Abstract
Winter oilseed rape (Brassica napus L.) is the main source of domestic oil in central and northern Europe, bringing profits to farmers, but the plants are often damaged by stem canker, caused by two fungal species belonging to the genus Leptosphaeria. Due to environmental concerns, the benefits of fungicide applications must outweigh disadvantages. The aim of this work was to determine the effect of stem canker on seed yield and its quality and find out the best timing of fungicide application. The multi-year field experiments were done at two sites in south-west Poland, where the disease is regarded as a serious problem. The fungicide treatments with the azole-containing preparation followed the same scheme each year; a single application was made at one-week intervals, starting in late September through mid-November for a total of eight treatments. Seed yield, oil and protein content, mass of thousand seeds as well as indole-and alkenyl-glucosinolate contents in seeds were statistically unrelated with the incidence and severity of phoma leaf spotting and stem canker symptoms. The significant decrease of the seed yield was observed in three (site × year combinations) of eight, in which phoma leaf spotting and stem canker were severe. Yield loss was noted only in years with warm and wet autumns, when cumulative mean temperatures between BBCH14 and BBCH19 plant growth stages exceeded 60 °C and precipitation in this period exceeded 110 mm of rain. Under these conditions, fungicide treatments were highly effective when they were done between BBCH15–BBC16 growth stages (5–6 true leaves).
1. Introduction
Common concern about food security as well as supporting a healthy environment make plant protection a difficult task. From one side, the human population is growing fast and needs a substantial supply of food, and from the other side, citizens demand food, air, soil, and water to be free from unwanted xenobiotics. Nowadays, the requirement is to find optimal solutions for pesticide use in agricultural crops.
Winter oilseed rape (Brassica napus L.) is the main source of domestic oil in central and northern Europe. There have been many attempts to introduce other oil crops such as soybean (Glycine max L.) or sunflower (Helianthus annuus L.), but the thermal conditions favour crops that can grow in temperate zone, such as oilseed rape. Considerable efforts of plant breeders have resulted in numerous cultivars of oilseed rape that can withstand winter frosts, provided that the plants are subjected to the proper acclimation. On the other hand, spring droughts often affects yield of spring oilseed rape. Consequently, winter oilseed rape is predominant and the processing industry is built around this crop. The consumption of oilseed rape oil has been growing in Europe [1,2] and globally [3]. This oil is desirable for human consumption, due to its comparatively low content of saturated fatty acids and high level of mono- and poly- unsaturated fatty acids. A diet high in these fats is linked to the health benefits from reduced cholesterol and increased cardioprotection [4]. Moreover, the oil of oilseed rape contains other healthy compounds, such as sterols, carotenoids, vitamin K, and tocopherols [5]. Cake resulting from processing of oil provides high energy and valuable nutrients to animal feed, including some oil, crude proteins [6], and sulfur-containing amino acids and lysine [7]. The popularity of oilseed rape cultivation, due to its profitability for the farmers and important role in cereal-based crop rotations, combined with its very long vegetation growth period (11 months in the field) are associated with high disease pressure [8].
Among the main diseases of oilseed rape worldwide are phoma leaf spotting and stem canker (blackleg), caused by the fungi Leptosphaeria maculans and L. biglobosa. Yield losses of millions of tons of oilseed rape in Europe, North America, Australia, and Africa may occur [9]. Populations of L. maculans and L. biglobosa can be reduced by plant resistance as well as chemical and agronomical control methods such as tillage, changes of sowing date, decrease of plant density, or nitrogen management [10]. The main source of plant infections are ascospores originating from fruiting bodies of the sexual stage, produced on oilseed rape stubble from previous seasons [11], although in some countries, the expansion of the disease is also attributed to pycnidiospore dispersal [12]. Sexual recombination is important in the pathogen life cycle as it generates a high genetic variation and increases the risk of plant resistance genes being overcome rapidly [13]. Ascospores are mainly transmitted by the wind, thus they can travel long distances. Conversely, pycnidiospores are transported by rain-splash and move to much shorter distances, but they can be dispersed further in gusts of wind. In Canada, the recommended distance in order to minimize inoculum dissemination between fields is 50–100 m [12] whereas in Australia, the recommended distance between the fields is more than 400 m [14]. In both countries, the spring form of oilseed rape is cultivated. The pathogens causing stem canker survive on plant material in the soil as long as the crop residues remain. If there is a series of dry years, stubble decomposition lasts up to 4 years [15]. This period of dormancy of oilseed rape residues can vary due to the conditions causing the breakdown of the stubble.
The development of stem canker epidemics is greatly affected by the environmental conditions prevalent during the growing season [8,16,17,18,19]. The climates of particular continents differ substantially and intracontinental differences in temperature and rainfall have been implicated as major factors affecting the incidence and severity of stem canker. The local microclimate plays a key role in formation of ascospores and their release [20]. Pseudothecial maturation and ascospore release are dependent on temperature and rainfall [21]. Pérès et al. [22] reported that the optimum conditions for pseudothecia maturation in France was 14 °C with frequent heavy rainfall (2.3 mm every 3–4 days). In Canada, the optimum temperature for ascospore formation was 15 °C, although at 20 °C the peak sporulation occurred earlier in the season, and at 10 °C, it lasted longer [23]. The frequency of days with rainfall was more important than the total rainfall. In the UK, pseudothecia of L. maculans and L. biglobosa matured in a temperature range between 5 and 20 °C; the higher the temperature, the faster the pseudothecia maturation [24]. Huang et al. [25] reported that the optimum temperature for pseudothecia maturation was 14–15 °C. In Poland, cumulative precipitation in the last 10 days of September directly affected the ascospore release and resulted in higher stem canker epidemics [8].
The pathogen species L. maculans and L. biglobosa have a worldwide distribution. The current geographic range of these two species may reflect their worldwide spread through infected seeds [9]. The biological and epidemiological differences between L. maculans and L. biglobosa allow the two species to coexist through occupation of different ecological niches due to differences in time and site of infection or distinct infection strategy (lesion type) [11]. A mixed population of L. maculans and L. biglobosa occurs in the western European countries including France, Germany, Lithuania, Poland, and the UK, but regional variations in the frequencies of the two species have been reported [26,27,28,29]. In Canada, where L. biglobosa was initially widespread, the first isolation of L. maculans was recorded in Saskatchewan in 1975 [30]. However, by 1984, L. maculans had moved westwards to Alberta and an increase in severity of epidemics was associated with this spread [18]. Both L. maculans and L. biglobosa have been identified in the USA [9], Mexico [31], Brazil [32], and Argentina [33]. In Poland, there are differences in the Leptosphaeria pathogen population structure between regions and seasons [34]. The regional variation in the incidence of phoma stem canker may be related to differences in climate, as disease incidence is greater in the western part of Poland where the climate is milder as compared to the east of the country, which is associated with a cold climate [8].
According to previous studies, azole fungicides are very valuable for protecting oilseed rape against L. maculans and L. biglobosa, but are effective for the limited period of time due to the degradation of active ingredients, leaf expansion, and the production of new untreated leaves [35,36,37,38,39,40,41]. However, the toxicity of some fungicides makes them dangerous for the environment, including direct impacts on humans [42], animals [43], plants [44], soil [45], water [46], and air [47]. At present, the fungicide applications against stem canker of winter oilseed rape are arbitrarily done from one to three times in the autumn at different growth stages of winter oilseed rape and the time of fungicide application mainly depends on the use of plant growth regulators (PGRs) suppressing the shoot growth before the winter pause. In the context of the most recent EU policy called European Green Deal [48] with the aim of improving the well-being of people, numerous pesticides have already been or will soon be withdrawn. The idea is to replace toxic compounds with less or none-toxic, thus protecting our natural habitat and providing the farmers with disease forecasting systems, helping to pinpoint the optimal time of their application.
The aim of this work was to determine: (1) the effect of weather in the autumn period on disease incidence and severity before winter pause (phoma leaf spotting) and before harvest (stem canker), (2) the impact of stem canker on seed yield and quality of winter oilseed rape, (3) the best period of the azole fungicide application to manage stem canker of winter oilseed rape, (4) the frequency of L. maculans and L. biglobosa species isolated from phoma leaf spotting and stem canker symptoms (composition of Leptosphaeria population within a plant of oilseed rape). To achieve this goal, we performed multi-year field experiments at two sites in south-west Poland, where stem canker is usually severe. The experiments followed the same regime of fungicide treatments (eight single sprays at weekly intervals in the autumn and an unsprayed control). We observed disease symptoms in late autumn and before harvest, compared disease incidence and severity with seed yield and seed quality. The results are discussed in relation to meteorological data in the autumn, which is the crucial period for the onset of plant infection, i.e., phoma leaf spotting, leading to the stem canker phase before harvest in the following summer.
2. Materials and Methods
2.1. Location of Experimental Sites
The study was conducted in the Opole Region. The field experiments were designed to compare a single fungicide application, done at weekly intervals over 8 weeks, for efficacy against stem canker of winter oilseed rape over four consecutive growing seasons (from 2009/2010 to 2012/2013). Each vegetative growing season started at sowing time (mid- to end-August) and ended in the following summer (harvest in July). The field experiments were located in Glubczyce (50°12′0.339″N, 17°49′45.552″E) and Toszek (50°27′18.383″N, 18°31′5.017″E) located 65 km apart.
2.2. Meteorological Data
Meteorological data covering years of studies were provided by the weather station located close to the field (300–500 m, depending on the year and the experimental site). Selected meteorological variables were examined in this study, i.e., mean, maximal, minimal air temperature at 2 m, and rainfall.
2.3. Field Experiments
The field experiments were done using the winter oilseed rape hybrid cultivar PR46W10 (Pioneer Hi-Bred, Solihull, UK). According to previous studies, this cultivar is susceptible to stem canker [49]. The individual plots were 1.5-wide and 10-m long, replicated 3 times. The distance between rows was 30 cm and the sowing rate was 45 seeds m−2. Seeds were commercially pre-treated by Cruiser 322 FS (Syngenta Crop Protection AG, Bazylea, Switzerland). Each year, there were eight single fungicide treatments applied at weekly intervals in the autumn on individual plots. The starting point for fungicide applications was the fourth fully developed true leaf (BBCH 14) according to Zadoks [50] which was at late September (BBCH14), through 8 subsequent weeks (BBCH 14, 8 weeks), which lasted to mid-November when the growth stage of oilseed rape plants was 9 or more fully developed true leaves (BBCH 19), as shown in Figure 1 and Table 1. During this period, the plants of winter oilseed rape get infected with ascospores of L. maculans and L. biglobosa [51]. A fungicide containing 250 g/L flusilazole (Capitan 250 EW) at the dose of 0.5 L·ha−1 was prepared. This preparation was officially registered during field experiments and now is withdrawn from the market. The fungicides that are most commonly used in Europe to protect oilseed rape from the stem canker contain azole-based active substances, including flusilazole. In the event of rain, applications were shifted 1–2 days later.
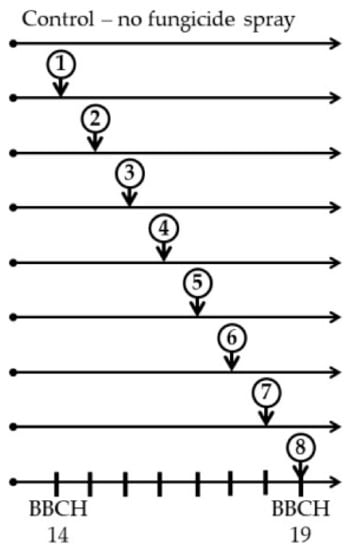
Figure 1.
Field experiment scheme, showing eight single application timings of winter oilseed rape cv. PR46W10 with the fungicide Capitan 250 EW at the dose of 0.5 L·ha−1, containing 250 g·L−1 flusilazole. The sprays were done at weekly intervals. Control was not treated with the fungicide.

Table 1.
Dates of sowing and fungicide applications of winter oilseed rape cv. PR46W10, in two experimental sites (Glubczyce and Toszek), Opole Region, Poland in four years.
The control variant was not treated with the fungicide. For each variant, the disease incidence (percent of infected plants) and disease severity (intensity of disease symptoms) were assessed on 30 randomly selected plants in each of three replicates. Before the winter vegetation pause (mid-November), phoma leaf spotting was evaluated according to the scale from 0 to 4, where 0 was no visible disease symptoms and 4 was numerous (over 10) leaf spots per plant [52]. The stem canker symptoms were assessed ca. 2 weeks before the harvest, according to a scale from 0 to 9, where 0 was no visible symptoms and 9 was a plant totally damaged by the disease i.e., dry, with visible crown canker (plant lying on the soil or easy to pull out by hand from the soil) [53]. Moreover, the percentage of plants with phoma leaf spotting in the autumn as well as plants with stem canker before harvest were also calculated and expressed as a percentage of infected plants (disease incidence). Before harvest, the borders of 0.5 m were removed from both ends of field plots by the combined harvester and the seeds were harvested from the area of 1.5 × 9 m.
2.4. Study of Seed Quality
Apart from seed yield, the mass of one thousand seeds was measured for each plot and re-calculated to 90% of dry matter content. Chemical analyses of seeds included oil content—measured with the Soxhlet method [54], and total protein content—measured based on the nitrogen content, using the Kjeldahl method [55]. Glucosinolates were extracted from whole seeds of oilseed rape using methanol and then applied to a DEAE A25 Sephadex column (Sigma Aldrich, St. Louis, MO, USA) where they were desulfated after the addition of the enzyme H1-type sulfatase (Merck Life Science UK Limited, Dorset, UK). The liquid phase was evaporated and, after silylation, the glucosinolates were determined by gas chromatography on Agilent 6890 chromatograph with FID detector and ChemStation software (Agilent Technologies, Santa Clara, CA, USA). The DB-23 capillary column working at 200 °C, with hydrogen as gas carrier, was applied (Agilent Technologies, Santa Clara, CA, USA). The injector and detector temperature was set to 220 °C. The internal standard was glucotropeoline (Merck Life Science UK Limited, Dorset, UK).
2.5. Sampling, Isolation, and Identification of Leptosphaeria Isolates from Plants
Isolates of Leptosphaeria spp. were obtained from 20 arbitrarily selected leaves of oilseed rape with visible symptoms of phoma leaf spotting. The sampling was done each autumn from control plots. In addition, 20 stems of oilseed rape with visible symptoms of phoma stem canker were collected before harvest, during disease assessment. Tissue samples with disease symptoms were surface disinfected with 1% of sodium hypochlorite for 2 min, followed by three washings in sterile distilled water for 2 min each. Small parts of disinfected plant fragments were placed on a Potato Glucose Agar medium (PDA, Merck Life Science UK Limited, Dorset, UK) supplemented with a 0.02% solution of streptomycin sulphate (Merck Life Science UK Limited, Dorset, UK). Fungal isolates were subcultured until they were free of bacteria and other contaminants in a shaken culture of the isolate grown on Czapek–Dox liquid medium (A&A Biotechnology, Gdansk, Poland). A hyphal tip of each isolate was collected with a sterile needle and a dissecting microscope and subcultured on a fresh PDA medium. In total, 147 isolates were obtained from leaves and 145 isolates originated from stems of oilseed rape. The taxonomic identification was based on culture morphology. The colonies of L. biglobosa were regular, circular, and the dark brown pigment was partially excreted to the agar medium and partially oozing from the mycelium in a form of dark brown droplets; pycnidia were relatively sparse. The cultures of L. maculans were less regular, white-beige, and speckled with numerous pycnidia.
2.6. Statistical Analyses
The statistical analyses were done using the programme Statistica version 13.0 (StatSoft Inc., Warsaw, Poland). Inference about the significance of differences between treatments (fungicide application variant × experimental site × year/season) was conducted using one way ANOVA and was determined using a post hoc Tukey test (p ≤ 0.05). For all variables with the same letter, the difference between the means was not statistically significant. To compare disease incidence, disease severity, and seed yield, the Spearman’s rank correlation coefficient was calculated (p ≤ 0.05) after checking, with Shapiro–Wilk test, the null hypothesis that a sample originates from a normally distributed population.
3. Results
3.1. Meteorological Data
The meteorological measurements for the autumn seasons of 2009–2012 are presented in the figures and in the Supplementary Materials (Figure 2 and Table S1—Glubczyce; Figure 3 and Table S2—Toszek). The overall analysis of mean temperature and rainfall helped to divide autumn seasons into four categories: (1) cold and wet, (2) cold and dry, (3) warm and wet, and (4) warm and dry. The autumn was regarded as cold when the mean temperature in the period starting with the first spray (Spray 1) and ending one week after the last spray (Spray 8) was below 7.5 °C, whereas the autumn was regarded as warm, when the mean air temperature was 7.5 °C and over. Very contrasted temperatures among the study years allowed easy categorizations into cold and warm autumns.
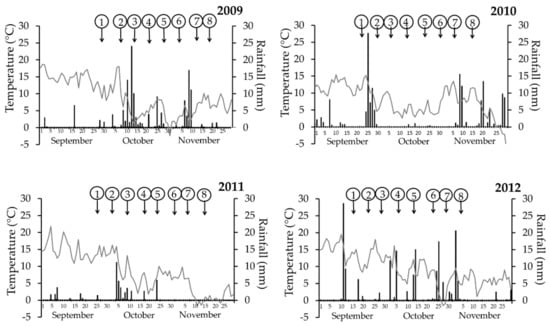
Figure 2.
Meteorological data obtained at Glubczyce experimental site in the autumn. Grey line—temperature (°C), black bars—rainfall (mm). Numbers in circles point out the days of fungicide sprays (flusilazole 250 g·L−1) against stem canker (Leptosphaeria maculans, L. biglobosa). Each experiment variant was a single application, and the sprays were done on winter oilseed rape cv. PR46W10, at weekly intervals.
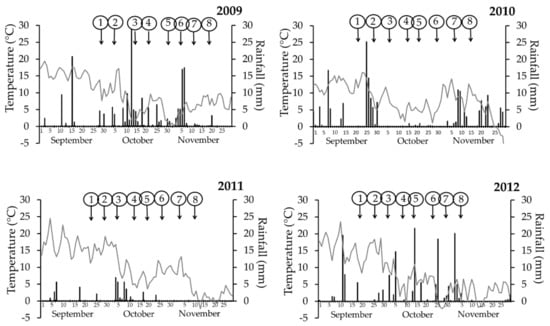
Figure 3.
Meteorological data obtained at Toszek experimental site in the autumn. Grey line—temperature (°C), black bars—rainfall (mm). Numbers in circles point out the days of fungicide sprays (flusilazole 250 g·L−1) against stem canker (Leptosphaeria maculans, L. biglobosa). Each experiment variant was a single application, and the sprays were done on winter oilseed rape cv. PR46W10, at weekly intervals.
Cold autumns were noted in Glubczyce 2009 and 2011 as well as Toszek 2009, the remaining autumn periods were classified as warm. Autumn was regarded as wet when the cumulative rainfall for the period starting with the first spray (Spray 1) and ending one week after the last spray (Spray 8) was over 100 mm, whereas autumn with rainfall below this value was classified as dry. Values for dry autumn were mostly in the range of 30–40 mm of rain. The exception was observed in 2010 at Glubczyce, where the very high rainfall was noted only at the very beginning and the end of the above mentioned period (Table S1); although it accounted for 104 mm, the autumn was mostly dry. Beside this case, dry autumns were noted in Glubczyce 2011 and Toszek 2011.
In summary, (1) cold and wet autumn periods were in Glubczyce 2009 and Toszek 2009, (2) cold and dry autumn was in Glubczyce 2011, (3) warm and wet autumn was in Glubczyce 2012, Toszek 2010, and Toszek 2012, and (4) warm and dry autumn was in Glubczyce 2010 and Toszek 2011.
3.2. Composition of the Pathogen Population
A total of 292 hyphal tip isolates were obtained from the lesions on the leaves (phoma leaf spots) and stems (crown cankers and upper parts of stems). Both L. maculans and L. biglobosa were isolated from the samples collected in Glubczyce and Toszek (Table 2). The proportions between the species strongly depended on the season. In the autumn, the predominant species causing leaf-spotting at both places was L. maculans (74.7%). Each summer, the dominant species on stems of oilseed rape was L. biglobosa; it was re-isolated from 72% of stem canker and upper stem symptoms.

Table 2.
Frequency (%) of Leptosphaeria spp. species isolated from winter oilseed rape plants in Glubczyce and Toszek in phoma leaf spot stage (autumn 2009–2012) and stem canker stage (summer 2010–2013).
3.3. Effect of Spray Time on Plant Health
A significantly higher disease incidence was observed on untreated plots or on plots where sprays were done late in the season (4–19 November). In the autumn in Glubczyce, the disease severity on leaves of untreated plants was from 2.7-fold (2011) to 45.7-fold (2010) higher than in plants treated by the fungicide (Table 3). At the same time in Toszek, the difference between the treated and untreated plots varied from 2.6 (2009) to 9.7 (2010), with more infection on control (untreated) plants.

Table 3.
Incidence (percent of infected plants) of phoma leaf spot and stem canker, caused by Leptosphaeria maculans and L. biglobosa at two experimental sites in Poland (Glubczyce and Toszek, Opole Region) in response to fungicide (flusilazole 250 g·L−1) application time. Each spray (1–8) was a single application on winter oilseed rape plants (cv. PR46W10) made at one-week intervals, starting from late September (BBCH 14) through mid-November (BBCH 19) (see Figure 1 for experiment scheme and Table 1 for spray dates).
The sprays done in November, i.e., late in the season, close to the winter pause, were the least effective. In this case, the percentage of infected plants ranged from 11.6% (2011) to 35% (2010) in Glubczyce and from 8% (2009) to 20.5% (2010) in Toszek.
Fungicide sprays with flusilazole had a significant impact on the severity of phoma leaf spotting and stem canker on winter oilseed rape plants. This effect was observed at both experimental sites, all years and seasons (autumn—phoma leaf spotting and summer—stem canker). The highest disease severity in a given season was always observed on all control variants, untreated with the fungicide. However, in some cases, namely Glubczyce 2010 and 2012 (Figure 4) as well as Toszek 2009, 2010, and 2012 (Figure 5), the severe symptoms of phoma leaf spotting were also observed on sprayed variants. The application of flusilazole at these times were not efficient. In the case of Glubczyce 2009 (Figure 4) and Toszek 2009 (Figure 5), the inefficient spray was S1, the earliest fungicide application in the season. The other cases concern the late (Toszek 2010, S5–S7) or the latest (Glubczyce 2012, S8) applications in the season. Similar effect of fungicide applications was found in respect to the symptoms of stem canker disease stage. The inefficient fungicide applications mostly concerned the same site × year combinations in Glubczyce (Figure 6) and Toszek (Figure 7).
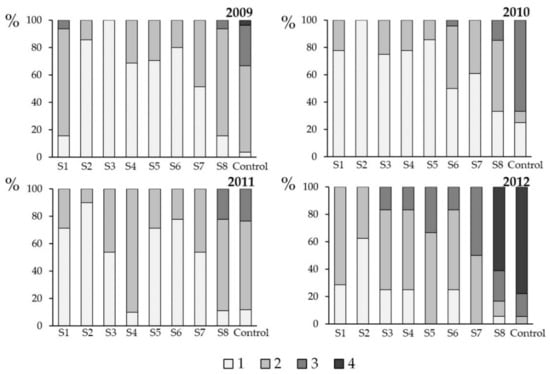
Figure 4.
Severity of phoma leaf spot (autumn 2009–autumn 2012), caused by Leptosphaeria maculans and L. biglobosa in Glubczyce (Opole Region, Poland) in response to fungicide applications (flusilazole 250 g·L−1) on winter oilseed rape plants (cv. PR46W10). Each spray (S1–S8) was a single application made at one-week intervals, starting from late September (BBCH 14) through mid-November (BBCH19) (see Figure 1 for experiment scheme and Table 1 for spray dates). The severity of phoma leaf spot was evaluated using a 1–4 scale (1: 1–2 leaf spots per plant, 2: 3–5 leaf spots per plant, 3: 6–9 leaf spots per plant, and 4: >10 leaf spots per plant). Control plants were not sprayed with the fungicide.
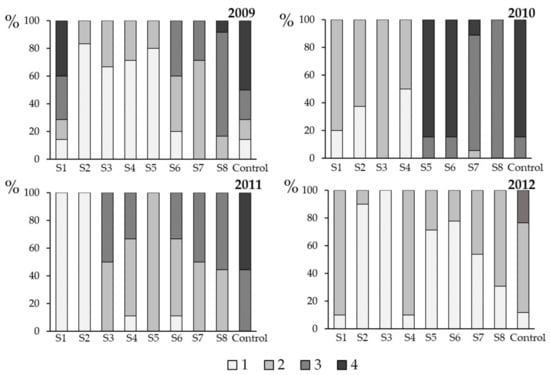
Figure 5.
Severity of phoma leaf spotting (autumn 2009–autumn 2012), caused by Leptosphaeria maculans and L. biglobosa in Toszek (Opole Region, Poland) in response to fungicide applications (flusilazole 250 g·L−1) on winter oilseed rape plants (cv. PR46W10). Each spray (S1–S8) was a single application made at one-week intervals, starting from late September (BBCH 14) through mid-November (BBCH 19) (see Figure 1 for experiment scheme and Table 1 for spray dates). The severity of phoma leaf spot was evaluated using a 1–4 scale (1: 1–2 leaf spots per plant, 2: 3–5 leaf spots per plant, 3: 6–9 leaf spots per plant, and 4: >10 leaf spots per plant). Control plants were not sprayed with the fungicide.
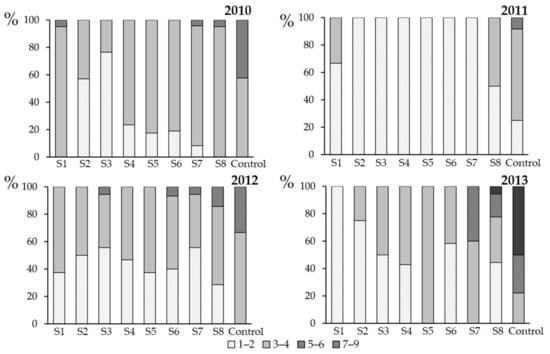
Figure 6.
Severity of stem canker (harvest: summer 2010–summer 2013), caused by Leptosphaeria maculans and L. biglobosa in Glubczyce (Opole Region, Poland) in response to fungicide applications (flusilazole 250 g·L−1) on winter oilseed rape cv. PR46W10 plants. Each spray (S1–S8) was a single application; the sprays were done in the previous autumn at weekly intervals (see Figure 1 for experiment scheme and Table 1 for spray dates). The severity of stem canker symptoms were evaluated using a 1–9 scale (1: <10% girdling of the stem, 2: 10% girding, 3: 11–20% girdling, 4: 21–30% girdling, 5: 31–50% girdling, 6: 51–75% girding, 7: 76–100%, 8: girdling + stem weakness, and 9: 100% girdling and stem death). In the Figure, the evaluations are grouped as follows: 1–2 (low severity), 3–4 (moderate severity), 5–6 (high severity), and 7–9 (very high severity). Control plants were not sprayed with the fungicide.
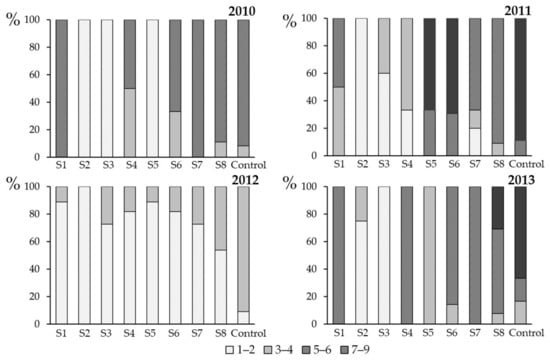
Figure 7.
Severity of stem canker (harvest: summer 2010–summer 2013), caused by Leptosphaeria maculans and L. biglobosa in Toszek (Opole Region, Poland) in response to fungicide applications (flusilazole 250 g·L−1). Each spray (S1–S8) was a single application, and the sprays were done on winter oilseed rape cv. PR46W10 plants, in the previous autumn at weekly intervals (see Figure 1 for experiment scheme and Table 1 for spray dates). The severity of stem canker symptoms were evaluated using a 1–9 scale (1: <10% girdling of the stem, 2: 10% girding, 3: 11–20% girdling, 4: 21–30% girdling, 5: 31–50% girdling, 6: 51–75% girding, 7: 76–100%, 8: girdling + stem weakness, and 9: 100% girdling and stem death). In the Figure, the evaluations are grouped as follows: 1–2 (low severity), 3–4 (moderate severity), 5–6 (high severity), and 7–9 (very high severity). Control plants were not sprayed with the fungicide.
The Spearman’s rank correlation coefficients between the incidence and severity of phoma leaf spotting ranged from 0.41 (Glubczyce, autumn 2009) to 0.86 (Toszek, autumn 2012) (Supplementary Materials, Table S3).
The results depended on the way of calculation and they were usually, but not always, higher when all assessed plants were considered (severity of all plants, with and without phoma leaf spotting), and slighly lower (by 0.09 on average) when the calculation concerned only the plants with disease symptoms (severity of infected plants). The Spearman’s rank correlation coefficients between the incidence and severity of stem canker symptoms ranged from 0.52 (Toszek, summer 2012) to 0.97 (Toszek, summer 2013) (Supplementary Materials, Table S3). The differences between the calculations for the severity of all observed plants (with and without the symptoms of stem canker) and the severity of plants with stem canker symptoms was slightly higher 0.13), and it ranged from 0.02 (Toszek, summer 2009) to 0.28 (Toszek, summer 2013) (Table S1).
3.4. Effect of Spray Time on Seed Yield
The relationship between the time of fungicide application and seed yield were significant in three out of eight site × year combinations (Glubczyce 2013, Toszek 2011, Toszek 2013). Significantly higher yield was obtained in the variants with the lowest percentage of infected plants and the lowest disease severity. In the summer 2013 in Glubczyce, the increase in yield in plots treated at the optimal time was 7.2 dt·ha−1 as compared to untreated controls (Table 4). In the summer 2011 in Toszek, the increase of yield was 5.2 dt·ha−1 (Table 5). The Spearman’s rank correlation coefficients between disease incidence and yield ranged from −0.09 (Glubczyce, summer 2012) to −0.93 (Toszek, autumn 2010) (Table S3). The highest correlations were obtained for Glubczyce vegetative season 2012/2013 as well as for Toszek 2010/2011 and Toszek 2012/2013. In these vegetative seasons, the autumn periods were always warm and wet (see the results presented in Section 3.1).

Table 4.
The effect of fungicide application (flusilazole 250 g·L−1) on seed yield quantity and quality of oilseed rape cv. PR46W10 F1, in 2009–2012, Glubczyce, Opole Region, Poland. Each spray (1–8) was a single application, done in the autumn at weekly intervals (see Figure 1 for experiment scheme and Table 1 for spray dates). Control plots were not treated with the fungicide.

Table 5.
The effect of fungicide application (flusilazole 250 g·L−1) on seed yield quantity and quality of winter oilseed rape cv. PR46W10 F1, in 2009–2012, Toszek, Opole Region, Poland. Each spray (1–8) was a single application, done in the autumn at weekly intervals (see Figure 1 for experiment scheme and Table 1 for spray dates). Control plots were not sprayed with the fungicide.
3.5. Effect of Spray Time on Seed Quality
Oil content in the seeds ranged from 46.01% to 48.57% in Glubczyce (Table 4) and from 44.13% to 48.62% in Toszek (Table 5). In comparison to seeds from untreated plots only in the season 2009/2010 in Glubczyce oil content from plots treated with the fungicide was significantly higher (Table 4). In all remaining site × year combinations tested, this parameter was not affected by the time of fungicide application.
Differences in seed protein concentration in relation to time of fungicide application were also statistically insignificant. Only in the season 2009/2010 in Glubczyce (Table 4) and 2010/2011 in Toszek (Table 5), the percent of proteins in seeds was significantly higher than in seeds from untreated plots. The effect of the fungicide application on the mass of thousand seeds was observed at one site-year. In season 2011/2012 in Toszek, the difference was very high and it reached 1.32 g per one thousand seeds (Table 5).
Fungicide treatments had no clear relation to the amount of glucosinolates in harvested seeds (Table 6 and Table 7). There were no statistically significant relationships between variants treated with flusilazole and the control. The level of indole glucosinolates was higher in a few variants treated with the fungicide as compared to control. For example, in Glubczyce, for seeds harvested in 2011 and 2012, fungicide treatment in few variants coincided with the lower content of gluconapine, and for seeds harvested in 2011, it was linked with less progoitine (Table 6). In some cases, fungicide treatment coincided with the lower content of alkenyl glucosinolates as compared to the control. In Glubczyce seeds harvested in 2010 and 2012 from fungicide-treated plots had lower levels of glucobrassicanapine and seeds harvested in 2011 had lower content of napoleiferine (Table 6). In Toszek, such relationship was not found (Table 7).

Table 6.
The content of glucosinolates (μMg−1) in the seeds of winter oilseed rape cv. PR46W10 in 2009–2012, in Glubczyce (Opole Region, Poland) depending on the time of fungicide application (flusilazole 250 g·L−1). Each spray (1–8) was a single application, and the sprays were done in the autumn at weekly intervals (see Figure 1 for experiment scheme and Table 1 for spray dates). Control plots were not sprayed with the fungicide.

Table 7.
The content of glucosinolates (μMg−1) in the seeds of winter oilseed rape cv. PR46W10 in 2009–2012, in Toszek (Opole Region, Poland), depending on the time of fungicide application (flusilazole 250 g·L−1). Each spray (1–8) was a single application, and the sprays were done in the autumn at weekly intervals (see Figure 1 for experiment scheme and Table 1 for spray dates). Control plots were not sprayed with the fungicide.
4. Discussion and Conclusions
In our study, in three out of eight site × year combinations, the seed yield of oilseed rape was significantly higher after a single fungicide treatment made in the autumn, at the plant rosette stage. The significant impact of stem canker on the decrease of winter oilseed rape yield was noted only in years with warm and wet autumn, when a cumulative mean temperature of the autumn weeks between BBCH14 and BBCH19 plant growth stages (8 weeks) exceeded 60 °C and the precipitation in this period exceeded 110 mm of rain. Stem canker affected seed yield only when these two parameters were combined. Under these conditions, the highest seed yield, significantly differing from the unsprayed control, was obtained two times when the application of the fungicide was done between BBCH14–BBCH16 (four to six true leaves) and one time when fungicide application was done between BBCH15–BBCH17 (five to seven true leaves). Fungicide applications at BBCH15–BBCH16 (5–6 true leaves) are recommended.
It is widely known that the weather directly affects plant disease development, and this phenomenon is also true for stem canker of winter oilseed rape [8,9]. In recent years, a lot of attention has been paid to the effects of climate change on the development of crop plant epidemics. In the UK, the predictions showed a high probability of much earlier start of the stem canker stage in 2050, as compared to 1990 [21]. Moreover, the predicted severities of stem canker at harvest increased to reach 2.3 in 2050, on the 0–4 scale. In Poland, the prolonged plant vegetation caused by higher air temperature has been increasing in the last years; in the period of 2000–2010, there were two years with an average autumn temperature higher than the multi-year average, whereas in 2011–2020, the number of warm autumns doubled as compared to the previous 10 years (https://www.imgw.pl/sites/default/files/2021-02/imgw-pib-klimat-polski-2020-opracowanie.pdf accessed on 7 June 2021). One may speculate that weather change, if continued in the current direction, will increase the risk of stem canker of oilseed rape in Poland, and generally in central and eastern Europe. By now, the disease was mitigated by cold autumns and winter frosts, whereas warmer autumns and mild winters, accompanied by rain and no snow cover, may dramatically increase the progression of the infections caused by L. maculans and L. biglobosa. It may also contribute to higher and longer periods of the inoculum occurrence. The evidence that warm and wet autumns will affect seed yield is supported by the observations that stem canker epidemics are most severe in countries with Mediterranean climates [9,21].
In our experiment, the incidence of phoma leaf spotting in the autumn, in the seasons when the seed yield increase resulting from plant treatment with azole fungicide was significant, ranged between 2 and 21% of infected plants, whereas in fungicide untreated controls, it ranged between 11 and 22% of infected plants. In general, the disease severity (percentage of infected plants) showed higher correlation with seed yield, as compared to the disease incidence, both in the case of phoma leaf spotting and stem canker stage. Phoma leaf spotting and stem canker symptoms, in five out of eight site x year combinations studied by our team, did not lead to the decrease of seed yield of winter oilseed rape.
High disease severity leads to high yield losses [56]. Fungicide application may decrease the severity of the disease, but it will not necessarily increase the seed yield [57]. Huang et al. [58] showed that fungicides applied to the plants with phoma leaf spotting prevented the pathogen from further developing and no hyphae was visible along the leaf petiole; these results were obtained in vitro and their transposition to field settings remain unclear. Studies done at different climatic regions of Poland demonstrated a very strong relationship between the fungicide treatments and health status of oilseed rape plants [41,59]. However, the best time for fungicide application varied from year to year and it differed between the weather zones [59].
In the UK, the application of fungicides significantly reduced the number of leaf lesions, when the incidence of L. maculans leaf spotting reached 10% of plants. Additional fungicide treatments further decreased the number of leaf lesions, but the effect was much smaller as compared to the earlier fungicide application [60]. In Poland, since 2017 the fungicide applications are recommended when 10% of oilseed rape plants show phoma leaf spots in the autumn or 15% of plants have such leaf lesions in the following spring [61]. However, in this study, even 30% of plants with phoma leaf spots did not lead to the significant yield loss, if the severity of leaf symptoms was low and the autumn was cold (as was the case of Glubczyce 2009/2010) or dry (Glubczyce 2010/2011). Spring droughts often encountered in Poland [62] can further change the severity of phoma stem canker.
Optimization of the timing of fungicide application based on the percentage of affected plants relies upon regular disease assessments and there is a threat that the pathogen can quickly reach the stem [10,36]. Such case is likely, when a protective treatment is delayed, e.g., due to weather conditions that are not suitable for the fungicide spray. The use of accurate system to predict the risk of plant infection or the model to predict the onset of ascospores allow effective deployment of disease control measures [39]. The decision on fungicide applications should be made on a local or farm scale [34].
The application of flusilazole-based fungicide done in this study had little or no effect on seed quality parameters. The oil content increased in the seeds of winter oilseed rape after a fungicide application only in the season where the incidence of phoma leaf spotting and stem canker was the highest among the studied site × year combinations (over 30% of infected plants both in the autumn and at harvest time). However, there was a clear tendency to increase oil content after the azole-fungicide treatment. In contrast, McCartney and collaborators found that phoma stem canker may affect oil content in seeds of oilseed rape, but there were differences between double-low and high erucic acid cultivars [63]. Seeds harvested from the fungicide-untreated plots of double-low cultivars usually had lower oil contents (up to 5%) than corresponding fungicide-treated plots. Furthermore, severe disease symptoms caused changes in the fatty acid composition. Similarly to oil content, the changes depended on the cultivar, the local disease pattern, and harvesting year peculiarities [63].
The glucosinolate content depends on genetic factors [64], agrotechnical procedures [65,66], and environmental conditions [67]. Moreover, some authors pointed out that fungicide treatments had an effect on the amount of glucosinolates in harvested seeds [41,68]. Paclobutrazole significantly reduced glucosinolates in the seeds of oilseed rape in comparison with untreated control plants [68]. In our previous studies, fungicide treatment coincided with the increase of indole glucosinolates and decreased content of alkenyl glucosinolates [41]. In this study, such correlation was significant only in some cases.
This experiment confirmed our previous findings about the seasonal changes in the populations of L. maculans and L. biglobosa inhabiting plant tissues. Both studied species of Leptosphaeria were present at different frequencies, depending on the stage of disease development. In the autumn, L. maculans was predominant on leaves of oilseed rape plants (phoma leaf spotting stage) and the species L. biglobosa was more frequent on stems, which is a possible consequence of its faster progression from the leaf lamina to the stem [39,40]. Similar findings were obtained in the Polish province of Lower Silesia [41]. In the Czech Republic, in autumn seasons, the dominant pathogen was L. maculans. This was confirmed in 98% of samples of infected oilseed rape leaves [68]. In the UK, in 2001–2003, when isolates from stem cankers were visually identified as L. maculans or L. biglobosa based on cultural morphological characteristics, 70% were L. maculans and 30% were L. biglobosa [39]. The quantities of L. maculans DNA were greater in cankers from southern England and those of L. biglobosa DNA were greater in northern England [69]. Moreover, the use of fungicides affects pathogen populations, since L. maculans and L. biglobosa differ in their sensitivities to active substances present in fungicides, with L. biglobosa being less sensitive to fungicides than L. maculans [13,38,40]. This suggests that regional variation in regimes of fungicide application may affect the occurrence of L. maculans and L. biglobosa. Both species are distributed worldwide and they coexist in many regions [11]. There is evidence that L. maculans was invading areas previously colonized only by L. biglobosa [70]. In the 1990s in Poland, L. biglobosa prevailed (95%) [71], whereas in this study, both species populations were equal, but differed along the season, with L. maculans prevailing on leaves and L. biglobosa dominating on stems. Fitt and collaborators [9] concluded that L. biglobosa predates L. maculans in evolutionary terms, thus it may be adapted to a wider range of environmental conditions.
Although both species differ in their sensitivity to fungicides [38,39,40], they are sensitive to the same active substances. Sewell and collaborators [72] demonstrated that penthiopyrad used jointly with picoxystrobin and prothioconazole can decrease phoma stem canker of winter oilseed rape. In the studies done by Zamani-Noor and Knüfer [57], the disease severity was the lowest after the application of fluxapyroxad combined with tebuconazole. The application of these fungicides was the most effective treatment in reducing yield losses, but it did not significantly affect seed quality. Other studies in controlled environment and field conditions investigated effects of the fungicide containing flusilazole and carbendazim on the growth of L. maculans and L. biglobosa and great differences were found between the reactions of these two species [72]. In controlled environment experiments, fungicide treatment decreased the lesion size and amount of pathogen DNA in leaves, but only in the case of L. maculans. Similarly to our experiment, fungicide treatment decreased phoma leaf spotting and stem canker severity, but the yield increase was found only in three out of eight cases. Huang and collaborators [73] concluded that the interplay between L. maculans and L. biglobosa may impact the final disease symptoms. In general, our experiment as well as the experiment by Huang et al. [73] have demonstrated the efficacy of flusilazole. Unfortunately, recent studies proved its toxicity to the endocrine system [74].
Due to their effectiveness toward L. maculans and L. biglobosa, azole fungicides that are less toxic to the environment remain a useful component of the integrated control of phoma stem canker [72]. Apart from flusilazole, used in this study, and tebuconazole, mentioned above [57], there are several other azole fungicides, including difenoconazole, prothioconazole, metconazole, paclobutrazole, and tetraconazole currently registered to protect oilseed rape against the stem canker. They all belong to the same group of active substances and their common target is lanosterol 14-α demethylase—a key enzyme in the biosynthesis of ergosterol in fungi. Sewell and coauthors [72] hypothesized that different sensitivity to azoles plays a role in pathogen frequency change in crops. The authors compared homologous protein models, identified highly conserved residues, particularly at the azole binding site, and only minor differences were found in sensitivity checked in vitro and in planta. The authors [72] concluded that both proteins are similarly sensitive to azole fungicides.
Based on our findings, we concluded that seed yield loss caused by phoma stem canker of oilseed rape was significant only in the years with warm and wet autumn. Many fungicide treatments against stem canker of winter oilseed rape could be avoided if the fungicide applications were made based on accurate local weather predictions. The precise pesticide applications can help maintain the profitability and sustainability of winter oilseed rape. Currently, much hope is also attributed to resistance breeding, as the specific resistance loci in plants (Rlm resistance genes) are highly efficient in controlling the pathogen populations, but improved resistance management for durable disease control is in demand [75]. Due to the ban of several substances—active against Leptosphaeria species, there are also great hopes and expectations connected with the new bio-based formulations.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/agronomy11061171/s1: Table S1 (Meteorological data at experimental site in Glubczyce (50°12′0.339″N, 17°49′45.552″E), Opole Region, Poland), Table S2 (Meteorological data at experimental site in Toszek (50°27′18.383″N, 18°31′5.017″E), Opole Region, Poland), Table S3 (Spearman’s rank correlation coefficients between the incidence and severity of phoma leaf spotting (autumn) and stem canker (summer) symptoms and seed yield of winter oilseed rape for the data obtained at Glubczyce and Toszek experimental sites, Opole Region, Poland).
Author Contributions
Conceptualization, A.B. and M.J.; methodology, A.B., J.K., and M.J.; software, J.K.; validation, M.J.; formal analysis, J.K.; investigation, A.B. and J.K.; resources, A.B.; data curation, M.J.; writing—original draft preparation, A.B. and J.K.; writing—review and editing, M.J.; visualization, J.K.; supervision, M.J.; project administration, M.J.; funding acquisition, M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by DuPont Poland Ltd.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data presented in this paper are available from the Institute of Plant Genetics, Polish Academy of Sciences (M.J.—the corresponding author) upon reasonable request.
Acknowledgments
The authors thank the Central Cultivar Testing Station in Slupia Wielka for sharing with meteorological data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carre, P.; Pouzet, A. Rapeseed market, worldwide and in Europe. OCL 2014, 21, D102. [Google Scholar] [CrossRef]
- EU-28. Oilseeds and Products Annual 2018. Global Agricultural Information Network Report, 2018. Available online: https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Oilseeds%20and%20Products%20Annual_Vienna_EU-28_3-29-2018.pdf (accessed on 30 March 2021).
- Grain Central. Rapeseed Oil Consumption Growing Globally. Available online: https://www.graincentral.com/cropping/oilseeds/rapeseed-oil-consumption-growing-globally/ (accessed on 30 March 2021).
- Lin, L.; Allemekinders, H.; Dansby, A.; Campbell, L.; Durance-Tod, S.; Berger, A.; Jones, P. Evidence of health benefits of canola oil. Nutr. Rev. 2013, 71, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Gül, M.K.; Amar, S. Sterols and the phytosterol content in oilseed rape (Brassica napus L.). J. Cell Mol. Biol. 2006, 5, 71–79. [Google Scholar]
- Jakobsen, G.V.; Jensen, B.B.; Knudsen, K.E.B.; Canibe, N. Improving the nutritional value of rapeseed cake and wheat dried distillers grains with solubles by addition of enzymes during liquid fermentation. Anim. Feed Sci. Technol. 2015, 208, 198–213. [Google Scholar] [CrossRef]
- Mansour, E.H.; Dworschák, E.; Lugasi, A.; Gaál, Ö.; Barna, É.; Gergely, A. Effect of processing on the antinutritive factors and nutritive value of rapeseed products. Food Chem. 1993, 47, 247–252. [Google Scholar] [CrossRef]
- Dawidziuk, A.; Kaczmarek, J.; Podlesna, A.; Kasprzyk, I.; Jedryczka, M. Influence of meteorological parameters on Leptosphaeria maculans and L. biglobosa spore release in central and eastern Poland. Grana 2012, 51, 240–248. [Google Scholar] [CrossRef]
- Fitt, B.D.L.; Brun, H.; Barbetti, M.J.; Rimmer, S.R. World-wide importance of phoma stem canker (Leptosphaeria maculans and L. biglobosa) on oilseed rape (Brassica napus). Eur. J. Plant Pathol. 2006, 114, 3–15. [Google Scholar] [CrossRef]
- Aubertot, J.N.; Pinochet, X.; Doré, T. The effects of sowing date and nitrogen availability during vegetative stages on Leptosphaeria maculans development on winter oilseed rape. J. Crop Prot. 2004, 23, 635–645. [Google Scholar] [CrossRef]
- Fitt, B.D.L.; Huang, Y.J.; van den Bosch, F.; West, J.S. Coexistence of related pathogen species on arable crops in space and time. Annu. Rev. Phytopathol. 2006, 44, 163–182. [Google Scholar] [CrossRef]
- Guo, X.W.; Fernando, W.G.D.; Entz, M. Effects of crop rotation and tillage on blackleg disease of canola. Can. J. Plant Pathol. 2005, 27, 53–57. [Google Scholar] [CrossRef]
- Eckert, M.; Gout, L.; Rouxel, T.; Blaise, F.; Jedryczka, M.; Fitt, B.; Balesdent, M.H. Identification and characterization of polymorphic minisatellites in the phytopathogenic ascomycete Leptosphaeria maculans. Curr. Genet. 2005, 47, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Marcroft, S.J.; Sosnowski, M.R.; Scott, E.S.; Ramsey, M.D.; Salisbury, P.A.; Howlett, B.J. Brassica napus plants infected by Leptosphaeria maculans after the third to fifth leaf growth stage in south-eastern Australia do not develop blackleg stem canker. Eur. J. Plant Pathol. 2005, 112, 289–292. [Google Scholar] [CrossRef]
- Fernando, W.; Chen, Y.; Ghanbarnia, K. Breeding for blackleg resistance: The biology and epidemiology. Adv. Bot. Res. 2007, 45, 271–311. [Google Scholar]
- Salam, M.U.; Fitt, B.D.L.; Aubertot, J.N.; Diggle, A.J.; Huang, Y.J.; Barbetti, M.J.; Gladders, P.; Jedryczka, M.; Khangura, R.K.; Wratten, N.; et al. Two weather-based models for predicting the onset of seasonal release of ascospores of Leptosphaeria maculans or L. biglobosa. Plant Pathol. 2007, 56, 412–423. [Google Scholar] [CrossRef]
- Savage, D.; Barbetti, M.J.; MacLeod, W.; Salam, M.U.; Renton, M. Seasonal and diurnal patterns of spore release can significantly affect the proportion of spores expected to undergo long-distance dispersal. Microb. Ecol. 2012, 63, 578–585. [Google Scholar] [CrossRef]
- West, J.S.; Kharbanda, P.D.; Barbetti, M.J.; Fitt, B.D.L. Epidemiology and management of Leptosphaeria maculans (phoma stem canker) on oilseed rape in Australia, Canada and Europe. Plant Pathol. 2001, 50, 10–27. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Evans, N.; Li, Z.-Q.; Eckert, M.; Chevre, A.-M.; Renard, M.; Fitt, B.D.L. Temperature and leaf wetness duration affect phenotypic expression of Rlm6-mediated resistance to Leptosphaeria maculans in Brassica napus. New Phytol. 2006, 170, 129–141. [Google Scholar] [CrossRef]
- Sadys, M.; Kaczmarek, J.; Grinn-Gofron, A.; Rodinkova, V.; Prikhodko, A.; Bilous, E.; Strzelczak, A.; Herbert, R.J.; Jedryczka, M. Dew point temperature affects ascospore release of allergenic genus Leptosphaeria. Int. J. Biometeorol. 2018, 62, 979–990. [Google Scholar] [CrossRef]
- Evans, N.; Baierl, A.; Semenov, M.A.; Gladders, P.; Fitt, B.D.L. Range and severity of a plant disease increased by global warming. J. R. Soc. Interface 2008, 5, 525–533. [Google Scholar] [CrossRef]
- Pérès, A.; Poisson, B.; Le Sourne, V.; Maisonneuve, C. Leptosphaeria maculans: Effect of temperature, rainfall and humidity on the formation of pseudothecia. In Proceedings of the 10th International Rapeseed Congress, Canberra, Australia, 26–29 September 1999; Available online: http://www.regional.org.aa/au/gcirc/index.html (accessed on 30 March 2021).
- Petrie, G.A. Effects of temperature and moisture on the number, size and septation of ascospores produced by Leptosphaeria maculans (Blackleg) on rapeseed stubble. Can. Plant Dis. Surv. 1994, 74, 141–151. [Google Scholar]
- Toscano-Underwood, C.; Huang, Y.J.; Fitt, B.D.L.; Hall, A.M. Effects of temperature on maturation of pseudothecia of Leptosphaeria maculans and L. biglobosa on oilseed rape stem debris. Plant Pathol. 2003, 52, 726–736. [Google Scholar] [CrossRef]
- Huang, Y.J.; Liu, Z.; West, J.S.; Todd, A.D.; Hall, A.M.; Fitt, B.D.L. Effects of temperature and rainfall on date of release of ascospores of Leptosphaeria maculans (phoma stem canker) from winter oilseed rape (Brassica napus) debris in the UK. Ann. Appl. Biol. 2007, 151, 99–111. [Google Scholar] [CrossRef]
- Piliponyte-Dzikiene, A.; Kaczmarek, J.; Petraitiene, E.; Kasprzyk, I.; Brazauskiene, I.; Brazauskas, G.; Jedryczka, M. Microscopic and molecular detection of airborne ascospores of Leptosphaeria maculans and L. biglobosa in Lithuania and Poland. Zemdirbyste 2014, 101, 303–312. [Google Scholar] [CrossRef][Green Version]
- Penaud, A.; Jain, L.; Poisson, B.; Balesdent, M.H. Structure of populations of Leptosphaeria maculans in France. In Proceedings of the 10th International Rapeseed Congress, Canberra, Australia, 26–29 September 1999; Available online: http://www.regional.org.au/au/gcirc (accessed on 30 March 2021).
- Kuswinanti, T.; Koopmann, B.; Hoppe, H.H. Virulence pattern of aggressive isolates of Leptosphaeria maculans on an extended set of Brassica differentials. J. Plant Dis. Prot. 1999, 106, 12–20. [Google Scholar]
- Humpherson-Jones, F.M. Pathogenicity studies on isolates of Leptosphaeria maculans from brassica seed production crops in south-east England. Ann. Appl. Biol. 1983, 103, 37–44. [Google Scholar] [CrossRef]
- Gugel, R.K.; Petrie, G.A. History, occurrence, impact, and control of blackleg of rapeseed. Can. J. Plant Pathol. 1992, 14, 36–45. [Google Scholar] [CrossRef]
- Moreno-Rico, O.; Frias-Trevino, A.G.; Luna-Ruiz, J.J.; Manzano-Flores, D.E.; Romero-Cova, S.; Seguin-Swartz, G. Characterization and pathogenicity of isolates of Leptosphaeria maculans from Aguascalientes and Zacatecas, Mexico. Can. J. Plant Pathol. 2001, 23, 270–278. [Google Scholar] [CrossRef]
- Fernando, W.G.D.; Parks, P.S. First report of blackleg disease caused by Leptosphaeria maculans on canola in Brazil. Plant Dis. 2003, 87, 314. [Google Scholar] [CrossRef]
- Gaetan, S.A. First outbreak of blackleg caused by Phoma lingam in commercial canola fields in Argentina. Plant Dis. 2005, 89, 435. [Google Scholar] [CrossRef]
- Jedryczka, M.; Kaczmarek, J.; Dawidziuk, A.; Brachaczek, A. System for forecasting disease epidemics—Aerobiological methods in Polish agriculture. Asp. Appl. Biol. 2008, 89, 65–70. [Google Scholar]
- Lô-Pelzer, E.; Bousset, L.; Jeuffroy, M.H.; Salam, M.U.; Pinochet, X.; Boillot, M.; Aubertot, J.N. SIPPOM WOSR: A simulator for integrated pathogen population management of phoma stem canker on winter oilseed rape: I. Description of the model. Field Crop. Res. 2010, 118, 73–81. [Google Scholar] [CrossRef]
- West, J.S.; Evans, N.; Leech, P.K.; Fitt, B.D.L.; Welham, S.J.; Jedryczka, M.; Penaud, A. Predicting leaf infection by Leptosphaeria maculans on oilseed rape. Bull. IOBC 2000, 23, 23–27. [Google Scholar]
- Steed, J.M.; Baierl, A.; Fitt, B.D.L. Relating plant and pathogen development to optimise fungicide control of phoma stem canker (Leptosphaeria maculans) on winter oilseed rape (Brassica napus). Eur. J. Plant Pathol. 2007, 118, 359–373. [Google Scholar] [CrossRef]
- Stonard, J.F.; Latunde-Dada, A.O.; Huang, Y.J.; West, J.S.; Evans, N.; Fitt, B.D.L. Geographic variation in severity of phoma stem canker and Leptosphaeria maculans/L. biglobosa populations on UK winter oilseed rape (Brassica napus). Eur. J. Plant Pathol. 2010, 126, 97–109. [Google Scholar] [CrossRef]
- Eckert, M.R.; Rossall, S.; Selley, A.; Fitt, B.D.L. Effects of fungicides on in vitro spore germination and mycelial growth of the phytopathogens Leptosphaeria maculans and L. biglobosa (phoma stem canker of oilseed rape). Pest Manag. Sci. 2010, 66, 396–405. [Google Scholar] [CrossRef]
- Kaczmarek, J.; Jędryczka, M. Wpływ wybranych fungicydów oraz ich substancji aktywnych na wzrost grzybów Leptosphaeria maculans i L. biglobosa w warunkach in vitro. Prog. Plant Prot. 2010, 50, 648–651. [Google Scholar]
- Brachaczek, A.; Kaczmarek, J.; Jędryczka, M. Monitoring blackleg (Leptosphaeria spp.) ascospore release timing and quantity enables optimal fungicide application to improved oilseed rape yield and seed quality. Eur. J. Plant Pathol. 2016, 145, 643–657. [Google Scholar] [CrossRef][Green Version]
- Gupta, P.K. Toxicity of Fungicides. In Veterinary Toxicology, 3rd ed.; Gupta, R.C., Ed.; Academic Press: San Diego, CA, USA, 2018; Volume 3, pp. 569–580. [Google Scholar]
- Roberts, J.R.; Reigart, J.R. Fungicides. In Recognition and Management of Pesticide Poisonings, 6th ed.; U.S. Environmental Protection Agency: Washington, DC, USA, 2013; pp. 143–160. [Google Scholar]
- Petit, A.N.; Fontaine, F.; Vatsa, P.; Clément, C.; Vaillant-Gaveau, N. Fungicide impacts on photosynthesis in crop plants. Photosynth. Res. 2012, 111, 315–326. [Google Scholar] [CrossRef]
- Baćmaga, M.; Wyszkowska, J.; Kucharski, J. The influence of chlorothalonil on the activity of soil microorganisms and enzymes. Ecotoxicology 2018, 27, 1188–1202. [Google Scholar] [CrossRef]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Brühl, C.A.; Imfeld, G.; Knäbel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmüller, A.; et al. Fungicides: An overlooked pesticide class? Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef]
- Fustinoni, S.; Rosa Mercadante, R.; Polledri, E.; Rubino, F.M.; Mandic-Rajcevic, S.; Vianello, G.; Colosio, C.; Moretto, A. Biological monitoring of exposure to tebuconazole in winegrowers. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 643–649. [Google Scholar] [CrossRef] [PubMed]
- European Green Deal. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 30 March 2021).
- Brachaczek, A.; Kamiński, M.; Kaczmarek, J.; Jędryczka, M. Economic value of oilseed rape cultivars under production conditions with full fungicide protection technology using the SPEC forecasting system. Oilseed Crop. 2010, 31, 67–83. [Google Scholar]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Kaczmarek, J.; Jedryczka, M. Characterization of two coexisting pathogen populations of Leptosphaeria spp., the cause of stem canker of brassicas. Acta Agrobot. 2011, 64, 3–14. [Google Scholar] [CrossRef]
- Kaczmarek, J. Rozwój Stadium Generatywnego Grzybów Leptosphaeria maculans ([Desm.] Ces. et de Not.) i L. biglobosa (Shoemaker i Brun 2001) Oraz Ochrona Rzepaku Przed Tymi Patogenami. Ph.D. Thesis, Institute of Plant Genetics PAS, Poznan, Poland, 2010. [Google Scholar]
- Jedryczka, M. Epidemiology and damage caused by stem canker of oilseed rape in Poland. Phytopathol. Pol. 2007, 45, 73–75. [Google Scholar]
- Soxhlet, F. Die gewichtsanalytische Bestimmung des Milchfettes. Dinglers Polytech. J. 1879, 232, 461–465. [Google Scholar]
- Kjeldahl, J. Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern. Fresenius Zeitschriftf. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Sprague, S.J.; Kirkegaard, J.A.; Howlett, B.J.; Graham, J. Effect of root rot and stem canker caused by Leptosphaeria maculans on yield of Brassica napus and measures for control in the field. Crop Pasture Sci. 2010, 61, 50–58. [Google Scholar] [CrossRef]
- Zamani-Noor, N.; Knüfer, J. Effects of host plant resistance and fungicide application on phoma stem canker, growth parameters and yield of winter oilseed rape. Crop Prot. 2018, 112, 313–321. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Qi, A.; King, G.J.; Fitt, B.D. L Assessing quantitative resistance against Leptosphaeria maculans (phoma stem canker) in Brassica napus (oilseed rape) in young plants. PLoS ONE 2014, 9, e84924. [Google Scholar] [CrossRef]
- Kaczmarek, J.; Brachaczek, A.; Jedryczka, M. The effect of fungicide spray time on the incidence of stem canker of Brassicas and seed yield of winter oilseed rape in Pomerania. J. Plant Dis. Prot. 2014, 121, 58–63. [Google Scholar] [CrossRef]
- Sewell, T.R.; Hawkins, N.J.; Stotz, H.U.; Huang, Y.-J.; Kelly, S.L.; Kelly, D.E. Azole sensitivity in Leptosphaeria pathogens of oilseed rape: The role of lanosterol 14α-demethylase. Sci. Rep. 2017, 7, 15849. [Google Scholar] [CrossRef] [PubMed]
- Jajor, E.; Mrówczyński, M.; Bartkowiak-Broda, I.; Broniarz, J.; Danielewicz, J.; Dobrzycka, A.; Dworzańska, D.; Fiedler, Ż.; Gorzała, G.; Horoszkiewicz-Janka, J.; et al. Metodyka Integrowanej Ochrony i Produkcji Rzepaku Ozimego Oraz Jarego Dla Doradców; IOR–PIB: Poznań, Poland, 2017; pp. 1–264. [Google Scholar]
- Pińskwar, I.; Choryński, A.; Kundzewicz, Z.W. Severe drought in the spring of 2020 in Poland—More of the same? Agronomy 2020, 10, 1646. [Google Scholar] [CrossRef]
- McCartney, H.A.; Doughty, K.J.; Norton, G.; Booth, E.J.; Kightley, S.P.J.; Landon, G.; West, G.; Walker, K.C.; Thomas, J.E. A study of the effect of disease on seed quality parameters of oilseed rape. In Proceedings of the 10th International Rapeseed Congress, Canberra, Australia, 26–29 September 1999; Available online: http://www.regional.org.aa/au/gcirc/index.html (accessed on 5 June 2021).
- Wenda-Piesik, A.; Hoppe, S. Evaluation of hybrid and population cultivars on standard and high-input technology in winter oilseed rape. Acta Agric. Scand. Sect. B Soil Plant Sci. 2018, 68, 678–689. [Google Scholar]
- Jankowski, K.J.; Sokólski, M.; Szatkowski, A. The effect of autumn foliar fertilization on the yield and quality of winter oilseed rape seeds. Agronomy 2019, 9, 849. [Google Scholar] [CrossRef]
- Gugała, M.; Sikorska, A.; Zarzecka, K. The effect of fertilization with sulphur, boron, and amino acids on the content of glucosinolate in winter rape seeds. Agronomy 2020, 10, 519. [Google Scholar] [CrossRef]
- Varga, L.; Ložek, O.; Ducsay, L.; Kováčik, P.; Lošák, T.; Hlušek, J. Effect of top dressing with nitrogen and boron on the yield and quality of rapeseed. Acta Univ. Agric. Silvic. Mendel. Brun. 2014, 58, 391–398. [Google Scholar] [CrossRef]
- Baylis, A.D.; Hutley-Bull, P.D. The effects of a paclobutrazol based growth regulator on the yield, quality and ease of management of oilseed rape. Ann. Appl. Biol. 1991, 118, 445–452. [Google Scholar] [CrossRef]
- Poslušná, J.; Plachká, E.; Horáček, J.; Macháčková, I.; Ondráčková, E.; Šmirous, P., Jr.; Vrbovský, V. The harmfulness of phoma stem canker, Sclerotinia stem rot, and phytoplasma on winter oilseed rape with regard to Czech breeding programs. Agronomy 2019, 9, 75. [Google Scholar] [CrossRef]
- Stonard, J.F.; Marchant, B.P.; Latunde-Dada, A.O.; Liu, Z.; Evans, N.; Gladders, P.; Eckert, M.R.; Fitt, B.D.L. Geostatistical analysis of the distribution of Leptosphaeria species causing phoma stem canker on winter oilseed rape (Brassica napus) in England. Plant Pathol. 2010, 59, 200–210. [Google Scholar] [CrossRef]
- Jedryczka, M.; Rouxel, T.; Balesdent, M.H.; Mendes Pereira, E.; Bertrandy, J. Molecular characterization of Polish Phoma lingam isolates. Cereal Res. Commun. 1997, 25, 279–283. [Google Scholar] [CrossRef]
- Sewell, T.; Moloney, S.; Ashworth, M.; Ritchie, F.; Mashanova, A.; Huang, Y.-J.; Stotz, H.; Fitt, B.D.L. Effects of a penthiopyrad and picoxystrobin fungicide mixture on phoma stem canker (Leptosphaeria spp.) on UK winter oilseed rape. Eur. J. Plant Pathol 2016, 145, 675–685. [Google Scholar] [CrossRef]
- Huang, Y.J.; Hood, J.; Eckert, M.R.; Stonard, J.F.; Cools, H.J.; King, G.J.; Rosall, S.; Ashworth, F.; Fitt, B.D.L. Effects of fungicide on growth of Leptosphaeria maculans and L. biglobosa in relation to development of phoma stem canker on oilseed rape (Brassica napus). Plant Path. 2011, 60, 607–620. [Google Scholar] [CrossRef]
- Draskau, M.K.; Boberg, J.; Taxvig, C.; Pedersen, M.; Frandsen, H.L.; Christiansen, S.; Svingen, T. In vitro and in vivo endocrine disrupting effects of the azole fungicides triticonazole and flusilazole. Environ. Pollut. 2019, 255, 113309. [Google Scholar] [CrossRef] [PubMed]
- Aubertot, J.N.; West, J.S.; Bousset-Vaslin, L.; Salam, M.U.; Barbetti, M.J.; Diggle, A.J. Improved resistance management for durable disease control: A case study of phoma stem canker of oilseed rape (Brassica napus). Eur. J. Plant Pathol. 2006, 114, 91–106. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).