Micropropagation Supports Reintroduction of an Apulian Artichoke Landrace in Sustainable Cropping Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Introduction of Explants in In Vitro Culture
- BM, regularly used at the Laboratory of Micropropagation and Microscopy-Department of Agricultural and Environmental Science-University of Bari Aldo Moro (Puglia, Italy), for routine culture produced by stocks [19];
- Linsmaier & Skoog Modified Basal Medium (commercial product LS452-PhytoTech Labs, Inc. 14610 W. 106th St, Lenexa KS 66215) [20];
- Murashige and Skoog basal medium (commercial product MS519-PhytoTech Labs, Inc. 14610 W. 106th St, Lenexa KS 66215) [17];
- MS519 modified by the authors increasing calcium chloride and magnesium sulphate content (plus 120 mg L−1 and 190 mg L−1 respectively) and named by the authors MS519-A (A as artichoke).
2.2. Rooting and Acclimatization with Mycorrhizal Fungi Inoculation
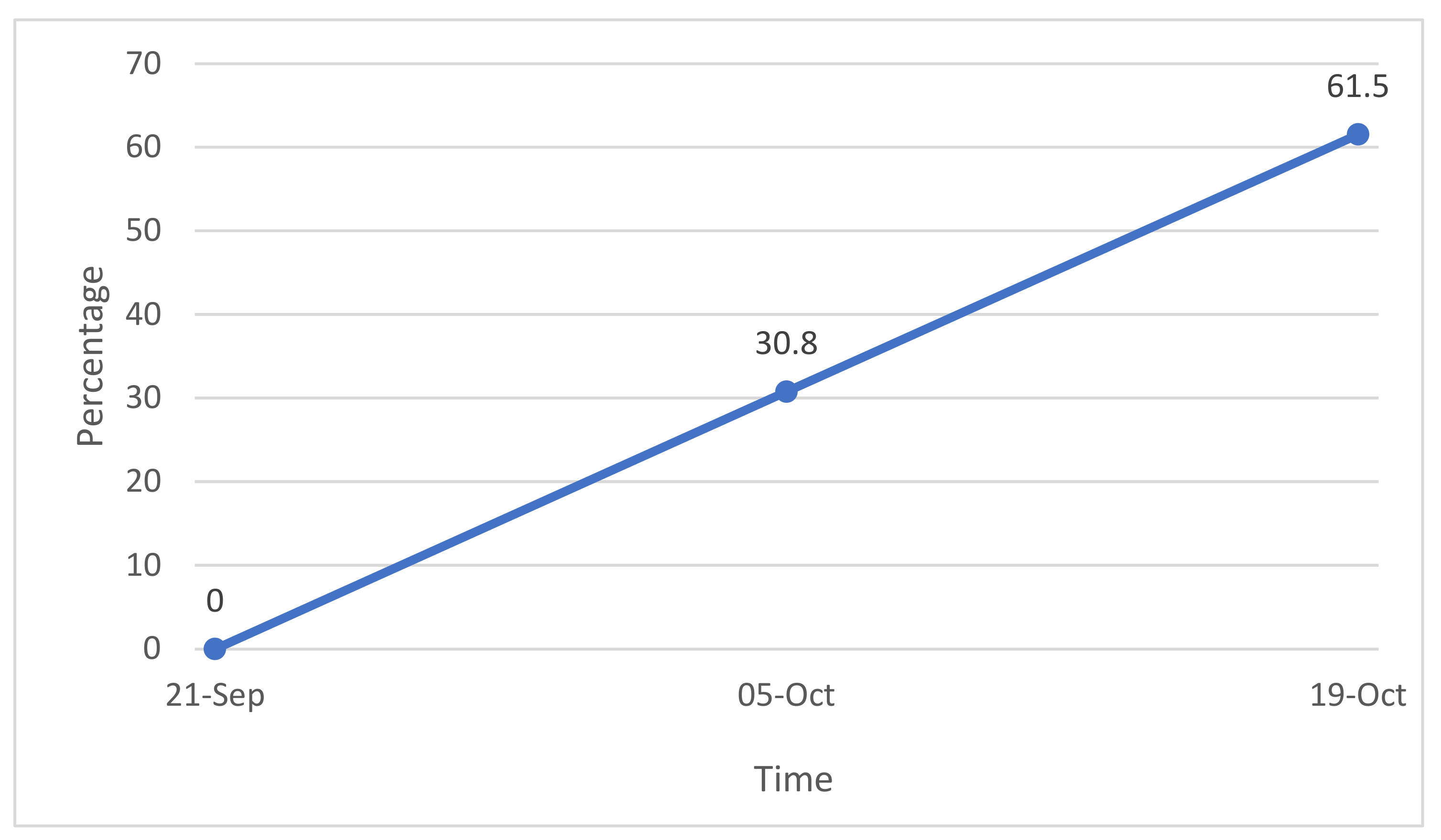
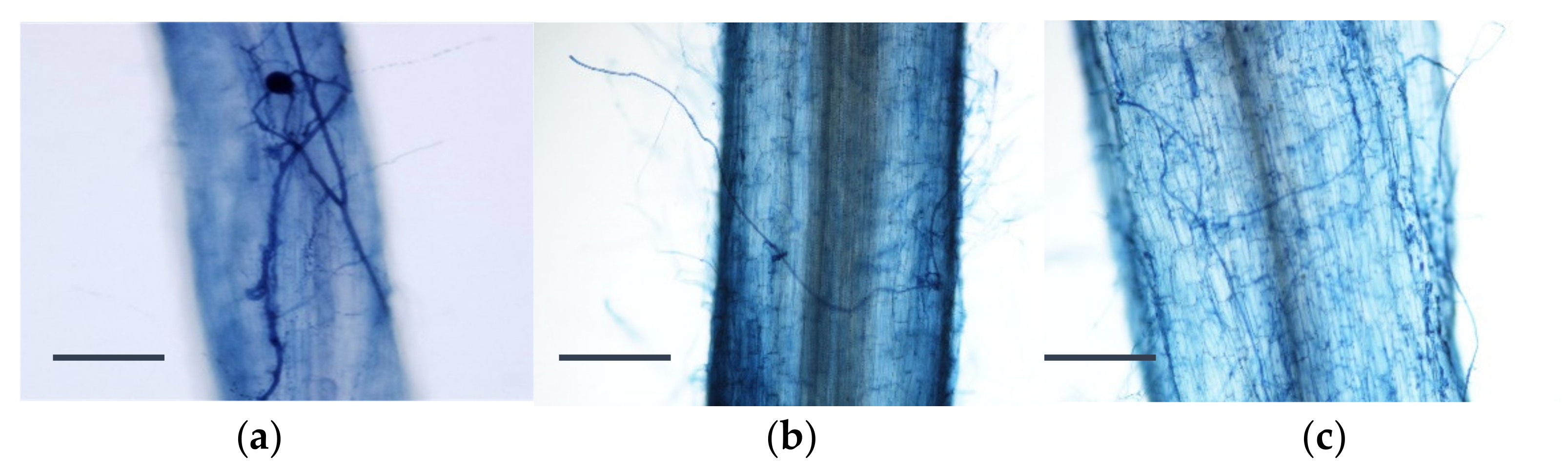
2.3. Evaluation of the Mycorrhizal Infection
2.4. Statistical Analysis
3. Results
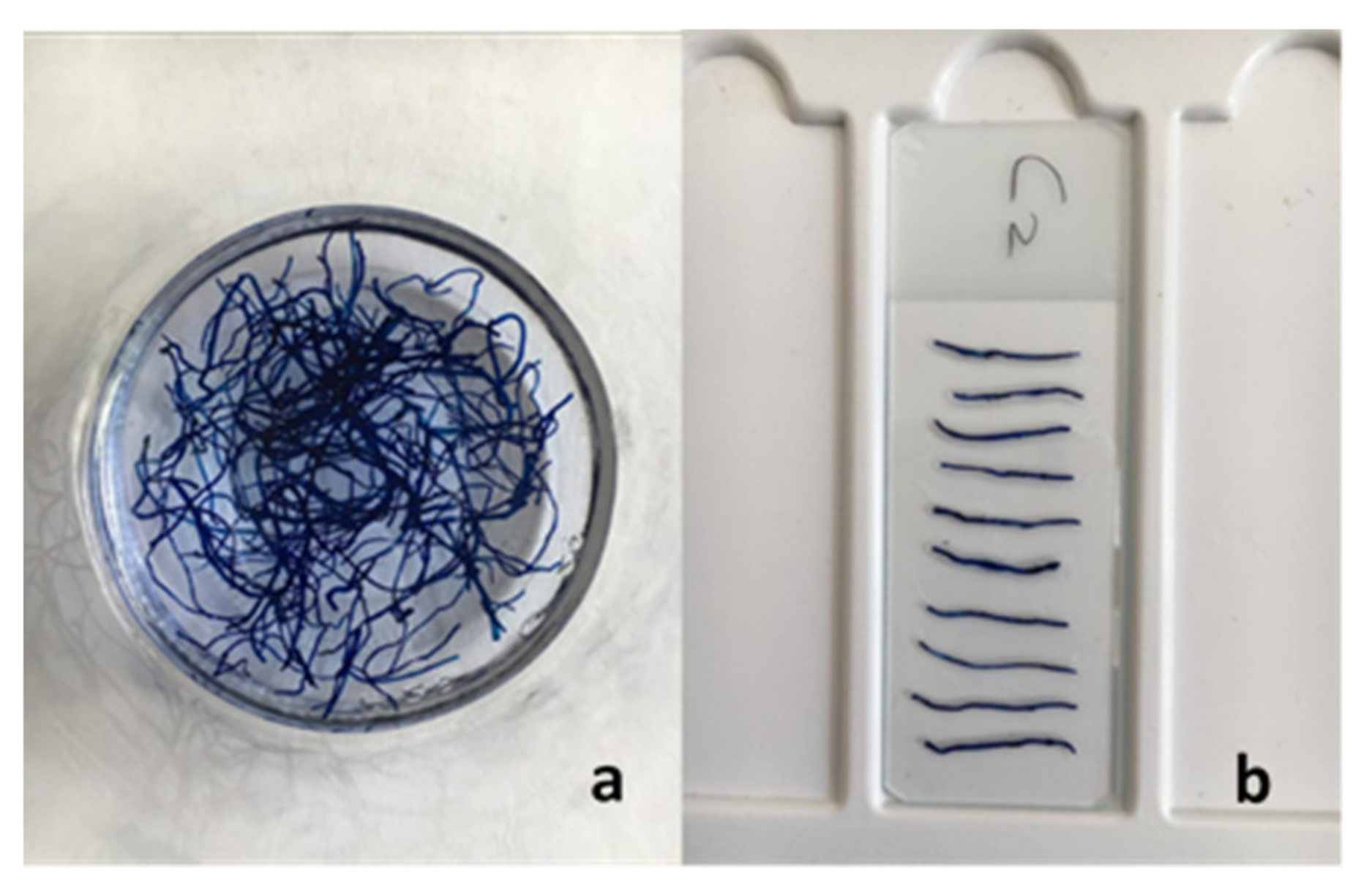
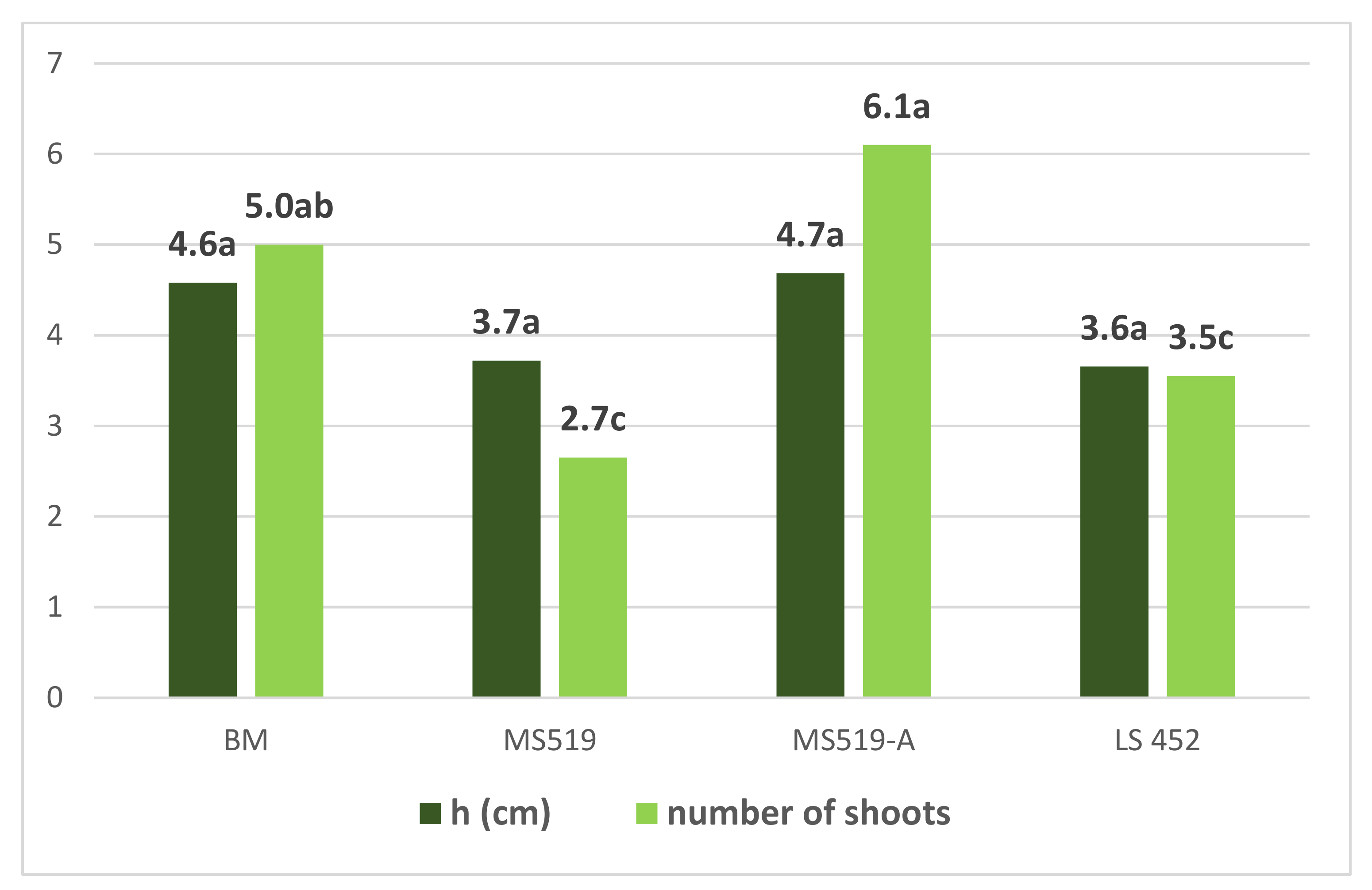
3.1. Introduction of Explants in In Vitro Culture
3.2. Rooting
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Falco, B.; Incerti, G.; Amato, M.; Lanzotti, V. Artichoke: Botanical, agronomical, phytochemical, and pharmacological overview. Phytochem. Rev. 2015, 14, 993–1018. [Google Scholar] [CrossRef]
- FAO STAT 2019. Available online: http://faostat.fao.org (accessed on 26 January 2021).
- Spanò, R.; Bottalico, G.; Corrado, A.; Campanale, A.; Di Franco, A.; Mascia, T. A Protocol for Producing Virus-Free Artichoke Genetic Resources for Conservation, Breeding, and Production. Agriculture 2018, 8, 36. [Google Scholar] [CrossRef]
- Duelli, P. Concepts of multifunctional agriculture and functional biodiversity. In Bio-Diversity Serving Agriculture; Biala, K., Paracchini, M.L., Terres, J.M., Pointereau, P., Pezet, J., Eds.; Institute for Environment and Sustainability: Ispra, Italy, 2006; p. 44. [Google Scholar]
- Pavan, S.; Curci, P.; Zuluaga, D.; Blanco, E.; Sonnante, G. Genotyping-by-sequencing highlights patterns of genetic structure and domestication in artichoke and cardoon. PLoS ONE 2018, 13, e0205988. [Google Scholar] [CrossRef]
- Costanzo, A.; Bàrberi, P. Functional agrobiodiversity and agroecosystem services in sustainable wheat production. A review. Agron. Sustain. Dev. 2014, 34, 327–348. [Google Scholar] [CrossRef]
- Mauromicale, G.; Licandro, P.; Ierna, A.; Morello, N.; Santoiemma, G. Planning of Globe Artichoke Plantlets Production in Nursery. Acta Hortic. 2005, 660, 279–284. [Google Scholar] [CrossRef]
- Bekheet, S.A. In vitro Preservation of Globe Artichoke Germplasm. Plant Tissue Cult. Biotechnol. 2007, 17, 1–9. [Google Scholar] [CrossRef]
- Ruta, C.; Tagarelli, A.; Campanelli, A.; De Mastro, G. Field performance of micropropagated and mycorrhizal early globe artichoke plants. Eur. J. Agron. 2018, 99, 13–20. [Google Scholar] [CrossRef]
- Martin, F.M.; Perotto, S.; Bonfante, P. Mycorrhizal Fungi: A fungal community at the interface between soil and roots. In The Rhizosphere: Biochemistry and Organic Substances at the Soil-Plant Interface; Pinton, R., Varanini, Z., Nannipieri, P., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 202–228. [Google Scholar]
- Green, J.J.; Baddeley, J.A.; Cortina, J.; Watson, C.A. Root development in the Mediterranean shrub Pistacia lentiscus as affected by nursery treatments. J. Arid Environ. 2005, 61, 1–12. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, L.R.; Zhu, H.H.; Chen, J.Z. Effect of arbuscular mycorrhizal fungal inoculation on root system architecture of trifoliate orange (Poncirus trifoliata L. Raf.) seedlings. Sci. Hortic. 2009, 121, 458–461. [Google Scholar] [CrossRef]
- Smith, S.E.; Facelli, E.; Pope, S.; Smith, F.A. Plant performance in stressful environments: Interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant Soil 2010, 326, 3–20. [Google Scholar] [CrossRef]
- Ciccarese, F.; Longo, O.; Paciolla, C.; Schiavone, D.; Morone Fortunato, I. Effetto di funghi micorrizici verso la verticillosi del carciofo. In Proceedings of the Il Carciofo: Dal Laboratorio al Mercato, Rome, Italy, 19–21 April, 2006; pp. 82–84. [Google Scholar]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Ceccarelli, N.; Curadi, M.; Martelloni, L.; Sbrana, C.; Picciarelli, P.; Giovannetti, M. Mycorrhizal colonization impacts on phenolic content and antioxidant properties of artichoke leaves and flower heads two years after field transplant. Plant Soil 2010, 335, 311–323. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Nitsch, J.P.; Nitsch, C. Haploid plants for pollen grains. Science 1969, 63, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Morone Fortunato, I.; Ruta, C.; Castrignanò, A.; Saccardo, F. The effect of mycorrhizal symbiosis on the development of micropropagated artichokes. Sci. Hortic. 2005, 106, 472–483. [Google Scholar] [CrossRef]
- Linsmaier, E.M.; Skoog, F. Organic growth factor requirement of tobacco tissues culture. Physiol. Plant. 1965, 18, 100–127. [Google Scholar] [CrossRef]
- Tarraf, W.; Ruta, C.; Tagarelli, A.; De Cillis, F.; De Mastro, G. Influence of arbuscular mycorrhizae on plant growth, essential oil production and phosphorus uptake of Salvia officinalis L. Ind. Crop. Prod. 2017, 102, 144–153. [Google Scholar] [CrossRef]
- Dalpè, Y.; Monreal, M. Arbuscular mycorrhiza inoculums to support sustainable cropping systems. Crop Manag. 2004, 10, 1094–1104. [Google Scholar] [CrossRef]
- Koske, R.E.; Gemma, J.N. A modified procedure for staining roots to detect VA mycorrhizas. Mycol. Res. 1989, 92, 486–488. [Google Scholar] [CrossRef]
- Ancora, G.; Belli, M.L.; Cuozzo, L. Globe artichoke plants from shoot apices through rapid in vitro micropropagation. Sci. Hortic. 1981, 14, 207–213. [Google Scholar] [CrossRef]
- Ancora, G. Globe artichoke (Cynara scolymus L.). In Biotechnology in Agriculture & Forestry, 2. Crops; Bajai, Y.P.S., Ed.; Springer Verlag: Berlin, Germany, 1986; pp. 471–484. [Google Scholar]
- Harbaoui, Y.; Debergh, P. Multiplication in vitro de clones selectionne’s l’artichaut (Cynara scolymus L.). In Application de la Colture In Vitro a L’amelioration des Plantes Potageres; Eucarpia: Wageningen, The Netherlands, 1980; pp. 1–7. [Google Scholar]
- Campanelli, A.; Ruta, C.; Tagarelli, A.; Morone-Fortunato, I.; De Mastro, G. Effectiveness of mycorrhizal fungi on globe artichoke (Cynara cardunculus L. var. scolymus) micropropagation. J. Plant Interact. 2014, 9, 100–106. [Google Scholar] [CrossRef]
- Benoit, H.; Ducreux, G. Etude de quelques aspects de la multiplication végétative in vitro de l’artichaut (Cynara scolymus L.). Agronomie 1981, 1, 225–230. [Google Scholar] [CrossRef]
- Morzadec, J.M.; Hourmant, A. In vitro rooting improvement of globe artichoke (cv. Camus de Bretagne) by GA3. Sci. Hortic. 1997, 72, 59–62. [Google Scholar] [CrossRef]
- López-Pérez, A.J.; Martínez, J.A. In vitro Root Induction Improvement by Culture in Darkness for Different Globe Artichoke Cultivars. In Vitro Cell. Dev. Biol. Plant 2015, 51, 160–165. [Google Scholar] [CrossRef]
- Draoui, N.; Ghorbel, A.; Kchouk, M.E. In vitro culture of globe artichoke (Cynara scolymus L.) in Tunisia: Utilization of vitro methods in artichoke improvement. Agric. Mediterr. 1993, 123, 139–145. [Google Scholar]
- Iapichino, G. Micropropagation of Globe Artichoke (Cynara scolymus L.) from underground dormant buds (ovoli). In Vitro Cell. Dev. Biol. Plant 1996, 32, 249–252. [Google Scholar] [CrossRef]
- Pacifici, S.; Lucchesini, M.; Curadi, M.; Graifenberg, A. Influence of medium composition and vessel ventilation during the micropropagation stages of Cynara scolymus L. cv. Grato 1. Adv. Hortic. Sci. 2007, 21, 83–88. [Google Scholar]
- Bedini, L.; Lucchesini, M.; Bertozzi, F.; Graifenberg, A. Plant Tissue Cultures from Four Tuscan Globe Artichoke Cultivars. Cent. Eur. J. Biol. 2012, 7, 680–689. [Google Scholar] [CrossRef]
- El Boullani, R.; Elmoshli, A.; El Finti, A.; El Mousadik, A.; Serghini, M.A. Improved in vitro Micropropagation of Artichoke (Cynara cardunculus var. scolymus L.). Eur. J. Sci. Res. 2012, 80, 430–436. [Google Scholar]
- Ercan, N. Effects of Various Growth Regulators on in vitro Rooting of Globe Artichoke (Cynara scolymus L.). J. Agric. Sci. Technol. A 2016, 6, 335–340. [Google Scholar]
- Bigot, C.; Foury, C. In vitro Propagation of Globe Artichoke (Cynara scolymus L.) from Seedlings: Field Comparison of Some Clones with Their Parent Lines. Agronomie 1984, 4, 699–710. [Google Scholar] [CrossRef]
- Ozsan, T.; Onus, A.N. A protocol on in vitro rooting of ‘Bayrampaşa’ artichoke (Cynara scolymus L.). Mediterr. Agric. Sci. 2019, 32, 129–134. [Google Scholar]
- Brutti, C.; Apostolo, N.M.; Ferrarotti, S.A.; Llorente, B.E.; Krymkiewicz, N. Micropropagation of Cynara scolymus L. employing cyclodestrins to promote rhizogenesis. Sci. Hort. 2000, 83, 1–10. [Google Scholar] [CrossRef]
- Ruta, C.; Campanelli, A.; Tagarelli, A.; Morone-Fortunato, I. Mantenimento e propagazione in vitro di ecotipi autoctoni di carciofo. Italus Hortus 2011, 1, 82–84. [Google Scholar]
- Pandino, G.; Lombardo, S.; Antonino, L.M.; Ruta, C.; Mauromicale, G. In vitro micropropagation and mycorrhizal treatment influences the polyphenols content profile of globe artichoke under field conditions. Food Res. Int. 2017, 99, 385–392. [Google Scholar] [CrossRef]
- Ozsan, T.; Onus, A.N. Comparative study on in vitro micropropagation response of seven globe artichoke (Cynara cardunculus var. scolymus (L.) Fiori) cultivars: Open-pollinated cultivars vs F1 hybrids. Not. Bot. Horti Agrobot. 2020, 48, 1210–1220. [Google Scholar] [CrossRef]
- Tavazza, R.; Papacchioli, V.; Ancora, G. An improved medium for in vitro propagation of globe artichoke (Cynara scolymus L.) cv. Spinoso sardo. Acta Hortic. 2004, 660, 91–97. [Google Scholar] [CrossRef]
- Ordas, R.; Tavazza, R.; Ancora, G. In vitro morphogenesis in the globe artichoke (Cynara scolymus L.). Plant Sci. 1990, 71, 233–237. [Google Scholar] [CrossRef]
- Binet, M.N.; Lemoine, M.C.; Martin, C.; Chambon, C.; Gianinazzi, S. Micropropagation of olive (Olea europaea L.) and application of mycorrhiza to improve plantlet establishment. In Vitro Cell Dev. Plant. 2007, 43, 473–478. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N.; Wang, G.Y. Arbuscular mycorrhizal fungi and acclimatization of micropropagated citrus. Comm. Soil Sci. Plant Anal. 2011, 42, 1825–1832. [Google Scholar] [CrossRef]
- Yadav, K.; Singh, N.; Aggarval, A. Arbuscular mycorrhizal technology for the growth enhancement of micropropagated Spilanthes acmella Murr. Plant Prot. Sci. 2012, 48, 31–36. [Google Scholar] [CrossRef]
- Ruta, C.; Perrini, R.; Tagarelli, A.; Morone-Fortunato, I. Use of arbuscular mycorrhiza for acclimatization of micropropagated plantlets of melon, oregano, artichoke and spanish broom. Acta Hortic. 2009, 812, 473–478. [Google Scholar] [CrossRef]
- Yadav, K.; Aggarwal, A.; Singh, N. Arbuscular mycorrhizal fungi induced acclimatization and growth enhancement of Glycyrrhiza glabra L.: A potential medicinal plant. Agric. Res. 2013, 2, 43–47. [Google Scholar] [CrossRef]
- Kapoor, R.; Sharma, D.; Bhatnagar, A.K. Arbuscular mycorrhizae in micropropagation systems and their potential applications. Sci. Hortic. 2008, 116, 227–239. [Google Scholar] [CrossRef]
- Castiglione, V.; Cavallaro, V.; Di Silvestro, I.; Barbera, A.C. Acclimatization of micropropagated globe artichoke (Cynara cardunculus L. subsp. scolymus (L.) Hegi) plantlets as affected by mycorrhizal inoculum, transplantation time and genotype. Acta Hortic. 2009, 812, 461–466. [Google Scholar] [CrossRef]
- Ruta, C.; Tagarelli, A.; Vancini, C.; De Mastro, G. Evaluation of commercial arbuscular mycorrhizal inoculants on micropropagated early globe artichoke during the acclimatization stage. Acta Hortic. 2016, 1147, 369–373. [Google Scholar] [CrossRef]
- Finlay, R.D. Ecological aspects of mycorrhizal symbiosis: With special emphasis on the functional diversity of interactions involving the extraradical mycelium. J. Exp. Bot. 2008, 59, 1115–1126. [Google Scholar] [CrossRef] [PubMed]





| Growth Media (mg L−1) | ||||
|---|---|---|---|---|
| Compounds | MS519 | MS519-A | LS452 | BM |
| Ammonium nitrate | 1650 | 1650 | 1650 | 1650 |
| Calcium Chloride, Anhydrous | 332.2 | 450 | 332.2 | 450 |
| Cobalt Chloride × 6H2O | 0.025 | 0.025 | 0.025 | |
| Cupric Sulfate × 5H2O | 0.025 | 0.025 | 0.025 | 0.025 |
| FeNaEDTA | 40.0 | |||
| Na2EDTA × 2H2O | 37.26 | 37.26 | 37.26 | |
| Ferrous Sulfate × 7H2O | 27.8 | 27.8 | 27.8 | |
| Magnesium Sulfate, Anhydrous | 180.7 | 370 | 180.7 | 370 |
| Manganese Sulfate × H2O | 16.9 | 16.9 | 16.9 | 25 |
| Molybdic Acid (Sodium Salt) × 2H2O | 0.25 | 0.25 | 0.25 | 0. 25 |
| Potassium Iodide | 0.83 | 0.83 | 0.83 | |
| Potassium Nitrate | 1900 | 1900 | 1900 | 1900 |
| Potassium Phosphate, Monobasic | 170 | 170 | 170 | 170 |
| Potassium Hydroxide | 100 | |||
| Zinc Sulfate × 7H2O | 8.6 | 8.6 | 8.6 | 10 |
| Boric Acid | 6.2 | 6.2 | 6.2 | 10 |
| Glycine (Free Base) | 2.0 | 2.0 | ||
| Nicotinic Acid (Free Acid) | 0.5 | 0.5 | ||
| Pyridoxine × HCl | 0.5 | 0.5 | ||
| Thiamine × HCl | 0.1 | 0.1 | 0.4 | 0.4 |
| Myo-Inositol | 100 | 100 | 100 | 100 |
| MES (Free Acid) | 1000 | |||
| Agar | 7000 | 7000 | 7000 | 7000 |
| sucrose | 20,000 | 20,000 | 30,000 | 20,000 |
| BAP | 0.05 | 0.05 | 0.05 | 0.05 |
| Treatment | Ex Vitro | Leaves | Roots | Root/Leaf * | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Survival | Number (n) | Length (cm) | Length (cm) | Colonization (%) | ||||||||||||
| % | T | 3M | T | 3M | T | 3M | 3M | 3M | ||||||||
| Control | 79 | c | 8.08 | a | 10.75 | a | 8.0 | a | 17.90 | c | 4.60 | a | 35.73 | c | 0b | 0.19 |
| S. viscosum | 95 | a | 8.05 | a | 13.90 | a | 8.3 | a | 33.40 | a | 4.50 | a | 104.25 | a | 90a | 0.22 |
| F. mosseae | 85 | b | 8.09 | a | 11.87 | a | 7.6 | a | 26.50 | b | 4.50 | a | 61.86 | bc | 80a | 0.20 |
| Comm. mix | 90 | ab | 8.08 | a | 13.00 | a | 7.7 | a | 33.10 | a | 4.60 | a | 89.53 | ab | 90a | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ancona, S.; De Mastro, G.; Jenderek, M.M.; Ruta, C. Micropropagation Supports Reintroduction of an Apulian Artichoke Landrace in Sustainable Cropping Systems. Agronomy 2021, 11, 1169. https://doi.org/10.3390/agronomy11061169
Ancona S, De Mastro G, Jenderek MM, Ruta C. Micropropagation Supports Reintroduction of an Apulian Artichoke Landrace in Sustainable Cropping Systems. Agronomy. 2021; 11(6):1169. https://doi.org/10.3390/agronomy11061169
Chicago/Turabian StyleAncona, Simona, Giuseppe De Mastro, Maria M. Jenderek, and Claudia Ruta. 2021. "Micropropagation Supports Reintroduction of an Apulian Artichoke Landrace in Sustainable Cropping Systems" Agronomy 11, no. 6: 1169. https://doi.org/10.3390/agronomy11061169
APA StyleAncona, S., De Mastro, G., Jenderek, M. M., & Ruta, C. (2021). Micropropagation Supports Reintroduction of an Apulian Artichoke Landrace in Sustainable Cropping Systems. Agronomy, 11(6), 1169. https://doi.org/10.3390/agronomy11061169








