Abstract
Biochemical conversion of wheat straw was investigated using hydrothermal pretreatment, enzymatic saccharification, and microbial fermentation. Pretreatment conditions that were compared included autocatalyzed hydrothermal pretreatment at 160, 175, 190, and 205 °C and sulfuric-acid-catalyzed hydrothermal pretreatment at 160 and 190 °C. The effects of using different pretreatment conditions were investigated with regard to (i) chemical composition and enzymatic digestibility of pretreated solids, (ii) carbohydrate composition of pretreatment liquids, (iii) inhibitory byproducts in pretreatment liquids, (iv) furfural in condensates, and (v) fermentability using yeast. The methods used included two-step analytical acid hydrolysis combined with high-performance anion-exchange chromatography (HPAEC), HPLC, ultra-high performance liquid chromatography-electrospray ionization-triple quadrupole-mass spectrometry (UHPLC-ESI-QqQ-MS), and pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS). Lignin recoveries in the range of 108–119% for autocatalyzed hydrothermal pretreatment at 205 °C and sulfuric-acid-catalyzed hydrothermal pretreatment were attributed to pseudolignin formation. Xylose concentration in the pretreatment liquid increased with temperature up to 190 °C and then decreased. Enzymatic digestibility was correlated with the removal of hemicelluloses, which was almost quantitative for the autocatalyzed hydrothermal pretreatment at 205 °C. Except for the pretreatment liquid from the autocatalyzed hydrothermal pretreatment at 205 °C, the inhibitory effects on Saccharomyces cerevisiae yeast were low. The highest combined yield of glucose and xylose was achieved for autocatalyzed hydrothermal pretreatment at 190 °C and the subsequent enzymatic saccharification that resulted in approximately 480 kg/ton (dry weight) raw wheat straw.
1. Introduction
Growing global energy demand and the need to replace fossil fuels with renewable fuels are the major challenges of modern society [1]. The current production of liquid biofuels, such as bioethanol, is dominated by biochemical conversion routes mainly based on food-related feedstocks, such as corn starch and sugarcane sugar. Lignocellulosic materials can serve as additional resources for the biofuel sector [1,2]. Agricultural and agroindustrial residues, e.g., wheat straw, corn stover, and sugarcane bagasse, are lignocellulosic materials of interest in many countries due to their availability [3,4]. Co-utilization of lignocellulosic residues with starch or sugar can further boost bioethanol production through integration into 1.5 G processes [5].
Wheat (Triticum aestivum L.) is the world’s most widely cultivated crop [6]. Based on the data on wheat production worldwide (FAOSTAT) and on a residue/crop ratio of 1.3 [7], around 980 million tons of wheat straw are estimated to be available on a yearly basis. Dry wheat straw consists mainly of cellulose (30–40%), hemicelluloses (20–30%), and lignin (10–20%) [8]. Theoretically, bioconversion processes can yield around 270 L of ethanol per ton of dry wheat straw [9]. Biochemical conversion of agricultural residues, such as corn stover and wheat straw, has been significantly improved [7,10], but further research is still required for designing highly competitive and sustainable processes for the production of biofuels and other bio-based commodities.
The production of biofuels from lignocellulosic biomass comprises several steps, such as pretreatment, enzymatic saccharification, and microbial fermentation [11,12]. For achieving effective enzymatic saccharification of cellulose and, consequently, a higher release of fermentable sugars, a pretreatment step is indispensable. By disrupting the lignocellulosic matrix, pretreatment exposes cellulose and makes it more reactive towards cellulases [13]. Among different existing pretreatment methods, hydrothermal processing is an attractive option for agricultural residues, and it has good potential for industrial implementation [14]. Hydrothermal pretreatment (HTP) can be catalyzed by either hydronium ions generated by water autoionization or externally added acid species [15]. In HTP, moist lignocellulosic biomass is heated to around 200 °C for a certain period of time. Under those conditions, degradation of hemicelluloses and relocation of lignin can occur, and that leads to better accessibility of cellulose, which facilitates enzymatic hydrolysis [15,16].
While aiming at producing pretreated biomass with highly digestible cellulose, most pretreatment methods unavoidably lead to the formation of byproducts, resulting mainly from partial degradation of polysaccharides and lignin [14]. The formed byproducts can negatively affect the efficiency of enzymatic saccharification and microbial fermentation [14,17]. Certain groups of substances that inhibit microorganisms, such as aliphatic carboxylic acids, furan aldehydes, and phenolic compounds, have been extensively studied [18], while the importance of other groups of inhibitors has only recently started to emerge. Recent studies [19,20] have shown the significance of small aliphatic aldehydes as inhibitors of microbes used for biochemical conversion of biomass. Furthermore, the presence of benzoquinones in pretreatment liquids of different materials and their inhibitory effects on Saccharomyces cerevisiae have recently been discovered [21]. With regard to the inhibition of enzymes, aromatic substances such as phenolics have been found to play a role [14]. Pseudolignin, another byproduct, consists of thermal degradation products of carbohydrates. Pseudolignin remains insoluble and is accounted for as Klason lignin in the analytical two-step treatment with sulfuric acid (TSSA). The formation of pseudolignin can affect the enzymatic saccharification process [22,23]. In previous studies on hydrothermal pretreatment of wheat straw, the inhibition problem has generally been limited to substances such as furfural, HMF (5-hydroxymethylfurfural), acetic acid, formic acid, and certain phenolic compounds [8,9,24], while newly discovered inhibitors, such as aliphatic aldehydes and benzoquinones, have not been considered.
There are still several issues about hydrothermal pretreatment of wheat straw that require further research and innovation efforts. Issues that have so far not received enough attention include (i) how autocatalyzed (A-HTP) and sulfuric acid-catalyzed (SA-HTP) hydrothermal pretreatments affect the formation of byproducts, including pseudolignin and newly discovered inhibitors, (ii) how the pretreatment liquids inhibit the enzymatic hydrolysis of cellulose, and (iii) how xylan and lignin affect the digestibility of pretreated solids. In the current study, hydrothermal pretreatment of wheat straw under different temperatures between 160 and 205 °C, using two catalytic approaches, auto-catalysis and catalysis with sulfuric acid, was investigated. The investigation covered the evaluation of the effects of pretreatment conditions on (i) release of sugars and bioconversion inhibitors, (ii) chemical composition and enzymatic digestibility of pretreated solids, and (iii) inhibitory effects of pretreatment liquids on enzymatic saccharification and yeast fermentation. The results were backed by advanced analytical techniques, such as pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS), high-performance anion-exchange chromatography (HPAEC), and ultra-high performance liquid chromatography-electrospray ionization-triple quadrupole-mass spectrometry (UHPLC-ESI-QqQ-MS).
2. Materials and Methods
2.1. Materials
Wheat straw was provided by RISE Processum AB (Örnsköldsvik, Sweden). The wheat straw was previously collected by the Swedish University of Agricultural Sciences (SLU) from Mälardalen, Sweden, in the autumn of 2015. The moisture content of the biomass was determined using an HG63 moisture analyzer (Mettler-Toledo, Greifensee, Switzerland).
2.2. Pretreatment of Wheat Straw
The severity factor (SF; Equation (1)) of different pretreatment conditions was determined as the logarithm of the reaction ordinate, as proposed by Overend and Chornet [25] (Equation (2)).
where t is the holding time of pretreatment in minutes, and Tr is the pretreatment temperature in °C.
For each pretreatment batch, 37.5 g of wheat straw (on dry weight (DW) basis) was mixed with tap water (for A-HTP) or aqueous sulfuric acid (for SA-HTP) at a 7:1 liquid-to-solid ratio in a 1-L reactor (Parr 4520, Moline, IL, USA). The loading of sulfuric acid (96%) was 0.5 g per 100 g wheat straw (DW). The reaction time was 15 min. For A-HTP, the temperature settings were 160, 175, 190, and 205 °C, which correspond to SF values of 2.9, 3.4, 3.8, and 4.3, respectively. For SA-HTP, the temperature settings were 160 and 190 °C, which correspond to SF values of 2.9 and 3.8, respectively. The pretreatment conditions were chosen within the ranges typically reported for wheat straw [8,9,10]. At the cooling stage of pretreatment, when the temperature was approximately 90 °C, a sample was carefully taken from the gas phase on the top of the reaction suspension. The sample was received under a water column in a 15-mL Falcon tube, and it was analyzed immediately (Section 2.6). After pretreatment, the solid and liquid phases were separated by vacuum filtration, and the solid phase was washed with 1 L water. A portion of the filter cake was stored frozen for analytical enzymatic saccharification assay, and the rest was air-dried for around one week; the yield of pretreated solids was determined gravimetrically based on DW. The weight of the portion used for enzymatic saccharification was included in the calculation of the DW of pretreated solids (DW of PSs).
The yield of pretreated solids (PSs) was calculated as follows:
2.3. Compositional Analysis of the Raw Material and Pretreated Solids
The content of extractives in raw wheat straw and pretreated solids was determined by extracting aliquots of each material with 200 mL ethanol in a Soxhlet system (Extraction System B-811, Büchi, Flawil, Switzerland). Structural carbohydrates and lignin were determined using TSSA. TSSA was performed essentially according to an NREL protocol [26] but using HPAEC for quantification of monosaccharides. HPAEC was performed using a Dionex ICS-5000 (Sunnyvale, CA, USA) instrument with pulsed amperometric detection (PAD), a separation column (4 × 250 mm), and a guard column (4 × 50 mm) (both CarboPac PA1, Dionex, Sunnyvale, CA, USA). Prior to the analysis, the samples were diluted with ultra-pure water and filtered through 0.2 μm Millex-GN nylon membranes (Merck Millipore Ltd., Cork, Ireland). Acid-insoluble (Klason) lignin was determined gravimetrically and acid-soluble lignin (ASL) was determined spectrophotometrically at λ 240 nm. Ash content was determined using an NREL protocol [27]. All analyses were performed in triplicates. Values are stated as mass fractions of initial masses of samples (DW).
The recoveries of glucan and xylan were calculated using the following equations:
Here, GPS and XPS are the mass fractions of glucan and xylan, respectively, in pretreated solids (PS), and GRM and XRM are their mass fractions in the raw material.
Total lignin recovery was calculated using the following equation:
Here, KL is the mass fraction of Klason lignin, and ASL is the mass fraction of acid-soluble lignin in pretreated solids (PSs) and raw material (RM).
2.4. Pyrolysis-Gas Chromatography/Mass Spectrometry (Py-GC/MS)
Py-GC/MS analysis was performed at the Biopolymer Analytical Facility of the KBC Chemical–Biological Center (Umeå, Sweden). Prior to Py-GC/MS analysis, the pretreated and raw samples were freeze-dried and ball-milled (mixer mill MM400, Retsch, Haan, Germany). Then, 50 μg biomass powder was applied to a pyrolyzer equipped with an autosampler (PY-2020iD and AS-1020E, Frontier Lab, Fukushima, Japan) connected to a GC/MS machine (7890A/5975C, Agilent Technologies AB, Kista, Sweden). The pyrolysate was separated and analyzed, as described by Gerber et al. [28]. Areas were determined for peaks assigned as carbohydrates (C), guaiacyl (G), syringyl (S), p-hydroxyphenyl (H), generic phenolic constituents (P), known spectra unknown identification (U), and unknown spectra (0). Values are stated as percentages of total peak areas (C + G + S + H + P + U + 0). The fraction of lignin (L) corresponds to the sum of the fractions of G, S, H, and P.
2.5. Analytical Enzymatic Saccharification of Pretreated Solids
The enzymatic digestibility of pretreated solids and the inhibitory effect of the pretreatment liquids on the cellulolytic enzymes were evaluated following an analytical enzymatic saccharification procedure [15]. In one set of assays, aliquots of 250 mg (DW) of pretreated solids or raw wheat straw were suspended in 50 mM citrate buffer (pH 5.2) in 15-mL Falcon tubes, with a total reaction mixture of 5 mL. In another set of assays, the pretreated solids were instead suspended in pretreatment liquids, whose pH had previously been adjusted to pH 5.2. No citrate buffer was included in the assays with pretreatment liquids as carboxylic acids present in the pretreatment liquid make the addition of buffer unnecessary. In all assays, cellulolytic enzymes (Cellic CTec2 enzyme blend, Sigma-Aldrich Chemie GmbH, Steinheim, Germany) were added so that the loading was 100 CMCase units/g biomass. The reaction mixtures were incubated at 50 °C and 180 rpm for 72 h in an Ecotron orbital incubator (INFORS HT, Bottmingen, Switzerland). After 72 h, the tubes were centrifuged, and the supernatants were collected for further analysis using HPLC. Enzymatic digestibility was determined using the following equation:
Here, GH is the amount of hydrolyzed glucan based on the glucose concentration in the enzymatic hydrolysate, and GAPS is the amount of glucan in pretreated solids.
The degree of inhibition of enzymatic saccharification of glucan by the pretreatment liquids was determined using Equation (8).
Here, is the enzymatic digestibility of glucan (%) resulting from enzymatic hydrolysis of pretreated solids suspended in sodium citrate buffer, and is the enzymatic digestibility of glucan (%) resulting from enzymatic hydrolysis of pretreated solids suspended in pretreatment liquid.
2.6. Analysis of Sugars and Inhibitors in Liquid Samples
Analysis of monosaccharides (glucose, xylose, arabinose, galactose, and mannose) was performed using HPAEC (as described in Section 2.3). Acid posthydrolysis of pretreatment liquids with 4% (w/w) sulfuric acid was performed in order to hydrolyze oligosaccharides to allow their quantification.
Determination of furfural and HMF was carried out using an HPLC system (Dionex UltiMate 300; ThermoFisher, Waltham, MA, USA) with a diode-array detector and a 3 × 50 mm, 1.8-μm Zorbax RRHT SB-C 18 column. The temperature was set to 40 °C.
Determination of formaldehyde, acetaldehyde, p-benzoquinone, vanillin, coniferyl aldehyde, syringaldehyde, p-hydroxybenzaldehyde, and acetovanillone was performed using UHPLC-ESI-QqQ-MS after derivatization with 2,4-dinitrophenylhydrazine (DNPH). A 1290 Infinity system (Agilent Technologies, Santa Clara, CA, USA) coupled to a 6490 triple-quadrupole mass spectrometer (QqQ-MS) was used. The MS parameters were set as follows: gas temperature 290 °C, gas flow 20 L/min, nebulizer 30 psi, sheath gas temperature 400 °C, sheath gas flow 12 L/min, capillary voltage—3000 V, and nozzle voltage—2000 V. A 2.1 × 150 mm XTerra MS C18 column was used. Eluent A consisted of aqueous 0.1% (v/v) formic acid, and Eluent B was a 75:25 (v/v) mixture of acetonitrile and 2-propanol with 0.1% formic acid. Elution was performed using a gradient profile containing the following fractions of Eluent B: 0.0–4.5 min 30–40%, 4.5–9.0 min 40–50%, 9.0–11.0 min 50–70%, 11.0–11.01 min 70–95%, 11.01–15.0 min 95%, 15.0–15.01 min 95–30%, 15.01–18.0 min 30%, and, at the end, two min post-time with 30% for further re-equilibration. Data evaluation was done with MassHunter Quant software. The calibration and derivatization processes were based on previous studies [19,21].
The determination of aliphatic carboxylic acids (formic acid, acetic acid, and levulinic acid) was performed by MoRe Research Örnsköldsvik AB, Sweden. Acid determination was done using HPAEC.
Total aromatic content (TAC) in pretreatment liquids was determined by measuring the absorbance at 280 nm using a UV1800 spectrophotometer (Shimadzu, Kyoto, Japan). Total carboxylic acid content (TCAC) was determined by titration from pH 2.8 to pH 7.0 using an aqueous solution of sodium hydroxide (200 mM).
The determination of total phenolic compounds was carried out by using the Folin–Ciocalteu method [29], with vanillin as the calibration standard. A SpectraMax i3x (Molecular Devices, LLC, San Jose, CA, USA) multimode microplate reader was used for reading the absorbance at 760 nm of the color generated after the incubation of reaction mixtures containing the sample and Folin–Ciocalteu reagent at 23 °C for two hours. Reactions were performed in triplicates.
2.7. Fermentability of Pretreatment Liquids
Fermentability tests with freeze-dried S. cerevisiae yeast (Ethanol Red, Fermentis, Marcq en Baroeul, France) were performed using microtiter plates (maximum well volume 330 μL; Nunc, Roskilde, Denmark). Freeze-dried yeast was rehydrated by suspending it in 5 times its weight of sterile tap water for 30 min at 35 °C, and it was then inoculated at an initial loading of 0.2 g (DW)/L. Prior to fermentation, the pH of the pretreatment liquids was adjusted to 5.5 using a 1-M solution of NaOH. The final volume in each well was 300 μL. The mixture contained either 120 μL pretreatment liquid (diluted with deionized water to 40% of the initial concentration) or 234 μL pretreatment liquid (100% of the initial concentration), 6 μL of a nutrient solution (consisting of 150 g/L yeast extract, 75 g/L (NH4)2HPO4, 3.75 g/L MgSO4·7 H2O, and 238.2 g/L NaH2PO4·H2O), and deionized water. Glucose was added to all the media until a final concentration of 20 g/L. Reference fermentations without any pretreatment liquid were performed, while control samples with only culture medium (no yeast) were also processed in order to confirm that there was no cross-contamination. The microtiter plates were incubated in an orbital shaker at 150 rpm and 30 °C.
Yeast growth was monitored by measurements of optical density (OD) at 600 nm using the SpectraMax i3x multimode microplate reader after 0, 12, 24, 36, and 48 h. Growth medium without yeast was used as blank. All experiments were performed in triplicates, and mean values were used in the evaluation. The growth rate in each medium was calculated with the following equation:
The relative growth rate (RGR) of cultures with a medium of pretreatment liquid was calculated by comparing the growth rate of those cultures to the average growth rate of the reference cultures with sugar-based medium (Equation (10)).
2.8. Statistical Processing of the Results
Two-way analysis of variance (ANOVA) and Tukey’s posthoc test were applied to evaluate the statistical difference between the results of enzymatic saccharification experiments corresponding to different pretreatment conditions and different liquid media (either sodium citrate buffer or pretreatment liquids) used in the assays. Levene’s test for homogeneity of variance and Shapiro’s test for normal distribution of residuals were used for verifying that the data met the model assumptions. Statistical tests were run using R package stat [30].
3. Results
3.1. Chemical Composition of Raw and Pretreated Solids
The wheat straw used in this study contained 39.8% glucan, 24.3% hemicellulosic carbohydrates, 22.8 ± 0.1% lignin, 5.1 ± 0.3% ash, and 4.7 ± 0.1% ethanol extractives (Table 1). That composition is in agreement with previous reports on wheat straw, showing similar values [31].

Table 1.
Chemical composition of raw wheat straw and pretreated solids, mass fractions in % (dry weight) a.
The pretreatment led to different degrees of reduction of the gravimetric yield of the resulting solid material with respect to the starting amount of wheat straw (Figure 1). Depending on the pretreatment conditions, the yields of pretreated solids ranged between 64.3% and 87.6%, and a steady decrease was observed with increasing temperature and increasing severity factor. The yield of pretreated solids resulting from the most severe autocatalyzed pretreatment (the one performed at 205 °C) was 27% lower than that of the least severe one (at 160 °C). The yields were lower for SA-HTP than for A-HTP, but the differences were relatively small. The most noticeable difference was found for 190 °C (69.4% for SA-HTP and 73.0% for A-HTP), while the values for 160 °C (85.5% for SA-HTP and 87.6% for A-HTP) were closer. It is noteworthy that the differences in yield between the pretreatment approaches (A-HTP and SA-HTP) at a given temperature were smaller than the differences in yield between two consecutive temperatures in the A-HTP series, for instance, 190 °C (73.0%) and 205 °C (64.3%) or 175 °C (80.3%) and 190 °C (73.0%). Evidently, a temperature increase of 15 °C had a stronger effect than the addition of sulfuric acid.
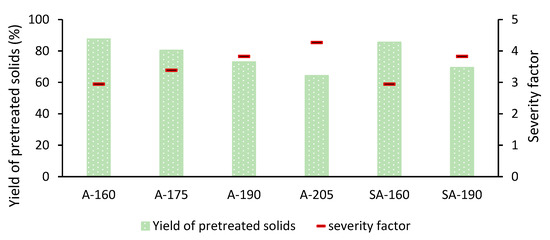
Figure 1.
Gravimetric yield of pretreated solids after autocatalyzed hydrothermal pretreatment (A-HTP) at 160 °C (A-160), 175 °C (A-175), 190 °C (A-190), and 205 °C (A-205), and sulfuric acid-catalyzed (SA-HTP) hydrothermal pretreatment at 160 °C (SA-160) and 190 °C (SA-190).
The glucan content was higher in the pretreated solids than in raw wheat straw, and, for both pretreatment approaches, it increased with the increase in temperature (Table 1). For A-HTP, the glucan content increased from 42.5% at 160 °C to 57.3% at 205 °C, while for SA-HTP, it increased from 43.5% at 160 °C to 54.8% at 190 °C. For the same pretreatment temperature, A-HTP and SA-HTP did not exhibit any major difference in glucan content. The same applies for glucan recovery, which was comparable for both pretreatment approaches, and displayed values above 92% for all experimental conditions (Figure 2).
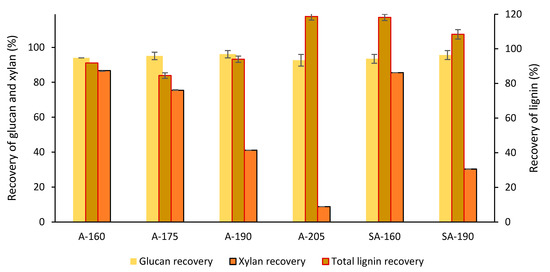
Figure 2.
Recovery of glucan, xylan, and total lignin (Klason lignin plus acid-soluble lignin (ASL)) in the pretreated solids. The codification of the experimental conditions is the same as in Table 1.
The xylan content decreased with increasing temperature (Table 1). Independently of the catalytic approach, the xylan content of solids pretreated at 160 °C was comparable to that of raw wheat straw. After pretreatment at 205 °C, the xylan content was below 3 ± 0.1%. The xylan recovery for pretreatments at 160 °C corresponded to approx. 87 ± 0.1%, whereas the recovery at 205 °C was below 9% (Figure 2). At 160 °C, the xylan recovery was not affected by acid addition, whereas at 190 °C, the value for A-HTP (41.1%) differed substantially from that of SA-HTP (30.3%). Other hemicellulosic carbohydrates were readily solubilized already at 160 °C as no arabinose, galactose, or mannose were detected in the analytical acid hydrolysates of the pretreated solids (Table 1).
A gradual increase of Klason lignin content in the pretreated solids was observed with increasing temperature. For experiments performed at the same temperature, the Klason lignin content was higher for SA-HTP than for A-HTP (Table 1). The content of acid-soluble lignin (ASL) did not display a clear trend regarding pretreatment temperature, but it was lower for SA-HTP than for A-HTP.
For the autocatalyzed pretreatment at 205 °C and for both acid-catalyzed pretreatments, the recovery of total lignin was higher than the theoretically possible value (Figure 2), indicating the formation of pseudolignin. To clarify this issue, Py-GC/MS analysis was performed. For the A-HTP experiments at 190 and 205 °C and for the SA-HTP experiments, Py-GC/MS revealed lower lignin content than the fraction of total lignin determined by the TSSA procedure (Klason lignin + acid-soluble lignin; Table 2). Higher ΔLignin factor values (Table 2) than for raw wheat straw indicate pseudolignin formation. The increase in ΔLignin factor value was especially apparent after pretreatment at 205 °C. At the same temperature, the ΔLignin factor was higher for SA-HTP than for A-HTP, indicating that increasing acidity stimulated pseudolignin formation.

Table 2.
Pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS) analysis of raw wheat straw and pretreated solids.
Negative ΔLignin factor values, particularly for raw wheat straw (Table 2), can be explained by the fact that the wheat straw contains relatively large fractions of ash (5.1%) and extractives (4.7%) (Table 1). While these substances are included in initial mass values used for TSSA calculations (Table 1), they (especially ash/inorganic substances) might not be covered by total peak areas used for Py-GC/MS calculations (Table 2). This explanation is supported by the fact that for raw wheat straw, it was not only the TSSA value for total lignin (22.8%) that was lower than the Py-GC/MS counterpart (27.8%); the TSSA value for the sum of carbohydrates (64.1%) was also lower than the Py-GC/MS counterpart (70.4%).
Py-GC/MS analysis of the G:S:H ratio (Table 2) indicated that pretreatment caused a relative increase of G (guaiacyl) units, a relative decrease of S (syringyl) units, and a relative increase of H (p-hydroxyphenyl) units. The relative decrease of S units might be associated with the cleavage of β-O-4 linkages in lignin, whereas corresponding increases for G and H units might be indirect effects.
The ash content was not much affected by the pretreatment but was slightly higher for the highest pretreatment temperature (A-HTP at 205 °C; Table 1). The increase in ash content at high temperatures can be an indirect effect of the decrease in hemicellulosic carbohydrates.
The mass fraction of extractives, which was 4.7% in raw wheat straw, decreased slightly for pretreatments at low temperature (160/175 °C; Table 1). At higher temperatures (190/205 °C), there was an increase. The decrease at lower temperatures can be explained by the solubilization of original extractives during pretreatment, whereas the increase at higher temperatures can be explained by the formation of new extractives due to the fragmentation of carbohydrates and lignin.
3.2. Carbohydrates in the Pretreatment Liquids
Carbohydrates in the pretreatment liquids are reported as monosaccharides and oligosaccharides (Table 3), with disaccharides included among the latter. Xylose, arabinose, and glucose were the main monosaccharides identified, whereas there were very little or no galactose and mannose. The concentrations of xylose, glucose, galactose, and mannose increased with increasing pretreatment temperatures. However, for the A-HTP series, the arabinose concentration was highest at intermediate temperatures (175/190 °C).

Table 3.
Concentrations of monosaccharides and oligosaccharides (OS) (g/L) and pH in the pretreatment liquids a.
For monosaccharides derived from oligosaccharides/disaccharides, the highest concentrations were 15.0 ± 0.3 g/L for Xylo-OS (xylose derived from oligosaccharides/disaccharides) and 2.7 ± 0.1 g/L for Gluco-OS (glucose derived from oligosaccharides/disaccharides) (Table 3). The maximum concentrations of the others (Arabino-OS, Galacto-OS, and Manno-OS) were around 1 g/L or lower. For A-HTP, Xylo-OS, Gluco-OS, and Manno-OS reached the highest concentrations at 190 °C, whereas the highest concentrations of Arabino-OS and Galacto-OS were instead found at 175 °C. This indicates that oligosaccharides containing arabinose and galactose units were more heat-labile. This is supported by data from the SA-HTP series showing higher concentrations of Xylo-OS, Gluco-OS, and Manno-OS for SA-190 than for SA-160, whereas Arabino-OS and Galacto-OS do not follow that pattern. In most cases, the concentrations of monosaccharides derived from oligosaccharides/disaccharides were higher than the concentrations of the corresponding monosaccharides in the same pretreatment liquid (Table 3).
The pH of the A-HTP liquids decreased with pretreatment temperature, from 5.0 for 160 °C to 3.5 for 205 °C (Table 3). As further discussed below, the decrease of pH with increasing pretreatment temperature results from the formation of carboxylic acids. For the SA-HTP series, the pH was only slightly (0.2–0.5 pH units) lower than for the corresponding pretreatment liquids in the A-HTP series.
3.3. Effects of Pretreatment Conditions on Byproduct Formation
For both the A-HTP and SA-HTP series, the furan aldehyde concentrations always increased with increasing pretreatment temperature (Table 4). The furfural concentrations (≤77.1 ± 2.4 mM) in the pretreatment liquids were always higher than the corresponding HMF concentrations (≤2.4 mM). The very low values after pretreatment at 160 °C are difficult to compare, but furfural values after pretreatment at 190 °C show clearly higher concentration for SA-HTP (24.1 ± 0.5 mM) than for A-HTP (14.4 mM). As acid conditions promote hydrolysis of hemicelluloses to pentose sugars, the precursors of furfural, this is an expected result. The condensate of A-205 was found to contain furfural that had been volatilized during the pretreatment.

Table 4.
Concentration of bioconversion inhibitors in the pretreatment liquids a.
The main aliphatic carboxylic acid in the pretreatment liquids was acetic acid (11.7–66.4 mM). Pretreatment liquids also contained formic acid (≤24.1 ± 0.1 mM) and levulinic acid (≤0.4 mM). The concentrations of acids increased with the pretreatment temperature, and there were only minor differences between the A-HTP and SA-HTP series. As expected, TCAC values were higher than the sum of concentrations of acetic acid, formic acid, and levulinic acids and followed the same trend.
The formaldehyde concentrations were mostly below the detection level. A reason for this can be that during hydrothermal pretreatment, formaldehyde is probably formed mainly from lignin, and wheat straw has rather low lignin content compared to, for example, softwood [19,20].
The concentration of total phenolics ranged from 1.0–7.2 g/L (Table 4). The SA-HTP series exhibited slightly higher values than corresponding pretreatment liquids in the A-HTP series. The concentrations of total phenolics and individual phenolics with a one-carbon side chain (i.e., vanillin, syringaldehyde, and p-hydroxybenzaldehyde) increased with increasing pretreatment temperature (Table 4). Phenolics with two- or three-carbon side chains, such as acetovanillone, coniferyl aldehyde, and p-coumaraldehyde, increased with the pretreatment temperature up to 190 °C, but then decreased for A-205. This suggests that phenolics with longer side chains are more susceptible to degradation at higher temperatures, whereas phenolics with one-carbon side chains are more stable.
p-Benzoquinone was not detected in any of the pretreatment liquids (Table 4). Studies of yeast have shown that relevant toxic concentrations of p-benzoquinone are several orders of magnitude lower than for other inhibitors in Table 4, as concentrations in the micromolar range have clearly inhibitory effects [21]. The methodology used would have also revealed concentrations in that range.
The TAC measurement covers a wide range of aromatics, including phenylic compounds, such as phenolic and nonphenolic aromatics, and heteroaromatics, such as furan aldehydes. The TAC values increased with the temperature (Table 4). At corresponding temperatures, the TAC values for the SA-HTP series were identical to those of the A-HTP series.
3.4. Enzymatic Saccharification of Pretreated Wheat Straw
Since the susceptibility to enzymatic saccharification is a key issue in the bioconversion of lignocellulosic biomass into bio-based products, the impact of pretreatment conditions on enzymatic saccharification was carefully examined using reaction mixtures with both buffer and pretreatment liquids. Reaction mixtures with buffer provide a clean analytical view of the susceptibility of pretreated solids to enzymatic saccharification. Reaction mixtures with pretreatment liquid provide a more complex and, perhaps, more industrially relevant view, where both the susceptibility of the solid phase and the influence of potential enzyme inhibitors in the pretreatment liquid are taken into account.
All pretreatment conditions resulted in a clear enhancement of saccharification of glucan, as revealed by the increase of enzymatic digestibility from 18% for raw wheat straw to 45–98% for pretreated solids (Figure 3). For A-HTP solids suspended in sodium citrate buffer, the enzymatic digestibility increased with the pretreatment temperature (from 46 ± 0.5% for A-160 to 98 ± 0.8% for A-205). For SA-HTP, the enzymatic digestibility values were comparable with those from A-HTP at corresponding temperatures. Xylan contained in the pretreated solids was also hydrolyzed during saccharification trials (data not shown).
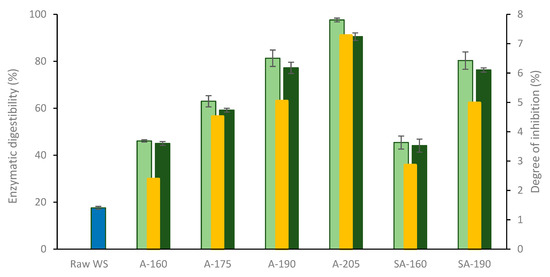
Figure 3.
Enzymatic digestibility of glucan in cut raw wheat straw (WS) in buffer (blue bar) and pretreated solids suspended in either sodium citrate buffer (light green bars) or pretreatment liquid (dark green bars). Yellow bars represent the degree of inhibition (DI) of enzymatic saccharification by the pretreatment liquids. Same codification of experimental conditions as in Table 1.
In the experiments with the pretreated solids suspended in pretreatment liquids, the enzymatic digestibility of glucan was lower than in the series with the buffer (Figure 3). The enzymatic digestibility ranged from 44 ± 0.8% for SA-160 to 90 ± 1.6% for A-205. Based on the differences between the hydrolysis series, the degree of inhibition (DI) by the pretreatment liquids was calculated. For A-HTP, the DI was 2.4 ± 0.1% for A-160, and it gradually increased with the temperature, reaching up to 7.3 ± 0.3% for A-205. At 160 °C, the DI was slightly higher for SA-HTP than for A-HTP, but at 190 °C, no discernible difference was found.
The statistical significance of the differences between enzymatic digestibility values achieved in saccharification trials of pretreated solids from different pretreatment conditions (p < 0.001), suspended in different liquid media (p < 0.001), and their interactions (p = 0.0155) was confirmed by two-way analysis of variance (Table 5) and Tukey’s posthoc test. Levene’s test for homogeneity of variance (F = 1.210, p = 0.333), and Shapiro’s test for normal distribution of residuals (W = 0.957, p = 0.173) confirmed that the data met the model assumptions.

Table 5.
Two-way ANOVA for enzymatic digestibility in saccharification trials with pretreatment solids suspended in either sodium citrate buffer or pretreatment liquids for all the pretreatment conditions.
Potential correlations between the enzymatic convertibility of glucan and the contents of xylan and lignin in the pretreated solids were investigated (Figure 4). For both the A-HTP and SA-HTP series, enzymatic digestibility displayed an obvious negative correlation (R2 0.92) with xylan content (Figure 4a). For lignin content, there was a slightly positive trend (Figure 4b) that can be attributed to indirect effects (hemicellulose removal leading to both higher lignin content and better digestibility).
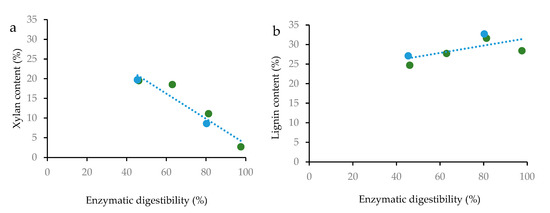
Figure 4.
Correlation between enzymatic digestibility of glucan with the content of xylan (a) and lignin (b) in the pretreated solids. The green markers are for A-HTP and the blue markers are for SA-HTP. R2 = 0.92 (xylan) and R2 = 0.45 (lignin). The plotted lignin content was determined using Py-GC/MS.
In order to have a better picture of the effectiveness of different pretreatment conditions on the saccharification of wheat straw, the yield of sugars per ton of raw material was calculated. For A-HTP, the combined glucose yield (after pretreatment and enzymatic saccharification) increased almost proportionally with the temperature, giving the highest value (407 ± 13 kg/t) at 205 °C (Figure 5). The xylose yield reached a peak (127 ± 8 kg/t) at 190 °C, including the amount solubilized during pretreatment and that resulting from enzymatic hydrolysis. The xylose yield sharply decreased to only 21 ± 1 kg/ton raw wheat straw at 205 °C, mostly due to losses in the pretreatment step. For SA-HTP, glucose and xylose yields at 160 °C were higher than those observed for A-HTP, whereas at 190 °C, they were slightly lower. Putting together glucose and xylose, the highest total sugar yield, 480 ± 12 kg per ton of wheat straw, was observed for A-HTP at 190 °C. That yield was higher than the one achieved by SA-HTP at the same temperature.
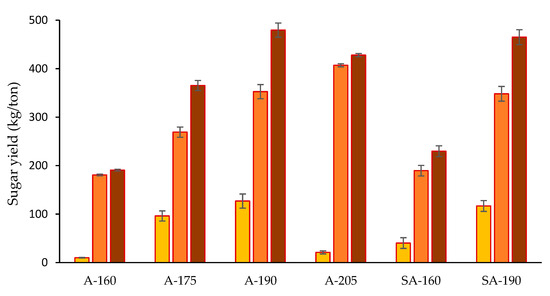
Figure 5.
Yield of xylose (yellow bars), glucose (orange bars), and total sugar (brown bars) after pretreatment and enzymatic hydrolysis. Values are given in kg per ton raw wheat straw. Same codification of experimental conditions as in Table 1.
3.5. Fermentability of Pretreatment Liquids
For elucidating the effect of the inhibitors formed during pretreatment on ethanolic fermentation, the fermentability of the pretreatment liquids by S. cerevisiae was investigated. A strong inhibitory effect on yeast cell growth was observed for the A-HTP liquid from pretreatment at 205 °C (Figure 6a). Albeit at a lower degree, the cell growth in the medium with SA-190 was also inhibited. Some inhibition was also observed for the pretreatment liquid from A-HTP at 190 °C, but it was hardly noticeable after 12 h. The inhibitory effects exerted by the other pretreatment liquids were minor and restricted to the beginning of the fermentation.
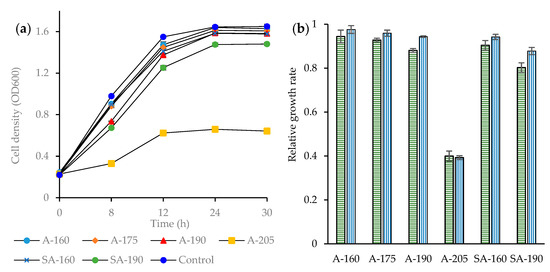
Figure 6.
Cell growth of S. cerevisiae in the fermentation of the pretreatment liquids (a) and relative growth rate (b) after 12 h (horizontal green pattern bars) and 24 h (vertical blue pattern bars). Same codification of experimental conditions as in Table 1.
The inhibition pattern of the different pretreatment liquids is better revealed by comparing the relative growth rates (RGRs), which were calculated as the ratio between the growth rates in each pretreatment liquid and the reference. The remarkable inhibition of the 205 °C A-HTP liquid is highlighted by RGR values around 0.40 ± 0.06 after both 12 and 24 h of fermentation (Figure 6b). On the other hand, the fermentability of the 160 °C A-HTP liquid was comparable with that of the control, as indicated by a relative growth rate of 0.98 ± 0.06 after 24 h of fermentation. The other pretreatment liquids displayed RGR values between 0.88 ± 0.03 and 0.96 ± 0.04 after 24 h. Pretreatment liquids from SA-HTP were more inhibitory than those from A-HTP at the same temperature.
4. Discussion
4.1. Chemical Composition of Raw and Pretreated Solids
Although different issues related to hydrothermal pretreatment of wheat straw for bioconversion have already been well investigated, there are other aspects that remain to be addressed. The effects of pretreatment temperature, time and equipment configuration on the yield of solids, solubilization of the main components, and recovery of sugars have been widely discussed [32,33,34,35], but important details linking pretreatment conditions with byproduct formation, enzymatic digestibility, and inhibition of biochemical conversion are still lacking. The current study assesses the effects of different pretreatment temperatures and catalytic approaches on the formation of bioconversion inhibitors, the enzymatic digestibility of pretreated solids, and the inhibition of enzymatic saccharification and fermentation by pretreatment liquids.
Increasing the pretreatment temperature from 160 to 205 °C while holding the reaction time at 15 min resulted in increased solubilization of wheat straw constituents and led to a reduction of the gravimetric yield of pretreated solids (Figure 1). The high pretreatment yield (almost 88%) under low-severity pretreatment (160 °C, SF 2.9) indicates that under these conditions, wheat straw was only marginally affected, while the low yield (around 64%) under the most severe pretreatment (205 °C, SF 4.3) revealed major solubilization of the raw material. These values are within the typical range for wheat straw. Min et al. [33] showed a decrease in the yield of pretreated solids from 86% to 80% while increasing the SF from 3.2 to 3.9, and Chen et al. [32] reported yields of approximately 60% in pretreatment at 200 °C for 30 min (SF 4.4).
The analysis of the composition of pretreated solids and pretreatment liquids indicates that the main factor contributing to the reduction of the gravimetric yield is the solubilization of hemicelluloses. The increase of solubilization of hemicelluloses with the temperature range studied is in agreement with previous reports of hydrothermal pretreatment of wheat straw [34]. The fact that the yield of solids was more affected by the stepwise increase of temperature than by the use of sulfuric acid can be explained by the rather low acid-loading that was used.
The observed temperature-related increase of the glucan content in the pretreated solids was a consequence of hemicellulose solubilization (Table 1). Cellulose was not affected to any major extent by the different pretreatment conditions, as indicated by the high glucan recoveries achieved (Figure 2). The lack of substantial differences in glucan recovery between A-HTP and SA-HTP for similar temperatures might be explained by the rather low sulfuric acid-loading used in SA-HTP. In previous studies on hydrothermal pretreatment of sugarcane bagasse using a higher sulfuric acid loading (0.5 g/100 g of reaction mixture instead of 0.5 g/100 g dry biomass), glucan recovery was higher for A-HTP than for SA-HTP under the same temperature and time [35,36].
The decrease of xylan content with the increase of the temperature is a logical result, considering that a temperature-dependent increase of hemicellulose solubilization is normally expected for hydrothermal pretreatment [32]. In contrast to previous reports on studies in which higher sulfuric acid-loadings or longer reaction times were used [37], pretreatment at 160 °C was not effective for xylan solubilization, even when sulfuric acid was used (Table 1). At 190 °C, there was some sulfuric-acid-promoted xylan solubilization, as can be interpreted from the decrease of xylan recovery from around 41% with A-HTP to nearly 30% with SA-HTP (Figure 2).
The observed arabinose solubilization at 160 °C (Table 1) matches well with previous information on the hydrothermal deconstruction of different hemicellulosic components. Under mild conditions, arabinose side-chain moieties are cleaved faster than xylose units [38]. Xylan backbone is initially fragmented to a minor degree, and only after subsequent scissions, a major release of xylo-oligosaccharides and xylose occurs [39]. However, the solubilization of galactose and mannose at 160 °C is surprising considering that mannan and galactan typically exhibit comparable dissolution kinetics to xylan [40], and it would have been expected that they behave similarly under HTP at 160 °C.
The gradual increase of lignin content in the pretreated solids with the increase of the temperature (Table 1) is primarily a consequence of hemicellulose solubilization, as typically observed for hydrothermal processing and acid-based pretreatment methods [13]. As confirmed by combining TSSA and Py-GC/MS analysis, lignin recovery of over 100%, detected for A-HTP at 205 °C and for both SA-HTP experiments (Figure 1), was due to pseudolignin formation, a phenomenon that has not been well-studied previously and which is typically difficult to quantify. The lower lignin fraction found by using Py-GC/MS (Table 2) in comparison with the fraction determined by TSSA (Table 1) for A-205, SA-160, SA-190, and A-190 is due to the fact that TSSA does not distinguish differences between real lignin and Klason-positive partially degraded carbohydrates, i.e., pseudolignin. Py-GC/MS analysis will detect Klason-positive partially degraded carbohydrates as carbohydrates. The lignin recoveries exceeding 100% in Figure 1 can, with certainty, be attributed to pseudolignin formation. For estimating pseudolignin formation, we used the ΔLignin value, a factor we recently introduced based on TSSA analyses of lignin and biomass characterization using Py-GC/MS [35,36]. As shown by the negative value for raw wheat straw, TSSA and Py-GC/MS do not give identical values, and it is, therefore, a possibility that also some of the pretreated samples, with low negative ΔLignin values, contain small fractions of pseudolignin. As hydrothermal pretreatment mainly targets hemicelluloses, the lignin showed minor compositional changes, as shown by small changes observed for the G:S:H ratio. There was an evident decrease in the relative abundance of syringyl units (Table 2).
The high ash content of the raw material is within the range previously determined for wheat straw [33,34]. The ash content increased slightly with the temperature as other components were removed and minerals remained in the pretreated solids. These results are in agreement with previous studies showing an increase in ash content with the temperature in HTP of wheat straw [33,34].
The increase of the mass fraction of extractives observed at 190 and 205 °C (Table 1) might be due to the fact that the solvent used in the analytical procedure extracted not only native extractives present in untreated wheat straw but also lignin fragments deposited on the solid phase during pretreatment. This is further strengthened by the finding that at similar temperatures, the content of extractives was higher for SA-HTP, the pretreatment approach that is expected to cause more extensive lignin fragmentation, condensation, and redeposition [41] than for A-HTP, and that the difference was more obvious at 190 °C than at 160 °C.
4.2. Carbohydrates in the Pretreatment Liquids
The carbohydrate profiles of the pretreatment liquids, i.e., relatively high concentrations of xylose, xylo-oligosaccharides (Xylo-OS), glucose, and gluco-oligosaccharides (Gluco-OS) (Table 3), are as expected, considering the polysaccharide content of the raw material (Table 1) and considering that most of the glucan is cellulose, which is more resistant to pretreatment than hemicellulose. The increase of xylose and glucose concentrations with the increase of temperature is typical for hydrothermal pretreatment of wheat straw [31,32] and other herbaceous feedstocks such as sugarcane bagasse [42] and corn stover [43]. The discrepancy between the increase of xylose and the decrease of Xylo-OS for A-HTP at 205 °C compared to 190 °C point towards degradation reactions. This type of reaction has been discussed elsewhere [14].
The relatively high arabinose formation at 160 °C agrees well with the observations regarding the composition of pretreated solids (Section 4.1) and with the literature on the hydrolysis of hemicelluloses [38]. The fast dynamics of arabino- and galacto-oligosaccharides are in agreement with previous studies of HTP under mild conditions. Chen et al. [32] reported maximal arabinose and galactose formation from wheat straw at 160 °C, which is lower than the temperature of the maximum concentrations in the current work (175 °C), but they applied a longer pretreatment time (30 min). A similar trend has also been reported for hydrothermal pretreatment of sugarcane bagasse, which displayed maximum arabinose formation at 180 °C, followed by a sequential reduction of its concentration at 185, 190, and 195 °C [42].
It was unexpected that the amount of monosaccharides released by SA-HTP was almost as low as that of A-HTP and that the amount of oligosaccharides was rather high for both pretreatment approaches. Typically, SA-HTP liquids are rich in monosaccharides, and A-HTP liquids are rich in oligosaccharides. For instance, xylose concentrations around 19 g/L were detected in SA-HTP liquids of sugarcane bagasse at 175 °C for 3.9 min [35], while a yield of 61.7 g xylo-OS per kg raw material was reported for A-HTP of wheat straw at 180 °C for 30 min [32]. That, together with the observation that the yield of pretreated solids was more affected by temperature increase than by the use of sulfuric acid, can be attributed to the low acid-loading, which was not enough to cause any major hydrolysis of hemicelluloses. Although compositional analysis of solids pretreated at 190 °C revealed more extensive xylan solubilization for SA-HTP than for A-HTP (Table 1, Figure 2), the total concentration of xylose and Xylo-OS was comparable for both pretreatment approaches (Table 3). That indicates that a fraction of the solubilized xylan was degraded, and, therefore, it could not be quantified in the pretreatment liquids either as xylose or Xylo-OS.
4.3. Effects of Pretreatment Conditions on the Formation of Bioconversion Inhibitors
Degradation reactions leading to byproducts that are inhibitory to microorganisms and enzymes is a common problem for different pretreatment methods, including hydrothermal processing [14]. In the current work, the formation of inhibitory compounds was rather moderate for pretreatment temperatures up to 190 °C, but a clear increase was observed when the pretreatment temperature was raised to 205 °C or when sulfuric acid was used as a catalyst at 190 °C (Table 4).
Increased formation of furan aldehydes for SA-190 can partially explain the previously discussed (Section 4.2) sugars that were not accounted for by carbohydrate analyses. Dehydration of sugars to furan aldehydes is a typical phenomenon for pretreatments performed under acidic conditions and at high temperatures, and the furans can be further degraded if the pretreatment is very severe [14]. There was a sharp increase in sugar degradation when the A-HTP temperature was increased to 205 °C, as indicated by high concentrations of furfural and HMF after pretreatment at 205 °C. At 205 °C, using a method that has not been reported before for hydrothermal pretreatment of wheat straw, furfural was also detected in condensate from the gas phase. The increase of formic acid and levulinic acid at 205 °C indicates further degradation of furans, as is expected for harsh conditions [44]. Acetic acid is formed mainly by the hydrolysis of acetyl groups of hemicelluloses. For the A-HTP series, the concentration of acetic acid continued to increase even up to 205 °C (Table 4), which agrees with the observation that there was still plenty of xylan left in the solid fraction after treatment at 190 °C (Table 1).
The formation pattern of phenolic substances was found to be different depending on the length of the side chain, as phenols with one-carbon side chains increased over the whole temperature range, whereas phenols with two- or three-carbon side chains exhibited a maximum within the temperature range (Table 4). Thermal degradation of phenols with a two- or three-carbon side chain can contribute to increased formation of the corresponding phenolic benzaldehydes. For instance, degradation of coniferyl aldehyde and p-coumaraldehyde can result in the formation of vanillin and p-benzaldehyde, respectively. Vanillin has been shown to be formed from cleavage of the Cα-Cβ bond of acetovanillone through radiolysis [45]. A similar trend was observed in studies of hydrothermal pretreatment of sugarcane bagasse, where the formation of vanillin, p-hydroxybenzaldehyde, and syringaldehyde increased with severity, while coniferyl aldehyde concentrations reached a maximum at intermediate severity (log R0 = 3.8) and then decreased when the severity increased further [35].
4.4. Enzymatic Saccharification of Pretreated Wheat Straw
The reports on enzymatic hydrolysis of pretreated wheat straw are often limited to studies where pretreated solids are suspended in a buffer [33,46], which does not provide information on the effect of the pretreatment liquids on enzymatic conversion. The experimental setup used in this work, with pretreated solids suspended in either buffer or pretreatment liquids, allowed us to investigate the enzymatic digestibility of glucan contained in the pretreated solids and evaluate the inhibitory effect of the pretreatment liquids on the saccharification of glucan.
The results showed that (i) all pretreatment conditions greatly (2.5- to 5.5-fold) enhanced the enzymatic saccharification of wheat straw glucan; (ii) the enzymatic digestibility of pretreated solids increased with the pretreatment temperature and was found to be inversely correlated with the xylan content; (iii) the use of 0.5% (on a DW basis) sulfuric acid in the pretreatment did not have any major impact on saccharification in comparison to autocatalyzed pretreatment; (iv) the pretreatment liquids had a small (2–7%) but clear inhibitory effect on enzymatic saccharification; (v) the degree of inhibition increased with temperature, with no clear differences between SA-HTP pretreatment liquids and A-HTP pretreatment liquids (Figure 3). While some of these results agree with previous studies [41], the finding on the inhibition of enzymatic hydrolysis by the pretreatment liquids is an important contribution of this work to the knowledge on hydrothermal pretreatment. The relatively high enzyme inhibition observed for the pretreatment liquid from A-HTP at 205 °C (7%) can be attributed to its higher content of solubilized aromatics [16], expressed as total phenolic compounds and TAC (Table 4).
The correlations between the enzymatic convertibility of glucan and the content of xylan and lignin in the pretreated solids revealed that for achieving efficient enzymatic hydrolysis of wheat straw glucan, it is important that the pretreatment removes as much xylan as possible, while the effect of lignin is less important. That is in agreement with previous studies of pretreated lodgepole pine, implicating that removal of hemicelluloses is more important than removal of lignin in order to achieve efficient enzymatic saccharification [47].
Since different biorefinery applications, with glucose-based routes and xylose-based routes, can be considered for wheat straw, the total yields of sugars per ton of raw material are an important indicator for a pretreatment method of relevance. This work evaluates the effect of HTP on total sugar yield, including glucose, mainly formed during enzymatic saccharification, and xylose, mainly formed during the pretreatment stage. Although A-HTP at 205 °C was very effective for producing glucose in enzymatic hydrolysis, it led to extensive xylose degradation in the pretreatment stage. Instead, moderate pretreatment conditions (A-HTP, 190 °C) resulted in the highest xylose yield and, even though the glucose yield in the enzymatic saccharification step was lower than after pretreatment at 205 °C, the combined sugar yield was the highest. Thus, the investigation has revealed that since the highest yield was obtained with A-HTP at 190 °C, no sulfuric acid and no temperatures over 200 °C are required for maximizing sugar production from wheat straw.
4.5. Fermentability of the Pretreatment Liquids
The inhibitory effects on S. cerevisiae by the pretreatment liquids were low except for A-205. Additionally, with regard to microbial inhibition, A-190 turned out to be a good initial step for the biorefining of wheat straw. The chemical analyses indicated that the inhibitory impact on the fermentability of pretreatment liquid from A-205 corresponded with high values for TAC, total phenolics, TCAC, and furfural (Table 4). TAC has recently been found to be a useful indicator of inhibition in studies of hydrothermal pretreatment of sugarcane bagasse and Norway spruce [36]. TAC values are related to the values of total phenolics and furfural, both of which contribute to TAC. The most inhibitory (A-205) and second-most inhibitory (SA-190) pretreatment liquids also exhibited the highest and second-highest values for TAC, total phenolics, and furfural. Regarding TCAC, it is noteworthy that the values were always < 100 mM. Previous experiments with S. cerevisiae yeast indicate that inhibitory effects were achieved when the total concentration of aliphatic acids was above 100 mM [48]. Therefore, it is reasonable to assume that it was aromatic and heteroaromatic substances that caused the inhibitory effects rather than carboxylic acids. The absence or very low concentrations of newly discovered inhibitors such as formaldehyde and p-benzoquinone can tentatively be attributed to the relatively low lignin content of the raw material.
5. Conclusions
This investigation clarifies correlations between operational conditions during hydrothermal pretreatment and critical aspects of biochemical conversion of wheat straw, including hemicellulose removal, susceptibility to enzymatic saccharification, total sugar yields, byproduct formation, and inhibitory effects on enzymatic saccharification and ethanolic fermentation. Pseudolignin formation in autocatalyzed hydrothermal pretreatment at 205 °C and in sulfuric-acid-catalyzed hydrothermal pretreatment at lower temperatures was demonstrated. The importance of the removal of hemicelluloses for achieving high enzymatic digestibility of glucan was shown. Hydrothermal pretreatment without using mineral acids and without exceeding 200 °C was found to be the best approach to achieve high sugar yields while minimizing byproduct formation.
Author Contributions
D.I., conceptualization, methodology, investigation, data curation, writing—original draft preparation; S.S., methodology, validation, formal analysis; L.J.J., conceptualization, methodology, supervision, writing—review and editing, project administration, funding acquisition; C.M., conceptualization, methodology, supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Swedish Energy Agency (P41285-1 and P47516-1), Bio4Energy (www.bio4energy.se, accessed on 4 March 2021), and the Kempe Foundations.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The support provided by Guochao Wu in the preparation of the fermentability experiment, Junko Takahashi Schmidt in the Py-GC/MS analysis, and the statistical assistance given by Francesca Pilotto are gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest. The funding agencies had no role in the design of the study, in the interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J., Jr.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Zech, K.M.; Meisel, K.; Brosowski, A.; Toft, L.V.; Müller-Langer, F. Environmental and economic assessment of the Inbicon lignocellulosic ethanol technology. Appl. Energy 2016, 171, 347–356. [Google Scholar] [CrossRef]
- Dias, M.O.S.; Lima, D.R.; Mariano, A.P. Techno-economic analysis of cogeneration of heat and electricity and second-generation ethanol production from sugarcane. In Advances in Sugarcane Biorefinery; Chandel, A.K., Silveira, M.H.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 197–212. [Google Scholar]
- Jiang, Y.; Xin, F.; Lu, J.; Dong, W.; Zhang, W.; Zhang, M.; Wu, H.; Ma, J.F.; Jiang, M. State of the art review of biofuels production from lignocellulose by thermophilic bacteria. Bioresour. Technol. 2017, 245, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Susmozas, A.; Martín-Sampedro, R.; Ibarra, D.; Eugenio, M.E.; Iglesias, R.; Manzanares, P.; Moreno, A.D. Process strategies for the transition of 1G to advanced bioethanol production. Processes 2020, 8, 1310. [Google Scholar] [CrossRef]
- FAOSTAT—Food and Agriculture Organization Corporate Statistical Database. Available online: http://www.fao.org/faostat/en/#search/wheat (accessed on 24 September 2020).
- Ericsson, K.; Nilsson, L.J. Assessment of the potential biomass supply in Europe using a resource focused approach. Biomass Bioenerg. 2006, 30, 1–15. [Google Scholar] [CrossRef]
- Thomsen, M.H.; Thygesen, A.; Thomsen, A.B. Hydrothermal treatment of wheat straw at pilot plant scale using a three-step reactor system aiming at high hemicellulose recovery, high cellulose digestibility and low lignin hydrolysis. Bioresour. Technol. 2008, 99, 4221–4228. [Google Scholar] [CrossRef]
- Thomsen, M.H.; Thygesen, A.; Thomsen, A.B. Identification and characterization of fermentation inhibitors formed during hydrothermal treatment and following SSF of wheat straw. Appl. Microbiol. Biotechnol. 2009, 83, 447–455. [Google Scholar] [CrossRef]
- Talebnia, F.; Karakashev, D.; Angelidaki, I. Production of bioethanol from wheat straw: An overview on pretreatment, hydrolysis and fermentation. Bioresour. Technol. 2010, 101, 4744–4753. [Google Scholar] [CrossRef]
- Galbe, M.; Wallberg, O. Pretreatment for biorefineries: A review of common methods for efficient utilisation of lignocellulosic materials. Biotechnol. Biofuels 2019, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- El-Zawawy, W.K.; Ibrahim, M.M.; Abdel-Fattah, Y.R.; Soliman, N.A.; Mahmoud, M.M. Acid and enzyme hydrolysis to convert pretreated lignocellulosic materials into glucose for ethanol production. Carbohyd. Polym. 2011, 84, 865–871. [Google Scholar] [CrossRef]
- Hendriks, A.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Gandla, M.L.; Martín, C.; Jönsson, L.J. Analytical enzymatic saccharification of lignocellulosic biomass for conversion to biofuels and bio-based chemicals. Energies 2018, 11, 2936. [Google Scholar] [CrossRef]
- Silva, J.P.A.; Carneiro, L.M.; Roberto, I.C. Treatment of rice straw hemicellulosic hydrolysates with advanced oxidative processes: A new and promising detoxification method to improve the bioconversion process. Biotechnol. Biofuels 2013, 6, 23. [Google Scholar] [CrossRef]
- Rasmussen, H.; Sørensen, H.R.; Meyer, A.S. Formation of degradation compounds from lignocellulosic biomass in the biorefinery: Sugar reaction mechanisms. Carbohydr. Res. 2014, 385, 45–57. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef]
- Cavka, A.; Stagge, S.; Jönsson, L.J. Identification of small aliphatic aldehydes in pretreated lignocellulosic feedstocks and evaluation of their inhibitory effects on yeast. J. Agric. Food Chem. 2015, 63, 9747–9754. [Google Scholar] [CrossRef] [PubMed]
- Martín, C.; Wu, G.; Wang, Z.; Stagge, S.; Jönsson, L.J. Formation of microbial inhibitors in steam-explosion pretreatment of softwood impregnated with sulfuric acid and sulfur dioxide. Bioresour. Technol. 2018, 262, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Stagge, S.; Cavka, A.; Jönsson, L.J. Identification of benzoquinones in pretreated lignocellulosic feedstocks and inhibitory effects on yeast. AMB Express 2015, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Normark, M.; Pommer, L.; Gräsvik, J.; Hedenström, M.; Gorzsás, A.; Winestrand, S.; Jönsson, L.J. Biochemical conversion of torrefied Norway spruce after pretreatment with acid or ionic liquid. Bioenergy Res. 2016, 9, 355–368. [Google Scholar] [CrossRef]
- Shinde, S.D.; Meng, X.; Kumar, R.; Ragauskas, A.J. Recent advances in understanding the pseudo-lignin formation in a lignocellulosic biorefinery. Green Chem. 2018, 20, 2192–2205. [Google Scholar] [CrossRef]
- Toquero, C.; Bolado, S. Effect of four pretreatments on enzymatic hydrolysis and ethanol fermentation of wheat straw. Influence of inhibitors and washing. Bioresour. Technol. 2014, 157, 68–76. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Phil. Trans. R. Soc. Lond. 1987, A321, 523–536. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Technical Report NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2012; p. 15. [Google Scholar]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass; Technical Report NREL/TP-510-42622; National Renewable Energy Laboratory: Golden, CO, USA, 2008; p. 12. [Google Scholar]
- Gerber, L.; Eliasson, M.; Moritz, T.; Sundberg, B. Multivariate curve resolution provides a high-throughput data processing pipeline for pyrolysis-gas chromatography/mass spectrometry. J. Anal. Appl. Pyrol. 2012, 95, 95–100. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Ambye-Jensen, M.; Thomsen, S.T.; Kádár, Z.; Meyer, A.S. Ensiling of wheat straw decreases the required temperature in hydrothermal pretreatment. Biotechnol. Biofuels 2013, 6, 116. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Sun, S.; Cao, X.; Sun, R. Co-production of oligosaccharides and fermentable sugar from wheat straw by hydrothermal pretreatment combined with alkaline ethanol extraction. Ind. Crops Prod. 2018, 111, 78–85. [Google Scholar] [CrossRef]
- Min, D.; Wei, L.; Zhao, T.; Li, M.; Jia, Z.; Wan, G.; Zhang, Q.; Qin, C.; Wang, S. Combination of hydrothermal pretreatment and sodium hydroxide post-treatment applied on wheat straw for enhancing its enzymatic hydrolysis. Cellulose 2018, 25, 1197–1206. [Google Scholar] [CrossRef]
- Merali, Z.; Ho, J.D.; Collins, S.R.A.; Le Gall, G.; Elliston, A.; Käsper, A.; Waldron, K.W. Characterization of cell wall components of wheat straw following hydrothermal pretreatment and fractionation. Bioresour. Technol. 2013, 131, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Ilanidis, D.; Stagge, S.; Jönsson, L.J.; Martín, C. Effects of operational conditions on auto-catalyzed and sulfuric-acid-catalyzed hydrothermal pretreatment of sugarcane bagasse at different severity factor. Ind. Crops Prod. 2021, 159, 113077. [Google Scholar] [CrossRef]
- Ilanidis, D.; Wu, G.; Stagge, S.; Martín, C.; Jönsson, L.J. Effects of redox environment on hydrothermal pretreatment of lignocellulosic biomass under acidic conditions. Bioresour. Technol. 2021, 319, 24211. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Shen, Z.; Wen, Y. Hydrolysis of wheat straw by dilute sulfuric acid in a continuous mode. Chem. Eng. J. 2015, 260, 20–27. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Salmi, T.; Holmbom, B.; Willför, S.; Murzin, D.Y. Synthesis of sugars by hydrolysis of hemicelluloses—A review. Chem. Rev. 2011, 111, 5638–5666. [Google Scholar] [CrossRef] [PubMed]
- Ibbett, R.; Gaddipati, S.; Davies, S.; Hill, S.; Tucker, G. The mechanisms of hydrothermal deconstruction of lignocellulose: New insights from thermal- analytical and complementary studies. Bioresour. Technol. 2011, 102, 9272–9278. [Google Scholar] [CrossRef] [PubMed]
- Jara, R.; Lawoko, M.; van Heiningen, A. Intrinsic dissolution kinetics and topochemistry of xylan, mannan, and lignin during auto-hydrolysis of red maple wood meal. Can. J. Chem. Eng. 2018, 97, 649–661. [Google Scholar] [CrossRef]
- Trajano, H.L.; Engle, N.L.; Foston, M.; Ragauskas, A.J.; Tschaplinski, T.J.; Wyman, C.E. The fate of lignin during hydrothermal pretreatment. Biotechnol. Biofuels 2013, 6, 110. [Google Scholar] [CrossRef]
- Rocha, G.J.M.; Silva, V.F.N.; Martín, C.; Gonçalves, A.R.; Nascimento, V.M.; Souto-Maior, A.M. Effect of xylan and lignin removal by hydrothermal pretreatment on enzymatic conversion of sugarcane bagasse cellulose for second generation ethanol production. Sugar Tech. 2013, 15, 390–398. [Google Scholar] [CrossRef]
- Lü, H.; Shi, X.; Li, Y.; Meng, F.; Liu, S.; Yan, L. Multi-objective regulation in autohydrolysis process of corn stover by liquid hot water pretreatment. Chin. J. Chem. Eng. 2017, 25, 499–506. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 1989. [Google Scholar]
- Madureira, J.; Leal, J.P.; Botelho, M.L.; Cooper, W.J.; Melo, R. Radiolytic degradation mechanism of acetovanillone. Chem. Eng. J. 2020, 382, 122917. [Google Scholar] [CrossRef]
- Wang, R.; Yue, J.; Jiang, J.; Li, J.; Zhao, J.P.; Xia, H.H.; Wang, K.; Xu, J.M. Hydrothermal CO2-assisted pretreatment of wheat straw for hemicellulose degradation followed with enzymatic hydrolysis for glucose production. Waste Biomass Valor 2021, 12, 1483–1492. [Google Scholar] [CrossRef]
- Leu, S.Y.; Zhu, J.Y. Substrate-related factors affecting enzymatic saccharification of lignocelluloses: Our recent understanding. Bioenerg. Res. 2013, 6, 405–415. [Google Scholar] [CrossRef]
- Larsson, S.; Palmqvist, E.; Hahn-Hägerdal, B.; Tengborg, C.; Stenberg, K.; Zacchi, G.; Nilvebrant, N.-O. The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzyme Microb. Tech. 1999, 24, 151–159. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).