by Potential Mineralization of Carbon and Nitrogen. AFNOR: Paris, France, 2009.
- Beauchamp, E.G.; Reynolds, W.D.; Brasche-Villeneuve, D.; Kirby, K. Nitrogen mineralization kinetics with different soil pretreatments and cropping histories. Soil Sci. Soc. Am. J. 1986, 50, 1478–1483. [Google Scholar] [CrossRef]
- Cabrera, M.L. Modeling the flush of nitrogen mineralization caused by drying and rewetting soils. Soil Sci. Soc. Am. J. 1993, 57, 63–66. [Google Scholar] [CrossRef]
- Tella, M.; Bravin, M.N.; Thuriès, L.; Cazevieille, P.; Chevassus-Rosset, C.; Collin, B.; Chaurand, P.; Legros, S.; Doelsch, E. Increased zinc and copper availability in organic waste amended soil potentially involving distinct release mechanisms. Environ. Pollut. 2016, 212, 299–306. [Google Scholar] [CrossRef]
- Thuriès, L.; Pansu, M.; Feller, C.; Herrmann, P.; Rémy, J.-C. Kinetics of added organic matter decomposition in a mediterranean sandy soil. Soil Biol. Biochem. 2001, 33, 997–1010. [Google Scholar] [CrossRef]
- Hernández, D.; Fernández, J.M.; Plaza, C.; Polo, A. Water-soluble organic matter and biological activity of a degraded soil amended with pig slurry. Sci. Total. Environ. 2007, 378, 101–103. [Google Scholar] [CrossRef]
- Cavalli, D.; Corti, M.; Baronchelli, D.; Bechini, L.; Marino Gallina, P. CO2 emissions and mineral nitrogen dynamics following application to soil of undigested liquid cattle manure and digestates. Geoderma 2017, 308, 26–35. [Google Scholar] [CrossRef]
- Reuland, G.; Sigurnjak, I.; Dekker, H.; Sleutel, S.; Meers, E. Assessment of the carbon and nitrogen mineralisation of digestates elaborated from distinct feedstock profiles. Agronomy 2022, 12, 456. [Google Scholar] [CrossRef]
- Dossa, E.; Khouma, M.; Diedhiou, I.; Sene, M.; Kizito, F.; Badiane, A.; Samba, S.; Dick, R. Carbon, nitrogen and phosphorus mineralization potential of semiarid sahelian soils amended with native shrub residues. Geoderma 2009, 148, 251–260. [Google Scholar] [CrossRef]
- Doelsch, E.; Masion, A.; Moussard, G.; Chevassus-Rosset, C.; Wojciechowicz, O. Impact of pig slurry and green waste compost application on heavy metal exchangeable fractions in tropical soils. Geoderma 2010, 155, 390–400. [Google Scholar] [CrossRef]
- Jaziri, S.; M’hamed, H.C.; Rezgui, M.; Labidi, S.; Souissi, A.; Rezgui, M.; Barbouchi, M.; Annabi, M.; Bahri, H. Long term effects of tillage–crop rotation interaction on soil organic carbon pools and microbial activity on wheat-based system in mediterranean semi-arid region. Agronomy 2022, 12, 953. [Google Scholar] [CrossRef]
- Miyittah, M.; Inubushi, K. Decomposition and CO2–C evolution of okara, sewage sludge, cow and poultry manure composts in soils. Soil Sci. Plant Nutr. 2003, 49, 61–68. [Google Scholar] [CrossRef]
- Zhao, N.-N.; Guggenberger, G.; Shibistova, O.; Thao, D.T.; Shi, W.J.; Li, X.G. Aspect-vegetation complex effects on biochemical characteristics and decomposability of soil organic carbon on the eastern qinghai-tibetan plateau. Plant Soil 2014, 384, 289–301. [Google Scholar] [CrossRef]
- Askri, A.; Laville, P.; Trémier, A.; Houot, S. Influence of origin and post-treatment on greenhouse gas emissions after anaerobic digestate application to soil. Waste Biomass Valorization 2016, 7, 293–306. [Google Scholar] [CrossRef]
- Levavasseur, F.; Lashermes, G.; Mary, B.; Morvan, T.; Nicolardot, B.; Parnaudeau, V.; Thuriès, L.; Houot, S. Quantifying and simulating carbon and nitrogen mineralization from diverse exogenous organic matters. Soil Use Manag. 2021, 38, 411–425. [Google Scholar] [CrossRef]
- Sjöberg, G.; Nilsson, S.I.; Persson, T.; Karlsson, P. Degradation of hemicellulose, cellulose and lignin in decomposing spruce needle litter in relation to N. Soil Biol. Biochem. 2004, 36, 1761–1768. [Google Scholar] [CrossRef]
- Fioretto, A.; Di Nardo, C.; Papa, S.; Fuggi, A. Lignin and cellulose degradation and nitrogen dynamics during decomposition of three leaf litter species in a mediterranean ecosystem. Soil Biol. Biochem. 2005, 37, 1083–1091. [Google Scholar] [CrossRef]
- Lashermes, G.; Nicolardot, B.; Parnaudeau, V.; Thuriès, L.; Chaussod, R.; Guillotin, M.L.; Linères, M.; Mary, B.; Metzger, L.; Morvan, T.; et al. Indicator of potential residual carbon in soils after exogenous organic matter application. Eur. J. Soil Sci. 2009, 60, 297–310. [Google Scholar] [CrossRef]
- Sarsan, S.; Pandiyan, A.; Rodhe, A.V.; Jagavati, S. Synergistic Interactions Among Microbial Communities. In Microbes in Microbial Communities: Ecological and Applied Perspectives; Singh, R.P., Manchanda, G., Bhattacharjee, K., Panosyan, H., Eds.; Springer Singapore: Singapore, 2021; pp. 1–37. ISBN 978-981-16-5617-0. [Google Scholar]
- Molina-Herrera, S.; Romanyà, J. Synergistic and antagonistic interactions among organic amendments of contrasted stability, nutrient availability and soil organic matter in the regulation of C mineralisation. Eur. J. Soil Biol. 2015, 70, 118–125. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, G.; Guo, T.; He, P.; Wang, X.; Zhou, W. Evident variations of fungal and actinobacterial cellulolytic communities associated with different humified particle-size fractions in a long-term fertilizer experiment. Soil Biol. Biochem. 2017, 113, 1–13. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, J.; Pan, G.; Liu, X.; Zhang, X.; Li, L.; Bian, R.; Cheng, K.; Jinwei, Z. Biochar decreased microbial metabolic quotient and shifted community composition four years after a single incorporation in a slightly acid rice paddy from southwest China. Sci. Total. Environ. 2016, 571, 206–217. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Li, J.; Kumar, S.; Awasthi, S.K.; Wang, Q.; Chen, H.; Wang, M.; Ren, X.; Zhang, Z. Effects of biochar amendment on bacterial and fungal diversity for co-composting of gelatin industry sludge mixed with organic fraction of municipal solid waste. Bioresour. Technol. 2017, 246, 214–223. [Google Scholar] [CrossRef]
- Li, S.; Wang, S.; Fan, M.; Wu, Y.; Shangguan, Z. Interactions between biochar and nitrogen impact soil carbon mineralization and the microbial community. Soil Tillage Res. 2020, 196, 104437. [Google Scholar] [CrossRef]
- Jensen, E.S. Nitrogen immobilization and mineralization during initial decomposition of15N-labelled pea and barley residues. Biol. Fertil. Soils 1997, 24, 39–44. [Google Scholar] [CrossRef]
- Morvan, T.; Nicolardot, B. Role of organic fractions on C decomposition and N mineralization of animal wastes in soil. Biol. Fertil. Soils 2009, 45, 477–486. [Google Scholar] [CrossRef]
- Abera, G.; Wolde-meskel, E.; Bakken, L.R. Carbon and nitrogen mineralization dynamics in different soils of the tropics amended with legume residues and contrasting soil moisture contents. Biol. Fertil. Soils 2012, 48, 51–66. [Google Scholar] [CrossRef]
- Yagüe, M.R.; Lobo, C.; García, P. Organic fertilization induces changes in soil nitrogen mineralization and enzyme activities. Plant Soil Environ. 2023, 69, 38–43. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Khera, T.S.; Doran, J.W. Mineralization and denitrification in upland, nearly saturated and flooded subtropical soilii. effect of organic manures varying in N content and C:N ratio. Biol. Fertil. Soils 2000, 31, 168–174. [Google Scholar] [CrossRef]
- Mendoza, O.; De Neve, S.; Deroo, H.; Sleutel, S. Mineralisation of ryegrass and soil organic matter as affected by ryegrass application doses and changes in soil structure. Biol. Fertil. Soils 2022, 58, 679–691. [Google Scholar] [CrossRef]
- Pathak, H.; Rao, D.L.N. Carbon and nitrogen mineralization from added organic matter in saline and alkali soils. Soil Biol. Biochem. 1998, 30, 695–702. [Google Scholar] [CrossRef]
- Parnaudeau, V.; Génermont, S.; Hénault, C.; Farrugia, A.; Robert, P.; Nicolardot, B. Measured and simulated nitrogen fluxes after field application of food-processing and municipal organic wastes. J. Environ. Qual. 2009, 38, 268–280. [Google Scholar] [CrossRef]
- Weyers, S.L.; Das, K.C.; Gaskin, J.W.; Liesch, A.M. Pine chip and poultry litter derived biochars affect C and N dynamics in two Georgia, USA, ultisols. Agronomy 2023, 13, 531. [Google Scholar] [CrossRef]
- Khalil, M.; Rosenani, A.; Van Cleemput, O.; Boeckx, P.; Shamshuddin, J.; Fauziah, C. Nitrous oxide production from an ultisol of the humid tropics treated with different nitrogen sources and moisture regimes. Biol. Fertil. Soils 2002, 36, 59–65. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Philippot, L. Insights into the resistance and resilience of the soil microbial community. FEMS Microbiol. Rev. 2013, 37, 112–129. [Google Scholar] [CrossRef]
- Gorliczay, E.; Boczonádi, I.; Kiss, N.É.; Tóth, F.A.; Pabar, S.A.; Biró, B.; Kovács, L.R.; Tamás, J. Microbiological effectivity evaluation of new poultry farming organic waste recycling. Agriculture 2021, 11, 683. [Google Scholar] [CrossRef]
- Craine, J.M.; Morrow, C.; Fierer, N. Microbial nitrogen limitation increases decomposition. Ecology 2007, 88, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- De Neve, S.; Hofman, G. Quantifying soil water effects on nitrogen mineralization from soil organic matter and from fresh crop residues. Biol. Fertil. Soils 2002, 35, 379–386. [Google Scholar] [CrossRef]
- Nahm, K.H. Evaluation of the nitrogen content in poultry manure. World’s Poult. Sci. J. 2003, 59, 77–88. [Google Scholar] [CrossRef]
- Santiago, S.; Geisseler, D. Effects of moisture contents in incorporated residues and soil on net nitrogen mineralization in a laboratory study. Agrosystems Geosci. Env. 2022, 5, e20268. [Google Scholar] [CrossRef]
- Bengtsson, G.; Bengtson, P.; Månsson, K.F. Gross nitrogen mineralization-, immobilization-, and nitrification rates as a function of soil C/N ratio and microbial activity. Soil Biol. Biochem. 2003, 35, 143–154. [Google Scholar] [CrossRef]
- Chu, H.; Fujii, T.; Morimoto, S.; Lin, X.; Yagi, K.; Hu, J.; Zhang, J. Community structure of ammonia-oxidizing bacteria under long-term application of mineral fertilizer and organic manure in a sandy loam soil. Appl. Env. Microbiol. 2007, 73, 485–491. [Google Scholar] [CrossRef]
- Andrianarisoa, K.S.; Zeller, B.; Dupouey, J.L.; Dambrine, E. Comparing indicators of N status of 50 beech stands (Fagus sylvatica L.) in northeastern france. For. Ecol. Manag. 2009, 257, 2241–2253. [Google Scholar] [CrossRef]
- Hestrin, R.; Enders, A.; Lehmann, J. Ammonia volatilization from composting with oxidized biochar. J. Env. Qual. 2020, 49, 1690–1702. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, H.; Lv, J. Evaluation of the applicability of organic amendments from microbially driven carbon and nitrogen transformations. Sci. Total Environ. 2022, 817, 153020. [Google Scholar] [CrossRef]


| Soil | CD | PL | SS | LGM | BG | BR | |
|---|---|---|---|---|---|---|---|
| TOC (g 100 g−1 DM) | 0.28 | 31.99 | 27.36 | 23.75 | 28.99 | 34.24 | 23.83 |
| TON (g kg−1 DM) | 0.20 | 19.70 | 65.00 | 25.50 | 11.00 | 9.70 | 6.90 |
| N-NO3 (mg kg−1 DM) | 0.80 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| N-NH4 (mg kg−1 DM) | 5.40 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| C: N | 13.00 | 16.24 | 4.21 | 9.31 | 26.35 | 35.30 | 34.54 |
| Total P (mg kg−1 DM) | 65 | 3493 | 12,941 | 9274 | 1164 | 580 | 452 |
| Assim. P (mg kg−1 DM) | 7 | 762 | 1141 | 196 | 360 | 192 | 162 |
| CEC (cmol(+) kg−1 DM) | 1.47 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| WC (g 100 g−1) | 3.32 | 68.74 | 8.12 | 3.74 | 56.66 | 59.00 | 64.12 |
| pH(H2O) | 6.67 | 7.65 | 7.57 | 6.98 | 7.09 | 7.28 | 7.87 |
| Sand (g 100 g−1 DM) | 83.50 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Silt (g 100 g−1 DM) | 9.50 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Clay (g 100 g−1 DM) | 5.40 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Names | Soil | OWP | BM | |||||
|---|---|---|---|---|---|---|---|---|
| Generic | Specific | CD | PL | SS | LGM | BG | BR | |
| Control | Control | x | ||||||
| S-OWPs | S-CD | x | x | |||||
| S-PL | x | x | ||||||
| S-SS | x | x | ||||||
| S-BMs | S-LGM | x | x | |||||
| S-BG | x | x | ||||||
| S-BR | x | x | ||||||
| S-OWPs-BMs | S-CD-LGM | x | x | x | ||||
| S-CD-BG | x | x | x | |||||
| S-CD-BR | x | x | x | |||||
| S-PL-LGM | x | x | x | |||||
| S-PL-BG | x | x | x | |||||
| S-PL-BR | x | x | x | |||||
| S-SS-LGM | x | x | x | |||||
| S-SS-BG | x | x | x | |||||
| S-SS-BR | x | x | x | |||||
| Bacteria (% OTU) | Groups | Genus | LGM | BG | BR |
| Proteobacteria | Acetobacter | 0.01 | 28.45 | 0.81 | |
| Acinetobacter | 0.01 | 0.01 | 10.79 | ||
| Pseudomonas | 0.01 | 0.01 | 3.09 | ||
| Enterobacter | 0.07 | 0.34 | 9.19 | ||
| Pantoea | 0.30 | 0.13 | 0.07 | ||
| Rhizobium | 0.81 | 0.05 | 0.22 | ||
| Methylobacterium | 0.04 | 0.01 | 0.003 | ||
| Stenotrophomonas | 0.01 | 0.01 | 17.82 | ||
| Burkholderia | 0.01 | 0.01 | 0.001 | ||
| Bradyrhizobium | 0.01 | 0.03 | 0 | ||
| Mesorhizobium | 0.20 | 0 | 0 | ||
| Devosia | 0.90 | 0.03 | 0.01 | ||
| Pseudoaminobacter | 0.01 | 0 | 0 | ||
| Actinobacteria | Mycobacterium | 0.60 | 0.01 | 0.02 | |
| Firmicutes | Lactobacillus | 61.51 | 40.00 | 6.95 | |
| Bacillus | 9.63 | 5.35 | 1.81 | ||
| Weissella | 1.87 | 5.08 | 0.28 | ||
| Paenibacillus | 1.17 | 0.27 | 0.47 | ||
| Clostridium | 0.01 | 8.01 | 0.28 | ||
| Lysinibacillus | 1.11 | 0.83 | 4.41 | ||
| Rummeliibacillus | 0.07 | 2.92 | 1.38 | ||
| Pediococcus | 1.21 | 0.74 | 0.03 | ||
| Virgibacillus | 1.62 | 0.01 | 0.003 | ||
| Bacteroidota | Sphingobacterium | 0.01 | 0.02 | 23.70 | |
| Chryseobacterium | 0 | 0 | 5.29 | ||
| Fungi (% OTU) | Ascomycota | Neoascochyta | 0.001 | 33.75 | 35.18 |
| Ascochyta | 2.85 | 3.59 | 10.98 | ||
| Myrothecium | 41.84 | 2.13 | 1.77 | ||
| Cladosporium | 24.82 | 0.01 | 0.024 | ||
| Colletotrichum | 10.67 | 0.05 | 0.56 | ||
| Basidiomycota | Yueomyces | 3.36 | 2.11 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noumsi-Foamouhoue, E.; Legros, S.; Fernandes, P.; Thuriès, L.; Assigbetsé, K.; Kane, A.; Feder, F.; Médoc, J.-M. Local Beneficial Microorganisms Impact Carbon and Nitrogen Mineralization in a Lixisol Incubated with Organic Waste Products. Agronomy 2023, 13, 2791. https://doi.org/10.3390/agronomy13112791
Noumsi-Foamouhoue E, Legros S, Fernandes P, Thuriès L, Assigbetsé K, Kane A, Feder F, Médoc J-M. Local Beneficial Microorganisms Impact Carbon and Nitrogen Mineralization in a Lixisol Incubated with Organic Waste Products. Agronomy. 2023; 13(11):2791. https://doi.org/10.3390/agronomy13112791
Chicago/Turabian StyleNoumsi-Foamouhoue, Emmanuel, Samuel Legros, Paula Fernandes, Laurent Thuriès, Komi Assigbetsé, Aboubacry Kane, Frédéric Feder, and Jean-Michel Médoc. 2023. "Local Beneficial Microorganisms Impact Carbon and Nitrogen Mineralization in a Lixisol Incubated with Organic Waste Products" Agronomy 13, no. 11: 2791. https://doi.org/10.3390/agronomy13112791
APA StyleNoumsi-Foamouhoue, E., Legros, S., Fernandes, P., Thuriès, L., Assigbetsé, K., Kane, A., Feder, F., & Médoc, J.-M. (2023). Local Beneficial Microorganisms Impact Carbon and Nitrogen Mineralization in a Lixisol Incubated with Organic Waste Products. Agronomy, 13(11), 2791. https://doi.org/10.3390/agronomy13112791







