Abstract
The paper explores the impact of electromagnetic stimulation of Ulstar alfalfa seeds on fresh mass yield, the quantum efficiency of the plants’ photochemical reactions, and the content of photosynthetic pigments in leaves. Before sowing, seeds were subjected to electromagnetic stimulation in the following configurations: control (C), no stimulation; stimulation with He–Ne laser light with the wavelength of 632.8 nm, surface power density of 3 mW·cm−2 and exposition time of 1 min (L1) or 5 min (L5); stimulation with alternating magnetic field with the induction of 30 mT and exposition time of 1 min (F1) or 5 min (F5). It was established that the variability of alfalfa yields at the onset of anthesis was dependent on weather conditions as well as, above all, on the electromagnetic stimulation employed. At the same time, the values of fresh mass yield of alfalfa and photosynthetic efficiency were higher in the first year of cultivation (2014) compared to the last year (2016). In terms of photosynthetic efficiency, the best results were observed for L1 and F5, respectively: 0.801 and 0.800. The significantly highest values in terms of chlorophyll a and b and carotenoid content were observed in 2014 at the onset of budding in the combination involving alternating magnetic field stimulation (F5), and were, respectively, 30%, 28% and 73% relative to the control.
1. Introduction
Medicago sativa L. (alfalfa) is a perennial flowering plant in the Fabaceae family. It is grown mainly as fodder to be used in the form of green fodder, hay, haylage, or dry material, as well as protein–xanthophylls (PX) concentrate or Lucerne leaf extract (EFL) used in human diet, etc. [,]. The plant is grown globally, its plantations covering a total of approximately 35 million hectares [,]. Alfalfa is characterized by high nutritional value, mainly due to its high protein and mineral content []. In agriculture, it plays an important proenvironmental role due to its ability to bind surface nitrogen as well as nitrogen in deeper layers of soil to its Rhizobia-infected roots [,].
Light is an essential factor influencing photosynthesis. The process takes place in plant chloroplasts (containing stroma and thylakoids). Light-dependent photosynthesis reactions take place in the thylakoid membranes, and light-independent reactions in the stroma. Literature reports suggest that the light-dependent phase takes place in two photosystems (i.e., photosystem I–PSI and photosystem II–PSII) []. Photosynthetic pigments include chlorophylls and carotenoids which are located in thylakoid membranes. PAR (photosynthetically active radiation 400–700 nm) sunlight is absorbed in antenna complexes by molecular chlorophyll and carotenoids [,,], which is why light plays such an important role in the growth and development of plants. Its deficit results in slower growth as well as lower chlorophyll content in leaves. Excess of high intensity light can also be harmful as it inhibits photosynthesis. The phenomenon is described as photoinhibition and occurs when a plant absorbs more light than it is capable of processing through photosynthesis, which leads to damaging the photosynthetic apparatus, mainly PSII. It is also noteworthy that photoinhibition may be either reversible, when it serves as a defense mechanism for the photosynthetic system, or irreversible, when it stems from damage thereto []. The process of photosynthesis utilizes under 5% of sunlight energy. The conditions of effective photosynthesis include the participation of sunlight and suitable ambient temperature (as well as an availability of light). The process of photosynthesis can take place at temperatures ranging from slightly below 0 °C (mountain plants) to nearly 50 °C (e.g., desert plants). In temperatures exceeding 50 °C cell membranes become fluid, allowing the escape of ions (protons) from within the thylakoids. Despite the continued function of electron transport, the proton gradient driving ATP synthesis in chloroplasts is not formed. Membrane fluidity is reduced in lower temperatures, which limits the mobility of electron carriers and consequently lowers the rate of photosynthesis [].
Measurements of chlorophyll a fluorescence allow one to determine the condition of the photosynthetic apparatus and overall physiological condition of higher plants []. For this purpose, different types of fluorometers can be used which are fairly easy to operate and provide a fast and noninvasive measurement []. Chlorophyll a absorbs light at approximately 650–670 nm, and after fluorescence excitation at 740 nm [,,,].
Plant chlorophylls are among the main photosynthetic pigments responsible for the plants’ green color. They come in two types: chlorophyll a and b. Carotenoids are considered auxiliary pigments and are also divided into two types: orange carotenes and orange-yellow xanthophylls. The latter absorb light at different wavelengths (violet and blue spectral range) than chlorophylls. Sunlight energy reaches the surface of the leaf in the form of a photon and is absorbed by chlorophyll molecules present in the photosynthetic antennae. Next, the absorbed energy is transferred to the PSII and PSI reaction centers where photochemical reaction is initiated. A portion of the energy is lost in the form of heat or emitted as chlorophyll a fluorescence. Chlorophyll fluorescence is the product of the return from the excited to the baseline energy state (i.e., only red light is emitted). On average, leaves contain three times more chlorophyll a than chlorophyll b [].
Various physical methods of presowing seed treatment are increasingly used in contemporary agriculture, including laser light [,,,,,], magnetic fields [,,,], static magnetic fields [,,,], and electromagnetic fields [,,,]. As follows from literature reports, depending on the parameters, the use of electromagnetic field stimulation can yield positive, negative, or nil effects at the respective stages of plant growth and development (also depending on the particular plant species). Numerous studies point to a significant increase observed in terms of leaf size, chlorophyll content, protein content, germination capacity, plant sprout rate, plant growth rate, reduced breathing, and increased sugar content, and consequently also improved yields [,,,,]. The reported effects in terms of yield improvement influence (positively) the overall level of plant production [].
He–Ne laser emits light at the wavelength of 632.8 nm, which corresponds to the color red. Specifically, the light’s wavelength falls within the far-red range, which is very significant for physiology in the processes of phytochrome activation. In turn, changes of the photochromic forms (Pr and Pfr) stimulate certain important life processes in plants (e.g., seed germination or plant growth and development) [,]. Agrophysical lasers utilize light energy to aid plants in chemically converting the energy absorbed and stored in the seeds. This energy then fuels the processes of germination, growth, and development []. Despite the rapid advances in contemporary science, the mechanisms responsible for the effects of laser and magnetic field stimulation are yet to be fully explained. Nonetheless, laser stimulation can demonstrably increase plant bioenergetic potential, which results in higher levels of photochromic activation stimulating biochemical and physiological processes [,]. The Earth is a natural source of an alternating magnetic field constantly affecting living organisms, with the field’s intensity depending on the particular latitude (within the range from 0 to 67 μT) [,]. One of the current theories evokes to the process observed by Aksenov et al. [], where under the influence of a low frequency magnetic field, release of the esterase enzyme was observed in swelling wheat seeds, connected with their quiescence. In turn, García Reina et al. presented a hypothesis [] of interaction between magnetic fields and ionic currents inside the germ cell. Changes in ion concentration and osmotic pressure under the influence of the magnetic field regulate the seed water intake.
Literature reports mention improvement in terms of germination efficiency and seed mass under the influence of laser light stimulation in certain plants, e.g., plums [], sprouting rate and yields in alfalfa [], protein, phosphorus, and molybdenum content in alfalfa dry mass, as well as reduced content of raw fiber in the plants []. Sujak et al. [] observed both a positive and negative impact of electromagnetic stimulation on the germination of alfalfa and lupine under laboratory conditions.
Stress may be caused by excessive electromagnetic radiation, therefore, the influence of electromagnetic factors on the efficiency of photosynthesis and the content of photosynthetic pigments in particular phases of development was considered here.
An analysis of the fluorescence parameters of chlorophyll a may provide a useful tool in the study of photosynthesis under stressful or unfavorable environmental conditions. The technique is widely employed in agriculture, horticulture, forest farming, seed production, plant farming, as well as storage and processing of fruit and vegetables [].
However, there have been few papers published worldwide reporting on multiannual studies on plants such as alfalfa, a fact that inspired us to approach the important research problem of monitoring plants at various stages of vegetation. Given the above, the presented study focused on alfalfa—a multiannual crop grown primarily as an excellent source of livestock fodder. We analyzed yield fluctuations (fresh mass), photosynthetic efficiency, and related parameters in plants grown from seeds previously subjected to electromagnetic stimulation (He–Ne laser light and alternating magnetic field). Fluorescence efficiency was measured using a Mini-PAM (Photosynthesis Yield Analyzer) fluorometer that facilitates quick assessment of the condition of crops and vegetables in a given stage of development. The study was also aimed at evaluating and comparing the response of fresh mass yield levels and the photosynthetic apparatus of multiannual plants to external stressors (e.g., changing weather conditions, different rainfall levels) and to correlate the same with the employed stimulation methods.
2. Materials and Methods
The research material constituted alfalfa leaves (Medicago sativa L.) of the Ulstar cultivar. Samples were collected and measurements were performed in three distinct periods corresponding to different developmental stages of plants (Table 1) cultivated in a field experiment conducted on microplots between 2014 and 2016 using the method of randomized blocks in four replicates on 2 m2 plots, in the amount of 1600 seeds per 2 m2, into rows spaced at 20 cm and at a depth of 1 cm. The experiment was conducted at the Experimental Farm in Felin (51°13′21.9″ N, 22°37′55.85″ E), on soil classified as good wheat complex. Before sowing, alfalfa seeds were subjected to electromagnetic stimulation in the following configurations: control (no stimulation)—C; stimulation with He–Ne laser light with the wavelength of 632.8 nm and surface power density of 3 mW·cm−2, with respective exposition times of 1 min (L1) and 5 min (L5); and stimulation with an alternating magnetic field with the magnetic induction of 30 mT and exposition time of 1 min (F1) and 5 min (F5). Each sample contained 160 seeds. The surface power density was measured with the use of a Power Meter Laser (Model CTL-2001, LaserInstruments, Poland). Laser light stimulation was conducted with the use of a device wherein the laser beam was projected from above onto a single layer of seeds placed in a vessel (Figure 1). Pietruszewski’s electromagnet was used for the alternating magnetic field stimulation. The construction and operating principle of the same were as described by Muszyński et al. [].

Table 1.
Dates of the alfalfa development stages according to the BBCH scale, mean temperatures, and total rainfall in the respective decades.

Figure 1.
Device for stimulating seeds with He–Ne laser light.
The study investigated the quantum efficiency of the photochemical reaction and the content of photosynthetic pigments in leaves. Measurements of the aforementioned parameters were conducted in the third leaf from the top obtained from 10 randomly selected plants for each of the combinations. The determination of pigment content was conducted in a laboratory on samples taken from those leaves.
The quantum photochemical efficiency Yield (II) and electron transport rate (ETR) were determined with the use of Mini-PAM 2000 by WALTZ Germany. The method involved measurement of the chlorophyll a fluorescence signal. The fluorometer passed a light beam (activated and deactivated at a high frequency) through the photosynthetic sample and registered the fluorescence signal emitted by excited chlorophyll a (PSII) molecules. The quantum photochemical efficiency corresponded to the ratio of quanta used in photochemical reactions to the total number of absorbed PAR quanta [,].
The Yield parameter formula is:
where Yield is the current quantum efficiency PSII, M is the maximal fluorescence yield (M = Fm or Fm’) measured during the last saturating light pulse triggered by START, and F is the momentary fluorescence yield displaying small fluctuations.
Yield = Y/1000 = (M − F)/M
Display of the current factor applied for the calculation of the relative electron transport rate (ETR) that for a standard leaf is defined as follows:
where ETR is the relative electron transport rate and PAR is the photosynthetically active radiation.
ETR = Yield × PAR × 0.5 × 0.84
The standard factor 0.84 corresponds to the fraction of incident light absorbed by the leaf. The preset value, which corresponds to an average observed in a variety of leaf species, can be modified via SET and the arrow keys.
The chlorophyll and carotenoid content was determined by isolating these from the leaves in acetone containing 0.01% BHT (butylated hydroxytoluene) in darkness to prevent oxidation. UV–Vis spectra were measured using a double beam Carry Bio 300 spectrophotometer (Agilent Technologies, CA, United States), while pigment concentrations were calculated on the basis of the procedure described by Lichtenhalter and Buschmann [].
Moreover, the mean value of alfalfa fresh mass yield was determined at the onset of anthesis over the three years of the experiment. Each research object was performed in four replicates during the measurement.
Table 1 presents the dates of the respective developmental stages for the plants (BBCH scale used by EU states to identify stages of crop plant development), during which Mini-PAM measurements were taken and leaf samples were collected for laboratory analyses, with the specification of mean air temperatures and total rainfall for the respective decade (10 days, one month corresponds to three decades) in the three years of alfalfa vegetation periods.
The weather conditions observed during the particular stages of alfalfa development during 2014, 2015, and 2016 (Figure 2 and Figure 3) were described based on the data obtained from the agrometeorological station in Felin.
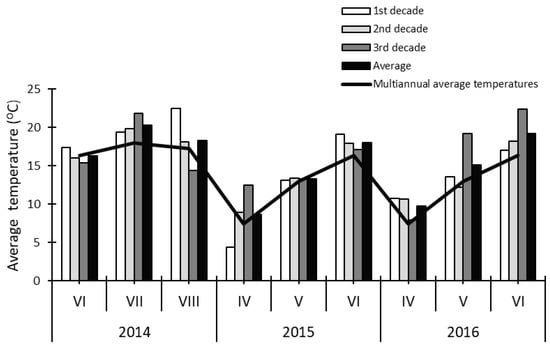
Figure 2.
Mean air temperature during the vegetation of first crop alfalfa in the year of sowing (2014) and the production years (2015, 2016) in relation to the multiannual average.
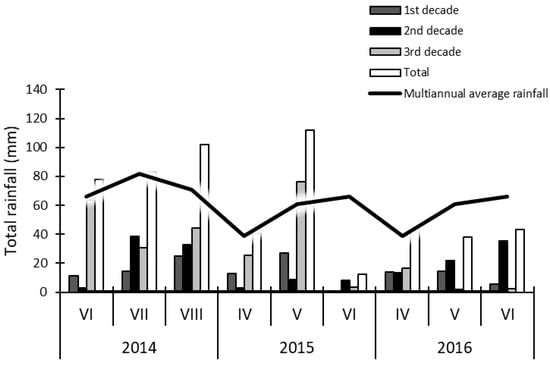
Figure 3.
Total rainfall during the vegetation of first crop alfalfa in the year of sowing (2014) and the production years (2015, 2016) in relation to the multiannual average.
The results obtained were processed by way of ANOVA analysis, single-factor repeated-measures ANOVA, LSD (littel statistical difference) NIR test at the significance level of α = 0.05 with the use of STATISTICA 13.1 software. The statistical analysis accounted for the control, electromagnetic factors, developmental stages, and years of harvest.
Single-factor repeated-measures ANOVA.
A repeated-measures ANOVA is a test similar to one-way ANOVA. The main difference is that we test related groups rather than independent groups, and the same groups of parameters are measured multiple times. In our study, time was the repeated factor [].
3. Results and Discussion
The mean air temperatures in July and August 2014, as well as in April and June of 2015 and 2016, were higher than the mean multiannual temperatures in the whole study period (Figure 2). The total rainfall in the aforementioned months was also higher in 2014 compared to the multiannual average. In May and June 2016, the recorded rainfall was lower than the multiannual average by 22.8 mm and 22.5 mm, respectively (Figure 3). Furthermore, the rainfall recorded in May 2015 was three times higher than the respective value in 2016. Rainfall and air temperature values during alfalfa vegetation observed in 2014 were higher than in other years, as it was the sowing year during which first-crop plants reached their respective developmental stages later than in the subsequent years.
Figure 4 presents the results for the obtained alfalfa fresh mass yield in the three experimental years, grown from seeds subjected to presowing electromagnetic stimulation. As we can see, the mean values of yield were decidedly higher in 2014, only to drop significantly in 2016. In the first year, the alfalfa yield under L1 and F1 stimulation was roughly the same. At the same time, they were also the highest values registered in 2014 exceeding those from the control by 24% (without statistical differences but noticeably higher yield). The fresh mass yield in the second year showed a significant increase by approximately 40% (F5) and 42% (L1). That year’s mean yield level for F1 was 17% higher compared to the control. In general, most physical factors, in particular weather conditions observed in 2014 and 2015, had a positive impact on the yields of alfalfa fresh mass, meanwhile a significant decrease of the same was observed in 2016. Weather data indicate that the significantly higher temperatures and heavy rainfall had a distinctly positive impact on the yields and the quality thereof in the first two years of cultivation. As follows from a study conducted by Ćwintal and Dziwulska-Hunek [], laser light stimulation (L) and alternating magnetic field stimulation (F) had an overall positive impact on fresh mass yields, particularly in the first year of cultivation, analogically to studies cited in this paper. In turn. Ni et al. [] reported that alfalfa yields may be significantly hampered by periods of drought. A similar correlation was observed in our study when, due to unfavorable weather conditions (reduced rainfall), the yield was significantly reduced. Another report published by Erice et al. [] reported that the activity of photosynthesis was reduced due to drought, which naturally translated to decreased yields.
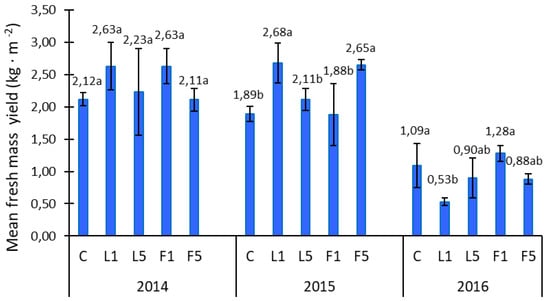
Figure 4.
Fresh mass yield alfalfa. C—control; L1—He–Ne laser, exposition time 1 min; L5—He–Ne laser, exposition time 5 min; P1—alternating magnetic field, exposition time 1 min; P5—alternating magnetic field, exposition time 5 min. Statistical differences were designated by lower-case letters a, b between control and electromagnetic stimulation.
As follows from literature, it is the Chl fluorescence index that determines the efficiency of photosynthesis. It allows one to assess the overall physiological condition of plants, but there is still no method that would allow one to determine the character of the stressor [,,,]. Fluorescence occurring with the participation of sunlight in metabolic or energetic processes may be reduced due to heat dissipation of energy [,].
The observed impact of electromagnetic stimulation of seeds on the photosynthetic efficiency in the particular stages of development and subsequent years of cultivation is presented in Table 2. The influence of electromagnetic stimulation included instances of both increase and decrease in photosynthetic activity in the particular years and stages of development. The highest and most consistent values of photosynthesis intensity were recorded at the onset of anthesis in 2014 for seeds stimulated with laser light (L1) and alternating magnetic field (F5), and were 0.801 and 0.800, respectively. For many plants grown in stress-free conditions, the ratio of Fv/Fm remained close to 0.83 [].

Table 2.
Influence of electromagnetic stimulation on photosynthetic efficiency.
The results of the present study indicate that in the year of sowing (2014), at the stage of full budding and the onset of anthesis, an upward trend in terms of photochemical efficiency was observed for all stimulation variants when compared to the control. On the other hand, in the third year of cultivation (2016), at the onset of budding, a significant decrease in photosynthetic efficiency was observed for object L5, while at the onset of anthesis, the efficiency of photosynthesis was reduced by half in the plants subjected to magnetic field stimulation (F5). However, the above may be due to stress related to significantly lower rainfall in 2016 as compared to that recorded in 2014. The decrease in photosynthetic efficiency under the described field conditions testifies to the high susceptibility of alfalfa to stress factors when subjected to the studied forms of stimulation. The fact that stressful conditions, particularly drought, do in fact impact plant growth and cause a decrease in photosynthetic activity was confirmed by Ni et al. [] and Erice et al. [].
At the onset of anthesis and in 2016 as a whole, a significant decrease in photosynthetic efficiency was observed for alternating magnetic field stimulation (F5) as compared to nonstimulated alfalfa by approximately 19% and 21%, respectively. Electromagnetic factors did not cause a significant differentiation in terms of the mean intensity of photosynthesis.
Electromagnetic stimulation did, however, have a significant effect on the differentiation of electron transport rates between the respective years (Table 3). In the year of sowing (2014), seed stimulation resulted in a decrease in electron transport relative to the control. Similar effects were observed in 2015 at the onset of budding. Meanwhile, at the stage of full budding and at the onset of anthesis in 2015, as well as in all developmental stages in 2016, an increase in electron transport was observed, with the same reaching significant levels for seeds stimulated with laser light L1 (full budding, 2015), laser light L5 (onset of anthesis, 2015), magnetic field F1 and F5 (full budding, 2016), and F5 (onset of anthesis, 2016).

Table 3.
Influence of electromagnetic stimulation on electron transport rate (ETR).
The highest increase in the ETR value, significantly different from the control, was observed for seeds subjected to magnetic field stimulation F5 (2016—onset of anthesis).
The research conducted by Li et al. [] with regard to photosynthetic efficiency and ETR relative to the watering of two alfalfa cultivars at various times, as well as to light intensity, indicated that photosynthetic efficiency decreased from 0.7 to 0.1–0.05 while ETR increased from 0 to 70. In another study including three alfalfa cultivars watered for 20 days, the photosynthetic efficiency and ETR values reported were 0.65–0.82 and 28–47, respectively []. In turn, Mouradi et al. [] reported, in a study on seed osmosis relative to water deficit levels (40% and 80%), that the fluorescence of chlorophyll (Fv/Fm) observed in alfalfa leaves was between 0.66 and 0.81, and ETR ranged from 20 to 160. In a study on the influence of mycorrhiza on the seeds of six alfalfa cultivars relative to chlorophyll fluorescence Fv/Fm, the latter was reported at the level of between 0.70 and 0.76 [].
The efficiency of photosynthesis in clover leaves relative to insolation was reported to range from 0.780 (control, shade) to 0.832 (90% insolation) []. The aforementioned results are similar to those reported herein.
An antenna complex comprises pigments (chlorophyll and carotenoids) bound to adequate proteins. When light energy is absorbed by chlorophyll a present therein, it undergoes numerous transformations and is ultimately stored and converted to chemical energy. Leaves emit fluorescent signals, 90% of which originate from the chlorophyll a molecule in photosystem II (PS II). The excited chlorophyll b energy is fully passed on to chlorophyll a (no fluorescence emission). Chlorophyll a is responsible for photosynthesis, while chlorophyll b and carotenoids are auxiliary pigment. A decrease in chlorophyll a content may lead to a reduction of photosynthetic efficiency [,,]. The study also involved the determination of the chlorophyll a and b content and carotenoid content after the use of the respective electromagnetic stimuli and relative to the year of cultivation and the phase of alfalfa development (Table 4, Table 5 and Table 6). The content of photosynthetic pigments was significantly varied relative to the analyzed factors.

Table 4.
Influence of electromagnetic stimulation on chlorophyll a content.

Table 5.
Influence of electromagnetic stimulation on chlorophyll b content.

Table 6.
Influence of electromagnetic stimulation on carotenoid content.
The concentration of chlorophyll a (Table 4) varied between 964 (C, budding, 2015) and 3584 μg ·g−1 (C, onset of anthesis, 2016). Electromagnetic stimulation showed a significant influence with regard to chlorophyll a content in the respective years of cultivation. The best effects observed in terms of increased chlorophyll a content were 19% (L1, 2016, onset of budding), 30% (F5, 2014, onset of budding, 73% (F1, 2015, full budding), and 29% for carotenoids (F5, 2016, full budding), 71% (L1, 2015, full budding), and 73% (F5, 2014, full budding). In other studies, Asghar et al. [], for example, observed that after stimulating soya seeds with a magnetic field and laser light, the content of chlorophyll in the leaves was significantly altered compared to the control. Furthermore, a study on the impact of electromagnetic stimulation on the content of chlorophyll a and carotenoids in the leaves of alfalfa, Radius (old) cultivar, revealed an increase, 2.5 (L)%–23 (F)% and 15 (L + F)%–32 (F)%, respectively, relative to the control [].
In the year of sowing, a significant increase in the chlorophyll a content was observed in alfalfa plants subjected to magnetic field stimulation F1 (full budding) and F5 (onset of and full budding). In the case of laser stimulation, a significant increase in chlorophyll a content was observed for the L1 combination at the budding stage and for L1 and L5 at the onset of anthesis.
In the second year of cultivation, at the onset of budding, a significant decrease in chlorophyll a content was observed for both alfalfa stimulated with laser light (L5) and magnetic field (F5). At the stage of full budding, an increase in the chlorophyll a content was observed for all variants of stimulation, while at the onset of anthesis the same was observed to decrease again but only in alfalfa subjected to magnetic field stimulation (F1 and F5).
In the third year of cultivation, all variants of stimulated alfalfa, with the exception of L1, were characterized by decreased chlorophyll a content either at the onset of budding (L5, F5) or at the onset of anthesis (L1, L5, F1).
Similar correlations were observed between electromagnetic stimulation and the content of chlorophyll b (Table 5).
The mean content of chlorophyll a and b varied significantly between the respective stages of alfalfa development. The lowest concentration of the same was recorded at the onset of anthesis.
The chlorophyll content in leaves primarily reflects the physiological condition of the plant, which can be responsible for reduced intensity of photosynthesis and can correlate with the decreased accumulation of organic compounds [].
The analysis of photosynthetic pigments (chlorophylls and carotenoids) conducted as part of the present study revealed instances of both decrease and increase in their respective concentrations under electromagnetic stimulation, depending on the year of cultivation and the particular stage of alfalfa development. A decrease in chlorophyll concentration was reported to result in lower photosynthetic efficiency and inhibited plant growth []. Similar effects were confirmed in the present study.
A significant discrepancy in terms of carotenoid content in alfalfa leaves under the influence of electromagnetic stimulation was observed in the respective years of cultivation (Table 6). It is noteworthy, however, that while in 2014 and 2015 increase in carotenoid content was more prevalent, in 2016 the opposite was true and decrease proved more common.
Irrespective of the electromagnetic stimuli, significant differentiation in terms of carotenoid content was observed between the respective stages of alfalfa development, with the highest values recorded for plants at the onset of anthesis. Furthermore, higher levels of the pigments were observed in alfalfa plants during production years (2015 and 2016) compared to the year of sowing (2014). As reported by Khavari-Nejad and Chaparzadeh [], the content of carotenoids in alfalfa was between 220 and 340 mg·g−1, while in a study by Owusu-Sekyere et al. [] it was between 169 and 243 mg·g−1.
Single-factor repeated-measures ANOVA.
3.1. Yield and ETR Analysis
Single-factor repeated-measures analysis of variance was employed to determine whether time had a significant impact on Yield (and, in a separate analysis, ETR), and whether changes were significant for the particular selected factor and relative to the stage of vegetation [,,].
The factor in the repeated measurements was time impacting the Yield index. The factor’s influence can be classified on three levels. The study was conducted in various vegetative stages and with the use of varying physical factors, hence the additional categorizing variables included the physical factor and stage of vegetation. The following research hypotheses were adopted:
- H0—Yield (ETR) does not change in time;
- H0—Yield (ETR) does not depend on the physical factor;
- Yield (ETR) does not depend on the stage of vegetation.
3.2. Yield Analysis
The results of the sphericity test (Mauchly’s test) indicated that the sphericity condition was satisfied (W = 0.812). For the “time” effect, the probability (p = 0.0) was lower than the adopted significance level, which indicated that time significantly influenced the Yield parameter. The Greenhouse–Geisser (G–G) and Huynh–Feldt (H–F) tests corroborated the results of single-parameter tests; the p for time was lower than the adopted significance level (α = 0.05). All the performed tests indicated that the physical factor and stage of vegetation also showed a significant impact on the changes of the Yield parameter. Figure 5 and Figure 6 present the interaction graph. Parallel (noncrossing) lines represent no interaction between the factors, while crossing lines indicate interaction between the corresponding parameters.
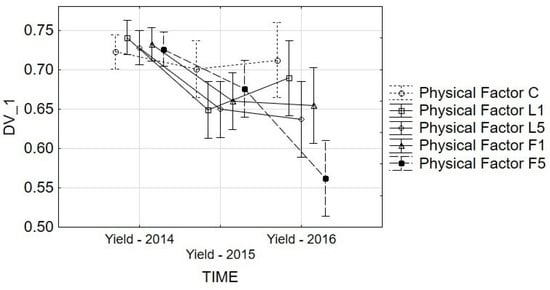
Figure 5.
Repeated-measures ANOVA for Yield: interaction graphs for time and physical factor. Vertical bars denote 0.95 confidence intervals. Estimated Marginal Means: TIME*Physical Factor. F(8, 270) = 3.2319, p = 0.00158. C—control; L1—He–Ne laser, exposition time 1 min; L5—He–Ne laser, exposition time 5 min; P1—alternating magnetic field, exposition time 1 min; P5—alternating magnetic field, exposition time 5 min.
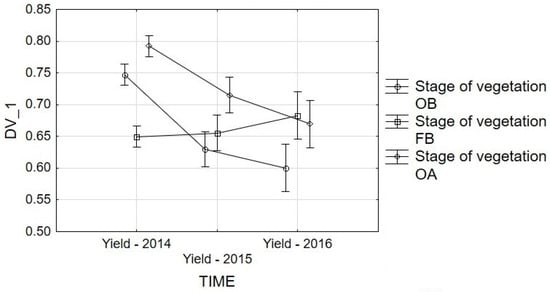
Figure 6.
Repeated-measures ANOVA for Yield: interaction graphs for time and stage of vegetation. Vertical bars denote 0.95 confidence intervals. Vertical bars denote 0.95 confidence intervals. Estimated Marginal Means: TIME*Stage of vegetation. F(4, 270) = 11.057, p = 0.00. Stages of vegetation: OB, onset of budding; FB, full budding; OA, onset of anthesis.
3.3. ETR Analysis
The results of the sphericity test (Mauchly’s test) indicated that the sphericity condition was satisfied (W = 0.939). For the “time” effect the probability (p = 0.11) was higher than the adopted significance level, which indicated that time did not significantly influence the ETR parameter. The Greenhouse–Geisser (G–G) and Huynh–Feldt (H–F) tests corroborated the results of single-parameter tests; the p for time was higher than the adopted significance level (α = 0.05).
All the performed tests indicated that the physical factor and stage of vegetation also showed a significant impact on the changes of the ETR parameter. Figure 7 and Figure 8 present the interaction graph. Parallel (noncrossing) lines represent no interaction between the factors, while crossing lines indicate interaction between the corresponding parameters.
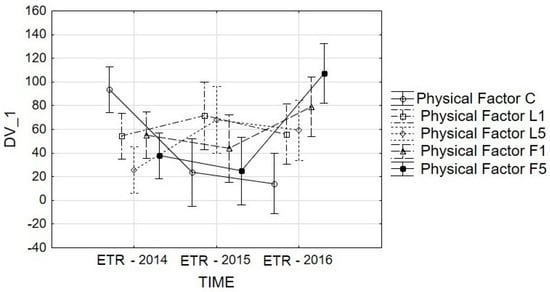
Figure 7.
Repeated-measures ANOVA for ETR: interaction graphs for time and physical factor. Vertical bars denote 0.95 confidence intervals. Estimated Marginal Means: TIME*Physical Factor. F(8,270) = 6.8785, p = 0.00. C—control; L1—He–Ne laser, exposition time 1 min; L5—He–Ne laser, exposition time 5 min; P1—alternating magnetic field, exposition time 1 min; P5—alternating magnetic field, exposition time 5 min.
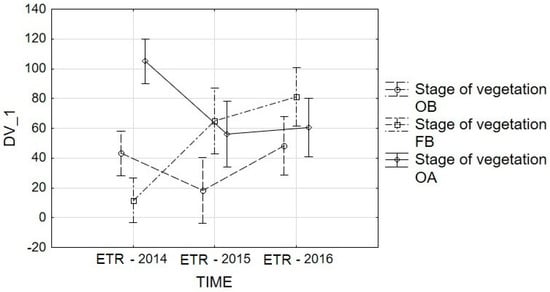
Figure 8.
Repeated-measures ANOVA for ETR: interaction graphs for time and stage of vegetation. Vertical bars denote 0.95 confidence intervals. Estimated Marginal Means: TIME*Stage of vegetation. F(4, 270) = 10.946, p = 0.00. Stages of vegetation: OB, onset of budding; FB, full budding; OA, onset of anthesis.
4. Conclusions
Fluctuations in terms of fresh mass yields and photosynthetic efficiency at the onset of anthesis depended both on weather conditions and, above all, the electromagnetic stimulation employed. It was observed that both parameters were higher on the first year of the experiment (2014) compared to the last year thereof (2016). Photosynthetic efficiency can therefore serve as a relatively good indicator of the plant’s expected yield. Moreover, the registered electron flow in the process of photosynthesis was also the highest in 2014 and the lowest in 2016, which also had a bearing on the yield. Fluctuations of fluorescence emission or its proportion (as well as estimation of the chlorophyll content) can serve as measures of the plant’s response to experience stressors. However, the interpretation of the results obtained does not, given the current state of the art, allow one to conclusively determine how the same impacts functional disorders of PSII.
We could also clearly observe that, depending on the year, Yield (II) decreased in the first and third stage of the plant development but observably increased in the second stage. This fact is clearly related to the energy needs of this alfalfa cultivar in the respective stages of its development. The applied stressors showed the highest impact (in terms of this parameter) in the first year of cultivation.
As shown, in the case of this alfalfa cultivar we observed a trend in the subsequent years (for the full budding phase) with regard to changes (increase) in electron transfer efficiency similar to that for Yield (II). This could suggest that as the plant develops (in subsequent years), a significant improvement of its condition may be observed, which in turn could affect its yield. Electromagnetic stimulation significantly increased the chlorophyll a and b content in the year of sowing but decreased the same in the subsequent years of cultivation. A significantly higher content of carotenoids in alfalfa leaves was observed at the onset of anthesis and in the second and third year of cultivation. Moreover, between the respective years the content of chlorophyll a and b in the budding phases increased observably, particularly between the second and third measurement years. Meanwhile, the carotenoid content consistently decreased in the first of the analyzed phases and increased in the second and third phase. Variability in terms of photosynthetic pigment content in the subsequent measurement years may also suggest good adaptation of this alfalfa cultivar to the changing environmental conditions of cultivation.
The use of electromagnetic stimulation could be effective to increase crop yields and improve photosynthetic performance, although more research is needed.
Author Contributions
Conceptualization, A.D.-H.; Data curation, A.D.-H., A.K.-G., A.N., and A.M.; Formal analysis, A.D.-H. and A.K.-G.; Funding acquisition, A.D.-H., A.N., and A.M.; Investigation, A.D.-H., A.K.-G., A.N., and A.M.; Methodology, A.N.; Project administration, A.D.-H. and A.M.; Resources, A.D.-H. and A.N.; Software, A.K.-G. and A.N.; Supervision, A.D.-H. and A.M.; Validation, A.K.-G.; Writing—original draft, A.D.-H., A.K.-G., A.N., and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The University of Life Sciences in Lublin.
Acknowledgments
The research of Agnieszka Niemczynowicz in publication was written as a result internship in Valencia, Spain, cofinanced by the European Union under the European Social Fund (Operational Program Knowledge Education Development), carried out in the project Development Program at the University of Warmia and Mazury in Olsztyn (POWR.03.05. 00-00-Z310/17). The authors Agnieszka Niemczynowicz and Arkadiusz Matwijczuk acknowledge the Cost project CA 15126.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Annicchiarico, P.; Pecetti, L.; Tava, A. Physiological and morphological traits associated with adaptation of lucerne (Medicago sativa) to severely drought-stressed and to irrigated environments. Ann. Appl. Biol. 2013, 162, 27–40. [Google Scholar] [CrossRef]
- Hayes, R.; Li, G.D.; Conyers, M.K.; Virgona, J.M.; Dear, B.S. Lime increases productivity and the capacity of Lucerne (Medicago sativa L.) and phalaris (Phalaris aquatic L.) to utilize stored soil water on an acidic soil in south-eastern Australia. Plant Soil 2016, 400, 29–43. [Google Scholar] [CrossRef]
- Le, X.H.; Franco, C.M.; Ballard, R.A.; Drew, E.A. Isolation and characterization of endophytic actinobacteria and their effect on the early growth and nodulation of lucerne (Medicago sativa L.). Plant Soil 2016, 405, 13–24. [Google Scholar] [CrossRef]
- He, S.; Liu, G.; Yang, H. Water Use Efficiency by Alfalfa: Mechanisms Involving Anti-Oxidation and Osmotic Adjustment under Drought. Russ. J. Plant Physiol. 2012, 59, 348–355. [Google Scholar] [CrossRef]
- Marley, C.L.; Fychan, R.; Theobald, V.J.; Cuttle, S.P.; Sanderson, R. Effects of a winter or spring sowing date on soil nitrogen utilization and yield of barley following a forage crop of red clover, lucerne or hybrid ryegrass. Agric. Ecosyst. Environ. 2013, 181, 213–222. [Google Scholar] [CrossRef]
- Bilodeau, S.E.; Wu, B.S.; Rufyikiri, A.S.; MacPherson, S.; Lefsrud, M. An update on plant photobiology and implications for cannabis production. Front. Plant Sci. 2019, 10, 296. [Google Scholar] [CrossRef]
- Hall, D.O.; Rao, K.K. Fotosynteza; WNT: Warszawa, Poland, 1999. (In Polish) [Google Scholar]
- Kalaji, M.H.; Łoboda, T. Fluorescencja Chlorofilu w Badaniach Stanu Fizjologicznego Roślin Wydawnictwo; SGGW: Warszawa, Poland, 2010. (In Polish) [Google Scholar]
- Alves, P.L.; Barja, P.R.; Magalhães, A.C.N. The phenomenon of photoinhibition of photosynthesis and its importance in reforestation. Bot. Rev. 2002, 68, 193–208. [Google Scholar] [CrossRef]
- Ptushenko, V.V.; Ptushenko, O.S.; Tikhonov, A.N. Chlorophyll fluorescence induction, chlorophyll content and chromaticity characteristics of leaves as in Arboreous plants. Biochemistry 2014, 79, 260–272. [Google Scholar]
- Sulkiewicz, M.; Ciereszko, I. Fluorescencja chlorofilu a–historia odkrycia i zastosowanie w badaniach roślin. KOSMOS Probl. Nauk Biol. 2016, 65, 103–115. (In Polish) [Google Scholar]
- Chen, Y.P.; Jia, J.F.; Yue, M. Effect of CO2 Laser Radiation on Physiological Tolerance of Wheat Seedlings Exposed to Chilling Stress. Photochem. Photobiol. 2010, 86, 600–605. [Google Scholar] [CrossRef]
- Hernandez, A.C.; Domínguez, P.A.; Cruz-Orea, A.; Ivanov, R.; Carballo, C.A.; Zepeda, B.R.; Galindo, S.L. Laser irradiation effects on field performance of maize seed genotypes. Int. Agrophys. 2009, 23, 327–332. [Google Scholar]
- Hernández, A.C.; Domínguez, P.A.; Cruz, O.A.; Ivanov, R.; Carballo, C.A.; Zepeda, B.R. Laser in agriculture. Int. Agrophys. 2010, 24, 407–422. [Google Scholar]
- Perveen, R.; Al, I.Q.; Ashraf, M.; Al-Qurainy, F.; Jamil, Y.; Ahmad, M.R. Effects of Different Doses of Low Power Continuous Wave He-Ne Laser Radiation on Some Seed Thermodynamic and Germination Parameters, and Potential Enzymes Involved in Seed Germination of Sunflower (Helianthus annuus L.). Photochem. Photobiol. 2010, 86, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.S.H.; Harith, M.A. Effects of laser biostimulation on germination of Acacia Farnesiana (L.). Willd. Acta Hortic.(ISHS) 2010, 854, 41–50. [Google Scholar] [CrossRef]
- Dziwulska-Hunek, A.; Kornarzyński, K.; Matwijczuk, A.; Pietruszewski, S.; Szot, B. Effect of laser and variable magnetic field simulation on amaranth seeds germination. Int. Agrophys. 2009, 23, 229–235. [Google Scholar]
- Matwijczuk, A.; Kornarzyński, K.; Pietruszewski, S. Effect of magnetic field on seed germination and seedling growth of sunflower. Int. Agrophys. 2012, 26, 271–278. [Google Scholar] [CrossRef]
- Moon, J.D.; Chung, H.S. Acceleration of germination of tomato seed by applying AC electric and magnetic fields. J. Electrost. 2000, 48, 103–114. [Google Scholar] [CrossRef]
- Nechitailo, G.; Gordeev, A. The use of an electric field in increasing the resistance of plants to the action of unfavorable space flight factors. Adv. Space Res. 2004, 34, 1562–1565. [Google Scholar] [CrossRef]
- Cakmak, T.; Rahmi, D.; Serkan, E. Acceleration of germination and early growth of wheat and bean seedlings grown under various magnetic field and osmotic conditions. Bioelectromagnetics 2010, 31, 120–129. [Google Scholar] [CrossRef]
- Kataria, S.; Baghel, L.; Guruprasad, K.N. Pre-treatment of seeds with static magnetic field improves germination and early growth characteristics under salt stress in maize and soybean. Biocatal. Agric. Biotechnol. 2017, 10, 83–90. [Google Scholar] [CrossRef]
- Vashisth, A.; Nagarajan, S. Exposure of Seeds to Static Magnetic Field Enhances Germination and Early Growth Characteristics in Chickpea (Cicer arietinum L.). Bioelectromagnetics 2008, 29, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Yano, A.; Ohashi, Y.; Hirasaki, T.; Fujiwara, K. Effects of a 60 Hz Magnetic Field on Photosynthetic CO2 uptake and Early Growth of Radish Seedlings. Bioelectromagnetics 2004, 25, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, P.A.; Hernandez, A.C.; Cruz, O.A.; Ivanov, R.; Carballo, C.A.; Zepeda, B.R.; Martínez, O.E. Influences of the electromagnetic field in maize seed vigor. Rev. Fitotec. Mex. 2010, 33, 183–188. (In Spanish) [Google Scholar]
- Galland, P.; Pazur, A. Magnetoreception in plants. J. Plant Res. 2005, 118, 371–389. [Google Scholar] [CrossRef]
- Hernandez, A.C.; Dominguez, P.A.; Carballo, C.A.; Cruz, O.A.; Ivanov, R.; López, B.J.L.; Valcarcel, M.J.P. Alternating magnetic field irradiation effects on three genotype maize seed field performance. Acta Agrophys. 2009, 170, 7–17. [Google Scholar]
- Zepeda, B.R.; Hernández, A.C.; Domínguez, P.A.; Cruz, O.A.; Godina, N.J.J.; Martínez, O.E. Electromagnetic field and seed vigour of corn hybrids. Int. Agrophys. 2010, 4, 329–332. [Google Scholar]
- Bujak, K.; Frant, M. Influence of pre-sowing seed stimulation with magnetic Field on spring wheat yielding. Acta Agrophys. 2009, 14, 19–29. (In Polish) [Google Scholar]
- Nimmi, V.; Madhu, G. Effect of pre-sowing treatment with permanent magnetic field on germination and growth of chilli (Capsicum annum. L.). Int. Agrophys. 2009, 23, 195–198. [Google Scholar]
- Pietruszewski, S.; Muszyński, S.; Dziwulska, A. Electromagnetic fields and electromagnetic radiation as non-invasive external simulations for seeds (selected methods and responses). Int. Agrophys. 2007, 21, 95–100. [Google Scholar]
- Greenebaum, B.; Barnes, F. Bioengineering and Biophysical Aspects of Electromagnetic Fields; CRC Press: Boca Raton, FL, USA, 2018; Available online: https://doi.org/10.1201/9781315186580 (accessed on 10 April 2020).
- Gutiérrez Cruz, D.; Zepeda Bautista, R.; Hernández Aguilar, C.; Domínguez Pacheoo, F.A.; Cruz Orea, A.; López Bonilla, J.L. Physical characteristic of grains of maize pre-sowing treated by electromagnetic fields. Acta Agrophys. 2011, 18, 17–31. [Google Scholar]
- Nobel, P. Physicochemical and Environmental Plant Physiology, 4th ed.; Akademic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Krawiec, M.; Dziwulska-Hunek, A.; Kornarzyński, K. The use of physical factors for seed quality improvement of horticultural plants. J. Hortic. Res. 2018, 26, 81–94. [Google Scholar] [CrossRef]
- Gładyszewska, B. Estimation of a laser biostimulation dose. Int. Agrophys. 2011, 25, 403–405. [Google Scholar]
- Vasilevski, G. Perspectives of the application of biophysical methods in sustainable agriculture. Bulg. J. Plant Physiol. 2003, 29, 179–186. [Google Scholar]
- Belyavskaya, N.A. Biological effects due to weak magnetic field on plants. Adv. Space Res. 2004, 34, 1566–1574. [Google Scholar] [CrossRef]
- Aksenov, S.I.; Buluchev, A.A.; Grunina, T.Y.; Turovetskii, V.B. Mechanisms of the action of a low-frequency magnetic field on the initial stages of germination of wheat seeds. Biophysics 1996, 41, 931–937. [Google Scholar]
- García Reina, F.; Arza Pascual, L.; Almanza Fundora, I. Influence of a stationary magnetic field on water relations in lettuce seeds. Part II: Experimental results. Bioelectromagnetics 2001, 22, 596–602. [Google Scholar] [CrossRef]
- Nasiri, A.A.; Mortazaeinezhad, F.; Taheri, R. Seed germination of medicinal sage is affected by gibberellic acid, magnetic field and laser irradiation. Electromagn. Biol. Med. 2018, 37, 50–56. [Google Scholar] [CrossRef]
- Ćwintal, M.; Dziwulska-Hunek, A. Effect of electromagnetic stimulation of alfalfa seeds. Int. Agrophys. 2013, 27, 391–401. [Google Scholar] [CrossRef][Green Version]
- Ćwintal, M.; Dziwulska-Hunek, A.; Wilczek, M. Laser stimulation effect of seeds on quality of alfalfa. Int. Agrophys. 2010, 24, 15–19. [Google Scholar]
- Sujak, A.; Dziwulska-Hunek, A.; Reszczyńska, E. Effect of electromagnetic stimulation on selected Fabaceae Plants. Pol. J. Environ. Stud. 2013, 22, 893–898. [Google Scholar]
- Muszyński, S.; Gagoś, M.; Pietruszewski, S. Short-term pre-germination exposure to ELF magnetic field does not influence seedling growth in Durum Wheat (Triticum durum). Pol. J. Environ. Stud. 2009, 18, 1065–1072. [Google Scholar]
- Center, M.D.; Dąbrowski, P.; Samborska, I.A.; Łukasik, I.; Sowczyna, T.; Pietkiewicz, S.; Bąba, W.; Kalaji, H.M. Application of chlorophyll fluorescence measurements in environmental studies (in Polish). Kosmos Probl. Nauk Biol. 2016, 65, 197–205. [Google Scholar]
- Lichtenthalter, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-Vis Spectroscopy. In Current Protocols in Food Analytical Chemistry; Wiley & Sons. Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Brereton, R.G. Chemometrics: Data Analysis for the Laboratory and Chemical Plant; John Wiley: Chichester, UK, 2003. [Google Scholar]
- Ni, Y.; Guo, Y.J.; Guo, Y.J.; Han, L.; Tang, H.; Conyers, M. Leaf cuticular waxes and physiological parameters in alfalfa leaves as influenced by drogught. Photosynthetica 2012, 50, 458–466. [Google Scholar] [CrossRef]
- Erice, G.; Louahlia, S.; Irigogen, J.J.; Sánchez-Díaz, M.; Alami, I.T.; Avice, J.C. Water use efficiency, transpiration and net CO2 exchange of four alfalfa genotypes submitted to progressive drought and subsequent recovery. Environ. Exp. Bot. 2011, 72, 123–130. [Google Scholar] [CrossRef]
- Dziwulska-Hunek, A.; Ćwintal, M.; Niemczynowicz, A.; Boroń, B.; Matwijczuk, A. Effect of stress caused by electromagnetic stimulation on the fluorescence lifetime of chlorophylls in alfalfa leaves. Pol. J. Environ. Stud. 2019, 28, 3133–3143. [Google Scholar] [CrossRef]
- Schurr, U.; Walter, A.; Rascher, U. Functional dynamic of plant growth and photosynthesis—From steady state to dynamics—From homogeneity to heterogeneity. Plant Cell Environ. 2006, 29, 340–352. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef]
- Li, W.; Zhang, S.; Shan, L. Responsibility of non-stomatal limitations for the reduction of photosynthesis—Response of photosynthesis and antioxidant enzyme characteristics in alfalfa (Medicago sativa L.) seedlings to water stress and rehydration. Front. Agric. China 2007, 1, 255–264. [Google Scholar] [CrossRef]
- Smethurst, C.; Garnett, T.; Shabala, S. Nutritional and chlorophyll fluorescence responses of lucerne (Medicago sativa) to waterlogging and subsequent recovery. Plant Soil 2005, 270, 31–45. [Google Scholar] [CrossRef]
- Mouradi, M.; Bouizgaren, A.; Farissi, M.; Latrach, L.; Qaddoury, A.; Ghoulam, C. Seed osmopriming improves plant growth, nodulation, chlorophyll fluorescence and nutrient uptake in alfalfa (Medicago sativa L.)—Rhizobia symbiosis under drought stress. Sci. Hortic. 2016, 213, 232–242. [Google Scholar] [CrossRef]
- Hwang, S.F.; Wang, H.; Gossen, B.D.; Chang, K.F.; Turnbull, G.D.; Howard, R.J. Impact of foliar diseases on photosynthesis, protein content and seed yield of alfalfa and efficacy of fungicide application. Eur. J. Plant Pathol. 2006, 115, 389–399. [Google Scholar] [CrossRef]
- Mauro, R.P.; Occhipinti, A.; Longo, A.M.G.; Mauromicale, G. Effects of shading on chlorophyll content, chlorophyll fluorescence and photosynthesis of subterranean clover. J. Agron. Crop Sci. 2011, 197, 57–66. [Google Scholar] [CrossRef]
- Cetin, M. Change in Amount of chlorophyll in some interior ornamental plants. Kast. Univ. J. Eng. Sci. 2017, 3, 11–19. [Google Scholar]
- Singh, S.K.; Reddy, V.R.; Fleisher, D.H.; Timlin, D.J. Relationship between photosynthetic pigments and chlorophyll fluorescence in soybean under varying phosphorus nutrition at ambient and elevated CO2. Photosynthetica 2017, 55, 421–433. [Google Scholar] [CrossRef]
- Asghar, T.; Jamil, Y.; Iqbal, M.; Abbas, M. Laser light and magnetic field stimulation effect on biochemical, enzymes activities and chlorophyll contents in soybean seeds and seedlings during early growth stages. J. Photochem. Photobiol. B Biol. 2016, 165, 283–290. [Google Scholar] [CrossRef]
- Bielinis, E.; Jóźwiak, W.; Robakowski, P. Modeling of the relationship between the SPAD values and photosynthetic pigments content in Queraus petraea and Prunus serotina leaves. Dendrobiology 2015, 73, 125–134. [Google Scholar] [CrossRef]
- Khavari-Nejd, R.A.; Chaparzadeh, N. The effects of NaCl and CaCl2 on photosynthesis and growth of alfalfa plants. Photosynthetica 1998, 35, 461–466. [Google Scholar] [CrossRef]
- Owusu-Sekyere, A.; Kontturi, J.; Hajiboland, R.; Rahmat, S.; Aliasgharzad, N.; Hartikainen, H.; Seppänen, M.M. Influence of selenium (Se) on carbohydrate metabolism, nodulation and growth in alfalfa (Medicago sativa L.). Plant Soil 2013, 373, 541–552. [Google Scholar] [CrossRef]
- Lúcio, A.D.C.; Couto, M.R.M.; Lopes, S.J.; Storck, L. Transformação box-cox em experimentos com pimentão em ambiente protegido. Hortic. Bras. 2011, 29, 38–42. [Google Scholar] [CrossRef][Green Version]
- Piepho, H.P. Data transformation in statistical analysis of field trials with changing treatment variance. Agron. J. 2009, 101, 865–869. [Google Scholar] [CrossRef]
- Sari, B.G.; Dal’Col Lúcio, A.; Santana, C.S.; Olivoto, T.; Diel, M.I.; Krysczun, D.K. Nonlinear growth models: An alternative to ANOVA in tomato trials evaluation. Eur. J. Agron. 2019, 104, 21–36. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).