Abstract
The soybean (Glycine max (L.) Merrill) is one of the world’s most important sources of food, feed, and fuel due to its high protein value and oil content. However, there exists a lack of soybean genotypes suitable for growth in diverse conditions as soybean breeders have developed their own varieties for specific purposes within their own unique environments. This, therefore, creates the need for soybean genotypes for different environments. The objectives of the experiment described herein were to determine the genotype magnitude through the environment interaction (GxE) of new soybean breeding lines, thereby identifying widely and/or specifically adapted genotypes under ten of Northeast Thailand’s typical environmental conditions from 2017 to 2019. Analyses of the environment (E) and GxE captured a large portion of the total sum of squares of grain yield and related traits, which demonstrated the influence of the two factors in evaluating soybean genotypes, thereby identifying the need for response analysis to identify superior genotypes in each environment. Based on the grain yields of three environments, four genotype groups were clustered. Within the high grain yield environment (EG1), we identified five genotypes with higher yield performance (35*sj-32 (3356 kg/ha), 38D*a-16 (3138 kg/ha), 42*Ly-50-2 (3122 kg/ha), 35*Lh-7 (3116 kg/ha), and 223*Lh-85 (3073 kg/ha)) of KK (3132 kg/ha), the recommended soybean variety for Northeast Thailand, than that of the CM60 (2606 kg/ha). These five top-yielding genotypes, however, produced unstable grain yields through varied environments as they were each narrowly adapted to a specific environment. Moreover, those genotypes may be grown within a rotational cropping system in a duo-environment (wet and dry season) of soybean production in Thailand’s northeast region.
1. Introduction
Soybean (Glycine max L. Merrill) is one of the most important grain crops for both humans and livestock, representing a staple source of nutritious vegetable proteins and oil, as well as viable industrial materials and biofuel. The diverse geographic distribution of wild soybean species throughout China indicates that China was the diversification center of the cultivated soybean [1], which gradually spread to Korea, Japan, India, Thailand, Indonesia, the Philippines, and other Asian countries over time [2,3,4]. As a result of such widespread distribution, a great number of soybean varieties were selected to grow under different environmental conditions that included soil characteristics, temperature, photoperiod, and rainfall.
Each area’s environmental conditions were also paired with the varied processing techniques developed by individual farmers [1], thereby leading to difficulties in identifying superior, stable cultivars for each region. Therefore, a single cultivar may perform differently according to the environment influenced by the presence of a significant genotype and environment interaction (GxE) [5,6,7,8]. In large-scale soybean production, seed production is a critical factor that will either limit or broaden the production area, thereby necessitating the identification and selection of soybean genotypes with greater adaptability and yield stability, together with diminished effects of a GxE interaction. Studies conducted worldwide [4,9,10,11,12,13] have stressed the necessity to develop environmentally adapted soybean genotypes.
In Thailand, soybeans are thought to have been introduced through Chinese migration in the 18th century. Thailand’s Department of Agriculture (DOA) created its soybean breeding program in 1960, which released 15 soybean cultivars throughout the country: SJ1, SJ2, SJ3, SJ4, SJ5, SukhoThai1, Sukhothai2, Sukhothai3, Srisamrong1, NS1, CM1, CM2, CM3, CM4, and CM60. Among these cultivars, SJ5 and CM60 have become the most widely accepted among farmers due to their productivity, adaptability, and consumption attributes [9], which are useful in creating several soy products, such as soymilk and tofu. For example, CM60 produces high protein content (36%) and a less “beany” taste most suitable for making soy milk. The predominance of these two soybean cultivars has led to few literary studies aimed at the development of other soybean cultivars, specifically, those addressing regions with dissimilar environmental conditions.
Thailand’s major area of soybean production lies in the northern region, followed by the northeast and central regions. In past years, soybean cropping systems across Thailand involved the alternate transfer of soybean seeds in the rainy season in upland areas of the north and the dry season in the lowland paddy fields of the central and northeast regions. Today, such cropping systems have changed due to several factors, such as (1) government policies that required a decrease in the crop production in mountainous areas; (2) lower profits of soybean production compared with other crops; (3) the substitution of other crops, such as vegetables in the lowland paddy fields of the central and northeast regions; (4) climate change, which adversely affects the cropping schedule in varied regions, as well as failed seed production; and (5) the aging society of soybean growers and the lack of advanced mechanized technology in soybean production. As a result, Thailand’s total soybean production area dramatically declined over the last two decades from 0.30 M ha in 2000 to 0.03 M ha in 2020 [14].
Based on the fluctuation of rainfall and the water status in major dams, the Thai DOA has requested that the second rice production in the lowland paddy fields of the central region during the dry season be replaced with less water-dependent crops, such as soybean and vegetables. However, soybean production is limited by the lack of specific adaptability to varied environments [15] and the low quality of yield and product that does not meet consumer, nutrition, and manufacturer preferences of the above-mentioned 15 soybean cultivars. Therefore, the evaluation of new soybean breeding lines with commercial soybean cultivars is needed to produce environmentally adaptable cultivars. The objective of this study, therefore, was to evaluate the yield potential of soybean cultivars and their stability and adaptability throughout the soybean production areas of Northeast Thailand.
2. Materials and Methods
2.1. Plant Material and Experimental Design
Twenty-four soybean genotypes comprising 6 commercial and 18 breeding lines derived from several crosses by pedigree selection since 2004 (Table 1) were grown in 10 environmentally contrasting locations. The environmental conditions were separated based on the different planting locations that cover three growing commercial soybean production areas in the northeastern part of Thailand. In each environment, there were differences in planting time, rainy day, soil type, and growing method that depended on the farmer’s practice. At each location, we employed a randomized complete-block design with three replications. The experimental units consisted of four parallel 4 m long rows per genotype spaced 25 cm between plants and 50 cm between rows, with 17 plants per row. Sowing took place from June to August for the wet season and from December to January for the dry season according to local practices. At 15 days after planting (DAP), plants were thinned to three plants per hole, and weeds were controlled by hand weeding. Fertilizer was initially applied at the rate of 14.06 kg/ha (N2-P2O5-K2O). At 30 DAP, a second fertilizer application was applied at the rate of 18.75 kgN2/ha, 37.50 kgP2O5/ha, and 18.75 kgK2O/ha. Herbicides and insecticides were used as necessary, as recommended by Thailand’s DOA, and irrigation was practiced throughout the dry season as required.

Table 1.
Variety type and source of soybean genotypes used in all experiments.
2.2. Data Collection
The amount of rainfall was recorded at each site by a weather station close to the experiment site (<2 km). Days to flowering (DTF) was estimated in each environment by recording the date in which 50% of the plants in each plot showed at least one fully bloomed flower. Grain yields were recorded in four random plants from the middle row in each plot and then weighed. Plant height, also measured in four random plants from the middle row, determined the distance from the soil surface to the top of the plant. The number of nodes and pods were counted from four random plants, and the pod heights of 10 random plants were measured from the soil surface to the node of the first pod. One hundred viable seeds were weighed. Harvesting took place from late October to early November in the wet season, and from late March to mid-April in the dry season, depending on their respective environmental conditions.
2.3. Statistical Analysis
A combined ANOVA for randomized complete-block design through each environment was carried out via R-Stat [16] for each trait. For all 10 environments, the 24 genotypes were combined, and the sum square percentage was calculated based on the mean square of environment (E), genotype (G), and environment by genotype interaction (GxE). Multiple comparisons were analyzed by Duncan’s Multiple Range Test (DMRT) using the Statistical Tool for Agricultural Research (STAR) software (http://bbi.irri.org/products). A group analysis obtained through Ward’s hierarchical clustering method generated a dendrogram, which was used to determine prior information involving the number of groups [17]. Clustering was performed for both the genotype and environmental groups based on the established between-group linkage distances. The dissimilarity measurement between genotypes was based on the Euclidian distance. These analyses were designed to improve the discrimination of superior genotypes and were used to compare the results from each environmental condition, as well as the stability of each genotype group. Cluster statistical analyses were performed using MEGA v.10 software [18].
3. Results
Ten contrasting location/year environments were evaluated, which included such factors as growing season, soil type, amount of rainfall, number of rainy days, and planting date (Table 2). These contrasting environments caused variations in grain yield, in which ENV1, ENV6, and ENV10 produced the highest grain yields (Table 2). Clustering based on grain yield resulted in the creation of three environmental groups: Group 1 (EG1) consisting of ENV1, ENV6, and ENV10; Group 2 (EG2) containing ENV3, ENV7, and ENV9; and Group 3 (EG3) made up of ENV2, ENV4, ENV5, and ENV8 (Figure 1). The groups were subsequently classified as having high (EG1), intermediate (EG2), and low (EG3) grain yields, which demonstrated the effects of different environmental conditions on the grain yields of various soybean genotypes.

Table 2.
Description of the 10 environments of 24 soybean genotypes grown within the 2017–2019 cropping season in Northeast Thailand.
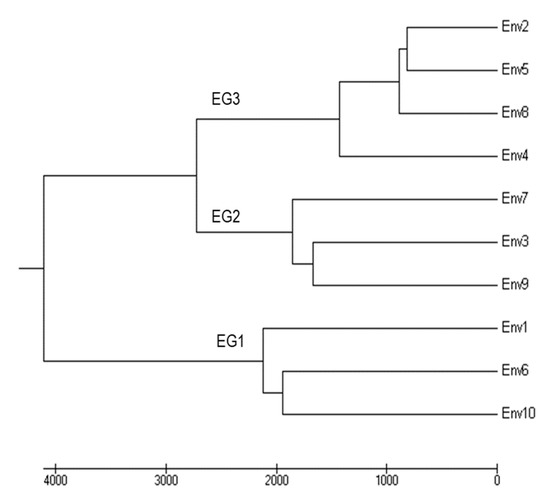
Figure 1.
Environmental clustering based on grain yield through Ward’s hierarchical clustering method.
The combined analyses of variance were calculated based on each trait from all 10 environments and 24 genotypes. The results showed for days to flowering (DTF), plant height, first-pod height, number of nodes, number of pods, seeds/pod, 100 seed weight, and grain yield (GY) of the 24 soybean genotypes under the 10 different environments that the principal effects (environment (E), genotype (G), and GxE interaction) were significant (Table 3 and Table 4). These environmental factors accounted for more than 80% of the total sum of squares in all traits, except for the number of seeds/pod, in which only 31.64% was attributed to environmental effects (Table 4). The effects of seeds per pod were determined primarily from the genotypes. The results indicated that the environment had a major effect on GY, DTF, plant height, first-pod height, number of nodes, number of pods, and 100 seed weight of each soybean genotype.

Table 3.
Combined analysis of variance for days to flowering (DTF), plant height, first-pod height, and the number of nodes of 24 soybean genotypes in 10 environments.

Table 4.
Combined analysis of variance for number of pods, seeds/pod, 100 seed weight, and grain yield (GY) of 24 soybean genotypes in 10 environments.
Grain yield differences among genotypes were highly significant, in which the average grain yield ranged from 541 kg/ha (ENV4) to 2983 kg/ha (ENV10), while the mean grain yield across the 10 environments was 1767 kg/ha (Table 2). Three environmental group (EG) classifications based on grain yield are presented in Figure 1, and their respective mean grain yields of 24 genotypes in 10 locations are summarized in Table 5. The three soybean breeding lines KKU74, 223*Lh-85, and 35*sj-32 produced higher grain yields than those of the check cultivars CM60 and SJ5 (Table 5). Based on grain yield, the soybean genotypes were classified into four groups: Group 1 (GG1) comprising NS1; Group 2 (GG2) consisting of 44*Ly-6-1-2-7, 44*Lh-4, and KKU35*m-7-2; Group 3 (GG3) containing KKU5e, 74-T4, SJ5, and CM6; and Group 4 (GG4) made up of 44*Ly-4E, 44*Ly-14E, 40*Ly-15, 42*Ly-50-2, 38D*a-16, KKU74, 76*B-14-1-3, 35*M-4, 35*Lh-7, 35*sj-32, 44*Lh-96, 42*Lh-1-1, 223*Lh-85, CM60, KKU35, and KK (Figure 2 and Table 6).

Table 5.
Grain yields of 24 soybean genotypes in each environment (Env) under three environmental groups.
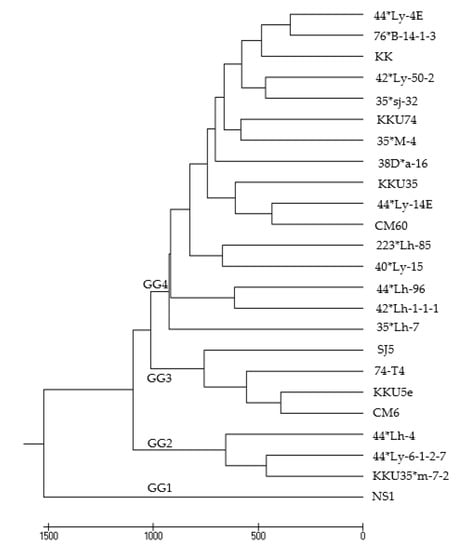
Figure 2.
Genotype clustering based on the grain yields of 10 environments.

Table 6.
Grain yields of all soybean genotypes in each environmental group (EG).
Based on the responses of the genotype groups (GGs) within the different environmental groups (EGs), the lowest grain yields across all groups were obtained in GG1, whereas the highest grain yields were produced by GG4 across all groups. The results further indicated that the soybean genotypes in GG4 adapted particularly well to the EG1 (high yielding environment) (Figure 3, Table 6). Interestingly, the contrasting environments of the EG1 included both wet and dry seasons, loamy clay and sandy clay soil, and diverse amounts of rainfall (Table 2). The genotype group responses to each environmental group were also useful for genotype selection. Several breeding lines within GG4, including the common Thai variety CM60, produced higher yields than those of commercial varieties.
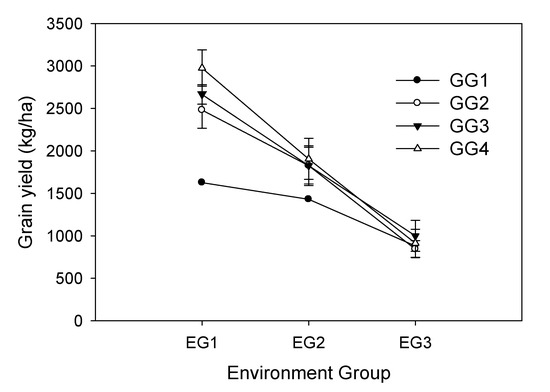
Figure 3.
Responses of four genotype groups (GGs) across three environmental groups (EGs). The bar depicts the standard deviation of each genotype in each group.
The high values of the environmental effects on grain yield and yield-related traits (Table 3 and Table 4) necessitate the need to study the stability of genotypes across each environment based on the GGE bi-plot, which is made difficult due to the lack of homogeneous data (data not shown). The results suggest that the superior genotype of each environmental group can be determined based on its genotype responses. We may, therefore, conclude that the higher grain yields of the Group 4 genotypes, ranging from 2574 kg/ha (KKU35) to 3356 kg/ha (35*sj-32) with an average grain yield of 2975 kg/ha (Table 6), are suitable for production, as well as seed rotation, in these areas in both the wet and dry seasons.
4. Discussion
Soybeans are considered a minor crop in Thailand’s vast agricultural production systems. Due to their ability to fixate nitrogen from the specific rhizobia bacteria in the soybean’s root nodule, soybeans are suitable for growth in rotational or intercropping systems, which contribute to increased farmer income and improved soil health [19,20]. Thailand’s two production seasons involve the common practice of planting soybeans in the wet season in the undulating topography of upland areas and the dry season in the lowland paddy fields. Each season presents several contrasting environmental factors that cause significantly different yields among soybean genotypes (Table 2, Table 3 and Table 4). In Northeast Thailand, sandy soil dominates both the upland field crops, such as cassava and sugarcane, as well as rice cultivated in the lowland rice paddies [21,22]. Sandy soil creates several problems with productivity due to its low fertility and water holding capacity, which adversely affect grain yields, particularly in the dry season (Table 2). Therefore, incorporating green-manure crops and plant residue in a rotational soybean cropping system represents an alternative way to improve an area’s soil properties.
Special attention was determined to be necessary for creating a successful cropping calendar as soybeans represent a minor crop, compared with the magnitude of rice and sugarcane production. In Thailand’s dry season, suitable sowing begins in early December due to the moisture content retained in the soil, where harvesting is expected to be completed in early April. Therefore, varieties with a moderated maturity of 90 to 100 days have proven suitable for cultivation. In our study, all breeding lines and check varieties were classified as having moderate maturity, except for the NS1 and KKU35 varieties, which represent early- and late-maturity varieties, respectively (Table 1). Early maturity varieties could complete the production process before the occurrence of growth-prohibiting stress factors. However, short-maturity genotypes have been associated with low yield potential due to their small plant canopy and short vegetative period [23]. In contrast, the late-maturity varieties achieved greater yields than those of the early- and moderate-maturity varieties. In the wet season, crop sowing begins in mid-June as rainfall is low, with harvesting being completed in mid-October in preparation for the upcoming cultivation of sugarcane. Planting soybeans during the wet season in the upland fields generally takes place during the sugarcane gap period between May and October, thereby making varieties with moderated maturity suitable for production. For several years, the KKU35 variety had been the most commonly planted soybean variety in Northeast Thailand; however, its production was limited due to its late maturity [24]. This level of maturity was limited as its harvest was interrupted by rain in the dry season and delayed in the wet season due to the land’s preparation for sugarcane cultivation (Figure 4). Our results determined that the cropping system, environmental conditions, and maturity were important criteria for soybean cultivar selection in Thailand.
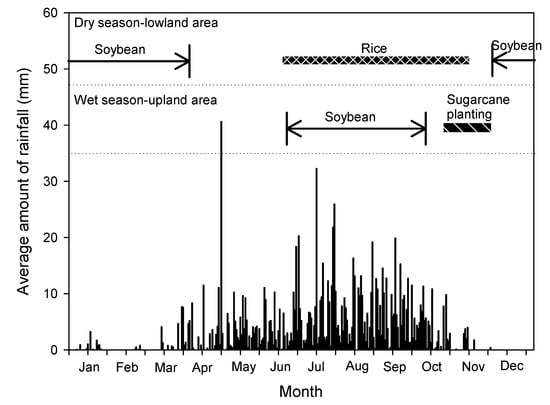
Figure 4.
Average rainfall in the wet and dry seasons in Northeast Thailand in 2015–2019.
Based on the combined analyses of variance for each trait under all 10 environments and 24 genotypes, it can be indicated that the environment had a major effect on GY, DTF, plant height, first-pod height, number of nodes, number of pods, and 100 seed weight (Table 3 and Table 4). In contrast, genotype had a major effect on the number of seeds/pod (Table 4). These results indicate that the cropping calendar, environmental condition, and crop maturity are important criteria for soybean production in Thailand due to the limitation of the expression of those traits [6,7,8]. Therefore, plant height and first-pod height can be considered important traits for new soybean production systems in this particular area. Another aspect besides the aging society of soybean growers in Thailand is that harvest machinery should be applied due to the harvesting operation being one of the most labor-intensive operations. During the harvesting time, the important growth traits that influence soybean harvest losses, such as plant length and first-pod height [25,26], are critical criteria for selection. In addition, Weber and Fehr [27] reported stem losses of 12.2% due to a higher cutting height of 16.5 cm. In Thailand, a rice combine harvester was applied to soybean harvesting; however, seed was still lost because of the height of the combine cutterbar, and the lowest cutting height was adjusted for 15 cm, which limited harvesting soybean. The development of soybean cultivars for high yield needs to focus on both plant height and first-pod height.
Soybean seeds are composed of roughly 20% lipids and are susceptible to qualitative deterioration processes via degradation of these compounds when improperly stored [28]. Long storage periods significantly reduce the rate of successful germination and negatively affect overall plant vigor [29,30]. Therefore, seed production is of critical importance for soybean production in Thailand. Traditional cropping systems involved seed rotation between seasons, in which seeds were rotated during the wet season in the north and the dry season in the central and northwest regions with the major varieties CM60 and SJ5 (Figure 5). These varieties have proven to be unsuitable in today’s farming practice due to their poor germination under high soil moisture conditions. We determined, herein, that environmental factors significantly affect grain yield (Table 2 and Table 4), thereby identifying the need to develop new soybean varieties. The study of the environmental groups (EGs) in both dry and wet seasons (Table 2 and Figure 1) demonstrated the necessity for duo-production locations.
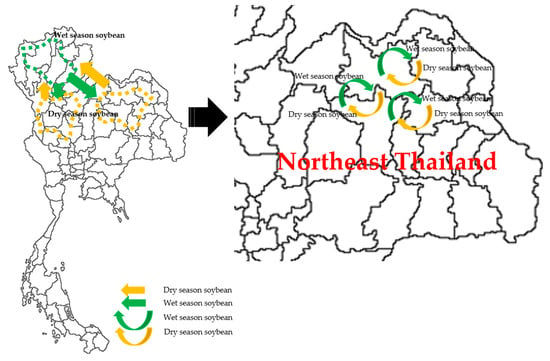
Figure 5.
Duo-locations of soybean cropping production areas among national regions (left) and within Northeast Thailand (right).
Five top-yielding genotypes in GG4 (35*sj-32, 38D*a-16, 42*Ly-50-2, 35*Lh-7, and 223*Lh-85) can be recommended for narrow adaptation to specific environmental areas (Table 6). Based on GE1, which comprised both the dry season in lowland paddy fields and the wet season in upland sugarcane fields (fallow sugarcane), the contrasting environments of soybean production can be used year-round as duo-planting areas. The top five soybean breeding lines are well-adapted to these fallow sugarcane areas, roughly 0.24 Mha, and thus have the potential to enhance soybean productivity in Northeast Thailand.
Author Contributions
Conceptualization, S.C., T.M., and J.S.; methodology, C.S.; software, T.M.; validation, C.S., T.M., and S.C.; formal analysis, T.M.; investigation, T.M.; resources, S.L., J.S., and S.S.; data curation, T.M.; writing—original draft preparation, S.C.; writing—review and editing, T.M. and S.C.; visualization, T.M.; supervision, S.C.; project administration, J.S.; funding acquisition, J.S. All authors have read and agreed to the published version of this manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
This research was supported by The Plant Breeding Research Center for Sustainable Agriculture, Khon Kaen University, and The Agricultural Research Development Agency (ARDA) through financial support (Project no. PRP6005020220).
Conflicts of Interest
The authors declare that no conflict of interest exists.
References
- Dong, S.Y.; Zhuang, C.B.; Zhao, L.M.; Sun, H.; He, M.Y. The genetic diversity of annual wild soybeans grown in China. Theor. Appl. Genet. 2001, 103, 98–103. [Google Scholar] [CrossRef]
- Wen, Z.; Ding, Y.; Zhao, T.; Gai, J. Genetic diversity and peculiarity of annual wild soybean (Glycine soja Sieb. et Zucc.) from various eco-regions in China. Theor. Appl. Genet. 2009, 119, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, Y.; Wang, Y.; Chen, J.; Li, Y.; Huang, H.; Qiu, L.; Wang, Y. Population structure of the wild soybean (Glycine soja) in China: Implications from microsatellite analyses. Ann. Bot. 2012, 110, 777–785. [Google Scholar] [CrossRef][Green Version]
- Lu, B.R. Conserving biodiversity of soybean gene pool in the biotechnology era. Plant Species Biol. 2004, 19, 115–125. [Google Scholar] [CrossRef]
- Adie, M.M.; Krisnawati, A. Soybean yield stability in eight locations and its potential for seed oil source in Indonesia. Energy Procedia 2015, 65, 223–229. [Google Scholar] [CrossRef]
- Polizel, A.C.; Juliatti, F.C.; Hamawaki, O.T.; Hamawaki, R.L.; Guimarães, S.L. Phenotypical adaptability and stability of soybean genotypes in the state of Mato Grosso. Bioscience 2013, 29, 910–920. [Google Scholar]
- Soares, I.O.; Bruzi, A.T.; Zambiazzi, E.V.; Guilherme, S.R.; Bianchi, M.C.; Silva, K.B.; Fronza, V.; Teixeira, C.M. Stability and adaptability of soybean cultivars in Minas Gerais. Genet. Mol. Res. 2017, 16, gmr16039730. [Google Scholar] [CrossRef] [PubMed]
- Susanto, G.W.A.; Adie, M.M. Adaptability of promising soybean lines at different environmental conditions. Penelit. Pertan. Tanam. Pangan 2010, 29, 166–170. [Google Scholar]
- Yothasiri, A.; Somwang, T. Stability of Soybean Genotypes in Central Plain Thailand. Kasetsart J. (Nat. Sci.) 2000, 34, 315–322. Available online: https://li01.tci-thaijo.org/index.php/anres/article/view/240321/163877 (accessed on 18 March 2021).
- Gurmu, F.; Mohammed, H.; Alemaw, G. Genotype X environment interactions and stability of soybean for grain yield and nutrition quality. Afr. Crop Sci. J. 2009, 17, 87–99. [Google Scholar] [CrossRef]
- Ramos Junior, E.U.; Brogin, R.L.; Godinho, V.P.C.; Botelho, F.J.E.; Tardin, F.D.; Teodoro, P.E. Identification of soybean genotypes with high stability for the Brazilian macro-region 402 via biplot analysis. Genet. Mol. Res. 2017, 16, gmr16039786. [Google Scholar] [CrossRef]
- Hamawaki, O.T.; Nogueira, A.P.O.; Teixeira, F.G.; Bicalho, T.F.; Jorge, G.L.; Hamawaki, R.L.; Machado Júnior, C.S.; Gomes, G.F.; Hamawaki, C.L. Adaptability and Stability of Soybean Genotypes in the States of Maranhão, Piauí, Tocantins and Bahia. Genet. Mol. Res. 2018, 17, gmr16039895. [Google Scholar] [CrossRef]
- Cover, J.E.; Aguiar, C.D.; Silva, A.V.; Silva, C.M.; Mielezrski, F. Productive potential and seed quality of soybean genotypes with different maturity groups. Aust. J. Crop Sci. 2019, 13, 1155–1161. [Google Scholar] [CrossRef]
- FAOSTAT. Database. 2020. Available online: http://www.fao.org/faostat/en/#data/SC (accessed on 2 January 2021).
- Srisomboon, S.; Kornthong, A.; Kaewmeechai, S.; Daengpradub., S. Genetic study of soybean. In Proceedings of the 4th Soybean Research Conference, Khon Kaen University, Khon Kaen, Thailand, 19–21 August 1992; pp. 20–30. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: http://www.R-project.org/ (accessed on 30 December 2020).
- Ward, J.H. Hierarchical grouping to optimize an objective junction. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Kumar, S.; Dudley, J.; Nei, M.; Tamura, K. MEGA: A biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinform. 2008, 9, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Miri, M.; Janakirama, P.; Held, M.; Ross, L.; Szczyglowski, K. Into the root: How cytokinin controls rhizobial infection. Trends Plant Sci. 2016, 21, 178–186. [Google Scholar] [CrossRef]
- Martins da Costa, E.; Almeida Ribeiro, P.R.; Soares de Carvalho, T.; Vicentin, R.P.; Balsanelli, E.; Maltempi de Souza, E.; Lebbe, L.; Willems, A.; de Souza Moreira, F.M. Efficient Nitrogen-Fixing Bacteria Isolated from Soybean Nodules in the Semi-arid Region of Northeast Brazil are Classified as Bradyrhizobium brasilense (Symbiovar Sojae). Curr. Microbiol. 2020, 77, 1746–1755. [Google Scholar] [CrossRef]
- Vityakon, P. Degradation and restoration of sandy soils under different agricultural land uses in northeast Thailand: A review. Land Degrad. Develop. 2007, 18, 567–577. [Google Scholar] [CrossRef]
- Arunrat, N.; Kongsurakan, P.; Sereenonchai, S.; Hatano, R. Soil Organic Carbon in Sandy Paddy Fields of Northeast Thailand: A Review. Agronomy 2020, 10, 1061. [Google Scholar] [CrossRef]
- Machikowa, T.; Laosuwan, P. Extension of Days to Flowering on Yield and Other Characters of Early Maturing Soybean. Suranareej. Sci. Technol. 2009, 16, 169–174. [Google Scholar]
- Lodthong, S. (Khon Kaen University, Khon Kaen, Thailand). Personal communication, 2017.
- Philbrook, B.D.; Oplinger, E.S. Soybean field losses as influenced by harvest delays. Agron. J. 1989, 81, 251. [Google Scholar] [CrossRef]
- Ramteke, R.; Singh, D.; Murlidharan, P. Selecting soybean (Glycine max) genotypes for insertion height of the lowest pod, the useful trait for combine harvester. Indian J. Agric. Sci. 2012, 82, 511–515. [Google Scholar]
- Weber, C.R.; Fehr, W.R. Seed Yield Losses from Lodging and Combine Harvesting in Soybeans. Agron. J. 1966, 58, 287. [Google Scholar] [CrossRef]
- Alencar, E.R.; Faroni, F.R.D. Storage of soybeans and its effects on quality of soybean sub-products. In Recent Trends for Enhancing the Diversity and Quality of Soybean Products; Krezhova, D., Ed.; IntechOpen: London, UK, 2011; pp. 48–66. [Google Scholar] [CrossRef]
- Matsue, Y.; Uchikawa, O.; Sato, H.; Tanaka, K. Productivity of the Soybean Seeds Stored for Various Periods. Plant Prod. Sci. 2005, 8, 393–396. [Google Scholar] [CrossRef]
- Panobianco, M.; Vieira, R.D. Electrical conductivity and deterioration of soybean seeds exposed to different storage conditions. Rev. Bras. Sementes 2007, 29, 97–105. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).