Infection Biology of Bipolaris oryzae in Rice and Defence Responses in Compatible and Less Compatible Interactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants
2.2. Inoculum Production and Inoculation
2.3. Quantitative Study of the Initial Stages of Infection
2.4. Qualitative Examination of Other Host Responses and Later Development of B. oryzae
2.5. Statistical Analyses
3. Results
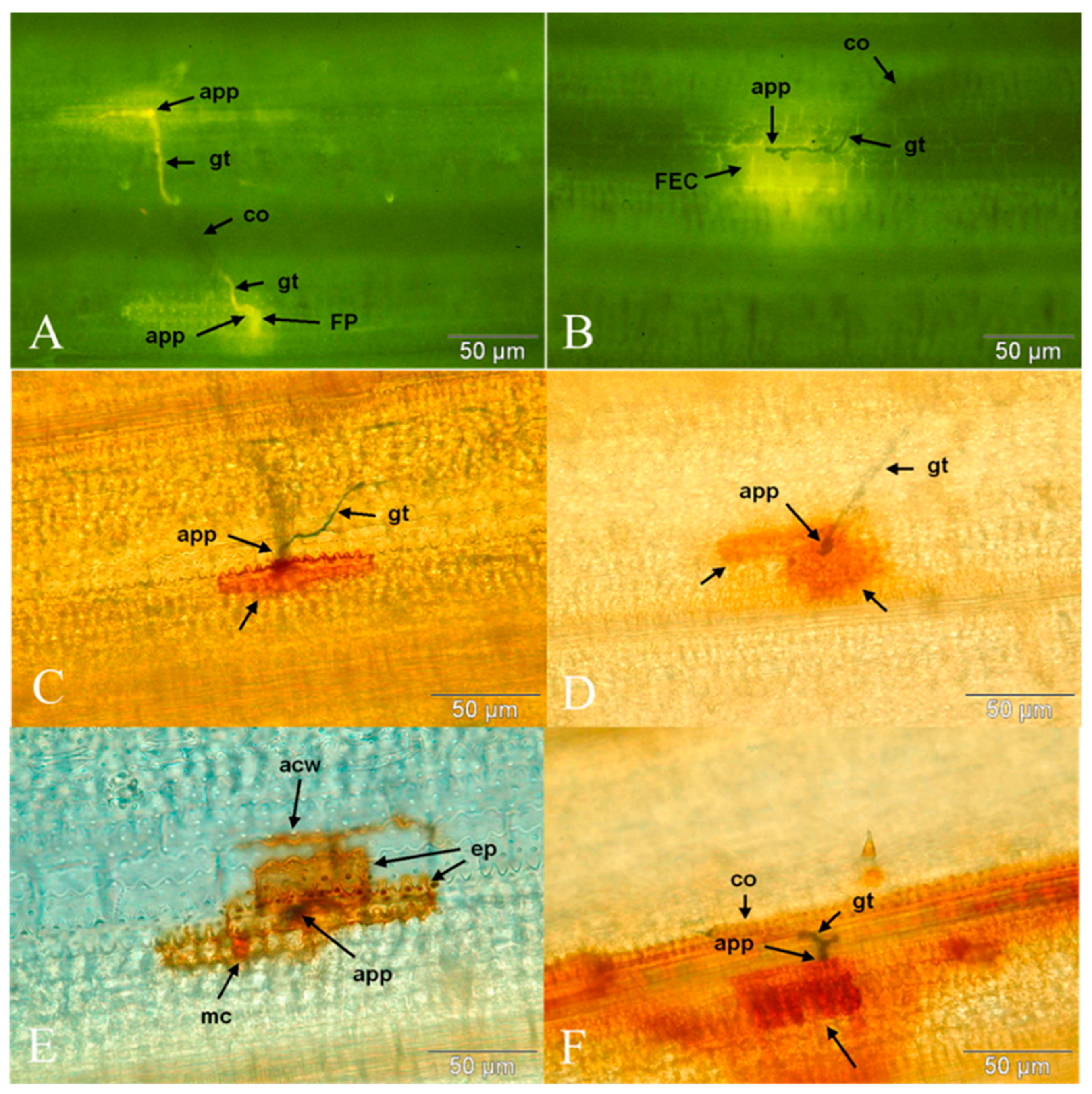
3.1. Quantitative Studies of the Initial Stages of Infection
| Time after Inoculation of MTL189 with Two Isolates of B. oryzae | ||||||
|---|---|---|---|---|---|---|
| Infection Process | 12 h | 24 h | ||||
| Process | Isolate K2 Less Compatible | Isolate B5 Compatible | Odds Ratio b | Isolate K2 Less Compatible | Isolate B5 Compatible | Odds Ratio b |
| Conidia germinated (%) | 95.7 | 97.6 | 1.81 NS | 93.9 | 96.6 | 1.86 NS |
| Conidia with branched germ tubes (%) | 29.5 | 69.0 | 5.58 *** | 33.0 | 69.5 | 4.64 *** |
| Conidia forming appressoria (%) | 82.5 | 15.5 | 0.04 *** | 76.0 | 45.5 | 0.24 *** |
| Conidia causing penetration (%) | 1.5 | 5.5 | 3.83 *** | 5.5 | 18.5 | 3.91 *** |
| Appressoria causing penetration (%) | 0.6 | 3.1 | 18.46 *** | 9.7 | 23.0 | 10.05 *** |
| Conidia causing FP c (%) | 2.0 | 0.0 | NS d | 1.5 | 0.0 | NS d |
| Conidia causing single FEC (%) | 8.0 | 0.0 | 0.00 *** | 1.0 | 0.5 | 0.49 NS |
| Conidia causing multiple FEC e (%) | 25.5 | 0.0 | 0.00 *** | 65.0 | 22.0 | 0.15 *** |
| Conidia causing FCW f (%) | 33.0 | 1.5 | 0.03 *** | 29.5 | 11.5 | 0.30 ** |
| Mean length of germ tubes per conidium (μm) | 211.9 | 398.8 | - g | 181.6 | 588.7 | - g |
| Mean diameter of appressoria (μm) | 12.1 | 7.5 | - h | 12.7 | 8.9 | - h |
| Mean number of appressoria per conidium | 1.7 | 1.4 | - i | 1.6 | 1.3 | - i |
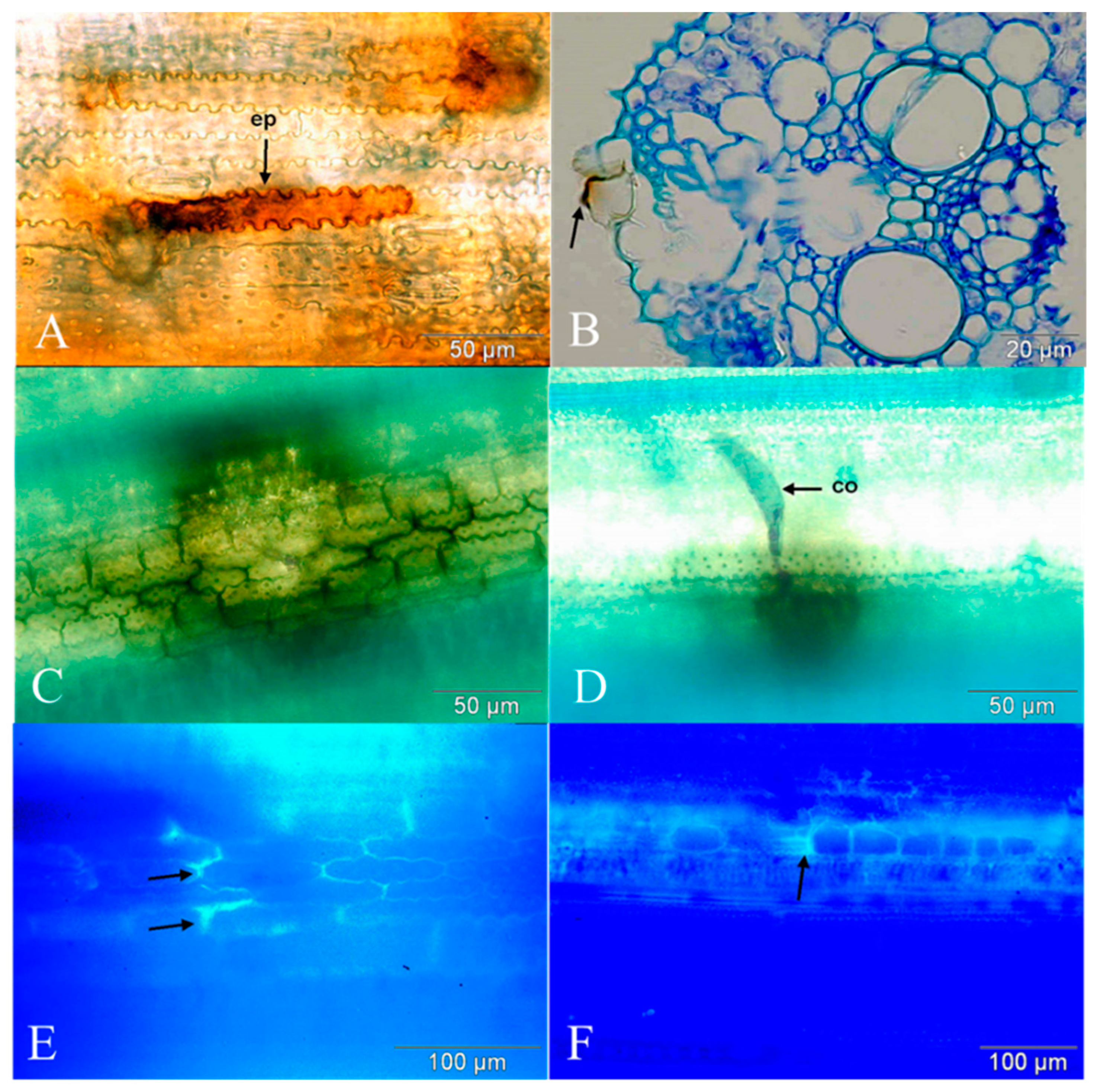
3.2. Qualitative Examination of the Later Development of Bipolaris oryzae and Host Responses
3.2.1. Hyphal Growth
3.2.2. Localisation of H2O2
3.2.3. Polyphenol Accumulation
3.2.4. Callose Accumulation
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barnwal, M.K.; Kotasthane, A.; Magculia, N.; Mukherjee, P.K.; Savary, S.; Sharma, A.K.; Singh, H.B.; Singh, U.S.; Sparks, A.H.; Variar, M.; et al. A review on crop losses, epidemiology and disease management of rice brown spot to identify research priorities and knowledge gaps. Eur. J. Plant Pathol. 2013, 136, 443–457. [Google Scholar] [CrossRef]
- Mizobuchi, R.; Fukuoka, S.; Tsushima, S.; Yano, M.; Sato, H. QTLs for resistance to major rice diseases exacerbated by global warming: Brown spot, bacterial seedling rot, and bacterial grain rot. Rice 2016, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Padmanabhan, S.Y. The great Bengal famine. Annu. Rev. Phytopathol. 1973, 11, 11–26. [Google Scholar] [CrossRef]
- Hoang, V.T. Determination of Causal Agents of Rice Diseases in the Mekong Delta. Bachelor’s Thesis, Department of Plant Protection, Can Tho University, Can Tho, Vietnam, 1979. (In Vietnamese). [Google Scholar]
- Thuy, T.T.T. Determination of Causal Agents of Rice, Sugarcane, Tobacco and Banana Diseases in the Mekong Delta. Bachelor’s Thesis, Department of Plant Protection, Can Tho University, Can Tho, Vietnam, 1980. (In Vietnamese). [Google Scholar]
- Thanh, N. Brown Spot Disease Infected More than 2,000 Hectares of Spring Rice in Ha Tinh. 2018. Available online: https://nongnghiep.vn/benh-dom-nau-tiem-lua-an-hon-2000-ha-lua-xuan-o-ha-tinh-d216544.html (accessed on 26 November 2022). (In Vietnamese).
- Moriwaki, A.; Kubo, E.; Arase, S.; Kihara, J. Disruption of SRM1, a mitogen-activated protein kinase gene, affects sensitivity to osmotic and ultraviolet stressors in the phytopathogenic fungus Bipolaris oryzae. FEMS Microbiol. Lett. 2006, 257, 253–261. [Google Scholar] [CrossRef]
- Dariush, S.; Darvishnia, M.; Ebadi, A.-A.; Padasht-Dehkaei, F.; Bazgir, E. Population structure, genetic diversity, and trait association analysis in rice (Oryza sativa L.) genotypes for brown spot disease resistance. Trop. Plant Pathol. 2021, 46, 265–281. [Google Scholar] [CrossRef]
- Sato, H.; Ando, I.; Hirabayashi, H.; Takeuchi, Y.; Arase, S.; Kihara, J.; Kato, H.; Imbe, T.; Nemoto, H. QTL analysis of brown spot resistance in rice (Oryza sativa L.). Breed. Sci. 2008, 58, 93–96. [Google Scholar] [CrossRef]
- Nisikado, Y.; Miyake, C. Studies on the Helminthosporiose of the rice-plant. Ber. Ōhara Inst. Landwirtsch. Forsch. 1922, 2, 133–195+plate III–plate IX. [Google Scholar]
- Horino, O.; Akai, S. Studies in the pathological anatomy of rice plants infected by Helminthosporium oryzae I. Behavior of the causal fungus on the coleoptile of rice seedlings and its ultrafine structure. Ann. Phytopathol. Soc. Jpn. 1968, 34, 51–55. [Google Scholar] [CrossRef]
- Hau, F.C.; Rush, M.C. Preinfectional interaction between Helminthosporium oryzae and resistant and susceptible rice plants. Phytopathology 1982, 72, 285–292. [Google Scholar] [CrossRef]
- Vidhyasekaran, P.; Borromeo, E.S.; Mew, T.W. Helminthosporium oryzae toxin suppresses phenol metabolism in rice plants and aids pathogen colonization. Physiol. Mol. Plant Pathol. 1992, 41, 307–315. [Google Scholar] [CrossRef]
- Van Bockhaven, J.; Spíchal, L.; Novák, O.; Strnad, M.; Asano, T.; Kikuchi, S.; Höfte, M.; De Vleesschauwer, D. Silicon induces resistance to the brown spot fungus Cochliobolus miyabeanus by preventing the pathogen from hijacking the rice ethylene pathway. New Phytol. 2015, 206, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Ongena, M.; Höfte, M. The cyclic lipopeptide orfamide induces systemic resistance in rice to Cochliobolus miyabeanus but not to Magnaporthe oryzae. Plant Cell Rep. 2017, 36, 1731–1746. [Google Scholar] [CrossRef]
- De Vleesschauwer, D.; Yang, Y.; Cruz, C.V.; Höfte, M. Abscisic acid-induced resistance against the brown spot pathogen Cochliobolus miyabeanus in rice involves MAP kinase-mediated repression of ethylene signaling. Plant Physiol. 2010, 152, 2036–2052. [Google Scholar] [CrossRef] [PubMed]
- Dorneles, K.R.; Refatti, J.P.; Pazdiora, P.C.; de Avila, L.A.; Deuner, S.; Dallagnol, L.J. Biochemical defenses of rice against Bipolaris oryzae increase with high atmospheric concentration of CO2. Physiol. Mol. Plant Pathol. 2020, 110, 101484. [Google Scholar] [CrossRef]
- Kariya, K.; Murata, K.; Kokubo, Y.; Ube, N.; Ueno, K.; Yabuta, Y.; Teraishi, M.; Okumoto, Y.; Mori, N.; Ishihara, A. Variation of diterpenoid phytoalexin oryzalexin A production in cultivated and wild rice. Phytochemistry 2019, 166, 112057. [Google Scholar] [CrossRef]
- Ahn, I.-P. Glufosinate ammonium-induced pathogen inhibition and defense responses culminate in disease protection in bar-transgenic rice. Plant Physiol. 2008, 146, 213–227. [Google Scholar] [CrossRef]
- Debona, D.; Rodrigues, F.A. A strobilurin fungicide relieves Bipolaris oryzae-induced oxidative stress in rice. J. Phytopathol. 2016, 164, 571–581. [Google Scholar] [CrossRef]
- Marwein, R.; Singh, S.; Maharana, J.; Kumar, S.; Arunkumar, K.P.; Velmurugan, N.; Chikkaputtaiah, C. Transcriptome-wide analysis of North-East Indian rice cultivars in response to Bipolaris oryzae infection revealed the importance of early response to the pathogen in suppressing the disease progression. Gene 2022, 809, 146049. [Google Scholar] [CrossRef]
- Jensen, B.; Lübeck, P.S.; Jørgensen, H.J.L. Clonostachys rosea reduces spot blotch in barley by inhibiting prepenetration growth and sporulation of Bipolaris sorokiniana without inducing resistance. Pest Manag. Sci. 2016, 72, 2231–2239. [Google Scholar] [CrossRef]
- Jørgensen, H.J.L.; Lübeck, P.S.; Thordal-Christensen, H.; de Neergaard, E.; Smedegaard-Petersen, V. Mechanisms of induced resistance in barley against Drechslera teres. Phytopathology 1998, 88, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Thuy, T.T.T. Infection Biology of Bipolaris oryzae in Rice and Its Pathogenic Variation in the Mekong Delta, Vietnam. Ph.D. Thesis, Plant Pathology Section, Department of Plant Biology, The Royal Veterinary and Agricultural University, Copenhagen, Denmark, 2002. [Google Scholar]
- Shetty, N.P.; Kristensen, B.K.; Newman, M.-A.; Møller, K.; Gregersen, P.L.; Jørgensen, H.J.L. Association of hydrogen peroxide with restriction of Septoria tritici in resistant wheat. Physiol. Mol. Plant Pathol. 2003, 62, 333–346. [Google Scholar] [CrossRef]
- Graham, E.T.; Joshi, P.A. Novel fixation of plant tissue, staining through paraffin with Alcian blue and Hematoxylin, and improved slide preparation. Biotech. Histochem. 1995, 70, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Shetty, N.P.; Jensen, J.D.; Knudsen, A.; Finnie, C.; Geshi, N.; Blennow, A.; Collinge, D.B.; Jørgensen, H.J.L. Effects of β-1,3-glucan from Septoria tritici on structural defence responses in wheat. J. Exp. Bot. 2009, 60, 4287–4300. [Google Scholar] [CrossRef] [PubMed]
- Collett, D. Modelling Binary Data; Chapman & Hall: London, UK, 2002. [Google Scholar]
- Cox, D.R.; Snell, E.J. Analysis of Binary Data. In Monographs on Statistics and Applied Probability, 2nd ed.; Chapman & Hall: London, UK, 1989; Volume 32. [Google Scholar]
- Ahn, I.-P.; Kim, S.; Kang, S.; Suh, S.-C.; Lee, Y.-H. Rice defense mechanisms against Cochliobolus miyabeanus and Magnaporthe grisea are distinct. Phytopathology 2005, 95, 1248–1255. [Google Scholar] [CrossRef]
- Bird, P.M.; Ride, J.P. The resistance of wheat to Septoria nodorum: Fungal development in relation to host lignification. Physiol. Plant Pathol. 1981, 19, 289–299. [Google Scholar] [CrossRef]
- Mumford, D.L. Factors associated with resistance in barley to spot blotch. Phytopathology 1966, 56, 79–82. [Google Scholar]
- Misra, A.P.; Chatterjee, A.K. Comparative study of two isolates of Helminthosporium oryzae Breda de Hann. Indian Phytopathol. 1963, 16, 275–281. [Google Scholar]
- Cholil, A.; de Hoog, G.S. Variability in Drechslera oryzae. Trans. Br. Mycol. Soc. 1982, 79, 491–496. [Google Scholar] [CrossRef]
- Vance, C.P.; Sherwood, R.T. Lignified papilla formation as a mechanism for protection in reed canarygrass. Physiol. Plant. Pathol. 1977, 10, 247–256. [Google Scholar] [CrossRef]
- Sherwood, R.T.; Vance, C.P. Resistance to fungal penetration in Gramineae. Phytopathology 1980, 70, 723–729. [Google Scholar] [CrossRef]
- Peng, Y.L.; Shishiyama, J. Timing of a cellular reaction in rice cultivars associated with different degrees of resistance to Pyricularia oryzae. Can. J. Bot. 1989, 67, 2704–2710. [Google Scholar] [CrossRef]
- Heath, M.C.; Valent, B.; Howard, R.J.; Chumley, F.G. Interactions of two strains of Magnaporthe grisea with rice goosegrass, and weeping lovegrass. Can. J. Bot. 1990, 68, 1627–1637. [Google Scholar] [CrossRef]
- Kumar, J.; Hückelhoven, R.; Beckhove, U.; Nagarajan, S.; Kogel, K.-H. A compromised Mlo pathway affects the response of barley to the necrotrophic fungus Bipolaris sorokiniana (teleomorph: Cochliobolus sativus) and its toxins. Phytopathology 2001, 91, 127–133. [Google Scholar] [CrossRef]
- Lindberg, G.D. Disease-induced toxin production in Helminthosporium oryzae. Phytopathology 1971, 61, 420–424. [Google Scholar] [CrossRef]
- Chattopadhyay, A.K.; Samaddar, K.R. Effects of Helminthosporium oryzae infection and ophiobolin on the cell membranes of host tissues. Physiol. Plant Pathol. 1976, 8, 131–139. [Google Scholar] [CrossRef]
- Xiao, J.Z.; Tsuda, M.; Doke, N.; Nishimura, S. Phytotoxins produced by germinating conidia of Bipolaris oryzae. Phytopathology 1991, 81, 58–64. [Google Scholar] [CrossRef]
- Voigt, C.A. Callose-mediated resistance to pathogenic intruders in plant defense-related papillae. Front. Plant Sci. 2014, 5, 168. [Google Scholar] [CrossRef]
- Shetty, R.; Jensen, B.; Shelton, D.; Jørgensen, K.; Pedas, P.; Jørgensen, H.J.L. Site-specific, silicon-induced structural and molecular defence responses against powdery mildew infection in roses. Pest Manag. Sci. 2021, 77, 4545–4554. [Google Scholar] [CrossRef]
- Thordal-Christensen, H.; Zhang, Z.; Wei, Y.; Collinge, D.B. Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Shetty, N.P.; Mehrabi, R.; Lütken, H.; Haldrup, A.; Kema, G.H.J.; Collinge, D.B.; Jørgensen, H.J.L. Role of hydrogen peroxide during the interaction between the hemibiotrophic fungal pathogen Septoria tritici and wheat. New Phytol. 2007, 174, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Poudel, A.; Navathe, S.; Chand, R.; Mishra, V.K.; Singh, P.K.; Joshi, A.K. Hydrogen peroxide prompted lignification affects pathogenicity of hemi-bio-trophic pathogen Bipolaris sorokiniana to wheat. Plant Pathol. J. 2019, 35, 287–300. [Google Scholar] [CrossRef]
- Shetty, N.P.; Jørgensen, H.J.L.; Jensen, J.D.; Collinge, D.B.; Shetty, H.S. Roles of reactive oxygen species in interactions between plants and pathogens. Eur. J. Plant Pathol. 2008, 121, 267–280. [Google Scholar] [CrossRef]
- Huysmans, M.; Lema, A.S.; Coll, N.S.; Nowack, M.K. Dying two deaths—Programmed cell death regulation in development and disease. Curr. Opin. Plant Biol. 2017, 35, 37–44. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thuy, T.T.T.; Lübeck, M.; Smedegaard-Petersen, V.; de Neergaard, E.; Jørgensen, H.J.L. Infection Biology of Bipolaris oryzae in Rice and Defence Responses in Compatible and Less Compatible Interactions. Agronomy 2023, 13, 231. https://doi.org/10.3390/agronomy13010231
Thuy TTT, Lübeck M, Smedegaard-Petersen V, de Neergaard E, Jørgensen HJL. Infection Biology of Bipolaris oryzae in Rice and Defence Responses in Compatible and Less Compatible Interactions. Agronomy. 2023; 13(1):231. https://doi.org/10.3390/agronomy13010231
Chicago/Turabian StyleThuy, Tran Thi Thu, Mette Lübeck, Viggo Smedegaard-Petersen, Eigil de Neergaard, and Hans J. L. Jørgensen. 2023. "Infection Biology of Bipolaris oryzae in Rice and Defence Responses in Compatible and Less Compatible Interactions" Agronomy 13, no. 1: 231. https://doi.org/10.3390/agronomy13010231
APA StyleThuy, T. T. T., Lübeck, M., Smedegaard-Petersen, V., de Neergaard, E., & Jørgensen, H. J. L. (2023). Infection Biology of Bipolaris oryzae in Rice and Defence Responses in Compatible and Less Compatible Interactions. Agronomy, 13(1), 231. https://doi.org/10.3390/agronomy13010231






