A Predictive Study on the Content of Epigallocatechin Gallate (EGCG) in Yunnan Large Leaf Tea Trees Based on the Nomogram Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Research Location’s General Situation
2.2. Experimental Materials
2.3. Statistical Analysis
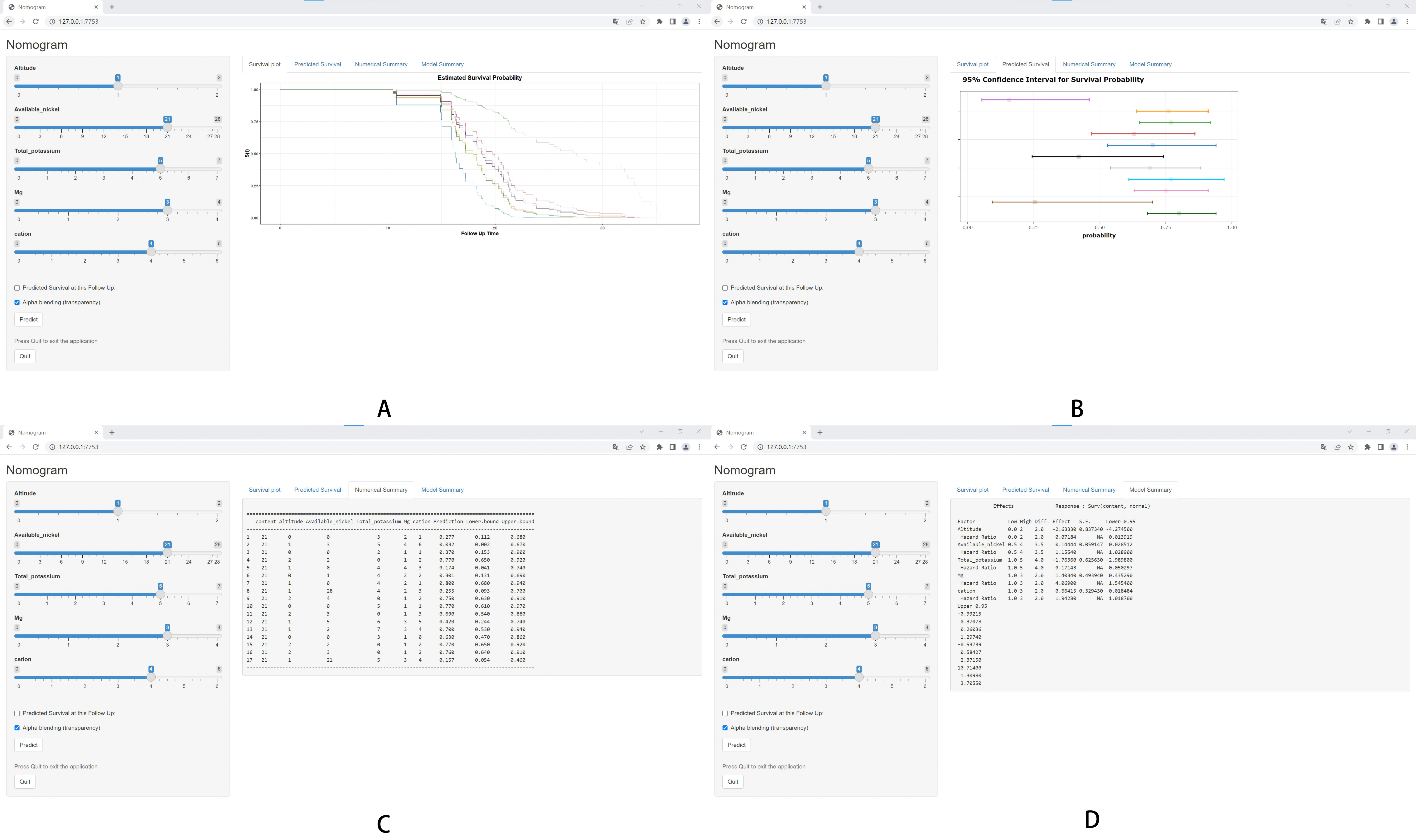
3. Results
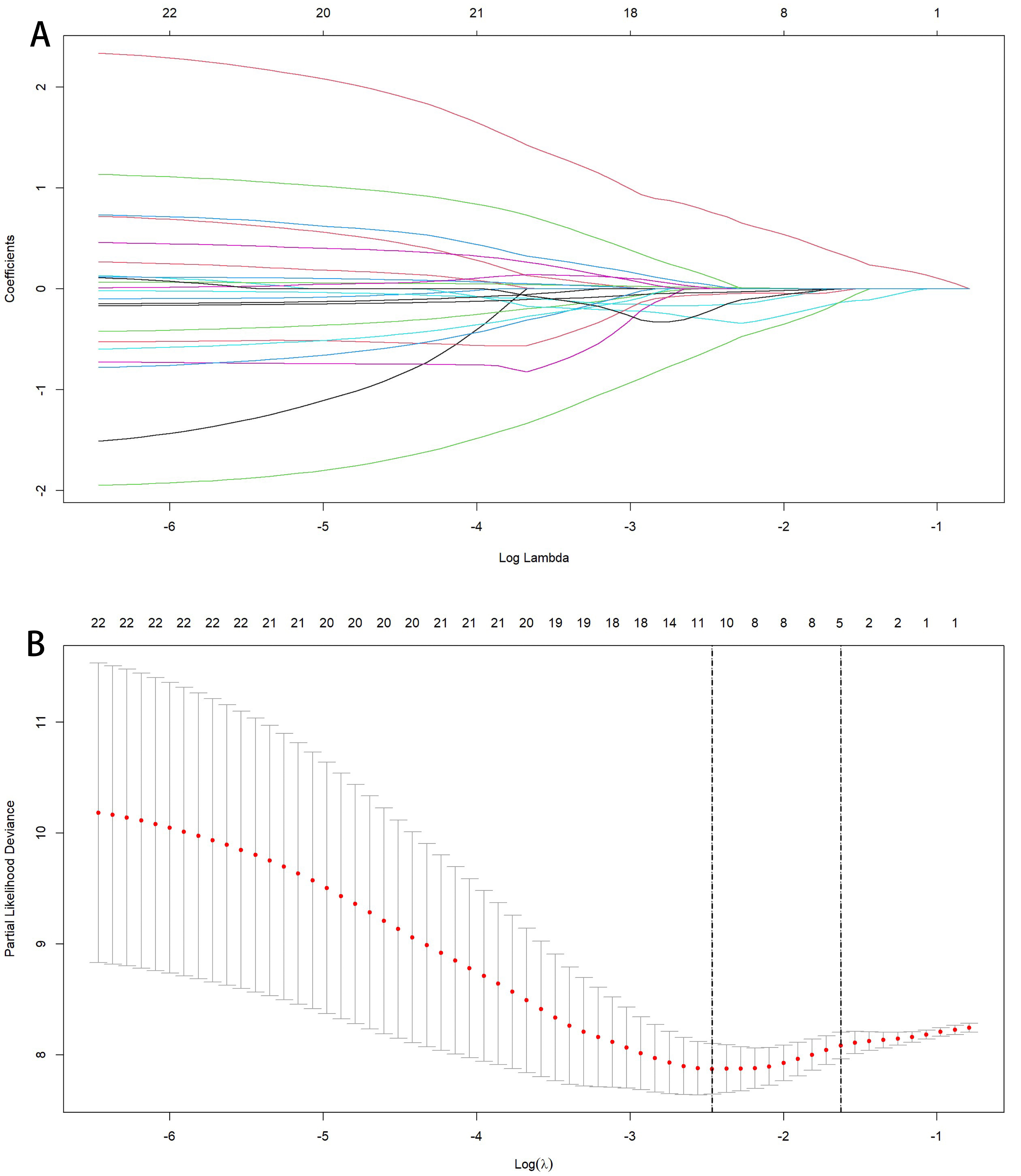
3.1. Model Construction Factor Selection
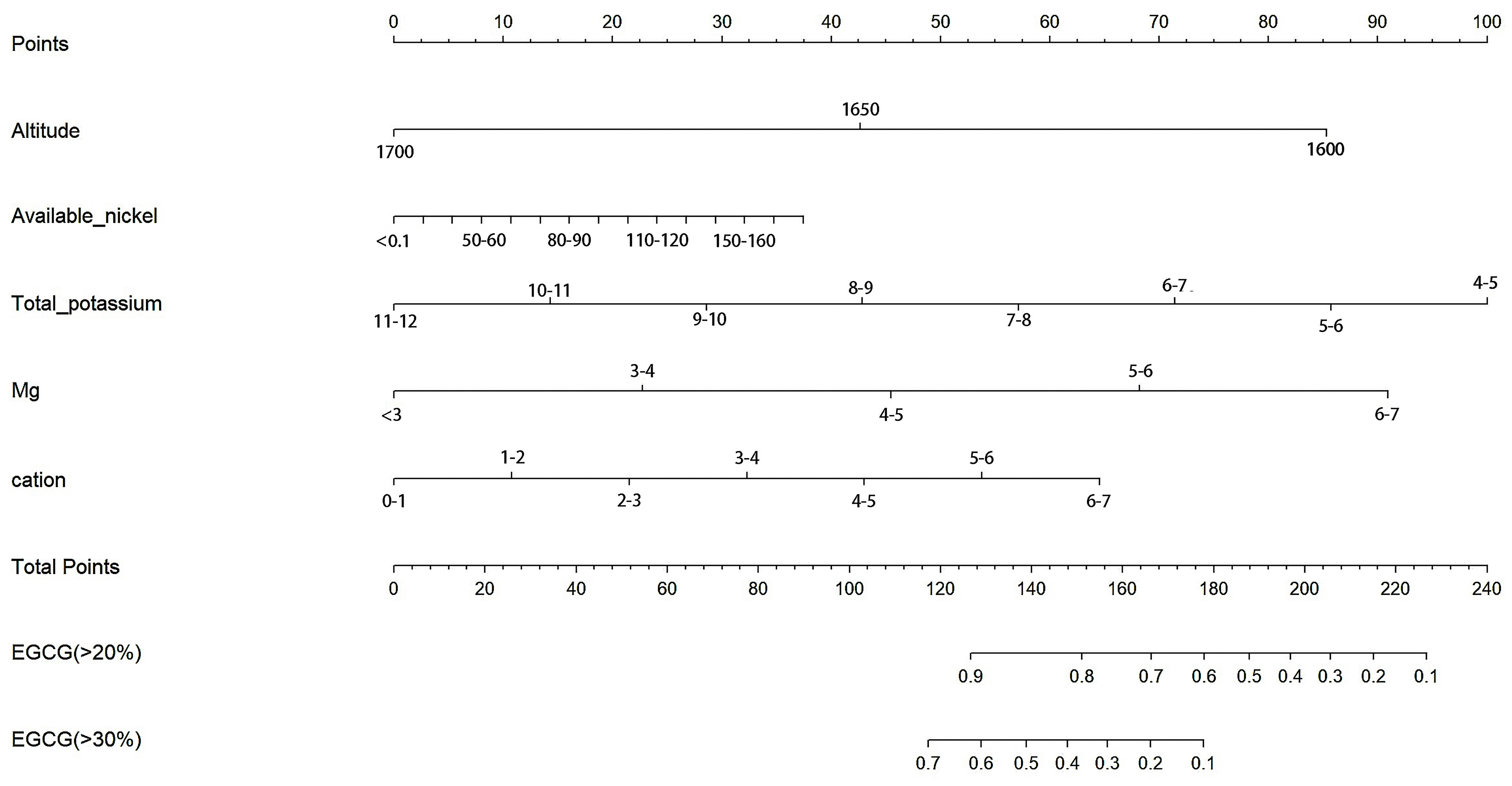
3.2. Factor Analysis and Model Construction
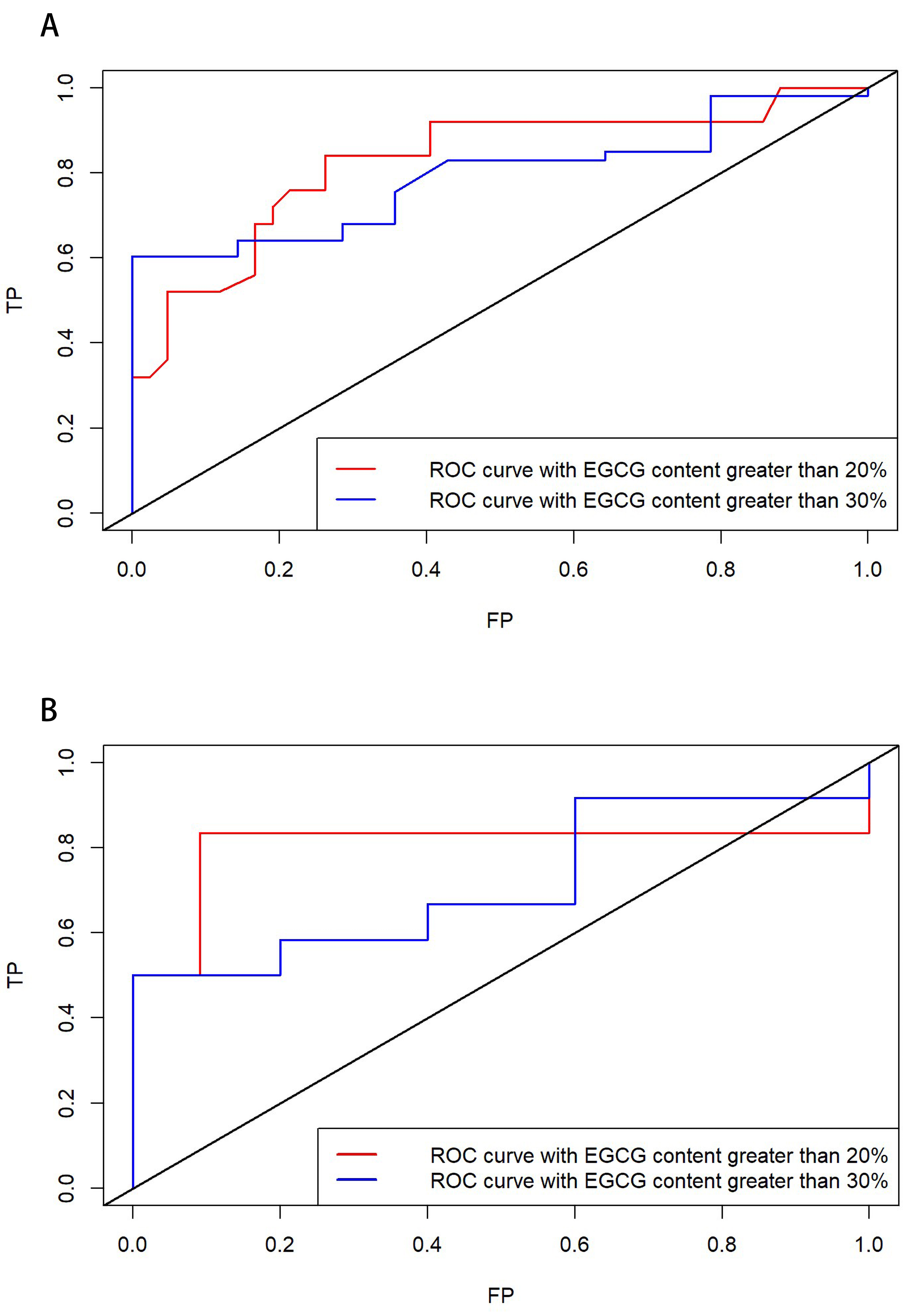
3.3. Model Accuracy Assessment
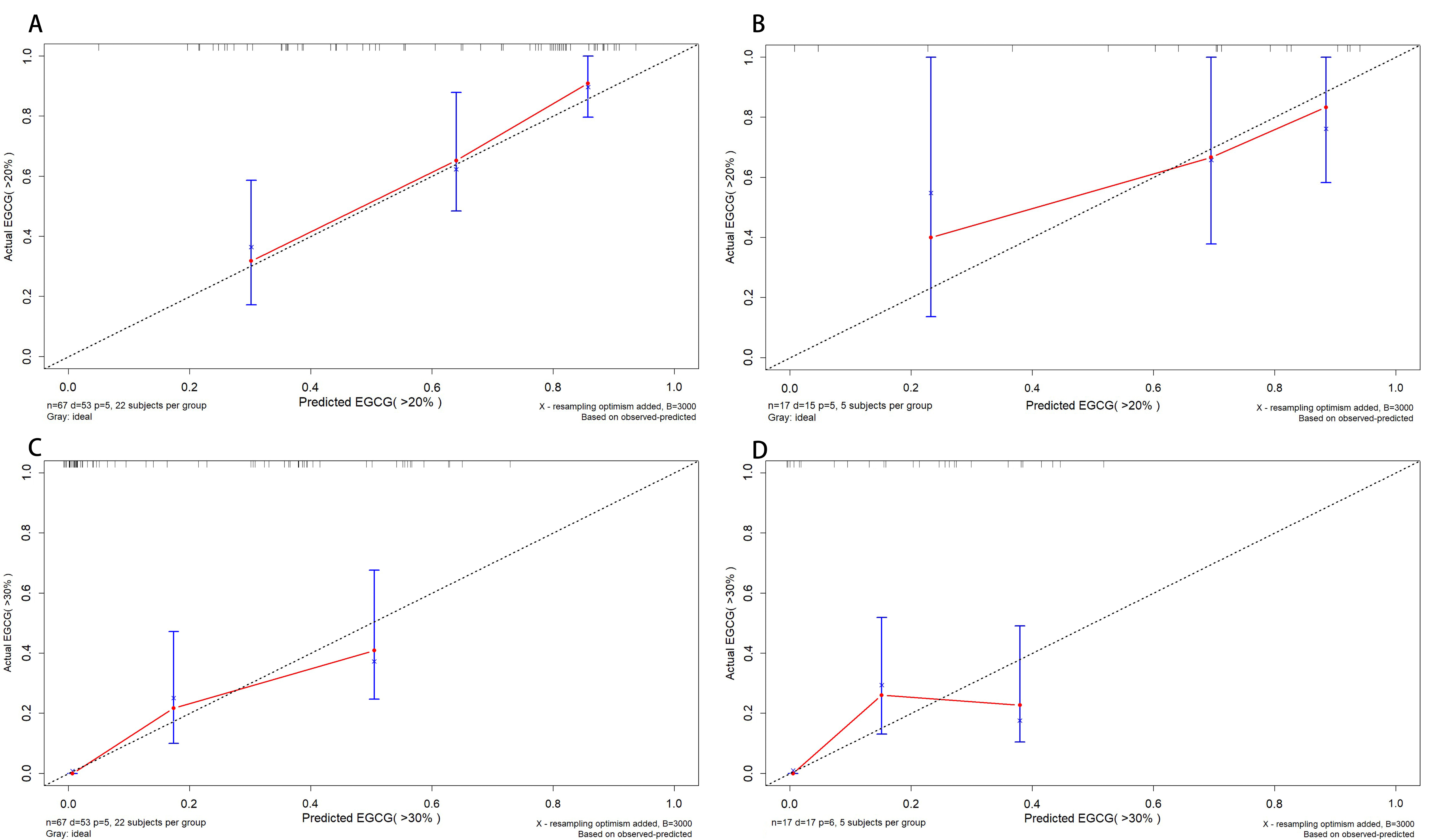
3.4. Evaluation of Model Stability
3.5. System Construction and Model Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xie, J.; Yu, H.; Song, S.; Fang, C.; Wang, X.; Bai, Z.; Sheng, J. Pu-erh Tea Water Extract Mediates Cell Cycle Arrest and Apoptosis in MDA-MB-231 Human Breast Cancer Cells. Front. Pharmacol. 2017, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Song, J.L.; Kim, J.D.; Lee, J.S.; Park, K.Y. Fermented Pu-erh tea increases in vitro anticancer activities in HT-29 cells and has antiangiogenetic effects on HUVECs. J. Environ. Pathol. Toxicol. Oncol. 2013, 32, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Zheng, W.; Zhang, B.; Chen, X.; Zhang, J.; Pang, X.; Ma, B. Comparison of hypoglycemic effects of ripened pu-erh tea and raw pu-erh tea in streptozotocin-induced diabetic rats. RSC Adv. 2019, 9, 2967–2977. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Wang, L.; Tinshun, Z.; Nakamura, T.; Ashida, H. Fermented Tea Improves Glucose Intolerance in Mice by Enhancing Translocation of Glucose Transporter 4 in Skeletal Muscle. J. Agric. Food Chem. 2012, 60, 11366–11371. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zeng, Z.; Huang, Y.; Zhang, X. Chemical compositions of Pu’er tea fermented by Eurotium Cristatum and their lipid-lowering activity. LWT 2018, 98, 204–211. [Google Scholar] [CrossRef]
- Ni, Y.; Zhao, L.; Yu, H.; Ma, X.; Bao, Y.; Rajani, C.; Jia, W. Circulating Unsaturated Fatty Acids Delineate the Metabolic Status of Obese Individuals. Ebiomedicine 2015, 2, 1513–1522. [Google Scholar] [CrossRef]
- Lv, H.P.; Zhu, Y.; Tan, J.F.; Guo, L.; Dai, W.D.; Lin, Z. Bioactive compounds from Pu-erh tea with therapy for hyperlipidaemia. J. Funct. Foods 2015, 19, 194–203. [Google Scholar] [CrossRef]
- Zeng, L.; Yan, J.; Luo, L.; Zhang, D. Effects of Pu-erh tea aqueous extract (PTAE) on blood lipid metabolism enzymes. Food Funct. 2015, 6, 2008–2016. [Google Scholar] [CrossRef]
- Vu, H.T.; Song, F.V.; Tian, K.V.; Su, H.; Chass, G.A. Systematic characterisation of the structure and radical scavenging potency of Pu’Er tea () polyphenol theaflavin. Org. Biomol. Chem. 2019, 17, 9942–9950. [Google Scholar] [CrossRef]
- Swen, W. Effects of green tea and EGCG on cardiovascular and metabolic health. J. Am. Coll. Nutr. 2007, 26, 373S–388S. [Google Scholar]
- Wei, R.; Wirkus, J.; Yang, Z.; Machuca, J.; Esparza, Y.; Mackenzie, G.G. EGCG sensitizes chemotherapeutic-induced cytotoxicity by targeting the ERK pathway in multiple cancer cell lines. Arch. Biochem. Biophys. 2020, 692, 108546. [Google Scholar] [CrossRef] [PubMed]
- La, X.; Zhang, L.; Li, Z.; Li, H.; Yang, Y. (-)-Epigallocatechin Gallate (EGCG) enhances the sensitivity of colorectal cancer cells to 5-FU by inhibiting GRP78/NF-κB/miR-155-5p/MDR1 pathway. J. Agric. Food Chem. 2019, 67, 2510–2518. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Hao, S.; Gao, J.; Jiang, P. EGCG Alleviates Obesity-Exacerbated Lung Cancer Progression by STAT1/SLC7A11 Pathway and Gut Microbiota. J. Nutr. Biochem. 2023, 120, 109416. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xu, H.; Wu, S.; Guo, Y.; Zhao, G.; Wang, D. Mechanisms Underlying the Effects of the Green Tea Polyphenol EGCG in Sarcopenia Prevention and Management. J. Agric. Food Chem. 2023, 71, 9609–9627. [Google Scholar] [CrossRef] [PubMed]
- Betts, J.W.; Hornsey, M.; Higgins, P.G.; Lucassen, K.; Wille, J.; Salguero, F.J.; La Ragione, R.M. Restoring the activity of the antibiotic aztreonam using the polyphenol epigallocatechin gallate (EGCG) against multidrug-resistant clinical isolates of Pseudomonas aeruginosa. J. Med. Microbiol. 2019, 68, 1552–1559. [Google Scholar] [CrossRef]
- Mao, S.; Ren, Y.; Wei, C.; Chen, S.; Ye, X.; Jinhu, T. Development of novel EGCG/Fe loaded sodium alginate-based packaging films with antibacterial and slow-release properties. Food Hydrocoll. 2023, 145, 109032. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, C.; Sheng, Y.; Liu, X.; Yuan, W.; Deng, X.; Wang, B. A Nomogram Model for Predicting the Polyphenol Content of Pu-Erh Tea. Foods 2023, 12, 2128. [Google Scholar] [CrossRef]
- Xie, D.; Dai, W.; Lu, M.; Tan, J.; Zhang, Y.; Chen, M.; Lin, Z. Nontargeted metabolomics predicts the storage duration of white teas with 8-C N-ethyl-2-pyrrolidinone-substituted flavan-3-ols as marker compounds. Food Res. Int. 2019, 125, 108635. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.M.; Zhang, M.N.; Yao, J.; Song, B. Nomogram Models for Distinguishing Intraductal Carcinoma of the Prostate from Prostatic Acinar Adenocarcinoma Based on Multiparametric Magnetic Resonance Imaging. Korean J. Radiol. 2023, 24, 668–680. [Google Scholar] [CrossRef]
- Peiyuan, G.; Ganlin, G.; Xu, Y.; Zining, L.; Jiachao, H.; Bin, Y.; Guiying, W. Construction and validation of a nomogram model for predicting the overall survival of colorectal cancer patients. BMC Surg. 2023, 23, 182. [Google Scholar] [CrossRef]
- Wu, X.; Tang, F.; Li, H.; Chen, C.; Zhang, H.; Liu, X.; Ye, Z. Development and validation of a nomogram model for medication non-adherence in patients with chronic kidney disease. J. Psychosom. Res. 2023, 171, 111385. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xie, B.C.; Lyu, P.J.; Wang, H.X.; Li, Y.; Wang, C.H.; Yu, P. Clinical value of nomogram model in evaluating the prognosis of cholangiocarcinoma after interventional therapy. Zhonghua Yi Xue Za Zhi 2023, 103, 1217–1224. [Google Scholar] [PubMed]
- Shen, Y.; Xiong, Y.; Cao, Q.; Li, Y.; Xiang, W.; Wang, L.; Hong, D. Construction and validation of a nomogram model to predict symptomatic intracranial hemorrhage after intravenous thrombolysis in severe white matter lesions. J. Thromb. Thrombolysis 2023, 56, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, S.; Li, H.; Luo, L.; Zeng, L. Identification of the key phytochemical components responsible for sensory characteristics of Hunan fuzhuan brick tea. J. Food Compos. Anal. 2023, 120, 10589. [Google Scholar] [CrossRef]
- Wang, M.; Cui, X.; Li, S.; Yang, X.; Ma, A.; Zhang, Y.; Yu, B. DeepMal: Accurate prediction of protein malonylation sites by deep neural networks. Chemom. Intell. Lab. Syst. 2020, 207, 104175. [Google Scholar] [CrossRef]
- Brester, C.; Voutilainen, A.; Tuomainen, T.P.; Kauhanen, J.; Kolehmainen, M. Epidemiological predictive modeling: Lessons learned from the Kuopio Ischemic Heart Disease Risk Factor Study. Ann. Epidemiol. 2022, 70, 1–8. [Google Scholar] [CrossRef]
- Farvahari, A.; Gozashti, M.H.; Dehesh, T. The Usage of Lasso, Ridge, and Linear Regression to Explore the Most Influential Metabolic Variables that Affect Fasting Blood Sugar in Type 2 Diabetes Patients. Rom. J. Diabetes Nutr. Metab. Dis. 2019, 26, 371–379. [Google Scholar]
- Hadi, R.S.; Saeedeh, P.; Seyyed, M.T.A. Identifying the Prognosis Factors in Death after Liver Transplantation via Adaptive LASSO in Iran. J. Environ. Public Health 2016, 2016, 7620157. [Google Scholar]
- Cella, L.; Oh, J.H.; Deasy, J.O.; Palma, G.; Liuzzi, R.; D’avino, V.; Conson, M.; Picardi, M.; Salvatore, M.; Pacelli, R. Predicting radiation-induced valvular heart damage. Acta Oncol. 2015, 54, 1796–1804. [Google Scholar] [CrossRef]
- Jia, M.; Pi, J.; Zou, J.; Feng, M.; Chen, H.; Lin, C.; Yang, S.; Deng, Y.; Xiao, X. A Nomogram Model Based on Neuroendocrine Markers for Predicting the Prognosis of Neuroendocrine Carcinoma of Cervix. J. Clin. Med. 2023, 12, 1227. [Google Scholar] [CrossRef]
- Wei, Y.; Sun, P.; Chang, C.; Tong, Y. Ultrasound-based Nomogram for Predicting the Pathological Nodal Negativity of Unilateral Clinical N1a Papillary Thyroid Carcinoma in Adolescents and Young Adults. Acad. Radiol. 2023, 30, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, D.; Shen, L.; Xie, Y.; Yan, S. Prognostic Value of a CT Radiomics-Based Nomogram for the Overall Survival of Patients with Nonmetastatic BCLC Stage C Hepatocellular Carcinoma after Stereotactic Body Radiotherapy. J. Oncol. 2023, 2023, 1554599. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wu, J.; Wang, Y.; Jin, Y. Development and Validation of an Ultrasound-Based Radiomics Nomogram for Identifying HER2 Status in Patients with Breast Carcinoma. Diagnostics 2022, 12, 3130. [Google Scholar] [CrossRef] [PubMed]
- Rani, L.; Gogia, A.; Singh, V.; Kumar, L.; Sharma, A.; Kaur, G.; Gupta, R. Comparative assessment of prognostic models in chronic lymphocytic leukemia: Evaluation in Indian cohort. Ann. Hematol. 2018, 98, 437–443. [Google Scholar] [CrossRef]
- Zhou, Z.; Xie, X.; Hao, N.; Diao, D.; Song, Y.; Xia, P.; Dang, C.; Zhang, H. Different lymph node staging systems for patients with adenocarcinoma of esophagogastric junction. Curr. Med. Res. Opin. 2018, 34, 963–970. [Google Scholar] [CrossRef]
- Matthew, M.C.; Richa, A.; Dana, P.E. The value of vital sign trends for detecting clinical deterioration on the wards. Resuscitation 2016, 102, 1–5. [Google Scholar]
- Fan, M.; Zhang, P.; Wang, Y.; Peng, W.; Wang, S.; Gao, X.; Xu, M.; Li, L. Radiomic analysis of imaging heterogeneity in tumours and the surrounding parenchyma based on unsupervised decomposition of DCE-MRI for predicting molecular subtypes of breast cancer. Eur. Radiol. 2019, 29, 4456–4467. [Google Scholar] [CrossRef]
- Zhang, C.; Mao, M.; Guo, X.; Cui, P.; Zhang, L.; Xu, Y.; Li, L.; Han, X.; Peltzer, K.; Xiong, S.; et al. Nomogram based on homogeneous and heterogeneous associated factors for predicting bone metastases in patients with different histological types of lung cancer. BMC Cancer 2019, 19, 238. [Google Scholar] [CrossRef]
- Bianchi, L.; Schiavina, R.; Borghesi, M.; Bianchi, F.M.; Briganti, A.; Carini, M.; Terrone, C.; Mottrie, A.; Gacci, M.; Gontero, P.; et al. Evaluating the predictive accuracy and the clinical benefit of a nomogram aimed to predict survival in node-positive prostate cancer patients: External validation on a multi-institutional database. Int. J. Urol. 2018, 25, 574–581. [Google Scholar] [CrossRef]
- Feng, Z.; Lou, S.; Zhang, L.; Zhang, L.; Lan, W.; Wang, M.; Shen, Q.; Hu, Z.; Chen, F. New Preoperative Nomogram Using the Centrality Index to Predict High Nuclear Grade Clear Cell Renal Carcinoma. Cancer Manag. Res. 2019, 11, 10921–10928. [Google Scholar] [CrossRef]
- Yan, L.; Deng, W.; Guan, L.; Xu, H. Nomogram forecasting 3-, 5-, and 8-year overall survival and cancer-specific survival of gingival squamous cell carcinoma. Cancer Med. 2020, 9, 8266–8274. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Wang, S.; Gao, L.; You, H. A nomogram incorporated lifestyle indicators for predicting nonalcoholic fatty liver disease. Medicine 2021, 100, e26415. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhao, J.; Guo, Z.; Wang, X. Determination of caffeine content and main catechins contents in green tea (Camellia sinensis L.) using taste sensor technique and multivariate calibration. J. Food Compos. Anal. 2010, 23, 353–358. [Google Scholar] [CrossRef]
- Liao, S.; Lin, J.; Liu, J.; Chen, T.; Xu, M.; Zheng, J. Chemoprevention of elite tea variety CFT-1 rich in EGCG against chemically induced liver cancer in rats. Food Sci. Nutr. 2019, 7, 2647–2665. [Google Scholar] [CrossRef]
| Characteristics | HR | CI | p | Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|---|---|---|
| Alkaline hydrolysable Nitrogen | 1.27 | 0.57–1.1 | 0.164 | |||
| Altitude | 1.46 | 0.5–0.94 | 0.018 | 0.27 | 0.12–0.61 | 0.002 |
| Arsenic | 1.27 | 0.67–0.93 | 0.005 | |||
| Available cadmium | 1.01 | 0.96–1.02 | 0.347 | |||
| Available chromium | 1.02 | 0.76–1.25 | 0.846 | |||
| Available nickel | 0.96 | 1.01–1.07 | 0.008 | 1.04 | 1.01–1.08 | 0.015 |
| Available phosphorus | 0.97 | 0.78–1.34 | 0.845 | |||
| Available potassium | 1.29 | 0.54–1.11 | 0.171 | |||
| Cations | 0.8 | 1.04–1.48 | 0.016 | 1.39 | 1.01–1.92 | 0.044 |
| Cu | 0.92 | 0.8–1.48 | 0.594 | |||
| Fluoride | 1.07 | 0.71–1.23 | 0.632 | |||
| Lead | 0.9 | 1–1.24 | 0.042 | |||
| Mercury | 1.65 | 0.32–1.15 | 0.125 | |||
| Mg | 0.6 | 1.33–2.11 | 0 | 2.02 | 1.24–3.27 | 0.004 |
| Nickel | 1.99 | 0.33–0.76 | 0.001 | |||
| Organic_matter | 1.11 | 0.82–0.98 | 0.018 | |||
| PH | 1.01 | 0.87–1.14 | 0.936 | |||
| Total_chromium | 1.05 | 0.8–1.14 | 0.578 | |||
| Total_nitrogen | 1.24 | 0.66–0.99 | 0.036 | |||
| Total_phosphorus | 1.15 | 0.68–1.12 | 0.283 | |||
| Total_potassium | 0.86 | 1.05–1.3 | 0.005 | 0.64 | 0.47–0.87 | 0.005 |
| Zn | 1.02 | 0.79–1.24 | 0.898 |
| EGCG (%) | Altitude (m) | Available Nickel (mg/kg) | Total Potassium (mg/kg) | Mg (mg/kg) | Cations (mg/kg) | Grade | Is It Correct |
|---|---|---|---|---|---|---|---|
| 15.03 | 1600 | 0.086 | 7.85 | 3.57 | 1.4 | 0.256 | √ |
| 10.81 | 1650 | 0.345 | 9.9 | 5.18 | 6.1 | 0.02 | √ |
| 19.04 | 1600 | 0.065 | 6.57 | 2.75 | 1.6 | 0.33 | |
| 22.61 | 1700 | 0.262 | 4.72 | 2.34 | 2.1 | 0.76 | √ |
| 18.27 | 1650 | 0.072 | 8.06 | 5.81 | 3.6 | 0.174 | √ |
| 20.76 | 1600 | 0.112 | 8.21 | 3.68 | 2.1 | 0.278 | √ |
| 32.96 | 1650 | 0.091 | 8.27 | 3.25 | 1.9 | 0.82 | √ |
| 22.83 | 1650 | 2.84 | 8.25 | 3.21 | 3.3 | 0.257 | |
| 29.71 | 1700 | 0.411 | 4.66 | 2.72 | 2.4 | 0.74 | √ |
| 23.84 | 1600 | 0.06 | 9.89 | 2.59 | 1.1 | 0.78 | √ |
| 33.67 | 1700 | 0.381 | 4.88 | 2.64 | 3.6 | 0.65 | |
| 27.63 | 1650 | 0.515 | 10.8 | 4.84 | 5.3 | 0.42 | √ |
| 23.81 | 1650 | 0.271 | 11.4 | 4.76 | 5 | 0.73 | √ |
| 21.33 | 1600 | 0.069 | 7.51 | 2.87 | 0.8 | 0.63 | √ |
| 32.15 | 1700 | 0.281 | 4.7 | 2.17 | 2.6 | 0.75 | |
| 14.91 | 1650 | 2.11 | 9.94 | 4.58 | 4.8 | 0.157 | √ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Yang, C.; Zhang, S.; He, J.; Deng, X.; Gao, J.; Li, L.; Wu, Y.; Fan, Z.; Xia, Y.; et al. A Predictive Study on the Content of Epigallocatechin Gallate (EGCG) in Yunnan Large Leaf Tea Trees Based on the Nomogram Model. Agronomy 2023, 13, 2475. https://doi.org/10.3390/agronomy13102475
Wang B, Yang C, Zhang S, He J, Deng X, Gao J, Li L, Wu Y, Fan Z, Xia Y, et al. A Predictive Study on the Content of Epigallocatechin Gallate (EGCG) in Yunnan Large Leaf Tea Trees Based on the Nomogram Model. Agronomy. 2023; 13(10):2475. https://doi.org/10.3390/agronomy13102475
Chicago/Turabian StyleWang, Baijuan, Chunhua Yang, Shihao Zhang, Junjie He, Xiujuan Deng, Jun Gao, Lei Li, Yamin Wu, Zongpei Fan, Yuxin Xia, and et al. 2023. "A Predictive Study on the Content of Epigallocatechin Gallate (EGCG) in Yunnan Large Leaf Tea Trees Based on the Nomogram Model" Agronomy 13, no. 10: 2475. https://doi.org/10.3390/agronomy13102475
APA StyleWang, B., Yang, C., Zhang, S., He, J., Deng, X., Gao, J., Li, L., Wu, Y., Fan, Z., Xia, Y., Guo, Q., Yuan, W., & Wang, Y. (2023). A Predictive Study on the Content of Epigallocatechin Gallate (EGCG) in Yunnan Large Leaf Tea Trees Based on the Nomogram Model. Agronomy, 13(10), 2475. https://doi.org/10.3390/agronomy13102475






