Abstract
Early maturity is a highly important factor in the interrelations between yield, grain moisture, and plant density, contributing to cost-efficient maize production. Landraces conserved in gene banks present a promising basis for enriching the diversity of early maize breeding material. To start and speed up the mobilization of the maize genetic resources maintained in the ex situ Maize Research Institute Zemun Polje gene bank collection, which are currently scattered, little studied, and underused, 63 landraces were selected as new potential sources of early maturity; their test-cross performance with two divergent early testers was evaluated. The majority of the landraces with a prevailed flint type (29) exhibited heterosis for yield when crossed with the Iowa Stiff Stalk Synthetic—Iowa Dent tester (102NS), out of which 20 top crosses expressed grain moisture below the defined threshold value (21.1%). The best performing landraces can be used as a starting point for a new pre-breeding programme for the broadening of flint maize breeding material. In parallel, nine landraces expressed simultaneous heterosis when crossed with the flint tester (14NS), exhibiting grain moisture above the threshold value. A simultaneous heterotic effect with two divergent inbred testers implies the existence of an independent heterotic pool. These findings will contribute to the broadening of maize breeding material for early maturity and low grain moisture at harvest, which are important goals in maize breeding.
1. Introduction
Maize (Zea mays L.) is one of the most important crops in the world and in the Republic of Serbia, where it is cultivated on about one million hectares annually. Its yield and stability have a significant impact on agriculture in general and on the gross domestic product [,]. Breeders have almost unlimited access to germplasm diversity. However, competition between seed companies for high-yield varieties imposes a broad use of a small number of the top-ranked hybrids, which leads to increased genetic vulnerability [].
An assessment of the genetic diversity that exists in the available germplasm is fundamental for the improvement of cultivated species to ensure continuous and long-term progress in breeding, thus meeting the new market requirements and increasingly frequent challenges in crop growth conditions [,]. The direct incorporation of genetic resources in breeding programmes is inefficient, due to difficulties in gathering all the desirable characteristics in a small number of generations while maintaining all the positive characteristics of the elite material.
Lowering grain moisture at harvest becomes an increasingly important aim, especially in temperate maize breeding []. The final grain moisture at harvest is mostly determined by the grain moisture at maturity and the dry-down rate in the field. The former is primarily controlled by genetic factors [,], while the grain dry-down rate is mainly affected by the temperature and relative humidity of the environment [].
Nowadays, the increase in plant density is a key feature of maize yield improvements [,,]. In addition to crop architecture that enables efficient use of crops’ surrounding areas [], the maize maturity group is one of the most important factors that influences the yield–density relationship []. Although early-maturing genotypes are less productive than late-maturing ones, they have the advantages of better tolerance to higher plant densities, the ability to survive drought, and lower grain moisture at harvest.
Knowledge of the heterotic pattern of parental lines is crucial in hybrid maize breeding programmes. Given that lines developed from local landraces most often retain the heterotic pattern of their ancestral populations [], it is very important to determine the combining ability of local landraces before they are incorporated into commercial breeding programmes. In addition, information regarding the traits of the landraces per se is also necessary [], as only initial breeding material with high frequencies of desirable alleles, i.e., desirable traits, can produce progenies with the desired characteristics.
The size of a collection, in terms of the large number of accessions maintained in the gene banks, hinders the efficient selection of desirable genotypes for the improvement of crops through breeding []. In order to start and speed up the mobilization of the currently scattered, little studied, and underused maize genetic resources that are maintained in the ex situ Maize Research Institute Zemun Polje (MRIZP) gene bank collection (https://mrizp.rs/emdb/default.htm, accessed on 15 May 2021), data generated during the long-term pre-breeding programme [,,,] were used. Accordingly, 63 early landraces were selected with the following aims: (i) to evaluate their heterotic potential by crossing with two divergent early maturing tester lines, and (ii) to identify the best performing landraces as new sources of early maturity and low grain moisture at harvest.
2. Materials and Methods
2.1. Plant Material
According to pre-breeding activities, a gene pool of 321 MRIZP gene bank local maize landraces was previously designated as a possible source of drought tolerance [], and then evaluated for the traits important for breeding []. Out of these 321 landraces, 63 early local landraces were chosen for crossing with two divergent early testers, 102NS (Stiff Stalk Synthetic-Iowa—Dent BSSS-ID; dent type) and 14NS, representing the early flint heterotic group (of the F2 inbred line type). The landraces were collected in the hilly and mountainous area of the former Yugoslavia (Figure 1).
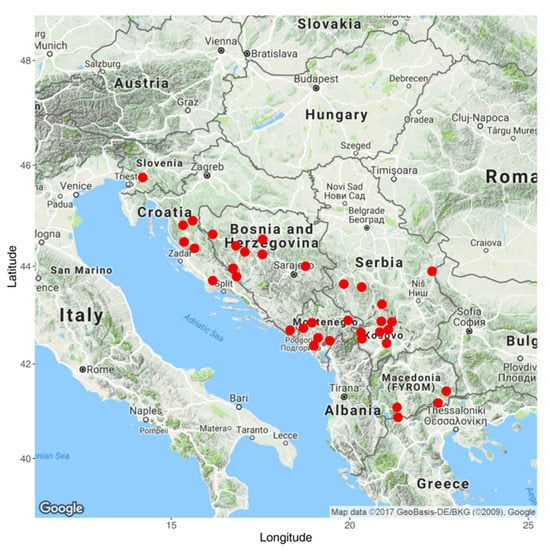
Figure 1.
Map of collection sites of the evaluated maize landraces.
In 2017, the crosses with testers were made under spatial isolation at the Institute of Field and Vegetable Crops, Novi Sad, Serbia. A crossing with the tester was considered successful when ten ears with full seed sets per landrace were obtained. Nine landraces were excluded due to poor performance, such as those that contained a higher number of albino plants, broken and lodged plants, large tassel size, and a higher number of tillers. Seventeen landraces exhibited poor test-crossing performance, producing small ears and seed amounts. Among the landraces that were successfully crossed with both testers were prevailed flints (24) and semi-flints (7); meanwhile, there were far fewer dents (3) and semi-dents (3) with shorter vegetation periods (Table 1). An equal seed amount from each ear was used for further yield trials.

Table 1.
Passport and morphological data of the evaluated maize landraces and testers used. Abbreviations: GD: genotype designation; AN: accession number; CC: country of collection; DT: days to tasseling; DS: days to silking; PH: plant height; EH: ear height; KRN: number of kernel rows; KNR: number of kernels per row; EL: ear length; KH: kernel hardiness; KD: kernel dentiness; KW: 1000 kernel weight; GY: grain yield (t ha−1); CRO: Croatia; MNE: Montenegro; SRB: Serbia; BIH: Bosnia and Herzegovina; MAC: Republic of Northern Macedonia; SLO: Slovenia.
2.2. Field Experiment and Statistical Analysis
To test the top-cross hybrids’ performance, field trials were conducted at three locations: Zemun Polje (44°51′ N, 20°18′ E, 73 m a.s.l.), Rimski Šančevi (44°88′ N, 20°77′ E, 78 m a.s.l.), and Sremska Mitrovica (45°02′ N, 19°64′ E, 88 m a.s.l.). According to the European Environmental Stratification [], the experimental sites are assigned to the Pannonian 3 zone within the temperate continental climate. The experimental trials were laid out according to the partially balanced incomplete block design []. Each genotype was sown in two 5 m long rows, with intra- and inter-row spacing of 0.20 m and 0.75 m, respectively (i.e., plant density was 66,700 plants ha−1). Standard cropping practices were applied.
In addition to the 74 top-cross hybrids, two commercial F1 hybrids were included in the field trials as checks (St1—FAO 100 and St2—FAO 200).
For each replication, the following traits were measured on ten randomly chosen plants per genotype: plant height (PH) (from ground level to the tip of the tassel), ear height (EH), the number of kernel rows (KRN), the number of kernels per row (KNR), and 1000 kernel weight (KW). The obtained values were then averaged. Grain yield (GY) per plot and grain moisture (GM) were measured at harvest. For the comparability of the test crosses, GY and KW were calculated at 14% water content.
The linear model for the partially balanced incomplete block design was fitted for grain yield as the response variable. The significance of the model terms was assessed using the Wald test. Differentials in environment experimental precision were accommodated for in the model by estimating the separate residual error variances and compared by the Akaike Information Criterion (AIC) with the model, assuming the homogeneous error variance model. A violin plot was used to visualize the data distribution of the measured traits for progenies obtained from crossings of the landraces with each tester separately. In addition, Principal Component Analysis (PCA) was performed to better understand the relationships among the examined traits of the top-cross hybrids. The expression of landraces’ specific combining ability for grain yield was defined by the threshold value of 75% of an average grain yield obtained by the check hybrids (St1 and St2) []. The average percentage of grain moisture at harvest of both checks was taken as the grain moisture threshold value. All analyses were conducted using R software [] and the mixed model Echidna software [].
3. Results
The diversity of the 102NS and 14NS testers, representing the BSSS-ID (dent type) and F2 (flint type) heterotic groups, respectively, clearly differentiated top-cross hybrids regarding their evaluated performance. The coefficient of variation of the evaluated traits ranged from 4.5% for plant height to 17.9% for grain yield. The progenies from the crosses of the local landraces and the 102NS tester had greater average values of grain yield, number of rows per ear, kernels per row, plant and ear height, and ear length, compared to the progenies of the 14NS tester, while the values of 1000 kernel weight and grain moisture were lower. The PCA showed positive correlations between GY, PH, and EH, which contributed most to the first principal component. The KRN, KW, and GM formed the second PC axis, with KRN being negatively correlated with KW and GM. The third PC axis was defined by positively correlated EL and KNR (Table 2).

Table 2.
Rotated component matrix (a) of the tested traits in top-cross hybrids. Abbreviations: KRN: number of kernel rows; KNR: number of kernels per rows; EL: ear length; KW: 1000 kernel weight; GY: grain yield (t ha−1); PH: plant height; EH: ear height; GM: grain moisture. Extraction method: principal component analysis. Rotation method: Varimax with Kaiser Normalization. Rotation converged in eight iterations.
With few exceptions, the PCAs clearly divided the progenies into two groups (Figure 2A,B). The distinctive differentiation of the progenies of different testers can be partly attributed to the general combining ability (GCA) of the testers, but also to their specific combining ability (SCA), and the fact that the chosen landraces were predominately early flints, which often showed heterosis in crosses with the dent 102NS tester.
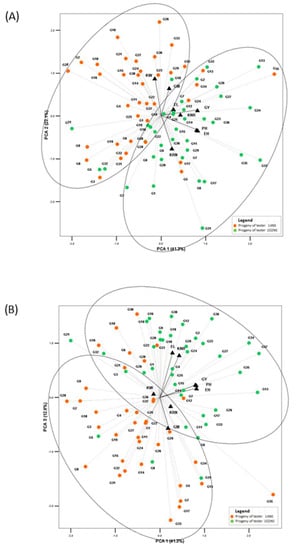
Figure 2.
(A) PC1 vs. PC2 biplot of maize landrace top-crosses for eight quantitative traits; (B) PC1 vs. PC3 biplot of maize landrace top-crosses for eight quantitative traits.
To provide additional information on the evaluated quantitative traits, violin plots were used to visualize the data distribution for each tester’s progeny (Figure 3). The violin plots integrate the box-plot and the probability density function, simultaneously showing measures of central tendency and frequency distributions, i.e., the dispersion of data for the same trait in the progeny of each tester. For EH and KW, the progeny of each tester differed in median, maximum, and minimum values, while their frequency distributions were similar. Regarding GY, the trait of the greatest interest for the present research, half of the progenies of the landraces crossed with the 14NS tester achieved a median value of up to 6.0 t ha−1, while the median for the progenies obtained with the 102NS tester was 7.7 t ha−1. A wider probability density function indicates that the value occurs more frequently. The highest yield frequency for the progenies of the 14NS tester was around 5.8 t ha−1, while for the progenies of 102NS it was about 8.0 t ha−1, with a far more pronounced peak. Half of the progenies of the 14NS tester had about 22% grain moisture at harvest, with the highest frequency around that value. The progenies of the 102NS tester also had the highest frequency around the median, but at a lower level (20.7%). In crosses with the 102NS tester, the largest number of examined landraces produced progenies with desirable traits, i.e., higher yield and lower grain moisture at harvest.
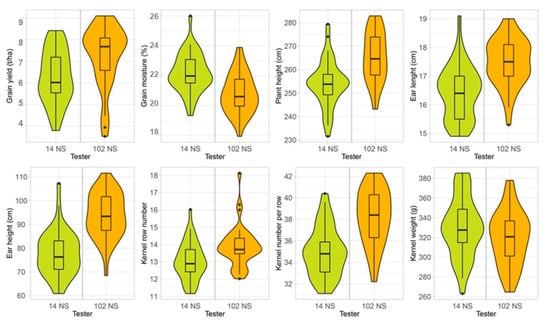
Figure 3.
Violin plots of the evaluated quantitative traits.
Analysis of variance shows that the effects of landrace, tester, and landrace by tester interaction were highly significant (p ≤ 0.001) (Table S1). The highest average grain yield was obtained in crossings with landrace 35 (8.91 t ha−1). The grain yields of crosses with landraces 13, 26, and 34 do not differ significantly according to the LSD test (Table S2). It can be concluded that these landraces showed the best GCA for grain yield. When comparing a large number of means, the LSD test often does not give sufficiently reliable results []. Therefore, a comparison of the differences in the effect of landrace on grain yield (presented in a heat map; Figure S1) with the results of the LSD test (Table S2) was conducted. It was clearly observed that the landrace 35 achieved the highest yield in crossings with both testers, significantly differing from the other landraces. This finding points to the best GCA for grain yield (Figure S1). Moreover, the landraces 13, 26, and 34 (next in rank that stand out in the quadrants of similar colours), could be considered good general combiners for yield. The correlation between landrace grain yield per se (Table 1) and the corresponding effect of landrace on the average grain yield in crossings with testers (Table S2) was significant (r = 0.605, p ≤ 0.01).
The grain yield vs. grain moisture biplot shows the variations across maize landrace top-crosses (Figure 4). The threshold value for grain yield heterosis of the progenies was defined as 75% of the mean grain yield of both St1 and St2 checks (6.77 t ha−1). A large number (27) of progenies from the crosses of landraces with the 102NS tester (BSSS-ID) achieved a GY above the threshold; of these top-crosses, a smaller number, namely G6, G33, G15, G2, G27, G11, G13, G35, and G26, achieved a GY higher than the St1 check, while none of the top-cross hybrids outperformed the better St2 check.
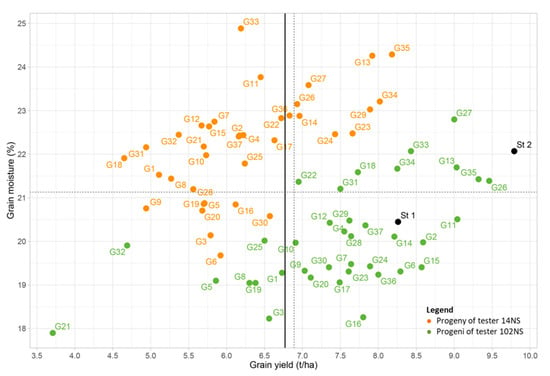
Figure 4.
Scatterplot of grain yield vs. grain moisture for evaluated top-crosses.
The most promising landraces that should be introduced into the flint heterotic group are those that had a greater GY and lower moisture content in crosses with the dent tester 102NS; this was observed in top-crosses from the lower right quadrant (G9, G20, G30, G17, G23, G7, G16, G24, G36, G6, G15, G10, G12, G4, G29, G28, G37, G14, G2, and G11). In this context, the landraces 9, 20, 6, 16, and 30 are particularly important, since they did not express heterosis in crossings with the 14NS tester. Moreover, emphasis should be put on the early flint landraces 6, 15, 2, and 11; with the 102NS tester, these top-cross hybrids achieved a GY above St1 and grain moisture below St1. Top-cross hybrids from crossings between the landraces 22, 31, 18, 34, 33, 27, 13, 35, and 26 and the 102NS tester exhibited heterosis for GY, although they had higher grain moisture at harvest compared to the average of both checks.
The progenies of the 14NS tester have a lower GY and higher grain moisture at harvest (Figure 3 and Figure 4). Top-cross hybrids G26, G14, G27, G24, G23, G29, G13, G34, and G35, obtained from the landraces crossed with the 14NS tester, expressed yield heterosis, although they had grain moisture above the average value for both checks and the better-performing check St2 per se (Figure 4). Dent germplasm (dentiness > 2.5) was present in the majority of the landraces, except in the landraces 27 and 29 of the flint germplasm (Table 1). All six dent and semi-dent landraces with kernel dentiness > 3 showed heterosis with the 14NS tester, as did the F2-type inbred line from the flint heterotic group. On the other hand, all of the landraces that expressed heterosis with the 14NS tester also expressed heterosis with the 102NS tester. Increasing the threshold for heterosis to 75% of the better yielding St2 check, which amounted to 7.4 t ha−1, still resulted in heterosis of landraces 23, 29, 13, 34, and 35 in crossings with both testers.
Our results indicate that landraces 26, 14, 27, 24, 23, 29, 13, 34, and 35 have a good combining ability for gain yield, but also that they can be considered as an independent heterotic source of lines that combine well with both of the used heterotic groups. Their performance per se (Table 1) demonstrates that they are high yielding landraces. They can be used to broaden the genetic bases of both heterotic gene pools, without disturbing their heterotic patterns. The landraces with prevailed flint germplasm (24, 27, 29 and 34) could primarily be used to increase yields and the general combining ability of the heterotic group of the early flint type, such as the F2 line, while the semi-dent and dent landraces (14, 26, 23, 13, and 35) could be used for incorporation into the BSSS and ID gene pools.
On the other hand, the top-cross hybrids of the 14NS line and landraces in which the measured grain moisture was lower than the checks’ average (21.15%) did not simultaneously show heterosis for grain yield (as seen in the lower left quadrant).
4. Discussion
Early maize genotypes better tolerate higher planting density and express faster transition of the phenological phases, thus avoiding drought in the most crucial developmental stages, and achieving lower grain moisture at harvest. Accordingly, the breeding of early maturing maize germplasm is an essential part of the management strategy for current and future maize production in terms of increasing mechanized harvests and reducing production costs and risks [,,,].
In maize breeding programmes, many different traits need to be considered. The results of the given research indicated a positive correlation between the yield and the plant and ear height, as well as kernel numbers per row and ear length. Maize plant height and ear height, and their ratio, are important parts of maize-plant-type structures and have played an important role in the improvement of maize lodging resistance and historical increases in grain yields []. Negative correlations were found between the number of rows and kernel weight and grain moisture, thus complicating the selection of the desired genotype [].
European flint landraces are natural candidates for introgression into the flint heterotic pool [,,,], because this traces back to lines extracted from a small number of European flint landraces at the beginning of hybrid breeding []. Before the incorporation of landraces from gene banks into commercial breeding gene pools, their heterotic pattern has to be defined, as this is of vital importance for successful hybrid breeding programmes []. Due to the importance of information related to the combining ability of the flint landraces in test crosses with representative elite tester lines from the dent heterotic pool [,], in this study, the selected early landraces, mostly flints (Table 1), were crossed with two divergent testers: representatives of the dent (BSSS-ID) and flint (F2 line type) heterotic groups.
Landrace 35, with the highest GY per se, gave the most productive progeny with both testers. The statistical significance of the correlation between landraces GY per se, and the average GY performance of landraces in crosses with inbred testers, indicated an additive gene effect as the main GCA. A positive linear relationship between the grain yield of the populations per se and their corresponding GCA values has also been reported [].
The largest number of landraces (29) showed heterosis for yield in crosses with the BSSS-ID tester (102NS), taking as a measure of heterosis 75% of the yield of the checks. In the population, change in vigour is directly proportional to the change in heterozygosity being highly correlated with GY. The probability of an allele giving a heterozygote in crossing with a population which is at equilibrium (p = q = 0.5) for the locus (i.e., 25%AA: 50%Aa: 25%aa) to inbred with a fixed locus (i.e., 100% AA or 100% aa) is 75% []. Given that the GY is a complex trait and that not all of the alleles that determine population yield are in equilibrium, top-cross hybrids could exhibit lower/higher yield values compared to the referent [].
Simultaneously, 20 top-crosses had grain moisture below the defined threshold value (21.1%) (in the lower right quadrant). Two groups can be selected: (1) landraces 6, 15, 2, and 11, where the top-cross hybrids had higher yields and lower grain moisture than St1, and (2) landraces 6, 16, 20, 9, and 30, where the progenies of both testers had low grain moisture at harvest, but expressed no heterosis with the flint 14NS tester (lower left and right quadrants). Accordingly, the best performing landraces can be used as a starting point for new pre-breeding programmes for improving the maize breeding material for early growth and low grain moisture at harvest, which are important goals for maize breeding.
The landraces with a greater or lesser portion of dent germplasm showed heterosis with both testers, where the landraces 14, 26, 27, 13, and 35 had more productive progenies with the dent tester (Figure 4). A simultaneous heterotic effect with two divergent inbred testers implied the existence of an independent heterotic pool within the evaluated maize landraces’ gene pool []. Because of the narrow genetic base of the commercial maize gene pool, using local germplasms to search for alternative heterotic patterns becomes highly interesting []. In early maize material, the production of three-way cross hybrids is more frequent due to the more frequent occurrence of poor agronomic characteristics in the parental lines []. Accordingly, the landraces can be used to form an independent heterotic gene pool, as well as to broaden the genetic basis of both of the divergent gene pools used. These landraces can be primarily used to improve the agronomic traits of the flint heterotic group (of the F2 inbred line type), without disturbing its heterotic pattern in relation to the BSSS-ID gene pool.
Pre-breeding that uses landraces as the starting material is a cost-demanding process, with a low change of achieving commercial results in a shorter period of time []. Inbred lines that are developed directly from landraces are often used as donors for numerous traits of interest, but also are characterised by a large performance gap compared to current varieties [,,]. In cases where the performance gap between the donors released from pre-breeding and elites is too large, and when the direct introductions are not converted into genetic gain, one may consider a buffer population between donors and elites before introducing them into the elite breeding population []. The authors propose considering genomic selection and optimal cross selection to recurrently improve genetic resources (i.e., pre-breeding), to bridge the improved genetic resources with elites (i.e., bridging), and to manage introductions into the elite breeding population. Therefore, several cycles of improvement of the most important agronomic traits in the selected groups of landraces are planned, in order to enhance their performance to a level similar to that of the elite breeding material. Thereafter, the best performing progenies could be incorporated into commercial breeding programmes without fear of compromising years of work focused on improving the agronomic characteristics of commercial material.
5. Conclusions
The maize maturity group is one of the most important factors influencing the yield–density–grain moisture relationship. A small number of initial landraces participate in the creation of early European maize inbred lines, especially flint ones. Therefore, a new pre-breeding programme was started in order to expand the genetic basis of early material by selecting a set of early, mostly flint, landraces. Test-cross evaluation with the dent tester highlighted the set of landraces that constitute a promising source for broadening the flint gene pool, producing high-yielding progenies with low grain moisture at harvest. Moreover, test-cross evaluation with both dent and flint testers underlined the landraces of independent heterotic pools. Because of the narrow genetic base of commercial early maize germplasm, identification of alternative heterotic patterns becomes highly interesting.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12081939/s1. Figure S1: Heat map of the differences in effect of landrace on grain yield; Table S1: Analysis of variance for grain yield; Table S2: Significance of differences in the effect of landraces on grain yield in crossings with both testers.
Author Contributions
Conceptualization, V.B. and D.S.; methodology, V.B. and D.S.; software, M.Z.; validation, S.M.; formal analysis, M.Z.; investigation, V.B., N.K., B.M. and D.S.; resources, V.A. and D.S.; data curation, V.B. and M.Z.; writing—original draft preparation, V.B.; writing—review and editing, N.K. and S.M.; visualization, V.B.; supervision, N.K.; funding acquisition, B.M. and V.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
This research was supported by the Ministry of Education, Science and Technological Development, Republic of Serbia, under Grant no. 451-03-68/2022-14/200040 and 451-03-68/2022-14/200032.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Secur. 2011, 3, 307–327. [Google Scholar] [CrossRef]
- Babić, V.; Pavlov, M.; Boćanski, J. Status and perspective of plant breeding and seed industry in Serbia. Sel. I Semen. Plant Breed. Seed Prod. 2016, 22, 19–27. [Google Scholar]
- Masuka, B.; Magorokosho, C.; Olsen, M.; Atlin, G.N.; Bänziger, M.; Pixley, K.V.; Vivek, B.S.; Labuschagne, M.; Matemba-Mutasa, R.; Burgueño, J.; et al. Gains in Maize Genetic Improvement in Eastern and Southern Africa: II. CIMMYT Open-Pollinated Variety Breeding Pipeline. Crop Sci. 2017, 57, 180–191. [Google Scholar] [CrossRef]
- Popović, A.; Kravić, N.; Prodanović, S.; Filipović, M.; Sečanski, M.; Babić, V.; Miriţescu, M. Characterisation and evaluation towards selection of maize landraces with the best per se performances. Rom. Agric. Res. 2020, 37, 49–58. Available online: https://www.incda-fundulea.ro/rar/nr37/rar37.7.pdf (accessed on 10 February 2021).
- Babić, V.; Andjelkovic, V.; Jovovic, Z.; Babic, M.; Vasic, V.; Kravic, N. Diversity Assessment of the Montenegrin Maize Landrace Gene Pool Maintained in Two Gene Banks. Plants 2021, 10, 1503. [Google Scholar] [CrossRef]
- Li, W.; Yu, Y.; Wang, L.; Luo, Y.; Peng, Y.; Xu, Y.; Liu, X.; Wu, S.; Jian, L.; Hu, J.; et al. The genetic architecture of the dynamic changes in grain moisture in maize. Plant Biotechnol. J. 2021, 19, 1195–1205. [Google Scholar] [CrossRef]
- De-Jager, B.; Roux, C.Z.; Kühn, H.C. An evaluation of two collections of south african maize (Zea mays L.) germplasm: 2. the genetic basis of dry-down rate. S. Afr. J. Plant Soil 2004, 21, 120–122. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Xu, C.; Tan, W.; Wang, P.; Meng, Q. Decreased kernel moisture in medium-maturing maize hybrids with high yield for mechanized grain harvest. Crop Sci. 2019, 59, 2794–2805. [Google Scholar] [CrossRef]
- Martinez-Feria, R.A.; Licht, M.A.; Ordóñez, R.A.; Hatfield, J.L.; Coulter, J.A.; Archontoulis, S.V. Evaluating maize and soybean grain dry-down in the field with predictive algorithms and genotype-by-environment analysis. Sci. Rep. 2019, 9, 7167. [Google Scholar] [CrossRef]
- Raymond, D.J.; Sessions, S.L.; Sobel, A.H.; Fuchs, Ž. The mechanics of gross moist stability. J. Adv. Model. Earth Syst. 2009, 1, 20. [Google Scholar] [CrossRef]
- Assefa, Y.; Carter, P.; Hinds, M.; Bhalla, G.; Schon, R.; Jeschke, M.; Paszkiewicz, S.; Smith, S.; Ciampitti, I.A. Analysis of long term study indicates both agronomic optimal plant density and increase maize yield per plant contributed to yield gain. Sci. Rep. 2018, 8, 4937. [Google Scholar] [CrossRef]
- Burton, A.B.; Kemanian, A.R. Maize yield in response to alternating low- and high-density rows of diverse hybrids. Eur. J. Agron. 2022, 135, 126472. [Google Scholar] [CrossRef]
- Lauer, S.; Hall, B.D.; Mulaosmanovic, E.; Anderson, S.R.; Nelson, B.; Smith, S. Morphological changes in parental lines of pioneer brand maize hybrids in the U.S. Corn Belt. Crop Sci. 2012, 52, 1033–1043. [Google Scholar] [CrossRef]
- Lindsey, A.J.; Thomison, P.R. Drought-tolerant corn hybrid and relative maturity yield response to plant population and planting date. Agron. J. 2016, 108, 229–242. [Google Scholar] [CrossRef]
- Brauner, P.C.; Schipprack, W.; Utz, H.F.; Bauer, E.; Mayer, M.; Schön, C.C.; Melchinger, A.E. Testcross performance of doubled haploid lines from European flint maize landraces is promising for broadening the genetic base of elite germplasm. Theor. Appl. Genet. 2019, 132, 1897–1908. [Google Scholar] [CrossRef]
- Vancetovic, J.; Bozinovic, S.; Ignjatovic-Micic, D.; Delic, N.; Kravic, N.; Nikolic, A. A diallel cross among drought tolerant maize populations. Euphytica 2015, 205, 1–16. [Google Scholar] [CrossRef]
- Van Hintum, T.J.L. Duplication within and between germplasm collections. III. A quantitative model. Genet. Resour. Crop Evol. 2000, 47, 507–513. [Google Scholar] [CrossRef]
- Babić, V.; Vančetović, J.; Prodanović, S.; Andjelković, V.; Babić, M.; Kravić, N. The identification of drought tolerant maize accessions by the two-step cluster analysis. Rom. Agric. Res. 2012, 29, 53–61. Available online: https://www.incda-fundulea.ro/rar/nr29/rar29.8.pdf (accessed on 1 March 2020).
- Babić, V.; Vančetović, J.; Prodanović, S.; Kravić, N.; Babić, M.; Anđelković, V. Numerical Classification of Western Balkan Maize Drought Tolerant Landraces. J. Agric. Sci. Technol. 2015, 17, 455–468. Available online: http://jast.modares.ac.ir/article_12232_8711c875192edd074d9cf7792c6a0ce2.pdf (accessed on 7 March 2021).
- Popović, A.; Kravić, N.; Babić, M.; Prodanović, S.; Sečanski, M.; Babić, V. Breeding potential of maize landraces evaluated by their testcross performance. Zemdirbyste 2020, 107, 153–160. [Google Scholar] [CrossRef]
- Metzger, M.J.; Brus, D.J.; Bunce, R.G.H.; Carey, P.D.; Gonçalves, J.; Honrado, J.P.; Jongman, R.H.G.; Trabucco, A.; Zomer, R. Environmental stratifications as the basis for national, European and global ecological monitoring. Ecol. Indic. 2013, 33, 26–35. [Google Scholar] [CrossRef]
- Ghosh, D.K.; Divecha, J. Two associate class partially balanced incomplete block designs and partial diallel crosses. Biometrika 1997, 84, 245–248. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Gilmour, A.R. Echidna Mixed Model Software. In Proceedings of the World Congress on Genetics Applied to Livestock Production, Auckland, New Zealand, 11–16 February 2018. [Google Scholar]
- Meier, U. A note on the power of Fisher’s least significant difference procedure. Pharm. Stat. 2006, 5, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Kosgey, J.R.; Moot, D.J.; Fletcher, A.L.; McKenzie, B.A. Dry matter accumulation and post-silking N economy of ‘stay-green’ maize (Zea mays L.) hybrids. Eur. J. Agron. 2013, 51, 43–52. [Google Scholar] [CrossRef]
- Antonietta, M.; Fanello, D.D.; Acciaresi, H.A.; Guiamet, J.J. Senescence and yield responses to plant density in stay green and earlier-senescing maize hybrids from Argentina. Field Crops Res. 2014, 155, 111–119. [Google Scholar] [CrossRef]
- Liu, W.; Liu, G.; Yang, Y.; Guo, X.; Ming, B.; Xie, R.; Liu, Y.; Wang, K.; Hou, P.; Li, S. Spatial variation of maize height morphological traits for the same cultivars at a large agroecological scale. Eur. J. Agron. 2021, 130, 126349. [Google Scholar] [CrossRef]
- Rana, M.; Sood, A.; Hussain, W.; Kaldate, R.; Sharma, T.R.; Gill, R.K.; Kumar, S.; Singh, S. Chapter 6—Gene Pyramiding and Multiple Character Breeding. In Lentils: Potential Resources for Enhancing Genetic Gains, 1st ed.; Singh, M., Ed.; Academic Press: London, UK, 2019; pp. 83–124. [Google Scholar] [CrossRef]
- Reif, J.C.; Hamrit, S.; Hechenberger, M.; Schipprack, W.; Maurer, H.P.; Bohn, M.; Melchinger, A.E. Trends in genetic diversity among European maize cultivars and their parental components during the past 50 years. Theor. Appl. Genet. 2005, 111, 838–845. [Google Scholar] [CrossRef]
- Wilde, K.; Burger, H.; Prigge, V.; Presterl, T.; Schmidt, W.; Ouzunova, M.; Geiger, H.H. Testcross performance of doubled-haploid lines developed from European flint maize landraces. Plant Breed. 2010, 129, 181–185. [Google Scholar] [CrossRef]
- Gouesnard, B.; Negro, S.; Laffray, A.; Glaubitz, J.; Melchinger, A.; Revilla, P.; Moreno Gonzalez, J.; Madur, D.; Combes, V.; Tollon Cordet, C.; et al. Genotyping-by-sequencing highlights original diversity patterns within a European collection of 1191 maize flint lines, as compared to the maize USDA genebank. Theor. Appl. Genet. 2017, 130, 2165–2189. [Google Scholar] [CrossRef]
- Böhm, J.; Schipprack, W.; Mirdita, V.; Utz, H.F.; Melchinger, A.E. Breeding potential of European flint maize landraces evaluated by testcross performance. Crop Sci. 2014, 54, 1665–1672. [Google Scholar] [CrossRef]
- Böhm, J.; Schipprack, W.; Utz, H.F.; Melchinger, A.E. Tapping the genetic diversity of landraces in allogamous crops with doubled haploid lines: A case study from European flint maize. Theor. Appl. Genet. 2017, 130, 861–873. [Google Scholar] [CrossRef]
- Melani, M.D.; Carena, M.J. Alternative maize heterotic patterns for the Northern Corn Belt. Crop Sci. 2005, 45, 2186–2194. [Google Scholar] [CrossRef]
- Hallauer, A.R.; Carena, M.J.; Miranda Filho, J.B. Quantitative genetics in maize breeding. In Handbook of Plant Breeding, 3rd ed.; Prohens, J., Nuez, F., Carena, M.J., Eds.; Springer: New York, NY, USA, 2010; Volume 6, pp. 1–663. [Google Scholar] [CrossRef]
- Chassaigne-Ricciulli, A.A.; Mendoza-Onofre, L.E.; Córdova-Téllez, L.; Carballo-Carballo, A.; San Vicente-García, F.M.; Dhliwayo, T. Effective Seed Yield and Flowering Synchrony of Parents of CIMMYT Three-Way-Cross Tropical Maize Hybrids. Agriculture 2021, 11, 161. [Google Scholar] [CrossRef]
- Allier, A.; Teyssèdre, S.; Lehermeier, C.; Claustres, B.; Maltese, S.; Melkior, S.; Moreau, L.; Charcosset, A. Assessment of breeding programs sustainability: Application of phenotypic and genomic indicators to a North European grain maize program. Theor. Appl. Genet. 2019, 132, 1321–1334. [Google Scholar] [CrossRef]
- Melchinger, A.E.; Munder, S.; Mauch, F.J.; Merdita, V.; Böhm, J.; Müller, J. High-throughput platform for automated sorting and selection of single seeds based on time-domain nuclear magnetic resonance (TD-NMR) measurement of oil content. Biosyst. Eng. 2017, 164, 213–220. [Google Scholar] [CrossRef]
- Allier, A.; Teyssèdre, S.; Lehermeier, C.; Moreau, L.; Charcosset, A. Optimized breeding strategies to harness genetic resources with different performance levels. BMC Genomics 2020, 21, 349. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).