Abstract
The use of spent mushroom substrate (SMS) in new cultivation cycles has already been reported due to its economic and environmental viability. When considering the application of the circular economy concept in the production of edible mushrooms, the re-use of the SMS within the same process is highly attractive, because it allows a better use of the biomass and the energy involved in the process and, therefore, tends to improve energy efficiency and resource conservation. However, this alternative generates important challenges, which derive from maintaining the quality standards of the mushrooms produced and, at the same time, not incurring excessive costs that are detrimental to the process itself. In our opinion, the main difficulty of the process in achieving success is regarding the biological and agronomic parameters that involve the production of the mushroom. It is useless to apply SMS in new cycles if the mushroom harvest is impaired and farms become non-viable. However, numerous examples are reported here where SMS was recycled into new substrates for either the same or different mushroom species without negatively affecting yield compared with using substrates prepared from 100% fresh raw materials. Thus, we suggest that each farm has its own specific technological study, since a small variation in the raw material of the compost, and mushroom cultivation practices and casing layer used, can influence the entire viability of the mushroom circular economy.
1. Introduction
Mushroom cultivation has a relationship with the conversion of agricultural and agro-industrial waste into food of high nutritional value; it stands out as an environmentally sustainable option [1]. This metabolic capacity of fungi takes place through degradative microbiological processes, which to achieve their highest economic viability, and optimal chemical, physical, environmental and technological process/conditions must be controlled (Figure 1).
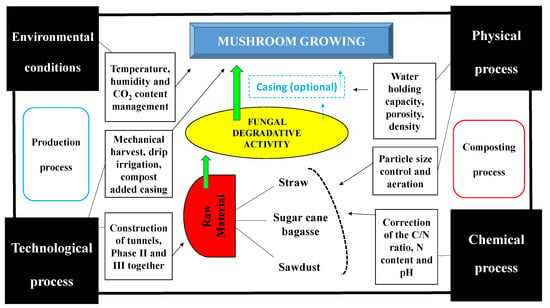
Figure 1.
Scheme of mushroom cultivation and the main chemical, physical, environmental and technological process/conditions, which can influence the most important stages of the production.
Currently mushroom cultivation is carried out worldwide, adopting the most diverse technologies possible, from the simplest to the most technologically advanced [2]. It should be noted that the degree of economic investment and the local social conditions of each country are factors that affect the technological degree of cultivation [3]. Despite this, the entire production process starts from the selection of the raw materials that will be used for the production of the compost/substrate.
There is a specific criterion in the choice of raw materials that is related to the nutrition of mushrooms, which are classified as primary and secondary decomposers. Primary decomposers require materials with higher C/N ratio and lignin content and lower nitrogen content (sawdust, sugarcane bagasse and straw), whereas secondary decomposers require materials with lower C/N ratio and higher cellulose, hemicellulose and nitrogen contents (manure and compost “mixture of various agricultural wastes”) [4,5,6]. Another specific aspect is that the secondary decomposers develop in substrate degraded by bacteria or other fungi [7]. Even so, the versatility of mushrooms can allow them to be primary and secondary decomposers at the same time, as is the case of Pleurotus spp., which can be grown in sawdust/wheat straw or in substrate (sugar cane bagasse, Brachiaria straw, wheat and rice bran and limestone). This factor allows species to be disseminated and cultivated in different regions with greater ease [8]. Therefore, in mushroom cultivation, special attention should be paid to which agricultural or agro-industrial wastes will be used to attain successful production and economic viability in the activity [9]. In this review, we will discuss the use of SMS in new mushroom crops.
2. Circular Economy
The circular economy’s concept is not only to correctly choose the raw materials that will be the basis of the activity, but also to define correctly all the actions involved during each production phase, in order to apply again the wastes generated in the activity on the same company [10]. Using this concept in mushrooms production, the main concern would be the spent mushroom substrate (SMS), the principal waste generated in the cultivation [11]. However, some research has already been developed aiming at the use of SMS as a complementary material to raw materials for new substrate formulations, and as a casing layer for the cultivation of Agaricaceous mushrooms [12,13,14,15]. Figure 2 illustrates the dynamics of the process in the mushroom circular economy, where the only waste during the crop cycle would be the plastic bags/bottle, which are used to grow certain species (Lentinula edodes, Pleurotus spp., Volvariella volvacea, Flammulina velutipes, and others).
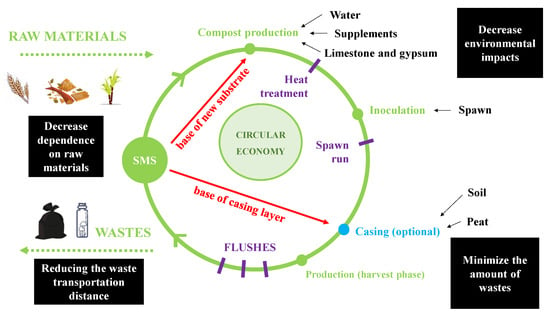
Figure 2.
Mushroom circular economy representing the beginning of the process with the introduction of raw materials, going through compost production, inoculation and production phase (three harvest flushes) until the end of cultivation, where the only waste of the cycle is the plastic bags/bottle. Red letters and arrows indicate the reuse of SMS in the formulation of new substrates and in the casing layer.
Finally, another waste in mushroom production, certainly generated in a smaller quantity than SMS, is the mushroom stipe base (bottom of the stipe which is in contact with the substrate/casing layer), which will be described briefly in item 6, since it is not the general aim of this review.
3. Spent Mushroom Substrate
After mushroom production, the substrate plus the cultivated mycelium generate the SMS byproduct or waste [16], which is widely utilized in the agronomic sector [6]. In the cultivation of Agaricaceous mushrooms, a casing layer based on peat or mineral soil + limestone is incorporated in the SMS.
Considering a commercial mushroom cultivation, i.e., Agaricus bisporus that can attain a yield of 40% of substrate weight (ratio of fresh mushroom weight harvested divided by the fresh weight of the substrate, multiplied by 100), every 1 kg of fresh mushroom generates 2.5 kg of fresh SMS. Emphasizing that in the cultivation of A. bisporus a casing layer is positioned over the compost, every 1 kg of fresh mushrooms generates 3.24 kg of fresh SMS, including the spent peat + limestone casing [17]. Mushrooms that have a lower conversion efficiency than A. bisporus can result in 5 kg of fresh SMS for every 1 kg of fresh mushrooms produced [18]. Significant amounts of wastes are therefore available to be used again in mushroom production.
Among the possible uses for the substrates remaining after mushrooms cultivation, there are references to its use in bioremediation (purification of air, water, soils and degradation of pesticides), for cultivating other crops (greenhouse flowers and vegetables, fruit and vegetables in the field, among others), as a general amendment/fertilizer for soils, in nurseries and landscaping, as animal feed or in aquaculture, in pest and disease management, as energy feedstock (alternative fuel, production of biogas), in vermiculture, as source of degradative enzymes, and other diverse uses. Its reuse in the cultivation of mushrooms, as component of casing material or ingredient of new growth substrates for further mushroom growing cycles, is also considered [6,19,20,21,22,23].
3.1. Spent Mushroom Substrate as Bases of New Substrates
To achieve maximum yield in commercial production of edible mushrooms, it is necessary to know the natural ecological habitats of mushrooms in wood and straw (primary decomposers) or compost (secondary decomposers), the nature of substrate materials and their preparation, the appropriate control of physical, chemical and biological parameters and the proper management of mushroom cultivation beds [24].
As for the ecological habitats, cultivated mushrooms have two kinds of saprophytic nutrition. Most of them are primary decomposers that can be cultivated on pasteurized or sterilized lignocellulosic substrates. The others are leaf-litter secondary decomposers cultivated on composts prepared from various agricultural wastes including manures [25].
Mushrooms can produce a wide range of hydrolytic and oxidative enzymes. The proposal for the reuse of SMS in new growing cycles is based on the different enzymatic activity of various species, highlighting the importance of their sequence of introduction. For the use of these materials in the preparation of substrates for the cultivation of different species of mushrooms, aspects such as their enrichment and combination with other materials, the treatments to be applied and the results obtained must be taken into account. These applications allow the integration of these types of materials, through new formulations and methodologies, with the double advantage of lowering production costs and reducing environmental impact.
3.1.1. SMS of Agaricus spp.
A. bisporus and Agaricus subrufescens (syn A. blazei and A. brasiliensis) are classic examples of secondary decomposers. These mushrooms typically grow on composted material, and they rely on the previous activity of other microorganisms to partially break down a substrate to a state wherein they can thrive. The actions of other fungi, actinomycetes, bacteria, and yeasts all operate within compost. Once these microorganisms (especially actinomycetes) have completed their life cycles, the compost is susceptible to colonization by a select secondary decomposer such as an Agaricus species [26]. However, only a proportion of the nutrient content of the substrate is utilized by an Agaricus mushroom crop, leaving the remainder available for use in subsequent crops. Table 1 contains references on reuse of SMS of Agaricus spp. in new substrate formulations.

Table 1.
Reuse of SMS of Agaricus spp. in new substrate formulations.
According to Till [27], A. bisporus SMS can be reused as a new substrate for Agaricus if it is autoclaved and enriched with cottonseed meal and soybean meal. Murphy [29] developed a successful SMS-based compost formula by supplementing SMS with corncobs and cottonseed meal prior to pasteurization. Schisler [30] studied the behavior in new A. bisporus growing cycles of SMS to which the commercial supplement Spawnmate II and Bonaparte peat were added. Different works refer to the use of mixtures of non-composted substrate (NCS), based on oak sawdust, millet, and SMS of A. bisporus. These substrate mixtures of NCS and SMS produced mushroom yields comparable to standard non-supplemented phase I compost [34,62]. A mushroom yield of 27.2 kg m2 was obtained with a 50/50 mixture of NCS and SMS supplemented with 10% (dry wt) of a commercial delayed release nutrient at casing [33,34]. Moisture content of substrates significantly influenced mushroom yield [63]. Other works abound in the concept of double-cropping. This consists of adding, after the 1st, 2nd, or 3rd flush, materials such as hydrolyzed protein, commercial supplements, crystalline amino acids or phase II compost [12,35,36,38,40,41]. Double-cropping, and even triple-cropping, offers growers an opportunity to increase bio-efficiency, reduce production costs, and increase profitability [36]. Besides that, Royse [37] explored the effect of using various levels of SMS as an ingredient in compost formulations, incorporated at the beginning of phase I or at the time of filling the tunnel for phase II composting (at fill).
Loehr [42] evaluated the use of SMS added as an ingredient to milled corn stover at fill in the development of a phase II-only composting protocol. Warnstrom [43] and Bishop et al. [45] investigated the reuse of A. bisporus SMS as an ingredient in the preparation of fresh mushroom composts based on straw-bedded horse manure and other comparatively higher lignocellulose-rich materials. Zisopoulos et al. [17] compared the performance of a conventional industrial mushroom production chain with a mushroom production chain where part of the compost waste was recycled and reused as raw material. Recently, Ahlawat and Kaur [46] studied the use of SMS in partial replacement of wheat straw in compost formulation for A. bisporus cultivation.
In the UK, Noble et al. [64] removed the peat casing layer (which has no nutrient value) from the cooked-out SMS during emptying of a mushroom crop. The separated SMS was then used in a phase I composting process. Substrate prepared with 25%w/w of the wheat straw and poultry manure substituted with SMS produced the same mushroom yield as the wheat straw + poultry manure substrate. The separated casing layer was partially recycled with fresh casing for new mushroom crops (Section 3.2). Machinery for separating the casing and compost layers on shelf mushrooms farms has been developed by the Dutch company MushComb [65].
It should be noted that the addition of SMS in the production of compost/substrate must follow strict control of steps during compost (phase I), being applied in the correct amount, mixed homogeneously and moistened to the ideal point. It should be noted that compost (phase II) follows the standards recommended for pasteurization (57–60 °C for 8–12 h) and physical, chemical and biological conditioning (45–48 °C for 3 to 5 days). After all these processes, the spawn must be added to the compost and the mycelium run time will occur in an appropriate manner with approximately 15 days, without the presence of contamination. The absence of contamination is due to pasteurization carried out in the cooking out (before the removal of the SMS from the cultivation chambers) and to the phase II of composting mentioned previously.
The use of Agaricus SMS in the production of different Pleurotus species has also been described [47,48,49,50]. In Pleurotus ostreatus cultivation, substrates based on combinations of Pleurotus spent substrate and Agaricus SMS mixed in proportions of 9:1 and 8:2 (w/w) provided a BE of 36.0% and 39.7%, respectively, which was not significantly different from the values obtained with the commercial substrate used as a control [48]. Picornell et al. [49] evaluated the quality parameters of sporphores of P. ostreatus obtained from A. bisporus and P. ostreatus spent substrates mixed in different amounts. Mueller et al. [50] used P. ostreatus spent substrate in the production of Pleurotus sajor–caju.
Oei [51,52] refers to the use in Taiwan of Agaricus SMS mixed with cotton waste, fermented between 2 and 4 days and pasteurized, for the production of Volvariella “straw mushroom”. Poppe [53,54] also suggests that spent substrate can be used to grow successive crops of mushrooms, like Agaricus SMS amended with cotton waste for satisfactory cultivation of Volvariella. Oei et al. [22], refer to Fan and Lu [55], who indicated that in some provinces of China, growers commonly cultivated straw mushroom Volvariella with A. bisporus SMS. There are also references in the use of SMS of A. bisporus in the production of other fungi, such as Auricularia polytricha [47], L. edodes [58] and A. blazei [59].
In Poland, the research of Jasinska and Smolna [61] proposed the use of sawdust or wheat straw substrate with addition of SMS, after A. brasiliensis cultivation, for P. ostreatus, Agrocybe cylindracea, Stropharia rugosoannulata and Hericium erinaceus.
3.1.2. SMS of Pleurotus spp.
Oyster mushrooms (Pleurotus spp.) are examples of primary decomposers. These mushrooms are typically fast-growing, sending out ropy strands of mycelium that quickly attach to and decompose plant tissue. Each species has developed specific sets of enzymes to break down lignin-cellulose, the structural components of most plant cells, so that once the enzymes of one mushroom species have broken down the lignin-cellulose to its fullest potential, other saprophytes utilizing their own repertoire of enzymes can reduce this material even further [26]. References on reuse of SMS of Pleurotus spp. in new substrate formulations are compiled in Table 2.

Table 2.
Reuse of SMS of Pleurotus spp. in new substrate formulations.
SMS of Pleurotus spp. has been used in the cultivation of Pleurotus sajor-caju [68], A. polytricha [68,69] and S. rugosoannulata [19,21,53,70]. Specifically, SMS of P. ostreatus has been used for P. ostreatus [48,49,72,81,95,96], P. florida [74] and A. blazei [82].
In Spain, the Mushroom Research, Experimentation and Services Center (CIES) has carried out different investigations on the reuse of a wheat straw-based substrate, previously used in growing cycles of P. ostreatus, in new production cycles of the same fungus (double-cropping) [48,76,77,78,79,80,81]. For this, the effect of the addition of different enrichment materials, such as commercial supplements, wheat straw and wheat bran, as well as combinations with SMS of A. bisporus has been studied. In the case of A. bisporus SMS, the material used was heat treated in the growing room before emptying (cooking-out). P. ostreatus SMS was heat treated and neutralized with calcium carbonate (15–20 g kg−1) prior to use. Gypsum (50 g kg−1) was added to the formulations as a structural agent. After mixing and moistening, the new substrate was pasteurized at 60–65 °C for 8 h before cooling over a period of 15 h to the spawning temperature (25 °C). Table 3 lists some successful formulations with indications of BE.

Table 3.
Some formulations of new substrates, based on SMSs, for cultivation of Pleurotus ostreatus.
SMS of P. pulmonarius has been used for Pleurotus abalonus and A. polytricha (alone or mixed with rubber sawdust) [83], A. blazei (mixed with sunflower seed hulls) [13] and A. cylindracea (alone or mixed with rubber sawdust) [84].
SMS of P. sajor-caju for P. sajor-caju (double cropping with extra organic nitrogen in the form of oil seed cakes) [85], A. blazei (supplemented with urea, rice bran or ammonium sulfate) [82] and Pleurotus citrinopileatus (with sawdust of Mangifera indica and rice bran) [86].
SMS of Pleurotus eryngii for P. ostreatus (with poplar sawdust, beet pulp and cotton seeds) [87], Volvariella volvacea (composted substrate and non-composted substrate supplemented with wheat straw) [88]; mixed with cotton waste [56,57], P. eryngii (double cropping combined with cottonseed hulls, ramie byproduct or ramie root mediums) [89], Agrocybe chaxingu (with wheat bran and Tenebrio molitor feces) [90], and Pleurotus geesteranus (with sawdust, bran, sugar, gypsum and lime at different rates) [91]; mixed with cottonseed shell and corncob [92]. SMS of Pleurotus cornucopiae for P. cornucopiae (double cropping) and P. ostreatus [93]. SMS of Pleurotus eous for P. sajor-caju, P. florida and P. flabellatus (mixed with fresh wheat straw) [94].
3.1.3. SMS of Lentinula edodes
Shiitake (L. edodes) is another example of a primary decomposer. Traditionally, shiitake has been cultivated on freshly cut logs, usually from the oak family. Heat treated substrates consist of a mixture of sawdust and/or other cellulose-containing materials supplemented with grain, bran or other sources of carbohydrates and nitrogen [97]. References on reuse of SMS of L. edodes in new substrate formulations are presented in Table 4.

Table 4.
Reuse of SMS of Lentinula edodes in new substrate formulations.
Stamets [26] proposed the recycling of substrates by sequencing mushroom species on the same substrate. After shiitake mushrooms stopped producing, sterilized substrate was used in P. ostreatus, P. eryngii and Grifola frondosa cultivation. After the second species in sequence had run its course, sterilized substrate could be used for S. rugosoannulata or Coprinus comatus cultivation.
The use of the sawdust from waste shiitake bed logs with rice bran or wheat bran as an additive was investigated as a resource in the cultivation of P. cornucopiae, P. ostreatus and Flammulina velutipes [99,100,102]. Rodríguez and Jaramillo [98] proposed to combine the residual substrate of the shiitake crop with coffee stalk sawdust for the cultivation of Pleurotus spp. and Ganoderma lucidum.
L. edodes spent substrate has been recycled in the production of other mushroom species: supplemented with wheat bran, white millet and ground soybean for P. sajor-caju cultivation [101], mixed into Agaricus substrate [19,103], partially replacing sawdust as substrate component for the cultivation of P. citrinopileatus [86], or partially replacing corn cob component in C. comatus substrate [106].
3.1.4. SMS of Volvariella volvacea and Other Mushroom Species
References on reuse of SMS of other edible mushrooms in new substrate formulations are compiled in Table 5. Paddy straw mushroom (V. volvacea) is considered a primary decomposer. It prefers high cellulose-, low lignin-containing substrate and produces a range of cellulolytic enzymes. However, common methods for cultivation use composted substrates based on cotton waste and/or paddy straw [4,107]. According to Oei et al. [22], in Fujian province, China, many farmers use spent mushroom compost from straw mushroom cultivation to grow Agaricus mushrooms, so they can save on the cost of straw and cow manure as these are becoming too expensive. Oei et al. [22] cites Zeng [108] who states that straw mushrooms use little nutrition from straw, which can then be refermented with cow manure to produce a good yield of C. comatus. After growing straw mushrooms, the spent compost can also be mixed with rice bran to grow Pleurotus spp. [67,109] or dried and saved for autumn to grow P. sajor-caju [4].

Table 5.
Reuse of SMS of other mushroom species in new substrate formulations.
Winter mushroom or enokitake (Flammulina velutipes), a primary decomposer, was initially cultivated on wood logs, but now sawdust, corncob meal and cottonseed hulls are used as the base ingredients and mixed with nutritional supplements [4,119]. Ohga et al. [113] evaluated the cultural waste from enokitake mushroom cultivation as a substitute for hardwood in shiitake production. Additionally, Chai et al. [114] investigated the recycling and reutilization of enokitake cultural waste as cultivating substrates, supplemented with fresh rice bran and oak sawdust for shiitake production, and supplemented with rice bran to enhance a second flush of enokitake. Utilization of spent straw generated after the cultivation of F. velutipes has been recommended for the production of other mushrooms, such as A. bisporus or C. comatus [19,110,111]. SMS of F. velutipes, supplemented with unfermented cow dung, gypsum, and calcium superphosphate was tested for the cultivation of A. bisporus [44]. Combinations of SMS of F. velutipes with corncob or with poplar sawdust, beet pulp and cotton seeds were evaluated in P. ostreatus production [87,112].
Reishi mushroom (Ganoderma lucidum), a species with numerous pharmacological effects, is produced using wood-log cultivation technology or on substitutes using cottonseed husks, corncobs, sawdust and bagasse as main ingredients [120]. Utilization of SMS generated after the cultivation of G. lucidum has been recommended for the production of A. bisporus and C. comatus [19,110,111].
Buna-shimeji (Hypsizygus marmoreus), another primary decomposer, is commonly cultivated on a sterilized substrate of sawdust and/or corncobs [119]. SMS of H. marmoreus supplemented with cottonseed hulls and wheat bran has been used in a second growing cycle [115]. It has also been utilized to substitute for cottonseed hulls as the substrate for P. ostreatus cultivation [116], and mixed with cotton waste to cultivate V. volvacea [56,57].
3.2. Spent Mushroom Substrate as a Base of the Casing Layer
In commercial cultivation of Agaricaceous mushrooms and others species (P. eryngii, C. comatus and G. lucidum), fructification occurs in the casing layer, material used as a top covering of the compost usually after the substrate is colonized by mushroom mycelium, to induce the transition from vegetative to reproductive growth [121,122]. A compost that is completely colonized by mycelium will not on its own produce mushrooms.
Although the role of the casing layer has been imprecisely defined, it must have particular physical, chemical and microbiological properties for it to function [123]. An ideal casing layer would be high in porosity and water-holding capacity, and low in density and salt concentration [124,125]. Moreover, casing layers, made of materials of a very diverse nature, are an important source of variation in terms of the yield, quality and uniformity of commercial cropping.
The success of using SMS as a casing layer has been reported by several authors [14,126,127]. The environmental gain in replacing peat with SMS in its entirety or in percentage brings numerous environmental and economic benefits. However, SMS should not be used directly as a casing layer, it must be submitted to some processes (thermal treatment, maturation, washing), as shown in Figure 3.
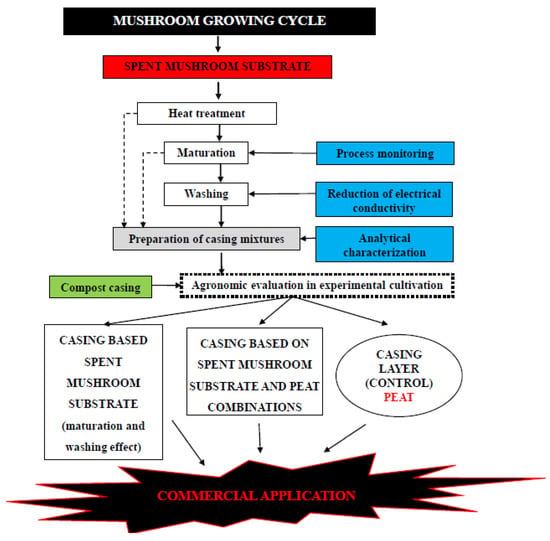
Figure 3.
Steps that must be followed carefully before applying SMS as a casing layer.
Heat treatment (cooking out) is done with the substrate still inside the cultivation rooms, at the end of the harvest phase. The air temperature must be maintained for 70 °C at 6–8 h [128,129]. This process guarantees a significant reduction of pathogens and pests in the compost and cultivation chambers; however, it represents a high-energy cost and can be considered an expensive practice. Therefore, we have one more reason to use this SMS in new cultivation cycles of A. bisporus.
Then the SMS must be taken to undergo a maturation and washing process, which takes 4 to 20 weeks, and requires specific facilities [130,131]. The area used for this maturation must be close to the mushroom industry, facilitating the transport of materials. Finally, some factors influence the time for reusing SMS as casing, such as the origin material (raw materials used in the composting, presence/absence of the casing layer and type of casing), the conditions of recomposting (natural maturation/accelerated controlled process, conditions of aerobiosis or anaerobiosis, frequency of turning and the weather), and performing an artificial washing, grinding or screening [132].
Some quality indicators represent the ideal point of physical and chemical characteristics of casing, such as electrical conductivity (EC) below 1600 µS cm−1, pH between 6.8 and 9 (ideal 7.8), water retention capacity above 45%, porosity above 50% and absence of mites, nematodes and competitor molds [15]. If the SMS does not reach these quality parameters, some materials can be added to obtain an optimal casing layer, for example peat, coconut fiber, limestone, and marl.
Pardo-Giménez et al. [14] studying the effect of sphagnum peat (SP) and SMS verified that mixtures of SP + SMS in the proportions of 4:1, 3:2 and 2:3 had similar A. bisporus yield to the control casing layer (SP + limestone). The mixture of SP + SMS (1:4) produced a significantly lower yield due to the high EC value (2426 µS cm−1).
The importance of the washing process was confirmed using two preparation methods of SMS as the sole material in the casing layer. The first method (washed once based on its volume—W1) produced an EC of 1616 µS cm−1 and the second method (washed twice based on its volume—W2) produced an EC of 1306 µS cm−1. The Dutch commercial casing (Topterra) was used as a control and it was found that mushroom yield was not significantly affected: Topterra (17.61 kg m−2), W1 (17.13 kg m−2), and W2 (16.78 kg m−2). With SMS casing, there was an increase in the dry matter of mushrooms harvested: W1 (79.2 g kg−1) and W2 (78.3 g kg−1), compared to Topterra (67.0 g kg−1) [133]. This is a particular benefit for mushroom destined for the canning industry, where there is reduced weight loss during processing.
To obviate the need for maturation and washing of the SMS, casing has been separated from the underlying compost at the end of mushroom crops. Nair and Bradley [134] and Jablonsky and Srb [135] obtained similar mushroom yields from fresh casing and recycled casing. Noble and Dobrovin-Pennington [65] separated the spent peat + lime casing from the underlying compost after cook out of a mushroom crop. After fully rewetting, the separated casing was mixed at 25% v/v with fresh peat + lime casing and produced the same mushroom yield as 100% fresh casing. Subsequent tests (R. Noble, unpublished) using 33% recycled casing showed a 4 to 5% (but not statistically significant) reduction in mushroom yield compared with using 100% fresh casing.
Another option regarding a circular economy is the use of SMS for producing worm compost (vermicompost) and then using it as a substrate supplement in the cultivation of further mushrooms. García et al. [71] used a non-conventional, non-composted substrate to evaluate the influence of adding vermicompost produced with SSP in the cultivation of A. bisporus. They applied the vermicompost in both the substrate (0–12%) and the casing layer (0–100%) to cultivate brown A. bisporus and reported BE values as a high as 96% in three flushes. The vermicompost was produced in three months, after finishing the cultivation of P. ostreatus, by adding worms to the SPP at 2000 m2 [136].
4. Facilities that Promote the Use of Spent Mushroom Substrate
Modern mushroom facilities and processes are already in place to allow the use of SMS in new growing cycles of A. bisporus. Examples of this refer to the construction of bunkers and shelves in the commercial cultivation. When SMS is added to the new substrate of A. bisporus, it must be properly mixed with the raw materials, before being deposited in the bunkers (Figure 4A–C). This practice is recommended so that good homogenization occurs between the materials and the instability of humidity between 70 to 75%. In Figure 4A, it is possible to see a tractor that deposits the raw materials in two different mixers (indicated by the red arrows). In Figure 4B, it stands out for the addition of water, for the correction of compost moisture and Figure 4C shows the general view of the compost (already added SMS) being translocated through mats for the bunkers. Soon this mixture goes to phase II of the composting process, following the normal patterns of pasteurization and conditioning. In the case of using SMS as a casing layer, the addition occurs in shelves (Dutch type) with mechanized nylon nets to move the compost with casing into the beds, without the use of plastic films/bags (Figure 4D–E). Filling always takes place from the backdoor (seen in Figure 4D) to the entrance door (used daily for harvesting) of the chambers.


Figure 4.
(A): Mechanical machinery used for different material mixtures, i.e., straw, SMS, manure, bran, etc., which are deposited by a tractor in phase I of composting.; (B): Irrigation system used to moisten the compost; (C): Compost being driven to bunker after being mixed and moistened; (D): Modern equipment for mechanicalized filling of phase III compost plus supplement and casing together into growing rooms. The truck with compost and the truck with casing soil from different companies arrive together and one hour later the room is completely filled [137]; (E): Nylon nets that are below the compost and casing (red arrow).
In the case of other species such as A. subrufescens, L. edodes, G. frondosa, Pleurotus spp., Agrocybe aegerita, A. polytricha where production is based on plastic bags, it is recommended that they are biodegradable. For F. velutipes, H. marmoreus, P. eryngii and P. nameko where production is with plastic bottles, it is recommended that they be reusable (after washing and disinfestation). Nevertheless, there is a great challenge in the reuse and RECYCLING of plastics for the mushroom circular economy.
5. Spent Mushroom Substrate Impact Assessment
Resources are getting scarce, ecosystems are threatened, and the consequences of climate change have a large impact on the living environment [9]. Thus, strategies aimed at sustainable production are currently being proposed. As with all food products, mushrooms require energy, material, and water inputs for their production [138].
Zisopoulos et al. [17] evaluating the efficiency assessment of the industrial mushroom production chain found that the critical exergy loss points (CEPs) identified were the cooking-out process of the SMS, and the phase I composting process which were related to chemical and physical energy losses, respectively. In this sense, the use of SMS as a complementary material in the formulation of new cultivation substrates can be efficient because it reduces the composting time in phase I, since it is already degraded and has a high microbial community, which helps in the start of the fermentation process during the phase I compost process.
Despite this, another high production cost in mushroom cultivation refers to transport. Several authors mention that the ideal raw material used as a base for compost production must be close to mushroom farms and available throughout the year. However, the cost of transport of raw materials is 36% of the total transportation expense of mushroom farms [138]. Peat casing accounts for 69% of the raw materials transport costs [138], which further justifies the reuse of SMS as a casing layer, especially after the high cost incurred in the cooking-out stage.
Finally, another advantage of using SMS in new mushroom crops is to avoid long distance transport for disposal as a soil conditioner, as in the case of Netherlands, which generates about 800,000 tons of SMS per year [9,139]. Banasik et al. [9] verified that adopting closing loop technologies in industrial mushroom production by reusing SMS has the potential to increase total profitability of the chain by almost 11% while the environmental performance improves by almost 28%.
6. Other Waste of Mushroom Cultivation
In Brazil, the Mushroom Study Center (CECOG) has carried out different investigations with waste mushroom stipes: bottom 1 to 2 cm of the stipe which is in contact with the substrate/casing layer and is removed with peat remnants during harvesting. Due to being part of the mushroom, nutrients absorbed from the compost and necessary for mushroom growth are present in this biomass, which can be used as a supplement in new mushroom growing cycles, if dehydrated and crushed (Figure 5). As the quantity is insufficient for use with A. bisporus substrate supplement, we are conducting studies for the supplementation of Pleurotus spawn, with A. bisporus stipe base + peat. However, adaptations of technologies still need to be improved, such as the amount of mushroom stipe base to be added in the spawn, the possibility of using the mushroom stipe base + soil as the base of the casing layer, and the addition of mixtures of stipe base from different mushrooms species.
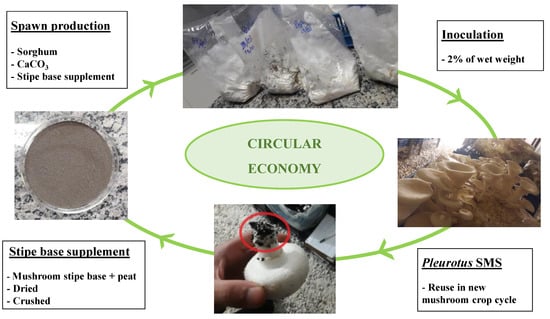
Figure 5.
Use of A. bisporus stipe base as a supplement to spawn production in the cultivation of P. ostreatus, a second example of the application of mushroom waste in a circular economy.
7. Conclusions
The use of SMS in new mushroom cultivation cycles has already been reported due to its cost saving and environmental viability but, in our opinion, the main difficultly of the process is to identify the biological and agronomic parameters that affect mushroom yield. It is useless to apply SMS in new cycles if the mushroom harvest is impaired and farms become non-viable. However, numerous examples are reported here where SMS was recycled into new substrates for either the same or different mushroom species without negatively affecting yield compared with using substrates prepared from 100% fresh raw materials. Thus, we suggest that each farm has its own specific technological study, since a small variation in the raw material of the compost, and mushroom cultivation practices and casing layer used, can influence the entire viability of the mushroom circular economy.
Author Contributions
Writing: D.C.Z., J.E.S., R.N., A.P.-G.; conception and design: D.C.Z., A.P.-G.; technical support: D.C.Z., J.E.S., R.N., A.P.-G.; and critical revision of the manuscript: D.C.Z., J.E.S., R.N., A.P.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This research was supported by the Fundação de Amparo à pesquisa do Estado de São Paulo in Brazil (FAPESP 2018/21492-2, 2015/15306-3) and the Diputación Provincial de Cuenca in Spain.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pontes, M.V.A.; Patyshakuliyeva, A.; Post, H.; Jurak, E.; Hildén, K.; Altelaar, M.; Mäkelä, M.R. The physiology of Agaricus bisporus in semi-commercial compost cultivation appears to be highly conserved among unrelated isolates. Fungal Genet. Biol. 2018, 112, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Dhar, B.L. Mushroom Farm Design and Technology of Cultivation. In Edible and Medicinal Mushrooms: Technology and Applications; Zied, D.C., Pardo-Gimenez, A., Eds.; Wiley-Blackwell: West Sussex, UK, 2017; pp. 271–308. [Google Scholar]
- Zhang, Y.; Geng, W.; Shen, Y.; Wang, Y.; Dai, Y.C. Edible mushroom cultivation for food security and rural development in China: Bio-innovation, technological dissemination and marketing. Sustainability 2014, 6, 2961–2973. [Google Scholar] [CrossRef]
- Chang, S.T.; Miles, P.G. Mushrooms: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004; p. 451. [Google Scholar]
- Mattos-Shipley, K.M.J.; Ford, K.L.; Alberti, F.; Banks, A.M.; Bailey, A.M.; Foster, G.D. The good, the bad and the tasty: The many roles of mushrooms. Stud. Mycol. 2016, 85, 125–157. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Wösten, H.A. Mushroom cultivation in the circular economy. Appl. Microbiol. Biotechnol. 2018, 102, 7795–7803. [Google Scholar] [CrossRef]
- Imtiaj, A.; Rahman, S.A. Economic viability of mushrooms cultivation to poverty reduction in Bangladesh. Trop. Subtrop. Agroecosyst. 2008, 8, 93–99. [Google Scholar]
- Corrêa, R.C.G.; Brugnari, T.; Bracht, A.; Peralta, R.M.; Ferreira, I.C. Biotechnological, nutritional and therapeutic uses of Pleurotus spp. (Oyster mushroom) related with its chemical composition: A review on the past decade findings. Trends Food Sci. Technol. 2016, 50, 103–117. [Google Scholar] [CrossRef]
- Banasik, A.; Kanellopoulos, A.; Claassen, G.D.H.; Bloemhof-Ruwaard, J.M.; van der Vorst, J.G. Closing loops in agricultural supply chains using multi-objective optimization: A case study of an industrial mushroom supply chain. Int. J. Prod. Econ. 2017, 183, 409–420. [Google Scholar] [CrossRef]
- Kopnina, H. Green-washing or best case practices? Using circular economy and Cradle to Cradle case studies in business education. J. Clean. Prod. 2019, 219, 613–621. [Google Scholar] [CrossRef]
- Levanon, D.; Danai, O. Chemical, physical and microbiological considerations in recycling spent mushroom substrate. Compost Sci. Util. 1995, 3, 72–79. [Google Scholar] [CrossRef]
- Royse, D.J.; Chalupa, W. Effects of spawn, supplement, and phase II compost additions and time of recasing second break compost on mushroom (Agaricus bisporus) yield and biological efficiency. Bioresour. Technol. 2009, 100, 5277–5282. [Google Scholar] [CrossRef]
- González-Matute, R.; Figlas, D.; Curvetto, N. Agaricus blazei production on non-composted substrate based on sunflower seed hulls and spent oyster mushroom substrate. World J. Microbiol. Biotechnol. 2011, 27, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Giménez, A.; Pardo-González, J.E.; Zied, D.C. Evaluation of harvested mushrooms and viability of Agaricus bisporus growth using casing materials made from spent mushroom substrate. Int. J. Food Sci. Technol. 2011, 46, 787–792. [Google Scholar] [CrossRef]
- Zied, D.C.; Pardo-González, J.E.; Minhoni, M.T.A.; Pardo-Giménez, A. A reliable quality index for mushroom cultivation. J. Agric. Sci. 2011, 3, 50–61. [Google Scholar]
- Ribas, L.C.C.; Mendonça, M.M.; Camelini, C.M.; Soares, C.H.L. Use of spent mushroom substrates from Agaricus subrufescens (syn. A. blazei, A. brasiliensis) and Lentinula edodes productions in the enrichment of a soil-based potting media for lettuce (Lactuca sativa) cultivation: Growth promotion and soil bioremediation. Bioresour. Technol. 2009, 100, 4750–4757. [Google Scholar] [PubMed]
- Zisopoulos, F.V.; Becerra, H.A.; van der Goot, A.J.; Boom, R.M. A resource efficiency assessment of the industrial mushroom production chain: The influence of data variability. J. Clean. Prod. 2016, 126, 394–408. [Google Scholar] [CrossRef]
- Hanafi, F.H.M.; Rezania, S.; Taib, S.M.; Din, M.F.M.; Yamauchi, M.; Sakamoto, M.; Hara, H.; Park, J.; Ebrahimi, S.S. Environmentally sustainable applications of agro-based spent mushroom substrate (SMS): An overview. J. Mater. Cycles Waste Manag. 2018, 20, 1383–1396. [Google Scholar] [CrossRef]
- Rinker, D.L. Handling and using “spent” mushroom substrate around the world. In Mushroom Biology and Mushroom Products; Sánchez, J.E., Huerta, G., Montiel, E., Eds.; Universidad Autónoma del Estado de Morelos: Cuernavaca, México, 2002; pp. 43–60. [Google Scholar]
- Rinker, D.L. Usos del sustrato degradado de los hongos. In Cultivo, Mercadotecnia e Inocuidad Alimenticia de Agaricus Bisporus; Sánchez, J.E., Royse, D.J., Leal, H., Eds.; Ecosur: Tapachula, México, 2007; pp. 135–149. [Google Scholar]
- Rinker, D.L. Spent mushroom substrate uses. In Edible and Medicinal Mushrooms: Technology and Applications; Zied, D.C., Pardo-Gimenez, A., Eds.; Wiley-Blackwell: West Sussex, UK, 2017; pp. 427–454. [Google Scholar]
- Oei, P.; Zeng, H.; Liao, J.; Dai, J.; Chen, M.; Cheng, Y. Alternative uses of spent mushroom compost. In Mushroom Biology and Mushroom Products; Lelley, J.I., Buswell, J.A., Eds.; GAMU GmbH: Bonn, Germany, 2008; pp. 231–245. [Google Scholar]
- Jasińska, A. Spent mushroom compost (SMC)—Retrieved added value product closing loop in agricultural production. Acta Agrar. Debreceniensis 2018, 150, 185–202. [Google Scholar] [CrossRef]
- Chang, S.T. Mushrooms and mushroom cultivation. In Van Nostrand’s Scientific Encyclopedia, 10th ed.; Considine, G.D., Kulik, P.H., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 3552–3555. [Google Scholar]
- Savoie, J.-M.; Foulogne-Oriol, M.; Barroso, G.; Callac, P. Genetics and genomics of cultivated mushrooms, application to breeding of Agarics. In The Mycota XI, Agricultural Applications, 2nd ed.; Esser, K., Kempken, F., Eds.; Springer: Heidelberg, Germany, 2013; pp. 3–33. [Google Scholar]
- Stamets, P. Growing Gourmet and Medicinal Mushrooms, 3rd ed.; Ten Speed Press: Berkeley, CA, USA, 2000; 614p. [Google Scholar]
- Till, O. Champignonkultur auf sterilisiertem nährsubstrat und die wiederwendung von abgetragenem compost. Mushroom Sci. 1963, 5, 127–133. [Google Scholar]
- Flegg, P.B.; Randle, P.E. Re-use of mushroom bed compost. MGA Bull. 1968, 223, 363–378. [Google Scholar]
- Murphy, W.S. Development of a mushroom production medium without phase I composting. Mushroom News 1972, 20, 4–22. [Google Scholar]
- Schisler, L.C. Why mushroom production declines with each successive break and the production of a second crop of Agaricus mushrooms on “spent” compost. Mushroom News 1988, 36, 6–11. [Google Scholar]
- Schisler, L.C. Why mushroom production declines with each successive break, and the production of a second crop of Agaricus mushrooms on ‘spent’ compost. Appl. Agric. Res. 1990, 5, 44–47. [Google Scholar]
- Rinker, D.L.; Alm, G. Cultivation of commercial mushrooms on spent compost. In Abstracts in Horticultural Research in Canada—1990; Canadian Horticultural Council: Nepean, ON, Canada, 1990; pp. 27–28. [Google Scholar]
- Mamiro, D.P.; Royse, D.J.; Beelman, R.B. Yield, size, and mushroom solids content of Agaricus bisporus produced on non-composted substrate and spent mushroom compost. World J. Microbiol. Biotechnol. 2007, 23, 1289–1296. [Google Scholar] [CrossRef]
- Mamiro, D.P. Non-Composted and Spent Mushroom Substrates for Production of Agaricus bisporus. Ph.D. Thesis, The Pennsylvania State University, The Graduate School, College of Agricultural Sciences, State College, PA, USA, 2006. [Google Scholar]
- Royse, D.J. Double cropping Agaricus bisporus by re-supplementing and re-casing compost. In Mushroom Biology and Mushroom Products; Lelley, J.I., Buswell, J.A., Eds.; GAMU GmbH: Bonn, Germany, 2008; pp. 48–53. [Google Scholar]
- Royse, D.J. Effects of fragmentation, supplementation and the addition of phase II compost to 2nd break compost on mushroom (Agaricus bisporus) yield. Bioresour. Technol. 2010, 101, 188–192. [Google Scholar] [CrossRef]
- Royse, D.J. Use of spent mushroom substrate (SMS) as an ingredient in mushroom compost formulations. Mushroom Sci. 2012, 18, 709–713. [Google Scholar]
- Royse, D.J.; Sánchez, J.E. Supplementation of first break mushroom compost with hydrolyzed protein, commercial supplements and crystalline amino acids. World J. Microbiol. Biotechnol. 2008, 24, 1333–1339. [Google Scholar] [CrossRef]
- Royse, D.J.; Sánchez, J.E. Supplementation of 2nd break mushroom compost with isoleucine, leucine, valine, pheylalanine, Fermenten and SoyPlus. World J. Microbiol. Biotechnol. 2008, 24, 2011–2017. [Google Scholar] [CrossRef]
- Royse, D.J.; Sánchez, J.E. Suplementación de la composta después de la primer, segunda o tercera cosecha para mejorar la producción de hongos (Agaricus bisporus). In Hongos Comestibles y Medicinales en Iberoamérica: Investigación y Desarrollo en un Entorno Multicultural; Sánchez, J.E., Mata, G., Eds.; El Colegio de la Frontera Sur: Tapachula, México, 2012; pp. 99–105. [Google Scholar]
- Royse, D.J.; Sánchez, J.E.; Beelman, R.B.; Davidson, J. Re-supplementing and re-casing mushroom (Agaricus bisporus) compost for a second crop. World J. Microbiol. Biotechnol. 2008, 24, 319–325. [Google Scholar] [CrossRef]
- Loehr, S.M. Minimally Composted Substrate for the Production of Agaricus bisporus. Master’s Thesis, The Pennsylvania State University, The Graduate School, Plant Pathology Department, State College, PA, USA, 2010. [Google Scholar]
- Warnstrom, E.L. Reuse of Spent Mushroom Compost for Production of Agaricus bisporus. Master’s Thesis, The Pennsylvania State University, The Graduate School, Plant Pathology Department, State College, PA, USA, 2013. [Google Scholar]
- Zhang, W.; Niu, X.; Zhang, W.; Liu, Z.; Yuan, S. The cultivation of Agaricus bisporus on the spent substrate of Flammulina velutipes. Afr. J. Agric. Res. 2013, 8, 4860–4863. [Google Scholar]
- Bishop, E.L.; Pecchia, J.A.; Wilkinson, V.; Albert, I.; Royse, D.J. Effects of Spent Mushroom Compost (SMC) as an Ingredient in Phase I Compost on Production of Agaricus bisporus. Compost Sci. Util. 2016, 24, 246–258. [Google Scholar] [CrossRef]
- Ahlawat, O.P.; Kaur, H. Re-use of Agaricus bisporus spent compost for commercial scale compost making of the succeeding crop of A. bisporus. Int. J. Curr. Microbiol. App. Sci. 2019, 8, 177–186. [Google Scholar] [CrossRef]
- Sharma, V.P.; Jandaik, C.L. Recycling of spent compost for growing Auricularia polytricha and Pleurotus species. Mushroom Inf. 1994, 10–11, 15–20. [Google Scholar]
- Pardo-Giménez, A.; Pardo-González, J.E. Elaboración de nuevos sustratos para cultivo de Pleurotus ostreatus (Jacq.) P. Kumm. basados en sustratos degradados por el cultivo de hongos. ITEA Prod. Veg. 2009, 105, 89–98. [Google Scholar]
- Picornell, M.R.; Pardo-Giménez, A.; De Juan, A. Agronomic assessment of spent substrates for mushroom cultivation. Biotechnol. Agron. Soc. 2016, 20, 363–374. [Google Scholar]
- Mueller, J.C.; Gawley, J.R.; Hayes, W.A. Utilization of spent alder compost as a substrate for cultivation of Pleurotus sajor-caju. Mushroom News Trop. 1984, 5, 3–7. [Google Scholar]
- Oei, P. Environmental care: An integrated approach. In Manual on Mushroom Cultivation; Tool Foundation: Amsterdam, The Netherlands, 1991; pp. 43–46. [Google Scholar]
- Oei, P. Environmental care: An integrated approach. In Mushroom Cultivation; Tool Publications: Leiden, The Netherlands, 1996; pp. 38–43. [Google Scholar]
- Poppe, J. Use of agricultural waste materials in the cultivation of mushrooms. Mushroom Sci. 2000, 15, 3–23. [Google Scholar]
- Poppe, J. Agricultural wastes as substrates for oyster mushroom. In Mushroom Growers’ Handbook 1. Oyster Mushroom Cultivation; MushWorld: Seoul, Korea, 2004; pp. 75–85. [Google Scholar]
- Fan, G.Q.; Lu, X.F. Key techniques of using spent mushroom compost to cultivate Volvariella volvacea continuously. Edible Fungi China 2005, 2, 29–30. [Google Scholar]
- Li, Z.; Bao, D.; Tan, Q. Cultivation of Volvariella volvacea using spent mushroom substrates. In Proceedings of the 8th International Conference on Mushroom Biology and Mushroom Products Book of Abstracts, WSMBMP, New Delhi, India, 19–22 November 2014; p. 75. [Google Scholar]
- Li, Z.; Yu, C.; Huang, J.; Bao, D.; Li, Y.; Zhou, F. Cultivation of Volvariella volvacea using spent mushroom substrates. Acta Edulis Fungi 2016, 23, 27–30. [Google Scholar]
- Kilpatrick, M.; Murray, D.J.; Ward, F. Influence of substrate formulation and autoclave treatment on Lentinula edodes production. Mushroom Sci. 2000, 15, 803–810. [Google Scholar]
- Chen, Z.M.; Qiu, C.J.; Li, B.Q.; Lin, J.Y. Sequential cultivation of Agaricus blazei Murrill with waste from Agaricus bisporus cultivation. Fujian J. Agric. Sci. 2011, 26, 989–993. [Google Scholar]
- Flick, M. Using spent mushroom compost for growing other edible fungi. Champignon 1981, 244, 22–26. [Google Scholar]
- Jasińska, A.; Smolna, M. Influence of Agaricus brasiliensis (Wesser) spent mushroom substrate supplemented with AD digestate on the growth of mycelium of several species of edible and medicinal mushrooms. In Nauka dla Środowiska; Dawidowicz, L., Cłapa, T., Eds.; Wydawnictwo Naukowe GSP: Zgorzelec, Poland, 2020; Volume IV. [Google Scholar]
- Mamiro, D.P.; Royse, D.J. The influence of spawn type and strain on yield, size and mushroom solids content of Agaricus bisporus produced on non-composted and spent mushroom compost. Bioresour. Technol. 2008, 99, 3205–3212. [Google Scholar] [CrossRef] [PubMed]
- Mamiro, D.P.; Royse, D.J. Yield and mushroom solids of Agaricus bisporus as influenced by moisture content of substrates. In Mushroom Biology and Mushroom Products; Lelley, J.I., Buswell, J.A., Eds.; GAMU GmbH: Bonn, Germany, 2008; pp. 83–90. [Google Scholar]
- Noble, R.; Dobrovin-Pennington, A.; Turnbull, W. Mushrooms: Carbon and Nitrogen Sources for Organic and Odourless Mushroom Composts; Final Report M 43; Horticultural Development Council: Kenilworth, UK, 2008; 22p. [Google Scholar]
- Noble, R.; Dobrovin-Pennington, A. Developing Alternatives to Peat in Casing Materials for Mushroom Production; Final Report M 60; Agricultural and Horticultural Development Board: Kenilworth, UK, 2015; 59p. [Google Scholar]
- Sharma, V.P.; Jandaik, C.L. Studies on recycling of Pleurotus waste. Mushroom J. Trop. 1985, 6, 13–15. [Google Scholar]
- Quimio, T.H.; Chang, S.T.; Royse, D.J. Technical Guidelines for Mushroom Growing in the Tropics; FAO Plant Production and Protection Paper 106; Food and Agricultural Organization of the United Nations: Roma, Italy, 1990. [Google Scholar]
- Sharma, V.P.; Jandaik, C.L. Recycling of mushroom industry waste for growing Pleurotus sajor-caju and Auricularia polytricha. Indian J. Mycol. Pl. Path. 1992, 22, 182–186. [Google Scholar]
- Sharma, V.P.; Jandaik, C.L. Supplementation of wheat straw for the improved yields of black ear mushroom (Auricularia polytricha). Mushroom Res. 1992, 1, 57–58. [Google Scholar]
- Poppe, J. Cultivation of Edible Mushrooms on Tropical Agricultural Wastes; Biennial Training Course, ABOS & VLIR; University Ghent: Ghent, Belgium, 1995. [Google Scholar]
- García, B.S.; Royse, D.J.; Sánchez, J.E. Vermicompost and casing formulas for the production of brown Agaricus bisporus. In Proceedings of the Fifth International Conference on Mushroom Biology and Mushroom Products, Shanghai, China, 8–12 April 2005; Tan, Q., Zhang, J., Chen, M., Cao, H., Buswell, J.A., Eds.; pp. 243–248. [Google Scholar]
- Jo, W.S.; Kim, J.S.; Cho, D.H.; Park, S.D.; Jung, H.Y. Fruitbody development of Pleurotus ostreatus via bottle cultivation using recycled substrate. Mycobiology 2008, 36, 157–160. [Google Scholar] [CrossRef]
- Pardo-Giménez, A.; Picornell, M.R.; De Juan, J.A.; Pardo-González, J.E.; Zied, D.C. Cultivation of Pleurotus ostreatus using supplemented spent oyster mushroom substrate. Acta Hortic. 2012, 933, 267–272. [Google Scholar] [CrossRef]
- Ashrafi, R.; Mian, M.H.; Rahman, M.M.; Jahiruddin, M. Recycling of spent mushroom substrate for the production of oyster mushroom. Res. Biotechnol. 2014, 5, 13–21. [Google Scholar]
- Herliyana, E.N.; Febrianti, M.; Munif, A.; Lioe, H.N. Kultivasi jamur Pleurotus ramah lingkungan dengan mendaur ulang limbah substrat jamur dan penambahan pupuk organik [Cultivation of Pleurotus environtmental friendly by recycling substrate waste of fungus and organic fertilizer addition]. J. Silvikultur Trop. 2015, 6, 33–42. [Google Scholar]
- Picornell, M.R.; Pardo-Giménez, A.; De Juan, A. Reuse of degraded Pleurotus ostreatus substrate through supplementation with wheat bran and Calprozime® quantitative parameters. Agron. Colomb. 2015, 33, 261–270. [Google Scholar] [CrossRef]
- Picornell, M.R.; Pardo-Giménez, A.; De Juan, A. Assessing reuse of degraded Pleurotus ostreatus (Jacq.) P. Kumm. substrate. Outlook Agric. 2016, 45, 94–99. [Google Scholar] [CrossRef]
- Picornell, M.R.; Pardo-Giménez, A.; De Juan, A. Agronomic qualitative viability of spent Pleurotus substrate and its mixture with wheat bran and a commercial supplement. J. Food Qual. 2016, 39, 533–544. [Google Scholar] [CrossRef]
- Picornell-Buendía, M.R.; Pardo-Giménez, A.; De Juan-Valero, A. Reutilización del sustrato degradado de Pleurotus ostreatus. Parámetros cuantitativos. ITEA-Inf. Tec. Econ. Agrar. 2016, 112, 357–374. [Google Scholar] [CrossRef]
- Picornell, M.R.; Pardo-Giménez, A.; De Juan, A. Reuse of degraded Pleurotus ostreatus (Jacq.) P. Kumm. substrate by supplementation with wheat bran. Quantitative parameters. Mycology 2016, 7, 53–63. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Picornell, M.R.; Pardo-Giménez, A.; De Juan, A. Spent substrates in new cultivation cycles of Pleurotus ostreatus. Sydowia 2017, 69, 73–79. [Google Scholar]
- Gern, R.M.M.; Libardi, N.; Patricio, G.N.; Wisbeck, E.; Chaves, M.B.; Furlan, S.A. Cultivation of Agaricus blazei on Pleurotus spp. spent substrate. Braz. Arch. Biol. Technol. 2010, 53, 939–944. [Google Scholar] [CrossRef]
- Sripheuk, P. Use of spent mushroom compost in a cultivation of abalone mushroom (Pleurotus abalonus Han, Chen et Cheng) and jew’s ear mushroom (Auricularia polytricha (Mont.) Sacc.) in plastic bags. Khon Kaen Agric. J. 2007, 35, 356–363. [Google Scholar]
- Noonsong, V.; Puttakun, N.; Tinsirisuk, M.; Seephueak, P. Recycling of spent Pleurotus compost for production of the Agrocybe cylindracea. Mycosphere 2016, 7, 36–43. [Google Scholar] [CrossRef]
- Shashirekha, M.; Rajarathnam, S.; Bano, Z. Enhancement of bioconversion efficiency and chemistry of the mushroom, Pleurotus sajor-caju (Berk and Br.) Sacc. produced on spent rice straw substrate, supplemented with oil seed cakes. Food Chem. 2002, 76, 27–31. [Google Scholar] [CrossRef]
- Liang, Z.C.; Wu, C.Y.; Wang, J. The evaluation of using mushroom sawdust wastes for cultivation of Pleurotus citrinopileatus. Fung. Sci. 2005, 20, 27–34. [Google Scholar]
- Cheong, J.C.; Lee, C.J.; Shin, P.G.; Suh, J.S. Recycling post-harvest medium from bottle cultivation for Oyster mushroom (Pleurotus ostreatus). J. Mushroom Sci. Prod. 2012, 10, 167–173. [Google Scholar]
- Sun, X.; Xu, H.; Liang, H.; Li, P.; Qiao, D.; Cao, Y.; Zhang, L. Chemical composition of spent Pleurotus eryngii mushroom substrate and its reuse for Volvariella volvacea production. Asian J. Chem. 2013, 25, 10504–10508. [Google Scholar] [CrossRef]
- Yan, L.; Xie, C.; Zhu, Z.; Li, Z.; Hu, Z.; Peng, Y. Study on the cultivation of Pleurotus eryngii using its spent substrates. J. Hunan Agric. Univ. (Nat. Sci.) 2015, 41, 156–160. [Google Scholar]
- Zeng, X.I.; Han, F.; Ye, J.L.; Zhong, Y.M. Recycling spent Pleurotus eryngii substrate supplemented with Tenebrio molitor feces for cultivation of Agrocybe chaxingu. Int. J. Recycl. Org. Waste Agric. 2017, 6, 275–280. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, M.; Li, P. Formulation research on cultivating Pleurotus geesteranus by fungus chaff of P. eryngii. J. Changjiang Veg. 2016, 10, 75–77. [Google Scholar]
- Wang, L.; Zang, C.; Liu, W.; Cui, J. Cultivating Pleurotus geesteranus by using Pleurotus eryngii residues. J. Agric. 2018, 8, 68–71. [Google Scholar]
- Nayaka, M.; Yoneyama, S.; Kato, Y.; Harada, A. Recycling of Cultural Waste of Pleurotus cornucopiae for cultivation of P. cornucopiae and P. ostreatus. 2000. Available online: http://www.agro.nl/pc/isms/posters/pos169.htm (accessed on 16 August 2000).
- Siddhant, C.; Singh, S. Recycling of spent oyster mushroom substrate to recover additional value. Kathmandu Univ. J. Sci. Eng. Technol. 2009, 5, 66–71. [Google Scholar]
- Picornell, M.R. Reutilización de sustratos postcultivo de hongos comestibles en el cultivo de Pleurotus Ostreatus (jacq.) p. Kumm. 2010. Master’s Thesis, Universidad de Castilla-La Mancha, The Graduate School, State College, Cuenca, Spain, 2010. [Google Scholar]
- Picornell, M.R.; Pardo-Giménez, A.; De Juan, A. Qualitative parameters of Pleurotus ostreatus (Jacq.) P. Kumm. mushrooms grown on supplemented spent substrate. J. Soil Sci. Plant Nutr. 2016, 16, 101–117. [Google Scholar]
- Przybylowicz, P.; Donoghue, J. Shiitake Growers Handbook, the Art and Science of Mushroom Cultivation; Kendall: Dubuque, IA, USA, 1990; 217p. [Google Scholar]
- Rodríguez, N.; Jaramillo, C. Cultivo de Hongos Medicinales en Residuos Agrícolas de la Zona Cafetera; FNC-Cenicafé: Chinchinás, CO, USA, 2005; 72p. [Google Scholar]
- Nayaka, M.; Yoneyama, S.; Kato, Y.; Yamamura, T.; Ohga, S. Recycling of waste mushroom substrate for mushroom cultivation. II. Cultivation of Pleurotus ostreatus and Flammulina velutipes using the sawdust from waste Shiitake bed logs. Mushroom Sci. Biol. 1998, 6, 95–99. [Google Scholar]
- Nayaka, M.; Yoneyama, S.; Kato, Y.; Harada, A. Cultivation of some important edible mushrooms using the sawdust from waste Shiitake bed logs. In Mushroom Biology and Mushroom Products; Broderick, A., Nair, T., Eds.; AMGA: Sydney, Australia, 1999; pp. 321–332. [Google Scholar]
- Royse, D.J. Recycling of spent shiitake substrate for production of the oyster mushroom, Pleurotus sajor-caju. Appl. Microbiol. Biotechnol. 1992, 38, 179–182. [Google Scholar] [CrossRef]
- Nayaka, M.; Yoneyama, S.; Yamamura, T. Recycling of waste mushroom substrate for mushroom cultivation. I. Cultivation of Pleurotus cornucopiae using the sawdust from waste shiitake bed log. Mushroom Sci. 1997, 4, 9–13. [Google Scholar]
- Rinker, D.L. Recycling spent shiitake substrate. In Mushroom Growers’ Handbook 2. Shiitake Cultivation; MushWorld: Seoul, Korea, 2005; pp. 186–189. [Google Scholar]
- Babcock, G. Reuse of substrate in specialty mushroom production. Mushroom Sci. 2004, 16, 559–563. [Google Scholar]
- Babcock, G. Recycled substrate in specialty mushroom production. Mushroom News 2007, 55, 12–17. [Google Scholar]
- Xu, Y.; Ren, Z.; Xia, X.; Lu, Y.; Zhang, G. Cultivation of Coprinus comatus using spent mushroom substrates and spent grain from a winery. Acta Edulis Fungi 2012, 19, 44–46. [Google Scholar]
- Ahlawat, O.P.; Tewari, R.P. Cultivation Technology of Paddy Straw Mushroom (Volvariella volvacea); National Research Centre for Mushroom (ICAR): Chambaghat, Solan, 2007; 36p. [Google Scholar]
- Zeng, H. Cultivation of Coprinus comatus on spent straw mushroom compost. Edible Fungi Zhejiang 1993, 2, 13. [Google Scholar]
- Quimio, T.H. Continuous recycling of rice straw in mushroom cultivation for animal feed. In Recent Advances in Biotechnology and Applied Microbiology; Chang, S.T., Chan, K., Woo, N.Y.S., Eds.; Chinese University Press: Hong Kong, China, 1988; pp. 595–602. [Google Scholar]
- Xiao, C. Studies on mushroom re-cultivation on use compost waste. In Proceedings of the International Symposium, Science and Cultivation of Mushrooms, Nanjing, China, 12–15 October 1998; 56p. [Google Scholar]
- Sánchez, C. Cultivation of Pleurotus ostreatus and other edible mushrooms. Appl. Microbiol. Biotechnol. 2010, 85, 1321–1337. [Google Scholar] [CrossRef]
- Li, C. Optimization research on cultivate formula of Pleurotus ostreatus using cultural residue in Flammulina velutiper (Fr.) Sing industrial production. North. Hortic. 2013, 37, 157–159. [Google Scholar]
- Ohga, S.; Yano, S.; Kira, K. Availability of enokitake mushroom, Flammulina velutipes cultural waste for use as a substrate in the sawdust-based cultivation of shiitake, Lentinus edodes. Mokuzai Gakkaishi 1993, 39, 1443–1448. [Google Scholar]
- Chai, J.K.; Kim, Y.; Lee, S. Reutilization of Enokitake Cultural Waste as Cultivating Substrates for Production of Shiitake, Lentinus edodes and Enokitake, Flammulina velutipes; Report of Ministry of Agriculture & Forestry; Ministry of Agriculture & Forestry: Gwacheon, South Korea, 2000; 113p. [Google Scholar]
- Weng, L.; Zhang, K.; Chen, Y.; Yin, P.-Y. Comparative study on cultivation of Hypsizygus marmoreus with corn stover and spent mushroom substrate. North. Hortic. 2014, 38, 143–145. [Google Scholar]
- Wang, S.; Xu, F.; Li, Z.; Zhao, S.; Song, S.; Rong, C.; Geng, X.; Liu, Y. The spent mushroom substrates of Hypsizigus marmoreus can be an effective component for growing the oyster mushroom Pleurotus ostreatus. Sci. Hortic. Amsterdam 2015, 186, 217–222. [Google Scholar] [CrossRef]
- Togashi, I. Effects of using Armillaria species cultural waste as a substrate in the bottle cultivation of hiratake mushrooms, Pleurotus ostreatus. Mokuzai Gakkaishi 1995, 41, 956–962. [Google Scholar]
- Akamatsu, Y. Reutilization of culture wastes of Pleurotus ostreatus and Phollota nameko for cultivation of Lyophyllum decastes. J. Wood Sci. 1998, 44, 417–420. [Google Scholar] [CrossRef]
- Yamanaka, K. Cultivation of mushrooms in plastic bottels and small bags. In Edible and Medicinal Mushrooms: Technology and Applications; Zied, D.C., Pardo-Gimenez, A., Eds.; Wiley-Blackwell: West Sussex, UK, 2017; pp. 309–338. [Google Scholar]
- Zhou, X.-W. Cultivation of Ganoderma lucidum. In Edible and Medicinal Mushrooms: Technology and Applications; Zied, D.C., Pardo-Gimenez, A., Eds.; Wiley-Blackwell: West Sussex, UK, 2017; pp. 385–413. [Google Scholar]
- Noble, R. Manipulation of temperature and controlled CO2 level to synchronise the flushing pattern of the mushroom Agaricus bisporus. Sci. Hortic. Amst. 1991, 46, 177–184. [Google Scholar] [CrossRef]
- Bechara, M.A.; Heinemann, P.H.; Walker, P.N.; Demirci, A.; Romaine, C.P. Evaluating the addition of activated carbon to heat-treated mushroom casing for grain-based and compost-based substrates. Bioresour. Technol. 2009, 100, 4441–4446. [Google Scholar] [CrossRef]
- Pardo-Giménez, A.; De Juan, J.A.; Pardo, J.E. Performance of composted vine shoots as a peat alternative in casing materials for mushroom cultivation. J. Food Agric. Environ. 2003, 1, 209–214. [Google Scholar]
- Noble, R.; Dobrovin-Pennington, A.; Evered, C.E.; Mead, A. Properties of peat-based casing soils and their influence on the water relations and growth of the mushroom (Agaricus bisporus). Plant Soil 1999, 297, 1–13. [Google Scholar]
- Seaby, D. The influence on yield of mushrooms (Agaricus bisporus) of the casing layer pore space volume and ease of water uptake. Compost Sci. Util. 1999, 7, 56–65. [Google Scholar] [CrossRef]
- Jarial, R.S.; Shandilya, T.R.; Jarial, K. Casing in mushroom beds—A review. Agric. Rev. 2005, 26, 261–271. [Google Scholar]
- Barry, J.; Doyle, O.; Grant, J.; Grogan, H. Influence of irrigation management on the quantity and quality of Agaricus bisporus produced on spent mushroom substrate (SMS) based casings. In Proceedings of the XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes, Brisbane, Australia, 17 August 2014; pp. 299–306. [Google Scholar]
- Carlsson, F.; Edman, M.; Holm, S.; Eriksson, A.M.; Jonsson, B.G. Increased heat resistance in mycelia from wood fungi prevalent in forests characterized by fire: A possible adaptation to forest fire. Fungal Biol. 2012, 116, 1025–1031. [Google Scholar] [CrossRef]
- Caslin, B.; Cirillo, M.; Finnan, J.; Forristal, D.; Gaffney, M.; McCutcheon, G.; Murphy, M.; Sproule, I.; Upton, J. Energy Use in Agriculture; Teagasc-Irish Agriculture and Food Development Authority: Carlow, Ireland, 2011; 86p. [Google Scholar]
- Lohr, V.I.; Wang, S.H.; Wolt, J.D. Physical and chemical characteristics of fresh and aged spent mushroom compost. HortScience 1984, 19, 681–683. [Google Scholar]
- Beyer, D.M. Polishing up and managing your casing. Mushroom News 2004, 52, 10–21. [Google Scholar]
- Pardo-Giménez, A. Reutilización del sustrato agotado en la producción de hongos comestibles cultivados. ITEA Prod. Veg. 2008, 104, 360–368. [Google Scholar]
- Pardo-Giménez, A.; Zied, D.C.; Pardo-González, J.E. Utilización de compost agotado de champiñón como capa de coberturas en nuevos ciclos de producción. Pesqui. Agropecu. Bras. 2010, 45, 1164–1171. [Google Scholar] [CrossRef]
- Nair, N.G.; Bradley, J.K. Recycling waste plant products as casing materials in mushroom cultivation. Mushroom Sci. 1981, XI, 147–152. [Google Scholar]
- Jablonsky, I.; Srb, A. Recycled casing soil in the culture of Agaricus bisporus. Mushroom Sci. 1989, XII, 433–443. [Google Scholar]
- Sánchez, J.E.; Moreno-Ruiz, L.; Andrade-Gallegos, R.H.; Cuevas-González, R. Diversificación de la producción del cafetal: La producción de hongos comestibles, una alternativa ecológica y sustentable. In Caminar el Cafetal. Perspectivas Socioambientales del Café y su Gente; Primera edición; Ecosur: Ciudad de México, Mexico, 2019. [Google Scholar]
- Buth, J. The Mushroom Industry in the Netherlands. In Edible and Medicinal Mushrooms: Technology and Applications; Zied, D.C., Pardo-Gimenez, A., Eds.; Wiley-Blackwell: West Sussex, UK, 2017; pp. 197–209. [Google Scholar]
- Robinson, B.; Winans, K.; Kendall, A.; Dlott, J.; Dlott, F. A life cycle assessment of Agaricus bisporus mushroom production in the USA. Int. J. Life Cycle Ass. 2019, 24, 456–467. [Google Scholar] [CrossRef]
- Phan, C.W.; Sabaratnam, V. Potential uses of spent mushroom substrate and its associated lignocellulosic enzymes. Appl. Microbiol. Biotechnol. 2012, 96, 863–873. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).