Abstract
The P genome of Agropyron cristatum Gaertn. contains many desirable genes that can be utilized as genetic resources to improve wheat. In this research, we used both the gametocidal chromosome 2Cc and the pairing homologous gene (Ph1b) mutant to induce structural aberrations and translocations between wheat and the 4P, 5P, and 6P genome chromosomes. By using the two approaches, a total of 19 wheat-A. cristatum translocations have been identified, in which 13 were induced by the Triticum aestivum cv. Chinese Spring (CS) ph1b mutant (CS ph1b) and six were induced by gametocidal chromosome 2Cc from Aegilops cylindrica Host. The wheat-4P, -5P and -6P A. cristatum translocations were characterized by in situ hybridization and by a set of conserved orthologous set (COS) molecular markers. The aberrations included centromeric translocations, terminal translocations, dicentric translocations, and deletions. The average induction frequency of chromosome structural aberrations was 10.9% using gametocidal 2Cc chromosome and 8.8% using ph1b mutant. The highest frequency obtained was for chromosome 4P using both approaches. All the wheat-A. cristatum translocation lines obtained were valuable for identifying A. cristatum chromosome 4P, 5P, and 6P related genes. In addition, these lines provided genetic resources and new germplasm accessions for the genetic improvement of wheat.
1. Introduction
Agropyron cristatum L. Gaertn (genome P) is a species in the Triticeae tribe, which has diploid (2n = 2X = 14), tetraploid (2n = 4X = 28), and hexaploid (2n = 6X = 42) forms [1]. A. cristatum is a valuable donor of important agronomic traits for wheat improvement such as resistance to leaf rust [2], resistance to stripe rust [3], resistance to powdery mildew [4,5], enhanced-grain number per spike and spike length [6], high salt-tolerance [7], and drought stress tolerance [8]. With the objective to introgress these agronomic traits into wheat, hybrids and fertile amphiploids between Triticum and A. cristatum have been successfully obtained [9,10,11,12,13], allowing the development of both durum and common wheat-A. cristatum introgressions [2,6,14,15,16].
Recombination between cultivated and alien chromosomes is essential for incorporating beneficial alleles into crop plants from their wild relatives. To enhance recombination between donor chromosomes and the wheat genome to produce wheat-alien chromosome translocations, different strategies were designed, such as ionizing irradiation, induction by Ph1 mutant, and gametocidal chromosomes. Since Sears’ [17] pioneering work transferring leaf rust resistance from Aegilops umbellulata to wheat, ionizing irradiation has been widely used to induce translocations between wheat and different Triticeae species such as rye (Secale cereale L.) [18], A. intermedium [19], and Aegilops spp. [20]. The pairing homoeologous (Ph1b) gene mapped on chromosome 5BL regulates chromosome pairing and recombination in common wheat [21,22]). In the homozygous ph1b locus deletion mutant, the alien chromosomes can pair and recombine with wheat chromosomes at a low frequency [23]. Resistances or tolerances to biotic and abiotic stress into common wheat have been successfully introgressed using the Ph1-deficient genetic stocks including nullisomic for chromosome 5B and mutation of the Ph1b gene [24,25,26,27,28,29]. Gametocidal chromosomes (Gc) from certain wild species in the genus Aegilops introduced into common wheat under monosomic conditions induces chromosome rearrangements in the gametes lacking Gc chromosomes [30]. The Gc system proved to be effective in inducing structural rearrangements in barley [31], rye [32], Haynaldia villosa L. [33], Leymus racemosus (Lam.) Tzvelev [34]), and H. chilense [35,36,37] chromosomes added to common wheat.
Both ionizing irradiation and gametocidal chromosome 2C methods have been previously used to produce wheat-A. cristatum translocations [2,15,16,38,39,40]. However, as far as we know no reports have been published about using a ph1b deletion to obtain wheat-A.cristatum chromosome translocations. In this work we present the development and characterization of structural rearrangements between wheat and chromosomes 4P, 5P, and 6P from A.cristatum using both the action of gametocidal 2Cc genes and ph1b deletion.
2. Materials and Methods
2.1. Plant Material
The plant materials used in this study included the common wheat (Triticum aestivum L.) cultivar ‘Chinese Spring’ (CS), A. cristatum accession PI222957 (2n = 4x = 28; PPPP) provided by USDA (United States Department of Agriculture), the CS/A. cristatum disomic addition lines 4P, 5P, and 6P, and the ditelosomic 4PS, 5PL, 6PS, and 6PL addition lines [10].
2.1.1. Gametocidal 2Cc Chromosome
The disomic addition lines were used as female parents in crosses with the wheat disomic addition line carrying the gametocidal chromosome 2Cc from Aegilops cylindrica Host. The F1 plants monosomic for both the A. cristatum chromosome (4P, 5P, or 6P) and the gametocidal chromosome 2Cc were selfed to obtain F2 plants.
2.1.2. Ph1b Mutant
The disomic addition lines were crossed to the homozygous CS ph1b mutant. The F1 plants monosomic for the A. cristatum chromosome (4P, 5P, or 6P) and heterozygous for the Ph1b gene were backcrossed to CS ph1b and the derived BC1F1 individuals were screened using molecular markers to identify individuals with the genotype ph1bph1b and P chromatin in their genetic background. The individuals that were homozygous for ph1b and heterozygous for the A. cristatum chromosome were selfed to obtain the BC1F2 generation. The BC1F2 progeny were characterized by cytological and molecular markers analysis.
Figure 1 shows the breeding procedures used to obtain structural changes in A. cristatum chromosomes in a common wheat background.
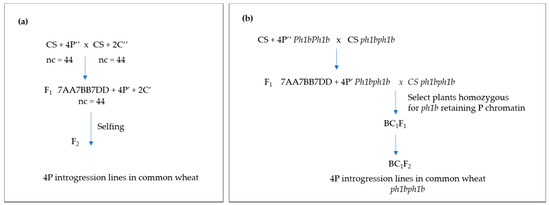
Figure 1.
The breeding procedures used to obtain structural changes in A. cristatum chromosomes in wheat background (i.e., 4P introgressions). (a) Crosses between the A. cristatum disomic addition lines in CS background and the gametocidal 2Cc disomic addition line in CS; (b) Crosses between the A. cristatum disomic addition line in CS and the ph1b mutant were developed and backcrosses to the ph1b mutant to develop A. cristatum introgression lines in common wheat in the ph1b mutant background.
2.2. Molecular Marker Characterization
Total genomic DNA was isolated from young leaf tissue using the CTAB method [41]. Samples were stored at −20 °C until PCR amplification was carried out. The concentration of each sample was estimated using a NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
A specific molecular marker (AcOPX11-817) from A. cristatum developed by Wu et al. [42] was used to detect P genome introgressions in the wheat background. PCR conditions for the AcOPX11-817 marker were as follows: 5 min at 95 °C, followed by 34 cycles of 1 min at 94 °C, 1 min at 53 °C, and 90 s at 72 °C. Amplification products were analyzed on 2% agarose gels in 1 X TBE buffer and visualized by ethidium bromide staining.
Conserved orthologous set (COS) markers [43] were used to identify chromosome arms involved in the translocations. Primer sequences for these markers and the annealing temperature (Ta) are given in Supplementary Table S1. These COS markers were selected for being polymorphic between A. cristatum and wheat CS and having previously transferred and determined arm locations on A. cristatum chromosomes [4,44]. PCR was performed with 40 ng of template DNA in a 25 μL reaction mixture containing 5 μL of 1× PCR buffer, 0.5 μM of each primer, 1.5 mM MgCl2, 0.3 mM dNTPs, and 0.625 U of Taq DNA polymerase (Promega, Madison, WI, USA). PCR conditions for the COS markers were as follows: 4 min at 94 °C, followed by 35 cycles of 45 s at 94 °C, 50 s at 58 °C, and 50 s at 72 °C. The PCR products were analyzed on polyacrylamide gels (10% w/v, C: 2.67%) stained with ethidium bromide.
A PCR assay described by Wang et al. [45] was used to verify the presence of Ph1. Each 30 μL PCR reaction contained 20 ng template DNA, 1x PCR buffer with MgCl2, 0.25 mM dNTPs, 0.17 μM primers, and 0.02 U/μL of Taq DNA polymerase (Promega, Madison, WI, USA). The reaction was first denatured at 94 °C for 5 min, and then subjected to 35 cycles of 94 °C for 60 s, 51 °C for 60 s, and 72 °C for 60 s, followed by a final extension at 72 °C for 7 min). The PCR products were electrophoretically separated through a 1% agarose gel and visualized by ethidium bromide staining.
2.3. Fluorescence In Situ Hybridization (FISH)
Chromosome spreads were prepared from root tip cells. Seeds were germinated on wet filter paper in the dark for 3 days at 4 °C, followed by a period of 24 h at 25 °C. Root tips from germinating seeds were excised and pretreated in ice water for 24 h and then fixed in freshly prepared ethanol-acetic acid (3:1 v/v) and stored at 4 °C for at least 1 month.
The plants were grown at greenhouse with a 16 h photoperiod and a day/night temperature of 25 °C/20 °C. The in situ hybridization protocol was that described by Cabrera et al. [46]. Both pAs1 and GAA-satellite probes were used to identify wheat chromosomes. The pAs1 probe hybridized primarily on the D-genome chromosomes and the GAA satellite sequence hybridized on B-genome chromosomes from wheat. Genomic DNA from A. cristatum was used as a probe to identify P genome introgressions. Total DNA of A. cristatum and GAA-satellite sequences were labeled with biotin-16-dUTP or digoxigenin-11-dUTP (Roche) using nick translation. The pAs1 probe was labeled with biotin-16-dUTP (Roche). Biotin- and digoxigenin-labeled probes were detected with streptavidin-Cy3 conjugates (Sigma, St. Louis, MO, USA) and anti-digoxigenin-FITC antibodies (Roche), respectively. Chromosome preparations were hybridized with A. cristatum genomic DNA probe or simultaneously with both pAs1 and A. cristatum genomic DNA probes. After examination of metaphases, preparations were re-probed with the GAA-satellite sequence. The chromosomes were counterstained with 4’,6-diamidino-2-phenylindole (DAPI) and mounted in Vectashield (Vector Laboratories, Inc., Burlingame, CA, USA). Hybridization signals were visualized using a Leica DMRB epifluorescence microscope and the images were captured with a Leica DFC7000T camera equipped with an exposimeter Spot Leica WildMPS52 and were processed with LEICA application suite v4.0 software (Leica, Germany).
3. Results
3.1. Induction of Wheat-A. cristatum Introgressions by Gametocidal 2Cc Chromosome
A total of 86, 90, and 217 F2 plants were established from the crosses between the CS/A. cristatum disomic addition line for chromosomes 4P, 5P, and 6P, respectively and the CS/Ae. cylindrica disomic addition line for the 2Cc gametocidal chromosome (Table 1). All 393 F2 plants were screened for the presence of P genome chromatin using specific AcOPX11-817 molecular marker from A. cristatum (Figure 2a). Approximately 30% of these F2 plants retained A. cristatum chromatin. The number of F2 plants containing P genome introgressions varied in the offspring of the three different crosses, namely, 21 (24.4%), 32 (35.6%), and 66 (30.4%) F2 plants were found with chromosome 4P, 5P, and 6P, respectively (Table 1). A cytogenetic analysis using FISH was carried out to identify the P genome introgression of the 119 wheat F2 plants that were positive for the AcOPX11-817 molecular marker. Overall, approximately 4% were disomic and 80% were monosomic. Telosomic chromosomes, which most likely resulted from univalent misdivisions, were observed in six plants affecting chromosomes 4P, 5P, and 6P (Table 2). A total of 13 (10.9%) plants contained wheat-A. cristatum translocations: four involving chromosome 4P (19.0%), four involving chromosome 5P (12.5%), and five (7.6%) involving chromosome 6P (Table 2). Most of these wheat-A. cristatum rearrangements were whole-arm translocations involving A genome chromosomes from wheat, as demonstrated by the absence of both pAs1 and GAA hybridization signals on wheat chromosome arms involved in the translocations (Figure 3b,d,e,l,m). A dicentric chromosome involving chromosome 5P (Figure 3h), as well as wheat-A. cristatum chromosomes carrying small translocated fragments from 4PS and 5PS chromosome arms were also observed (Figure 3i,j). A deletion in chromosome 4P was also found (Figure 3k). The A. cristatum chromosome arm involved in the wheat-A. cristatum translocations was identified using chromosome specific COS molecular markers transferred from wheat to A. cristatum (Figure 4).

Table 1.
Progeny retaining A. cristatum chromatin. (a) F2 plants from crosses between the disomic addition line for chromosomes 4P, 5P and 6P, respectively, and the CS disomic addition line for gametocidal 2Cc chromosome; (b) BC1F1 and BC1F2 plants from crosses between the disomic addition line for chromosomes 4P, 5P and 6P, respectively, and CS ph1 mutant.
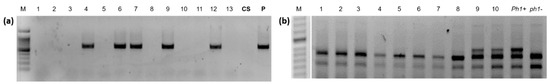
Figure 2.
Genotypic assays for the presence of A. cristatum introgressions and Ph1. (a) The presence of P genome introgression from A. cristatum (lanes 4, 6, 7, 9 and 12) is marked by the successful amplification of the AcOPx11-817 fragment. (b) The absence of Ph1 is marked by the ABC920 SCAR marker (individuals 1-8). M: size marker; CS: Triticum aestivum cv Chinese Spring; P: Agropyron cristatum; Ph1+: wild type wheat CS; ph1-: the parental ph1b mutant.

Table 2.
Transmission of P-genome chromatin and wheat-A. cristatum translocations. (a) F2 plants from crosses between the disomic addition line for chromosomes 4P, 5P and 6P, respectively, and the CS disomic addition line for gametocidal 2Cc chromosome; (b) BC1F2 plants from crosses between the disomic addition line for chromosomes 4P, 5P and 6P, respectively and CS ph1 mutant.
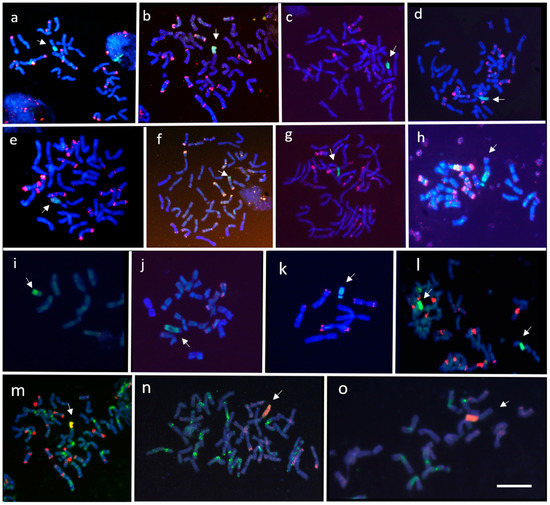
Figure 3.
FISH with A. cristatum genomic DNA (green) and pAs1 repetitive (red) probes to mitotic metaphase of wheat-A.cristatum introgression lines. (a) wheat-4PS centromeric translocation; (b) wheat-4PL centromeric translocation; (c) wheat-5PS centromeric translocation; (d) wheat-5PL centromeric translocation; (e) wheat-6PS centromeric translocation; (f) terminal wheat-6PS translocation; (g) wheat-6PL centromeric translocation; (h) dicentric wheat-A.cristatum involving chromosome 5P (i) and (j) small wheat-A.cristatum translocations; (k) deficient 4P chromosome (l) wheat-5PS centromeric translocations hybridized with genomic A.cristatum genomic DNA (green) and GAA repetitive sequence (red); (m) the same cell as in (b) reprobed with GAA repetitive sequence (green); (n) and (o) centromeric wheat-6PL and wheat-6PS centromeric translocation, respectively, hybridized with A. cristatum genomic DNA (red) and GAA repetitive sequence (green). Chromosome rearrangements were arrowed. The wheat genetic background is ‘Chinese Spring’. Wheat chromosomes are counterstained with DAPI. (a,c,f,g,l,n,o) obtained by Ph1b mutant; (b,d,e,h,i,j,k,m) obtained by gametocidal 2Cc chromosome. Scale bar, 10 µm.
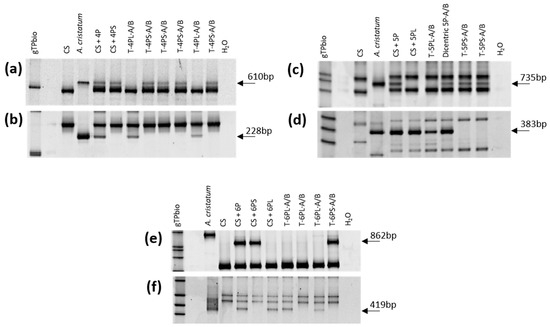
Figure 4.
Molecular characterization of wheat-A.cristatum translocation lines using chromosome-specific COS markers. (a) C:757404-4PS; (b) C:739175-4PL; (c) C:728036-5PS; (d) C:762245-5PL; (e) C:741456-6PS; (f) C:755292-6PL. The differences in the size of the amplified COS fragment in the A. cristatum genome used as control and the A. cristatum chromosome present in the addition lines for some markers are due to genetic differences between the A. cristatum accession used in this study and the accession used to obtain the addition lines.
3.2. Induction of Wheat-A. cristatum Introgressions by ph1b Mutant
A total of 97, 94, and 140 BC1F1 plants were established from the crosses between the CS/A cristatum disomic addition line for chromosomes 4P, 5P, and 6P, with CS ph1 mutant (Table 1). These 331 BC1F1 plants were genotyped using P-specific AcOPX11-817 molecular marker (Figure 2a). The transmission frequencies of A. cristatum chromosomes through female gametes varied in the offspring of the three different crosses: 36 (37.1%), 45 (47.8%), and 57 (38.0%) plants for chromosome 4P, 5P, and 6P, respectively (Table 1). Overall, approximately 41% of the progeny retained A. cristatum chromatin. Zygosity at the Ph1 locus was predicted using a PCR assay (Figure 2b). Based on an analysis using the ph1b diagnostic ABC920 SCAR marker, 63 of the 138 BC1F1 plants were identified to be homozygous for ph1b and heterozygous for the A. cristatum chromosome: 17 (47.2%) for 4P, 24 (53.3%) for 5P, and 22 (38.6%) for 6P, respectively. Among the derived BC1F2 plants, 156 were genotyped using the P-specific AcOPX11-817 molecular marker and 68 retained P chromatin. These BC1F2 plants were analyzed by FISH and specific COS molecular markers to detect and characterize wheat-A. cristatum chromosome arrangements in the background of the ph1b mutant (Table 2). Overall, approximately 13% were disomic and 65% were monosomic. Mono-telosomic and di-telosomic chromosome arrangements most likely resulted from univalent misdivisions and were observed in nine plants in total. A total of six individuals harbored translocations involving A. cristatum: three (15.8%) for chromosome 4P, one for chromosome 5P (4.2%) and two (8.0%) for chromosome 6P. The average induction frequency of chromosome structural aberrations was 8.8%. Among these recombinants, most of the observed aberrations were whole arm translocations (Figure 3a,c,g). The absence of both pAs1 and GAA hybridization signals on wheat chromosome arms involved in the centromeric translocations suggest that these chromosome arms were from genome A (Figure 3a,c,g,n,o). One small terminal introgression involving the 6PS chromosome arm and 6D wheat chromosome was obtained (Figure 3f). The P chromosome arm involved in the wheat-A. cristatum translocation was identified using chromosome specific COS molecular markers transferred from wheat to A. cristatum (Supplementary Table S1). Examples of PCR amplification profiles used for identifying wheat-A. cristatum translocations are shown in Figure 4.
4. Discussion
Chromosome 2Cc from Ae. cylindrica induces chromosomes breaks in CS wheat [30] and some of its relatives [31,32,35,36,37]. In the present study, wheat-A. cristatum translocations were successfully obtained utilizing the gametocidal chromosome 2Cc from Ae. cylindrica. Most of the translocation lines obtained by both gametocidal and Ph1b mutation approaches were whole-arm translocations, indicating that both the A. cristatum and wheat chromosomes were more easily broken at the centromere. In addition, chromosome breakages were also found in the interstitial regions of the chromosome arms as demonstrated by the wheat-A. cristatum dicentric translocation detected in the F2 progeny from the CS + 4P × CS + 2Cc cross (Figure 3h), as well as the terminal 6P-6D translocation detected in the progeny from the CS + 6P × CSph1 cross (Figure 3f). Chromosome breakages in the interstitial regions of the chromosome arms also resulted in deficient chromosomes (Figure 3k).
Chromosome translocations between wheat and the P genome have not been widely reported. Luan et al. [15] used the wheat-A. cristatum disomic addition line for chromosome 6P as bridge materials to produce wheat-A. cristatum 6P translocation lines induced by gametocidal chromosomes. Luan et al. [15] found that the frequency of the translocation induced by the gametocidal chromosome was 5.08%, which is similar to the frequency found for individuals carrying wheat-6P chromosome translocations generated by the gametocidal chromosome 2Cc (7.6%) in this work. Liu et al. [38] obtained a translocation frequency of 3.75% between wheat an A. cristatum chromosome 4P induced by gametocidal 2Cc, which was lower than the 19.0% obtained in the present study.
Attempts to induce translocations between A. cristatum and wheat genomes using the ph1b mutation have been previously carried out by Jubault et al. [47]. The authors [47] found that allosyndetic association between P and ABD genomes in F1 hybrids are very rare and that the low frequency of pairing is likely due to divergence between Agropyron and wheat genomes. It has been also suggested that P chromosomes might carry a genetic system that inhibits the Ph gene in wheat [48,49]. The A. cristatum Ph1b suppressor system appears to be polygenic with all chromosomes, except 2P, promoting homoeologous pairing [49]. However, the genes involved in the A. cristatum suppression system are not efficient enough to induce homoeologous pairing between A. cristatum and wheat genomes and its effect on the Ph genetic system is much weaker than the effect of the ph1b deletion [49]. In this work, no wheat-A. cristatum translocations were observed in plants with genotype Ph1bPh1b (data not shown), which agrees with these findings.
In this study the frequency of A. cristatum–wheat translocations induced by the ph1b deletion was 15.8% for chromosome 4P, 4.2% for chromosome 5P, and 8.0% for chromosome 6P. In Hordeum chilense, a species from the tertiary gene pool of wheat (similar to A. cristatum), the frequency of Hordeum chilense-wheat translocations induced using ph1b mutant ranged from 3.3% to 8% depending of the H. chilense chromosome involved in the translocation [50]. These results are similar to the frequency that we observed for the A. cristatum–wheat translocation induced by the ph1b deletion. Most of the wheat-A. cristatum translocations were obtained with wheat genome A chromosomes, as demonstrated by the absence of both pAs1 and GAA hybridization signals in the wheat chromosomes involved in these translocations (see Figure 3). These results suggested that the P genome from A. cristatum may be more closely related to the wheat A genome than B or D genome. Zhou et al. [51] suggested that, compared with the wheat A and B genomes, the P and D genomes had a greater variant density and a longer evolutionary distance, suggesting that A. cristatum is more distantly related to the wheat D genome. Unfortunately, the absence of GAA hybridization signals on wheat chromosomes arms involved in the translocations hampered the identification of the wheat chromosome involved in the translocations. Further studies should be conducted to identify the wheat chromosome involved in the translocations.
It has been suggested that, among the different strategies developed to produce wheat- alien chromosome translocations, induced homoeologous pairing is the method of choice if gene synteny is conserved [23]. A highly conserved synteny between wheat and A. cristatum has been found. For example, a high transferability of COS markers between wheat and A. cristatum has been reported [4,44,52]. By sequencing the transcriptome of a tetraploid A. cristatum accession, Zhou et al. [53] found that the sequence similarity of most A. cristatum and wheat genes were greater than 95%. The synteny in homoeologous group 5 is also conserved between A. cristatum and wheat, as shown for agronomically important genes, such as the grain hardness (Ha) locus (mapped on the short arm of chromosome 5P) [54] and the vernalization (VRN-1) locus (mapped on the long arm of chromosome 5P) [55], indicating that these loci are collinear which those located on the short and long arms, respectively of chromosome 5 in each of the wheat A, B, and D genomes.
In conclusion, the genetic stocks characterized here included new wheat-A. cristatum recombinations useful for genetic studies and for transferring agronomics traits from the tertiary gene pool to wheat cultivars.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4395/11/2/277/s1, Supplemental Table S1.
Author Contributions
A.C. conceived and designed the study; A.C.-P. and C.P. performed the experiments; A.C. and A.C.-P. analyzed the data; A.C. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grant RTI2018-093367-B100 from the Ministerio de Economía y Competitividad, co-financed by the European Regional Development Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dewey, D.R. The genomic system of classification as a guide to intergeneric hybridization with the perennial Triticeae. In Gene Manipulation in Plant Improvement; Gustafson, J.P., Ed.; Stadler Genetics Sympo, sium Series; Springer: Boston, MA, USA, 1984; pp. 209–279. [Google Scholar]
- Ochoa, V.; Madrid, E.; Said, M.; Rubiales, D.; Cabrera, A. Molecular and cytogenetic characterization of a common wheat-Agropyron cristatum chromosome translocation conferring resistance to leaf rust. Euphytica 2015, 201, 89–95. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, L.; Han, H.; Zhou, S.; Zhang, J.; Yang, X.; Li, X.; Liu, W.; Li, L. Physical localization of a locus from Agropyron cristatum conferring resistance to stripe rust in common wheat. Int. J. Mol. Sci. 2017, 18, 2403. [Google Scholar] [CrossRef] [PubMed]
- Copete, A.; Cabrera, A. Chromosomal location of genes for resistance to powdery mildew in Agropyron cristatum and mapping of conserved orthologous set molecular markers. Euphytica 2017, 213, 189. [Google Scholar] [CrossRef]
- Li, H.; Jiang, B.; Wang, J.; Lu, Y.; Zhang, J.; Pan, C.; Yang, X.; Li, X.; Liu, W.; Li, L. Mapping of novel powdery mildew resistance gene(s) from Agropyron cristatum chromosome 2P. Theor. Appl. Genet. 2017, 130, 109–121. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Liu, W.; Han, H.; Lu, Y.; Yang, X.; Li, X.; Li, L. Introgression of Agropyron cristatum 6P chromosome segment into common wheat for enhanced thousand-grain weight and spike length. Theor. Appl. Genet. 2015, 128, 1827–1837. [Google Scholar] [CrossRef]
- McGuire, P.E.; Dvorák, J. High salt-tolerance potential in wheatgrasses. Crop Sci. 1981, 21, 702–705. [Google Scholar] [CrossRef]
- Bayat, H.; Nemati, H.; Tehranifar, A.; Gazanchian, A. Screening different crested wheatgrass (Agropyron cristatum (L.) Gaertner.) accessions for drought stress tolerance. Arch. Agron. Soil Sci. 2016, 62, 769–780. [Google Scholar] [CrossRef]
- Limin, A.E.; Fowler, D.B. An interspecific hybrid and amphiploid produced from Triticum aestivum crosses with Agropyron cristatum and Agropyron desertorum. Genome 1990, 33, 581–584. [Google Scholar] [CrossRef]
- Chen, Q.; Jahier, J.; Cauderon, Y. Production and cytogenetic analysis of BC1, BC2, and BC3 progenies of an intergeneric hybrid between Triticum aestivum (L.) Thell. and tetraploid Agropyron cristatum (L.) Gaertn. Theor. Appl. Genet. 1992, 84, 698–703. [Google Scholar] [CrossRef]
- Martín, A.; Rubiales, D.; Cabrera, A. Meiotic pairing in a trigeneric hybrid Triticum tauschii-Agropyron cristatum-Hordeum chilense. Hereditas 1998, 129, 113–118. [Google Scholar] [CrossRef][Green Version]
- Martín, A.; Cabrera, A.; Esteban, E.; Hernández, P.; Ramírez, M.C.; Rubiales, D. A fertile amphiploid between diploid wheat (Triticum tauschii) and crested wheatgrass (Agropyron cristatum). Genome 1999, 42, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.H.; Rubiales, D.; Cabrera, A. A Fertile Amphiploid between durum wheat (Triticum turgidum) and the ×Agroticum amphiploid (Agropyron cristatum × T. tauschii). Hereditas 2001, 135, 183–186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soliman, M.H.; Cabrera, A.; Sillero, J.C.; Rubiales, D. Genomic constitution and expression of disease resistance in Agropyron cristatum x durum wheat derivatives. Breed. Sci. 2007, 57, 17–21. [Google Scholar] [CrossRef][Green Version]
- Luan, Y.; Wang, X.; Liu, W.; Li, C.; Zhang, J.; Gao, A.; Wang, Y.; Yang, X.; Li, L. Production and identification of wheat-Agropyron cristatum 6P translocation lines. Planta 2010, 232, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lv, M.; Song, L.; Zhang, J.; Gao, A.; Li, L.; Liu, W. Production and identification of wheat-Agropyron cristatum 2P translocation lines. PLoS ONE 2016, 11, e0145928. [Google Scholar] [CrossRef]
- Sears, E.R. The transfer of leaf rust resistance from Aegilops umbellulata to wheat. Brookhaven Symp. Biol. 1956, 9, 1–22. [Google Scholar]
- Mukai, Y.; Friebe, B.; Hatchett, J.H.; Yamamoto, M.; Gill, B.S. Molecular cytogenetic analysis of radiation-induced wheat-rye terminal and intercalary chromosomal translocations and the detection of rye chromatin specifying resistance to Hessian fly. Chromosoma 1993, 102, 88–95. [Google Scholar] [CrossRef]
- Friebe, B.; Jiang, J.; Gill, B.S.; Dyck, P.L. Radiation-induced nonhomologous wheat Agropyrum intermedium chromosomal translocations conferring resistance to leaf rust. Theor. Appl. Genet. 1993, 86, 141–149. [Google Scholar] [CrossRef]
- Sharma, P.; Sheikh, I.; Kumar, S.; Verma, S.K.; Kumar, R.; Vyas, P.; Dhaliwal, H.S. Precise transfers of genes for high grain iron and zinc from wheat-Aegilops substitution lines into wheat through pollen irradiation. Mol. Breed. 2018, 38, 81. [Google Scholar] [CrossRef]
- Riley, R.; Chapman, V. Genetic control of the cytologically diploid behaviour of hexaploid wheat. Nature 1958, 182, 713–715. [Google Scholar] [CrossRef]
- Sears, E.R. An induced mutant with homoeologous pairing in common wheat. Can. J. Genet. Cytol. 1977, 19, 585–593. [Google Scholar] [CrossRef]
- Qi, L.L.; Friebe, B.; Zhang, P.; Gill, B.S. Homoeologous recombination, chromosome engineering and crop improvement. Chromosome Res. 2007, 15, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Friebe, B.; Jiang, J.; Raupp, W.J.; McIntosh, R.A.; Gill, B.S. Characterization of wheat-alien translocations conferring resistance to diseases and pests: Current status. Euphytica 1996, 91, 59–87. [Google Scholar] [CrossRef]
- Xin, Z.Y.; Zhang, Z.Y.; Chen, X.; Lin, Z.S.; Ma, Y.Z.; Xu, H.J.; Banks, P.M.; Larkin, P.J. Development and characterization of common wheat-Thinopyrum intermedium translocation lines with resistance to barley yellow dwarf virus. Euphytica 2001, 119, 163–167. [Google Scholar] [CrossRef]
- Hajjar, R.; Hodgkin, T. The use of wild relatives in crop improvement: A survey of developments over the last 20 years. Euphytica 2007, 156, 1–13. [Google Scholar] [CrossRef]
- Qi, L.L.; Pumphrey, M.O.; Friebe, B.; Chen, P.D.; Gill, B.S. Molecular cytogenetic characterization of alien introgressions with gene Fhb3 for resistance to Fusarium head blight disease of wheat. Theor. Appl. Genet. 2008, 117, 1155–1166. [Google Scholar] [CrossRef]
- Mullan, D.J.; Mirzaghaderi, G.; Walker, E.; Colmer, T.D.; Francki, M.G. Development of wheat-Lophopyrum elongatum recombinant lines for enhanced sodium exclusion during salinity stress. Theor. Appl. Genet. 2009, 119, 1313–1323. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, H.; Xiao, J.; Bie, T.; Cheng, S.; Jia, Q.; Yuan, C.; Zhang, R.; Cao, A.; Chen, P.; et al. Induction of 4VS chromosome recombinants using the CS ph1b mutant and mapping of the wheat yellow mosaic virus resistance gene from Haynaldia villosa. Theor. Appl.Genet. 2013, 126, 2921–2930. [Google Scholar] [CrossRef]
- Endo, T.R. Gametocidal chromosomes and their induction of chromosome mutations in wheat. Jpn. J. Genet. 1990, 65, 135–152. [Google Scholar] [CrossRef]
- Shi, F.; Endo, T. Genetic induction of structural changes in barley chromosomes added to common wheat by a gametocidal chromosome derived from Aegilops cylindrica. Genes Genet. Syst. 1999, 74, 49–54. [Google Scholar] [CrossRef]
- Friebe, B.; Kynast, R.G.; Gill, B.S. Gametocidal factor-induced structural rearrangements in rye chromosomes added to common wheat. Chromosome Res. 2000, 8, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Cao, A.; Qi, Z.; Zhang, W.; Chen, P. Structural changes of 2V chromosome of Haynaldia villosa induced by gametocidal chromosome 3C of Aegilops triuncialis. Agric. Sci. China 2008, 7, 804–811. [Google Scholar] [CrossRef]
- Chen, P.D.; Liu, W.X.; Yuan, J.H.; Wang, X.; Zhou, B.; Wang, S.L.; Zhang, S.Z.; Feng, Y.G.; Yang, B.J.; Liu, G.X.; et al. Development and characterization of wheat- Leymus racemosus translocation lines with resistance to Fusarium Head Blight. Theor. Appl. Genet. 2005, 111, 941–948. [Google Scholar] [CrossRef]
- Said, M.; Cabrera, A. A physical map of chromosome 4Hch from H. chilense containing SSR, STS and EST-SSR molecular markers. Euphytica 2009, 167, 253–259. [Google Scholar] [CrossRef]
- Cherif-Mouaki, S.; Said, M.; Alvarez, J.; Cabrera, A. Sub-arm location of prolamin and EST-SSR loci on chromosome 1Hch from Hordeum chilense. Euphytica 2011, 178, 63–69. [Google Scholar] [CrossRef]
- Said, M.; Recio, R.; Cabrera, A. Development and characterisation of structural changes in chromosome 3Hch from Hordeum chilense in common wheat and their use in physical mapping. Euphytica 2012, 188, 429–440. [Google Scholar] [CrossRef]
- Liu, W.H.; Luan, Y.; Wang, J.C.; Wang, X.G.; Su, J.J.; Zhang, J.P.; Yang, X.M.; Gao, A.N.; Li, L.H. Production and identification of wheat—Agropyron cristatum (1.4P) alien translocation lines. Genome 2010, 53, 472–481. [Google Scholar] [CrossRef]
- Song, L.; Jiang, L.; Han, H.; Gao, A.; Yang, X.; Li, L.; Liu, W. Efficient induction of Wheat-Agropyron cristatum 6P translocation lines and GISH detection. PLoS ONE 2013, 8, e69501. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, H.; Zhang, J.; Zhou, S.; Han, H.; Liu, W.; Li, X.; Yang, X.; Li, L. Molecular cytogenetic characterization of an Agropyron cristatum 6PL chromosome segment conferring superior kernel traits in wheat. Euphytica 2018, 214, 198. [Google Scholar] [CrossRef]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic. Acids. Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, J.P.; Wang, J.C.; Yang, X.M.; Gao, A.N.; Zhang, X.K.; Liu, W.H.; Li, L.H. Cloning and characterization of repetitive sequences and development of SCAR markers specific for the P genome of Agropyron cristatum. Euphytica 2010, 172, 363–372. [Google Scholar] [CrossRef]
- Quraishi, U.M.; Abrouk, M.; Bolot, S.; Pont, C.; Throude, M.; Guilhot, N.; Bortolini, F.; Praud, S.; Murigneux, A.; Charmet, G.; et al. Genomics in cereals: From genome-wide conserved orthologous set (COS) sequences to candidate genes for trait dissection. Funct. Integr. Genomics 2009, 9, 473–484. [Google Scholar] [CrossRef]
- Said, M.; Copete, A.; Gaál, E.; Molnár, I.; Cabrera, A.; Doležel, J.; Vrána, J. Uncovering macrosyntenic relationships between tetraploid Agropyron cristatum and bread wheat genomes using COS markers. Theor. Appl. Genet. 2019, 132, 2881–2898. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lai, J.; Liu, G.; Chen, F. Development of a Scar marker for the Ph1 locus in common wheat and its application. Crop Sci. 2002, 42, 1365–1368. [Google Scholar] [CrossRef]
- Cabrera, A.; Martín, A.; Barro, F. In-situ comparative mapping (ISCM) of Glu-1 loci in Triticum and Hordeum. Chromosome Res. 2002, 10, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Jubault, M.; Tanguy, A.M.; Abelard, P.; Coriton, O.; Dusautoir, J.C.; Jahier, J. Attempts to induce homoeologous pairing between wheat and Agropyron cristatum genomes. Genome 2006, 49, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yang, X.; Wang, R.; Gao, A.; Li, L.; Liu, W. The inhibiting effect of 1.4 recombinant P chromosome of wheat-Agropyron cristatum addition line on the Ph gene. Sci. Bull. 2010, 55, 153–157. [Google Scholar] [CrossRef]
- Jauhar, P.P. Chromosome pairing in hybrids between hexaploid bread wheat and tetraploid crested wheatgrass (Agropyron cristatum). Hereditas 1992, 116, 107–109. [Google Scholar] [CrossRef]
- Rey, M.-D.; Calderon, M.C.; Prieto, P. The use of the ph1b mutant to induce recombination between the chromosomes of wheat and barley. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, J.; Che, Y.; Liu, W.; Lu, Y.; Yang, X.; Li, X.; Jia, J.; Liu, X.; Li, L. Construction of Agropyron Gaertn. genetic linkage maps using a wheat 660K SNP array reveals a homoeologous relationship with the wheat genome. Plant Biotechnol. J. 2018, 16, 818–827. [Google Scholar] [CrossRef]
- Linc, G.; Gaál, E.; Molnár, I.; Icsó, D.; Badaeva, E.; Molnár-Láng, M. Molecular cytogenetic (FISH) and genome analysis of diploid wheatgrasses and their phylogenetic relationship. PLoS ONE 2017, 12, e0173623. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yan, B.; Li, F.; Zhang, J.; Zhang, J.; Ma, H.; Liu, W.; Lu, Y.; Yang, X.; Liu, X.; et al. RNA-Seq analysis provides first insights into the phylogenetic relationships and interespecific variation between Agropyron cristatum and wheat. Front. Plant Sci. 2017, 8, 1644. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, A.; Castellano, L.; Recio, R.; Alvarez, J.B. Chromosomal location and molecular characterization of three grain hardness genes in Agropyron cristatum. Euphytica 2019, 215, 165. [Google Scholar] [CrossRef]
- Cabrera, A.; Copete-Parada, A.; Madrid, E. Cloning and characterization of a putative orthologue of the wheat vernalization (VRN1) gene in perennial wheatgrass (Agropyron cristatum). Plant Breed. 2020. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).