Abstract
Desert truffles are edible hypogeous (forming fruit bodies below ground) fungi that grow in semi-arid and arid areas. They are highly valued for both their culinary and medicinal properties in the Mediterranean basin, the Middle East and the Gulf areas. Desert truffles form mycorrhizae mostly with plants belonging to the Cistaceae family, mainly with Helianthemum species. These truffles are still, usually, collected from the wild, but loss of habitats due to urbanization, desertification, intensive agriculture and global warming, along with an urgent need to develop new crops adapted to arid conditions, are currently hastening efforts towards their domestication. Here, we sum up the successful research leading to cultivation of this crop, based on plots that were established in sandy to silt soils with high pH values and low mineral contents. We report suitable methods for production of mycorrhized seedlings and preferred planting methods. We found that under natural conditions yields are affected by water availability, so irrigation regimes to ensure good yields were sought. Although good yields were indeed obtained in some years, fluctuations in yields over the years were significant; the reasons for this are not entirely clear and are currently under study. This crop is particularly well suited to relatively marginal conditions but prospects for establishment of desert truffles as a niche crop for arid and semi-arid areas depend on further improvements in yields.
1. Introduction
The term ‘desert truffles’ is used to describe edible hypogeous fungi growing in arid areas around the world. Although different from European forest truffles, they are true truffles, namely, Ascomycete underground fungi—as opposed to Basidiomycete underground fungi, termed false truffles []. In the Mediterranean basin, the Middle East and the Gulf areas, desert truffles are well known and highly valued for both their culinary and medicinal properties [,,]. In nutritional terms, the dry matter (about 20% by weight) consists of 20–27% protein, some 85% of which is digestible by humans; 3–7.5% fat, including unsaturated and saturated fatty acids; 7–13% crude fiber; about 60% carbohydrates; and appreciable amounts (2–5%) of ascorbic acid [,]. High levels of potassium and phosphate and fair amounts of iron have been reported []. Medicinally, it has been shown that the belief of Saudi desert dwellers that truffle extract is effective against the eye disease, trachoma, does indeed have a scientific basis []. In addition, evidence of broad antibiotic activity and antioxidant properties has been reported for truffle extracts [,,,,]. Anticancer and immunomodulatory activities were also lately reported for Terfezia boudieri Chatin [] and Terfezia claveryi Chatin [].
Desert truffles were traditionally classified as members of the Terfeziaceae family within the order Tuberales. However, molecular phylogenetic studies have demonstrated that certain hypogeous fungi show a greater similarity to epigeous members of the Pezizales order than to other hypogeous species [,,]. Thus, the 38 genera of the Ascomycete hypogeous fungi (i.e., true truffles) are now classified into six Pezizales families []. The best-known genera of desert truffles, Terfezia and Tirmania, have indeed been shown to be members of the Pezizaceae family [,]
Our current knowledge indicates that underground members of the Pezizaceae are mycorrhizal, forming mostly a distinct type of mycorrhiza known as ectendomycorrhiza (EEM). EEMs are characterized by the co-occurrence of an intercellular Hartig net, intracellular hyphae penetrating the hosts’ cortex cells, where they form coil-like structures, and—sometimes—a thin and disordered fungal mantle surrounding the colonized roots [,,,,,,]. Whether mycorrhizal structures are intercellular or intracellular depends on several factors: in vitro conditions, i.e., high auxin, high phosphate, and/or high-water content favor the intercellular mycorrhizal type, whereas field conditions, i.e., low auxin, low phosphate and/or low water availability favor the intracellular mycorrhizal type [,,,,,]. Species of Terfezia and Tirmania form mycorrhizas mainly with members of the Cistaceae family, such as different species of the genus Helianthemum e.g., [,,,], but they may also form mycorrhizas with other symbionts.
Desert truffles mostly inhabit sandy soils [], with the best-known species of the genera Terfezia and Tirmania developing in high pH calcareous soils [,], but several other species are found in soils with acid pH values [,].
Desert truffles require relatively little water [,]. Although many climatic factors have recently been shown to be involved in determining desert truffle yields [], the main parameters determining yields in the wild are rainfall amount and distribution during the rainy season. As little as 200–250 mm per season may produce a good yield []. The fruit bodies—truffles—appear in the rainy season, which normally runs from January/February to April/May, depending on the area.
To this day, desert truffles are mainly collected from the wild by desert dwellers. Nonetheless, efforts to domesticate them as a niche crop—not a trivial task—are being met with a certain degree of success. For decades, the cultivation of desert truffles has not been given priority in agricultural R&D, but global warming and the attendant urgent need to develop new crops adapted to arid conditions are currently hastening domestication efforts. The demand for novel cuisine products is also contributing to the growing interest in desert truffles. The first report of a successful desert truffle plantation came from Spain where the first Terfezia claveryi Chatin fruit bodies were collected in symbiosis with Helianthemum almeriense Pau in 2001 []. Since that time, cultivation efforts have been undertaken by three groups of researchers—one in Spain (mainly T. claveryi) [,,,], one in Tunisia (T. boudieri) [], and one in Israel (T. boudieri) as described here.
In this article, we review practices for desert truffle cultivation of two species of Terfezia, T. claveryi and T. boudieri, mycorrhizing two species of Helianthemum, H. almeriense and Helianthemum sessiliflorum (Desf.) Pers, respectively, under different soil and climatic conditions, with both symbiotic pairs being suitable for cultivation under arid conditions. The rationale underlying the review is to report successful practices adopted by our two groups (Spanish and Israeli), based on our latest findings, and to provide recommendations for establishing desert truffle plots, with the aim to expand farmers’ options for crops suitable for arid and semi-arid conditions.
2. Plot Selection and Preparation
Plot sites were selected close to naturally producing areas on the basis of initial data collected concerning weather and soil (Table 1). True to the nature of deserts, the conditions in these areas were characterized by high summer temperatures and low annual rainfall. Soils were sandy in Israel and rather silty and clayey loam in Spain (Table 1). However, as reported by Bonifacio and Morte [], both soil types exhibited high pH values and low mineral contents (Table 1).

Table 1.
Weather, main soil attributes and initial mineral contents in soils of successful truffle plots.
Plot Preparation
Among the approaches that were tried for plot design, Honrubia et al. [] concluded that the approach giving the optimal truffle yield is a block design with 12 plants per block, 1 m between plants (to form a root community conducive of fructification) and 5 m between blocks. Studies in Israel have provided support for this recommendation. Another successful plantation design consisted of planting seedlings at a spacing of 1.5 × 1.5 m in 4–5 rows forming a block, with 2–3 m separating the blocks []. Nonetheless, other formations enabling the creation of underground root fungal communities may also be effective (Figure 1).

Figure 1.
Productive blocks. (Left) H. sessiliflorum in a 12-plant block. Block A and B with 5 m space between blocks. (Right) H. almeriense, spaced at 1.5 × 1.5 m and arranged alternately within the rows.
Before transferring seedlings to the plots, the soil should be tilled to eliminate weeds and aerate the soil. An irrigation system is essential in areas with erratic rainfall. In Israel, drip irrigation is used, with the best results being obtained with drippers of 1.6 L/h, placed every 50 cm. Sprinkler irrigation may also be used, but extensive weeding then becomes necessary. In Spain, under semi-arid conditions an irrigation system of drippers or sprinklers is also recommended for use in dry years, with irrigation being used only when necessary (see Cultivation Practices section). Fertilization of the plots is not necessary.
3. Preparation of Mycorrhized Seedlings
The easiest and cheapest method would be to sow seeds and spores together directly into pre-prepared blocks. However, this method is highly unreliable, with the major problem being erratic germination of both seeds and spores. Therefore, it is necessary to prepare mycorrhized seedlings in nursery, for planting in the cultivation plots. Several approaches may be adopted, depending on available facilities and funds. Here, we describe only the most cost effective and successful.
3.1. Host Plant Selection and Seed Germination
The first step is selection of the suitable host—a perennial species rather than an annual species. Each desert truffle species forms mycorrhizal associations with several host species (e.g., [,]), but there is usually an abundant host in the area to be cultivated, and this species should initially be the one chosen. In Israel’s Negev desert, for example, the indigenous most common host species for T. boudieri is H. sessiliflorum. In Spain, H. almeriense was initially chosen as the host for T. claveryi, but other species, such as H. violaceum (Cav.) Pau and H. hirtum (L.) Miller, were tried later and found to be successful hosts for the same fungal species at different elevation ranges.
In general, seed scarification (gentle abrasion) is necessary to increase germination rates of all Helianthemum species []. In Israel, H. sessiliflorum seeds are germinated on a low nutrient medium in perforated cones that allow lateral roots to spread out, thereby being readily exposed to spore access, thus promoting mycorrhization. In Spain, bacteria isolated from mycorrhizosphere soil and the peridium of desert truffles are added to the seed-germination soil to enhance the survival of the germinating seedlings [].
3.2. Truffle Selection and Use as Inoculum
Germinated seedlings may be successfully mycorrhized using either mycelia or spores, with the two alternatives having different advantages and disadvantages [,]. The use of spores is cheaper in the long run. In addition, it affords a wider gene pool that includes strains with different mating-type genes. The recent discovery of MAT genes in the genome of heterothallic Terfezia species implies that planting a mixture of genotypes is crucial for the sexual reproduction of desert truffles []. We, therefore, describe only the spore inoculation methods that we found to be cost effective and to provide the best inoculation rates.
Good spore quality is essential, as unripe spores do not germinate well. Thus, samples of fruit bodies from which spores are to be extracted should be examined under a light microscope to evaluate the number of spores and the degree of spore maturation. After examining dozens of ripe fruit bodies over the years, we concluded that the key for successful inoculation by spores is selecting fruit bodies which contain a large number of mature spores.
A huge variability of spore content—as much as 1000-fold—exists among fruit bodies. An example of four differing ones is presented in Figure 2. We chose to present fruit bodies containing mature spores. Spores should be brown in color and have fully developed spikes (T. boudieri) or reticules (T. claveryi).
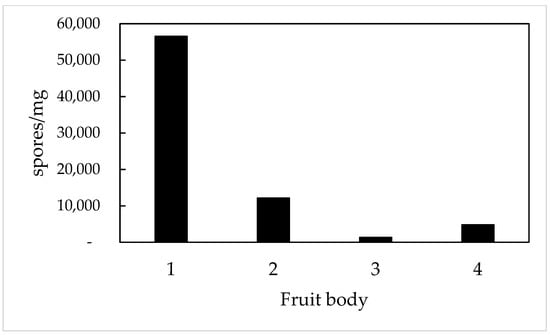
Figure 2.
Variability of spore yields in four different fruit bodies of T. boudieri. Four tissue samples were taken from each fruit body and ground (with pestle and mortar) in water. The extracted spores were counted under a light microscope.
Ripe or overripe fruit bodies should be carefully chosen, dried, and then ground to a fine powder. The fine powder is then rehydrated in a nutrient broth, with stirring overnight, to support development of mycelia from the germinating spores. A study of the effect of the concentration of the nutrient solution and the germination protocol on T. boudieri spore germination revealed the best protocol to be gently stirring the spore solution in Fontana × 3 [] for 12 h, prior to use for inoculation (Figure 3).
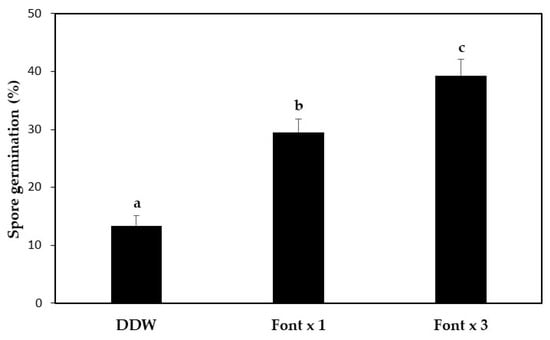
Figure 3.
Spore germination of T. boudieri following 12 h of imbibition in different solutions. Spore germination was determined by counting emerging hyphal tips under a microscope, at the end of the imbibition period. DDW—doubly distilled water; Font × 1—Fontana [] nutrient solution; Font × 3—Fontana solution concentrated × 3. Significant differences were tested by one-way ANOVA. A P value of < 0.05 was considered to be a statistically significant difference. Values are mean ± SE (n = 4 experiments, three samples examined for each treatment), letters denote significant differences.
In Israel, when the lateral roots of the seedlings emerge out of the growth cones (after about 2–3 months), the seedlings are transferred to small pots (~250 mL) and laid on a spore-containing sand layer. The optimal concentration of spores is about 5–10 g/L (~106 spores) [], and each pot receives about 10 mL of the spore mixture. In Spain, the spore solution is usually added to different carriers, where the spores are attached to their surface [,]. The use of this carrier technique facilitates a 40% reduction in the number of spores needed for optimal mycorrhization [].
In general, mycorrhized seedlings are ready for transfer to pre-prepared blocks about 4–5 months after inoculation. Checks of mycorrhiza establishment of a batch should be carried out before transfer. For this purpose, we suggest that 1% of plants (with a minimum of 10 plants) should be examined, and we consider a mycorrhization percentage exceeding 33% as a quantitative indicator of good plant quality [].
4. Cultivation Practices
4.1. Irrigation
In Israel, under the Negev desert conditions, irrigation is started at the beginning of November and is given twice a week for two hours until April (drippers of 1.6 L/h). Thereafter, irrigation is reduced to once a week for the month of April and to once every two weeks for the month of May, after which no irrigation is given until the following November. Light soil raking before the irrigation season starts is recommended. In Spain, in semi-arid areas, irrigation was found to be helpful only if supplied in autumn (September–October) and spring (end of March), with irrigation being provided according to an aridity index (calculated as precipitation divided by evapotranspiration) or to the soil water potential of the plot [].
4.2. Fertilization
To date, no fertilization—organic or inorganic—is supplied to the plots. In Spain, as a means of weed control, sheep are allowed to graze the plot areas. The sheep consume the weeds but do not harm the Helianthemum plants. They also leave behind their droppings, which provide minimal natural organic fertilization. In Israel, the issue of minimal organic fertilization is still under research. Attempts are being made to understand whether there is a need for mineral fertilization of any kind. To this end, mineral concentrations in the soil of the planted blocks in Israel were monitored over the years 2017 (initial) and 2019 (Table 2). As is evident from the table, phosphate levels remained under the detection threshold during the entire measurement period. Nitrite levels went up over the years, but not by much, while ammonia levels went up by six times the initial level. The ammonia level also increased somewhat in the control soil, probably because this soil was also irrigated. Similarly, soluble potassium levels increased with time. It is interesting to note that the levels of NPK were similar between productive blocks and nonproductive ones (Table 2). These results pertaining to nutrient levels in the soil indicate that mineral fertilization is not necessary.

Table 2.
Changes in levels of soil NPK in plots of H. sessiliflorum mycorrhized by T. boudieri.
An examination of Table 2 also indicates that bacterial activity probably affects the N and K contents of the soil, and some plant-beneficial bacteria were indeed isolated from the rhizosphere of mycorrhizal plants in both Spain and Israel [], Sitrit, unpublished results. Among these bacteria, nitrogen-fixing as well as potassium- and phosphorous-solubilizing bacteria were identified. In experiments conducted in Spain, it was found that some of these bacteria had a positive effect on seed germination and survival of H. almeriense plants, with an increase of 40–122% in comparison with the treatment without bacteria. In addition, it was found that auxin-producing bacteria were highly important during the mycorrhization stage in that they facilitated an increase in the root-stem ratio and in colonization percentages (by 47–154%) in comparison with plants not inoculated with bacteria []. In particular, a strain of Pseudomonas mandelii considerably increased mycorrhizal colonization, but not plant growth, and may therefore be considered as mycorrhiza-helper-bacteria (MHB) []. These results indicate that the effects of plant-beneficial bacteria on the growth of mycorrhized plants and on truffle fruiting should be studied further.
In Israel, a strain of Bacillus spp, a known plant growth promoting bacterium, was isolated from the rhizosphere of mycorrhized H. sessiliflorum growing in the Negev dunes (Sitrit unpublished). Some Bacillus species are known to fix atmospheric nitrogen []. A greenhouse pot experiment was conducted, to see whether improved nitrogen supply is the cause for the bacterium beneficial effect. Bacteria were added to the rhizosphere of Helianthemum ledifolium (L.) Mill. plants along with PK, and PK or NPK fertigation was supplied to control plants. Compared to plants fertigated with NPK or PK, plants supplemented with the bacterium, exhibited higher contents of chlorophylls a and b and of total carotenoids (Figure 4). The finding that levels of chlorophylls and carotenoids were higher in bacteria-treated plants compared to PK and NPK, may indicate that the bacterium affects the physiological performances of the plant beyond simply supplying N. The data clearly demonstrate that the formation of tripartite plant–bacteria–mycorrhiza complexes will contribute to establishing healthier plantations that will produce earlier (see next section) and higher yields.
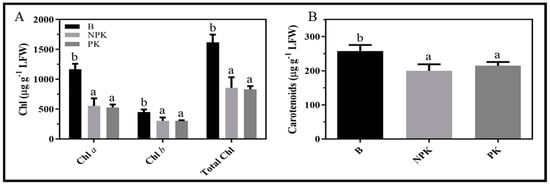
Figure 4.
Application of Bacillus spp (designated B) to H. ledifolium roots increased the contents of chlorophylls (A), and carotenoids (B) in plant leaves. Plants were grown in pots. PK—Control. Pots were treated only with 100 ppm PK. NPK—Nitrogen enrichment treatment. Pots were supplied with 100 ppm NPK. B—Bacillus spp treatment. Pots were treated with 100 ppm PK + 107 Bacillus cells/g soil. All treatments received similar amount of water per irrigation. Data are means; error bars indicate ±SE’s (n = 5). Significance is indicated by lower case letters and was calculated according to Tukey’s multiple comparisons test (α ≤ 0.05).
5. Factors Affecting Yields
5.1. Harvest Consideration
Fructification may occur, under the preparation practices described above, as early as six months after planting (Figure 5), but it may be delayed, depending on the quality of the mycorrhized seedlings and field management practices, mainly irrigation and weed control. Yields may, to some extent, be dependent on the time of collection. It is our experience that delaying collection of a discovered truffle (thus enabling further development) can increase its weight considerably (Figure 6 and Table 3). However, the timing of fruit body collection after discovery should be determined according to temperature and humidity, as high temperature accelerates ripening and infection by fly maggots.

Figure 5.
Fruit bodies under Helianthemum bushes. (Left) T. boudieri under a six-month-old H. sessiliflorum plant growing in sandy dune soil in Israel. (Right) T. claveryi under a H. almeriense bush in a plantation in Spain.
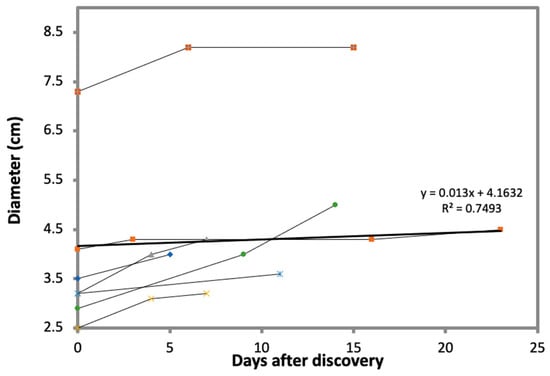
Figure 6.
Increase in truffle diameter (cm) when the T. boudieri truffles are left to mature on site. Fruit bodies were left intact in the soil after discovery, and the change in diameter was monitored for up to two weeks. Orange squares and the heavy line represent the average of all fruit bodies. Any increase in radius (x), (x + y) culminates in a higher degree (x) 3, (x + y)3 of difference in volume and weight.

Table 3.
Calculated increase in the weight of a single T. boudieri truffle, from discovery until collection. The volume and increase in weight were calculated on the assumption that the fruit body has a perfect ball shape.
Yield records revealed a reduction in T. boudieri plot production, which became rather pronounced after four years (Figure 7).
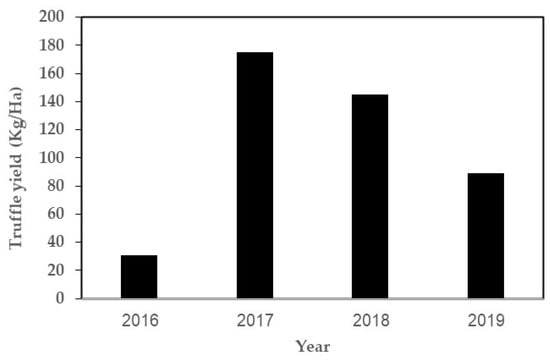
Figure 7.
Yields of desert truffles (T. boudieri) per year in Israel (2019 = 2019/2020 season).
An examination of plant root samples suggested that the reductions could have occurred due to a drop in mycorrhiza levels. However, root samples were checked in summer when fresh young roots are rarely found. Mycorrhiza levels should be checked again during spring to determine the levels in roots developed during the wet season—in order to verify this observation.
Efforts were undertaken to renew and enhance mycorrhiza in plots. Several methods to increase yields were tried; these included reinoculation with fresh spores and—following a published practice used in stands of the Périgord black truffle, Tuber melanosporum Vittad.—trapping truffles in holes []. However, the results obtained thus far are not reproducible, and much more work is needed to reveal the factors that drive the reduction in productivity when water is not deficient.
5.2. Climate Factors Affecting Yields
The first agricultural plot of desert truffles established in Murcia, Spain, was followed for 20 years, from 2001 to 2020. Through the years yields fluctuated strongly. The cumulative average of annual crop yield of the plot increased almost linearly until 2009 (Figure 8), when it reached a cumulative average harvest of 379 kg/ha, and it remained almost constant, with a standard deviation of ±14 kg/ha, throughout the remaining years. By the end of the study, the average desert truffle yield was 376 kg/ha/year. However, the yearly yield showed large inter-annual fluctuations, with a standard deviation of ±318 kg/ha. The yield fell to zero (2014) or to less than 2 kg/ha (2005, 2006, 2016, 2018) in very dry years. The largest harvest was in 2009 with 1069 kg/ha (Figure 8). A study aimed at analyzing meteorological factors potentially affecting yields was therefore conducted, and significant correlations were indeed found between yield and several parameters [], particularly the aridity index (AI) (Table 4).
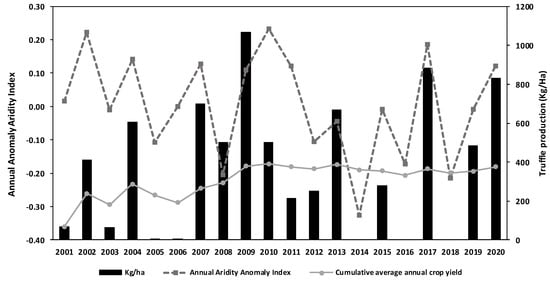
Figure 8.
Annual desert truffle (T. claveryi) yields (kg/ha) 2001–2020 in Spain. The solid line shows the average annual harvest (kg-ha-year) since planting. The dotted line shows the annual aridity anomaly index (AAI): AAI = AIy − AIaverage20, where AIy is the AI of a certain year and IAaverage20 is the mean for last 20 years in the plantation. The bars represent the total annual truffle production per year (kg/ha). There are no bars when the yield was zero (2014) or less than 2 kg/ha (2005, 2006, 2016, 2018).

Table 4.
Means of annual aridity anomaly index and production of T. claveryi desert truffles between 2001 and 2020 (all years), in high (>376 kg/ha) and low (<376 kg/ha) harvest years of a plantation in Murcia (Spain).
6. Conclusions
Although attempts aimed at cultivating desert truffles are still in their infancy as compared to efforts invested in cultivating Tuber spp. truffles, a considerable body of knowledge has accumulated since the first successful efforts reported by Honrubia et al. [] in Spain. Here we sum up, rather succinctly, the recommended practices for the establishment and maintenance of agricultural desert truffle-producing plots under two different sets of conditions: the arid conditions of the Israeli Negev desert and the semi-arid conditions of Murcia, Spain.
The profitability of the crop depends on a host of factors, including the cost of seedling preparation, the levels and stability of the yields, maintenance costs and the market price. These should be carefully considered before undertaking any truffle cultivation project. At present, relatively low yields and fluctuations in annual yields constitute the main limitations to a further increase in commercial plantations for truffle production, at least under the conditions in Israel. It is important to note that in recent years there has been increased interest in developing truffles as new exotic crops. Two elements have contributed to this trend: (i) global warming, which has led to a drastic decrease of truffle yield in natural habitats, clearly contributing to increases in truffle prices and market demands, and (ii) the loss of natural habitats to conventional agricultural activity, on the one hand, and to desertification, on the other. The realization that these two processes may eventually cause the loss of natural habitats have led us to propose that a possible route to conservation of the hosts and fungi would be development of truffles as a new crop for arid lands, especially as it is a crop suited to relatively marginal conditions: poor soils, low water need, and low levels (if at all) of mineral and organic matter fertilization. As an intermediate step towards developing desert truffles as a commercial crop, two possible routes can be taken: 1. Planting mycorrhized plants in already existing tree orchards thus establishing a dual crop. 2. Truffle farming could be integrated with a tourist activity in what is termed “truffle hunting” or “mycotourism”.
The prospects and future of this crop depend on further improvements with time, acquired through research and hands-on farming experience. In addition, we firmly believe that the way is now opened to extending cultivation to other species of desert truffles.
Author Contributions
V.K.-Z. and Y.S. wrote the text for desert truffle cultivation in Israel and A.M. and A.N.-R. for cultivation in Spain. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by AEI/FEDER, UE, grant No. CGL2016-78946-R; Fondo Europeo Agrícola de Desarrollo Rural, Programa de Desarrollo Rural de la Región de Murcia 2014-2020, grant No. G73977902 Grupo Operativo Turmicultura; FEDER and Programa Regional de Fomento de la Investigación -Plan de Actuación 2018- de la Fundación Séneca, Agencia de Ciencia y Tecnología of the Region of Murcia, Spain, grant No. 20866/PI/18; and the Israel Ministry of Agriculture, grant No. 16-13-0008.
Acknowledgments
The authors are grateful to the funding institutions listed above.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bunyard, B.A. Truffles and false truffles: A primer. Fungi 2008, 3, 13–15. [Google Scholar]
- Shavit, E. The History of Desert Truffle Use. In Desert Truffles; Kagan-Zur, V., Roth-Bejerano, N., Sitrit, Y., Morte, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 38, pp. 217–241. [Google Scholar]
- Shavit, E.; Shavit, E. The Medicinal Value of Desert Truffles. In Desert Truffles; Kagan-Zur, V., Roth-Bejerano, N., Sitrit, Y., Morte, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 38, pp. 323–340. [Google Scholar]
- Zambonelli, A.; Donnini, D.; Rana, G.L.; Fascetti, S.; Benucci, G.M.N.; Iotti, M.; Morte, A.; Khabar, L.; Bawadekji, A.; Piattoni, F.; et al. Hypogeous fungi in Mediterranean maquis, arid and semi-arid forests. Plant Biosyst. 2014, 148, 392–401. [Google Scholar] [CrossRef]
- Martínez-Tomé, M.; Maggi, L.; Jiménez-Monreal, A.M.; Murcia, M.A.; Marí, J.A.T. Nutritional and Antioxidant Properties of Terfezia and Picoa. In Desert Truffles; Kagan-Zur, V., Roth-Bejerano, N., Sitrit, Y., Morte, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 38, pp. 261–273. [Google Scholar]
- Al-Laith, A.A.A. Nutritional and Antioxidant Properties of the white desert truffle Tirmania nivea (Zubaidi). In Desert Truffles; Kagan-Zur, V., Roth-Bejerano, N., Sitrit, Y., Morte, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 38, pp. 275–297. [Google Scholar]
- Janakat, S.; Al-Fakhiri, S.; Sallal, A.K. A promising peptide antibiotic from Terfezia claveryi aqueous extract against Staphylococcus aureus in vitro. Phytother. Res. 2004, 18, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Janakat, S.M.; Al-Fakhiri, S.M.; Sallal, A.-K.J. Evaluation of antibacterial activity of aqueous and methanolic extracts of the truffle Terfezia claveryi against Pseudomonas aeruginosa. Saudi Med. J. 2005, 26, 952–955. [Google Scholar] [PubMed]
- Hamza, A.; Jdir, H.; Zouari, N. Nutritional, antioxidant, and antibacterial properties of Tirmania nivea, a wild edible truffle from Tunisia arid zone. Med. Aromat. Plants 2016, 5, 4–258. [Google Scholar] [CrossRef]
- Al Obaydi, M.F.; Hamed, W.M.; Al Kury, L.T.; Talib, W.H. Terfezia boudieri: A Desert truffle with anticancer and immunomodulatory Activities. Front. Nutr. 2020, 7, 38. [Google Scholar] [CrossRef]
- Dahham, S.S.; Al-Rawi, S.S.; Ibrahim, A.H.; Abdul Majid, A.S.; Abdul Majid, A.M.S. Antioxidant, anticancer, apoptosis properties and chemical composition of black truffle Terfezia claveryi. Saudi J. Biol. Sci. 2018, 25, 1524–1534. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, K.; Cigelnicl, E.; Weber, N.S.; Trappe, J.M. Phylogenetic relationships among ascomycetous truffles and the true and false morels inferred from 18S and 28S ribosomal DNA sequence analysis. Mycologia 1997, 89, 48–65. [Google Scholar] [CrossRef]
- Hansen, K.; Læssøe, T.; Pfister, D.H. Phylogenetics of the Pezizaceae, with an emphasis on Peziza. Mycologia 2001, 93, 958–990. [Google Scholar] [CrossRef] [Green Version]
- Hansen, K. Cup fungi go underground. Newsl. Friends Farlow 2006, 47, 1–4. [Google Scholar]
- Norman, J.E.; Egger, K.N. Molecular phylogenetic analysis of Peziza and related genera. Mycologia 1999, 91, 820–829. [Google Scholar] [CrossRef]
- Percudani, R.; Trevisi, A.; Zambonelli, A.; Ottonello, S. Molecular phylogeny of truffles (Pezizales: Terfeziaceae, Tuberaceae) derived from nuclear rDNA sequence analysis. Mol. Phylogenet. Evol. 1999, 13, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Morte, M.A.; Cano, A.; Honrubia, M.; Torres, P. In vitro mycorrhization of micropropagated Helianthemum almeriense plantlets with Terfezia claveryi (desert truffle). Agric. Food Sci. 1994, 3, 309–314. [Google Scholar] [CrossRef]
- Yu, T.E.; Egger, K.N.; Peterson, L.R. Ectendomycorrhizal associations characteristics and functions. Mycorrhiza 2001, 11, 167–177. [Google Scholar] [CrossRef]
- Morte, A.; Honrubia, M. Morphological characterization of the mycorrhiza formed by Helianthemum almeriense Pau with Terfezia claveryi Chatin and Picoa lefebvrei (Pat.) Maire. Mycorrhiza 2003, 13, 299–307. [Google Scholar] [CrossRef]
- Zaretsky, M.; Kagan-Zur, V.; Mills, D.; Roth-Bejerano, N. Analysis of mycorrhizal associations formed by Cistus incanus transformed root clones with Terfezia boudieri isolates. Plant Cell Rep. 2006, 25, 62–70. [Google Scholar] [CrossRef]
- Navarro-Ródenas, A.; Pérez-Gilabert, M.; Torrente, P.; Morte, A. The role of phosphorus in the ectendomycorrhiza continuum of desert truffle mycorrhizal plants. Mycorrhiza 2012, 22, 565–575. [Google Scholar] [CrossRef]
- Navarro-Ródenas, A.; Bárzana, G.; Nicolás, E.; Carra, A.; Schubert, A.; Morte, A. Expression analysis of aquaporins from desert truffle mycorrhizal symbiosis reveals a fine-tuned regulation under drought. Mol. Plant Microbe Interact. 2013, 26, 1068–1078. [Google Scholar] [CrossRef] [Green Version]
- Roth-Bejerano, N.; Navarro-Ródenas, A.; Gutiérrez, A. Types of mycorrhizal association. In Desert Truffles; Kagan-Zur, V., Roth-Bejerano, N., Sitrit, Y., Morte, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 38, pp. 69–80. [Google Scholar]
- Marqués-Gálvez, J.E.; Miyauchi, S.; Paolocci, F.; Navarro-Ródenas, A.; Arenas, F.; Pérez-Gilabert, M.; Morin, E.; Auer, L.; Barry, K.W.; Kuo, A.; et al. Desert truffle genomes reveal their reproductive modes and new insights into plant–fungal interaction and ectendomycorrhizal lifestyle. New Phytol. 2021, 229, 2917–2932. [Google Scholar] [CrossRef]
- Awameh, M.S. The response of Helianthemum salicifolium and H. ledifolium to infection by the desert truffle Terfezia boudieri. Mushroom Sci. 1981, 11, 843–853. [Google Scholar]
- Dexheimer, J.J.; Gerard, J.P.; Leduc, J.P.; Chevalier, G. Étude ultrastructurale comparée des associations symbiotiques my-corhiziennes Helianthemum salicifolium–Terfezia claveryi et Helianthemum salicifolium–Terfezia leptoderma. Can. J. Bot. 1985, 63, 582–591. [Google Scholar] [CrossRef]
- Roth-Bejerano, N.; Livne, D.; Kagan-Zur, V. Helianthemum–Terfezia relations in different growth media. New Phytol. 1990, 114, 235–238. [Google Scholar] [CrossRef]
- Bonifacio, E.; Morte, A. Soil properties. In Desert Truffles; Kagan-Zur, V., Roth-Bejerano, N., Sitrit, Y., Morte, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 38, pp. 57–67. [Google Scholar]
- Crous, P.; Wingfield, M.; Burgess, T.; Hardy, G.; Gené, J.; Guarro, J.; Baseia, I.; García, D.; Gusmão, L.; Souza-Motta, C.; et al. Fungal Planet description sheets: 716–784. Pers. Mol. Phylogeny Evol. Fungi 2018, 40, 239–392. [Google Scholar] [CrossRef]
- Giovannetti, G.; Roth-bejerano, N.; Zanini, E.; Kaganzur, V. Truffles and Their Cultivation. Hortic. Rev. 1994, 17, 71–107. [Google Scholar] [CrossRef]
- Bordallo, J.-J.; Rodríguez, A.; Muñoz-Mohedano, J.M.; Suz, L.M.; Honrubia, M.; Morte, A. Five new Terfezia species from the Iberian Peninsula. Mycotaxon 2013, 124, 189–208. [Google Scholar] [CrossRef]
- Bordallo, J.J.; Rodríguez, A.; Kounas, V.; Camello, F.; Honrubia, M.; Morte, A. Two new Terfezia species from Southern Europe. Phytotaxa 2015, 230, 239. [Google Scholar] [CrossRef] [Green Version]
- Morte, A.; Lovisolo, C.; Schubert, A. Effect of drought stress on growth and water relations of the mycorrhizal association Helianthemum almeriense-Terfezia claveryi. Mycorrhiza 2000, 10, 115–119. [Google Scholar] [CrossRef]
- Turgeman, T.; Ben Asher, J.; Roth-Bejerano, N.; Kagan-Zur, V.; Kapulnik, Y.; Sitrit, Y. Mycorrhizal association between the desert truffle Terfezia boudieri and Helianthemum sessiliflorum alters plant physiology and fitness to arid conditions. Mycorrhiza 2011, 21, 623–630. [Google Scholar] [CrossRef]
- Andrino, A.; Navarro-Ródenas, A.; Marqués-Gálvez, J.E.; Morte, A. The crop of desert truffle depends on agroclimatic parameters during two key annual periods. Agron. Sustain. Dev. 2019, 39, 51. [Google Scholar] [CrossRef]
- Bradai, L.; Bissati, S.; Chenchouni, H.; Amrani, K. Effects of climate on the productivity of desert truffles beneath hyper-arid conditions. Int. J. Biometeorol. 2014, 59, 907–915. [Google Scholar] [CrossRef]
- Honrubia, M.; Gutierrez, A.; Morte, A. Desert truffle plantation from south-east Spain. In Proceedings of the 2nd International Conference on Edible Mycorrhizal Mushrooms, Christchurch, New Zealand, 3–6 July 2001. [Google Scholar]
- Morte, A.; Honrubia, M.; Gutiérrez, A. Biotechnology and Cultivation of Desert Truffles. In Mycorrhiza; State of the Art Genetics and Molecular Biology, Eco-Function, Biotechnology, Eco-Physiology, Structure and Systematics; Varma, A., Ed.; Springer: Berlin, Germany, 2008; pp. 467–483. [Google Scholar] [CrossRef]
- Morte, A.; Andrino, A.; Honrubia, M.; Navarro-Ródenas, A. Terfezia cultivation in arid and semiarid soils. In Edible Ectomycorrhizal Mushrooms; Zambonelli, A., Bonito, G.M., Eds.; Springer: Berlin, Germany, 2012; pp. 241–263. [Google Scholar] [CrossRef]
- Morte, A.; Pérez-Gilabert, M.; Gutiérrez, A.; Arenas, F.; Marqués-Gálvez, J.E.; Bordallo, J.J.; Rodríguez, A.; Berná, L.M.; Lozano-Carrillo, C.; Navarro-Ródenas, A. Basic and applied Research for Desert Truffle Cultivation. In Mycorrhiza Eco-Physiology, Secondary Metabolites, Nanomaterials; Varma, A., Prasad, R., Tuteja, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 23–42. [Google Scholar]
- Morte, A.; Gutiérrez, A.; Navarro-Ródenas, A. Advances in desert truffle mycorrhization and cultivation. In Mushrooms, Humans and Nature in a Changing World; Pérez-Moreno, J., Guerin-Laguette, A., Flores Arzú, R., Yu, F.-Q., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 205–219. [Google Scholar]
- Slama, A.; Fortas, Z.; Boudabous, A.; Neffati, M. Cultivation of an edible desert truffle (Terfezia boudieri Chatin). Afr. J. Microbiol. Res. 2010, 4, 2350–2356. [Google Scholar]
- Honrubia, M.; Andrino, A.; Morte, A. Preparation and maintenance of both man-planted and wild plots. In Desert Truffles; Kagan-Zur, V., Roth-Bejerano, N., Sitrit, Y., Morte, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 38, pp. 367–387. [Google Scholar]
- Chevalier, G.; Dupre, C.; Riousset, L.; Dexhemier, J. Synthese mycorrhizienne entre Terfezia leptoderma Tul et diverses Cistaceae. Agronomie 1984, 4, 210–211. [Google Scholar]
- Morte, A.; Andrino, A. Domestication: Preparation of mycorrhizal seedlings. In Desert Truffles; Kagan-Zur, V., Roth-Bejerano, N., Sitrit, Y., Morte, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 38, pp. 343–365. [Google Scholar]
- Pérez-García, F.; González-Benito, M. Seed germination of five Helianthemum species: Effect of temperature and presowing treatments. J. Arid. Environ. 2006, 65, 688–693. [Google Scholar] [CrossRef]
- Navarro-Ródenas, A.; Berná, L.M.; Lozano-Carrillo, C.; Andrino, A.; Morte, A. Beneficial native bacteria improve survival and mycorrhization of desert truffle mycorrhizal plants in nursery conditions. Mycorrhiza 2016, 26, 769–779. [Google Scholar] [CrossRef]
- Arenas, F.; Navarro-Ródenas, A.; Chávez, D.; Gutiérrez, A.; Pérez-Gilabert, M.; Morte, A. Mycelium of Terfezia claveryi as inoculum source to produce desert truffle mycorrhizal plants. Mycorrhiza 2018, 28, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, P.F.; Fontana, A. Sulla Nutrizione del Micelio di Tuber Melanosporum Vitt in Coltura; Atti della Accademia delle scienze di Torino: Torino, Italy, 1973; Volume 107, pp. 713–741. [Google Scholar]
- Andrino, A.; Morte, A.; Honrubia, M. Method for Producing Plants of the Cistaceae Family that Establish Mycorrhiza with Different Desert Truffle Species. Patent ES2386990B1, 19 July 2013. [Google Scholar]
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D. Bacillus species in soil as a natural resource for plant health and nutrition. J. Appl. Microbiol. 2019, 128, 1583–1594. [Google Scholar] [CrossRef] [Green Version]
- Murat, C.; Bonneau, L.; de la Varga, H.; Olivier, J.M.; Fizzala, S.; Le Tacon, F. Trapping truffle production in holes: A promising technique for improving production and unravelling truffle life cycle. Ital. J. Mycol. 2016, 45, 47–53. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).