Abstract
Betaine is one of the most competitive compounds that accumulate in different cellular compartments to adjust osmotic balance. Among the various stressors, salinity stress often leads to osmotic and ionic stress in plants, either increasing or decreasing certain secondary plant metabolites. In this study, different concentrations of NaCl, betaine, and combined NaCl and betaine were used in time-course experiments to investigate growth pattern variation and accumulation of phenylpropanoid compounds in buckwheat sprouts. A significant increase in growth was observed with the application of 0.1–1.0 mM betaine. Although overall, the total phenylpropanoid compounds were lower compared to the control, the sole application of 50 mM NaCl and 1.0 mM betaine especially enhanced the accumulation of some of these compounds in comparison to others. Betaine application at lower concentrations was found to enhance the growth of buckwheat sprouts slightly. The results of this study show that phenylpropanoid content did not increase significantly in any of the treatments. However, it was proven that the phenylpropanoid biosynthetic pathway is stimulated under abiotic stress, resulting in a higher accumulation of various phenylpropanoid compounds. This suggests that the level of accumulation of phenylpropanoid compounds due to abiotic stress may be species-dependent.
1. Introduction
Salinity in soil hinders plant growth and development and decreases crop yields worldwide. When plants are damaged by high salinity, the excessive uptake of sodium ions (Na+) and chloride ions (Cl−) causes water stress, nutritional imbalance, and cytotoxicity to occur. Research into salinity stress commonly focuses on oxidative stress from the generation of reactive oxygen species [1,2,3]. Plant salinity responses have two main steps. Initially, a quick (minutes to days) decrease in growth takes place, driving repression of cell expansion and stomatal closure [4,5,6]. The following step occurs over a period of days or weeks and comprises the accumulation of cytotoxic ions, delay of metabolic reactions, and enhancement of early senescence processes, ultimately leading to cell death [7,8].
Salinity stress causes osmotic and ionic stress, as well as decreasing or increasing certain secondary plant metabolites. For example, alfalfa, a halophyte, accumulates proline quickly in its roots, but the accumulation of proline in glycophytes is slow [9]. Additionally, research has shown that endogenous jasmonic acid increases under salinity stress in tomato plants (Solanum lycopersicum) [10]. In contrast to most grains, buckwheat (Fagopyrum esculentum), a member of the Polygonaceae family, is commonly utilized in alternative crop systems. Buckwheat seeds can be saved without clear chemical variation. Additionally, buckwheat seeds contain more rutin than most plants. Rutin, a flavonoid and secondary metabolite of plants, has important biological properties, including antioxidant, anti-inflammatory, and anticarcinogenic properties, and it can diminish the blood vessel weakness associated with hemorrhagic and hypertension illnesses in humans [11]. Two types of buckwheat are cultivated globally: common buckwheat (F. esculentum) and Tartary buckwheat (F. tataricum Gaertn.). The main cultivators of common buckwheat are the USA, Canada, Europe, Australia, South Africa, and Brazil. Japan, Korea, and the central and northern parts of China are considered to cultivate the most buckwheat in Asia. Tartary buckwheat, also known as bitter buckwheat, contains more flavonoids than common buckwheat and contains the most rutin.
Phenylpropanoids originate from the six-carbon phenyl group and are the largest group of organic compounds that are biosynthesized from phenylalanine and tyrosine [12]. Phenylpropanoids are found throughout the plant kingdom and mediate plant–pollinator interactions, including those relating to floral pigments and fragrance compounds. In addition, they protect against herbivores, pathogens, and ultraviolet light [13,14]. Flavonoids, which are a large subgroup of phenylpropanoids, primarily contain compounds that are based on sources such as flavonols, anthocyanins, and proanthocyanins [15,16].
Glycinebetaine (GB) is an N-methyl-substituted derivative of glycine, which is a type of quaternary ammonium alkaloid. It is also a functional compound that regulates stress responses, protects proteins and enzymes, and maintains cell osmotic pressure. It is usually found in a variety of microorganisms, animals, and higher plants [17,18]. The formation of GB is a two-step process. First, choline is synthesized by choline monooxygenase (CMO), leaving the hydrate form of betaine aldehyde. Then, GB is synthesized from betaine aldehyde by betaine aldehyde dehydrogenase (BADH) [17]. There is a positive relationship between GB accumulation and improved stress tolerance, especially to salt and drought, in crop plants such as spinach, sorghum, and barley [19]. Nevertheless, the effect of betaine hydrochloride treatment on phenylpropanoid biosynthesis and salt stress responses has not been well studied, especially for buckwheat. Therefore, in the present study, we evaluate the effects of betaine hydrochloride treatment on Tartary buckwheat sprout growth and phenylpropanoid accumulation under NaCl stress.
2. Materials and Methods
2.1. Plant Materials
Tartary buckwheat seeds were purchased from Asia Seed Co. Ltd. (Seoul, Korea). One hundred seeds were sown in an 11 × 11 cm plastic pot and kept in a plant growth chamber at 25 °C under a 16 h light/8 h dark photocycle. To determine growth characteristics and optimal concentrations in buckwheat sprouts under different NaCl concentrations, treatments of 30, 50, 70, and 100 mM NaCl were applied for six days. In addition, to determine the growth characteristics of buckwheat sprouts under different glycine betaine concentrations, treatments of 0.1, 0.5, 1, 5, 10, 20, and 30 mM betaine hydrochloride were applied for six days. To determine the effect of betaine hydrochloride under NaCl stress, six-day-old Tartary buckwheat sprouts were treated with 50 mM NaCl as the control and treated with 50 mM NaCl in addition to 0.1, 0.5, 1, 5, 10, 20, or 30 mM betaine hydrochloride in the experimental groups. After determining the optimal concentration of treatment, a time-course experiment was performed. Six-day-old sprouts were treated with 0 mM NaCl as the control and were treated with 50 mM NaCl, 1 mM betaine, or 50 mM NaCl with 1 mM betaine in the experimental groups. Sprouts were harvested after four, five, seven, and nine days.
2.2. Phenylpropanoid Extraction
To extract phenylpropanoids, samples were frozen using liquid nitrogen and lyophilized at −70 °C for 72 h immediately after harvest. Lyophilized samples were ground to a fine powder using a pestle. Powder samples were weighed to 100 mg and added to 3 mL of 80% MeOH. The mixture was vortexed vigorously for 3 min and sonicated for 1 h at 36 °C. The sonicated samples were centrifuged at 12,000 rpm at 4 °C for 10 min to obtain the supernatant. The supernatant samples were transferred to a new tube and filtered using 0.45 µm Whatman No. 42 filter paper and injected into a vial for high-performance liquid chromatography (HPLC) analysis.
2.3. HPLC Analysis of Phenylpropanoid Content
Phenylpropanoids were quantified using a Futecs model NS-4000 apparatus (Futecs Co. Ltd., Daejeon, Korea) with a 250 × 4.6 mm, 5-µm C18 column (RStech Co. Ltd., Daejeon, Korea). For HPLC analysis, the column temperature was maintained at 30 °C and detection was performed under a 280 UV wavelength. The flow rate was 1.0 mL/min, and the injection volume was 10 µL. The mobile phase was a mixture of (A) water to acetic acid (99.85:0.15 v/v) and (B) 100% MeOH. The initial mobile phase composition was as follows: 5% solvent B, followed by a linear gradient from 5–80% solvent B over 93 min, then holding at 5% solvent B for an additional 5 min. The phenylpropanoid content was calculated based on the calibration curve and the peak area of the standard compounds for each sample.
2.4. Statistical Analysis
All the growth parameter values are stated as the mean ± SD of five samples. The values of phenylpropanoid content in Tartary buckwheat sprouts are expressed as the mean ± SD of three samples. Both growth and phenylpropanoid data were analyzed statistically using Statistical Analysis System software (SAS, system 9.4, 2013; SAS Institute Inc., Cary, NC, USA). Statistical significance was evaluated using Duncan’s multiple range test (DMRT) with a significance level of p ≤ 0.05. All data are represented as the mean ± standard deviation of triplicate tests.
3. Results
3.1. Effect of NaCl Treatment on the Growth of Tartary Buckwheat Sprouts
In this study, we investigate the effect of different concentrations of NaCl (0, 30, 50, 70, and 100 mM) on growth patterns of six-day-old Tartary buckwheat sprouts, using fresh weight, shoot length, and root length indices. We found that growth was significantly influenced by NaCl concentration (Table 1). As NaCl concentration increased, all growth parameters were gradually reduced. Fresh weight of sprouts treated with 30, 50, 70, and 100 mM NaCl was 21.79, 33.66, 67.0, and 77.36% lower than that of the control, respectively. Shoot lengths were 34.44, 49.46, 87.87, and 89.85% lower than that of the control when treated with 30, 50, 70, and 100 mM NaCl, respectively. Similarly, root lengths were 12.86, 39.08, 46.07, and 69.29% lower than the control, following treatment with 30, 50, 70, and 100 mM NaCl, respectively.

Table 1.
Effect of NaCl treatment on the growth of Tartary buckwheat sprouts.
Different concentrations of betaine (0.1, 0.5, 1, 5, 10, 20, and 30 mM) were also used to examine the growth parameters (shoot fresh weight and shoot and root length) of Tartary buckwheat sprouts. A control group with no betaine addition was used for comparison. We found that the growth of Tartary buckwheat sprouts was markedly enhanced with betaine addition. A significant increase in growth was observed in the 0.1–1 mM betaine range, with growth declining as betaine increased between 5–30 mM (Table 2). It is notable that the fresh weight of Tartary buckwheat sprouts were higher in all concentrations of betaine (0.1–30 mM) compared to the control. The fresh weight of sprouts was 40.34, 50.92, and 52.61% higher than that of the control when treated with 0.1, 0.5, and 1.0 mM betaine, respectively.

Table 2.
Effect of betaine hydrochloride treatment on the growth of Tartary buckwheat sprouts.
The growth trend of shoot length was similar to that previously described: a significant increasing trend for shoot length was observed from 0.1–1 mM betaine, which then started to decline from 5–30 mM (Table 2). It was observed that the shoot length was higher than that of the control up to a concentration of 10 mM, after which shoot length was lower. Shoot lengths were 19.54, 25.29, and 32.18% higher than those of the control group when treated with 0.1, 0.5, and 1.0 mM betaine, respectively.
Increasing the concentration of betaine reduced the root length of Tartary buckwheat sprouts significantly. At lower concentrations of betaine (up to 1 mM), the trend of root length decrease was slow. At higher concentrations, the decreasing trend was much more amplified. The root length of sprouts was 6% lower than the control up to a concentration of 1 mM betaine. Root length then decreased very sharply, being 54.55% lower than the control with the highest concentration (30 mM) of betaine.
3.2. Effect of 50 mM NaCl Combined with Different Concentrations of Betaine Hydrochloride on the Growth of Tartary Buckwheat Sprouts
Whether 50 mM NaCl combined with different concentrations of betaine hydrochloride had an influence on the growth parameters of six-day-old Tartary buckwheat sprouts were investigated (Table 3). Results indicated that none of the tested combinations led to a significant increase in growth. A slight increase in shoot fresh weight of 2.23% and 0.15% was observed with the treatment of 50 mM NaCl and 0.5 and 1 mM betaine, respectively. Furthermore, fresh weight was reduced by 18.45, 26.79, 27.23, and 66.82% compared to the control when treated with 50 mM NaCl and 5, 10, 20, and 30 mM betaine, respectively.

Table 3.
Effect of NaCl 50 mM with different concentrations of betaine hydrochloride treatment on the growth of Tartary buckwheat sprouts.
Shoot and root lengths were also suppressed by the application of NaCl and betaine hydrochloride. As betaine increased (in application with 50 mM NaCl), shoot and root lengths decreased linearly. Shoot lengths were 28.40, 31.84, 37.35, and 73.15% lower than the control when treated with 50 mM NaCl and 5, 10, 20, and 30 mM betaine, respectively. The trend for root length was found to be similar: a root length reduction of 28.61, 31.65, 41.27, and 65.06% was noted compared to the control when treated with 50 mM NaCl and 5, 10, 20, and 30 mM betaine, respectively.
3.3. Growth of Tartary Buckwheat Sprouts under a Time-Course Experiment
Tartary buckwheat seedlings were harvested four, five, seven, and nine days after treatment with 50 mM NaCl, 1 mM betaine hydrochloride, and 50 mM NaCl + 1 mM betaine, at which point the fresh weight, shoot length, and root length were measured (Figure 1). Tartary buckwheat sprouts showed a significant difference per treatment (Figure 1A). At each sampling time, these growth parameters changed significantly. Treatment with 1 mM betaine improved fresh weight at all sampling dates except at four days post-treatment. In this case, a significant decrease in fresh weight was observed compared to the control (Figure 1B). The fresh weight at four days post-treatment was 1.28, 14.10, and 28.21% lower than the control when treated with betaine, 50 mM NaCl + 1 mM betaine, and 50 mM NaCl, respectively. For the second sampling (five days after treatment), the fresh weight under 1 mM betaine and 50 mM NaCl + 1 mM betaine treatments was 14.29% and 11.90% higher, respectively, compared to the control (Figure 1B). However, when treated with 50 mM NaCl, the fresh weight of seedlings five days after treatment was reduced by 27.38% compared to that of the control. At seven and nine days post-treatment, a significant increase in fresh weight of 34.68 and 44.53%, respectively, was observed with 1 mM betaine treatment compared to the control. In contrast, on these sampling days, fresh weight was lower than that of the control with 50 mM NaCl + 1 mM betaine and 50 mM NaCl treatments.
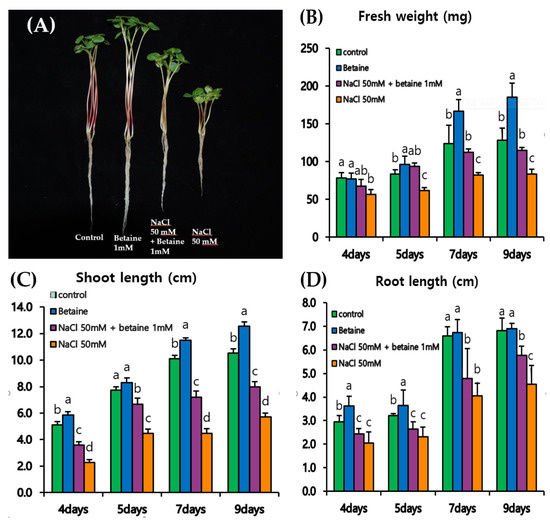
Figure 1.
Time course of Tartary buckwheat sprout development after treatment of 50 mM NaCl and 1 mM betaine hydrochloride. Different letters indicate a significant difference (p < 0.05) between areas for that parameter using DMRT (Duncan’s multiple range test; n ≥ 5, mean ± SD). (A) Picture of Tartary buckwheat sprouts at 9 days of betaine hydrochloride and NaCl treatment; (B) graph of fresh weight in treated Tartary buckwheat sprouts; (C) graph of shoot weight in treated Tartary buckwheat sprouts; (D) graph of root length in treated Tartary buckwheat sprouts.
Treatment with 1 mM betaine significantly increased both the shoot and root lengths of buckwheat seedlings at all sampling dates (Figure 1C,D). Shoot length of seedlings treated with 1 mM betaine was 15.69, 7.79, 13.86, and 19.05% higher than that of the control at four, five, seven, and nine days after treatment, respectively. The trend observed for root length was similar to that for shoot length at all sampling dates. A root length increase of 20.0, 12.50, 1.52, and 1.47% compared to the control was observed after treatment with 1 mM betaine at four, five, seven, and nine days after treatment, respectively.
3.4. Effect of 50 mM NaCl, 1 mM Betaine, and Their Combination on Phenylpropanoid Content (mg/g Dry wt.) of Buckwheat Sprouts at Different Harvest Times
HPLC analysis was performed to explore whether the level of phenylpropanoid compounds in buckwheat sprouts was affected by harvest time (four, five, seven, and nine days post-treatment) or treatment type (50 mM NaCl, 1 mM betaine, 50 mM NaCl + 1 mM betaine, and a control). The phenylpropanoid compounds gallic acid, chlorogenic acid, epicatechin, p-coumaric acid, ferulic acid, benzoic acid, rutin, trans-cinnamic acid, quercetin, and kaempferol were detected in different amounts at different sampling times (Table 4).

Table 4.
Phenylpropanoid compound contents (mg/g dry wt.) in Tartary buckwheat sprouts.
At the first sampling time (four days post-treatment), 1 mM betaine treatment led to a slightly higher (1.24%) total content of phenylpropanoid compounds compared to the control. Total phenylpropanoid compound content was 33.77, 34.19, 30.22, and 33.66 (mg/g dry wt.) under control, 1 mM betaine, 50 mM NaCl, and 50 mM NaCl + 1 mM betaine treatments, respectively. Treatment with 1 mM betaine led to a higher accumulation of epicatechin, p-coumaric acid, rutin, transcinnamic acid, and kaempferol (26.64, 12, 0.3, 50, and 4.44% higher than the control, respectively). Treatment with 50 mM NaCl also increased transcinnamic acid, quercetin, and kaempferol (50, 53.49, and 6.67% higher than the control, respectively). Moreover, 50 mM NaCl + 1 mM betaine treatment increased the accumulation of rutin, quercetin, and kaempferol (4.95, 20.93, and 28.89% higher than the control, respectively).
At the second sampling time (five days post-treatment), the total phenylpropanoid compound content was higher in the control than in all other treatments. The total phenylpropanoid compound content was 47.98, 47.90, 39.52, and 39.53 (mg/g dry wt.) under the control, 1 mM betaine, 50 mM NaCl, and 50 mM NaCl + 1 mM betaine treatments, respectively. Although the total content was highest in the control group, some individual treatments led to an increase in the amounts of individual phenylpropanoid compounds. Treatment with 1 mM betaine led to a higher accumulation of p-coumaric acid, rutin, and quercetin (7.14, 0.18, and 27.66% higher than the control, respectively). Application of 50 mM NaCl produced higher levels of p-coumaric acid, ferulic acid, and quercetin (42.86, 50, and 65.96% higher than the control, respectively). Additionally, 50 mM NaCl + 1 mM betaine treatment enhanced the accumulation of p-coumaric acid, ferulic acid, and quercetin (28.57, 40, and 38.30% higher than the control, respectively).
At seven days post-treatment, the total phenylpropanoid compound content was higher in the control than in all other treatments. The total phenylpropanoid compound content was 49.44, 48.09, 37.27, and 39.69 (mg/g dry wt.) under the control, 1 mM betaine, 50 mM NaCl, and 50 mM NaCl + 1 mM betaine treatments, respectively. Although the total content was highest in the control group, some individual treatments led to an increase in the amounts of individual phenylpropanoid compounds. Treatment with 1 mM betaine led to a higher accumulation of rutin, quercetin, and kaempferol (0.05, 1.77, and 7.25% higher than the control, respectively). Application of 50 mM NaCl produced a higher amount of gallic acid, p-coumaric acid, and ferulic acid (33.33, 54.55, and 40% higher than the control, respectively). The combined application of 50 mM NaCl + 1 mM betaine enhanced the accumulation of p-coumaric acid, ferulic acid, and kaempferol (18.18, 50, and 1.45% higher than the control, respectively).
Nine days after treatment, the total phenylpropanoid compound content was much higher in the control than in all other treatments. The total accumulation of phenylpropanoid compounds was 43.77, 39.16, 30.74, and 33.63 (mg/g dry wt.) under the control, 1 mM betaine, 50 mM NaCl, and 50 mM NaCl + 1 mM betaine treatments, respectively. Although the total content was highest in the control group, some individual treatments led to an increase in the amounts of individual phenylpropanoid compounds. Treatment with 1 mM betaine led to a higher accumulation of p-coumaric acid and kaempferol (8.33 and 44.93% higher than the control, respectively), as did the 50 mM NaCl treatment (41.67 and 34.78% higher than the control, respectively). The combined application of 50 mM NaCl + 1 mM betaine did not enhance the accumulation of any individual phenylpropanoid compounds.
4. Discussion
In this study, we investigated the effect of different concentrations of NaCl (0, 30, 50, 70, and 100 mM) on growth patterns of six-day-old Tartary buckwheat sprouts, using fresh weight, shoot length, and root length indices. It is reported that salinity causes a significant reduction in growth in a number of plant species, including wheat [20], Sesamum indicum [21], Vigna radiata [22], Cassia angustifolia [23], Pak-choi [24], and Pisum sativum [25]. In addition, salinity results in a decrease in vertical shoot growth rate, shoot and root fresh weight, chlorophyll (Chl) content, superoxide dismutase (SOD) activity, catalase (CAT) activity, and ascorbate peroxidase (APX) activity in perennial ryegrass [26]. The findings of our study are consistent with the existing scientific literature on this subject. High salinity causes water imbalance, which may decrease osmotic adjustment and reduce plant growth [27].
It has previously been reported that there is a direct relationship between salinity and exogenous application of GB, with a significant influence on plant growth and accumulation of secondary metabolites. For example, both the shoot fresh weight of Pokkali rice and shoot and root dry weight of IR-28 rice were lower under salinity stress but increased with exogenous GB application [28]. Accumulation of GB is associated with improved stress tolerance in crop plants, including spinach, sorghum, and barley under salt and drought stress, as reported by Ashraf and Foolad [19]. Many other studies have reported on GB effects on salt stress responses in different plants, including rice [29], turnip [30], tobacco [31], maize [32,33], wheat [34], ryegrass [26], mung bean [35], and tomato [36]. The findings of our study are consistent with these studies where the exogenous application of betaine and GB enhanced and, in some cases, reduced the accumulation of secondary metabolites.
The growth rate of buckwheat sprouts did not respond positively with increased treatment time in this study. These findings are consistent with previous studies demonstrating that, under NaCl treatment, growth was reduced in seedlings of Triticum aestivum [37], Brassica juncea [38], and Schizonepeta tenuifolia [39].
Due to osmotic and ionic stress, abiotic stressors cause either an increase or decrease in certain secondary plant metabolites. Red peppers (Capsicum annuum L.) under moderate salinity exhibited an increase in total phenolic compound content, whereas green and turning peppers showed a decrease in these compounds [40]. The findings of our study are also consistent with the existing scientific literature, which shows the exogenous application of NaCl and betaine can enhance and reduce the accumulation of secondary metabolites.
Cuong et al. [20] found that the accumulation of phenylpropanoids was the highest in wheat sprouts treated with 50 mM NaCl. Additionally, the application of GB reduces proline content in salt-stressed perennial ryegrass [26]. Furthermore, the protein content of IR-28 rice decreased under saline conditions, whereas it increased with GB application [28]. Our results are consistent with these and other studies, including those relating to NaCl treatment of Solanum nigrum [41], maize [42], and red pepper [40], which show slightly higher phenylpropanoid contents under salt treatments than the control. Various studies have shown that both salinity stress [41,43,44] and light stress [45] increase total plant phenylpropanoid content. Other studies have also revealed that some phenylpropanoid compounds fail to accumulate, irrespective of salt treatment, in lettuce [46] and broccoli [47]. This suggests that variation in the content of phenylpropanoid compounds due to salt stress may be species-dependent.
5. Conclusions
The growth of six-day-old Tartary buckwheat sprouts was inhibited by NaCl treatment. A significant increase in growth was observed with the application of a lower concentration of betaine, with the highest growth increase observed with 1 mM betaine treatment. The application of 50 mM NaCl with different betaine concentrations did not lead to any positive growth responses. No single treatment enhanced the accumulation of phenylpropanoid compounds, except for 1 mM betaine. Although total phenylpropanoid compound content was highest in the control group, some individual treatments, especially 50 mM NaCl, led to an increase in the accumulation of individual phenylpropanoid compounds. The growth indices of buckwheat sprouts decreased under different stress-related treatments, except with lower concentrations of betaine. In addition, 50 mM NaCl enhanced the accumulation of some phenylpropanoid compounds. Previous studies confirm that phenylpropanoids are influenced by abiotic stress. Our findings provide additional information regarding the accumulation of secondary metabolites in the sprouts of buckwheat under different abiotic stressors and, therefore, may be helpful for studying secondary metabolites in other plant species.
Author Contributions
Y.S.C. and S.U.P. designed the experiments and analyzed the data. M.C.K., N.S.K. and Y.B.K. performed the experiments and analyzed the data. N.S.K., C.M.K. and Y.S.C. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2019R1F1A1061916), and (NRF-2019K2A9A2A06024347). We thank the Sustainable Agriculture Research Institute (SARI) at Jeju National University for providing the experimental facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tsugane, K.; Kobayashi, K.; Niwa, Y.; Ohba, Y.; Wada, K.; Kobayashi, H. A recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification. Plant Cell 1999, 11, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.A.; Ferrer, M.A.; Jiménez, A.; Barceló, A.R.; Sevilla, F. Antioxidant systems and O2.−/H2O2 production in the apoplast of pea leaves. Its relation with salt-induced necrotic lesions in minor veins. Plant Physiol. 2001, 127, 817–831. [Google Scholar] [CrossRef]
- Isayenkov, S. Physiological and molecular aspects of salt stress in plants. Cytol. Genet. 2012, 46, 302–318. [Google Scholar] [CrossRef]
- Munns, R.; Passioura, J. Hydraulic resistance of plants. III. Effects of NaCl in barley and lupin. Funct. Plant Biol. 1984, 11, 351–359. [Google Scholar] [CrossRef]
- Munns, R.; Termaat, A. Whole-plant responses to salinity. Funct. Plant Biol. 1986, 13, 143–160. [Google Scholar] [CrossRef]
- Rajendran, K.; Tester, M.; Roy, S.J. Quantifying the three main components of salinity tolerance in cereals. Plant Cell Environ. 2009, 32, 237–249. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Roy, S.J.; Negrão, S.; Tester, M. Salt resistant crop plants. Curr. Opin. Biotechnol. 2014, 26, 115–124. [Google Scholar] [CrossRef]
- Petrusa, L.M.; Winicov, I. Proline status in salt-tolerant and salt-sensitive alfalfa cell lines and plants in response to NaCl. Plant Physiol. Biochem. 1997, 35, 303–310. [Google Scholar]
- Pedranzani, H.; Racagni, G.; Alemano, S.; Miersch, O.; Ramírez, I.; Peña-Cortés, H.; Taleisnik, E.; Machado-Domenech, E.; Abdala, G. Salt tolerant tomato plants show increased levels of jasmonic acid. Plant Growth Regul. 2003, 41, 149–158. [Google Scholar] [CrossRef]
- Oomah, B.D.; Mazza, G. Flavonoids and antioxidative activities in buckwheat. J. Agric. Food Chem. 1996, 44, 1746–1750. [Google Scholar] [CrossRef]
- Barros, J.; Serrani-Yarce, J.C.; Chen, F.; Baxter, D.; Venables, B.J.; Dixon, R.A. Role of bifunctional ammonia-lyase in grass cell wall biosynthesis. Nature Plants 2016, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tossi, V.; Amenta, M.; Lamattina, L.; Cassia, R. Retracted: Nitric oxide enhances plant ultraviolet-B protection up-regulating gene expression of the phenylpropanoid biosynthetic pathway. Plant Cell Environ. 2011, 34, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sarma, B.K.; Upadhyay, R.S.; Singh, H.B. Compatible rhizosphere microbes mediated alleviation of biotic stress in chickpea through enhanced antioxidant and phenylpropanoid activities. Microbiol. Res. 2013, 168, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Routaboul, J.-M.; Dubos, C.; Beck, G.; Marquis, C.; Bidzinski, P.; Loudet, O.; Lepiniec, L. Metabolite profiling and quantitative genetics of natural variation for flavonoids in Arabidopsis. J. Exp. Bot. 2012, 63, 3749–3764. [Google Scholar] [CrossRef]
- Saito, K.; Yonekura-Sakakibara, K.; Nakabayashi, R.; Higashi, Y.; Yamazaki, M.; Tohge, T.; Fernie, A.R. The flavonoid biosynthetic pathway in Arabidopsis: Structural and genetic diversity. Plant Physiol. Biochem. 2013, 72, 21–34. [Google Scholar] [CrossRef]
- Rhodes, D.; Hanson, A. Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu. Rev. Plant Biol. 1993, 44, 357–384. [Google Scholar] [CrossRef]
- Mansour, M.M.F. Protection of plasma membrane of onion epidermal cells by glycinebetaine and proline against NaCl stress. Plant Physiol. Biochem. 1998, 36, 767–772. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.; Ashraf, M.; Foolad, M. Improving plant abiotic-stress resistance by exogenous application of osmoprotectants glycine, betaine and proline. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Cuong, D.M.; Kwon, S.-J.; Nguyen, B.V.; Chun, S.W.; Kim, J.K.; Park, S.U. Effect of Salinity Stress on Phenylpropanoid Genes Expression and Related Gene Expression in Wheat Sprout. Agronomy 2020, 10, 390. [Google Scholar] [CrossRef]
- Koca, H.; Bor, M.; Özdemir, F.; Türkan, İ. The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ. Exp. Bot. 2007, 60, 344–351. [Google Scholar] [CrossRef]
- Saha, P.; Chatterjee, P.; Biswas, A.K. NaCl pretreatment alleviates salt stress by enhancement of antioxidant defense system and osmolyte accumulation in mungbean (Vigna radiata L. Wilczek). Indian J. Exp. Biol. 2010, 48, 593–600. [Google Scholar] [PubMed]
- Agarwal, S.; Pandey, V. Antioxidant enzyme responses to NaCl stress in Cassia angustifolia. Biol. Plant. 2004, 48, 555–560. [Google Scholar] [CrossRef]
- Jamil, M.; Bae, D.; Yong, K.; Ashraf, M.; Chun, L.; Shik, R. Effect of salt (NaCl) stress on germination and early seedling growth of four vegetables species. J. Cent. Eur. Agric. 2006, 7, 273–282. [Google Scholar]
- Noreen, Z.; Ashraf, M. Assessment of variation in antioxidative defense system in salt-treated pea (Pisum sativum) cultivars and its putative use as salinity tolerance markers. J. Plant Physiol. 2009, 166, 1764–1774. [Google Scholar] [CrossRef]
- Hu, L.; Hu, T.; Zhang, X.; Pang, H.; Fu, J. Exogenous glycine betaine ameliorates the adverse effect of salt stress on perennial ryegrass. J. Am. Soc. Hortic. Sci. 2012, 137, 38–46. [Google Scholar] [CrossRef]
- Bohnert, H.J.; Jensen, R.G. Strategies for engineering water-stress tolerance in plants. Trends Biotechnol. 1996, 14, 89–97. [Google Scholar] [CrossRef]
- Demiral, T.; Türkan, I. Exogenous glycinebetaine affects growth and proline accumulation and retards senescence in two rice cultivars under NaCl stress. Environ. Exp. Bot. 2006, 56, 72–79. [Google Scholar] [CrossRef]
- Harinasut, P.; Tsutsui, K.; Takabe, T.; Nomura, M.; Takabe, T.; Kishitani, S. Exogenous glycinebetaine accumulation and increased salt-tolerance in rice seedlings. Biosci. Biotechnol. Biochem. 1996, 60, 366–368. [Google Scholar] [CrossRef]
- Mäkelä, P.; Peltonen-Sainio, P.; Jokinen, K.; Pehu, E.; Setälä, H.; Hinkkanen, R.; Somersalo, S. Uptake and translocation of foliar-applied glycinebetaine in crop plants. Plant Sci. 1996, 121, 221–230. [Google Scholar] [CrossRef]
- Banu, M.N.A.; Hoque, M.A.; Watanabe-Sugimoto, M.; Matsuoka, K.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Proline and glycinebetaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. J. Plant Physiol. 2009, 166, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, C. Photosynthesis is improved by exogenous glycinebetaine in salt-stressed maize plants. Physiol. Plant. 2005, 124, 343–352. [Google Scholar] [CrossRef]
- Nawaz, K.; Ashraf, M. Exogenous application of glycinebetaine modulates activities of antioxidants in maize plants subjected to salt stress. J. Agron. Crop Sci. 2010, 196, 28–37. [Google Scholar] [CrossRef]
- Raza, S.H.; Athar, H.R.; Ashraf, M.; Hameed, A. Glycinebetaine-induced modulation of antioxidant enzymes activities and ion accumulation in two wheat cultivars differing in salt tolerance. Environ. Exp. Bot. 2007, 60, 368–376. [Google Scholar] [CrossRef]
- Hossain, M.A.; Fujita, M. Evidence for a role of exogenous glycinebetaine and proline in antioxidant defense and methylglyoxal detoxification systems in mung bean seedlings under salt stress. Physiol. Mol. Biol. Plants 2010, 16, 19–29. [Google Scholar] [CrossRef]
- Heuer, B. Influence of exogenous application of proline and glycinebetaine on growth of salt-stressed tomato plants. Plant Sci. 2003, 165, 693–699. [Google Scholar] [CrossRef]
- Grieve, C.; Francois, L.; Poss, J. Effect of salt stress during early seedling growth on phenology and yield of spring wheat. Cereal Res. Commun. 2001, 29, 167–174. [Google Scholar] [CrossRef]
- Pandey, M.; Penna, S. Time course of physiological, biochemical, and gene expression changes under short-term salt stress in Brassica juncea L. Crop J. 2017, 5, 219–230. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, N.; Huang, L.; Zhao, Y.; Tang, X.; Wang, K. Effects of salt stress on plant growth, antioxidant capacity, glandular trichome density, and volatile exudates of Schizonepeta tenuifolia Briq. Int. J. Mol. Sci. 2018, 19, 252. [Google Scholar] [CrossRef]
- Navarro, J.M.; Flores, P.; Garrido, C.; Martinez, V. Changes in the contents of antioxidant compounds inpepper fruits at different ripening stages, as affected by salinity. Food Chem. 2006, 96, 66–73. [Google Scholar] [CrossRef]
- Ben Abdallah, S.; Aung, B.; Amyot, L.; Lalin, I.; Lachaal, M.; Karray-Bouraoui, N.; annoufa, A. Salt stress (NaCl) a_ects plant growth and branch pathways of carotenoid and flavonoid biosyntheses in Solanum nigrum. Acta Physiol. Plant. 2016, 38, 72. [Google Scholar] [CrossRef]
- Hichem, H.; Mounir, D.; Naceur, E. Di_erential responses of two maize (Zea mays L.) varieties to salt stress: Changes on polyphenols composition of foliage and oxidative damages. Ind. Crop. Prod. 2009, 30, 144–151. [Google Scholar] [CrossRef]
- Kim, N.S.; Kwon, S.J.; Cuong, D.M.; Jeon, J.; Park, J.S.; Park, S.U. Accumulation of phenylpropanoids in tartary buckwheat (Fagopyrum tataricum) under salt stress. Agronomy 2019, 9, 739. [Google Scholar] [CrossRef]
- Yun, Y.; Jung, H.J.; Rahim, M.A.; Park, N.K.; Kuk, Y. Molecular analysis of genes related to phenylpropanoid and ascorbate biosynthesis in salt and UV-B treated pak choi grown under LEDs. Botany 2019, 97, 513–519. [Google Scholar] [CrossRef]
- Thwe, A.A.; Kim, J.K.; Li, X.; Kim, Y.B.; Uddin, M.R.; Kim, S.J.; Suzuki, T.; Park, N.I.; Park, S.U. Metabolomic analysis and phenylpropanoid biosynthesis in hairy root culture of tartary buckwheat cultivars. PLoS ONE 2013, 8, 6. [Google Scholar] [CrossRef]
- Kim, H.J.; Fonseca, J.M.; Choi, J.H.; Kubota, C.; Kwon, D.Y. Salt in irrigation water a_ects the nutritional and visual properties of romaine lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2008, 56, 3772–3776. [Google Scholar] [CrossRef]
- Lopez-Berenguer, C.; Martinez-Ballesta, M.D.; Moreno, D.A.; Carvajal, M.; Garcia-Viguera, C. Growing hardier crops for better health: Salinity tolerance and the nutritional value of Broccoli. J. Agric. Food Chem. 2009, 57, 572–578. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).