NaCl and Na2SO4 Salinities Have Different Impact on Photosynthesis and Yield-Related Parameters in Rice (Oryza sativa L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
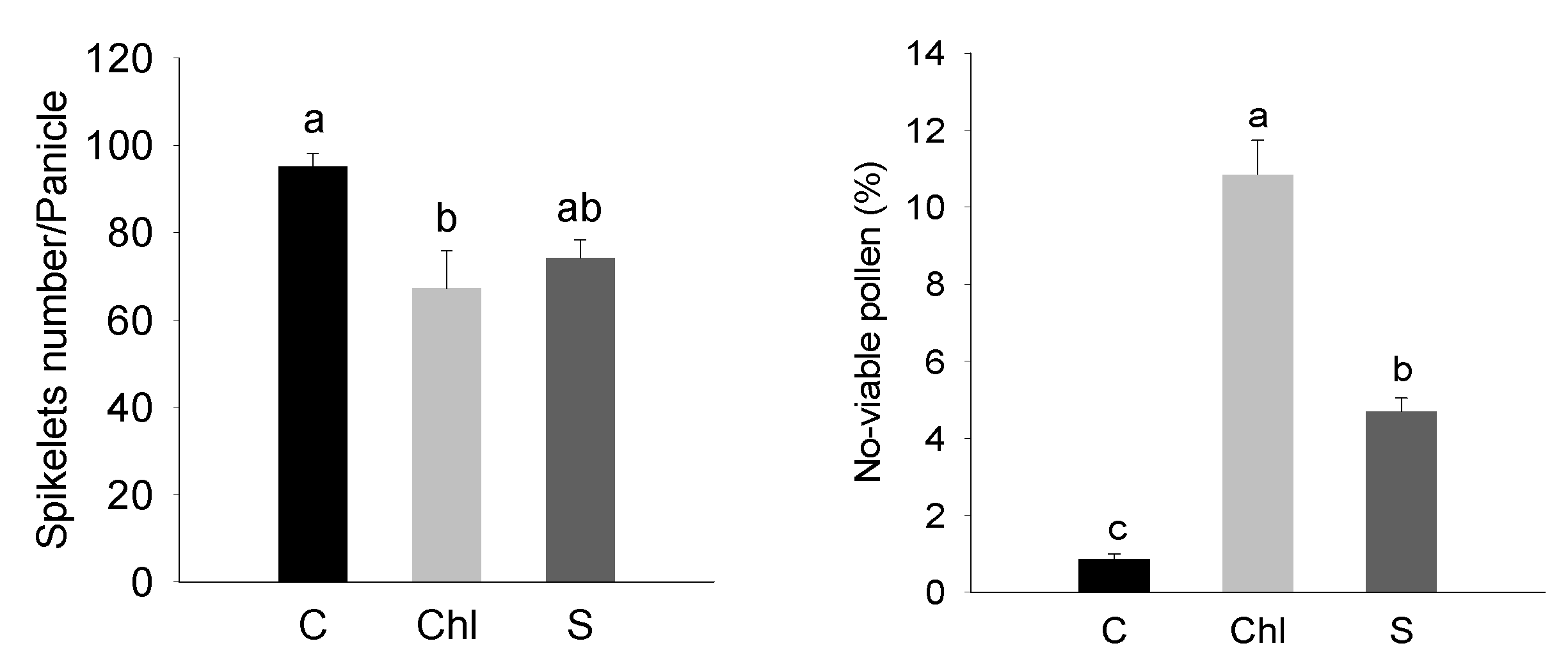
2.2. Estimation of Pollen Viability and Grain Ion Content
2.3. Photosynthesis-Related Parameters
2.4. Statistical Treatment of the Data
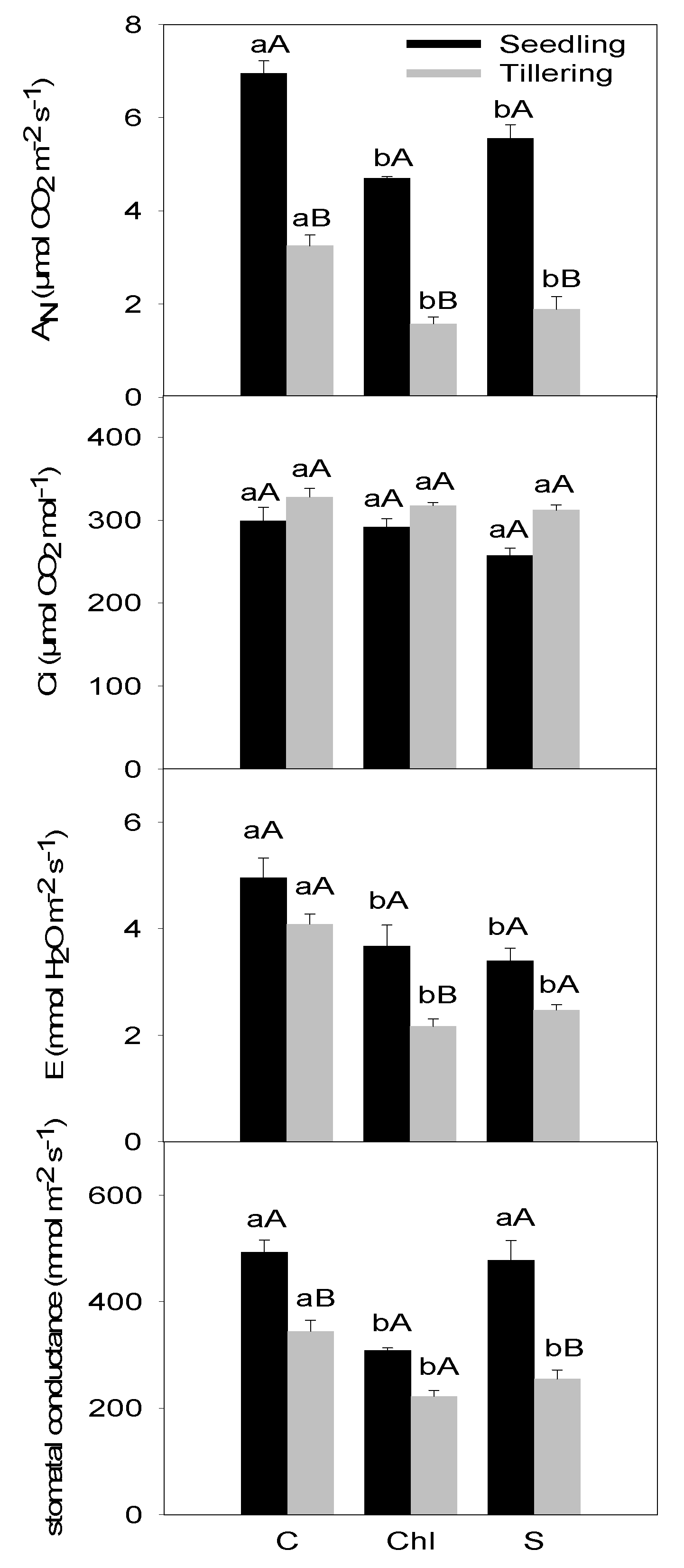
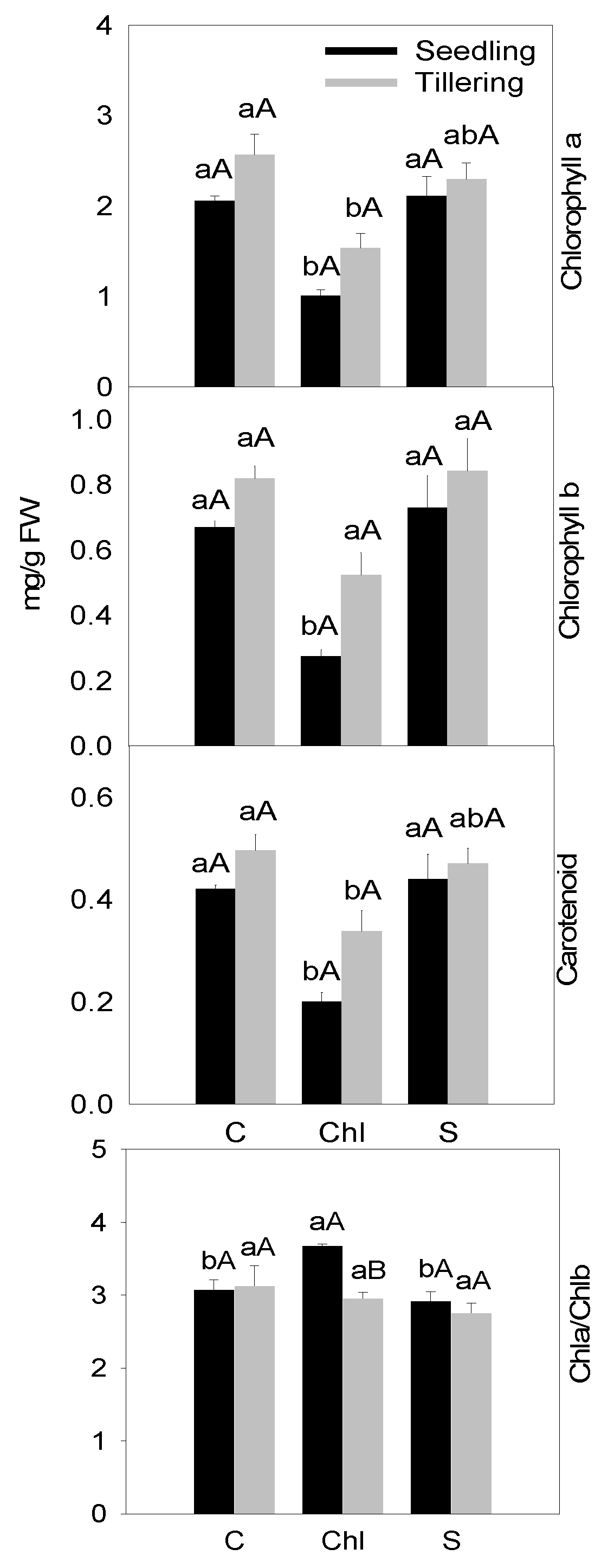
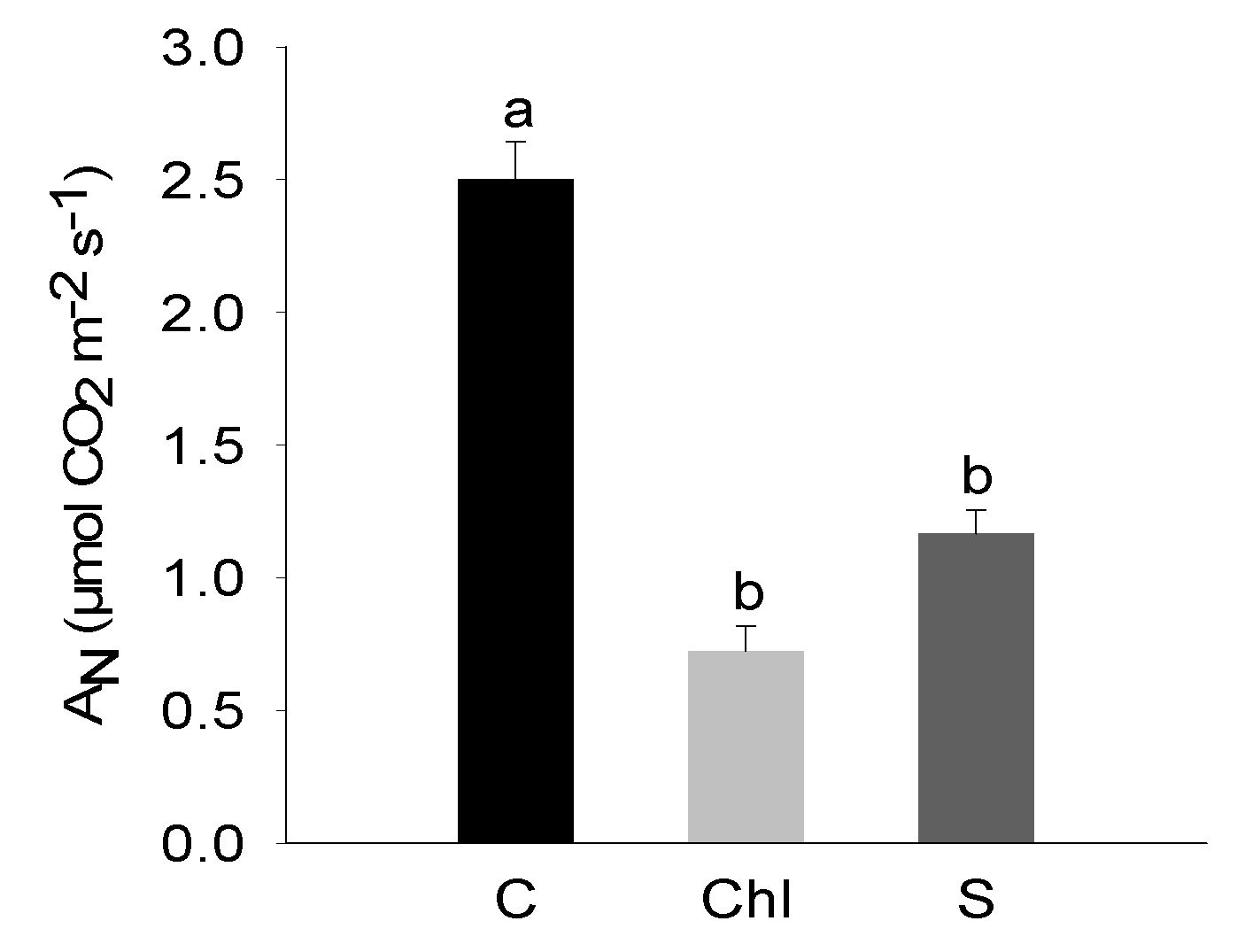
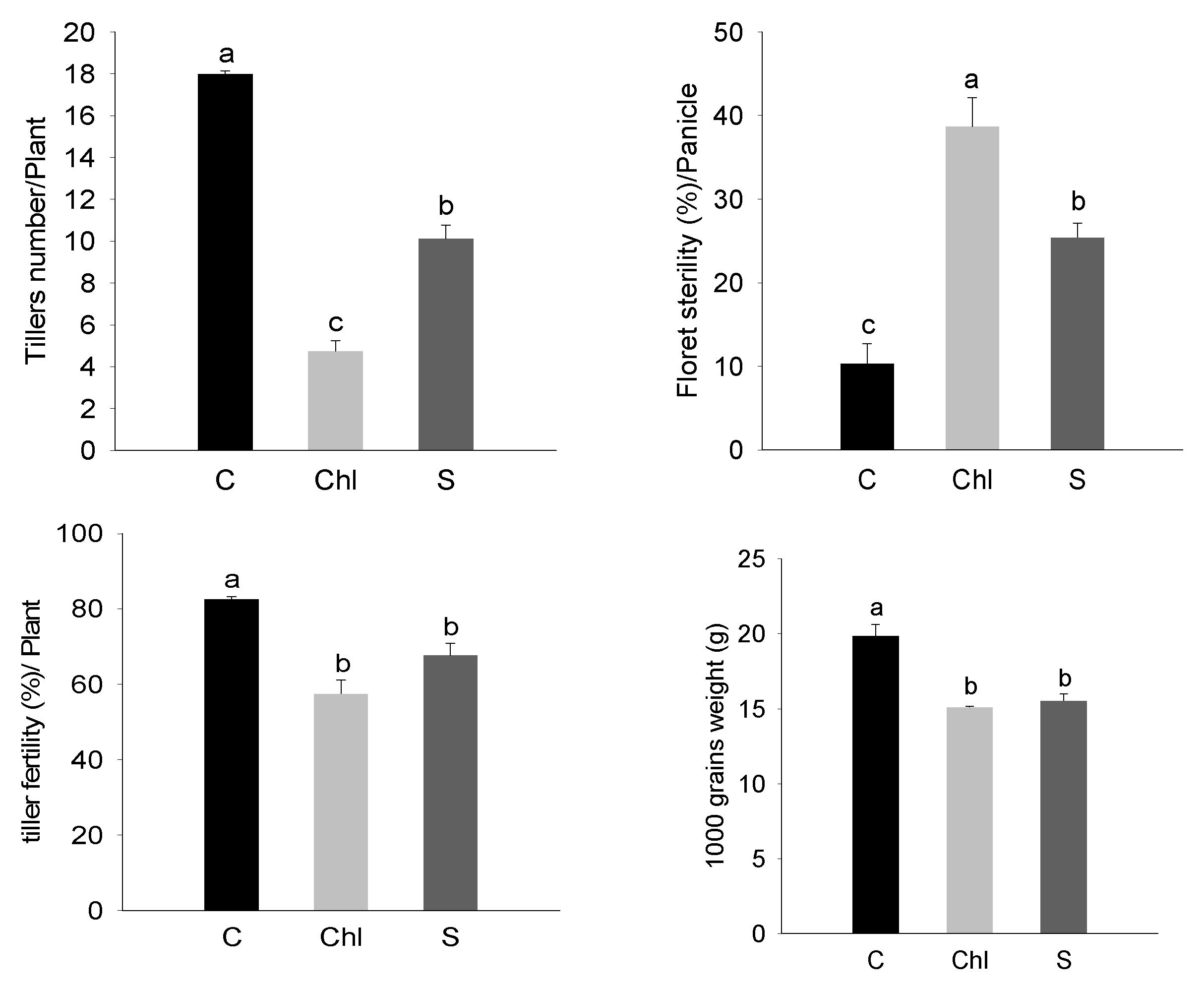
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bui, E. Soil salinity: A neglected factor in plant ecology and biogeography. J. Arid Environ. 2013, 92, 14–25. [Google Scholar] [CrossRef]
- Wassmann, R.; Nguyen, X.H.; Chu, T.H.; Tuong, T.P. Sea level rise affecting the Vietnamese Mekong Delta: Water elevation in the flood season and implications for rice production. Clim. Chang. 2004, 66, 89–107. [Google Scholar] [CrossRef]
- Grattan, S.R.; Zeng, L.; Shannon, M.C.; Roberts, S.R. Rice is more sensitive to salinity than previously thought. Calif. Agric. 2002, 56, 189–198. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. Changes in plant response to NaCl during development of rice (Oryza sativa L.) varieties differing in salinity resistance. J. Exp. Bot. 1995, 46, 1843–1852. [Google Scholar] [CrossRef]
- Moradi, F.; Ismail, A.M. Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann. Bot. 2007, 99, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Shannon, M.C. Salinity effects on seedling growth and yield components of rice. Crop. Sci. 2000, 40, 996–1003. [Google Scholar] [CrossRef]
- Zhu, G.; Kinet, J.M.; Lutts, S. Characterisation of rice (Oryza sativa) F3 populations selected for salt resistance. 2. Relationships between yield-related parameters and physiological properties. Austr. J. Exp. Agric. 2004, 44, 333–342. [Google Scholar] [CrossRef]
- Zhu, G.; Kinet, J.M.; Lutts, S. Characterization of rice (Oryza sativa L.) F3 populations selected for salt resistance. I. Physiological behaviour during vegetative growth. Euphytica 2001, 121, 251–263. [Google Scholar] [CrossRef]
- Abdullah, Z.; Khan, M.A.; Flowers, T. Causes of sterility in seed set of rice under salinity stress. J. Agron. Crop Sci. 2001, 187, 25–32. [Google Scholar] [CrossRef]
- Rao, P.S.; Mishra, B.; Gupta, S.R.; Rathore, A. Reproductive stage tolerance to salinity and alkalinity stresses in rice genotypes. Plant Breed. 2008, 127, 256–261. [Google Scholar] [CrossRef]
- Sultana, N.; Ikeda, T.; Itoh, R. Effect of NaCl salinity on photosynthesis and dry matter accumulation in developing rice grains. Environ. Exp. Bot. 1999, 42, 211–220. [Google Scholar] [CrossRef]
- Zeng, L.; Lesch, S.M.; Grieve, C.M. Rice growth and yield respond to changes in water depth and salinity stress. Agric. Water Manag. 2003, 59, 67–75. [Google Scholar] [CrossRef]
- Zeng, L.; Shannon, M.C.; Lesch, S.M. Timing of salinity stress affects rice growth and yield components. Agric. Water Manag. 2001, 48, 191–206. [Google Scholar] [CrossRef]
- Flowers, T.; Yeo, A.R. Variability in the resistance of sodium chloride salinity within rice (Oryza sativa L.) varieties. New Phytol. 1981, 88, 363–373. [Google Scholar] [CrossRef]
- Kaddah, M.T. Salinity effects on growth of rice at the seedling and inflorescence stages of development. Soil Sci. 1963, 96, 105–111. [Google Scholar] [CrossRef]
- Pardo, J.M. Biotechnology of water and salinity stress tolerance. Curr. Opin. Biotechnol. 2010, 21, 185–196. [Google Scholar] [CrossRef]
- Roy, S.J.; Negrão, S.; Tester, M. Salt resistant crop plants. Curr. Opin. Biotechnol. 2014, 26, 115–124. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 1996, 78, 389–398. [Google Scholar] [CrossRef]
- Castillo, E.G.; Tuong, T.P.; Ismail, A.M.; Inubushi, K. Response to salinity in rice: Comparative effects of osmotic and ionic stresses. Plant Prod. Sci. 2007, 10, 159–170. [Google Scholar] [CrossRef]
- Khatun, S.; Flowers, T. Effects of salinity on seed set in rice. Plant Cell Environ. 1995, 18, 61–67. [Google Scholar] [CrossRef]
- Samson, M.E.; Fortin, J.; Pepin, S.; Caron, J. Impact of potassium sulfate salinity on growth and development of cranberry plants subjected to overhead and subirrigation. Can. J. Soil Sci. 2016, 97, 20–30. [Google Scholar] [CrossRef]
- Khatun, S.; Flowers, T. The estimation of pollen viability in rice. J. Exp. Bot. 1995, 46, 151–154. [Google Scholar] [CrossRef]
- Aghajanzadeh, T.A.; Reich, M.; Kopriva, S.; De kok, L.J. Impact of chloride (NaCl, KCl) and sulphate (Na2SO4, K2SO4) salinity on glucosinolate metabolism in Brassica rapa. J. Agron. Crop. Sci. 2018, 204, 137–146. [Google Scholar] [CrossRef]
- Gao, Y.; Li, D.; Chen, Y. Differentiation of carbonate, chloride, and sulfate salinity responses in tall fescue. Sci. Hort. 2012, 139, 1–7. [Google Scholar] [CrossRef]
- Irakoze, W.; Vanpee, B.; Rufyikiri, G.; Dailly, H.; Nijimbere, S.; Lutts, S. Comparative effects of chloride and sulfate salinities on two contrasting rice cultivars (Oryza sativa L.) at the seedling stage. J. Plant Nutr. 2019, 42, 1001–1015. [Google Scholar] [CrossRef]
- Cock, J.; Yoshida, S.; Forno, D.A. Laboratory Manual for Physiological Studies of Rice; International Rice Research Institute: Los Baños, Philippines, 1976. [Google Scholar]
- Alexander, M. Differential staining of aborted and nonaborted pollen. Stain Technol. 1969, 44, 117–122. [Google Scholar] [CrossRef]
- Hamrouni, L.; Hanana, M.; Abdelly, C.; Ghorbel, C. Exclusion du chlorure et inclusion du sodium: Deux mécanismes concomitants de tolérance à la salinité chez la vigne sauvage Vitis vinifera subsp. sylvestris (var.’Séjnène.). Biotech. Agron. Soc. Environ. 2011, 15, 387. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 350–382. [Google Scholar] [CrossRef]
- Krishnamurthy, S.L.; Gautam, R.K.; Sharma, P.C.; Sharma, D.K. Effect of different salt stresses on agro-morphological traits and utilisation of salt stress indices for reproductive stage salt tolerance in rice. Field Crop. Res. 2016, 190, 26–33. [Google Scholar] [CrossRef]
- Devinar, G.; Llanes, A.; Masciarelli, O.; Luna, V. Different relative humidity conditions combined with chloride and sulfate salinity treatments modify abscisic acid and salicylic acid levels in the halophyte Prosopis strombulifera. Plant Growth Regul. 2013, 70, 247–256. [Google Scholar] [CrossRef]
- Han, L.; Gao, Y.; Li, D. Ion uptake in tall fescue as affected by carbonate, chloride, and sulfate salinity. PLoS ONE 2014, 9, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Reich, M.; Aghajanzadeh, T.; Helm, J.; Parmar, S.; Hawkesford, M.J.; De kok, L.J. Chloride and sulfate salinity differently affect biomass, mineral nutrient composition and expression of sulfate transport and assimilation genes in Brassica rapa. Plant Soil 2017, 411, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.; Grieve, C.; Shannon, M. The response of lucerne (Medicago sativa L.) to sodium sulphate and chloride salinity. Plant Soil 1998, 202, 271–280. [Google Scholar] [CrossRef]
- Bilski, J.J.; Nelson, D.; Conlon, R.L. Response of six wild potato species to chloride and sulfate salinity. Amer. Pot. J. 1988, 65, 605–611. [Google Scholar] [CrossRef]
- Chunthaburee, S.; Sanitchon, J.; Pattanagul, W.; Theerakulpisut, P. Effects of salt stress after late booting stage on yield and antioxidant capacity in pigmented rice grains and alleviation of the salt-induced yield reduction by exogenous spermidine. Plant Prod. Sci. 2015, 18, 32–42. [Google Scholar] [CrossRef]
- Lekklar, C.; Pongpanich, M.; Suriya-arunroj, D.; Chinpongpanich, A.; Tsai, H.; Comai, L.; Chadchawan, S.; Buaboocha, T. Genome-wide association study for salinity tolerance at the flowering stage in a panel of rice accessions from Thailand. BMC Genom. 2019, 20, 76–85. [Google Scholar] [CrossRef]
- Thitisaksakul, M.; Tananuwong, K.; Shoemaker, C.F.; Chun, A.; Tanadul, O.; Labavitch, J.M.; Beckles, D.M. Effects of timing and severity of salinity stress on rice (Oryza sativa L.) yield, grain composition, and starch functionality. J. Agric. Food Chem. 2015, 63, 2296–2304. [Google Scholar] [CrossRef]
- Ma, Y.; Shabala, S.; Li, C.; Liu, C.; Zhang, W.; Zhou, M. Quantitative trait loci for salinity tolerance identified under drained and waterlogged conditions and their association with flowering time in barley (Hordeum vulgare L). PLoS ONE 2015, 10, 456–469. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, W.Y.; Yun, D.J. A role for GIGANTEA: Keeping the balance between flowering and salinity stress tolerance. Plant Signal. Behav. 2013, 8, 24820. [Google Scholar] [CrossRef]
- Ghanem, M.E.; Elteren, J.V.; Albacete, A.; Quinet, M.; Martínez-Andújar, C.; Kinet, J.M.; Pérez-Alfocea, F.; Lutts, S. Impact of salinity on early reproductive physiology of tomato (Solanum lycopersicum) in relation to a heterogeneous distribution of toxic ions in flower organs. Funct. Plant Biol. 2009, 36, 125–136. [Google Scholar] [CrossRef]
- Zonia, L.; Cordeiro, S.; Tupý, J.; Feijó, J.A. Oscillatory chloride efflux at the pollen tube apex has a role in growth and cell volume regulation and is targeted by inositol 3, 4, 5, 6-tetrakisphosphate. Plant Cell 2002, 14, 2233–2249. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, N.; Usuda, H.; Nakamoto, H.; Ishihara, K. Changes in the rate of photosynthesis during grain filling and the enzymatic activities associated with the photosynthetic carbon metabolism in rice panicles. Plant Cell Physiol. 1990, 31, 835–844. [Google Scholar] [CrossRef]
- Vromman, D.; Lutts, S.; Lefèvre, I.; Somer, L.; De Vreese, O.; Šlejkovec, Z.; Quinet, M. Effects of simultaneous arsenic and iron toxicities on rice (Oryza sativa L.) development, yield-related parameters and As and Fe accumulation in relation to As speciation in the grains. Plant Soil 2013, 371, 199–217. [Google Scholar] [CrossRef]
| mmol CO2 Uptake/mmol H2O Loss | ||||||
|---|---|---|---|---|---|---|
| Seedling | Tillering | |||||
| C | Chl | S | C | Chl | S | |
| WUE | 1.4 ± 0.09 aA | 1.3 ± 0.14 Aa | 1.6 ± 0.12 aA | 0.8 ± 0.08 aB | 0.7 ± 0.09 aB | 0.8 ± 0.08 aB |
| µmol g−1 DW | ||||||
|---|---|---|---|---|---|---|
| Grain Hull | Grain | |||||
| Ion | C | Chl | S | C | Chl | S |
| Na+ | 42.8 ± 3.5 c | 503.4 ± 3.2 a | 314 ± 36.5 b | 5.4 ± 0.06 c | 51.7 ± 2.3 a | 22.9 ± 3.5 b |
| K+ | 920 ± 30 a | 534.9 ± 15 b | 550 ± 24 b | 226 ± 1.5 a | 141 ± 3.6 b | 146 ± 7 b |
| Cl− | 199.3 ± 13 b | 1455.6 ± 27 a | 188 ± 14 b | 6.7 ± 0.08 b | 64.6 ± 3 a | 7 ± 0.7 b |
| S6+ | 48.7 ± 5.6 b | 57.2 ± 4.3 b | 284 ± 17 a | 16.4 ± 1 b | 15 ± 0.1 b | 25 ± 2 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irakoze, W.; Prodjinoto, H.; Nijimbere, S.; Rufyikiri, G.; Lutts, S. NaCl and Na2SO4 Salinities Have Different Impact on Photosynthesis and Yield-Related Parameters in Rice (Oryza sativa L.). Agronomy 2020, 10, 864. https://doi.org/10.3390/agronomy10060864
Irakoze W, Prodjinoto H, Nijimbere S, Rufyikiri G, Lutts S. NaCl and Na2SO4 Salinities Have Different Impact on Photosynthesis and Yield-Related Parameters in Rice (Oryza sativa L.). Agronomy. 2020; 10(6):864. https://doi.org/10.3390/agronomy10060864
Chicago/Turabian StyleIrakoze, Willy, Hermann Prodjinoto, Séverin Nijimbere, Gervais Rufyikiri, and Stanley Lutts. 2020. "NaCl and Na2SO4 Salinities Have Different Impact on Photosynthesis and Yield-Related Parameters in Rice (Oryza sativa L.)" Agronomy 10, no. 6: 864. https://doi.org/10.3390/agronomy10060864
APA StyleIrakoze, W., Prodjinoto, H., Nijimbere, S., Rufyikiri, G., & Lutts, S. (2020). NaCl and Na2SO4 Salinities Have Different Impact on Photosynthesis and Yield-Related Parameters in Rice (Oryza sativa L.). Agronomy, 10(6), 864. https://doi.org/10.3390/agronomy10060864





