Abstract
RD6 is one of the most favorable glutinous rice varieties consumed throughout the north and northeast of Thailand because of its aroma and softness. However, blast disease and salt stress cause decreases in both yield quantity and quality during cultivation. Here, gene pyramiding via marker-assisted backcrossing (MAB) using combined blast resistance QTLs (qBl 1, 2, 11, and 12) and Saltol QTL was employed in solving the problem. To pursue our goal, the RD6 introgression line (RGD07005-12-165-1), containing four blast-resistant QTLs, were crossed with the Pokkali salt tolerant variety. Blast resistance evaluation was thoroughly carried out in the fields, from BC2F2:3 to BC4F4, using the upland short-row and natural field infection methods. Additionally, salt tolerance was validated in both greenhouse and field conditions. We found that the RD6 “BC4F4 132-12-61” resulting from our breeding programme successfully resisted blast disease and tolerated salt stress, while it maintained the desirable agronomic traits of the original RD6 variety. This finding may provide a new improved rice variety to overcome blast disease and salt stress in Northeast Thailand.
1. Introduction
Rice (Oryza sativa L.) is consumed as a staple food in Asia, especially in the southeast region. In Thailand, the indica rice variety RD6 developed from KDML105 through gamma irradiation is one of the most favorable glutinous rice consumed throughout the northeast of Thailand [1,2]. Because of its cooking quality, aroma, and softness, production demand has increased over time. However, its yield of 4.16 ton/ha fails to meet its potential, due to biotic and abiotic stress.
Rice blast disease caused by the fungus Pyricularia grisea (Cooke) Sacc. leads to crop losses up to 85% of total yield [3]. Disease symptoms occur in all stages of plant growth, beginning with blast discoloration and wilting of the foliage [4]. Neck blast can be found at the flowering stage, accelerating plant death [5]. Severe damage was also observed within areas of intensive planting with high doses of nitrogen application [6]. Development of new rice varieties resistant to blast fungus is an alternative approach to diminish or control the invasion of this pathogen. The resistance quantitative trait loci (QTL) have been investigated to achieve parental varieties, which are further used for gene pyramiding in breeding programmes. Currently, more than 100 blast-resistant genes have been identified, of which 22 genes structures have been cloned [7]. In Thailand, few studies of blast resistant genes have been conducted. Noenplab et al. [8] studied the relationship of leaf blast and neck blast of resistant genes in the Jao Hom Nil (JHN) variety, in which the resistant QTLs were detected on chromosomes 1 and 11. The resistant QTLs conferred resistance to both leaf blast and neck blast. Suwannual et al. [9] pyramided four blast-resistant QTLs, individually, on chromosomes 2 and 12 within the P0489 variety, and on chromosomes 1 and 11 carried by the JHN variety, resulting in the creation of new RD6 introgression lines. Their results demonstrated that the RD6 introgression lines carrying a high number of QTLs (achieved through pyramiding) reached a broader spectrum of blast resistance to the blast pathogens prevalent in the region.
In addition to rice blast fungus, salt stress is a crucial constraint for RD6 production. Thailand’s northeast region is the country’s largest rice-producing area, and it is comprised of two basins: Sakon Nakhon and Nakhon Ratchasima. In those basins containing an understructure of accumulated salt rock, the salt-affected range covers approximately 1.84 Mha [10]. Evaporation during the dry season tends to raise salinity from the subsoil to the surface, thereby increasing salinity intensity and increasing salt stress from 2–4 dS/m to 8–16 dS/m [10,11,12]. Rice is a salt-sensitive crop, capable of tolerating salinity at moderate levels of electrical conductivity (4–8 dS/m) [13]. Therefore, rice produced under rain-fed, lowland conditions is usually exposed to high levels of soil salinity. The RD6 variety, which is well known, was identified as a geographical indication (GI) within the Tung Gula Rong Hai of the Northeast, Thailand. Specifically, RD6 requires optimal soil salinity to enhance rice seed aroma [14]. However, an abundance of salinity can reduce rice plant growth, tiller number, and seed set-up [15], and the stress caused by excessive salt can significantly reduce total crop yield and result in plant death [16].
The Pokkali variety, derived from the International Rice Research Institute (IRRI), has become a well-known source of salinity tolerance worldwide, attributed to the salt-tolerant QTL located on rice chromosome 1 (Saltol) [17,18,19,20]. Therefore, several researchers have attempted to develop salt-tolerant rice varieties using the Saltol QTL [21,22,23,24,25,26].
The marker-assisted backcrossing (MAB) method has been employed to obtain beneficial QTLs from donor parents via introgression between the qualitative and quantitative traits from landraces and wild relatives [27] due to the precision method with shortened time frame in both foreground and background selection. MAB provides effective gene selection and/ or QTLs for pyramiding multi-genes/QTLs within the rice population. These benefits further support breeding practices for improved resistance and tolerance [28,29,30,31,32]. The objective of this study was to determine the blast resistance and salt tolerance levels within the RD6 introgression lines by pyramiding four blast-resistant and one salt-tolerant QTL into the RD6 rice variety in both greenhouse and field conditions.
2. Materials and Methods
2.1. Plant Materials and Marker-Assisted Backcrossing Selection (MABS)
Three parental varieties/lines were used to generate the BC4F4 population, representing a pseudo-backcrossing approach to increasing the recurrent genetic background of a pyramiding population, comprised of the RD6 (recurrent parent), Pokkali (obtained for the saltol QTL present on chromosome 1), and RGD07005-12-165-1 (the RD6 near-isogenic line obtained from the Rice Gene Discovery Unit, Kasetsart University, Thailand). The RGD07005-12-165-1 obtained four blast-resistant QTLs from the JHN and P0489 varieties on chromosomes 1 and 11, and chromosomes 2 and 12, respectively. The breeding program was subsequently improved within the population through MAB, by crossing the RGD07005-12-165-1 with the Pokkali variety to improve salt tolerance. The F1 was then backcrossed with RGD07005-12-165-1through BC1F1, whereas BC1F2 was utilized as a marker-assisted selection (MAS) in the blast-resistant and salt-tolerant QTLs. In this step, the flanking marker RM3412/RM10748 was used for the selected Saltol QTL [33], RM319/RM212 and RM114/RM224 were used for selected blast-resistant QTLs on chromosomes 1 and 11, respectively [8], and RM48/RM207 and RM313/RM277 were used for selected blast-resistant QTLs on chromosomes 2 and 12, respectively [9], as shown in Figure 1.

Figure 1.
Breeding schematics for the development and validation of the RD6 NILs populations.
Total genomic DNA from young leaves of individual plants, lines, and their parents was extracted according to the method described by Dellaporta et al. [34] with slight modifications. The PCR reactions for SSR markers were carried out in a volume of 10 µL, containing 25 ng of genomic DNA, 1 × PCR buffer, 1.8 mM MgCl2, 0.2 mM dNTP, 0.2 µM forward and reverse primer, and 0.05 unit Taq DNA polymerase. DNA amplification was performed in a DNA thermal cycle for five minutes at 95 °C, followed by 35 cycles of 30 s at 95 °C, 30 s at 55 °C, and two min at 72 °C, with a final extension of seven minutes at 72 °C. The amplification products were separated via 4.5% polyacrylamide gel electrophoresis [32]. The selected lines in the BC1, BC2, and BC3 generations were backcrossed with the RD6 variety, using MAS for saltol and blast-resistant QTL selection. Trait qualities; such as glutinous type, aromatic character, and gelatinization temperature (GT) in the BC2F2 populations were fixed through MAS using glutinous 23 primer on chromosome 6, badh2 on chromosome 8, and RM190 on chromosome 6 (Table S1). Each backcross generation within the BC4F4 populations was evaluated for salt tolerance and blast resistance (Figure 1).
2.2. Evaluation of Salt Tolerance and Blast Resistance in the BC2F2:3 Populations (Exp. 1)
The evaluation of salt tolerance and blast resistance of the BC2F2:3 lines, as well as the parental and check varieties, were conducted at the Department of Agronomy, Faculty of Agriculture, Khon Kaen University, Khon Kaen, Thailand. The salt tolerance evaluation was performed through two methods, salt solution and artificial soil salinity. The salt solution method was laid out in a completely randomized design (CRD), with four replications. Seedlings were transplanted at seven days to 50 × 57 cm2 Styrofoam sheets with 1.5 cm diameter holes [17]. Fertilizer was applied with Yoshida nutrient solution [35] at three to twenty-one days of age. NaCl was then added to reach the electrical conductivity (EC) of 4 dS/m. EC was subsequently increased at three-day intervals, reaching EC 8 and 12 dS/m. When the susceptible checks (IR29) presented salt injury symptoms, salt tolerance data were recorded following the standard evaluation score (SES) [36]. The artificial soil salinity method was also laid out in CRD with four replications. Seedlings were transplanted at seven days and transferred from the spent soil trays to the water trays. At 14 days after transplanting, the experimental trays were treated with NaCl, which adjusted the water solution to EC 8dS/m, which increased to EC 12dS/m after two days. Salt tolerance data were recorded similarly in both methods.
Blast resistance was also evaluated via the upland short-row method at the Sakon Nakhon Rice Research Center, Sakon Nakhon, Thailand. The experiment was laid out in CRD with three replications. Seeds of each BC2F2:3 line were sown in rows (approximately) 50 cm long and 10 cm apart. A susceptible KDML105 variety was planted alternately with every two testing varieties. Blast resistance scores were recorded following the SES method [36].
2.3. Evaluation of Salt Tolerance in the BC3F4 Populations (Exp. 2)
The BC3F4 lines and parental varieties were evaluated for salt tolerance in field conditions. The experiment was conducted at the Ban Daeng Village, Ban Fhang, Khon Kaen, Thailand. The experiment was laid out in a randomized complete block design (RCBD) with three replications. Germinated seeds were sown on seedbeds; then, at thirty days, the seedlings were transplanted to the field. Plot sizes were 1 × 1.5 m2, in three rows, spaced 25 × 25 cm between and within rows. The RD6 variety was planted between every five plots within the test lines to ensure that salinity occurred uniformly. Fertilizer (23.44 kg/ha of N, P2O5, and K2O) was applied at four days after transplanting, and hand weeding and chemical application for disease and insect control were performed as needed. When the susceptible check (RD6) presented salt injury symptoms, salt tolerance data were recorded following SES [37]. Moreover, the agronomic traits including 1000/seed weight, seed length, seed width, and seed shape were recorded.
2.4. Evaluation of Salt Tolerance and Blast Resistance Evaluations in the BC4F3 Populations (Exp. 3)
The salt tolerance evaluation of the BC4F3 lines was conducted in greenhouse conditions at the Department of Agronomy, Faculty of Agriculture, Khon Kaen University, Khon Kaen, Thailand. The experiment was laid out in CRD with three replications, as described in the salt solution method of Exp. 1. The experiment for blast resistance of the BC4F3 lines was conducted at the Department of Agronomy, Faculty of Agriculture, Khon Kaen University, Khon Kaen, Thailand through field and upland short-row experiments. The upland short-row method was conducted similarly to Exp. 1. The field experiment was laid out in RCBD with three replications, in plots 0.5 × 1 m2, in three rows, spaced 25 × 25 cm between and within rows. Seedlings were transplanted at 30 days of age. Symptoms of natural blast infection were identified when seedlings began to show signs of infection, following the protocol of the SES [37], in which both leaf and neck blast symptoms were recorded.
Agronomic trait data, such as plant height (PH) and panicle length (PL), were recorded at pre-harvest; whereas post-harvest data recorded 4/panicle seed weight (SW4P), 1000/seed weight (1000SW), total dry weight (TDW), total seed weight (TSW), harvest index (HI), seed length (SL), seed width (SW), and seed shape (SS) (ratio of SL/SW). Additionally, seed qualities of the BC4F4 seeds, such as seed morphology, including SL, SW, SS and seed color of brown and paddy rice and aromatic traits, were evaluated and compared with the RD6 variety. The seed aromatic evaluation of each line was achieved through the quantitative determination of 2-acetyl-1-pyrroline (2AP) content using automated headspace gas chromatography following the methods as prescribed by Sriseadka et al. [38]. In brief, polished seed (1.00 g) were ground and then placed in a 20 mL headspace vial. The headspace vials were immediately sealed with PTFE/silicone septa and aluminum caps prior to analysis by static headspace-gas chromatography. A static headspace (Model 7697A, Agilent Technologies, Santa Clara, CA, USA) coupled to an Agilent 7890B Series GC system equipped with an Agilent 5977B GC/MSD system was used. A series of 2AP standard solutions with concentrations of 1.22, 2.45, 4.90, 9.79, and 19.67 ppm in isopropanol were prepared, which was added to headspace vials containing 1.00 g of non-aromatic rice seed (cv. Chai Nat 1) used as the external standard. The optimum headspace operating conditions were oven temperature 110 °C, loop temperature 120 °C, transfer line temperature 130 °C, vial equilibration time 10 min with high speed shaking, pressurizing time 0.15 min, loop equilibration time 0.40 min, and inject time 0.50 min. The headspace volatiles were separated using an HP-5 (25 m × 250 µm × 0.25 µm film thickness) column (J&W Scientific, Folsom, CA, USA). The optimum GC conditions were achieved using an HP-5 column with a splitless injection at 210 °C. The column temperature programme began at 50 °C and increased to 200 °C at 10 °C/min. Purified helium was used as the GC carrier gas at a flow rate of 1.2 mL/min. A calibration curve for 2AP analysis by headspace was generated by spiking known concentrations of 2AP into a non-fragrant rice variety (Chai Nat 1). Samples were run in triplicate, and the concentration of 2AP was calculated based upon the relative peak area of external standard.
2.5. Evaluation of Salt Tolerance in the BC4F4 Populations (Exp. 4)
The salt tolerance evaluations of the BC4F4 lines were conducted in greenhouse conditions at the Department of Agronomy, Faculty of Agriculture, Khon Kaen University, Khon Kaen, Thailand, via the salt solution method. Laid out in CRD with four replications, the planting methods and experiment protocol were similar to those described in Exp1, except that the EC in Exp. 4 was adjusted to 18 dS/m. The leaf, stem, root, and total dry weight data were recorded. Dry weight of the seedlings was determined by oven-drying the seedlings at 80 °C for 3 days, and the percentages of Na+ and K+ in the leaves, stems, and roots also were determined, according to the flame photometric method [39]. In addition, the percentages of Na+ and K+ in the leaves, stems, and roots also were determined, according to the flame photometric method [39]. In brief, pounding sample volume 0.5 g of each tissues were digest by 10 mL nitric acid (HNO3) and 5 mL perchloric acid (HClO4), then incubated at 200 °C. The contents were covered to reflux acid fumes generated during digestion until digest appeared translucent. After cooling down, 100 mL deionized water was added to each digestion tube. Contents were then vortexed and passed through qualitative cellulose filter paper (Whatman No.1, Sigma-Aldrich®, St. Louis, MO, USA) and measurement the K+ (768 nm wavelength) and Na+ (589 nm wavelength) by flame photometer (Model 410 Flame Photometer, Sherwood Scientific Limited, Cambridge, UK). The K+ and Na+ concentrations of samples were compared with the known standard solutions of 0.0, 5.0, 10.0, 15, and 20 ppm from a calibration curve with a correlation coefficient (r2) = 0.999. Finally, the amount of K+ and Na+ were transformed to percentage when compared with dry weight of raw materials.
2.6. Data Analysis
Salt tolerance scores, blast resistance scores, and agronomic trait data were analyzed via the STATISTIC 10© program (1985–2013) (Analytical Software, Tallahassee, FL, USA). Means were compared by the least significant difference (LSD) at p < 0.05.
3. Results
3.1. Development of Populations Through MABS
The F1 population (RGD07005-12-165-1 × Pokkali) was backcrossed with RGD07005-12-165-1 using MAS through BC2F2, in which the MAS contained genes of the glutinous type, aromatic, and gelatinization temperature (GT); and the BC2F2:3 populations were then evaluated for salt tolerance and blast resistance. One BC2F2:3 line (no. 74) obtained all QTLs required to obtain the target traits necessary for generation advancement (Table S2). In developing the BC3 generation, BC2F2:3 (no. 74) was crossed back to RD6 to produce the BC3F1, in which the BC3F1 was developed through the BC3F4 population by MAS. Thirty-one BC3F4 lines were evaluated for salt tolerance and agronomic traits within the salted field. Among the BC3F4 lines, the BC3F4 (no. 132) demonstrated salt tolerance and agronomic characteristics similar to those of the RD6 variety and were subsequently selected for the development of the BC4F1 through BC4F3. The BC4F3-132-12-61 line was successfully developed via the MABS, consisting of one QTL for salt tolerance and four QTLs for blast resistance. BC4F3 lines with varied QTL combinations were also validated in the tests.
3.2. Evaluation of Salt Tolerance and Blast Resistance in the BC2F2:3 Populations
Eight of the BC2F2:3 lines were evaluated for salt tolerance in the seedling stage through the salt solution and artificial soil salinity methods. The results concluded that the BC2F2:3 lines presented as highly significant at EC 12 dS/m. BC2F2:3 (nos. 23, 67, and 74), demonstrated tolerance (T) in both methods, similar to that of the tolerant check (Pokkali); while the remaining five BC2F2:3 lines showed moderate tolerance (MT) under both the salt solution and artificial soil salinity tests (Table 1). The BC2F2:3 lines were evaluated for blast resistance via the upland short-row method, through which the blast resistance reaction (R) was similar to that of the resistance checks (JHN and P0489). Their resistance proved greater than that of the susceptible check (RD6), due to the presence of blast-resistant QTLs, and superior to that of the original RD6 (Table 1).

Table 1.
Salt tolerance scores at EC 12 Ds/m in salt solution, artificial soil salinity, and blast resistance within the BC2F2:3 populations.
3.3. Evaluation of Salt Tolerance in the BC3F4 Populations
BC2F2:3 (No.74) was selected for the next backcross cycle with the RD6, and MAS was performed through the BC3F4 population. Thirty-one BC3F4 lines were screened for salt tolerance and agronomic performance. The results showed that the 21 BC3F4 lines were salt-tolerant (T), whereas the 10 BC3F4 lines proved only moderately tolerant (MT). Agronomic traits of the BC3F4 lines were similar to those of the RD6 variety, which was in accordance with our objectives (Table 2). Five BC3F4 lines (nos.22, 36, 115, 129, and 132) demonstrated superior tolerance (T) and agronomic traits (1000/SW, SL, SW, and SS), again, similar to those of the original RD6, and were selected as donor parents for the development of the BC4. The results showed that BC3F4 (no. 132) produced the best performance for pollination, and was therefore selected for development of the BC4 population.

Table 2.
Salt tolerance scores and agronomic traits within the BC3F4 populations.
3.4. Evaluation of Salt Tolerance and Blast Resistance in the BC4F3 Populations
This experiment evaluated eight BC4F3 lines obtained via Saltol QTL from MAS, specifically, the combination lines, donor parents, and tolerance, and susceptible checks were screened for salt tolerance via the salt solution method (EC 12 dS/m). The results showed highly significant scores within the BC4F3 population and the tolerant check (Pokkali), with salt tolerance scores less than 5.0, indicated as tolerant (T). The recurrent parent (RD6) presented moderate tolerance (MT) with a score of 6.5 (Table 3). The results indicated that the Saltol QTL within the BC4F3 population demonstrated superior performance in salt tolerance to that of the recurrent parent (RD6).

Table 3.
Blast resistance scores of leaf blast neck blast and salt tolerant evaluations in the field and upland short-row methods in the BC4F3 populations.
The BC4F3 populations were evaluated for blast resistance in field conditions through natural infection. Since blast infection in the field was not severe, leaves with severe symptoms from the surrounding trap plants were collected and stored in a bag, under dark conditions, for twelve hours to induce spore formation. The natural inoculum was added with water, and spore suspension was then sprayed over the field. The BC4F3 populations and their parents showed a high level of blast resistance. A total of eight BC4F3 lines demonstrated high resistance (HR), similar to those of both the donor parents, whereas the recurrent parent (RD6) presented moderate susceptibility (MS) (Table 3). Importantly, some introgression lines were greater in resistance than the recurrent parent (RD6). The second peak of bimodal rain, which occurred in the flowering stage, initiated significant signs of neck blast. The results found that eight BC4F3 lines showed resistance (R) similar to that of the donor parent, whereas the RD6 was moderately susceptibility (MS) to neck blast (Table 3).
Additionally, blast resistance evaluation was also conducted via the upland short-row method in the seven BC4F3 lines, which presented highly significant blast-resistant levels. The BC4F3 lines showed resistance abilities similar to both donor parents, whereas the RD6 recurrent parent presented moderate resistance (MR). Notably, the blast-resistant genes in the BC4F3 populations provided blast resistance in the seedling stage similar to that of the tilling and grain filling stage, and evidenced greater resistance than that of the RD6 recurrent parent (Table 3).
The agronomic traits were also evaluated in the blast field experiments. The results were highly significant within the BC4F3 populations and their parents for ten traits: PH; PL; SW4P; 1000/SW; TDW; TSW; HI; SL; SW; and SS (Table 4). The BC4F3 132-12 maintained agronomic traits similar to those of the recurrent parent (RD6) for nine traits, except for the 1000/SW (1000 seed weight), in which the RD6 presented moderate susceptibility (MS) for leaf and neck blast, resulting in low grain filling (Table 4). The results indicate that the newly developed MAB population had greater resistance than that of the original RD6 variety while maintaining its desirable agronomic traits and satisfying consumer demand. The BC4F3 lines also showed improvements in seed length (SL), seed width (SW), and seed shape (SS) as long and slender type. Additionally, the color of paddy rice was straw yellow, similar to the original RD6 varieties (Figure 2).

Table 4.
Evaluation of agronomic traits and 2-acetyl-1-pyrroline (2AP) content from the blast fields of the BC4F3 populations.
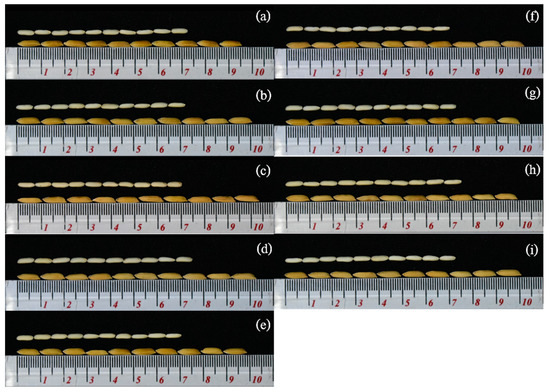
Figure 2.
Seed quality, seed length, seed shape, and seed color of 10 seeds of the BC4F3 populations compared with the RD6 variety. (a) RD6, (b) BC4F3 133-12-61, (c) BC4F3 133-25-18, (d) BC4F3 132-14, (e) BC4F3 132-98, (f) FC4F3 132-174, (g) BC4F3 132-51, (h) BC4F3 132-167, (i) BC4F3 132-276. The top row is brown rice and bottom row is paddy rice with straw color. The small scale is in millimeters.
Eight BC4F3 lines and the RD6 (recurrent parent) were evaluated for seed aroma through the determination of 2AP content via the automated headspace gas chromatography method. The results showed a significant difference among the BC4F3 lines and RD6 variety, with mean values of 2AP content of the BC4F3 exceeding 3.00 ppm (Table 4). BC4F3 132-98-87 presented the highest 2AP content (4.68 ppm), similar to that of the RD6 (Table 4). The BC4F3 lines and RD6 were determined to be similar in fragrance.
3.5. Evaluation of Salt Tolerance in the BC4F4 Population
Two BC4F4 lines, BC4F4 132-12 and BC4F4 132-167, presented as tolerant (T) in salt tolerance evaluations, similar to that of the Pokkali, whereas the remaining five BC4F4 lines and the recurrent parent (RD6) showed moderate tolerance (MT) (Table 5). Statistically, seven of the BC4F4 lines demonstrated EC values up to 18 dS/m. The dry weights of the seven BC4F4 lines, their parent, and KDML105 check varieties presented as highly significant, in which the tolerant check (Pokkali) presented the highest LDW, SDW, RDW, and TDW evident in the weights of leaf stems and roots, whereas the BC4F4 lines were similar to that of the recurrent parent (RD6) (Table 5). The results indicate that salinity significantly affected dry weight.

Table 5.
Leaf, stem, root, and total dry weights in the experiment 4.
Additionally, the percentages of Na+ and K+ in leaf stems and roots were also recorded and proved highly significant in all traits. The tolerant checks (Pokkali) presented the lowest Na+ in leaves (1.94), stems (1.75), and roots (4.94), whereas K+ in leaf stems were non-significant within the BC4F4 lines. Moreover, Pokkali also displayed low Na+-to-K+ ratios in rice shoots. The BC4F4 132-12 also showed low levels of Na+ in both stems and leaves, as well as low Na+-to-K+ ratios in rice shoots comparable to those of the Pokkali variety, yet lower than those of the RD6. We may infer that transferring the saltol QTL from the Pokkali line created greater tolerance in subsequent breeding lines that that of RD6 recurrent parent (Figure 3). Interestingly, the BC4F4 lines presented Na+ levels in leaves and stems similar to those in Pokkali, and significantly different in RD6. This confirmed the Pokkali saltol QTL’s ability to exclude Na+ from the leaf blade, expressing salt tolerance through the salt-excluder method. Moreover, the salt tolerance scores were negatively correlated to the leaf, stem, root, and total dry weights (r = −0.7899 **, r = −0.8136 **, r = −0.8140 **, r = −0.8065 **, respectively) and positively correlated with leaf and stem Na+ (r = 0.7670 ** and r = 0.8917 **, respectively) (Table 6). The results indicated that salt stress decreased plant growth in susceptible varieties, while growth was maintained in salt-tolerant varieties and that the accumulation of Na+ in leaves and stems was related to salt susceptibility (Table 6).
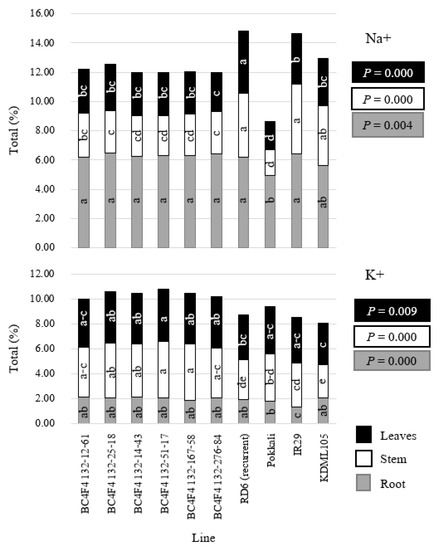
Figure 3.
Na+ and K+ on leaves, stems, and roots in the experiment 4. Different letters within a color bar show a mean significant difference of each line.

Table 6.
The correlation between leaf dry weight, stem dry weight, root dry weight, total dry weight, leaf Na+, leaf K+, stem Na+, stem K+, root Na+, root K+, and the salt score of seeds in the BC4F4 populations and check varieties in the experiment 4.
4. Discussion
Since its release in 1977, the RD6 glutinous rice variety has remained a staple food crop for domestic consumption in Thailand’s north and northeast regions. Comprising 83% of total glutinous rice production in these areas, consumers have developed a preference for its superior characteristics. However, the RD6 variety suffers from several production constraints, including biotic stress responsible for both rice blast [40] and bacterial blight disease [41]. Current research has attempted to eliminate sustainable infection-resistant production practices by pyramiding multiple resistant genes [42]. To date, an RD6 introgression line capable of resisting both biotic and abiotic stress has yet to be developed. Thailand’s salt rock basins of Sakon Nakhon and Nakhon Ratchasima have demonstrated that consistent levels of salinity can enhance the fragrance of the RD6 rice variety [14] and increase production.
This study proposes the successful introgression of blast-resistant QTLs (qBL 1, 2, 11, and 12) from RGD07005-12-165-1 and the Saltol QTL (Pokkali, chromosome 1) to improve the RD6 rice variety through the MAB method within BC4F4 populations. Trait evaluations were completed for the validation of progenies with desirable traits in each advanced population, based on the introgression of the genetic foregrounds and maintenance of the genetic backgrounds, respectively.
Salt salinity was absent in several areas of northeast Thailand, due to high levels of NaCl [43], and such factors as precipitation, soil type, and field management. In past research, the evaluation of salt tolerance was typically conducted through salt screening, hydroponic culture, and soil culture, as well as through pot and field methods [33]. The current study assessed salt tolerance within the breeding populations studied through salt solution, artificial salt culture, and field condition evaluations. Based on the results, salt evaluation under field conditions produced the lowest capability among the tested rice lines (Table 1, Table 2 and Table 3 and 5), due to the inherent difficulties and uncertainties present under field conditions. Kranto et al. [33] reported that effective alternative screening approaches must be proven to correlate with results produced within the early phases of growth in both greenhouse and field conditions. Within the present study, visual symptom scores of salt stress generated through the salt solution method proved to be the most appropriate method with which to confirm tolerance abilities within a breeding population (Table 5), suggesting that the salt solution method could, therefore, substitute salt tolerance score analysis in field conditions (Table 5). However, we acknowledge the necessity to evaluate RD6 plant types, yield performances, and agronomic traits within the field. The introgression lines developed within our study were evaluated for similarity with the original RD6 agronomic traits, namely plant height, panicle length, 4/panicle seed weight, 1000/seed weight, total dry weight, total seed weight, harvest index, seed length, seed width, and seed shape, as well as seed qualities, such as seed morphology (Table 2 and Table 4).
The Saltol QTL on chromosome 1 from the Pokkali rice variety has been commonly used for rice improvement in several studies [21,22,23,24,25,26]. In our results, the Saltol QTLs from the Pokkali variety produced the greatest salt tolerance within the RD6 introgression lines (Table 1, Table 2, Table 3, and Table 5). This saltol QTL also contributed to the maintenance of low Na+, high K+, and low Na+/K+ homeostasis levels in rice stems, further resulting in increased salt tolerance [24,44] (Figure 3). The Pokkali variety was classified to balance the influx of Na+ and K+ for dilution in the mechanism, creating the ability to exclude Na+ from leaf blades and stems [45,46]. As the water up-take mechanisms in rice accept both nutrients and salt together, the Pokkali variety thereby demonstrated the highest and most significant differences in leaf, stem, root, and total dry weights when compared with other breeding lines (Table 5). However, the BC4F4 lines presented the agronomic traits (above) more closely matched to the RD6 than to the Pokkali (Table 5), due to the advance generation and visual selection of the trait performances (Table 2 and Table 4). RD6 performance is very important for farmer acceptance and crop adaptation in our test areas. For example, excessively tall RD6 rice plants present problems in the grain filling stages as a result of heavy wind or rain [47]. Visual selection may explain the differences in percentages of Na+ of the RD6 introgression lines with those of Pokkali (Table 5, Figure 3).
As a photosensitive rice variety, the RD6 grows once a year, during Thailand’s rainy season from late May to November [48]. These bimodal rain patterns produce favorable conditions for the occurrence of blast disease, causing damage in all stages of growth. Leaf blast generally occurs during the seedling and tilling stages, whereas neck blast usually occurs during the reproductive phase [4]. In our study, introgression lines were evaluated for blast disease in both the field and upland short-row evaluations.
In this study, the upland short-row method displayed greater incidences of blast disease, due to the favorable microclimate and moisture contents around the experimental plots (Table 1 and Table 3) [49]. The experimental field was influenced by bimodal rain, capable of inducing leaf and neck blast symptoms (Table 3), further indicating the resistance of the QTLs [42,50]. Noenplab et al. [8] also reported that the blast QTL on chromosome 11 in the JHN variety successfully contributed to leaf and neck blast resistance. Pyramiding of four blast-resistant QTLs through MAS achieved high levels of blast resistance and broad-spectrum resistance to pathogens prevalent in the region [9]. Moreover, the testing of RD6 introgression lines for durable blast resistance and no-yield penalties were observed [42]. The results, herein, further demonstrated that neck blast disease caused direct yield loss during the grain filling phase [51], as well as lower 1000/SW within the original RD6 variety compared with those of the RD6 introgression lines (Table 4).
The resistance/tolerance abilities of the RD6 introgression lines represent the foreground genetics capable of enhancing plant breeding programs. However, maintaining the background of the original RD6 variety is also desirable; therefore, the quality and performance of the RD6 within the QTL introgression was also a consideration. The BC4F4 populations, herein, were achieved through the introgression of blast-resistant QTLs (qBL 1, 2, 11, and 12) from RGD07005-12-165-1 and Saltol QTL (Pokkali) and improved the RD6 rice variety through MAB. Consequently, the performance of the RD6 introgression lines was similar to that of the original RD6 variety (Table 4, Figure 2). The results indicate that foreground and background selection, together with visual selection, accurately depicts the efficiency of MAB.
5. Conclusions
Improvement of the RD6 rice variety for salt tolerance and blast resistance was successfully achieved utilizing the Saltol QTL and qBl (1, 2, 11, and 12) through marker-assisted backcrossing, together with phenotypic selection. The resulting BC4F4 132-12 introgression line exhibited superior salt tolerance, blast resistance, and reduced neck blast and was capable of maintaining higher qualities and agronomic performances than that of the original RD6 variety.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4395/10/8/1118/s1, Table S1: QTL traits and primer sequences of the SSR markers for blast resistance and salt tolerance, Table S2: Genotype of the BC2F2 populations derived from MAB.
Author Contributions
K.T. and S.K. conceived the study. T.M., S.C., and J.S. designed the experiments. K.T. and S.K. performed the experiments. J.S. and S.C. supervised the study. K.T., T.M., S.C., and J.S. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This research was supported by The Plant Breeding Research Center for Sustainable Agriculture and The Salt-Tolerance Rice Research Group, Khon Kaen University, Khon Kaen, Thailand. Our gratitude is also extended to the Sakon Nakhon Rice Research Center for their support in our field experiments and Ubon Ratchathani Rice Research Center for their support in 2AP analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Keeratipibul, S.; Luangsakul, N.; Lertsatchayarn, T. The effect of Thai glutinous rice cultivars, grain length and cultivating locations on the quality of rice cracker (arare). Food Sci. Technol. 2008, 41, 1934–1943. [Google Scholar] [CrossRef]
- Chairote, E.; Jannoey, P.; Chairote, G. Improvement of Monacolin K and minimizing of citrinin content in Korkor 6 (RD 6) red yeast rice. World Acad. Sci. Eng. Technol. 2015, 9, 43–46. [Google Scholar]
- Raj, S.V.; Saranya, R.S.; Kumar, D.S.; Chinnadurai, M. Farm-level economic impact of rice blast: A Bayesian approach. Agric. Econ. Res. Rev. 2018, 31, 141. [Google Scholar] [CrossRef]
- Mentlak, T.A.; Kombrink, A.; Shinya, T.; Ryder, L.S.; Otomo, I.; Saitoh, H.; Terauchi, R.; Nishizawa, Y.; Shibuya, N.; Thomma, B.P.H.J.; et al. Effector-mediated suppression of chitin-triggered immunity by magnaporthe oryzae is necessary for rice blast disease. Plant Cell 2012, 24, 322–335. [Google Scholar] [CrossRef]
- Chumpol, A.; Chankaew, S.; Saepaisan, S.; Monkham, T.; Sanitchon, J. New sources of rice blast resistance obtained from Thai indigenous upland rice germplasm. Euphytica 2018, 214, 183. [Google Scholar] [CrossRef]
- Greer, C.A.; Webster, R.K. Occurrence, distribution, epidemiology, cultivar reaction, and management of rice blast disease in California. Plant Dis. 2001, 85, 1096–1102. [Google Scholar] [CrossRef]
- Wang, D.; Guo, C.; Huang, J.; Yang, S.; Tian, D.; Zhang, X. Allele-mining of rice blast resistance genes at AC134922 locus. Biochem. Biophys. Res. Commun. 2014, 446, 1085–1090. [Google Scholar] [CrossRef]
- Noenplab, A.; Vanavichit, A.; Toojinda, T.; Sirithunya, P.; Tragoonrung, S.; Sriprakhon, S.; Vongsaprom, C. QTL mapping for leaf and neck blast resistance in Khao Dawk Mali105 and Jao Hom Nin recombinant inbred lines. Sci. Asia 2006, 32, 133–142. [Google Scholar] [CrossRef]
- Suwannual, T.; Chankaew, S.; Monkham, T.; Saksirirat, W.; Sanitchon, J. Pyramiding of four blast resistance QTLs into Thai rice cultivar RD6 through marker-assisted selection. Czech J. Genet. Plant Breed. 2017, 53, 1–8. [Google Scholar] [CrossRef]
- Arunin, S.; Pongwichian, P. Salt-affected soils and management in Thailand. Bull. Soc. Sea Water Sci. 2015, 69, 319–325. [Google Scholar]
- Wongsomsak, S. Salinization in Northeast Thailand. Southeast Asian Stud. 1986, 24, 133–153. [Google Scholar]
- Arunin, S. Salt effect soil in Southeast Asia. In Proceedings of the International Symposium on Salt Affected Lagoon Ecosystem, Valencia, Spain, 18–25 September 1995. [Google Scholar]
- Akbar, M. Breeding for salinity resistance in rice. In Prospects for Bio-Saline Research; Ahmed, R., Pietro, A.S., Eds.; Department of Botany, University of Karachi: Karachi, Sindh, Pakistan, 1986; pp. 37–55. [Google Scholar]
- Summart, J.; Thanonkeo, P.; Panichajakul, S.; Prathepha, P.; McManus, M.T. Effect of salt stress on growth, inorganic ion and proline accumulation in Thai aromatic rice, KhaoDawk Mali 105, callus culture. Afr. J. Biotechnol. 2010, 9, 145–152. [Google Scholar]
- Kumar, K.; Kumar, M.; Kim, S.-R.; Ryu, H.; Cho, Y.-G. Insights into genomics of salt stress response in rice. Rice 2013, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.I.; Yamaji, N.; Yamamoto, H.; Okubo, K.; Ueno, H.; Costa, A.; Tanoi, K.; Matsumura, H.; Fujii-Kashino, M.; Horiuchi, T.; et al. OsHKT1;5 mediates Na+ exclusion in the vasculature to protect leaf blades and reproductive tissues from salt toxicity in rice. Plant J. 2017, 91, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, G.B.; Senadhira, D.; Mendoza, R.D. Screening rice for salinity tolerance. IRRI Discuss. Pap. Ser. 1997, 22, 2–23. [Google Scholar]
- Bhowmik, S.K.; Islam, M.M.; Emon, R.M.; Begum, S.N.; Siddika, A.; Sultana, S. Identification of salt tolerant rice cultivars via phenotypic and marker-assisted procedures. Pak. J. Boil. Sci. 2007, 10, 4449–4454. [Google Scholar]
- Kavitha, P.G.; Miller, A.J.; Mathew, M.K.; Maathuis, F.J.M. Rice cultivars with differing salt tolerance contain similar cation channels in their root cells. J. Exp. Bot. 2012, 63, 3289–3296. [Google Scholar] [CrossRef]
- Ferreira, L.J.; Azevedo, V.S.; Marôco, J.P.; Oliveira, M.M.; Santos, A.P. Salt tolerant and sensitive rice varieties display differential methylome flexibility under salt stress. PLoS ONE 2015, 10, e0124060. [Google Scholar] [CrossRef]
- Mohammadi-Nejad, G.; Arzani, A.; Rezai, A.M.; Singh, R.K.; Gregorio, G.B. Assessment of rice genotypes for salt tolerance using microsatellite markers associated with the saltol QTL. Afr. J. Biotechnol. 2008, 7, 730–736. [Google Scholar]
- Huyen, L.T.N.; Cuc, L.M.; Ham, L.H.; Khanh, T.D. Introgression the Saltol QTL into Q5BD, the elite variety of Vietnam using marker assisted selection (MAS). Am. J. Biosci. 2013, 1, 80–84. [Google Scholar] [CrossRef]
- Ali, S.; Gautam, R.K.; Mahajan, R.; Krishnamurthy, S.L.; Sharma, S.K.; Singh, R.K. Stress indices and selectable traits in Saltol QTL introgressed rice genotypes for reproductive stage tolerance to sodicity and salinity stresses. Field Crops Res. 2013, 154, 65–73. [Google Scholar] [CrossRef]
- Waziri, A.; Kumar, P.; Purty, R.S. Saltol QTL and their role in salinity tolerance in rice. Austin. J. Biotechnol. Bioeng. 2016, 3, 1067. [Google Scholar]
- Ganie, S.A.; Borgohain, M.J.; Kritika, K.; Talukdar, A.; Pani, D.R.; Mondal, T.K. Assessment of genetic diversity of Saltol QTL among the rice (Oryza sativa L.) genotypes. Physiol. Mol. Boil. Plants 2016, 22, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Aala, W.F.; Gregorio, G.B. Morphological and molecular characterization of novel salt-tolerant rice germplasms from the philippines and bangladesh. Rice Sci. 2019, 26, 178–188. [Google Scholar] [CrossRef]
- Tanksley, S.; Nelson, J. Advanced backcross QTL analysis: A method for simultaneous discovery and transfer of valuable QTLs from unadapted germplasm into elite breeding lines. Theor. Appl. Genet. 1996, 92, 191–203. [Google Scholar] [CrossRef]
- Steele, K.; Price, A.H.; Shashidhar, H.E.; Witcombe, J.R. Marker-assisted selection to introgress rice QTLs controlling root traits into an Indian upland rice variety. Theor. Appl. Genet. 2005, 112, 208–221. [Google Scholar] [CrossRef]
- Neeraja, C.N.; Maghirang-Rodriguez, R.; Pamplona, A.; Heuer, S.; Collard, B.C.; Septiningsih, E.M.; Vergara, G.; Sanchez, D.; Xu, K.; Ismail, A.M.; et al. A marker-assisted backcross approach for developing submergence-tolerant rice cultivars. Theor. Appl. Genet. 2007, 115, 767–776. [Google Scholar] [CrossRef]
- Sundaram, R.M.; Vishnupriya, M.R.; Biradar, S.K.; Laha, G.S.; Reddy, G.A.; Rani, N.S.; Sarma, N.P.; Sonti, R.V. Marker assisted introgression of bacterial blight resistance in Samba Mahsuri, an elite indica rice variety. Euphytica 2007, 160, 411–422. [Google Scholar] [CrossRef]
- Iftekharuddaula, K.M.; Newaz, M.A.; Salam, M.A.; Ahmed, H.U.; Mahbub, M.A.A.; Septiningsih, E.M.; Collard, B.C.; Sanchez, D.L.; Pamplona, A.M.; Mackill, D. Rapid and high-precision marker assisted backcrossing to introgress the SUB1 QTL into BR11, the rainfed lowland rice mega variety of Bangladesh. Euphytica 2010, 178, 83–97. [Google Scholar] [CrossRef]
- Srichant, N.; Chankaew, S.; Monkham, T.; Thammabenjapone, P.; Sanitchon, J. Development of Sakon Nakhon rice variety for blast resistance through marker assisted backcross breeding. Agronomy 2019, 9, 67. [Google Scholar] [CrossRef]
- Kranto, S.; Chankaew, S.; Monkham, T.; Theerakulpisuta, P.; Sanitchon, J. Evaluation for salt tolerance in rice using multiple screening methods. J. Agric. Sci. Technol. 2016, 18, 1921–1931. [Google Scholar]
- Dellaporta, S.L.; Wood, J.; Hicks, J.B. A plant DNA minipreparation: Version II. Plant Mol. Boil. Rep. 1983, 1, 19–21. [Google Scholar] [CrossRef]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Gomez, K.A. Routine procedure for growing rice plants in cultures solution. Lab. Man. Physiol. Stud. Rice 1976, 17, 60–65. [Google Scholar]
- International Rice Research Institute. Standard Evaluation System Manual; International Rice Research Institute: Manila, Philippines, 1996. [Google Scholar]
- International Rice Research Institute. Standard Evaluation System for Rice (SES). Leaf blast; International Rice Research Institute: Manila, Philippines, 2002; p. 15. [Google Scholar]
- Sriseadka, T.; Wongpornchai, S.; Kitsawatpaiboon, P. Rapid method for quantitative analysis of the aroma impact compound, 2-Acetyl-1-pyrroline, in fragrant rice using automated headspace Gas chromatography. J. Agric. Food Chem. 2006, 54, 8183–8189. [Google Scholar] [CrossRef] [PubMed]
- Havre, G.N. The flame photometric determination of sodium, potassium and calcium in plant extracts with special reference to interference effects. Anal. Chim. Acta 1961, 25, 557. [Google Scholar] [CrossRef]
- Wongsaprom, C.; Sirithunya, P.; Vanavichit, A.; Pantuwan, G.; Jongdee, B.; Sidhiwong, N.; Lanceras-Siangliw, J.; Toojinda, T. Two introgressed quantitative trait loci confer a broad-spectrum resistance to blast disease in the genetic background of the cultivar RD6 a Thai glutinous jasmine rice. Field Crop. Res. 2010, 119, 245–251. [Google Scholar] [CrossRef]
- Pinta, W.; Toojinda, T.; Thummabenjapone, P.; Sanitchon, J. Pyramiding of blast and bacterial leaf blight resistance genes into rice cultivar RD6 using marker assisted selection. Afr. J. Biotechnol. 2013, 12, 4432–4438. [Google Scholar] [CrossRef]
- Nan, M.S.A.; Janto, J.; Sribunrueang, A.; Monkham, T.; Sanitchon, J.; Chankaew, S. Field evaluation of RD6 introgression lines for yield performance, blast, bacterial blight resistance, and cooking and eating Qualities. Agronomy 2019, 9, 825. [Google Scholar] [CrossRef]
- Suwanich, K. Geology and geological structure of potash and rock salt deposits in Chalerm Phrakiat District, Nakhon Ratchasima Province in Northeastern Thailand. Kasetsart J. (Nat. Sci.) 2010, 44, 1058–1068. [Google Scholar]
- Chunthaburee, S.; Dongsansuk, A.; Sanitchon, J.; Pattanagul, W.; Theerakulpisut, P. Physiological and biochemical parameters for evaluation and clustering of rice cultivars differing in salt tolerance at seedling stage. Saudi J. Boil. Sci. 2015, 23, 467–477. [Google Scholar] [CrossRef]
- Suriya-arunroj, D.; Supapoj, N.; Vanavichit, A.; Toojinda, T. Screening and selection for physiological characters contributing to salinity tolerance in rice. Kasetsart J. (Nat. Sci.) 2005, 39, 174–185. [Google Scholar]
- Kao, C.H. Mechanisms of salt tolerance in rice plants: Na+ transporters. Crop Environ. Bioinform. 2015, 12, 113–119. [Google Scholar]
- Miyakawa, S. Expansion of an improved variety into rain-fed rice cultivation in Northeast Thailand. Southeast Asian Stud. 1995, 33, 187–203. [Google Scholar]
- Jongdee, B.; Pantuwan, G.; Fukai, S.; Fischer, K. Improving drought tolerance in rainfed lowland rice: An example from Thailand. Agric. Water Manag. 2006, 80, 225–240. [Google Scholar] [CrossRef]
- Vasudevan, K.; Cruz, C.M.V.; Gruissem, W.; Bhullar, N.K. Large scale germplasm screening for identification of novel rice blast resistance sources. Front. Plant Sci. 2014, 5, 505. [Google Scholar] [CrossRef]
- Wang, R.; Fang, N.; Guan, C.; He, W.; Bao, Y.-M.; Zhang, H. Characterization and fine mapping of a blast resistant gene Pi-jnw1 from the japonica rice landrace Jiangnanwan. PLoS ONE 2016, 11, e0169417. [Google Scholar] [CrossRef]
- Goto, K. Estimating losses from rice blast in Japan. In The Rice Blast Disease; Johns Hopkins Press: Baltimore, MD, USA, 1965; pp. 195–202. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).