Abstract
The aim of the study was to examine the effects of application of plant-growth regulators (PGR) on the growth of paulownia plants and evaluate their usefulness in paulownia nurseries. The experiment was carried out on the paulownia interspecific hybrid (Paulownia elongata × Paulownia fortunei) ‘Oxytree’. Micropropagated plants grown in pots were sprayed with PGR solutions. Gibberellins (GA3, GA4+GA7), 6-benzyladenine (BA), triclopyr (TPA), ethephon (ETH), daminozide (DA), prohexadione-Ca (PH), and trinexapac-ethyl (TE) were used. They were included in commercial products (Florgib 245 mg dm−3, Gibb plus 11 SL 5 cm3 dm−3, Globaryll 100 SL 1.5 cm3 dm−3, Topper 10 ST 200 mg dm−3, Agrostym 480 SL 2 cm3 dm−3, B-Nine 85 SG 2 g dm−3, Regalis plus 10 WG 2.5 g dm−3, Moddus 250 EC 1 cm3 dm−3, respectively). None of the studied preparations modified positive correlations between the longitudinal vs. transverse growth of stems and growth of leaves. Not only GA3, GA4+GA7, but also BA (cytokinin) and unexpectedly TE (retardant) stimulated stem elongation and thickening. Furthermore, the effect of TE lasted longer than influence of GAx and BA. Leaves of TE-treated plants were distinguished by a higher chlorophyll a/b ratio, and a lower relative chlorophyll content and efficiency of photosystem II (Fv/Fm, Fv/F0). TPA strongly deformed the stems and leaves of liners. Other regulators (ETH, DA, PH) retarded the diameter growth of stems and leaf expansion. Some results obtained by GAx, BA, and especially by TE treatment, might be beneficial for paulownia grown as ornamental and/or timber tree.
Keywords:
acylcyclohexanediones; auxin; cytokinin; ethylene; gibberellins; retardants; multipurpose trees 1. Introduction
Paulownia sp. is a genus of increasing economic importance [1] which includes trees native to central China, but widely distributed throughout China, Korea, and Japan [2]. They belong to the fastest growing trees in the world [2]. However, in the opinion of Young and Lundgren [3], the mechanism behind their rapid growth remains unknown and paulownias are not C4 plants. Currently having risen in popularity, paulownias are grown in many countries, including North America, Australia, and western and south Europe, not only as ornamental but as multi-purpose trees [1]. They are considered medicinal plants, used for phytoremediation, reclamation, and reforestation, and grown for biomass and timber [1,2]. An increased demand for paulownia nursery plants to establish new plantations has been observed in Poland. Paulownia plants can be propagated generatively and vegetatively [4,5,6,7]. As paulownias are open-pollinating plants, propagation by seeds does not ensure the maintenance of the cultivar’s characteristics in progeny. Additionally, in the case of paulownia interspecific hybrids, like ‘Oxytree’ (Paulownia elongata × Paulownia fortunei), generative propagation is not possible because they do not produce fruits and seeds. Therefore, valuable paulownia cultivars should be propagated vegetatively (cloned). The conventional method is propagation by root cuttings prepared from juvenile plants. However, it is unreliable and inefficient, especially in the case of mature trees. Thus, it does not provide a sufficient number of uniform nursery plants of good quality. An alternative is propagation through in vitro axillary shoot cultures. This method of micropropagation of various Paulownia species was elaborated in the early 1980s [1,8] and has been improved in many laboratories subsequently [6,7,9,10,11,12,13,14,15,16]. Now it is easy and very efficient, although expensive, and allows one to produce large quantities of healthy plants and to deliver them at the desired time. There are some other problems with production of paulownia plantlets of high quality well prepared to establish field plantations in the spring in Poland. Paulownia is fast growing, light-demanding genus [2]. Therefore, paulownia liners grown in late winter/early spring in glasshouses in unfavorable light conditions (short days, low light intensity) are subject to partial etiolation. They produce long shoots which are fragile and prone to damage during transportation and planting in the field. The question arises whether it is possible to counteract this phenomenon and obtain more compact plants using PGRs, as in the case of ornamental plants [17,18]. Plant-growth regulators (PGRs) are a group of phytohormones (among others: auxins, cytokinins, gibberellins, abscisic acid, ethylene), and their synthetic analogues and inhibitors [19]. They are small signaling molecules that profoundly modulate the growth and development of plants, affecting cell division and enlargement, growth traits, metabolic processes, vascular patterning, flowering, and fruit and seed development [19,20]. They can change regarding photoassimilate distribution, nutrient acquisition, stress tolerance, etc. There is a large quantity of information about the properties of individual PGRs, their biological activity, and practical applications. Some of these are worth attention and study because they may improve the quality of paulownia nursery plants and their field performance. It is well-known that various plant-growth regulators strongly influence development and dormancy of plants and modify elongation, thickness, and lignification of shoots [17,21,22,23,24,25,26,27,28] The retardants and ethephon are widely used to limit vegetative growth and improve the compactness of ornamentals and fruit woody plants or prevent the lodging of cereals [17,29,30]. On the other hand, some growth stimulants, like cytokinins and gibberellins, may induce vegetative growth of shoots and prolong the (pseudo)juvenile plant status [31,32]. Application of cytokinins, gibberellins, and retardants may increase the tolerance of abiotic and biotic stresses [17,19,22,29,32,33,34,35]. Therefore, the aim of the present study was to examine the effects of application of some PGR-containing commercial products and to assess their usefulness for paulownia nurseries and plantations. To our best knowledge, information on the response of paulownias on plant-growth regulators (except for in vitro cultures) is not presented. It is also scarce for other deciduous trees.
2. Materials and Methods
2.1. Plant Material and Treatments
The experiment was carried out on the paulownia interspecific (Paulownia elongata × Paulownia fortunei) hybrid ‘Oxytree’(in vitro clone 112). The plantlets were micropropagated by ‘Viver i Laboratori’ in Spain (https://invitro.es/, accessed on 1 April 2018) and kindly provided by Oxytree Solutions Poland S.A. Details of the micropropagation procedure were not provided. Nevertheless, to the best of our knowledge, paulownias are micropropagated in a very similar way in many laboratories. This is generally based on the multiplication of axillary shoots on MS medium supplemented with BA (0.5–2.0 mg dm−3), NAA, IAA, or IBA, and sometimes GA3 (all at 0.1 mg dm−3), sucrose (20–30 g dm−3), pH 5.7, solidified with agar (7.0–8.0 g dm−3). The in vitro cultures were grown in a growth chamber at 25–26 °C/20–23 °C day/night (d/n) temperatures and a 16 h/8 h (d/n) photoperiod. Light was provided by cool white fluorescent lamps (26–60 µmol m−2 s−1 PPFD). The obtained shoots (about 2–3 cm long) were rooted in in vitro MS basal medium without PGRs. The rooted plantlets were transplanted into pots filled with peat substrate and acclimatized in vivo [6,7,8,9,10,11,13,14,15].
The provided paulownia plantlets were about 10 cm high and grew in small pots (7 cm × 7 cm × 8 cm). They were replanted into 2.1 dm−3 (10 cm × 10 cm × 21 cm) pots filled with a commercial peat-moss substratum (pHH2O 5.5–6.5; salinity 1.5–2.5 g NaCl dm−3; N 144 mg dm−3, P 132 mg dm−3, K 216 mg dm−3) designed for vegetable liners. Subsequently, the old shoots were cut off and only one new shoot accretion was allowed to grow after plant regrowth. As the experiment lasted 3 months (beginning of February–end of April 2019) and visible symptoms of nutrient deficit were not observed, the plants were not fertilized additionally except for one foliar spray (0.1%) with YARA KristalonTM green (N-18%, P-18%, K-18%, Mg-3%, S-5%, B-0.025%, Cu-0.01%, Fe-0.07%, Mn-0.04%, Mo-0.004%, Zn 0.025%) on 12 March.
They were grown in the greenhouse at 20 ± 5 °C/15 ± 5 °C (day/night) under a 14 h/10 h (day/night) photoperiod. The sunlight were supplemented with artificial metal-halogen light (85 µmol m−2 s−1 PAR) when solar radiation dropped under 130 µmol m−2 s−1 PAR. The plants were treated with solutions of commercially available preparations containing plant-growth regulators (PGR) which are allowed to be used in agricultural or horticultural practice in Poland. The most important reason for choosing specific PGRs was to obtain more compact liners (ethepon, retardants, cytokinin), as in the case of many ornamental plants. The second was to counteract the sometimes-occurring phenomenon of premature dormancy and cessation of growth in plants (gibberellins, cytokinin, auxin). Thus, the idea of the present work was to improve the quality of paulownia nursery plants. They were diluted in deionised water (6.6 µS cm−1) and applied immediately after preparation. Table 1 presents the doses of preparations and PGRs. They were chosen based on the results of preliminary experiments carried out on other paulownia clones. They were conducted on a small number of plants (4 per treatment). The plants were not measured, only observed and assessed. Thus, the obtained results are not presented. The only triclopyr dose was set on the basis of the average concentration given in the preparation leaflet. The adjuvants, Agrigent Flipper (0.5 cm3 dm−3) and Agrigent Activ 5 (1 cm3 dm−3), were added into the working mixtures. The control plants were sprayed with a solution of the aforementioned adjuvants. The acidity of the working solutions was in the range of 2.3–3.6 pH (Table 1). Before treatment, in the morning, the plants were watered abundantly. Ten potted plants growing together in a plastic tray were simultaneously sprayed with solutions of PGRs and adjuvants with a ‘Kwazar’ pump sprayer at a pressure of 0.3–0.35 MPa on 1 March 2018. A volume of 200 cm3 of working solution per tray (average 20 cm3 per plant) was applied. The few drops falling from the leaves went mainly into pots or a tray. To improve the effectiveness of the uptake of PGRs, the treatment was performed at dusk (after 3.30 p.m.) at 18 °C and about 30% relative air humidity. The application of PGRs was not repeated. The content of active chemicals in the plants after treatment was not determined.

Table 1.
The list of tested preparations/plant-growth regulators.
2.2. Observations and Measurements
Nine groups consisting of 40 similar plants were created before treatment to reduce variability. The basic measurements of shoots (length, diameter) and leaves (length, width, length/width ratio) was performed on 28 February 2018. The relative chlorophyll content expressed in SPAD units was determined using a portable SPAD-502 Plus chlorophyll meter. The second (from the top) fully developed leaf was chosen. Similar measurements were conducted about one and five weeks after treatment (9 March, 6 April 2018). Additionally, the real chlorophyll and carotenoid content was measured and chlorophyll fluorescence analyses were performed. They were performed on 16 plants typical for each treatment. The content of photosynthetic dyes was determined by the spectrophotometric method [36]. Chlorophyll fluorescence was determined on dark-adapted (at least 30 min) leaf material using an IMAGING-PAM M-Series chlorophyll fluorimeter (MINI version manufactured by the Heinz Walz). The initial (F0) and maximal fluorescence (Fm), as well as their derivatives, variable fluorescence (Fv), maximal quantum yield of PSII (Fv/Fm), and potential activity of PSII (Fv/F0), were considered.
2.3. Data Analyses
Forty plants (4 replications × 10 plants) represented each treatment. The collected data were submitted to ANOVA and LSD mean separation test at p < 0.05 significance level. Cluster analysis according to Ward’s method was also performed. The following traits were used for agglomeration: shoot length and diameter, leaf length and width, relative chlorophyll content, real chlorophyll and carotenoid content, maximal quantum yield of PSII (Fv/Fm), and potential activity of PSII (Fv/F0) The Statistica v.12 computer software was used.
3. Results
Some symptoms of PGR toxicity were found four days after treatment. Small light spots and/or slight necrosis were sporadically observed on the older leaves sprayed with solutions of retardants (daminozide, prohexadione-Ca, trinexapac-ethyl). They were not noticed on young leaves nor on plants treated with other regulators. Strong distortions of stems and leaf petioles were observed in the case of triclopyr application (Figure 1a). In an additional experiment, it was found that triclopyr did not work phytotoxically only in a dose four times lower (Figure 1h). Cytokinin BA moderately counteracted apical domination, as the intensive growth of side shoots was not observed (Figure 1b,f).

Figure 1.
Paulownia ‘Oxytree’ plants, 5 weeks after PGR treatment: (a)—distortion of shoots-TPA (Topper, 200 mg dm−3), (b)—activation of axillary buds-BA (Globaryll, 1.5 cm3 dm−3), (c)—control (without growth regulators), (d)—GA4+GA7 (Gibb plus, 5 cm3 dm−3), (e)—GA3 (Florgib, 245 mg dm−3), (f)—BA (Globaryll, 1.5 cm3 dm−3), (g)—TPA (Topper, 200 mg dm−3), (h)—TPA (Topper, 0/12/25/50/100/200 mg dm−3, respectively), (i)—ETH (Agrostym, 2 cm3 dm−3), (j)—DA (B-Nine, 2 g dm−3), (k)—PH (Regalis plus, 2.5 g dm−3), (l)—TE (Moddus, 1 cm3 dm−3); abbreviations: BA—6-benzyladenine, DA—daminozide, ETH—ethephon, GAx—gibberellins, PH—prohexadione-Ca, TPA—triclopyr, TE—trinexapac-ethyl. The length of bar: 10 cm (a), 1 cm (b), 20 cm (c–l).
3.1. Shoot Growth
The groups of plants in this study did not differ in terms of stem and leaf size before treatment (Table 2 and Table 3). During the first week after application, both gibberellins (GAx) and cytokinin (BA) stimulated the elongational growth of paulownia shoots (Table 2). The shoot extension of plants treated with other preparations were similar to control. However, more differences were found in the four weeks after treatment. Elongation of shoots was significantly more intense in the case of plants treated with gibberellins (GAx), 6-benzyladenine (BA), and trinexapac-ethyl (TE) when compared with control (Figure 1d–f,l). The stem extensions of plants subjected to TE were the strongest and surpassed control by 40 % (Table 2). A similar result (+31%) was obtained for plants treated with GA3. Accretions of stems treated with other retardants, daminozide (DA), prohexadione-Ca (PH), and ethephon (ETH), were similar to control (Table 2, Figure 1i–k). Triclopyr (TPA) strongly (−51%) and significantly inhibited shoot elongation (Figure 1g). One week after PGR application, the stems of plants treated with growth stimulators (BA, GAx) became thicker than control (Table 2). However, this effect was short-lived, as after five weeks any significant differences among these plants were not proven. On the other hand, growth retardants and ethepon did not affect the transverse growth of shoots in the first week after treatment, whereas distinct differences were found after five weeks. Two retardants (DA, PH), and especially ethephon (ETH), inhibited shoot thickening. The opposite effect was observed in the case of plants treated with trinexapac-ethyl (TE), in which shoots became the most thick (109% of control). Treatment with only plant gibberellins (GA4+7) increased leaf length, and in the first week of growth only (Table 3). Ethephon (ETH) inhibited the elongation of leaf blades. Shortened leaf blades were also observed in the case of triclopyr (TPA) and prohexadione-Ca (PH) treatment. Ethephon and triclopyr strongly reduced the width of the leaf blade also (Table 3). A gentler but still distinct reduction was also observed in the case of two retardants (DA, PH), and cytokinin (BA) treatment. The shape of leaves was unaffected by the applied regulators after one week. However, the leaf blades of plants treated with growth stimulators (GAx, BA, TPA) were relatively wider than control and plants treated with retardants and ethephon after five weeks of growth.

Table 2.
Growth of paulownia ‘Oxytree’ shoots after PGR treatment.

Table 3.
Growth of paulownia ‘Oxytree’ leaves after PGR treatment.
3.2. Chosen Aspects of Photosynthesis
The groups of plants designed to study PGR influence did not differ in term of relative chlorophyll content (RChlC) before treatment (Table 4). However, after one week, the leaves of plants sprayed with gibberellins (GAx), cytokinin (BA), and trinexapac-ethyl (TE) contained relatively less chlorophyll (Table 4). Other treatments did not influence RChlC values when compared with control. Some differences among studied plants were found after five weeks.

Table 4.
Relative and real content of photosynthetic pigments in paulownia ‘Oxytree’ leaves after PGR treatment.
The leaves of plants treated with GA3 and TE contained relatively less photosynthetically active pigments, whereas those subjected previously to ethephon contained significantly and relatively more (Table 4). Contrary to RChlC results, only the leaves of plants sprayed with BA contained distinctly less chlorophylls and carotenoids than control after one week (Table 4). The LSD0.05 test indicated lower carotenoid content after five weeks also. Nevertheless, the studied treatments changed the chlorophyll a (CHLa) and chlorophyll b (CHLb) contents in various ways. The leaves of plants sprayed with gibberellins GA4+7 and trinexapac-ethyl (TE) contained significantly more CHLa than control after one week (Table 4). Other plants did not differ distinctly from control when such traits were considered. Any significant differences (based on the ANOVA SL result) among the studied plants were not proven after five weeks of growth. However, LSD0.05 test marked lower CHLa content in the leaves treated previously with 6-benzyladenine (BA). The BA-treated leaves contained less CHLb than control, whereas those subjected to triclopyr had significantly more after one week (Table 4). Any significant differences between the studied plants and control were not proved by two measurement dates with the exception of trinexapac-ethyl (TE) treatment (distinctly lowered CHLb content after five weeks). As a result, some treatments changed the CHLa/CHLb ratios (Table 4). The leaves of plants treated previously with BA and TE contained relatively more CHLa than CHLb in comparison with control after one week. Similar results were found in the case of plants subjected to TE, as well as GA4+7 and TPA after five weeks of growth.
Relatively few differences between the studied plants and control were found when chlorophyll fluorescence parameters were considered (Table 5). The plants treated previously with prohexadione-Ca (PH) were only distinguished from control on higher values of initial (F0) and maximum fluorescence (Fm). Nevertheless, higher values of variable fluorescence (Fv) were determined also for plants subjected to gibberellins GA4+7, triclopyr (TPA), and ethephon (ETH). One week after PGR treatment, the plants treated with TPA or ETH distinguished themselves with higher values of maximal quantum yield of PSII (Fv/Fm) and potential activity of PSII (Fv/F0) when compared to control (Table 5). This feature remained in the case of plants treated with ETH after five weeks. At that time, the Fv/Fm and Fv/F0 values determined for plants subjected to trinexapac-ethyl were significantly lower than control (Table 5).

Table 5.
Chlorophyll fluorescence of paulownia ‘Oxytree’ leaves after PGR treatment.
3.3. Similarity of Reaction on PGR Treatment
Cluster analysis designated three types of reaction to the tested preparations (Figure 2). The response of plants to two retardants (daminozide, prohexadione-calcium) was similar to control. The most divergent was the reaction of paulownia to gibberellins, which was close to the response to trinexapac-ethyl and, to certain extent, to cytokinin (6-benzyladenine). The third type of reaction, however, was closer to control, and was found in the case of triclopyr and ethephon treatment.
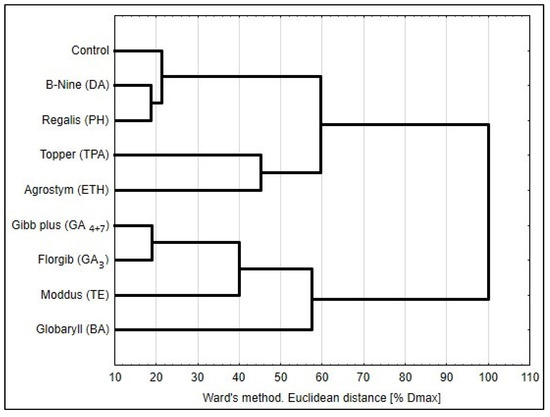
Figure 2.
Similarity of reaction of paulownia ‘Oxytree’ plants on tested growth regulators; abbreviations: BA—6-benzyladenine, DA—daminozide, ETH—ethephon, GAx—gibberellins, PH—prohexadione-Ca, TPA—triclopyr, TE—trinexapac-ethyl.
4. Discussion
The application of plant-growth regulators (PGRs) has become an extensive practice in horticulture and agriculture [17,31,32,37,38]. However, the information about the influence of PGR on deciduous trees is scarce, except for that concerning fruit trees and Populus. Any report describing response of paulownias—valuable and interesting, multi-purpose, but light-demanding, thermophilic trees—to such chemicals was not found. There are some reasons to suppose that the application of PGRs may solve problems met during nursery production. Therefore, the aim of the presented study was to examine and evaluate the effects caused by the chosen, commercially available PGRs on the growth of paulownia ‘Oxytree’ pot plants.
It was found in the present study that in some cases paulownia plants responded to the tested chemicals in a different way than expected. We observed similar effects on other paulownias (three P. tomentosa clones and one P. tomentosa × P. fortunei hybrid) in the preliminary experiments carried out on a smaller number of plants (data not presented). It seems that the reaction on gibberellins A3, A4, A7 was mainly typical and the same (Figure 2) despite GA3 not being a phytohormone and, in the opinion of Keswani et al. [39], GA4 and GA7 possessing different specific biological activities. All those chemicals stimulated the elongation of paulownia stems in comparison with the control (Table 2). Such an effect, as well as strengthening apical dominance, is a clearly visible symptom of gibberellin application in many woody plants [17,23,37,40,41]. Gibberellins (GAs) are especially involved in heterosis for shoot growth [42,43]. In the present study, GAs increased diameter (caliper) of paulownia stems also. A positive correlation between extension growth and tree caliper is common for fruit trees [31]. However, gibberellins stimulated stronger longitudinal than transverse growth of paulownia stems when compared to control (Table 2). A similar effect was observed in the case of a Populus interspecific hybrid [23]. However, the elongation of shoots lasted longer than their broadening. Thus it is possible that GA-treated paulownia nursery plants may be prone to damage and lodging.
Cytokinins usually stimulate the outgrowth of axillary shoots; thus, they could weaken the growth of the main shoot [17,31,32,44,45]. Cytokinins, by stimulating the growth of side/axillary shoots, often weaken the growth of the main shoot and plant height. This was observed in the case of many ornamental and fruit woody plants [44,45]. In spite of this, in the present study 6-benzylaminopurine (BA) did not strongly counteract apical domination, as it activated axillary buds but the growth of side shoots was weak (Figure 1b). It surprisingly induced, not slowed down, the elongation of the main shoot. As expected, BA broadened paulownia stems. Cytokinins, independently of gibberellins and auxins, which work synergistically, stimulate cambial activity and the growth of wood-forming tissues. This was found in these studies for other tree species [26,27,28]. However, similarly to gibberellins, the longitudinal growth of stems was relatively more intense and lasted longer than transverse growth in the case of BA treatment (Table 2).
Triclopyr (TPA) belongs to synthetic auxins, another class of plant stimulators. Such hormones are involved in many developmental processes, including stem growth, apical dominance, rhizogenesis, and wound healing. Triclopyr negatively influenced the growth of paulownia plants in concentrations higher than 5 mg dm−3 (Figure 1h). It retarded the growth of stems and leaves, especially in the weeks after treatment (Table 2 and Table 3). The stems and leaf petioles were often curved and malformed (Figure 1a). Similar symptoms were described by Judy at al. [46] on Tradescantia. However, contrary to their results triclopyr did not inhibit photosynthesis in paulownia leaves, and even increased chlorophyll b content as well as maximal quantum yield and potential activity of PSII (Table 4 and Table 5). Taha et al. [12] did not observe any negative effects of other auxins (IAA, IBA, NAA) on paulownia in vitro cultures. Those chemicals were used in relatively high concentrations (1 mg dm−3), as for in vitro cultures. In preliminary experiments on other paulownias (three P. tomentosa clones and one P. tomentosa × P. fortunei hybrid), we did not find negative effects of NAA and BNOA applied at a 5 mg dm−3 dose. It seems that paulownia is especially sensitive to triclopyr. It is an active ingredient of some herbicides (Garlon 3A, Garlon 4) which are used (as well as or instead of glyphosate) in the successful chemical control of paulownias, when such plants are considered invasive and/or undesirable species. However, it is recommended to apply 50× higher doses than that used in our study.
Ethephon (ethylene generator), next to chlormequat and trinexapac-ethyl, is used on cereals and other field crops to prevent lodging, by helping plants develop shorter, thicker, and stronger stems [17]. Unfortunately, such an effect was not obtained in the present research, as ethephon did not retard elongational but suppressed the transverse growth of stems (Table 2). Cluster analysis revealed a similar reaction in paulownia plants to ethephon and triclopyr (Figure 2). This supports the hypothesis that ethylene and auxins modulate each other regarding their synthesis, transport, and signaling. Stem and leaf malformations observed in the present study after the application of triclopyr are also typical for ethylene treatment. However, triclopyr could cause more intense and/or prolonged ethylene synthesis, as in the case of ethephon treatment distorted plants were not noticed.
The influence of three retardants, daminozide (DA), prohexadione-Ca (PH), and trinexapac-ethyl (TE), was also different than expected, especially in the case of TE. Plant-growth retardants are widely used as anti-lodging agents on cereals and other annual field crops, as plants treated with them develop shorter and thicker stems [17]. They also improve the compactness of ornamentals and fruit woody crops through a reduction in plant height [17,29,30]. Daminozide, before its withdrawal, was used in several fruit-tree species, particularly in apple, to reduce vegetative growth [17]. It was replaced by prohexadione-Ca, which in the opinion of many authors [38,47,48] is an efficient tool to reduce shoot growth. Elansary and El-Ansary [49] observed a similar effect on peach trees after the application of trinexapac-ethyl. However, little information is found on whether retardants stimulate or inhibit the transverse growth of woody plant stems. Carraa et al. [50] observed reduced length and diameter of pear lateral shoots after the application of prohexadione-Ca. Chaney [51] found reduced growth in the diameter of the trunk and branches of urban trees after soil application of paclobutrazol. The authors of other reports usually did not mention how retardants influenced trunk caliper. In our study, daminozide (DA), and prohexadione-Ca (PH) did not reduce stem elongation in five weeks after treatment, whereas they suppressed the diameter growth of stems (Table 2). Nevertheless, the proportions between elongational and transverse growth of paulownia stems remained similar to control (Table 2). Curiously, the effect of trinexapac-ethyl (TE) was completely adverse, as plants developed significantly longer and thicker stems than control and plants treated with other retardants (DA, PH). The paulownia plants treated with gibberellins (GAx) and TE resembled each other (Figure 1d,e,l and Figure 2). Those chemicals stimulated stronger elongational than transverse growth of stems. However, the TE-effect was visible in further weeks of plant growth. It lasted longer than that caused by gibberellins, especially when the growth of the stem in diameter was considered (Table 2). At first look, this seems unusual, as three studied retardants (daminozide, prohexadione-Ca, and trinexapac-ethyl) belong to the same group (acylcyclohexanediones). They influence the synthesis of different gibberellin forms in the same way—by blocking hydroxylation of inactive GA20 into active GA1 [4,29]. In addition, prohexadione-Ca (PH) and trinexapac-ethyl (TE) were applied at the same dose in the present study. However, it is worth mentioning that a GA-like effect of acylcyclohexanediones was reported for Matthiola incana plants treated with PH [52] and yellow pine after TE application [53]. Hisamatsu et al. [52] and Rademacher [17,39,54] suggested that PH may also inhibit the hydroxylation of active gibberellins via GA 2-oxidase into inactive GA8 and GA34. Thus, the same chemical (PH) acts contradictorily in the early and late stages of vegetation on the content of endogenously active gibberellins and the growth of plants [38]. However, both prohexadione-Ca (PH) and trinexapac-ethyl (TE) were applied at the same dose to plants and at the same phase of growth. Thus, their effect should be similar, which is contrary to observations made in our study. It seems that the reason for this is based on substantial differences in the time of activation and translocation of the aforementioned retardants inside plants. Prohexadione-Ca and trinexapac-ethyl are active only in their respective free acid form [54]. Free acid prohexadione is readily generated by dissolving its calcium salt in water and is primarily translocated acropetally [29,54]. The ester trinexapac-ethyl is comparatively easily taken up but translocated basipetally. What is more, the ester trinexapac-ethyl must be saponified by the plant metabolism prior to becoming active [29,54]. Such processes are time-consuming and temperature/light dependent. Possibly, the delayed reaction of paulownia plants on TE is caused by this mechanism. In the opinion of Rademacher [54], due to the aforementioned differences, products containing prohexadione-Ca (PH) and trinexapac-ethyl (TE) may perform differently in various plant species. This was confirmed in present study.
Some distinct correlations among plant size, relative chlorophyll content and fluorescence were found. It was especially visible in the case of TE-treated plants. They generally developed the strongest (both longest and thickest) stems after five weeks of growth, and were also characterized by a much higher chlorophyll a/b ratio, but distinctly lower relative chlorophyll content and efficiency of PSII (Fv/Fm, Fv/F0) than control and the majority of plants subjected to other growth regulators (Table 2, Table 4 and Table 5). The distinctly lowered relative chlorophyll content found for trinexapac-ethyl (TE)- and gibberellin (GAx)-treated plants, which was much greater than control, might be a first sign of mild nitrogen deficit. It also might be explained by the acclimatization of the leaves to a higher light intensity as TE-treated plants surpassed control plants by more than 20 cm and thus were closer to the artificial light. Nevertheless, plants treated with gibberellins (especially GA3) reached a similar stem size and SPAD values but, on the other hand, were characterized by a close-to-control CHL a/b ratio and fluorescence after five weeks of growth (Table 2, Table 4 and Table 5). Additionally, an increased CHL a/b ratio was also found in the case of triclopyr-treated plants, which developed the shortest stems and distinctly smaller leaves. Thus, the modified phenotype of TE-treated plants might be a combined effect of light acclimatization and compensation of traits of the photosynthetic apparatus, as well as a modified proportion of plant hormones (mainly gibberellins and ethylene). Rademacher [29] write that acylcyclohexanediones not only influenced gibberellin synthesis and accumulation but also reduced ethylene formation by inhibiting the conversion of 1-aminocyclopropane-1-carboxylic acid (ACC) into ethylene. That the application of ethephon usually gave the opposite result to TE in our study confirms this statement.
In general, none of the studied preparations produced an effect that would be advantageous for nurserymen, i.e., plants with shorter but thicker, stronger stems, and smaller, greener leaves. The aforementioned biometric traits were usually strongly and positively correlated and none of tested growth regulators broke this relationship. Paulownias possibly require more intense light, which is hard to provide in early spring in Poland. In the present study, paulownia plants were characterized by a lower-than-typical chlorophyll a/b ratio (0.9–1.9 vs. 3). It is well-known that shaded plants usually produce more chlorophyll b. Therefore, the excessive elongation of stems might be a result of the partial etiolation of nursery plants.
It seems that none of the studied preparations produced an effect that would be advantageous for nursery practice, i.e., compact liners that are easier to transport and plant in the field. The light shortage was possibly a much stronger factor influencing paulownia liner growth than any of the tested growth regulators. However, some results obtained by GA, BA, and TE treatment might be beneficial for paulownia grown as an ornamental and/or timber tree. PGRs are frequently employed in agriculture and horticulture for various benefits, such as improving plant morphological structure and increasing tolerance against biotic and abiotic stresses [18]. In the opinion of Wang and Shi [34], the spraying of exogenous PGRs is a simple and efficient method for affecting plant cultivation, and its use to improve plant resistance to stress has been attracting more attention. Plant-growth regulators play intrinsic roles in plant responses to stress. There has been an idea to try PGR products for cultivation and reclamation purposes in Canada [19]. Small and Degenhardt [19] assume that the use of PGRs may improve reclamation success by enhancing the growth of plants and transplanted seedlings and cuttings under environmentally stressful conditions, increasing adaptation and resiliency during climate change. Paulownia is thermophilic genus, native to central China, with a climate warmer than in central and north-eastern Europe. Therefore might be insufficiently winter-hardy in Poland. Paulownias are often planted in abandoned, devastated areas. Thus, it can therefore be assumed that paulownias are grown in unfavorable, stressful conditions and their cultivation for timber is risky. However, it can be assumed that the use of GAs, BA, and TE may facilitate cultivation of paulownias. This should make it easier to obtain a longer, thicker, and straighter trunk in the first years of cultivation, when the plants (seedlings or clones) are in the juvenile/pseudojuvenile stage of intensive growth. It can be assumed that the use of ‘plant youth hormones’—cytokinins and retardants (antigiberellins)—may extend this period [32]. In the case of gibberellins, the reaction may be different, because these hormones can both accelerate and delay generative induction [31,32,39]. Cytokinins have important roles in alleviating biotic and abiotic stresses [55]. It was found that foliar sprays of BA enhanced the tolerance of roses for extreme temperatures [34]. It is well-known that cytokinins increase the regenerative capacity of plants. However, it should be remembered that cytokinins, especially gibberellins, prevent plant dormancy and extend the vegetation period, which may increase frost damage to plants. Thus, the application of these stimulants to paulownias might be beneficial in the first part of the growing season. In the second part, it would probably be better to use a retardant (TE). It is known that retardants increase the dry-matter content in plants, which should increase the plants’ tolerance to low temperatures. That acylcyclohexanediones may increase the tolerance for abiotic (spring frost) and biotic (bacterial and fungal pathogens, sucking and chewing insects) stresses is also worth mentioning [33]. The reason for this is that prohexadione-calcium (PH) and trinexapac-ethyl (TE) inhibit also flavanone 3-hydroxylase, which is involved in the biosynthesis of flavonoids, thus changing their spectrum and possibly leading to the formation of phytoalexin-like compounds [33]. It is worth mentioning that the influence of TE lasted longer that GAs and BA, which should reduce the number of sprays with PGRs. Nevertheless, it should be strongly emphasized that these statements are only hypothetical. To our best knowledge, there are no results of field studies on the impact of PGRs on paulownia plants in field conditions. These were also not conducted in the present work. The plant reactions in the greenhouse and field conditions may be different. It is well-known that plant responses to plant-growth regulators are different and due to species, cultivars, climate, soil, and the specifics of the method applied [31]. Therefore, field experiments should be established to find the proper dose of growth regulators and the time of their application on paulownia plantations.
5. Conclusions
Summarizing the results of this study, it was found that none of the studied preparations changed positive correlations between longitudinal vs. transverse growth of stems and the growth of leaves at the tested dose. It seems that the light shortage was the much stronger factor influencing the growth of paulownia liners than each of the tested growth regulators. Gibberellins (GA3, GA4+GA7), cytokinin (BA), and one of retardants, trinexapac-ethyl (TE), stimulated stem elongation and thickening. The effect of TE appeared later but lasted longer than that created by GAs and BA. Two retardants (acylcyclohexanediones), prohexadione-Ca (PH) and trinexapac-ethyl (TE), applied at the same dose, performed extremely differently regarding paulownia plant growth. The leaves of TE-treated plants were distinguished by a higher chlorophyll a/b ratio and lower relative chlorophyll content and efficiency of PSII (Fv/Fm, Fv/F0). Triclopyr distorted the growth of plants. Other regulators (ethephon, daminozide, prohexadione-Ca) slowed down the diameter growth of stems and leaf expansion.
Author Contributions
Conceptualization, methodology, statistical analyses, manuscript writing, W.L.; plant care, measurements and analyses, data input, B.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Oxytree Solutions Poland S.A. (contract No. RARR/PPNT/1298/2017/AERO).
Data Availability Statement
The micropropagated plants of paulownia interspecific (Paulownia elongata × Paulownia fortunei) hybrid ‘Oxytree’ (in vitro clone 112) were kindly provided by Oxytree Solutions Poland S.A.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yadav, N.K.; Vaidya, B.N.; Henderson, K.; Lee, J.F.; Stewart, W.M.; Dhekney, S.A.; Joshee, N. A Review of Paulownia Biotechnology: A Short Rotation, Fast Growing Multipurpose Bioenergy Tree. Am. J. Plant Sci. 2013, 4, 2070–2082. [Google Scholar] [CrossRef]
- Woods, V.B. Paulownia as a novel biomass crop for Northern Ireland? A review of current knowledge. Occas. Publ. Agri-Food Biosci. Inst. 2008, 7, 1–47. Available online: https://www.doc-developpement-durable.org/file/Arbres-Bois-de-Rapport-Reforestation/FICHES_ARBRES/Paulownia/Paulownia%20as%20a%20novel%20biomass%20crop_Ireland.pdf (accessed on 1 June 2020).
- Young, S.N.R.; Lundgren, M.R. C4 photosynthesis in Paulownia? A case of inaccurate citations. Plants People Planet 2023, 5, 292–303. [Google Scholar] [CrossRef]
- Burger, D.W.; Liu, L.; Wu, L. Rapid micropropagation of Paulownia tomentosa. HortScience 1985, 20, 760–761. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Chao, C.J.; Lu, X.Y.; Xiong, Y.G. Paulownia in China: Cultivation and Utilization; Asian Network for Biological Science and International Development Research Centre: Singapore, 1986; pp. 37–46. [Google Scholar]
- Rao, C.D.; Goh, C.; Kumar, P.P. High frequency adventitious shoot regeneration from excised leaves of Paulownia spp. cultured in Vitro. Plant Cell Rep. 1996, 16, 204–209. [Google Scholar] [CrossRef]
- Ozaslan, M.; Can, C.; Aytekin, T. Effect of explant source on in Vitro propagation of Paulownia tomentosa Steud. Biotechnol. Biolechnological Equip. 2005, 19, 20–26. [Google Scholar] [CrossRef]
- Marcotrigiano, M.; Stimart, D.P. In Vitro organogenesis and shoot proliferation of Paulownia tomentosa Steud. (Empress Tree). Plant Sci. Lett. 1983, 31, 303–310. [Google Scholar] [CrossRef]
- Kumar, P.P.; Rao, C.D.; Goh, C.J. Influence of petiole and lamina on adventitious shoot initiation from leaf explants of Paulownia fortunei. Plant Cell Rep. 1998, 17, 886–890. [Google Scholar] [CrossRef]
- Ipekci, Z.; Altinkut, A.; Kazan, K.; Bajrovic, K.; Gozukirmizi, N. High frequency plant regeneration from nodal explants of Paulownia elongata. Plant Biol. 2001, 3, 113–115. [Google Scholar] [CrossRef]
- Rout, G.R.; Reddy, G.M.; Das, P. Studies on in Vitro clonal propagation of Paulownia tomentosa Steud. and evaluation of genetic fidelity through RAPD marker. Silvae Genet. 2001, 50, 208–212. [Google Scholar]
- Taha, L.S.; Soad, I.M.M.; Farahat, M.M. A micropropagation protocol of Paulownia kowakamii through in Vitro culture technique. Aust. J. Basis Appl. Sci. 2008, 2, 594–600. [Google Scholar]
- Rajbahak, S.; Sah, S.K. Micropropagation of Paulownia tomentosa through in Vitro culture technique. St. Xaviers J. Sci. 2010, 2, 15–20. [Google Scholar]
- Litwińczuk, W.; Bochnia, E. Development of royal paulownia (Paulownia tomentosa Steud.) in Vitro shoot cultures under the influence of different saccharides. Acta Sci. Pol. Hort. Cult. 2012, 11, 3–13. [Google Scholar]
- Markovic, M.; Vilotic, D.; Popovic, M. Propagation of Paulownia elongata S.Y. Hu by axillary shoots. Propag. Ornam. Plants 2013, 13, 73–77. [Google Scholar]
- Pożoga, M.; Olewnicki, D.; Jabłońska, L. In Vitro Propagation Protocols and Variable Cost Comparison in Commercial Production for Paulownia tomentosa × Paulownia fortunei Hybrid as a Renewable Energy Source. Appl. Sci. 2019, 9, 2272. [Google Scholar] [CrossRef]
- Rademacher, W. Plant Growth Regulators: Backgrounds and Uses in Plant Production. J. Plant Growth Regul. 2015, 34, 845–872. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Yadav, S.R.; Mochida, K.; Phan, L. Plant Growth Regulators: True Managers of Plant Life. Plant Cell Physiol. 2022, 63, 1757–1760. [Google Scholar] [CrossRef]
- Small, C.; Degenhardt, D. Plant growth regulators for enhancing revegetation success in reclamation: A review. Ecol. Eng. 2018, 118, 43–51. [Google Scholar] [CrossRef]
- Shah, S.H.; Islam, S.; Alamri, S.; Parrey, Z.A.; Mohammad, F.; Kalaji, H.M. Plant Growth Regulators Mediated Changes in the Growth, Photosynthesis, Nutrient Acquisition and Productivity of Mustard. Agriculture 2023, 13, 570. [Google Scholar] [CrossRef]
- Janowska, B.; Andrzejak, R. Plant Growth Regulators for the Cultivation and Vase Life of Geophyte Flowers and Leaves. Agriculture 2023, 13, 855. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, Y.; Sun, X.; Zhang, S. Effects of the Most Appropriate Proportion of Phytohormones on Tree-Ring Growth in Clones of Hybrid Larch. Sustainability 2023, 15, 6508. [Google Scholar] [CrossRef]
- Yuan, H.; Zhao, L.; Guo, W.; Yu, Y.; Tao, L.; Zhang, L.; Song, X.; Huang, W.; Cheng, L.; Chen, J. Exogenous Application of Phytohormones Promotes Growth and Regulates Expression of Wood Formation-Related Genes in Populus simonii × P. nigra. Int. J. Mol. Sci. 2019, 20, 792. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Y.; Guo, G.S.; Qiu, Z.F.; Li, X.D.; Zeng, B.S.; Fan, C.J. Exogenous GA3 Application Altered Morphology, Anatomic and Transcriptional Regulatory Networks of Hormones in Eucalyptus grandis. Protoplasma 2018, 255, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Xiaokang, F.; Huili, S.; Shuai, L.; Xuelian, D.; Changzheng, X.; Keming, L. Cytokinin signaling localized in phloem noncell-autonomously regulates cambial activity during secondary growth of Populus stems. New Phytol. 2021, 230, 1476–1488. [Google Scholar] [CrossRef]
- Fischer, U.; Kucukoglu, M.; Helariutta, Y.; Bhalerao, R.P. The dynamics of cambial stem cell activity. Annu. Rev. Plant Biol. 2019, 70, 293–319. [Google Scholar] [CrossRef]
- Nieminen, K.; Immanen, J.; Laxell, M.; Kauppinen, L.; Tarkowski, P.; Dolezal, K.; Tahtiharju, S.; Elo, A.; Decourteix, M.; Ljung, K. Cytokinin signaling regulates cambial development in poplar. Proc. Natl. Acad. Sci. USA 2008, 105, 20032–20037. [Google Scholar] [CrossRef]
- Immanen, J.; Nieminen, K.; Smolander, O.P.; Kojima, M.; Alonso, J.; Koskinen, P.; Zhang, J.; Elo, A.; Mahonen, A.P.; Street, N. Cytokinin and auxin display distinct but interconnected distribution and signaling profiles to stimulate cambial activity. Curr. Biol. 2016, 26, 1990–1997. [Google Scholar] [CrossRef]
- Rademacher, W. Growth retardants: Effects on gibberellin biosynthesis and other metabolic pathways. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 501–531. [Google Scholar] [CrossRef]
- Elfving, D.C.; Visser, D.B.; Lang, G.A. Effects of Prohexadione-Calcium and ethephon on growth and flowering of ‘Bing’ sweet cherry. Acta Hort. 2005, 667, 439–446. [Google Scholar] [CrossRef]
- Jacyna, T. Factors influencing lateral-branch formation in woody plants. Acta Agrobot. 2002, 55, 5–25. [Google Scholar] [CrossRef]
- Koprna, R.; De Diego, N.; Dundálková, L.; Spíchal, L. Use of cytokinins as agrochemicals Bioorganic & Medicinal Chemistry Bioorg. Med. Chem. 2016, 24, 484–492. [Google Scholar] [CrossRef]
- Spinelli, F.; Speakman, J.B.; Rademacher, W.; Halbwirth, H.; Stich, K.; Costa, G. Luteoforol, a flavan 4-ol, is induced in pome fruits by prohexadione-calcium and shows phytoalexin-like properties against Erwinia amylovora and other plant pathogens. Eur. J. Plant Pathol. 2005, 112, 133–142. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, L. Effects of Exogenous Salicylic Acid (SA), 6-Benzylaminopurine (6-BA), or Abscisic Acid (ABA) on the Physiology of Rosa hybrida ‘Carolla’ under High-Temperature Stress. Horticulturae 2022, 8, 851. [Google Scholar] [CrossRef]
- Castro-Camba, R.; Sánchez, C.; Vidal, N.; Vielba, J.M. Plant Development and Crop Yield: The Role of Gibberellins. Plants 2022, 11, 2650. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Quinlan, J.D.; Tobutt, K.R. Manipulating fruit tree chemically and genetically for improved performance. HortScience 1990, 25, 60–64. [Google Scholar] [CrossRef]
- Rademacher, W. Chemical regulation of shoot growth in fruit trees. Acta Hortic. 2004, 653, 29–32. [Google Scholar] [CrossRef]
- Keswani, C.; Singh, S.P.; García-Estrada, C.; Mezaache-Aichour, S.; Glare, T.R.; Borriss, R.; Rajput, V.D.; Minkina, T.M.; Ortiz, A.; Sansinenea, E. Biosynthesis and beneficial effects of microbial gibberellins on crops for sustainable agriculture. J. Appl. Microbiol. 2022, 132, 1597–1615. [Google Scholar] [CrossRef]
- Eriksson, M.E.; Moritz, T. Daylength and spatial expression of a gibberellin 20-oxidase isolated from hybrid aspen (Populus tremula L. × P. tremuloides Michx.). Planta 2002, 214, 920–930. [Google Scholar] [CrossRef]
- Ko, J.H.; Kim, W.C.h.; Cho, J.S.; Choi, Y.I.; Im, J.H.; Han, S.; Keathley, D.; Han, K.H. EliteTreeTM: An advanced biomass tree crop technology that features greater wood density and accelerated stem growth. Biofuels Bioprod. Bioref. 2017, 11, 521–533. [Google Scholar] [CrossRef]
- Ma, Q.; Hedden, P.; Zhang, Q. Heterosis in Rice Seedlings: Its Relationship to Gibberellin Content and Expression of Gibberellin Metabolism and Signaling Genes. Plant Physiol. 2011, 156, 1905–1920. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Thomas, B.R. Hormones and heterosis in hybrid balsam poplar (Populus balsmifera L.). Forests 2019, 10, 143. [Google Scholar] [CrossRef]
- Hrotko, K.; Magyar, L.; Yao, C.; Ronay, Z. Effect of BA (benzyladenine) concentration in repeated application on feathering of nursery trees of ‘Egri Piros’ apple. Hortic. Sci. 1997, 29, 40–45. [Google Scholar]
- Pobudkiewicz, A. The influence of growth retardants and cytokinins on flowering of ornamental plants. Acta Agrobot. 2008, 61, 137–141. [Google Scholar] [CrossRef]
- Judy, B.; Lower, W.; Miles, C.; Thomas, M.; Krause, G. Chlorophyll Fluorescence of a Higher Plant as an Assay for Toxicity Assessment of Soil and Water. In Plants for Toxicity Assessment; Wang, W., Gorsuch, J., Lower, W., Eds.; ASTM International: West Conshohocken, PA, USA, 1990; pp. 308–318. [Google Scholar] [CrossRef]
- Pasa, M.S.; Einhorn, T.C. Heading cuts and prohexadione-calcium affect the growth and development of ‘d’Anjou’ pear shoots in a high-density orchard. Sci. Hort. 2014, 168, 267–271. [Google Scholar] [CrossRef]
- Einhorn, T.; Pasa, M.; Turner, J. ‘D’Anjou’ pear shoot growth and return bloom, but not fruit size, are reduced by prohexadione-calcium. HortScience 2014, 49, 180–187. [Google Scholar] [CrossRef]
- Elansary, H.O.; El-Ansary, D.O. Trinexapac-ethyl application enhanced physiological performance of Prunus persica (L.) Batsch. under drought conditions. Bangladesh J. Bot. 2017, 46, 1367–1373. [Google Scholar]
- Carraa, B.; Pasab, M.S.; Fachinello, J.C.; Spagnola, D.; Abreua, E.S.; Giovanaz, M.A. Prohexadione calcium affects shoot growth, but not yield components, of ‘Le Conte’ pear in warm-winter climate conditions. Sci. Hort. 2016, 209, 241–248. [Google Scholar] [CrossRef]
- Chaney, W.R. Growth Retardants: A Promising Tool for Managing Urban Trees. Purdue Extension. Purdue University. 2008. Available online: https://www.extension.purdue.edu/extmedia/FNR/FNR-252-W.pdf (accessed on 31 May 2023).
- Hisamatsu, T.; Koshioka, M.; Kubota, S.; King, R.W. Effect of gibberellin A4 and GA biosynthesis inhibitors on growth and flowering of stock [Matthiola incana (L.) R. Br.]. Jpn. Soc. Hortic. Sci. 1998, 67, 537–543. [Google Scholar] [CrossRef]
- Jankowski, K.; Sosnowski, J.; Wilk, A.; Malinowska, E.; Wiśniewska-Kadżajan, W. Effect of growth regulators on selected morphological features of yellow pine. J. Ecol. Eng. 2014, 15, 105–108. [Google Scholar] [CrossRef]
- Rademacher, W. Prohexadione-Ca and Trinexapac-ethyl: Similarities in structure but differences in biological action. Acta Hort. 2014, 1042, 33–41. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Benková, E. Cytokinin cross-talking during biotic and abiotic stress responses. Front. Plant Sci. 2013, 4, 451. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).