Genetic and Molecular Characterization of a New EMS-Induced Mutant without the Third Glucose Moiety at the C-3 Sugar Chain of Saponin in Glycine max (L.) Merr.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. TLC of Saponin Composition
2.3. Liquid Chromatography with Photodiode Array and Tandem Mass Spectrometry (LC-PDA-MS/MS) of Saponin Composition
2.4. Extraction of Genomic DNA
2.5. Bulked Segregant Analysis and Sequencing of Sg-3
2.6. Multiple Alignment of UDP-glycosyltransferases
2.7. Segregation and Cosegregation Analysis of Sg-3
2.8. Expression Analysis of Sg-3
3. Results and Discussion
3.1. Phenotypic Characterization of a New EMS-Induced Mutant
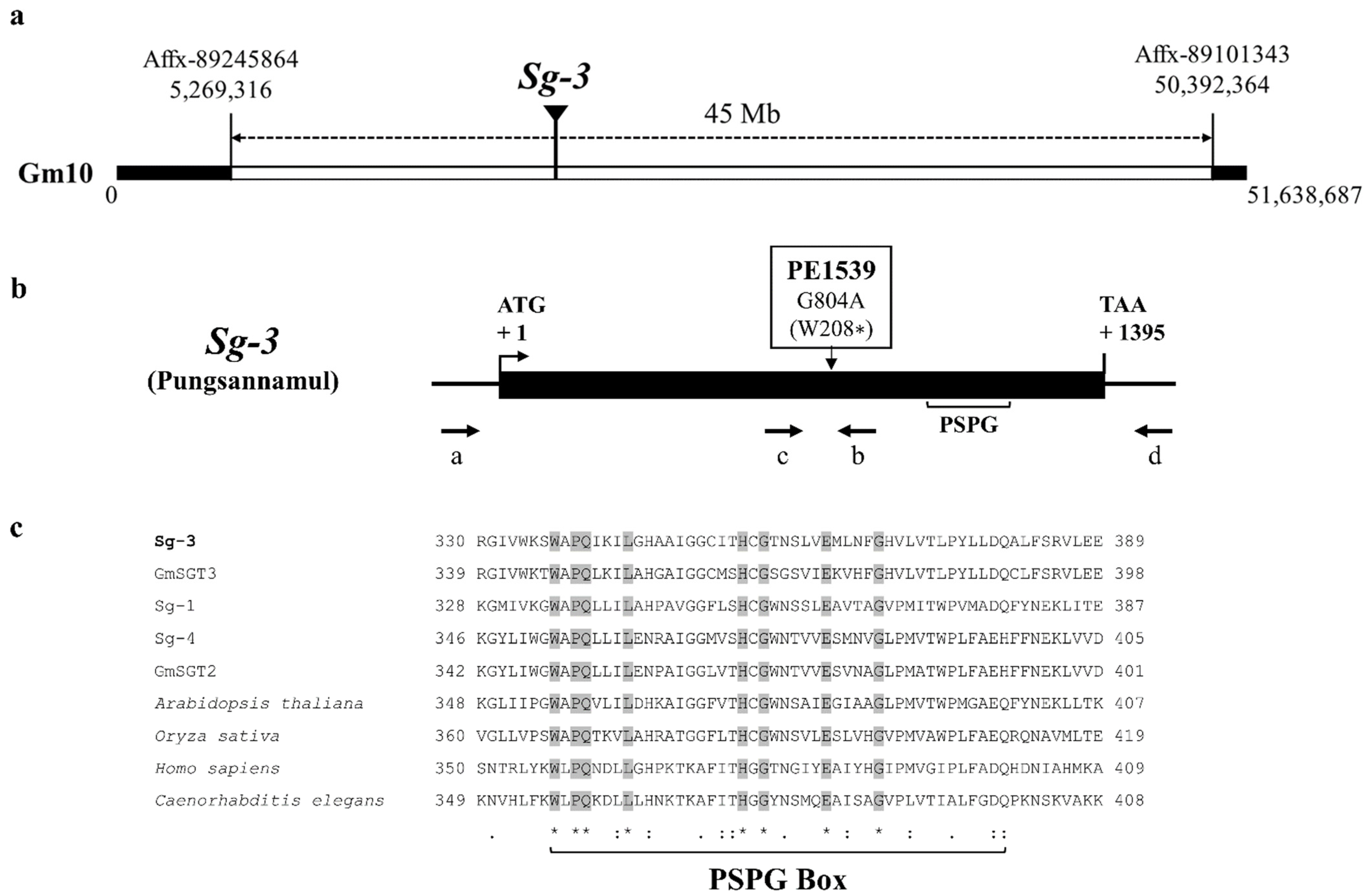
3.2. Physical Mapping and Molecular Characterization of PE1539
3.3. Cosegregation of the Sg-3 Polymorphism with the Saponin Phenotype
3.4. Expression Analysis of Sg-3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medic, J.; Atkinson, C.; Hurburgh, C.R. Current knowledge in soybean composition. J. Am. Oil Chem. Soc. 2014, 91, 363–384. [Google Scholar] [CrossRef]
- Isanga, J.; Zhang, G.-N. Soybean bioactive components and their implications to health—A review. Food Rev. Int. 2008, 24, 252–276. [Google Scholar] [CrossRef]
- Kim, S.-L.; Berhow, M.A.; Kim, J.-T.; Chi, H.-Y.; Lee, S.-J.; Chung, I.-M. Evaluation of soyasaponin, isoflavone, protein, lipid, and free sugar accumulation in developing soybean seeds. J. Agric. Food Chem. 2006, 54, 10003–10010. [Google Scholar] [CrossRef]
- Shimoyamada, M.; Harada, K.; Okubo, K. Saponin composition in developing soybean seed (Glycine max (L.) Merrill, cv. Mikuriyaao). Agric. Biol. Chem. 1991, 55, 1403–1405. [Google Scholar] [CrossRef][Green Version]
- Nakashima, H.; Okubo, K.; Honda, Y.; Tamura, T.; Matsuda, S.; Yamamoto, N. Inhibitory effect of glycosides like saponin from soybean on the infectivity of HIV in vitro. AIDS 1989, 3, 655–658. [Google Scholar] [CrossRef]
- Ellington, A.A.; Berhow, M.; Singletary, K.W. Induction of macroautophagy in human colon cancer cells by soybean B-group triterpenoid saponins. Carcinogenesis 2005, 26, 159–167. [Google Scholar] [CrossRef]
- Ellington, A.A.; Berhow, M.A.; Singletary, K.W. Inhibition of Akt signaling and enhanced ERK1/2 activity are involved in induction of macroautophagy by triterpenoid B-group soyasaponins in colon cancer cells. Carcinogenesis 2006, 27, 298–306. [Google Scholar] [CrossRef]
- Salyer, J.; Eswaranandam, S.; Lee, S.-O. Soyasaponin I, III, and soyasapogenol B inhibit proliferation and modulate PKC expression in caco-2 human colon cancer cells. J. Food Res. 2013, 2, 81. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, M.; Liu, X.; Liu, H.; He, Y.; Wang, B.; Ma, T. Evaluation of in vitro antioxidant activities of soyasaponins from soy hypocotyls in human HepG2 cell line. Chem. Pap. 2017, 71, 653–660. [Google Scholar] [CrossRef]
- Zha, L.; Chen, J.; Sun, S.; Mao, L.; Chu, X.; Deng, H.; Cai, J.; Li, X.; Liu, Z.; Cao, W. Soyasaponins can blunt inflammation by inhibiting the reactive oxygen species-mediated activation of PI3K/Akt/NF-kB pathway. Proc. Nalt. Acad. Sci. USA 2014, 9, e107655. [Google Scholar] [CrossRef]
- Yano, R.; Takagi, K.; Takada, Y.; Mukaiyama, K.; Tsukamoto, C.; Sayama, T.; Kaga, A.; Anai, T.; Sawai, S.; Ohyama, K. Metabolic switching of astringent and beneficial triterpenoid saponins in soybean is achieved by a loss-of-function mutation in cytochrome P450 72A69. Plant J. 2017, 89, 527–539. [Google Scholar] [CrossRef]
- Sundaramoorthy, J.; Park, G.T.; Komagamine, K.; Tsukamoto, C.; Chang, J.H.; Lee, J.D.; Kim, J.H.; Seo, H.S.; Song, J.T. Biosynthesis of DDMP saponins in soybean is regulated by a distinct UDP-glycosyltransferase. New Phytol. 2019, 222, 261–274. [Google Scholar] [CrossRef]
- Kudou, S.; Tonomura, M.; Tsukamoto, C.; Uchida, T.; Sakabe, T.; Tamura, N.; Okubo, K. Isolation and structural elucidation of DDMP-conjugated soyasaponins as genuine saponins from soybean seeds. Biosci. Biotechnol. Biochem. 1993, 57, 546–550. [Google Scholar] [CrossRef]
- Kudou, S.; Tonomura, M.; Tsukamoto, C.; Shimoyamada, M.; Uchida, T.; Okubo, K. Isolation and structural elucidation of the major genuine soybean saponin. Biosci. Biotechnol. Biochem. 1992, 56, 142–143. [Google Scholar] [CrossRef]
- Chitisankul, W.T.; Shimada, K.; Omizu, Y.; Uemoto, Y.; Varanyanond, W.; Tsukamoto, C. Mechanism of DDMP-saponin degradation and maltol production in soymilk preparation. LWT 2015, 64, 197–204. [Google Scholar] [CrossRef]
- Sayama, T.; Ono, E.; Takagi, K.; Takada, Y.; Horikawa, M.; Nakamoto, Y.; Hirose, A.; Sasama, H.; Ohashi, M.; Hasegawa, H. The Sg-1 glycosyltransferase locus regulates structural diversity of triterpenoid saponins of soybean. Plant Cell 2012, 24, 2123–2138. [Google Scholar] [CrossRef]
- Shibuya, M.; Nishimura, K.; Yasuyama, N.; Ebizuka, Y. Identification and characterization of glycosyltransferases involved in the biosynthesis of soyasaponin I in Glycine max. FEBS Lett. 2010, 584, 2258–2264. [Google Scholar] [CrossRef]
- Takada, Y.; Tayama, I.; Sayama, T.; Sasama, H.; Saruta, M.; Kikuchi, A.; Ishimoto, M.; Tsukamoto, C. Genetic analysis of variations in the sugar chain composition at the C-3 position of soybean seed saponins. Breed. Sci. 2012, 61, 639–645. [Google Scholar] [CrossRef]
- Takagi, K.; Yano, R.; Tochigi, S.; Fujisawa, Y.; Tsuchinaga, H.; Takahashi, Y.; Takada, Y.; Kaga, A.; Anai, T.; Tsukamoto, C. Genetic and functional characterization of Sg-4 glycosyltransferase involved in the formation of sugar chain structure at the C-3 position of soybean saponins. Phytochemistry 2018, 156, 96–105. [Google Scholar] [CrossRef]
- Yano, R.; Takagi, K.; Tochigi, S.; Fujisawa, Y.; Nomura, Y.; Tsuchinaga, H.; Takahashi, Y.; Takada, Y.; Kaga, A.; Anai, T. Isolation and characterization of the soybean Sg-3 gene that is involved in genetic variation in sugar chain composition at the C-3 position in soyasaponins. Plant Cell Physiol. 2018, 59, 797–810. [Google Scholar] [CrossRef]
- Tsukamoto, C.; Kikuchi, A.; Kudou, S.; Harada, K.; Kitamura, K.; Okubo, K. Group A acetyl saponin-deficient mutant from the wild soybean. Phytochemistry 1992, 31, 4139–4142. [Google Scholar] [CrossRef]
- Tsukamoto, C.; Kikuchi, A.; Harada, K.; Kitamura, K.; Okubo, K. Genetic and chemical polymorphisms of saponins in soybean seed. Phytochemistry 1993, 34, 1351–1356. [Google Scholar] [CrossRef]
- Chae, J.-H.; Dhakal, K.H.; Asekova, S.; Song, J.T.; Lee, J.-D. Variation of fatty acid composition in soybean ‘Pungsannamul’ mutation population from EMS treatment. Curr. Res. Agric. Life Sci. 2013, 31, 45–50. [Google Scholar]
- Krishnamurthy, P.; Tsukamoto, C.; Yang, S.H.; Lee, J.D.; Chung, G. An improved method to resolve plant saponins and sugars by TLC. Chromatographia 2012, 75, 1445–1449. [Google Scholar] [CrossRef]
- Sundaramoorthy, J.; Park, G.T.; Mukaiyama, K.; Tsukamoto, C.; Chang, J.H.; Lee, J.-D.; Kim, J.H.; Seo, H.S.; Song, J.T. Molecular elucidation of a new allelic variation at the Sg-5 gene associated with the absence of group A saponins in wild soybean. Proc. Nalt. Acad. Sci. USA 2018, 13, e0192150. [Google Scholar] [CrossRef]
- Takada, Y.; Sasama, H.; Sayama, T.; Kikuchi, A.; Kato, S.; Ishimoto, M.; Tsukamoto, C. Genetic and chemical analysis of a key biosynthetic step for soyasapogenol A, an aglycone of group A saponins that influence soymilk flavor. Theor. Appl. Genet. 2013, 126, 721–731. [Google Scholar] [CrossRef]
- Son, H.; Mukaiyama, K.; Omizu, Y.; Tsukamoto, C. A fragmentation database of soyasaponins by liquid chromatography with a photodiode array detector and tandem mass spectrometry. Anal. Sci. Technol. 2021, 34, 172–179. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Mackenzie, P.I.; Owens, I.S.; Burchell, B.; Bock, K.W.; Bairoch, A.; Belanger, A.; Fournel-Gigleux, S.; Green, M.; Hum, D.W.; Iyanagi, T. The UDP glycosyltransferase gene superfamily: Recommended nomenclature update based on evolutionary divergence. Pharmacogenetics 1997, 7, 255–269. [Google Scholar] [CrossRef]
- Gachon, C.M.; Langlois-Meurinne, M.; Saindrenan, P. Plant secondary metabolism glycosyltransferases: The emerging functional analysis. Trends Plant Sci. 2005, 10, 542–549. [Google Scholar] [CrossRef]
- Wang, X. Structure, mechanism and engineering of plant natural product glycosyltransferases. FEBS Lett. 2009, 583, 3303–3309. [Google Scholar] [CrossRef]
- Peccarelli, M.; Kebaara, B.W. Regulation of natural mRNAs by the nonsense-mediated mRNA decay pathway. Eukaryot. Cell 2014, 13, 1126–1135. [Google Scholar] [CrossRef]
- Muhlrad, D.; Parker, R. Aberrant mRNAs with extended 3′ UTRs are substrates for rapid degradation by mRNA surveillance. RNA 1999, 5, 1299–1307. [Google Scholar] [CrossRef]
- Kertesz, S.; Kerenyi, Z.; Merai, Z.; Bartos, I.; Palfy, T.; Barta, E.; Silhavy, D. Both introns and long 3′-UTRs operate as cis-acting elements to trigger nonsense-mediated decay in plants. Nucleic Acids Res. 2006, 34, 6147–6157. [Google Scholar] [CrossRef]
- Schwartz, A.; Komarova, T.; Skulachev, M.; Zvereva, A.; Dorokhov, Y.L.; Atabekov, J. Stability of plant mRNAs depends on the length of the 3′-untranslated region. Biochemistry 2006, 71, 1377–1384. [Google Scholar] [CrossRef]



| Parents and Their Progenies | Saponinphenotype | Observed | (Expected) | χ2 Value | Probability * | Genotype † | Observed | (Expected) | χ2 Value | Probability * |
|---|---|---|---|---|---|---|---|---|---|---|
| P1: Uram | Typical | W | ||||||||
| P2: PE1539 | Ab-γg rich | M | ||||||||
| F2 population | Typical | 132 | (129.75) | 0.156 | 0.69 | W | 41 | (43.25) | 0.468 | 0.79 |
| Ab-γg rich | 41 | (43.25) | H | 91 | (86.5) | |||||
| M | 41 | (43.25) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, J.; Sundaramoorthy, J.; Tsukamoto, C.; Park, J.; Lee, J.; Jo, H.; Lee, J.-D.; Seo, H.S.; Song, J.T. Genetic and Molecular Characterization of a New EMS-Induced Mutant without the Third Glucose Moiety at the C-3 Sugar Chain of Saponin in Glycine max (L.) Merr. Agronomy 2022, 12, 1598. https://doi.org/10.3390/agronomy12071598
Ko J, Sundaramoorthy J, Tsukamoto C, Park J, Lee J, Jo H, Lee J-D, Seo HS, Song JT. Genetic and Molecular Characterization of a New EMS-Induced Mutant without the Third Glucose Moiety at the C-3 Sugar Chain of Saponin in Glycine max (L.) Merr. Agronomy. 2022; 12(7):1598. https://doi.org/10.3390/agronomy12071598
Chicago/Turabian StyleKo, Jeongyun, Jagadeesh Sundaramoorthy, Chigen Tsukamoto, Junbeom Park, Jinwon Lee, Hyun Jo, Jeong-Dong Lee, Hak Soo Seo, and Jong Tae Song. 2022. "Genetic and Molecular Characterization of a New EMS-Induced Mutant without the Third Glucose Moiety at the C-3 Sugar Chain of Saponin in Glycine max (L.) Merr." Agronomy 12, no. 7: 1598. https://doi.org/10.3390/agronomy12071598
APA StyleKo, J., Sundaramoorthy, J., Tsukamoto, C., Park, J., Lee, J., Jo, H., Lee, J.-D., Seo, H. S., & Song, J. T. (2022). Genetic and Molecular Characterization of a New EMS-Induced Mutant without the Third Glucose Moiety at the C-3 Sugar Chain of Saponin in Glycine max (L.) Merr. Agronomy, 12(7), 1598. https://doi.org/10.3390/agronomy12071598









