Abstract
To verify the priming effects of Maillard reaction precursors on the microbial decomposition of rice straw at different incubation temperatures, the method of indoor incubation at a constant temperature was adopted. In the process, the addition of glucose, catechol or glycine solution alone or in mixed solution was conducted at incubation temperatures of 10 °C, 15 °C and 28 °C, respectively. The C content of humic-extracted acid (CHLE), humification index (the ratio of C content of humic-like acid to fulvic-like acid, CHLA/CFLA), ∆logK value of humic-like acid (HLA), and C content of humin-like acid (CHLu) were dynamically analyzed at 0, 30, 60, and 90 d, respectively. At the same time, the differences in the atomic ratio and FTIR spectra before and after incubation were systematically analyzed. The results showed that (1) the additions of glucose alone and mixed precursors were both beneficial to increasing the CHLE content at three tested temperatures, especially at two low temperatures (10 °C and 15 °C), and glucose alone manifested the most significant improvement in CHLE. In contrast, following the addition of glycine alone, the CHLE content decreased by 2.4% at 15 °C and 4.6% at 28 °C after incubation. (2) Glucose as the sole precursor was more beneficial to improving the quality of the humic substance (HS) at 28 °C, but only enhanced the condensation degree of HLA molecules at 15 °C. Compared with the results at 15 °C and 28 °C, the HLA molecules had the lowest condensation degree at 10 °C, regardless of whether a single precursor or mixed Maillard precursors were used. (3) After incubation, the amounts of N compounds in the HLA molecules decreased to varying degrees, especially at 28 °C. The O-containing functional groups, such as carboxyl groups, from HLA molecules decreased following the addition of a single precursor, while the mixed precursors resulted in an increase in O-containing functional groups. Increasing the catechol content directly enriched the unsaturated bonds of HLA. With the decomposition of rice straw, regardless of how the precursors were added, the polysaccharide content decreased to different degrees. The decomposition of polysaccharides in HLA was more temperature-sensitive, and an increase in temperature might encourage more polysaccharide consumption. Under each temperature, the molecular structure of HLA was simplified initially and then gradually became complex. Finally, the addition of glucose alone at 15 °C was more favorable for the complexity of HLA molecules, while at 28 °C, it could only alleviate the degree of simplification of the HLA molecular structure to a certain extent. (4) At the three tested temperatures, compared with the CK control, either one precursor or a mixture of three precursors could more effectively promote the decomposition of CHLu. Under the conditions of 10 °C and 15 °C, the addition of mixed precursors was more beneficial to the decomposition of CHLu, causing the CHLu content to decrease by 37.9% and 44.7%, respectively, followed by the addition of glucose alone.
1. Introduction
Rice straw is a byproduct of large-scale rice production. Large amounts of rice straw are often disposed in paddy fields in late autumn and early winter every year in northern China, which are difficult to decompose under natural low temperatures and then affect the normal cultivation of rice during the next year. To eliminate the adverse effects of the remaining rice straw in paddy fields following tillage, most farmers choose the incineration of rice straw, which cannot be returned to paddy fields to maintain soil fertility and causes harmful environmental effects [1]. Therefore, it is urgent to determine the positive factors of straw composting in a low-temperature environment, which is not limited to research on high-efficiency microbial agents. Although we believe that composting is a useful method for managing rice straw [2], microorganisms demonstrate poor activity at low temperatures, especially fungi that can play a leading role in straw decomposition; this is the main reason for the poor effect of straw return on fertilizing fields and the longer composting cycle.
Composting is a complex biochemical process in which abiotic and biotic pathways occur simultaneously for humic substance (HS) formation [3]. Biotic pathways play an important role in HS formation during composting, in which inoculating microorganisms and regulating environmental factors can accelerate the turnover of organic matter by improving microbial metabolic activities and then producing more HS precursors, such as reducing sugars, phenols and amino acids. These precursors can be condensed to form HS in abiotic pathways [4,5]. The Maillard reaction is a non-enzymatic browning reaction, which is an abiotic humification process of three precursors (glucose, catechol and glycine) catalyzed by δ-MnO2, resulting in the formation of dark substances that are highly similar to HS [6]. Maillard precursors contribute significantly to the formation of HS [7]. Tan et al. [8] noted that in the composting process of organic materials, the condensation reaction between degradation products and precursors such as amino acids, sugars and phenols represents the key to the formation of HS. Hardie et al. [9] suggested that glucose could promote the abiotic humification of catechol catalyzed by δ-MnO2. In addition, increasing the molar ratio of glucose to catechol and glycine could also promote the production of low-molecular substances that are similar to natural humic acids in the integrated catechol–Maillard system. It could be concluded that glucose significantly contributes to the formation and nature of HS. Wu et al. [10] studied the changes in polyphenols, amino acids and polysaccharides in the composting process and noted that each precursor significantly promoted the formation of HS through the Maillard reaction mechanism. The addition of exogenous amino acids or their ionic liquid was found to improve lignocellulose degradation and HS synthesis during straw biomass composting [11]. Zhang et al. [12] investigated the effect of MnO2 on the abiotic humification of glucose and glycine by adding catechol at different concentrations. The results showed that increasing the content of catechol could promote the formation of HS, and transform more unstable FLA (fulvic-like acid) into HLA (humic-like acid), while also reducing 0.73–1.87 mg of CO2 released per mg C content of FLA. Zhang et al. [13] added benzoic acid and soybean residue to a mixture of corn stover and chicken manure to study the effects of precursors on HS formed in compost. The results showed that the addition of precursors could promote the formation of HS and the accumulation of HA in the compost. Since glucose, glycine and catechol could form darkening substances that were similar to HS through the Maillard reaction under abiotic conditions, the change in the molar ratio between Maillard precursors could affect the amount of HS formed [7]. Then, in view of the priming effects of glucose, glycine and catechol on the humification process, we investigated whether the decomposition of rice straw could be strengthened through the addition of Maillard precursors at natural low temperatures and whether the humification process and the maturation degree of rice straw could be promoted to a certain extent. The relevant scientific problems remain to be revealed.
The incubation method of simulated low temperature was adopted, with rice straw serving as the test object, and three incubation temperatures of 10 °C, 15 °C and 28 °C were selected, among which the most suitable temperature of 28 °C for decomposition was used for comparative consideration. The C content of humic-like acid (CHLA), humification index (the ratio of C content of humic-like acid to C content of fulvic-like acid, CHLA/CFLA), atomic ratio, FTIR spectra and ∆logK value of humic-like acid (HLA), and the C content of humin-like (CHLu) rice straw decomposition products were dynamically analyzed during incubation for 90 days by adding glucose (Glu), catechol (Cat), glycine (Gly) solution and the mixed solution of these components. The objective of the present study was to (i) reveal the effects of different Maillard precursors on the decomposition process of rice straw and (ii) choose a suitable method from three Maillard precursors that could promote the decomposition of rice straw at a low temperature. The clarification of the above scientific problems could provide a theoretical reference for the biotic contribution of Maillard reaction precursors to the abiotic humification of rice straw and provide a practical basis for the formulation of technical measures for the more efficient decomposition of rice straw.
2. Materials and Methods
2.1. Materials
The tested rice straw was obtained from the experimental field of Jilin Agricultural Science and Technology University. After a series of impurities were removed, washed and dried, the rice straw was crushed and sifted through a 0.10 mm sieve. The total organic C, N, P2O5, K2O and pH values of rice straw powder were 60.2%, 7.85 g/kg, 3.51 g/kg, 33.0 g/kg and 6.34, respectively, and the rice straw was composed of cellulose (32.6%), hemicelluloses (21.5%), lignin (22.4%) and ash (15.6%).
The analytical reagents manganese dioxide (MnO2), glucose (C6H12O6), catechol (C6H6O2) and glycine (C2H5NO2) were all purchased from Sinopharm Group Co. Ltd., Shanghai, China.
Preparation of the microbial inoculum was carried out as follows: the commercial EM agent was produced by Tonghua Wanying Biotechnology Co., Ltd., Jilin, China, and contained lactic acid bacteria, a photosynthetic bacteria group, yeast group, actinomycetes and a linear bacteria group. Its total number of living microbes was ≥2 × 108 cfu/mL, with a pH of 3.5–4.5. The commercial EM agent was diluted with sterile and deionized water at a ratio of 1:100 to prepare the microbial inoculum.
2.2. Methods
The incubation method was adopted in the study. A number of glass triangular bottles of 100 mL were prepared, 15.0 g of accurately weighed rice straw powder was added into the triangular bottles in batches, and 0.5 g of MnO2 powder was added to each bottle. The C/N ratios of the above mixtures were adjusted with (NH4)2SO4 solution to 25:1. According to the test objectives, the following four treatments were designed: (1) Glu treatment, which included adding 15 mL of glucose solution with a concentration of 0.12 mol/L; (2) Cat treatment, which included adding 15 mL of catechol solution with a concentration of 0.12 mol/L; (3) Gly treatment, which included adding 15 mL of glycine solution with a concentration of 0.12 mol/L; (4) Ms treatment, which included adding the mixed solution composed of 5 mL each of glucose, catechol and glycine, each with a concentration of 0.12 mol/L, and an equal volume (15 mL) of sterile, deionized water was used as the CK control. After treatment, all glass triangular bottles were covered with sterile permeable films and then sterilized with high-pressure steam sterilization at 121 °C for 20 min. After cooling naturally, all bottles were inoculated with the microbial inoculum at a dose of 5 mL/vial under aseptic operating conditions, and the breathable films were sealed again after inoculation.
The incubation temperatures were set as 10 °C, 15 °C and 28 °C, and the total incubation period for a constant temperature was designated as 90 days. Sterile water was dynamically supplemented according to the loss of weight. The samples were taken out at 0, 30, 60 and 90 days. Based on the destructive sampling method, all the samples from each treatment, temperature and sampling time were repeated three times. The samples were removed, dried at 55 °C to stop microbial activity, and then ground through a 0.10 mm sieve to analyze the C content of humic-like acid (CHLA), humification index (the ratio of C content of humic-like acid to C content of fulvic-like acid, CHLA/CFLA), atomic ratio, FTIR spectra and ∆logK value of humic-like acid (HLA), and the C content of humin-like acid (CHLu).
2.3. Analysis
The samples were analyzed according to a modified humus composition method. The brief process was as follows: a 1.0 g sample was weighed in a 100 mL polyethylene centrifuge tube, and 30 mL of distilled water was added to the tube and stirred evenly. The centrifuge tubes with samples were extracted in a water bath oscillator, maintained at 70 °C for 1 h and centrifuged (3500 r/min, 15 min). The obtained supernatant was filtered into a 50 mL volumetric flask. The samples were extracted again using 20 mL of distilled water, and the extracts from two extractions were pooled together to yield the water-soluble substance (WSS). The residue in the centrifuge tube was extracted again with a mixed solution of 0.1 mol/L Na4P2O7·10H2O and 0.1 mol/L NaOH using the same procedure as above, and the extracted solution was the humic-extracted acid (HLE). The residue in the tube was rinsed with distilled water many times until the eluent was nearly neutral and then transferred into a drying oven at 50 °C until a constant weight was achieved, which was defined as humin-like (HLu), and ground through a 0.10 mm sieve.
The HLE solution (30 mL) was adjusted to a pH of 1.0–1.5 using H2SO4 at a concentration of 0.5 mol/L, transferred to a water bath, maintained at 70 °C for 1.5 h, and then kept overnight. The solution that contained flocculent matter was filtered into a volumetric bottle and filled to 50 mL, and the filter liquor was fulvic-like acid (FLA). The flocculent matter on the filter paper was rinsed repeatedly with a dilute acid solution and dissolved into a 50 mL volumetric bottle with a heated NaOH solution at a concentration of 0.05 mol/L. The obtained solution was humic-like acid (HLA). The organic C contents of WSS, HLE, HLA and HLu (CWSS, CHLE, CHLA and CHLu) were determined by the TOC analyzer, in which the CFLA (CFLA = CHLE − CHLA) and the humification index (CHLA/CFLA ratio) were calculated. The absorbances of the HLA liquid sample at 400 and 600 nm (A400 and A600) were measured by a UV–visible light spectrophotometer (TU-1900, Beijing Purkinje General Instrument Co., Ltd., Beijing, China), and then ∆logK was calculated by the following equation: ∆logK = lgA400 − lgA600 [14]. All analyses were carried out in triplicate, and the mean values are presented.
The extracted HLA liquid samples were freeze-dried to prepare solid HLA samples for further analysis. The percentages of C, H, O and N in the solid HLA samples were measured by an elemental analyzer (PerkinElmer PE 2400II CHNS/O), and the C/N, H/C, and O/C ratios were calculated. The spectroscopic characterization of solid HLA samples was measured by FTIR spectrophotometry (model: FTIR-850, Tianjin Guangdong Sci & Tech Development Co., Ltd., Tianjin, China). FTIR spectra were acquired over the wavelength region from 400 to 4000 cm−1, analyzed with FTIR 850 software and presented as graphs with Origin 8.0 software.
2.4. Statistical Analysis of the Data
Data analysis and spectrum processing were performed using Excel 2003 and Origin 8.0. All statistical tests were performed using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). The differences among the treatment means were determined using one-way analysis of variance (ANOVA) with the least significant difference (LSD) test. The significance was set at the p < 0.05 level.
3. Results
3.1. C content of Humic-Extracted Acid (CHLE)
As shown in Figure 1, different treatments exhibited different trends at each temperature. Under the incubation temperature of 10 °C, the CHLE from the Glu treatment showed a trend of increasing first and then tending to remain stable and the CHLE in the Cat, Gly and Ms treatments showed an overall trend of increasing first and then decreasing, while the CHLE of the CK control showed a trend of decreasing first and then increasing. Compared with the result at 0 d, the CHLE of all the treatments displayed different degrees of increase after the end of incubation, and the CHLE contents in the CK control, Glu, Cat, Gly and Ms treatments were increased by 4.7%, 45.6%, 37.6%, 15.9%, and 15.1%, respectively, among which the Glu treatment had the most obvious increase, followed by the Cat treatment. At 15 °C, the CHLE of the Glu treatment gradually increased, while the CHLE of the other treatments first increased and then decreased. Compared with the result at 0 d, the CHLE in the CK control and Cat treatment had no significant change after the end of incubation, but the CHLE of the Glu and Ms treatments increased by 53.3% and 31.3%, respectively. The Glu treatment had the most significant effect on CHLE, while the CHLE of the Gly treatment was merely reduced by 2.4%. At 28 °C, the CHLE in the Glu, Cat and Gly treatments showed a trend of increasing first and then decreasing, while the CHLE of the CK control gradually increased, and the CHLE in the Ms treatment showed a trend of decreasing first and then increasing. Compared with the result at 0 d, after the end of incubation, the CHLE contents of the CK control, Glu, Cat and Ms treatments increased by 11.5%, 22.2%, 34.8% and 25.6%, respectively, while the CHLE of the Gly treatment decreased by 4.6%. Thus, it could be determined that the addition of glucose alone and mixed Maillard precursors were both beneficial to increasing the CHLE of the rice straw decomposition material at three temperatures, and the addition of glucose alone yielded the most significant increase in CHLE at the two simulated low temperatures of 10 °C and 15 °C. In contrast, after the end of incubation, the CHLE of the rice straw decomposition material was decreased by 2.4% at 15 °C and 4.6% at 28 °C by the addition of glycine alone.
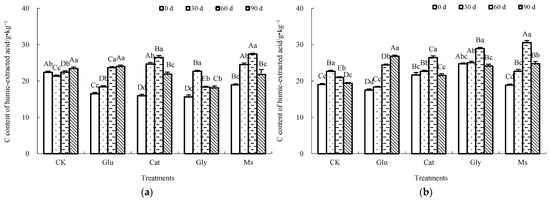
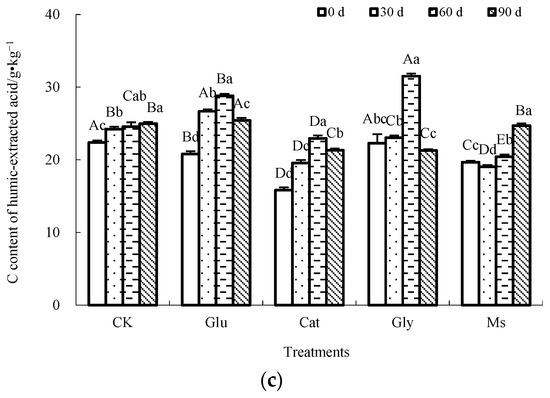
Figure 1.
Effect of Maillard reaction precursors on CHLE extracted from the decomposing material of rice straw at three incubation temperatures. Note: significant difference in the different treatments with the same number of incubation days is indicated by different capital letters, and significant difference in the different incubation days under the same treatment is expressed by different lowercase letters. (a) Incubated at 10 °C; (b) incubated at 15 °C; (c) incubated at 28 °C.
3.2. Humification Index (CHLA/CFLA Ratio)
The humification index (CHLA/CFLA ratio) is widely accepted as the best index that reflects the degree of compost maturity and polymerization or the degree of condensation of the aromatic nucleus of humus [1,15]. As shown in Figure 2, the CHLA/CFLA ratios of the decomposing material of rice straw in each treatment were different at the three incubation temperatures. Compared with the result at 0 d, the CHLA/CFLA ratio decreased to different degrees after the end of incubation. At 10 °C, the CHLA/CFLA of the Cat, Gly and Ms treatments declined initially and then increased progressively. The CHLA/CFLA ratio of the Glu treatment gradually decreased, while the CHLA/CFLA ratio of the CK control showed a fluctuating decrease. Compared with the result at 0 d, after the end of incubation, the CHLA/CFLA ratios decreased in the following order: Glu (73.8%) > Cat (71.7%) > Ms (59.3%) > Gly (36.7%) > CK (21.9%). At 15 °C, the CHLA/CFLA ratio of the CK control fluctuated, and the CHLA/CFLA ratio of the Glu treatment decreased first and then increased, while the CHLA/CFLA ratios of the Cat, Gly and Ms treatments gradually decreased with incubation. Compared with the result at 0 d, after the completion of incubation, the CHLA/CFLA ratios decreased in the following order: Ms (76.1%) > Gly (67.2%) > Cat (56.4%) > Glu (9.2%), while the CHLA/CFLA ratio of the CK control increased by 42.6%. At 28 °C, the CHLA/CFLA ratios for the CK control and Cat treatment gradually decreased, while the CHLA/CFLA ratios of the Glu, Gly and Ms treatments decreased first and then increased. Compared with the result at 0 d, the descending ranges of the CHLA/CFLA ratio after the end of incubation were as follows: Cat (64.6%) > CK (62.3%) > Ms (35.8%) > Gly (3.6%). In contrast, the CHLA/CFLA ratio of the Glu treatment increased by 14.7%. Under two low temperatures (10 °C and 15 °C), the single precursor or mixed Maillard precursors did not improve the quality of HS, while under incubation at 28 °C, the addition of glucose alone improved the HS quality of the decomposing material of rice straw after incubation. With the addition of glucose alone, different incubation temperatures greatly influenced the HS quality of the decomposing material of rice straw. Under the conditions of 10 °C and 15 °C, the CHLA/CFLA ratios were decreased by 73.8% and 9.2%, respectively, while at 28 °C, the CHLA/CFLA ratio was increased by 14.7%. With the addition of glucose alone, increasing the incubation temperature could gradually improve the polymerization degree and indicate an increase in the structural complexity of HS of the decomposing material of rice straw.
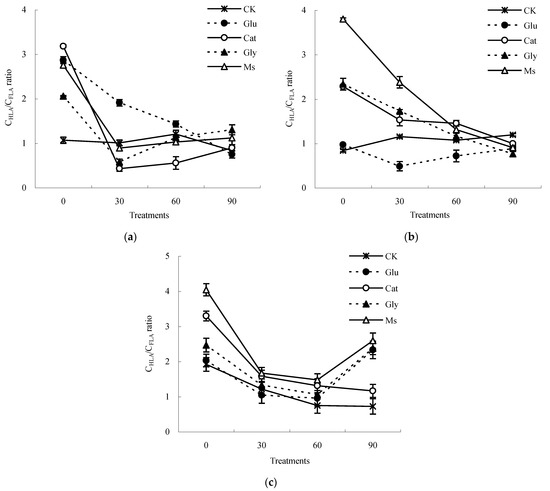
Figure 2.
Effect of Maillard reaction precursors on the CHLA/CFLA ratio of the decomposing material of rice straw at three incubation temperatures. (a) Incubated at 10 °C; (b) incubated at 15 °C; (c) incubated at 28 °C.
3.3. Atomic Ratio, FTIR Spectra of Humic-like Acid (HLA) and ΔlogK Value of HLA Liquid Sample
The H/C ratio represented the degree of aromatic condensation [16,17]. Based on the results of the elemental analysis presented in Table 1, after incubation at 10 °C and 28 °C, the H/C ratios of HLA molecules in each treatment were all significantly higher than those before incubation, reflecting a decreasing aromatization degree of HLA after the end of incubation [16]. However, at 15 °C, the H/C ratios of HLA molecules in the CK control and Glu treatment were both lower than those before incubation, indicating that more condensed aromatic structures formed in the molecular structure of HLA [16,17]. However, the H/C ratios from the other treatments (Cat, Gly, Ms) were significantly higher than those before incubation. In contrast, the treatments supplemented with Maillard precursors obtained the highest H/C ratio of HLA molecules at an incubation temperature of 10 °C. It was indicated by the above observations that the condensation degree of HLA molecules from the decomposed material of rice straw decreased to different degrees after incubation for 90 days at 10 °C and 28 °C, but the condensation degree of HLA molecules was enhanced under the Glu treatment and CK control at 15 °C. However, the condensation degree of HLA molecules was also reduced to varying degrees at 15 °C following the addition of catechol or glycine alone and a mixture of the three precursors. Additionally, compared with the results at 15 °C and 28 °C, the condensation degree of HLA molecules was the lowest at the incubation temperature of 10 °C, regardless of whether a single precursor or mixed Maillard precursors were used. At three temperatures, compared with the result before incubation, the C/N ratio of the decomposing material of rice straw under each treatment increased to varying degrees after the end of incubation, among which the C/N ratio from each treatment at 28 °C was significantly higher than the result of the other two temperatures. It was indicated in the above observations that after incubation, the amount of N compounds in the HLA molecules decreased; the decreased range was greater in magnitude than the loss of organic C due to mineralization, thus increasing the C/N ratio. Especially at 28 °C, the loss rate of N compounds in the HLA molecules was the highest in each treatment after incubation. The O/C ratio represented the proportion of O-containing functional groups [16]. At three temperatures, based on the addition of a single precursor, compared with the result before incubation, the O/C ratio of the HLA molecules was somewhat reduced after the end of incubation. In contrast, the O/C ratio upon treatment with the mixed Maillard precursors increased significantly at the end of incubation, resulting in an increase in the number of O-containing functional groups in the HLA molecules.

Table 1.
Atomic ratio of HLA extracted from the decomposing material of rice straw at different incubation temperatures.
The chemical characteristics of HLA extracted from the decomposing material of rice straw at different incubation temperatures were observed from the FTIR spectra in Figure 3. The spectra were characterized by (a) a broad peak at 3356–3462 cm−1, which represented the O–H stretching vibration from the hydroxyl groups of alcohols, phenols, organic acids, and other easily decomposed substances [2]; (b) the two weak bands at approximately 2922–2960 cm−1 and 2852 cm−1, which were ascribed to the aliphatic C–H stretching of CH3/CH2 groups; (c) a band at 1618–1659 cm−1, which was assigned to the C=C stretching vibration of lignin aromatic rings [9,18]; (d) a weak band at approximately 1510–1599 cm−1, ascribed to the N–H deformation and C=N stretching of amides or to lignin [18,19]; (e) a weak band at approximately 1452–1458 cm−1, assigned to the C–O asymmetric stretching of carboxyl groups [19]; (f) a band at 1385–1396 cm−1, attributed to the stretching vibration of COO- groups [20]; (g) a band observed at 1219–1265 cm−1, which corresponded with the C–O–H deformation and C–O stretching of phenolic compounds [21]; (h) a band at approximately 1045–1126 cm−1, mainly due to the C–O stretching of polysaccharides [19], which was also assigned to the C–O–C stretching vibration in cellulose and hemicellulose [22], implying high proportions of cellulose and hemicellulose.
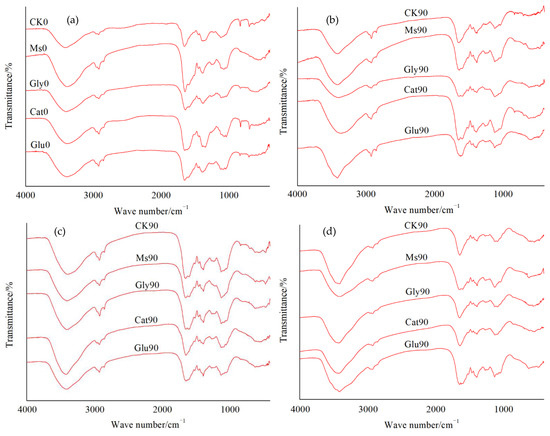
Figure 3.
FTIR spectra of HLA extracted from the decomposing material of rice straw at different incubation temperatures: (a) 0 days before the incubation; (b) incubated at 10 °C for 90 days; (c) incubated at 15 °C for 90 days; (d) incubated at 28 °C for 90 days.
The common features in the FTIR spectra are summarized in Table 2. Compared with the result at 0 d, the intensity of the absorption peak at 3356–3462 cm−1 of HLA extracted from the decomposing material of rice straw increased at each temperature in the Glu, Cat, Gly and Ms treatments, which showed the following trend: CK > Glu > Cat > Gly > Ms. The ratio of the aliphatic C–H stretching of CH3/CH2 groups (2922–2960 cm−1 and 2852 cm−1) to the C=C stretching vibration of aromatic rings (1618–1659 cm−1) could be expressed as the ratio c/(a + b). Compared with the result at 0 d, the c/(a + b) ratios of the CK control and Glu treatment at 15 °C were both enhanced, and the c/(a + b) ratios of the other treatments (Cat, Gly and Ms) at three temperatures were all decreased to different degrees. It was indicated that the addition of glucose alone enhanced the aromatization degree of HLA molecules at 15 °C; in contrast, the addition of glycine or catechol alone and mixed precursors could be helpful for the decomposition of rice straw and improve the degree of aliphatization at the three temperatures. At these three temperatures, compared with the result at 0 d, the intensity of the absorption peak at 1510–1599 cm−1 from each treatment decreased to different degrees following the completion of incubation. Moreover, based on the same treatment, the higher the incubation temperature, the greater the decreased range of the intensity of the peak at 1510–1599 cm−1. Under the condition of 10 °C, the peak at 1219–1265 cm−1 appeared only in HLA molecules treated with Cat, while at the temperatures of 15 °C and 28 °C, the absorption peak at 1219–1265 cm−1 appeared in all the treatments, and the vibration intensity of this peak under the Cat treatment was the highest among all the treatments. Compared with the result at 0 d, at different temperatures, the intensity of the peak at 1045–1126 cm−1, representing the polysaccharide, cellulose or hemicellulose constituents of HLA molecules in the Glu, Cat, Gly and Ms treatments, decreased to different degrees, indicating that the decomposition of cellulose and hemicellulose in the HLA [18] was accompanied by the consumption of polysaccharides. In addition, with increasing incubation temperature, the consumption of polysaccharides under the same treatment was also enhanced, so the decreased range of polysaccharides was magnified.

Table 2.
FTIR relative intensities (% of total area) for the HLA extracted from the decomposing material.
The ∆logK value was used in this study to describe the humification degree of HLA extracted from the decomposing material of rice straw. The increase in the humification degree and condensation aromatic structures of HLA corresponded to a decrease in the ∆logK value. As shown in Figure 4, the ∆logK value of the HLA liquid sample increased first and then decreased at three temperatures (10 °C, 15 °C and 28 °C), indicating that the structure of HLA molecules was simple at first, and then tended to become more complicated with incubation. Compared with the result at 0 d, after incubation for 90 d at 10 °C, the ∆logK values of HLA in the CK, Glu, Cat, Gly and Ms treatments were increased by 1.4%, 104.2%, 89.8%, 81.0% and 103.9%, respectively. At 15 °C, the ∆logK values of HLA in the CK control and Glu treatment were decreased by 17.2% and 3.5%, respectively, while the ∆logK values of HLA in the Cat, Gly and Ms treatments increased by 81.1%, 71.6% and 39.3%, respectively. At 28 °C, the ∆logK values of HLA treated with the CK control, Glu, Cat, Gly and Ms treatments were increased by 95.2%, 3.9%, 76.2%, 82.8% and 81.1%, respectively, among which that after treatment with glucose alone increased the most.
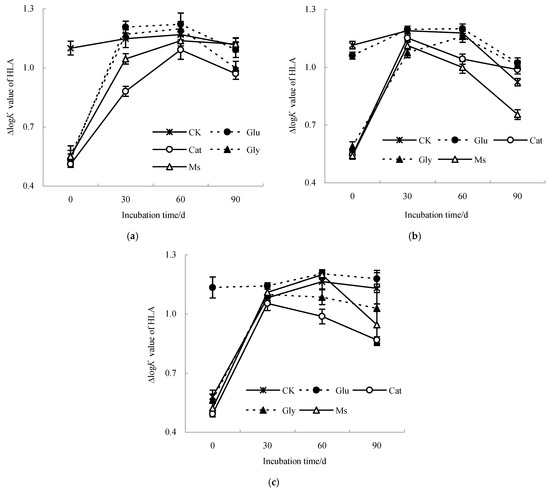
Figure 4.
∆logK value of humic-like acid liquid sample from the decomposing material of rice straw at different incubation temperatures. (a) Incubated at 10 °C; (b) incubated at 15 °C; (c) incubated at 28 °C.
3.4. C Content of Humin-like (CHLu)
As shown in Figure 5, the variation in the CHLu content of the decomposing material of rice straw in each treatment was slightly different at three incubation temperatures (10 °C, 15 °C and 28 °C). Under the condition of 10 °C, while the CHLu content under the CK control exhibited an increase first followed by a decrease, the CHLu contents from the other four treatments gradually decreased. Compared with the result at 0 d, after the end of incubation, the CHLu contents of each treatment decreased in the following order: Ms (37.9%) > Glu (27.6%) > Cat (18.8%) > Gly (17.9%) > CK control (10.3%). At 15 °C, the CHLu contents of the CK control and Glu treatment increased first and then decreased, while the CHLu contents from the other three treatments gradually decreased. Compared with the result at 0 d, at the end of incubation, the CHLu contents of each treatment decreased in the following order: Ms (44.7%) > Glu (40.7%) > Gly (31.6%) > Cat (28.1%) > CK control (4.0%). At 28 °C, the contents of CHLu in the CK control and all the treatments showed a gradual decrease. Compared with the result at 0 d, after the end of incubation, the decreased ranges of CHLu content in each treatment were as follows: Cat (69.2%) > Glu (53.0%) > Gly (33.7%) > Ms (25.7%) > CK (19.2%). It could be determined from the above rules that at three temperatures, compared with the CK control, either one precursor or a mixture of three Maillard precursors could more effectively promote the decomposition of CHLu. Under the conditions of 10 °C and 15 °C, the addition of mixed Maillard precursors was more beneficial to the decomposition of CHLu, causing the CHLu content to decrease by 37.9% and 44.7%, respectively, followed by the addition of glucose alone.
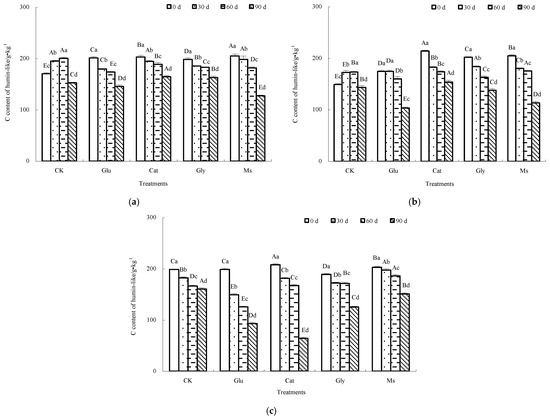
Figure 5.
Effect of Maillard reaction precursors on CHLu extracted from the decomposing material of rice straw at different incubation temperatures. Note: significant difference in the different treatments with the same number of incubation days is indicated by different capital letters, and significant difference in the different incubation days under the same treatment is expressed by different lowercase letters. (a) Incubated at 10 °C; (b) incubated at 15 °C; (c) incubated at 28 °C.
4. Discussion
After large-scale rice production, a large amount of rice straw is retained in paddy fields in late autumn and early winter in northern China, which is rich in lignin and difficult to decompose under natural low temperatures. In the past, many reports focused on the research of high-efficiency microbial agents, but these studies used the abiotic condensation of Maillard precursors to contribute to the humification process. In the present study, catechol, glycine and glucose were used as representative phenols, amino acids and sugars, respectively.
The precursors were recognized as the key factors that affect HS formation [13]. The additions of glucose alone and mixed precursors were both beneficial to increasing the CHLE contents of the decomposing material of rice straw at 10 °C, 15 °C and 28 °C, especially at two low temperatures (10 °C and 15 °C), among which glucose alone yielded the most significant improvement of CHLE, enabling the CHLE content to increase by 45.6% at 10 °C and 53.3% at 15 °C, respectively, values which were significantly higher than the results from the other treatments at the same temperature. In contrast, after incubation, the CHLE contents were decreased by 2.4% at 15 °C and 4.6% at 28 °C following the addition of glycine alone. This was consistent with the conclusion that amino acids were gradually consumed to form HS, but exhibited a negative relationship with HS formation [13]. The carboxyl C and especially alkyl C contents of glycine were incorporated into the humic polycondensates, which was the main mechanism of its combination with phenols to form HS. The synthesis of CHLE might be affected to a certain extent by the addition of glycine alone when exogenous phenols are missing [23]. The stable supply of glucose could enhance humification by indirectly providing microbial carbon sources, contributing to the formation of HS and the production of CHLE [24]. Phenol, carboxyl, reducing sugars and amino acids are the main functional groups used for precursor formation during humification [9]. Therefore, adding more precursors might promote the maturity of rice straw composting and enhance its CHLE content [13].
At temperatures of 10 °C and 15 °C, neither the sole precursor nor mixed Maillard precursors promoted the conversion of FLA to HLA or effectively improved the quality of HS, while at 28 °C, the addition of glucose alone improved the HS quality after incubation, increasing the CHLA/CFLA ratio by 14.7%. Therefore, the microorganisms were the crucial promoters of HS formation, and the quality of HS could be effectively improved by a sufficient supply of carbon (glucose) at a suitable temperature (28 °C) [25].
Only under the condition of 15 °C was the addition of glucose alone more conducive to improving the condensation degree of HLA molecules, while at 10 °C and 28 °C, the condensation degrees of HLA molecules from the decomposed material of rice straw decreased to different degrees after incubation for 90 d. Additionally, compared with the results observed at 15 °C and 28 °C, the condensation degree of HLA molecules was the lowest at the incubation temperature of 10 °C, regardless of whether a single precursor or mixed Maillard precursors were used. The condensation degree of HLA molecules was related to the balance of mineralization and humification during the decomposition of rice straw. A lower temperature (10 °C) was not conducive to humification, while a higher temperature (28 °C) could promote mineralization to a certain extent. Therefore, at 15 °C, humification was slightly more prevalent than mineralization, resulting in an increase in the condensation degree of HLA molecules [26]. Compared to biotic processes, the structure of HLA showed that the HS formed by abiotic processes was more complex and more quickly generated [25]. The main reason for this was that mineralization and humification simultaneously existed in the biotic process, which was sensitive to temperature.
The amount of N compounds in the HLA molecules decreased to varying degrees after incubation, especially at 28 °C, and the loss rate of N compounds was the highest in each treatment. As Zhang et al. reported [27], composting could result in the loss of substantial quantities of nitrogen (N). N loss is generally caused by the denitrification of NH4+–N in the form of volatilization [28]. The incubation temperature could affect and reflect the microbial activity, decomposition rate, and degree of humification of the compost product, and the increase in temperature possibly enhanced ammonia volatilization [27]. Furthermore, the higher temperature (e.g., 28 °C) might trigger the Maillard reaction, causing more proteins to be consumed [29]. The contents of O-containing functional groups of HLA molecules decreased after incubation with the addition of the sole precursor, indicating that the contents of O-alkyl and carboxylic acid functional groups in HLA decreased with incubation [16]. In contrast, the mixed precursors could result in an increase in the number of O-containing functional groups. Jointly adding exogenous precursors might promote the humification process during the decomposition of rice straw [13]. Furthermore, the slight increase in O-alkyl groups in HLA possibly occurred because the microorganisms preferentially utilized these mixed precursors, according to the preferential substrate use theory [30]. The increase in O-alkyl groups suggests that some of the undecomposed precursors might have been incorporated into HLA through hydrogen bonding [31]. When a single precursor was provided, it could not meet the needs of the microorganisms in order to decrease the contents of the O-containing functional groups in HLA molecules.
The hydroxyl content of HLA molecules was increased because of the addition of MnO2 and other easily decomposed substances [2], and the effects of glucose, catechol or glycine alone were higher than that of their mixed solution. Lignin was proposed to be the main contributor to aromatic C [32]. The amount of N compounds in the HLA molecules could be decreased to various degrees and was accompanied by lignin degradation after the completion of incubation, and the decreased range was larger for higher incubation temperatures [2]. The microbial activities affected by temperature indeed caused structural differences in HLA during HS formation [25]. The peaks at 1452–1458 cm−1 and 1385–1396 cm−1 were related to the carboxyl groups. In view of this, the two peaks were combined and expressed as the sum of d and e (d + e) to determine and evaluate the carboxyl content of each treatment. Compared with the result before the incubation, the d + e of the Glu, Cat and Gly treatments decreased to different degrees after the completion of incubation. In contrast, d + e increased to varying degrees in the Ms treatment. In addition, the order of d + e was Ms > Gly > Cat > Glu > CK at all incubation temperatures. As an important intermediate product derived from the process of HS formation, carboxyl groups play an important role in increasing the aliphatic compounds and unsaturation degree of HS [9]. The addition of glucose, catechol or glycine alone could result in a decrease in the carboxyl groups in the HLA molecules after incubation; in contrast, the mixed addition of these precursors could increase the number of carboxyl groups in HLA molecules after incubation. The significant increase in the carboxyl C of HLA might be related to the oxidation of lignin side chains, aromatic rings and polysaccharides and the accumulation of fatty acids during rice straw degradation [33]. The increase in the carboxylic (COOH and COO−) groups indicated a high degree of oxidation [34], so the rule was consistent with the change in O-containing functional groups in the HLA molecules during the decomposition of rice straw. Polyphenols are described in the lignin/phenol–protein theory, and the Maillard reaction is one of the most important precursors of HS [35]. Under the condition of 10 °C, the disappearance of the peak at 1219–1265 cm−1 under all treatments, except for the Cat treatment, might be attributed to the aerobic degradation of lignin by microbial inoculum via dehydration, demethylation, or cleavage of the β-O-4 bond, indicating that Maillard precursors could stimulate microbial activity, and in turn accelerate the decomposition of rice straw. The appearance of polyphenols in the HLA molecules might be caused by the degradation of cellulose and hemicellulose derived from the decomposition of rice straw and the exogenous addition of catechol [36]. Polyphenols might significantly promote the aromaticity of HS, leading to the more stable and recalcitrant structure of HS [37]. Increasing catechol could contribute to the increase in the degree of unsaturation in HLA [7], and the increase in its concentration was also capable of introducing more N-containing substances into HLA, which further accelerated the darkening effect of humification. Increasing the catechol content directly enriched the unsaturated bonds of HLA, suggesting that catechol provided the molecular C skeleton for precursors to generate complicated organic macromolecules of HLA [7]. As a major contributor to the formation of HS [15], polysaccharides are derived from the degradation of lignin, cellulose and hemicellulose [38] and can be utilized as a primary energy and C source by microbes. With the decomposition of rice straw, the polysaccharide content decreased to different degrees with the addition of a single precursor or mixed precursors. This could be attributed to the preferential degradation of polysaccharides used for the energy requirements of the microorganisms and the enhancement of microbial activity by the suitable incubation temperature [19]. Cáceres et al. [39] mentioned that an increase in temperature might contribute to the rapid decomposition of easily degradable organic matter. Microbial activity was controlled by C availability and other specific factors, including temperature. The enzyme activity was limited by low temperature, and substrates were sufficiently available. With increasing temperature, the substrates would be consumed more quickly and become exhausted [40]. The decomposition of polysaccharides in HLA was more temperature-sensitive [41]. Valmaseda et al. [42] found that the decomposition of wheat straw by ligninolytic fungi consisted of an initial colonization stage of consumption of free sugars and a subsequent degradation stage of catabolizing the polysaccharides and lignin.
Microbial metabolism is crucial in complicating HLA structures [25]. Under each temperature, the molecular structure of HLA was simplified initially and then gradually became complicated. The synthetic function of core bacteria to improve HS humification might be more prominent in the later stage of composting [43]. However, despite the observation that the molecular structure of HLA became complicated when glucose alone and the CK control were added and the treatments were carried out separately at 15 °C, the remaining structures of the HLA molecules under other treatments eventually became simpler. At 28 °C, although all the treatments simplified the molecular structure of HLA, the addition of glucose alone alleviated the degree of simplification of the HLA molecular structure to a certain extent.
In addition, carbon transformation related to HS formation was also involved in microbial energy transformation [44]. According to Qi et al. [45], the molecular weight and degree of polymerization in HLu were greater than those in HLA and FLA, indicating the higher stability and long-lasting impacts of HLu. At the three temperatures, compared with the CK control, either the sole precursor or a mixture of the three precursors could more effectively promote the decomposition of CHLu. As a major and non-reactive component of HS, the decomposition of HLu was more conducive to improving the generation of reactive HS. Under the conditions of 10 °C and 15 °C, the addition of mixed precursors was more beneficial to the decomposition of CHLu, causing the CHLu content to decrease by 37.9% and 44.7%, respectively, followed by the addition of glucose alone. Normally, these precursors are mainly formed by organic matter degradation and microbial polymerization [46]. However, in this experiment, exogenous precursors were added to further promote the progress of the decomposition reaction and, to some extent, compensated for the adverse effects of low temperature on the decomposition reaction.
5. Conclusions
- (1)
- The additions of glucose alone and mixed precursors were both beneficial to increase the CHLE content at three tested temperatures, especially at two low temperatures (10 °C and 15 °C), and glucose alone enabled the most significant improvement of CHLE. In contrast, following the addition of glycine alone, the CHLE content decreased by 2.4% at 15 °C and 4.6% at 28 °C after incubation.
- (2)
- The use of glucose as the sole precursor was more beneficial to improving the quality of HS at 28 °C, but only enhanced the condensation degree of HLA molecules at 15 °C. Compared with the results at 15 °C and 28 °C, the HLA molecules had the lowest condensation degree at 10 °C, regardless of whether a single precursor or mixed Maillard precursors were used.
- (3)
- After incubation, the amount of N compounds in the HLA molecules decreased to varying degrees, especially at 28 °C. The O-containing functional groups, such as carboxyl groups, from HLA molecules decreased following the addition of a single precursor, while the mixed precursors could result in an increase in O-containing functional groups. Increasing the catechol content directly enriched the unsaturated bonds of HLA. With the decomposition of rice straw, regardless of how the precursors were added, the polysaccharide content decreased to different degrees. The decomposition of polysaccharides in HLA was more temperature-sensitive, and an increase in temperature might encourage more polysaccharide consumption. Under each temperature, the molecular structure of HLA was simplified initially and then gradually became more complex. Finally, the addition of glucose alone at 15 °C was more favorable for the complexity of HLA molecules, while at 28 °C, it could only alleviate the degree of simplification of the HLA molecular structure to a certain extent.
- (4)
- At the three tested temperatures, compared with the CK control, either one precursor or a mixture of three precursors could more effectively promote the decomposition of CHLu. Under the conditions of 10 °C and 15 °C, the addition of mixed precursors was more beneficial to the decomposition of CHLu, causing the CHLu content to decrease by 37.9% and 44.7%, respectively, followed by the addition of glucose alone.
Author Contributions
Conceptualization, N.W. and S.W.; methodology, Y.Z.; software, L.S.; validation, N.W., Y.Z. and M.W.; formal analysis, Z.L.; investigation, Y.Z., X.Z., H.G. and Y.L.; resources, L.H.; data curation, N.W.; writing—original draft preparation, N.W.; writing—review and editing, S.W.; visualization, N.W.; supervision, S.W.; project administration, S.W.; funding acquisition, N.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Jilin Province Key R & D Program Project, grant number 20210202118NC, and the Jilin Province 18th Innovative and Entrepreneurial Talent Funding Project (Excellence Category), grant number 2023027.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bhattacharyya, P.; Bhaduri, D.; Adak, T.; Munda, S.; Satapathy, B.S.; Dash, P.K.; Padhy, S.R.; Pattanayak, A.; Routray, S.; Chakraborti, M.; et al. Characterization of rice straw from major cultivars for best alternative industrial uses to cutoff the menace of straw burning. Ind. Crop. Prod. 2020, 143, 111919. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, L. Addition of mature compost improves the composting of green waste. Bioresour. Technol. 2022, 350, 126927. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Gu, J.Y.; Zhang, J.; Chen, B.; Xu, Y.D.; Liu, D.Y.; Hu, H.W.; Huang, H.Y. Reduced pH is the primary factor promoting humic acid formation during hyperthermophilic pretreatment composting. J. Environ. Manag. 2022, 316, 115215. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Wei, Z.; Zhang, J.; Zhao, Y.; Wu, J.; Gao, X.; Liu, Z.; Li, Y. Effect of MnO2 on biotic and abiotic pathways of humic-like substance formation during composting of different raw materials. Waste Manag. 2019, 87, 326–334. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Yue, D.B.; Fang, D.; Dong, X.W.; Li, W.L. Enhanced darkening effect from the interaction of MnO2 and oxygen on the component evolution of amino-phenolic humic-like substances. Chemosphere 2021, 263, 127956. [Google Scholar] [CrossRef]
- Jokic, A.; Wang, M.C.; Liu, C.; Frenkel, A.I.; Huang, P.M. Integration of the polyphenol and Maillard reactions into a unified abiotic pathway for humification in nature: The role of δ-MnO2. Org. Geochem. 2004, 35, 747–762. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Yue, D.B.; Ma, H. Darkening mechanism and kinetics of humification process in catechol Maillard system. Chemosphere 2015, 130, 40–45. [Google Scholar] [CrossRef]
- Tan, K.H. Humic Matter in Soil and the Environment: Principles and Controversies; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Hardie, A.G.; Dynes, J.J.; Kozak, L.M.; Huang, P.M. The role of glucose in abiotic humification pathways as catalyzed by birnessite. J. Mol. Catal A Chem. 2009, 308, 114–126. [Google Scholar] [CrossRef]
- Wu, J.Q.; Zhao, Y.; Qi, H.S.; Zhao, X.Y.; Yang, T.X.; Du, Y.Q.; Zhang, H.; Wei, Z.M. Identifying the key factors that affect the formation of humic substance during different materials composting. Bioresour. Technol. 2017, 244, 1193–1196. [Google Scholar] [CrossRef]
- Lu, M.Y.; Feng, Q.; Li, X.; Xu, B.C.; Shi, X.S.; Guo, R.B. Effects of arginine modified additives on humic acid formation and microbial metabolic functions in biogas residue composting. J. Environ. Chem. Eng. 2022, 10, 108675. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Yue, D.B.; Wang, X.; Song, W.F. Mechanism of oxidation and catalysis of organic matter abiotic humification in the presence of MnO2. J. Environ. Sci. 2019, 77, 167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.C.; Zhao, Y.; Wang, R.X.; Lu, Q.; Wu, J.Q.; Zhang, D.Y.; Nie, Z.F.; Wei, Z.M. Effect of the addition of exogenous precursors on humic substance formation during composting. Waste Manag. 2018, 79, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wu, J.G.; Qu, X.J.; Li, J.M. Effects of organic wastes on structural characterizations of humic acid in semiarid soil under plastic mulched drip irrigation. Chemosphere 2018, 200, 313–321. [Google Scholar] [CrossRef]
- Wu, J.Q.; Zhao, Y.; Zhao, W.; Yang, T.X.; Zhang, X.; Xie, X.Y.; Cui, H.Y.; Wei, Z.M. Effect of precursors combined with bacteria communities on the formation of humic substances during different materials composting. Bioresour. Technol. 2017, 226, 191–199. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, K.; Shan, R.R.; Han, Z.; Shao, Y.Q.; Tian, C. The influence of humification degree of humic acid on its sorption of norfloxacin during sewage sludge composting. Water Air Soil Pollut. 2018, 229, 160. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Jin, Y.; Zou, S.; Li, C. Recovery of sludge humic acids with alkaline pretreatment and its impact on subsequent anaerobic digestion. J. Chem. Technol. Biotechnol. 2014, 89, 707–713. [Google Scholar] [CrossRef]
- Wu, Y.P.; Chen, Y.X.; Shaaban, M.; Zhu, D.W.; Hu, C.X.; Chen, Z.B.; Wang, Y. Evaluation of microbial inoculants pretreatment in straw and manure co-composting process enhancement. J. Clean. Prod. 2019, 239, 118078. [Google Scholar] [CrossRef]
- Song, C.H.; Li, M.X.; Xi, B.D.; Wei, Z.M.; Zhao, Y.; Jia, X.; Qi, H.; Zhu, C.W. Characterisation of dissolved organic matter extracted from the bio-oxidative phase of co-composting of biogas residues and livestock manure using spectroscopic techniques. Int. Biodeter. Biodegr. 2015, 103, 38–50. [Google Scholar] [CrossRef]
- Niemeyer, J.; Chen, Y.; Bollag, J.M. Characterization of humic acids, composts, and peat by diffuse reflectance Fourier-transform infrared spectroscopy. Soil Sci. Soc. Am. J. 1992, 56, 135–140. [Google Scholar] [CrossRef]
- Jung, A.V.; Frochot, C.; Parant, S.; Lartiges, B.S.; Selve, C.; Viriot, M.-L.; Bersillon, J.L. Synthesis of amino-phenolic humic-like substances and comparison with natural aquatic humic acids: A multi-analytical techniques approach. Org. Geochem. 2005, 36, 1252–1271. [Google Scholar] [CrossRef]
- Shen, D.; Liu, G.; Jing, Z.; Xue, J.; Guan, S.; Rui, X. Thermo-chemical conversion of lignin to aromatic compounds: Effect of lignin source and reaction temperature. J. Anal. Appl. Pyrolysis 2015, 112, 56–65. [Google Scholar] [CrossRef]
- Wang, M.C.; Huang, P.M. Cleavage of 14C-labeled glycine and its polycondensation with pyrogallol as catalyzed by birnessite. Geoderma 2005, 124, 415–426. [Google Scholar] [CrossRef]
- Zarkadas, I.S.; Pilidis, G.A. Anaerobic Co-Digestion of table olive debittering & washing Effluent, cattle manure and pig manure in batch and high volume laboratory anaerobic digesters: Effect of temperature. Bioresour. Technol. 2011, 102, 4995–5003. [Google Scholar] [PubMed]
- Wu, J.Q.; Yao, W.K.; Zhao, L.; Zhao, Y.; Qi, H.S.; Zhang, R.J.; Song, C.H.; Wei, Z.M. Estimating the synergistic formation of humus by abiotic and biotic pathways during composting. J. Clean. Prod. 2022, 363, 132470. [Google Scholar] [CrossRef]
- Zech, W.; Senesi, N.; Guggenberger, G.; Kaiser, K.; Lehmann, J.; Miano, T.M.; Miltner, A.; Schroth, G. Factors controlling humification and mineralization of soil organic matter in the tropics. Geoderma 1997, 79, 117–161. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X.; Tian, Y.; Gong, X.Q. Effects of brown sugar and calcium superphosphate on the secondary fermentation of green waste. Bioresour. Technol. 2013, 131, 68–75. [Google Scholar] [CrossRef]
- Jiang, T.; Schuchardt, F.; Li, G.; Guo, R.; Zhao, Y. Effect of C/N ratio, aeration rate and moisture content on ammonia and greenhouse gas emission during the composting. J. Environ. Sci. 2011, 23, 1754–1760. [Google Scholar] [CrossRef]
- Mu, D.C.; Qu, F.T.; Zhu, Z.C.; Wu, D.; Qi, H.S.; Mohamed, T.A.; Liu, Y.M.; Wei, Z.M. Effect of Maillard reaction on the formation of humic acid during thermophilic phase of aerobic fermentation. Bioresour. Technol. 2022, 357, 127362. [Google Scholar] [CrossRef]
- van der Wal, A.; de Boer, W. Dinner in the dark: Illuminating drivers of soil organic matter decomposition. Soil Biol. Biochem. 2017, 105, 45–48. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Yao, S.H.; Cao, X.Y.; Schmidt-Rohr, K.; Olk, D.C.; Mao, J.D.; Zhang, B. Structural evidence for soil organic matter turnover following glucose addition and microbial controls over soil carbon change at different horizons of a Mollisol. Soil Biol. Biochem. 2018, 119, 63–73. [Google Scholar] [CrossRef]
- Xu, Y.H.; Fan, J.L.; Ding, W.X.; Gunina, A.; Chen, Z.M.; Bol, R.; Luo, J.F.; Bolan, N. Characterization of organic carbon in decomposing litter exposed to nitrogen and sulfur additions: Links to microbial community composition and activity. Geoderma 2017, 286, 116–124. [Google Scholar] [CrossRef]
- Guan, S.; An, N.; Zong, N.; He, Y.T.; Shi, P.L.; Zhang, J.J.; He, N.P. Climate warming impacts on soil organic carbon fractions and aggregate stability in a Tibetan alpine meadow. Soil Biol. Biochem. 2018, 116, 224–236. [Google Scholar] [CrossRef]
- Wang, C.; Tu, Q.P.; Dong, D.; Strong, P.J.; Wang, H.L.; Sun, B.; Wu, W.X. Spectroscopic evidence for biochar amendment promoting humic acid synthesis and intensifying humification during composting. J. Hazard. Mater. 2014, 280, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.S.; Zhao, Y.; Lu, Q.; Feng, W.X.; Wang, L.Q.; Wei, Z.M. Evaluating differences in humic substances formation based on the shikimic acid pathway during different materials composting. Bioresour. Technol. 2022, 364, 128060. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.D.; Huang, H.Y.; Sun, E.H.; Butterly, C.; Xu, Y.D.; He, H.; Zhang, J.; Chang, Z.Z. Spectroscopic evidence for hyperthermophilic pretreatment intensifying humification during pig manure and rice straw composting. Bioresour. Technol. 2019, 294, 122131. [Google Scholar] [CrossRef]
- Watteau, F.; Villemin, G. Characterization of organic matter microstructure dynamics during co-composting of sewage sludge, barks and green waste. Bioresour. Technol. 2011, 102, 9313–9317. [Google Scholar] [CrossRef]
- Sánchez-Monedero, M.A.; Roig, A.; Cegarra, J.; Bernal, M.P. Relationships between water-soluble carbohydrate and phenol fractions and the humification indices of different organic wastes during composting. Bioresour. Technol. 1999, 70, 193–201. [Google Scholar] [CrossRef]
- Cáceres, R.; Flotats, X.; Marfà, O. Changes in the chemical and physicochemical properties of the solid fraction of cattle slurry during composting using different aeration strategies. Waste Manag. 2006, 26, 1081–1091. [Google Scholar] [CrossRef]
- Wei, L.; Razavi, B.S.; Wang, W.Q.; Zhu, Z.K.; Liu, S.L.; Wu, J.S.; Kuzyakov, Y.; Ge, T.D. Labile carbon matters more than temperature for enzyme activity in paddy soil. Soil Biol. Biochem. 2019, 135, 134–143. [Google Scholar] [CrossRef]
- Creamer, C.A.; de Menezes, A.B.; Krull, E.S.; Sanderman, J.; Newton-Walters, R.; Farrell, M. Microbial community structure mediates response of soil C decomposition to litter addition and warming. Soil Biol. Biochem. 2015, 80, 175–188. [Google Scholar] [CrossRef]
- Valmaseda, M.; Martínez, M.J.; Martínez, A.T. Kinetics of wheat straw solid-state fermentation with Trametes versicolor and Pleurotus ostreatus-lignin and polysaccharide alteration and production of related enzymatic activities. Appl. Microbiol. Biotechnol. 1991, 35, 817–823. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhuge, C.; Weng, Q.; Hu, B. Additional strains acting as key microbes promoted composting process. Chemosphere 2022, 287, 132304. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhao, Y.; Wang, X.; Wei, Z.; Zhang, X.; Wu, J.; Xie, X.; Kang, K.; Yang, H.; Shi, M.; et al. Manganese dioxide driven the carbon and nitrogen transformation by activating the complementary effects of core bacteria in composting. Bioresour. Technol. 2021, 330, 124960. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhao, Y.; Zhao, X.; Yang, T.; Dang, Q.; Wu, J.; Wei, Z. Effect of manganese dioxide on the formation of humin during different agricultural organic wastes compostable environments: It is meaningful carbon sequestration. Bioresour Technol. 2020, 299, 122596. [Google Scholar] [CrossRef]
- Wang, X.G.; Tian, L.; Li, Y.X.; Zhong, C.; Tian, C.J. Effects of exogenous cellulose-degrading bacteria on humus formation and bacterial community stability during composting. Bioresour. Technol. 2022, 359, 127458. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).