Abstract
With the forecasted dramatic growth of insect rearing in the near future, frass (insect excreta) has been increasingly considered a sustainable resource for managing plant nutrition in cropping systems and a promising alternative to conventional fertilizer. However, the impact of soil fauna on its fertilizing effect has not been investigated so far. In this study, we investigated the effect of earthworms (Lumbricus terrestris L.) on nitrogen (N), phosphorus (P), potassium (K) and calcium (Ca) uptake and crop growth in the presence of frass from mealworm (Tenebrio molitor L.). Using a pot experiment, we found that earthworms increased N, P, K and Ca concentration in barley (Hordeum vulgare L.) in the presence of frass, suggesting that earthworm activity enhances the short-term recycling of nutrients from frass. Compared to treatments with and without frass and earthworms, the specific leaf area of barley was the highest in the presence of both earthworms and frass. This confirms that earthworms and frass have a synergistic effect on soil fertility. Overall, our study shows that earthworms may improve the efficiency of organic fertilizers and argues therefore for the importance of developing sustainable agricultural practices that promote earthworm populations.
Keywords:
earthworms; frass; insect excreta; insect farming; nitrogen; phosphorus; soil fauna; soil fertility; waste management 1. Introduction
In the context of the massive increase in the human population at an unprecedented level, insect rearing represents an opportunity to answer the growing demand for proteins with a low ecological footprint [1]. Although insect production is highly efficient in converting by-products into biomass, it also yields a waste stream consisting especially of insect feces (“frass”). Given the “zero waste” context and the need to contribute to the circular economy, the possibility of recovering frass as a fertilizer has recently been considered by researchers [2,3,4]. For instance, Houben et al. [3] have found that frass from mealworm (Tenebrio molitor L.) might be as efficient as conventional mineral fertilizer to sustain crop growth due to its rapid mineralization after its incorporation into the soil and the presence of nutrients in a readily-available form. A couple of studies have suggested that microbial activity might partially control the effect of frass on soil fertility, either in natural conditions [5,6,7] or in cropping systems [2,3]. However, the impact of soil fauna has not been considered so far. It is known that soil fauna, especially earthworms, may positively affect plant growth [8,9] due to, among others, changes in soil structure and water regime [10], improvement of soil organic matter and nutrient cycling [11,12], and stimulation and dispersal of beneficial microorganisms [13]. Moreover, adding exogenous organic amendments generally stimulates earthworm activity which reciprocally improves the fertilizing effect of these amendments [13,14,15], even though some contradictory results have also been found [12,16].
Since frass is an organic amendment, it is therefore likely that its effect on soil fertility might also be mediated by earthworm activity. Therefore, the aim of this study was to investigate the impact of the earthworm presence on the fertilizer potential of frass. For this purpose, we carried out a pot experiment to determine the effect of earthworms on the frass fertilizing effect. Barley (Hordeum vulgare L.) was grown in greenhouse conditions with or without frass from mealworm (Tenebrio molitor L.) in the presence or absence of earthworms (Lumbricus terrestris L.).
2. Materials and Methods
A pot experiment was conducted to determine the effect of earthworms on the fertilizer potential of frass. Frass (ŸnFrass) from mealworm (Tenebrio molitor L.) was provided in the form of powder by Ÿnsect (Paris, France), an industrial company farming this insect at a large-scale. Chemical characteristics of frass are presented in Table 1.

Table 1.
Chemical characteristics of frass (data from Houben et al. [3]).
The studied soil was sampled in Beauvais (Northern France) and was classified as a Haplic Luvisol (IUSS Working Group WRB, 2015), a soil with properties suitable for soil fauna activity [17]. Soil characterization was carried out by Houben et al. [3] following the procedures described elsewhere [18] and revealed that organic C was 1.54%, total N was 0.18%, the cation exchange capacity (CEC) was 12.5 cmolc kg−1, and pH was 7.8.
The experimental device was based on our previous study which aimed at estimating the fertilizer potential of frass [3]. Briefly, plastic plant pots were filled with 3500 g of either soil or a mixture of soil and frass at a rate of 10 Mg dry matter ha-1 (hereafter called “Frass” treatment), or untreated soil (hereafter called “Control”). Three earthworms (Lumbricus terrestris L.) were added in half of the pots (hereafter called “Frass + earthworms” or “Control + Earthworms” treatments) representing biomass of 12.05 ± 0.24 g and 12.25 ± 0.17 g in Frass + Earthworms and Control + Earthworms treatments respectively, according to the recommendations by Vos et al. [19]. Each of the four treatments was replicated four times.
Eight seeds of barley (Hordeum vulgare L.) were sown in each pot. After 10 days, excess germinated seedlings were removed (first harvest) so that only four uniform plants per pot were allowed to grow for the following eight weeks (ca. 120 plants m−2). The trials were conducted under controlled greenhouse conditions (temperature 18–25 °C, 16 h photoperiod) with daily sprinkler watering to maintain the soil moisture at field capacity. After 9 weeks, the shoots were harvested with ceramic scissors. Three fully-grown young leaves per replicate were scanned at 600 dpi and then dried at 60 °C for 72 h to determine specific leaf area (SLA). All aboveground biomass was dried at 60 °C for 48 h in a similar manner and weighed. The concentrations of P, K, and Ca in aerial parts were analyzed by inductively coupled plasma-atomic emission spectroscopy (ICP-AES; Jarrell Ash) after aqua regia digestion. The concentration of N in aerial parts was analyzed using the Dumas combustion method. Earthworms were extracted from pots, counted, and weighed. As suggested by Coulis et al. [20], available P concentration in soil was assessed using water extraction (soil:water 1:60; w-v) following the procedure described by Sissingh [21]. Available K and Ca concentrations were determined using the acetate ammonium-ethylenediamine tetraacetic acid (AAEDTA) [18,22]. Soil pH was measured in water (soil:water 1:5; w-v).
All recorded data were analyzed using descriptive statistics (mean ± standard error) and normality was determined using the Shapiro-Wilk test. One-way ANOVAs and Tukey’s multiple comparison tests or Kruskal-Wallis and Mann-Whitney tests were used to compare biomass, SLA, and nutrient concentrations in the shoot and soil according to whether the distribution was normal or not, respectively. Pearson’s correlation coefficient was used to analyze the relationship between SLA and N concentration. All statistical analyses were performed using R software version 3.5.0 [23] and the package Rcmdr [24].
3. Results and Discussion
3.1. Earthworm Survival
At harvest, earthworm survival was 100% for all the treatments and their burrowing activity was clearly visible (Figure 1). In addition, their number and biomass per pot at the end of the experiment were not significantly (p > 0.05) different from that before their incorporation into the soil. This indicates that frass had no toxic effect on earthworms and allows us to ascribe the following results to the actual presence of earthworms. The similar earthworm biomass between the beginning and the end of the experiment contrasts with Sizmur et al. [25] who found in a 12-week microcosm experiment a continuous decrease of earthworm (L. terrestris) biomass in soil with no amendment or with organic amendments including farmyard manure, anaerobic digestate, and compost. However, Sizmur et al. [25] carried out their experiment without plants, which could possibly explain the discrepancy with our study. Since L. terrestris may feed on plant roots [26,27], it is likely that, by providing an additional source of food, the presence of plants contributed to maintaining earthworm biomass all over the experiment.

Figure 1.
Representative pictures of soil collected in pots at the plant harvest illustrating the intense burrowing activity of earthworms.
3.2. Impact of Earthworms on Nutrient Uptake and Crop Growth
Many studies have reported that earthworm activities significantly increase N concentration in plant tissues [13,28], predominantly due to an earthworm-induced stimulation of N mineralization, which in turn, enhances N availability for plants [29,30]. For instance, Amador et al. [31,32] showed higher N mineralization in the drilosphere of L. terrestris, leading to an accumulation of nitrate in earthworm burrow soil. This increase of nitrate can result in higher N uptake, as observed for oilseed rape grown in an earthworm-inoculated (Metaphire guillemi) soil [33]. In agreement with these researchers, our results showed that irrespective of the treatment, N concentration in barley shoot was higher with than without earthworms (Figure 2).
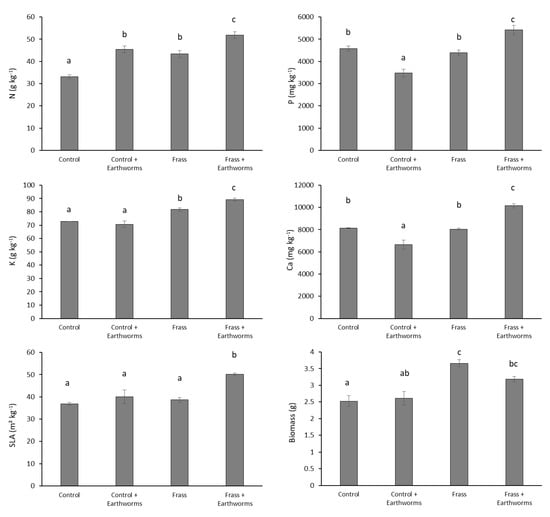
Figure 2.
Concentrations of N, P, K, and Ca, specific leaf area (SLA), and biomass of barley. Values are average (n = 4) ± standard error. Columns with the same letter do not differ significantly at the 5% level.
More importantly, our results suggest a synergistic effect between frass and earthworms since the Frass + Earthworms treatment displayed the highest N concentration in barley shoot (Figure 2). The positive effect of frass on N uptake by plants has been previously discussed and was attributed to its very rapid mineralization after its incorporation into the soil [3]. Here, our results indicate that earthworms induced a higher uptake of N in the presence of frass. Although the present study did not allow us to identify the pools from which N was taken up by plants, it is likely that the presence of earthworms stimulated the release of N from frass since earthworms generally promote N mineralization from organic fertilizer [8]. For instance, Postma-Blaauw [12] showed that L. terrestris enhanced the release of N from crop residue by increasing its mineralization while it had no effect on the mineralization of soil organic matter-derived N. Using 15N, Amador and Görres [34] found that L. terrestris could double the amount of litter-derived N taken up by maize grown in mesocosms. In another study, N uptake by maize was 26 and 74% higher from manure and compost treatments, respectively in the presence of earthworms (Pheretima hawayana) compared to control without earthworms [14]. This was attributed to an increase of the decomposition of organic N by earthworms which enhanced the N mineralization from the manure and compost treatments, as also observed by Rashid et al. [35]. Besides increasing microbial metabolic activity [3], frass, like other organic amendments, might also have promoted earthworm activity [36], which could further increase N mineralization.
Unlike N, the presence of earthworms decreased P concentration in the shoot of the control (Figure 2), which can be related to the decrease of available P concentration in soil (Figure 3). Earthworms have been reported to enhance P availability in the short run due to changes in complexes induced by competition for sorbing sites between orthophosphates and carboxyl groups of the mucus produced in the gut [37]. However, after three weeks of incubation, Le Bayon and Binet [38] found a dramatic decrease of P availability in the presence of L. terrestris, which was ascribed to the immobilization of P by microorganisms. Phosphorus availability may also be reduced due to soil pH increase brought about by earthworm activities [39]. Our results indicate, however, that soil pH was unaffected by the presence of earthworms (Figure 3), which therefore suggests that the lower P availability in the Control + Earthworm treatment would predominantly result from P immobilization by microorganisms. As reported by Houben et al. [3], application of frass to soil improved P nutrition (Figure 2), which is due to the presence of P in a readily available form as well as to the slightly acidifying effect of frass which can, in turn, increase P solubility (Figure 3). By contrast to the control, the presence of earthworms in the frass treatment increased P concentrations in shoots, suggesting that earthworms promoted the recycling of P from frass. Interestingly, available P concentration was not increased in the Frass + Earthworm treatment and pH was unaffected by earthworms (Figure 3), which indicates that the higher P concentration in barley shoot in this treatment would not result only from a change in the biogeochemical status of P. Improvement of P concentration in barley shoot might be explained by a better distribution of P within the soil due to earthworm activities. Earthworms facilitate P transfer of organic fertilizer within the soil [15,38,40], which can in turn increase the root accessibility to P, especially for plants such as barley, whose spatial soil exploration by roots plays an important role in the acquisition of P from organic fertilizer [41]. Similar to P, available Ca and K concentrations in soil in the presence of frass were not increased by earthworms (Figure 3) while, as for P, the Frass + Earthworm treatment showed the highest Ca and K concentrations in the shoot (Figure 3). This, therefore, suggests that mechanisms responsible for the earthworms-induced recycling of Ca and K from frass are similar to that for P.
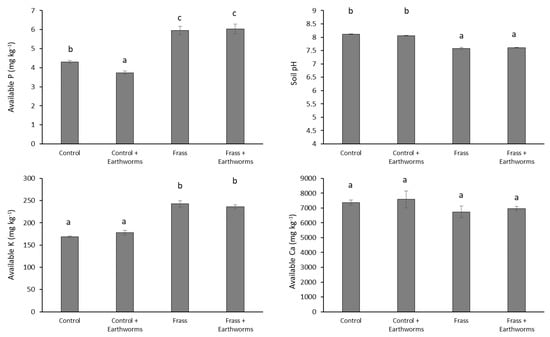
Figure 3.
Available P concentration (water extraction), available K and Ca concentrations (AA-EDTA extraction), and pH in the soil. Values are average (n = 4) ± standard error. Columns with the same letter do not differ significantly at the 5% level.
The synergistic effect between earthworms and frass on plant nutrition was reflected by an increase of SLA of barley shoot (Figure 2). Being related to the relative growth rate of plant species [42], SLA is widely used as a target trait to unravel plant responses to soil properties, especially those linked to soil fertility [43], and can explain plant productivity [44]. As a leaf functional trait, its characterization allows us to elucidate the plant response to changes in soil properties at an individual scale. SLA is usually well correlated to N availability and N concentration in plants [45,46], which was also found in our study (r = 0.82; p <0.001). Therefore, its improvement in the Frass + Earthworms treatment corroborates our findings that earthworms improve the fertilizer potential of frass. It is noteworthy that, unexpectedly, shoot biomass was not improved by the presence of earthworms in the frass treatment. Shoot biomass is known to be less sensitive than SLA to a change of soil fertility as many factors can drive it [47]. In the present study, the lack of biomass improvement in the Frass + Earthwoms treatment, in spite of a higher soil fertility status, could be explained by competition for light induced by the higher SLA [48,49]. The perspective is, therefore, to elucidate the plant density which optimizes canopy light interception, crop yield, and nutrient use efficiency from frass. The lack of biomass improvement could also have been due to herbivory by earthworms. Some studies reported L. terrestris to commonly consume roots [27], especially in situations of low litter availability [26], which might, in turn, reduce the aboveground biomass production, as shown for other organisms [50]. Therefore, another perspective will be to investigate how the root consumption by earthworms may be affected by organic amendments such as frass.
4. Conclusions
With the forecasted growth of insect farming in the near future, frass is increasingly considered a promising resource for the sustainable management of plant nutrition in cropping systems and an enticing alternative to conventional fertilizer. In this study, we evidenced that earthworms enhance the fertilizer potential of frass. Indeed, their activity increases soil fertility and nutrient (N, P, K and Ca) concentrations in barley in the presence of frass, likely by improving the short-term recycling of nutrients from frass. More generally, our study highlights that, as key biological agents in the transformation of organic matter and waste, earthworms may improve the efficiency of organic fertilizers. Coupled with the other well-documented ecosystem services delivered by earthworms, our findings further argue for the importance of developing sustainable agricultural practices that promote earthworm populations.
Author Contributions
Conceptualization, A.-M.D., G.D., M.-P.F. and D.H.; methodology, A.-M.D. and D.H.; investigation, A.-M.D. and D.H.; writing—original draft preparation, A.-M.D. and D.H..; writing—review and editing, G.D. and M.-P.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a private corporation, Ÿnsect.
Acknowledgments
We thank the “ASET 158” students, Céline Roisin, Aurore Coutelier and Vincent Hervé for technical assistance. L. Dulaurent is heartily acknowledged for his contribution to the weightless cloud.
Conflicts of Interest
Although the research was funded by a private corporation, Ÿnsect, we ensure the research is free of bias.
References
- Dicke, M. Insects as feed and the Sustainable Development Goals. J. Insects Food Feed 2018, 4, 147–156. [Google Scholar] [CrossRef]
- Poveda, J.; Jimenez-Gomez, A.; Saati-Santamaría, Z.; Usategui-Martín, R.; Rivas, R.; García-Fraile, P. Mealworm frass as a potential biofertilizer and abiotic stress tolerance-inductor in plants. Appl. Soil Ecol. 2019, 142, 110–122. [Google Scholar] [CrossRef]
- Houben, D.; Daoulas, G.; Faucon, M.-P.; Dulaurent, A.-M. Potential use of mealworm frass as a fertilizer: Impact on crop growth and soil properties. Sci. Rep. 2020, 10, 4659. [Google Scholar] [CrossRef]
- Schmitt, E.; de Vries, W. Potential benefits of using Hermetia illucens frass as a soil amendment on food production and for environmental impact reduction. Curr. Opin. Green Sustain. Chem. 2020. [Google Scholar] [CrossRef]
- Frost, C.J.; Hunter, M.D. Insect Canopy herbivory and frass deposition affect soil nutrient dynamics and export in oak mesocosms. Ecology 2004, 85, 3335–3347. [Google Scholar] [CrossRef]
- Kagata, H.; Ohgushi, T. Positive and negative impacts of insect frass quality on soil nitrogen availability and plant growth. Popul. Ecol. 2012, 54, 75–82. [Google Scholar] [CrossRef]
- Lovett, G.M.; Ruesink, A.E. Carbon and nitrogen mineralization from decomposing gypsy moth frass. Oecologia 1995, 104, 133–138. [Google Scholar] [CrossRef]
- van Groenigen, J.W.; Lubbers, I.M.; Vos, H.M.J.; Brown, G.G.; De Deyn, G.B.; van Groenigen, K.J. Earthworms increase plant production: A meta-analysis. Sci. Rep. 2014, 4, 6365. [Google Scholar] [CrossRef]
- Blouin, M.; Hodson, M.E.; Delgado, E.A.; Baker, G.; Brussaard, L.; Butt, K.R.; Dai, J.; Dendooven, L.; Peres, G.; Tondoh, J.E.; et al. A review of earthworm impact on soil function and ecosystem services. Eur. J. Soil Sci. 2013, 64, 161–182. [Google Scholar] [CrossRef]
- Blanchart, E.; Albrecht, A.; Alegre, J.; Duboisset, A.; Gilot, C.; Pashanasi, B.; Lavelle, P.; Brussaard, L. Effects of earthworms on soil structure and physical properties. In Earthworm Management in Tropical Agroecosystems; Lavelle, P., Brussaard, L., Hendrix, P., Eds.; CABI: New York, NY, USA, 1999; pp. 149–171. [Google Scholar]
- Chapuis-Lardy, L.; Le Bayon, R.-C.; Brossard, M.; Lopez-Hernandez, D.; Blanchart, E. Role of Soil Macrofauna in Phosphorus Cycling. In Phosphorus in Action: Biological Processes in Soil Phosphorus Cycling; Bünemann, E., Oberson, A., Frossard, E., Eds.; Soil Biology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 199–213. ISBN 978-3-642-15271-9. [Google Scholar]
- Postma-Blaauw, M.B.; Bloem, J.; Faber, J.H.; van Groenigen, J.W.; de Goede, R.G.M.; Brussaard, L. Earthworm species composition affects the soil bacterial community and net nitrogen mineralization. Pedobiologia 2006, 50, 243–256. [Google Scholar] [CrossRef]
- Medina-Sauza, R.M.; Alvarez-Jimenez, M.; Delhal, A.; Reverchon, F.; Blouin, M.; Guerrero-Analco, J.A.; Cerdan, C.R.; Guevara, R.; Villain, L.; Barois, I. Earthworms Building Up Soil Microbiota, a Review. Front. Environ. Sci. 2019, 7, 81. [Google Scholar] [CrossRef]
- Waqar, A.; Shah, G.M.; Bakhat, H.F.; Shahid, M.; Aslam, M.; Ashraf, M.R.; Hafeez, R.; Murtaza, B.; Rashid, M.I. The earthworm species Pheretima hawayana influences organic wastes decomposition, nitrogen mineralization and maize N recovery. Eur. J. Soil Biol. 2019, 90, 1–8. [Google Scholar] [CrossRef]
- Sharpley, A.; McDowell, R.; Moyer, B.; Littlejohn, R. Land application of manure can influence earthworm activity and soil phosphorus distribution. Commun. Soil Sci. Plant Anal. 2011, 42, 194–207. [Google Scholar] [CrossRef]
- Jouquet, P.; Plumere, T.; Thu, T.D.; Rumpel, C.; Duc, T.T.; Orange, D. The rehabilitation of tropical soils using compost and vermicompost is affected by the presence of endogeic earthworms. Appl. Soil Ecol. 2010, 46, 125–133. [Google Scholar] [CrossRef]
- Clause, J.; Barot, S.; Richard, B.; Decaëns, T.; Forey, E. The interactions between soil type and earthworm species determine the properties of earthworm casts. Appl. Soil Ecol. 2014, 83, 149–158. [Google Scholar] [CrossRef]
- Houben, D.; Meunier, C.; Pereira, B.; Sonnet, P. Predicting the degree of phosphorus saturation using the ammonium acetate–EDTA soil test. Soil Use Manag. 2011, 27, 283–293. [Google Scholar] [CrossRef]
- Vos, H.M.J.; Ros, M.B.H.; Koopmans, G.F.; van Groenigen, J.W. Do earthworms affect phosphorus availability to grass? A pot experiment. Soil Biol. Biochem. 2014, 79, 34–42. [Google Scholar] [CrossRef]
- Coulis, M.; Bernard, L.; Gerard, F.; Hinsinger, P.; Plassard, C.; Villeneuve, M.; Blanchart, E. Endogeic earthworms modify soil phosphorus, plant growth and interactions in a legume–cereal intercrop. Plant Soil 2014, 379, 149–160. [Google Scholar] [CrossRef]
- Sissingh, H.A. Analytical technique of the Pw method, used for the assessment of the phosphate status of arable soils in the Netherlands. Plant Soil 1971, 34, 483–486. [Google Scholar] [CrossRef]
- Gomez-Suarez, A.D.; Nobile, C.; Faucon, M.-P.; Pourret, O.; Houben, D. Fertilizer potential of struvite as affected by nitrogen form in the rhizosphere. Sustainability 2020, 12, 2212. [Google Scholar] [CrossRef]
- R Core Team. R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; ISBN 3-900051-07-0. Available online: https://www.R-project.org (accessed on 22 December 2019).
- Fox, J. The R Commander: A Basic-Statistics Graphical User Interface to R. J. Stat. Softw. 2005, 14, 1–42. [Google Scholar] [CrossRef]
- Sizmur, T.; Martin, E.; Wagner, K.; Parmentier, E.; Watts, C.; Whitmore, A.P. Milled cereal straw accelerates earthworm (Lumbricus terrestris) growth more than selected organic amendments. Appl. Soil Ecol. 2017, 113, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Griffith, B.; Türke, M.; Weisser, W.W.; Eisenhauer, N. Herbivore behavior in the anecic earthworm species Lumbricus terrestris L.? Eur. J. Soil Biol. 2013, 55, 62–65. [Google Scholar] [CrossRef]
- Cortez, J.; Bouche, M.B. Do earthworms eat living roots? Soil Biol. Biochem. 1992, 24, 913–915. [Google Scholar] [CrossRef]
- Scheu, S. Effects of earthworms on plant growth: Patterns and perspectives. Pedobiologia 2003, 47, 846–856. [Google Scholar] [CrossRef]
- Baker, G. Differences in nitrogen release from surface and incorporated plant residues by two endogeic species of earthworms (Lumbricidae) in a red–brown earth soil in southern Australia. Eur. J. Soil Biol. 2007, 43, S165–S170. [Google Scholar] [CrossRef]
- Lubbers, I.M.; Brussaard, L.; Otten, W.; Groenigen, J.W.V. Earthworm-induced N mineralization in fertilized grassland increases both N2O emission and crop-N uptake. Eur. J. Soil Sci. 2011, 62, 152–161. [Google Scholar] [CrossRef]
- Amador, J.A.; Gorres, J.H.; Savin, M.C. Effects of Lumbricus terrestris L. on nitrogen dynamics beyond the burrow. Appl. Soil Ecol. 2006, 33, 61–66. [Google Scholar] [CrossRef]
- Amador, J.A.; Gorres, J.H.; Savin, M.C. Carbon and nitrogen dynamics in Lumbricus terrestris (L.) burrow Soil. Soil Sci. Soc. Am. J. 2003, 67, 1755–1762. [Google Scholar] [CrossRef]
- Zhang, S.; Chao, Y.; Zhang, C.; Cheng, J.; Li, J.; Ma, N. Earthworms enhanced winter oilseed rape (Brassica napus L.) growth and nitrogen uptake. Agric. Ecosyst. Environ. 2010, 139, 463–468. [Google Scholar] [CrossRef]
- Amador, J.A.; Gorres, J.H. Role of the anecic earthworm Lumbricus terrestris L. in the distribution of plant residue nitrogen in a corn (Zea mays)–soil system. Appl. Soil Ecol. 2005, 30, 203–214. [Google Scholar] [CrossRef]
- Rashid, M.I.; de Goede, R.G.M.; Corral Nunez, G.A.; Brussaard, L.; Lantinga, E.A. Soil pH and earthworms affect herbage nitrogen recovery from solid cattle manure in production grassland. Soil Biol. Biochem. 2014, 68, 1–8. [Google Scholar] [CrossRef]
- Edwards, C.A. Earthworm Ecology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004; ISBN 978-0-429-12904-9. [Google Scholar]
- Lopez-Hernandez, D.; Lavelle, P.; Fardeau, J.C.; Nino, M. Phosphorus transformations in two P-sorption contrasting tropical soils during transit through Pontoscolex corethrurus (Glossoscolecidae: Oligochaeta). Soil Biol. Biochem. 1993, 25, 789–792. [Google Scholar] [CrossRef]
- Le Bayon, R.C.; Binet, F. Earthworms change the distribution and availability of phosphorous in organic substrates. Soil Biol. Biochem. 2006, 38, 235–246. [Google Scholar] [CrossRef]
- Vos, H.M.J.; Koopmans, G.F.; Beezemer, L.; de Goede, R.G.M.; Hiemstra, T.; van Groenigen, J.W. Large variations in readily-available phosphorus in casts of eight earthworm species are linked to cast properties. Soil Biol. Biochem. 2019, 138, 107583. [Google Scholar] [CrossRef]
- Li, H.; Xiang, D.; Wang, C.; Li, X.; Lou, Y. Effects of epigeic earthworm (Eisenia fetida) and arbuscular mycorrhizal fungus (Glomus intraradices) on enzyme activities of a sterilized soil–sand mixture and nutrient uptake by maize. Biol. Fertil. Soil 2012, 48, 879–887. [Google Scholar] [CrossRef]
- Nobile, C.; Houben, D.; Michel, E.; Firmin, S.; Lambers, H.; Kandeler, E.; Faucon, M.-P. Phosphorus-acquisition strategies of canola, wheat and barley in soil amended with sewage sludges. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Osone, Y.; Ishida, A.; Tateno, M. Correlation between relative growth rate and specific leaf area requires associations of specific leaf area with nitrogen absorption rate of roots. New Phytol. 2008, 179, 417–427. [Google Scholar] [CrossRef]
- Hodgson, J.G.; Montserrat-Martí, G.; Charles, M.; Jones, G.; Wilson, P.; Shipley, B.; Sharafi, M.; Cerabolini, B.E.L.; Cornelissen, J.H.C.; Band, S.R.; et al. Is leaf dry matter content a better predictor of soil fertility than specific leaf area? Ann. Bot. 2011, 108, 1337–1345. [Google Scholar] [CrossRef]
- Madani, N.; Kimball, J.S.; Running, S.W. Improving global gross primary productivity estimates by computing optimum light use efficiencies using flux tower data. J. Geophys. Res. Biogeosci. 2017, 122, 2939–2951. [Google Scholar] [CrossRef]
- Ordoñez, J.C.; Bodegom, P.M.V.; Witte, J.-P.M.; Wright, I.J.; Reich, P.B.; Aerts, R. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Glob. Ecol. Biogeogr. 2009, 18, 137–149. [Google Scholar] [CrossRef]
- Lambers, H.; Poorter, H. Inherent variation in growth rate between pigher Plants: A search for physiological causes and ecological consequences. In Advances in Ecological Research; Begon, M., Fitter, A.H., Eds.; Academic Press: Amsterdam, The Netherlands, 1992; Volume 23, pp. 187–261. [Google Scholar]
- Gong, H.; Gao, J. Soil and climatic drivers of plant SLA (specific leaf area). Glob. Ecol. Conserv. 2019, 20, e00696. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, Y.; Yi, X.; Zhang, X.; Zhang, W. Cotton responds to different plant population densities by adjusting specific leaf area to optimize canopy photosynthetic use efficiency of light and nitrogen. Field Crops Res. 2016, 188, 10–16. [Google Scholar] [CrossRef]
- Knops, J.M.; Reinhart, K. Specific leaf area along a nitrogen fertilization gradient. Am. Midl. Nat. 2000, 144, 265–272. [Google Scholar] [CrossRef]
- Ingham, R.E.; Detling, J.K. Effects of root-feeding nematodes on aboveground net primary production in a North American grassland. Plant Soil 1990, 121, 279–281. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).