Abstract
Soil amendments may decrease trace element accumulation in vegetables, improving food security and allowing the recovery of contaminated farmlands. Despite some promising results in the laboratory, validation of soil amendments in field conditions are scarce, especially in aerobic soils. Here, we assessed the effect of different potential soil amendments on arsenic (As) accumulation in lettuces. Then, we compared them in terms of food security and the associated investment (efficacy and efficiency, respectively). We also hypothesized that the soil amendments do not lead to side effects, such as yield decrease, phytotoxicity of Cu, or undesired changes in soil properties. Thereby, we assessed lettuces grown on untreated contaminated soils (C+), treated contaminated soils, and untreated uncontaminated soils (C−) in two contrasting soil types (sandy and loamy soils). The treated contaminated soils consisted of multiple soil amendments. Soil amendments were: diammonium phosphate (DP), iron sulfate (IS), ferrous phosphate (FP), calcium peroxide (CP), and organic matter (OM). We found that phosphate amendments (DP and FP) reduced the As in edible tissues of lettuce in both areas, while CP only reduced As accumulation in the sandy soils area. The As intake through lettuces grown on these amended soils was about 30% lower than on the unamended ones. Cu concentrations in lettuces above 25 mg kg−1 grown in contaminated soils without reducing growth were found, a result that differed from non-field studies.
1. Introduction
The intake of arsenic (As) in people by food consumption is an issue of global concern since it can induce several deleterious effects on human health [1]. To date, most As remediation efforts have focused on rice grown on soils under anaerobic conditions, where As is predominantly like arsenite ((AsO2)−) [2,3]. However, remediation experiences on crops grown in soils under aerobic conditions, where arsenate ((AsO4)3−) is the most common speciation of As (e.g., [4]), are much fewer in number. This knowledge gap is seen in Mediterranean areas such as central Chile despite their global importance due to the high diversity of food produced.
Chile, the world leader in copper (Cu) production [5], has developed its mining activities with several externalities to the environment, including contamination of agricultural soils [6]. Chile holds seven copper smelters, of which two are in the Valparaíso Region of central Chile: Ventanas Smelter (hereafter VS) and Chagres smelter (hereafter CS), both coexisting with agricultural activities. VS and CS have functioned constantly since 1964 and 1959, respectively, until nowadays. During this period, smelter atmospheric emissions have increased trace element concentrations in the surrounding agricultural soils, especially with As and Cu. During the 1990s, the smelters implemented significant environmental improvements [7], but chronic contamination of the surrounding soils is still a problem [8,9].
Our previous results showed that As concentrations in the edible tissues of vegetables grown near VS and CS are generally higher than in control areas (without copper smelter activities) [10,11,12]. The As concentration in lettuces surrounding VS and CS were 4.8- and 8.2-fold higher, respectively, than in control areas. Thus, the consumption of vegetables grown in these contaminated areas can be considered a human health risk [13]. In contrast, Cu has an improbable human health risk by food consumption [10,11,12]. While Cu is an essential micronutrient in plants, high concentrations could produce phytotoxicity, reducing farmers’ agricultural productivity [14,15].
The high concentration of As and Cu in the soils surrounding copper smelters should be remediated to restore agricultural activities. Arsenic is predominantly present as arsenate in soils, an oxyanion. Arsenate solubility could be reduced by soil acidification [16]. However, such management would increase Cu2+ availability and its potential phytotoxicity to crops [15]. Thus, the co-contamination of As and Cu makes the challenge of finding a solution more complex.
Some potential solutions to decrease As accumulation in vegetables are phosphate or iron soil amendments. Phosphate additions can decrease the absorption of arsenate by competition in plants (e.g., [17]). However, the opposite may occur [18] by anion exchange competition, complexation reactions, or by retention of oxides in the soil [19]. The latter mechanism could also be obtained by applying iron oxide precursors, such as iron sulfate [20]. In a recent field experiment, the use of an iron amendment diminished As extraction by plants (65% decrease). However, it also decreased their fresh biomass (decrease of 57%) due to nutrient immobilization (61% decrease of P in plants tissues) [21]. As an alternative, we hypothesized that ferrous phosphate (vivianite) would act with the effect of phosphate and iron oxides. However, to date, no studies aimed to analyze the effect of ferrous phosphate as a soil amendment to decrease As accumulation in plants.
Another potential alternative is the addition of calcium peroxide or organic matter. Calcium peroxide could stabilize As by oxidizing arsenite to arsenate (less toxic) and forming insoluble calcium arsenate precipitates [22]. On the other hand, the use of organic matter addition as a compost in agricultural soils can increase soil adsorption capacity, favoring As immobilization [23]. However, the effect of organic matter on As accumulation in plants has been inconsistent in previous studies [24].
To be adopted by farmers, soil amendments should reduce As accumulation in vegetables without side-effects on crop productivity. In this sense, field experiments are crucial but scarce [3]. Wan et al. [25] recently found two research gaps: (1) the number of laboratory experiments is still substantially higher than that of field trials, and (2) the evaluation or comparison of different soil remediation technologies, which is essential for decision-making. These knowledge gaps are relevant in the case of Chile, where mining and agricultural activities need to be balanced. However, evidence has revealed the vulnerability of agriculture in this mining country. Despite the consequences of soil contamination on food safety being referred to in our recent reports [10,11,12] and the existence of national funds for the recovery of degraded soils [26], there is no study concerning this issue.
Therefore, our research aimed to assess and compare the performance of multiple soil amendments to improve food safety without side effects on the productivity of crops grown on contaminated soils from central Chile. We used lettuce as a study model crop in two contrasting soils (sandy and loamy). We selected the following amendments: diammonium phosphate (DP), iron sulfate (IS), ferrous phosphate (FP), calcium peroxide (CP), and organic matter (OM). These soil amendments differ in the potential mechanisms of action, which is a crucial contribution of the present study.
We hypothesized that soil amendments could reduce As accumulation in lettuce. Then, we compared the soil amendments in terms of food security and the associated investment (efficacy and efficiency, respectively). On the other hand, we also hypothesized that the studied soil amendments do not lead to side effects, such as yield decrease, phytotoxicity of Cu, or undesired changes in soil properties.
2. Materials and Methods
2.1. Characterization of the Study Areas
Our research was assessed in two contrasting areas from the Valparaíso region in central Chile (Figure 1). The first area is the Puchuncaví Valley, where the VS is located (Figure 1a) and is characterized by a coastal area with predominantly sandy soils. The second area is in Catemu, where the CS is (Figure 1b) located near the Aconcagua River with predominantly loamy soils.
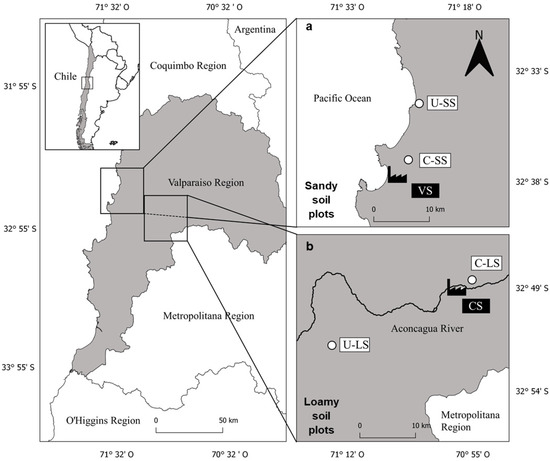
Figure 1.
The geographical location of the studied areas and the main trace element sources: (a) sandy soils area; and (b) loamy soils area. U-SS = uncontaminated sandy soil plot; C-SS = contaminated sandy soil plot; VS = Ventanas smelter; U-LS = uncontaminated loamy soil plot; C-LS = contaminated loamy soil plot; and CS = Catemu smelter.
Considering the importance of soil texture, as clay has a higher sorption capacity than coarser particles [27], we labeled the plots surrounding the VS as sandy soil plots and the plots surrounding the CS as loamy soil plots. These plots also differ in climatic characteristics [28] and the contamination magnitude and source; VS emissions have been characterized [29] but not CS. Thus, we will refer to sandy soil plots as contrasting compared to the loamy soil plots. Both study areas were selected based on previous knowledge of As accumulation in vegetables when no soil amendments are applied [10,11,12].
For each study area, two experimental plots were assessed. A contaminated (C-SS) (32°44′0″ S, 71°26′43″ W) and an uncontaminated one (U-SS) (32°38′32″ S, 71°25′28″ W) for the sandy soil, located at 4.6 and 14 km, respectively, from the VS, and a contaminated (C-LS) (32°47′22″ S, 70°56′13″ W) and an uncontaminated soil plot (U-LS) (32°53′43″ S, 71°12′24″ W) for the loamy soil, located at 2.5 and 25 km, respectively, from the CS. The potential impact of VS and CS is through atmospheric deposition [7]; thus, C-SS and C-LS were located to the northeast of the smelters (in the direction of the dominant winds). Before starting the experiment, all plots were characterized by analyzing three composite soil samples (≈25 cm depth) at each one (detailed later in Section 3.1).
2.2. Design of the Experiment
The field experiment included four experimental plots of 126 m2 (7 m × 18 m) corresponding to U-SS, C-SS, U-LS, and C-LS. Two rows were placed at the edges of each plot to grow lettuces without any treatment (to avoid edge-effect). Between those, six rows were used for the treatments, randomly placed and separated 1 m from each other (Figure S1). The treatments were an unamended row (C), and the other five were amended.
The amendments were diammonium phosphate ((NH4)2HPO4) (DP), iron sulfate (FeSO₄·7H₂O) (IS), ferrous phosphate (Fe3(PO4)2 8H₂O) (FP), calcium peroxide (CaO2) (CP), and organic matter addition as compost (OM). The chemical characteristics of compost are shown in Table S1. DP, IS, FP, and CP treatments were applied at 0.05% w/w (300 kg/ha), while OM treatment was applied at 4% w/w (120 t/ha). This amount of compost is recommended by US EPA to remediate soils [30], and it promoted the long-term revegetation of contaminated soils surrounding VS, as we observed in a previous field experiment [31]. However, the As accumulation in plants was not assessed in that study.
Soil amendments were purchased at a local farm supplies store, but FP was prepared in a laboratory mixing DP and IS according to Rosado et al. [32]. Amendments were added to the soil through manual tillage (≈0–25 cm depth). Then, soils were watered weekly for seven weeks to react soil As with the amendments.
Seedlings of “Victoriosa” var lettuce were used as a model plant. They were sown in the School of Agriculture, Pontificia Universidad Católica de Valparaíso, and transplanted to each plot 45 days after sown. The farmers grew lettuces according to traditional local management but without pesticides. A single nitrogen corrective fertilization was applied, detailed later (Section 4.1), but without additional fertilization.
2.3. Soil and Vegetable Sampling at the End of the Experiment and Sample Preparation
Lettuces were harvested 75 and 85 days after transplanting in the sandy and loamy soil areas, respectively. The rows of each experimental plot were divided into seven sub-areas of 1 m in length. Then, a composite sample of four lettuces was taken in each of the five middle subareas. At the same time, a composite soil sample was obtained by collecting soil adhered to the roots of the harvested lettuces (≈ 0–25 cm depth). This procedure was performed in both contaminated plots (C-SS and C-LS), whereas only the control rows were sampled in the uncontaminated plots (U-SS and U-LS).
Vegetables were immediately weighed (to register the fresh biomass under each treatment) and washed no later than 24 h afterwards. Specifically, lettuces were thoroughly washed in the following sequence: tap water, 0.1 M HCl, distilled water, 0.05 M EDTA, distilled water, and distilled water again [33]. HCl and EDTA were used to assure soil particles remotion from lettuces leaves, which is particularly complex for field-collected plants. Although we used an aggressive washing method, this is useful for analyzing only the trace elements within the plant tissues, which we aimed for in this and in previous research [10,11]. Then, the samples were put into paper bags and dried in an oven at 70 °C for 72 h. Later, the samples were weighed, ground, sieved through 18-mesh (aperture size 1 mm), and homogenized. On the other hand, soil samples were separated from vegetal and animal residues, put into paper bags, and dried in an oven at 40 °C for 48 h. The dry soil samples were ground, sieved through 10-mesh (aperture size 2 mm), and then homogenized.
2.4. Laboratory Analyses
Soil samples collected for the initial characterization of the plots were analyzed according to national standard procedures [34]. In contrast, soil samples collected at the end of the experiment were analyzed as follows: Soil pH was measured in a 1:2.5 w:v solution with CaCl2, electrical conductivity was measured in a saturated paste extract, and soil organic matter content was determined by the potassium dichromate method [35,36]. The concentrations of As and Cu of the collected soil samples at the end of the experiment were estimated using a portable X-ray fluorescence spectrometer (XRF, S1 Titan Bruker with GeoChem method) to check the homogeneity of the soil contaminants among treatments. Soil samples were prepared in the laboratory following US EPA protocol, method 6200 [37]. Each soil sample was analyzed in triplicates, and results were previously calibrated with soils from the studied area and previously measured through Flame-AAS.
The concentrations of As and Cu in lettuce were measured using standard methods [38]. Samples were digested in boiling nitric acid, followed by perchloric acid [39]. To prevent volatilization of As during the digestion process, a Teflon stopper with a 30-cm-long glass reflux tube was used (adapted from [40]). Then, total As concentration was determined by hydride generation–atomic fluorescence spectrometry (HG–AFS) under experimental conditions detailed in Vargas et al. [4]. On the other hand, the total concentration of Cu was determined by atomic absorption spectroscopy (Flame–AAS).
Quality was assured by analyzing duplicate certified reference samples obtained from Wageningen University, Netherlands. ISE-973 and ISE-859 were used for soil analyses, while IPE-907 (Spinach) and IPE-951 (Aubergine (leaf + fruit)) were used for plant analyses. The obtained results for the standard reference materials were within 10% of the certified values. Quality control was assured by introducing standards and blanks every 20 samples.
2.5. Statistical Analysis
Before starting the experiment, the parameters of soil samples collected in each studied plot were compared to characterize their differences. Parameters of soil and plant samples collected at the end of the experiment were compared to test the effect of the amendments. This analysis was performed separately for each studied area (sandy and loamy soils areas). For each studied area, we compared the results among the unamended row of the contaminated soil plots (C+ = contaminated control), the unamended row of the uncontaminated soil plots (C− = uncontaminated control), and the amended rows of the contaminated soil plots. Comparisons were performed using ANOVA, with Tukey’s post hoc test to compare means (p-value < 0.05).
Since the field experiment was carried out in farmers’ plots, a Mantel test was performed to check the spatial homogeneity of soil As and Cu concentrations inside each of the contaminated plots (C-SS and C-LS) at the end of the experiment. On the other hand, we correlated the fresh weight and the foliar Cu concentration of lettuces to assess if yield decreased as foliar Cu of lettuces increased in the contaminated plots.
ANOVA and correlation analyses were carried out using Minitab 16 software, and figures were created using the Microsoft Excel 365 software. The Mantel test was performed using the haversine formula of the geosphere package in RStudio.
2.6. Contamination and Food Safety Assessment
The As and Cu concentrations were assessed using each plot’s contamination factor analysis (Cf) (Equation (S1)). To assess the efficacy of the treatments in terms of food safety, we estimated the chronic daily intake for As (CDI) [41]. CDI was calculated for children (1–5 years old) since this is the most vulnerable age group due to the high rate between consumption and bulk bodyweight to dilute the contaminants [12]. CDI was determined based on measured concentrations of As in the leaves of lettuces.
where CDI is the average daily intake of As by consumption of lettuce (mg kg−1 of body weight-day); C is the concentration of As in lettuce (mg kg−1 fresh weight); IR is the ingestion rate of lettuces (kg meal−1); FI is the fraction ingested, EF is the exposure frequency (meals per year); ED is the exposure duration (years), BW is the average body weight of the studied population; and AT is the average exposure time (days), i.e., the number of days exposed.
The As concentration in lettuces (on a fresh weight basis) was obtained according to the measured concentrations in this experiment and the corresponding dry matter contents. In contrast, the studied population’s dietary habits and exposure scenarios were obtained from Lizardi et al. [12].
Then, the human health risk was assessed through the Hazard Quotient index (HQ) [41] by dividing CDI by the reference dose (RfD), which is 0.0003 for As [42]. Thereby, a value of HQ ≥ 1 represents a potential health risk [41].
3. Results
3.1. Characterization of Soils at the Beginning of the Experiment
Table 1 shows the main difference among the soils from the four experimental plots clustered by their corresponding geographic area. Soils collected in plots from the same study area showed similar characteristics. The most relevant differences were the higher available N, total Cu, and total As in C-SS than in U-SS, and the higher total Cu and total As in C-LS than in U-LS. C-SS and C-LS were considered contaminated by As and Cu according to contamination factor analysis (Cf) (Table S2). Specifically, soil total As and Cu concentrations were 1.5- and 3.6-fold higher than the background concentrations, respectively, in the C-SS plot. In contrast, they were 2.2- and 9.0-fold higher in the C-LS plot.

Table 1.
Average ± SD of general physicochemical properties of the studied soils before amendment application (n = 3).
Table 1 also compared soils from the contaminated plots of both studied areas (i.e., C-SS vs. C-LS). Soils from the C-SS plot were sandy loam and slightly acidic, while those from the C-LS were silty loam and slightly basic. We also found higher soil total As and Cu concentrations but lower SOM and available P in C-LS than in C-SS.
3.2. Food Safety Assessment: As Concentration in Lettuces
For the sandy and loamy soil plots, the As concentration in lettuces grown on C+ soils were 1.9- and 2.7-fold higher than those grown in C- soils. DP, FP, and CP significantly decreased the foliar As concentration in the C-SS plot (Figure 2a). Even the As concentration in lettuces grown on these treated soils was similar to C- treatment. In the loamy soil plots, only DP and FP decreased the foliar As concentration significantly compared to C+ treatment. However, only lettuces on the FP treatment reached a similar As concentration to lettuces grown in C- (Figure 2b).

Figure 2.
Arsenic concentration in the edible tissues of lettuces (in dry weight basis) grown in (a) sandy soils and (b) loamy soils. Bars are means ± SD (n = 5). Different letters above the means indicate significant differences between treatments based on Tukey multiple comparison test (p ≤ 0.05). Black bars represent contaminated soil under different treatments: C+ = Contaminated soil without amendment; DP = diammonium phosphate; FP = ferrous phosphate; IS = iron sulphate; OM = organic matter addition; CP= calcium peroxide. White bar represents uncontaminated soil: C- = uncontaminated soil without amendment. C-SS = contaminated sandy soil plot; U-SS = uncontaminated sandy soil plot; C-LS = contaminated loamy soil plot; and U-LS = uncontaminated loamy soil plot.
The consumption of lettuces grown on contaminated soils could supply 26% and 30% of the HQ for the C-SS and C-LS plots, respectively (Table S3). However, the amendments reduced the HQ values compared to the respective C+ treatments. The best amendment for C-SS was CP, and the best for C-LS was FP (reductions of 45% and 39%, respectively). However, DP and FP showed the best efficiency considering the investment.
3.3. Phytotoxicity Assessment: Yield and Cu Concentration in Lettuces
No amendment produced a significant change in yield and foliar Cu concentration of lettuces grown in C-SS (Figure 3a,c). In contrast, the yields of lettuces grown in C-SS were higher than the reference U-SS. On the other hand, the yield of lettuces grown in C-LS increased by applying DP (average increment of 79% regarding C+). Foliar Cu concentration of lettuces grown in C-LS was statistically higher in the OM treatment (Figure 3d). Other amendments did not generate a statistically significant effect (Figure 3b).
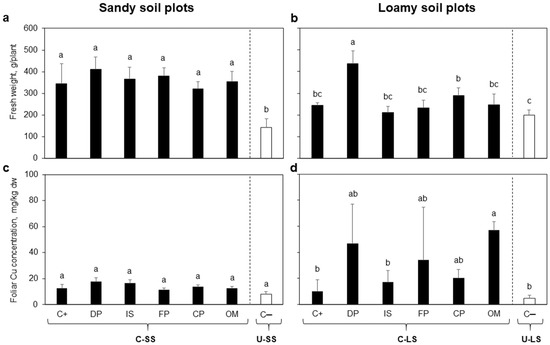
Figure 3.
Fresh weight of lettuces at harvest time and the corresponding copper concentration in their edible tissues (on a dry weight basis): (a,c) are the results of lettuces grown on sandy soils, while (b,d) are those for lettuce grown on loamy soils. Bars are means ± SD (n = 5). Different letters above the means indicate significant differences between treatments based on Tukey multiple comparison test (p ≤ 0.05). Black bars represent contaminated soil under different treatments: C+ = Contaminated soil without amendment; DP = diammonium phosphate; FP = ferrous phosphate; IS = iron sulfate; OM = organic matter addition; CP= calcium peroxide. White bar represents uncontaminated soil: C− = Uncontaminated soil without amendment. C-SS = Contaminated sandy soil plot; U-SS = Uncontaminated sandy soil plot; C-LS = contaminated loamy soil plot; and U-LS = Uncontaminated loamy soil plot.
The relation between yield (measured as fresh weight) and the foliar Cu concentration of lettuces grown in the contaminated plots (C-SS and C-LS) is shown in Figure 4. We also showed the effective concentrations (EC) stated by Verdejo et al. (2015) [43] using similar soils. These effective concentrations are 11, 16, and 21 mg kg−1, which would imply reductions in the lettuces’ shoot length of 10%, 25%, and 50%, respectively.

Figure 4.
Scatterplot between the fresh weight of lettuce and the Cu concentration in leaves, in the sandy soil plots (a), and the loamy soil plots (b). Dashed lines represent the theoretical phytotoxicity thresholds for foliar Cu according to Verdejo et al., 2015 [43]; yellow = EC10, orange = EC25, and red = EC50.
3.4. Soil Parameters after Amendments Application
The total concentrations of As and Cu in the contaminated soils were similar among the treatments except for soil Cu concentration in the C-LS plot (Table S4). Mantel test showed that no significant spatial autocorrelation was found between the total concentrations of As and Cu in the contaminated soils of the samples collected in C-SS and C-LS (p-value > 0.05) (Table S5).
The comparison of pH, electrical conductivity, and soil organic matter content in soils treated with different amendments is shown in Figure S2. Soil pH was only modified by the DP treatment in the C-LS plot (~0.4 units on average) (Figure S2b). On the other hand, electrical conductivity was not affected by any amendment, and only OM treatment in C-LS showed a significantly higher SOM content than the other treatments (Figure S2c–f).
4. Discussion
4.1. Comparison of the Studied Soils before the Application of Amendments
According to previous baselines [8,9], the high concentration of As and Cu found in soils of contaminated plots was as expected. Thus, our results confirm that soil contamination is still latent despite the environmental improvements in ore processing taken more than 20 years ago [7]. The higher SOM and available contents of N and P in C-SS than in U-SS are attributed to the farmer’s constant addition of manure in the soil. However, most of these differences were agronomically negligible as they do not suppose a limitation or excess [44]. The only exception was the potential nitrogen deficit in U-SS, U-LS, and C-LS plots. Consequently, they were fertilized with urea to reach up to 20 mg kg−1 of available nitrogen, the minimal adequate concentration for Chilean agricultural soils [45]. Thus, we obtained four plots representing two areas and two conditions (contaminated and uncontaminated).
Differences between C-LS and C-SS showed that they not only differ in soil texture but also in the nutrient content and the trace element concentration. These differences condition plant responses. Therefore, results from both cases should be understood as contrasting cases.
4.2. Effect of the Amendments on as Concentration in Lettuces and the Implications on Food Safety
Figure 2 showed significant differences in the As accumulation in lettuces among the treatments. These differences are attributed to the effect of the amendments, considering that we discarded a potential effect of the spatial pattern on soil conditions (Table S5). Phosphate amendments (DP and FP) decreased As concentration in the edible tissues of lettuce. These amendments were effective in both studied areas, even with different soil pH. This result disagrees with Wang et al. [46], who found that phosphate addition reduced As accumulation in lettuces grown in artificially spiked soils (with Na2HAsO4 solution) only in soils with a high pH. Our results also contrast with Cao and Ma [47], who studied the As accumulation in lettuce grown in field contaminated soils (with chromate copper arsenate) after adding phosphate. Cao and Ma [45] found that adding phosphate to soils increased As accumulation in lettuces by 2.4–10 due to increased soil water-soluble As. Differences between our results and Cao and Ma can be attributed to P concentration. Specifically, high P concentration in soil solution can facilitate the As translocation from root to shoot [48]. However, in the study of Cao and Ma [47], P addition was extremely high (about 4 g of P per kg of soil), which is at least 40-fold the dose of P we added.
FP was the unique amendment decreasing foliar As concentrations to a similar level of C- in both studied areas (Figure 2). Nevertheless, we could not find previous reports in the literature with the use of FP as a soil amendment to remediate As in agricultural soils (using WoS database). We only found iron phosphate (FePO4), but it was ineffective in reducing As availability [49]. Fe(II) (present in FePO4) does not act as a reactant but rather as a catalyst which is supported by the studies of Roberts et al. [50] and Hug and Leupin [51]. Specifically, they found that Fe(II) but nto Fe(III) catalyzed oxidation of As(III) to As (V) in the presence of oxygen. This reaction could explain our findings. However, those studies were done in water systems, and hence, further research should be performed to assess the mechanism in field experiments in agricultural soils.
Another promising amendment was CP (Figure 2). CP can form insoluble As-Ca complexes [52] and release H2O2 which favors the stabilization of As(V) instead of As(III) [53]. However, why did CP decrease As concentrations in lettuces grown on C-SS but not in C-LS? We propose three possible explanations to this finding: (1) C-SS has lower exchangeable Ca than the C-LS plot (Table 1). Hence, the addition of Ca would have a higher effect where Ca was less available. (2) C-SS plot had a lower soil pH than C-LS (Table 1), and soluble As(V) reaches a minimum around pH 6 at high concentration of major bivalent cations (e.g., Ca2+) [54], and (3) C-SS plot was constantly amended with manure, which constitutes a bulk of soil reduction capacity. Thus, the effect of H2O2 stabilizing As(V) would be higher in C-SS than in C-LS ([55], and references therein).
The IS and OM treatments were insufficient to decrease the As concentration in lettuces. IS has been widely studied as soil amendments for decreasing As availability in soils, even in a wide range of soil pH [20]. However, most studies are performed in laboratory conditions and/or with very high doses (≥1% w/w) with low feasibility for being used in field conditions ([20], and references therein). On the other hand, OM was ineffective in decreasing As accumulation in lettuces. In contrast, it seemed to increase it in the C-LS, which can be associated to dissolved organic matter mobilizing As from all solid phases [56]. However, this effect was not significantly higher than C+. The inefficacy of OM is a relevant result for proper land management. In previous research, we recommended the application of compost in soils from the sandy soil area to promote revegetation [31]. However, this management would not be helpful in farmlands.
HQ values of Table S3 imply a critical threat considering that people are also exposed to other food (e.g., list in [57]) and other multiple exposition sources (e.g., [58]). Lizardi et al. [12] stated that HQ value surpasses 1.0 for inorganic As considering the uptake of vegetable consumption and other sources (considered in [59]) for the inhabitants of between 1–5 years old from the Puchuncaví valley (where C-SS plot is located). As shown by HQ analysis, amendments improved the food security of lettuces grown in contaminated soils. We found that CP and FP were the best amendments in the sandy and loamy soils, respectively. Although, if we added the economic criteria, DP and FP would be the best alternative for contaminated agricultural areas of central Chile, while CP would be less affordable.
The present research remarks on the importance of studying multiple amendments in field conditions. It is necessary to consider that soils contaminated with As (V) are not only in Mediterranean areas but also in mild and semiarid areas. Thus, we encourage more field studies to test the efficacy of soil amendment in other regions, especially using FP. FP was remarkable as it showed significant efficacy and economic efficiency to manage As agricultural contaminated soils. However, this compound had never been studied before as a soil amendment.
4.3. Effect of the Amendments on Yield. Is There Phytotoxicity in Field Conditions?
Cu is an essential trace element for plant growth, but it can lead to phytotoxicity symptoms in plants grown of soils from the studied area [15]. However, the yield of lettuces was not affected by Cu contamination (Figure 3). Even, we found a higher fresh weight of lettuces grown in C-SS than in U-SS, although the highest macronutrient availability explains this in C-SS soils (Table 1).
The fresh weight and foliar Cu content of lettuces grown in loamy soil plots showed high heterogeneity. The highest average of foliar Cu in lettuces grown on C-LS was found in OM treatment, which is related to the increment of dissolved organic complexes with Cu [60]. However, the lowest yields were not the lettuces with the highest foliar Cu concentration. Surprisingly, these variables were positively correlated in both areas (Figure 4), showing no phytotoxicity symptoms. These results strongly contrast with those of Verdejo et al. [43], which evaluate lettuce performance on similar soils to those of the present study but in a growth chamber. Verdejo et al. [43] found a substantial decrease of biomass at higher foliar Cu concentrations, stating that lettuces lower a 50% shoot length with foliar Cu concentrations of 21 mg kg−1 dw. Nevertheless, our field-conditions results showed that lettuces’ growth did not diminish by the foliar Cu concentration. We even obtained a higher foliar Cu concentration than 21 mg kg−1 without changes in the biomass.
According to Kalra [38] and the reviewed references, a foliar Cu concentration of 25 mg kg−1 dw can be considered adequate. However, Broadley et al. [61] stated that for most crop species, the critical toxicity level of Cu in the leaves is above 20 to 30 mg kg−1 dw. These inconsistent statements are explained by the roots being affected before the aboveground plant tissues [62]. Therefore, the correlation seen in Figure 4 could be seen in the contrary sense, i.e., the higher plant growth allows more Cu accumulation in leaves. If this is the case, phytotoxicity thresholds of Cu stated in Verdejo et al. [43] for lettuce could be misleading. The concentration of Cu in leaves would not be a promising biomarker in field conditions. Consequently, it can over-alarm a situation with no food safety or phytotoxicity risks. This issue should be analyzed in further studies, remarking that a correlation does not imply causation.
The application of DP in the loamy soils was the only treatment that increased yield compared to the C+ treatment. High As concentration in plants trigger the replacement of phosphate in biomolecules like adenosine triphosphate (ATP), with negative impacts on the growth and metabolism of plants [63]. Therefore, the added phosphate in the DP treatment would mitigate the inhibitory effect of As. To assess the latter, we measured the foliar concentration of P in lettuces. DP treatment increased foliar P concentrations in C-LS (Figure S3). The results of Figure S4 also support this hypothesis. We found a negative correlation between fresh weight and foliar As concentrations in C-LS at the margin of statistical significance (p = 0.06), and significant positive relations between fresh weight and foliar P concentrations (p = 0.002). This effect would be seen only in the C-LS plot since it has a higher As concentration and lower available P in soils than the C-SS plot (Table 1). Also, the effect of the amendment on yield would be significant only in DP treatment due to the higher contribution of P than FP at the same rate of 0.05% w/w (P content of 23.4% and 14.1%, respectively).
4.4. Side-Effects of the Amendments Application
DP treatment acidified the C-LS, which is attributed to the high incorporation of ammonium in soils [64]. This side effect can be critical considering DP showed promising efficacy in reducing As accumulation and increasing the fresh weight of lettuces. For example, the effect on soil pH could modify the interaction between arsenic and other soil components [54]. Moreover, DP application can lead to environmental impacts associated with ammonium overfertilization and its volatilization capacity [65]. Although only slight changes were found in soils, long-term experiments are necessary to assess their persistence and discern if repeated applications are needed.
4.5. Towards a Holistic Remediation Approach
The present study is one of the first attempts to evaluate amendments to remediate contaminated soils in critical agricultural areas coexisting with the mining industry in central Chile. We obtained promising results, but these are not enough to fulfill a holistic remediation approach as is required nowadays by research programs and legislation worldwide [66,67]. A holistic remediation approach must consider the potential human health risks, the interlinks between contaminant exposure, the remediation technology, and the ecological effects, including climate change. Similarly, it must consider the soil-food-environment-health nexus to maintain soil health [68] and the environmental externalities associated with the production and distribution of the different amendment alternatives. Pending assessments are the potential leaching of contaminants, the side effects on soil functioning [69], and the potential CO2 sequestration after amendment application [70]. Thus, we expect our research to promote interdisciplinary efforts (laboratory and field studies) to remediate contaminated soils with a holistic approach.
5. Conclusions
The phosphate amendments (diammonium phosphate and ferrous phosphate) decreased the foliar As concentration in edible tissues of lettuce grown in contaminated soils in two contrasting areas (sandy and loamy soils). Calcium peroxide only decreased it in the sandy soils. Accordingly, a diet based on the consumption of lettuces grown in amended soils would decrease about 30% of the As intake than the unamended ones.
The use of diammonium phosphate involved good efficacy on decreased As accumulation in lettuce, increased productivity, and low investment cost. However, caution should be taken with this amendment due to soil acidification. In contrast, ferrous phosphate showed good efficacy with apparently no side effects, and the investment would be just a bit higher. We determined that ferrous phosphate is a promising compound that has never been studied before as a soil amendment.
We did not find Cu phytotoxicity despite the high concentrations of Cu in soils and lettuce leaves. This result differed from similar research developed in a growth chamber. Thus, we conclude that soil amendments would allow food security and productivity in agricultural soils with long-term contamination of arsenic and copper. The present study is one of the first studies aiming to improve food security in central Chile, an important agricultural area. It tried to unravel food safety and agricultural productivity uncertainties while opening scientific questions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12010221/s1, Table S1: Chemical characterization of compost, Table S2: Soil contamination factors (Cf) of the experimental plots before starting the experiment (n = 3), Table S3: Average amendment performance in terms of food safety and investment (n = 5), Table S4: Average concentrations ± SD of As and Cu in soils surrounding roots of the sampled lettuces (n = 5), Table S5: Mantel test applied to soil As and Cu concentrations in function of the spatial distance of the contaminated plots, Equation (S1): Calculation of concentration factor, Figure S1: Experimental plots, Figure S2: Chemical parameters of soils surrounding roots of lettuces, Figure S3: Phosphorus concentration in the edible tissues of lettuces (on a dry weight basis) grown on (a) sandy soils and (b) loamy soils, Figure S4: Scatterplot between the fresh weight of lettuces and the As and P concentration in their leaves.
Author Contributions
Conceptualization, P.M., W.Q., M.V. and J.L.C.-D.; methodology, P.M., W.Q. and J.L.C.-D.; investigation, P.M., P.V. and N.R.; resources, W.Q. and J.L.C.-D.; writing—original draft preparation, P.M.; writing—review and editing, J.L.C.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by ANID (Chile) FONDEF ID17AL0056. P.M. was funded by the ANID doctoral scholarship grant 21201569. P.V. was funded by the ANID postdoctoral scholarship grant 74200084. W.Q. was funded by FONDECYT 1191041. J.L.C.-D. was funded by ANID/PIA/ACT192027, and PUCV 039.431/2020 “Núcleo de Soluciones de Base Natural”. J.L.C.-D. is an Associated Researcher of the Instituto de Ecología y Biodiversidad (IEB-Chile) funded by ANID ACE210006. Funding sources had no involvement in study design, in the collection, analysis and interpretation of data, in the report’s writing, or in the decision to submit the article for publication.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
P.M. wants to acknowledge Christopher Ulriksen for his technical support in fieldwork and Javiera Díaz and Rodrigo Miranda for their support in laboratory analyses. We thank Alexander Neaman for funding acquisition and discussion of experimental design.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- Rodríguez-Eugenio, N.; McLaughlin, M.; Pennock, D. Soil Pollution: A Hidden Reality; FAO: Rome, Italy, 2018; p. 142. [Google Scholar]
- Hite, A.H. Arsenic and rice: A call for regulation. Nutrition 2013, 29, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Kumpiene, J.; Antelo, J.; Brännvall, E.; Carabante, I.; Ek, K.; Komárek, M.; Söderberg, C.; Wårell, L. In situ chemical stabilization of trace element-contaminated soil—Field demonstrations and barriers to transition from laboratory to the field—A review. J. Appl. Geochem. 2019, 100, 335–351. [Google Scholar] [CrossRef]
- Vargas, C.; Quiroz, W.; Bravo, M.; Neaman, A. Stability of arsenic during soil treatment and storage. J. Chil. Chem. Soc. 2015, 60, 3045–3048. [Google Scholar] [CrossRef] [Green Version]
- Comisión Chilena del Cobre (COCHILCO). Yearbook: Copper and other Mineral Statistics 2000–2019; COCHILCO: Santiago, Chile, 2020. [Google Scholar]
- Berasaluce, M.; Díaz-Siefer, P.; Rodríguez-Díaz, P.; Mena-Carrasco, M.; Ibarra, J.T.; Celis-Diez, J.L.; Mondaca, P. Social-Environmental Conflicts in Chile: Is There Any Potential for an Ecological Constitution? Sustainability 2021, 13, 12701. [Google Scholar] [CrossRef]
- Folchi, M. Las grandes fundiciones y la contaminación atmosférica: Chagres y Ventanas, 1959–2006. In Historia Ambiental de las Labores de Beneficio en la Minería del Cobre en Chile, Siglox XIX y XX; Folchi, M., Ed.; Barcelona: Catalonia, Spain, 2006. [Google Scholar]
- Aguilar, R.; Hormazábal, C.; Gaete, H.; Neaman, A. Spatial distribution of copper, organic matter and pH in agricultural soils affected by mining activities. J. Soil Sci. Plant Nutr. 2011, 11, 125–145. [Google Scholar]
- PGS. Muestreo de Suelos Para las Comunas de Quintero y Puchuncaví, Región de Valparaíso; PGS: Santiago, Chile, 2015. [Google Scholar]
- Aguilar, M.; Mondaca, P.; Ginocchio, R.; Vidal, K.; Sauve, S.; Neaman, A. Comparison of exposure to trace elements through vegetable consumption between a mining area and an agricultural area in central Chile. Environ. Sci. Pollut. Res. 2018, 25, 19114–19121. [Google Scholar] [CrossRef]
- Alekseev, I.; Neaman, A.; Lizardi, N.; Mondaca, P.; Aguilar, M. Assessment of potential health risk due to consumption of vegetables grown near a copper smelter in central Chile. Taurida Her. Agrar. Sci. 2018, 2, 9–14. [Google Scholar] [CrossRef]
- Lizardi, N.; Aguilar, M.; Bravo, M.; Fedorova, T.A.; Neaman, A. Human health risk assessment from the consumption of vegetables grown near a copper smelter in central Chile. J. Soil Sci. Plant Nutr. 2020, 20, 1472–1479. [Google Scholar] [CrossRef]
- Manjon, I.; Ramirez-Andreotta, M. A dietary assessment tool to estimate arsenic and cadmium exposures from locally grown foods. Environ. Geochem. Health 2020, 42, 2121–2135. [Google Scholar] [CrossRef]
- Küpper, H.; Andresen, E. Mechanisms of metal toxicity in plants. Metallomics 2016, 8, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Mondaca, P.; Catrin, J.; Verdejo, J.; Sauvé, S.; Neaman, A. Advances on the determination of thresholds of Cu phytotoxicity in field-contaminated soils in central Chile. Environ. Pollut. 2017, 223, 146–152. [Google Scholar] [CrossRef]
- Adriano, D.C. (Ed.) Arsenic. In Trace Elements in Terrestrial Environments: Biogeochemistry, Bioavailability, and Risks of Metals, 2nd ed.; Springer: New York, NY, USA, 2001; pp. 219–261. [Google Scholar]
- Wang, J.; Zhao, F.J.; Meharg, A.A.; Raab, A.; Feldmann, J.; McGrath, S.P. Mechanisms of arsenic hyperaccumulation in Pteris vittata. Uptake kinetics, interactions with phosphate, and arsenic speciation. Plant Physiol. 2002, 130, 1552–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karczewska, A.; Lewinska, K.; Galka, B. Arsenic extractability and uptake by velvetgrass Holcus lanatus and ryegrass Lolium perenne in variously treated soils polluted by tailing spills. J. Hazard. Mater. 2013, 262, 1014–1021. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.; Esteban, E.; Penalosa, J.M. The fate of arsenic in soil-plant systems. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2012; Volume 215, pp. 1–37. [Google Scholar]
- Komárek, M.; Vaněk, A.; Ettler, V. Chemical stabilization of metals and arsenic in contaminated soils using oxides—A review. Environ. Pollut. 2013, 172, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Baragaño, D.; Gallego, J.L.R.; Baleriola, G.; Forján, R. Effects of different in situ remediation strategies for an As-polluted soil on human health risk, soil properties, and vegetation. Agronomy 2020, 10, 759. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.B.; Kwon, E.E.; Baek, K. Mitigating translocation of arsenic from rice field to soil pore solution by manipulating the redox conditions. Sci. Total Environ. 2021, 762, 143124. [Google Scholar] [CrossRef] [PubMed]
- Arco-Lazaro, E.; Agudo, I.; Clemente, R.; Bernal, M.P. Arsenic(V) adsorption-desorption in agricultural and mine soils: Effects of organic matter addition and phosphate competition. Environ. Pollut. 2016, 216, 71–79. [Google Scholar] [CrossRef]
- Mehmood, T.; Bibi, I.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Wang, H.; Ok, Y.S.; Sarkar, B.; Javed, M.T.; Murtaza, G. Effect of compost addition on arsenic uptake, morphological and physiological attributes of maize plants grown in contrasting soils. J. Geochem. Explor. 2017, 178, 83–91. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M.; Chen, T. Review on remediation technologies for arsenic-contaminated soil. Front. Environ. Sci. Eng. 2020, 14, 24. [Google Scholar] [CrossRef]
- Servicio Agrícola y Ganadero (SAG). Programa de Recuperación de Suelos Degradados. Available online: http://www.sag.cl/ambitos-de-accion/programa-de-recuperacion-de-suelos-degradados (accessed on 16 July 2021).
- Abbasi, S.; Lamb, D.T.; Kader, M.; Naidu, R.; Megharaj, M. The influence of long-term ageing on arsenic ecotoxicity in soil. J. Hazard. Mater. 2021, 407, 124819–124825. [Google Scholar] [CrossRef] [PubMed]
- Santibáñez, F.; Santibáñez, P.; Caroca, C.; González, P. Atlas Agroclimático de Chile. Estado Actual y Tendencias del Clima. Tomo III: Regiones de Valparaíso, Metropolitana, O’Higgins y Maule; Universidad de Chile: Santiago, Chile, 2017. [Google Scholar]
- Parra, S.; Bravo, M.A.; Quiroz, W.; Moreno, T.; Karanasiou, A.; Font, O.; Vidal, V.; Cereceda, F. Distribution of trace elements in particle size fractions for contaminated soils by a copper smelting from different zones of the Puchuncaví Valley (Chile). Chemosphere 2014, 111, 513–521. [Google Scholar] [CrossRef] [PubMed]
- US EPA. The Use of Soil Amendments for Remediation, Revitalization, and Reuse; Environmental Protection Agency/National Service Center for Environmental Publications: Cincinnati, OH, USA, 2007; p. 52.
- Pardo, J.; Mondaca, P.; Celis-Diez, J.L.; Ginocchio, R.; Navarro-Villarroel, C.; Neaman, A. Assessment of revegetation of an acidic metal(loid)-polluted soils six years after the incorporation of lime with and without compost. Geoderma 2018, 331, 81–86. [Google Scholar] [CrossRef]
- Rosado, R.; Del Campillo, M.C.; Barrón, V.; Torrent, J. Inyección de vivianita al suelo para corregir la clorosis férrica en olivo. Edafología 2000, 7, 57–66. [Google Scholar]
- Steubing, L. Problems of bioindication and the necessity of standardization. In Monitoring Air Pollutants by Plants; Steubing, L., Jäger, H., Eds.; Dr. Junk Publishers: Amsterdam, The Netherlands, 1982; pp. 19–27. [Google Scholar]
- Sadzawka, A.; Flores, H.; Grez, R.; Carrasco, M.; Mora, M.; Neaman, A.; Demanet, R. Métodos de Análisis de Lodos y Suelos; Instituto de Investigaciones Agropecuarias: Santiago, Chile, 2007. [Google Scholar]
- Houba, V.J.G.; Temminghoff, E.J.M.; Gaikhorst, G.A.; van Vark, W. Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun. Soil Sci. Plant Anal. 2000, 31, 1299–1396. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnson, C.T.; Sumner, M.E. Methods of Soil Analysis. Part III: Chemical Methods; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996. [Google Scholar]
- US EPA. Method 6200: Field Portable X-ray Fluorescence Spectrometry for the Determination of Elemental Concentrations in Soil and Sediment; US EPA: Washington, DC, USA, 2007. [Google Scholar]
- Kalra, Y. Handbook of Reference Methods for Plant Analysis; CRC Press: Boca Raton, FL, USA, 1997; p. 320. [Google Scholar]
- Maxwell, J.A. Rock and Mineral Analysis; Interscience Publishers: New York, NY, USA, 1968. [Google Scholar]
- Verlinden, M. On the acid decomposition of human blood and plasma for the determination of selenium. Talanta 1982, 29, 875–882. [Google Scholar] [CrossRef]
- US EPA. Risk Assessment Guidance for Superfund. Human Health Evaluation Manual (Part A); US EPA: Washington, DC, USA, 1989; Volume 1. [Google Scholar]
- US EPA. Arsenic, Inorganic; CASRN 7440-38-2; US EPA: Washington, DC, USA, 1991. [Google Scholar]
- Verdejo, J.; Ginocchio, R.; Sauvé, S.; Mondaca, P.; Neaman, A. Thresholds of copper toxicity to lettuce in field-collected agricultural soils exposed to copper mining activities in Chile. J. Soil Sci. Plant Nutr. 2015, 15, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Osman, K.T. Management of Soil Problems; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- SOQUIMICH. Agenda del Salitre; SOQUIMICH Comercial: Santiago, Chile, 2001. [Google Scholar]
- Wang, J.; Zeng, X.; Zhang, H.; Li, Y.; Zhao, S.; Su, S.; Bai, L.; Wang, Y.; Zhang, T. Effect of exogenous phosphate on the lability and phytoavailability of arsenic in soils. Chemosphere 2018, 196, 540–547. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.D.; Ma, L.Q. Effects of compost and phosphate on plant arsenic accumulation from soils near pressure-treated wood. Environ. Pollut. 2004, 132, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Mahimairaja, S.; Kunhikrishnan, A.; Choppala, G. Phosphorus-arsenic interactions in variable-charge soils in relation to arsenic mobility and bioavailability. Sci. Total Environ. 2013, 463–464, 1154–1162. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Liu, R.L.; Zeng, X.B.; Lin, Q.M.; Bai, L.Y.; Li, L.F.; Su, S.M.; Wang, Y.N. Reduction of arsenic bioavailability by amending seven inorganic materials in arsenic contaminated soil. J. Integr. Agric. 2015, 14, 1414–1422. [Google Scholar] [CrossRef]
- Roberts, L.C.; Hug, S.J.; Ruettimann, T.; Billah, M.; Khan, A.W.; Rahman, M.T. Arsenic removal with iron(II) and iron(III) in waters with high silicate and phosphate concentrations. Environ. Sci. Technol. 2004, 38, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Hug, S.J.; Leupin, O. Iron-catalyzed oxidation of arsenic(III) by oxygen and by hydrogen peroxide: pH-dependent formation of oxidants in the Fenton reaction. Environ. Sci. Technol. 2003, 37, 2734–2742. [Google Scholar] [CrossRef]
- Moon, D.H.; Dermatas, D.; Menounou, N. Arsenic immobilization by calcium-arsenic precipitates in lime treated soils. Sci. Total Environ. 2004, 330, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.L.; Jean, J.S.; Yang, C.M.; Hseu, Z.Y.; Chen, Y.H.; Wang, H.L.; Das, S.; Chou, L.S. Inhibition of ethylenediaminetetraacetic acid ferric sodium salt (EDTA-Fe) and calcium peroxide (CaO2) on arsenic uptake by vegetables in arsenic-rich agricultural soil. J. Geochem. Explor. 2016, 163, 19–27. [Google Scholar] [CrossRef]
- Deng, Y.; Li, Y.; Li, X.; Sun, Y.; Ma, J.; Lei, M.; Weng, L. Influence of calcium and phosphate on pH dependency of arsenite and arsenate adsorption to goethite. Chemosphere 2018, 199, 617–624. [Google Scholar] [CrossRef]
- Husson, O. Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: A transdisciplinary overview pointing to integrative opportunities for agronomy. Plant Soil 2012, 362, 389–417. [Google Scholar] [CrossRef] [Green Version]
- Bauer, M.; Blodau, C. Mobilization of arsenic by dissolved organic matter from iron oxides, soils and sediments. Sci. Total Environ. 2006, 354, 179–190. [Google Scholar] [CrossRef]
- EFSA. Dietary exposure to inorganic arsenic in the European population. EFSA J. 2014, 12, 3597–3664. [Google Scholar] [CrossRef]
- Bacigalupo, C.; Hale, B. Human health risks of Pb and As exposure via consumption of home garden vegetables and incidental soil and dust ingestion: A probabilistic screening tool. Sci. Total Environ. 2012, 423, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Berasaluce, M.; Mondaca, P.; Schuhmacher, M.; Bravo, M.; Sauvé, S.; Navarro-Villarroel, C.; Dovletyarova, E.A.; Neaman, A. Soil and indoor dust as environmental media of human exposure to As, Cd, Cu, and Pb near a copper smelter in central Chile. J. Trace Elem. Med. Biol. 2019, 54, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Mondaca, P.; Neaman, A.; Sauvé, S.; Salgado, E.; Bravo, M. Solubility, partitioning, and activity of copper-contaminated soils in a semiarid region. J. Soil Sci. Plant Nutr. 2015, 178, 452–459. [Google Scholar] [CrossRef]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of nutrients: Micronutrients. In Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Gautam, S.; Anjani, K.; Srivastava, N. In vitro evaluation of excess copper affecting seedlings and their biochemical characteristics in Carthamus tinctorius L. (variety PBNS-12). Physiol. Mol. Biol. Plants 2016, 22, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Punshon, T.; Jackson, B.P.; Meharg, A.A.; Warczack, T.; Scheckel, K.; Guerinot, M.L. Understanding arsenic dynamics in agronomic systems to predict and prevent uptake by crop plants. Sci. Total Environ. 2017, 581–582, 209–220. [Google Scholar] [CrossRef]
- Wallace, A. Soil acidification from use of too much fertilizer. Commun. Soil Sci. Plant Anal. 2008, 25, 87–92. [Google Scholar] [CrossRef]
- Behera, S.N.; Sharma, M.; Aneja, V.P.; Balasubramanian, R. Ammonia in the atmosphere: A review on emission sources, atmospheric chemistry and deposition on terrestrial bodies. Environ. Sci. Pollut. Res. 2013, 20, 8092–8131. [Google Scholar] [CrossRef]
- Moreira, H.; Pereira, S.I.A.; Mench, M.; Garbisu, C.; Kidd, P.; Castro, P.M.L. Phytomanagement of metal(loid)-contaminated soils: Options, efficiency and value. Front. Environ. Sci. 2021, 9, 1–48. [Google Scholar] [CrossRef]
- Thakare, M.; Sarma, H.; Datar, S.; Roy, A.; Pawar, P.; Gupta, K.; Pandit, S.; Prasad, R. Understanding the holistic approach to plant-microbe remediation technologies for removing heavy metals and radionuclides from soil. Curr. Biotechnol. 2021, 3, 84–98. [Google Scholar] [CrossRef]
- Gu, B.; Chen, D.; Yang, Y.; Vitousek, P.; Zhu, Y.G. Soil-food-environment-health nexus for sustainable development. Research 2021, 2021, 9804807. [Google Scholar] [CrossRef] [PubMed]
- Giagnoni, L.; dos Anjos Borges, L.G.; Giongo, A.; de Oliveira Silveira, A.; Ardissone, A.N.; Triplett, E.W.; Mench, M.; Renella, G. Dolomite and compost amendments enhance Cu phytostabilization and Increase microbiota of the leachates from a Cu-contaminated soil. Agronomy 2020, 10, 719. [Google Scholar] [CrossRef]
- Beerling, D.J.; Kantzas, E.P.; Lomas, M.R.; Wade, P.; Eufrasio, R.M.; Renforth, P.; Sarkar, B.; Andrews, M.G.; James, R.H.; Pearce, C.R.; et al. Potential for large-scale CO2 removal via enhanced rock weathering with croplands. Nature 2020, 583, 242–248. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).