Abstract
Nitrogen (N) plays an important role in sugarcane (Saccharum spp. hybrids) growth and development; however, long-term effects of N application levels on cane and sugar production in different sugarcane cultivars under field conditions remain unclear. In this study, we investigate the agronomic, yield, and quality traits in three sugarcane cultivars (GT11, B9, and ROC22) under different N levels (0, 150, and 300 kg/ha urea) from 2015 to 2019. Continuous four-year field experiments of plant and ratoon crops were carried out by using two-factor split-plot design. The results showed that N fertilizer application improved the tillering rate, stalk diameter, plant height, stalk weight, millable stalks/ha, cane yield, sugar yield and juice rate of cane, and the difference between N application and non-N application was significant. The cane yield, millable stalks/ha, juice rate, and juice gravity purity increased with the increase of N application, but the milled juice brix and sucrose % cane decreased with the increase of N application. The sugar yield was the highest at 150 kg/ha urea application, while the cane yield was the highest at 300 kg/ha urea application. Different N fertilizer application levels significantly regulated the activities of glutamic pyruvic transaminase (GPT) and glutamic oxaloacetic transaminase (GOT) and the contents of chlorophyll and nitrate N in plant leaves, which reflected the regulation in nitrogen metabolism and alteration in dry matter production and distribution, cane yield and sugar accumulation in different sugarcane cultivars. During the four-year experiment duration, the cane yield and sugar yield generally showed ROC22 > B9 > GT11. These data suggested that 300 kg/ha urea application was suitable for the plant and first ratoon crops, and 150 kg/ha urea application was suitable for the second and third ratoon crops. Both cane and sugar yields could be the highest in a four-year production cycle under this circumstance.
1. Introduction
China is one of the main sugar producers in the world. Guangxi is the largest sugarcane and sugar-producing area in China, whose sugar production accounts for more than 60% of the total in China [1]. Most sugarcane in Guangxi is mainly planted on dry sloping land without irrigation conditions, and soil fertility is low. The raining season is hot, and the soil nutrients are easily lost. In addition, the amount of chemical fertilizer application is large, generally more than 600 kg/ha nitrogen (N), which is more than twice the world average level, and the utilization rate of N fertilizer is low [1,2]. In the past fifteen years, the main sugarcane cultivar in Guangxi was ROC22, whose planting area accounts for 68% of the total sugarcane planting area, even more than 95% in some areas. Now the cultivar ROC22 has been degenerating, and the occurrence of borer damage and smut is serious [3,4].
Previous studies have confirmed that proper application of N fertilizer is beneficial to the plant growth and yield formation in sugarcane, but the increasing application of N fertilizer will accelerate the loss of N in the farmland; only part of N fertilizer will be absorbed by sugarcane [5]. Usually, only 30%–50% of N fertilizer is absorbed and utilized by crops to maintain the normal growth of plants [6]. Excessive application of N fertilizer will reduce the nitrogen use efficiency (NUE), and make a large amount of N leached away through rainwater and other ways, polluting the environment, resulting in serious soil acidification, unbalanced nutrients, and potential negative impact on soil carbon cycle [7,8,9,10].
Sugarcane growth and development need various organic matters supplied by N metabolism. Glutamic pyruvic transaminase (GPT) and glutamic oxaloacetic transaminase (GOT) are the two key enzymes involved in transamination, and their activities can reflect the synthesis level of amino acids in the process of N metabolism in plants [11]. Nitrate N is an important N source for plants; it can be converted into ammonium N after being reduced, which is catalyzed by nitrate reductase to further synthesize amino acids or amides [11]. The content of nitrate N in plants can reflect the supply level of soil N and the N nutrition level in plants. Nitrate N content increased in wheat leaves with the N supply increasing [12]. N fertilizer plays an irreplaceable role in crop production [13]. Early application of N fertilizer can promote plant elongation and dry matter accumulation, which was beneficial to the formation of sugar yield in sugarcane [14,15]. There is a positive correlation between N uptake and dry matter accumulation in sugarcane [16]. The proportion of N in sugarcane stalks and dead leaves increased with the increase of N application rate. N would give priority to the shoot when N supply was sufficient [17]. The distribution of N and biomass in the leaves was very similar to that in the stalks in sugarcane under different N fertilization rates [18]. The more nitrogen was distributed to leaves, the more favorable for sugarcane growth under low N condition [19]. The biomass of leaves significantly increased with the increasing of N application rate within a certain extent [20]. Although many studies have reported the effects of N on the nutrient uptake and NUE in crops [13,21,22], the relationship between sugarcane yield and NUE remains unclear. It has been pointed out that there are significant differences in N uptake and utilization in different sugarcane cultivars, and even in the same organ at different growth stages [23,24,25]. The selection and application of cultivars with high NUE have become an effective way to decrease N fertilizer application, stabilize yield, and improve NUE [26,27]. It was reported that sugarcane yield showed an upward trend within a certain level of N fertilizer application rate and reached a peak at 276 kg/ha N application rate [28]. N fertilization is an important N nutrition source for the early growth stage, and up to 40% of N comes from fertilization during this stage, while only about 10% of N comes from fertilization at the mature stage in sugarcane [29,30]. N loss is more easily caused by a single application of N fertilizer as a basal dress, and the NUE is low [31].
B9 is a sugarcane cultivar selected by the Sugarcane Research Institute of Guangxi Academy of Agricultural Sciences. Field experiments showed that there was no significant difference in cane yield treated with urea between 150–300 kg/ha N application, but there was a significant difference between the N application and non-N application for the cultivar B9 [32]. Xing et al. [33] reported that nitrogenase activities in B9 were higher than other sugarcane cultivars. Zhang et al. [11] conducted a field experiment using 3 sugarcane cultivars viz. ROC22, GT11, and B9 under three levels of urea fertilization (150, 300, and 600 kg/ha) in 2013 (plant cane) and 2014 (ratoon cane), and found that the N metabolism in sugarcane was significantly enhanced with the increase of N level within the urea application rates of 150–300 kg/ha. The sugarcane cultivar B9 has a stronger regulative and adaptive ability in a low N environment, and 300 kg urea application might achieve the best comprehensive benefits for both cane and sugar production [11]. Yuan et al. [34] used three sugarcane cultivars (ROC22, GT11, and B9) as materials to carry out experiments with three different N levels (0, 150, 300 kg/ha urea), and found that the photosynthetic rate of sugarcane was closely related to chloroplast number, chloroplast ultrastructure, and grana lamella number, and these traits were affected by sugarcane cultivars and N application rates.
In the present study, three sugarcane cultivars (GT11, B9 and ROC22) and three N levels (0, 150, 300 kg/ha urea) were used to grow one-year plant crop and three-year ratoon crops to study the long-term effects of different N levels on growth, yield, quality, and N metabolism in different sugarcane cultivars. The purpose of this study is to provide a reference for N fertilizer application in sugarcane production, to reduce N application rate, improve N utilization efficiency, and reduce sugarcane production cost and environmental pollution caused by excessive application of N fertilizer.
2. Materials and Methods
2.1. Materials and Growth Conditions
Three sugarcane cultivars that are currently used in commercial production, GT11, B9, and ROC22 were selected. Urea (N 46%, product of Guangxi Hechi Chemical Fertilizer Company, China) was used as the nitrogenous fertilizer. Calcium magnesium phosphate fertilizer (P2O5 18.8%) and potassium chloride fertilizer (K2O 60%) were applied as recommended for common commercial sugarcane production. The present study was conducted in the experimental field of College of Agriculture, Guangxi University, Nanning, Guangxi, China (longitude: 108°19′ E; latitude: 22°49′ N) from 2015 to 2018. The experimental spot is located in the subtropical monsoon climate zone; the average temperature, rainfall, and sunshine from 2015 to 2018 are 22.0 °C, 1434.4 mm, and 1532.9 h, respectively. The precipitation from June to September accounted for 54% of the total precipitation (Figure 1). The soil is upland red loam and the former crop was sugarcane. The 5-point sampling method was used to take soil samples from row to row (0–30 cm depth) before fertilization in 2015, 2016, 2017, and 2018, respectively. Soil samples were dried, ground, and screened with a 1-mm sieve before analysis. The soil physical and chemical properties are shown in Table 1.
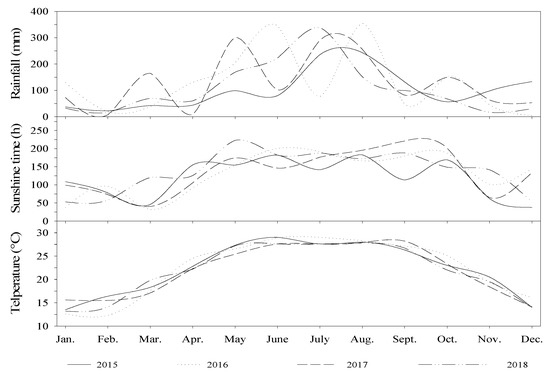
Figure 1.
The rainfall, temperature, and sunshine in the experimental field from 2015 to 2018.

Table 1.
The soil chemical properties before fertilization from 2015 to 2018.
2.2. Experimental Design
A two-factor split-plot design was adopted in this experiment. N application levels were set as the main plot, and three levels, 0, 150, and 300 kg/ha urea, were denoted as U1, U2, and U3, respectively. The same amounts of calcium magnesium phosphate fertilizer (600 kg/ha) and potassium chloride fertilizer (300 kg/ha) were applied for all the treatments. Three sugarcane cultivars (GT11, B9, and ROC22) were set as the subplot. Each subplot had 7 rows with 6.5 m in length, 1.2 m in spacing, and 54.6 m2 in area. Protective rows were set around the experimental field.
Sugarcane planting was carried out on 2 March 2015. Sugarcane seedcanes were cut into double bud setts and soaked in 50% carbendazim 800 times solution for 15 min before planting. The planting density was 105,000 buds/ha. Fertilizer was applied two times as basal and dressing fertilizations, respectively, in 2015 (plant crop). Then, 50% urea (75, 150 kg/ha for U2, U3, respectively) and all the calcium magnesium phosphate fertilizer (600 kg/ha) were applied as basal fertilizers. Additionally, the remaining 50% urea and all the potassium chloride fertilizer (300 kg/ha) were applied on 15 May 2015. For ratoon crops from 2016 to 2018, all the fertilizers, including urea, calcium magnesium phosphate, and potassium chloride, were applied at the same time in April of each year.
2.3. Investigation of Pests, Diseases, and Agronomic and Yield Traits
Investigations were done for withered heart incidence rate, smut incidence rate, emergence rate (shooting rate for ratoon crop), and tillering rate during the experiment. The plant shoots and withered heart and smut incidences were investigated from April to June every year. The withered heart incidence rate, smut incidence rate, emergence rate (shooting rate for ratoon crop), and tillering rate were calculated, respectively. Plant height, stalk diameter, growth speed, stalk weight, millable stalks/ha, cane yield, and sugar yield were investigated. Fifteen main shoots were selected from each subplot for observation, the plant height was measured, and the growth speed (cm/d) was calculated from April to October every year. Fifteen millable stalks were randomly cut from each subplot before harvesting, and their plant height and stalk diameter were measured. The actual cane yield in each subplot was weighed after harvest, and the millable stalks/ha and stalk weight were investigated.
2.4. Quality and Physiological Analyses
Field brix was measured in October, November, and December of each year by a double scale concentration meter (PAL-BX/RI, Atago, Japan). Fifteen sugarcane plants were selected randomly from each subplot every month to measured relative chlorophyll content (SPAD value) in leaf +1 (top visible dewlap leaf) by chlorophyll analyzer (SPAD-502Plus, Konica Minolta, Japan) from May to September. Six stalks were selected and cut randomly from each subplot every month in November, December, and January (next year) and sent to the Sugarcane Research Institute of Guangxi Academy of Agricultural Sciences for cane quality analysis.
Determination of GPT, GOT activity, and nitrate N content in sugarcane leaves were referred to Zhang et al. [11]. Determination of dry matter and total N content in sugarcane was operated as follows: Six plants were randomly selected from each subplot, and the leaves and stalks were separated and heated at 105 °C for 30 min, and then dried at 60 °C to constant weight. After weighing, they were crushed and sifted into a 0.15 mm mesh sieve and bagged. The samples were used to determine total N content with the Kjeldahl method. NUE of each treatment was determined using the formula [35] as follows:
NUE = (Total N accumulation at U2, U3 level−Total N accumulation at U1 level)/N application rate × 100%.
2.5. Data Analysis
Data analyses were performed using the SPSS 22.0 software package (IBM SPSS Statistics 22). Analysis of variance (ANOVA) was done to evaluate the effects of different N levels and sugarcane cultivars, and the N application level × cultivar interactions. The results were reported as the mean values of the experiments. Graphs were generated with Sigma Plot 10.0, Origin 2019b and Microsoft Excel 2010.
3. Results
3.1. Incidences of Withered Heart and Smut
It can be seen from Table 2 that withered heart seedlings mainly occurred in the plant crop, so the withered heart incidence rate was lower in the ratoon crops. The withered heart incidence rate and smut incidence rate in the ratoon crops increased first and then decreased. The mean of withered heart incidence rate showed a trend of U1 > U3 > U2, and significantly different between U1 and U2. For different crops, it showed significantly higher in U1 and U2 than U3 in the plant crop, but significantly higher in U3 than U1 and U2 in the first ratoon crop (2016). The mean of smut incidence rate showed a trend of U3 > U2 > U1, and significantly different between U1 and U3 although an exception was observed in the first ratoon crop which showed U2 > U3 > U1 (Table 2), suggesting that the smut incidence rate might in general increase with the increase of urea application rate. For different sugarcane cultivars, although the mean of withered heart incidence rate showed no significant differences, there were significant differences for different cultivars in different planting years. It was significantly higher in B9 than GT11 in the plant crop, in ROC22 than B9 and GT11 in the first and third ratoon crops, in GT11 than B9 in the first ratoon crop. The smut incidence rate showed a trend of GT11 > ROC22, B9 for different sugarcane cultivars.

Table 2.
The effects of N application level and cultivar on withered heart incidence and smut incidence rates in sugarcane.
There was an interaction between N fertilizer and sugarcane cultivars on the withered heart incidence rate (Table 2). It was found that the lowest withered heart incidence rate occurred in the combination U2 × B9 (sugarcane cultivar B9 applied with 150 kg/ha urea). There was no interaction between N fertilizer level and sugarcane cultivar for the smut incidence rate (Table 2), and the best combination was U1 × B9.
3.2. Emergence Rate, Shooting Rate, and Tillering Rate
There was no significant difference in the emergence rate in different treatments in the plant crop, but the shooting rate showed U3 < U1, U2 significantly (Table 3). Obviously, the highest N fertilization level (300 kg/ha urea) inhibited the bud germination and early growth in the ratoon crops. The tillering rate was significantly higher in U2 than U1. The tillering rate was positively correlated with the level of N fertilization in the plant crop, with significant differences among U1, U2, and U3, indicating that increasing applications of N fertilizer could promote the tillering in the plant crop. The tillering rate in the treatment U2 increased year by year in the ratoon crop, while that in U1 and U3 increased in the first ratoon and then decreased with time. For different cultivars, the tillering rate showed B9 > ROC22 > GT11, and significantly higher in B9 than ROC22 and GT11 (Table 3).

Table 3.
The effects of N application level and cultivar on emergence rate, shooting rate, and tillering rate in sugarcane.
In general, the effects of urea application level and sugarcane cultivar on the emergence rate, the shooting rate, and the tillering rate were independent of each other, and there was no interaction between the urea application level and cultivar in the three indexes (Table 3). The best performance in the emergence rate, the shooting rate, and the tillering rate was found in the U2 × B9 combination. However, there was an interaction between urea application level and sugarcane cultivar in the tillering rate in the plant crop (Table 3), and the best was found in the U3 × B9 combination by using DMRT.
3.3. Stalk Diameter and Plant Height
It can be seen from Table 4 that the stalk diameter and plant height showed the same as U2, U3 > U1 significantly in all the years except for the stalk diameter in 2018. The mean of stalk diameter in U2 and U3 was 2.6 cm and 2.5-fold larger than that in U1, respectively, and the mean of plant height in U2 and U3 was the same, 43 cm higher than that in U1. For different sugarcane cultivars, the stalk diameter showed GT11 > ROC22, B9, and the mean in GT11 was 2.5 and 2.1 mm significantly larger than that in B9 and ROC22, respectively (Table 4). For all the years, the plant height showed ROC22 > B9 > GT11 significantly, and the mean in ROC22 was 28 and 53 cm higher than B9 and GT11, respectively, and the mean in B9 was 25 cm higher than that in GT11.

Table 4.
The effects of N application level and cultivar on stalk diameter and plant height in sugarcane.
There was no interaction between urea application level and cultivar in stalk diameter and plant height of sugarcane (Table 4). The best performance for stalk diameter was found in the combination U2 × GT11 and for plant height in the combination U3 × ROC22.
3.4. Relative Chlorophyll Content (SPAD Value)
The data in Figure 2 showed that the SPAD values in sugarcane leaves were affected by both cultivar and urea application level. In general, the SPAD values were lower in U1 than U2 and U3, the same in different years, and slightly higher in U3 than U2 in most cases. The SPAD values were significantly lower in GT11 than B9 in both the plant or ratoon crops, and also significantly lower than ROC22 in the ratoon crops.
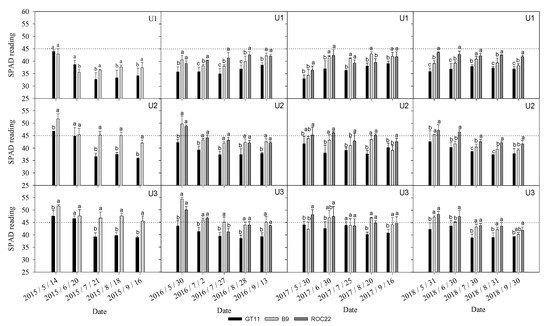
Figure 2.
SPAD values (relative chlorophyll content) of sugarcane leaves from May to September during the 4-year planting duration under different N levels. Data with different letters in the same column are significantly different at LSR 0.05 using Duncan′s multiple range test (DMRT).
3.5. Stalk Weight and Millable Stalks/ha
The data in Table 5 indicate that the mean of stalk weight showed U1 < U2, U3 significantly, which was 0.23 and 0.13 kg significantly higher in U2 and U3 than U1, respectively. It showed the same trend as the total mean in the plant crop but varied in different years of the ratoon crops.

Table 5.
The effects of N application level and cultivar on stalk weight and millable stalks/ha in sugarcane.
It was found in Table 5 that the mean of millable stalks/ha showed U1 < U2, U3 for different N levels, 26.9% and 38.6% significantly higher in U2 and U3 than that in U1, respectively. The millable stalks/ha was considerably higher in the plant crop than the ratoon crops. For different cultivars, the mean of millable stalks/ha was 36.4% and 34.1% significantly higher in B9 and ROC22 than GT11, respectively, and similar results were observed in all the years.
There was no interaction between N fertilizer application level and sugarcane cultivar in stalk weight (Table 5), and the best performance was observed in the U2 × GT11 combination. There was an interaction between N fertilizer application level and sugarcane cultivar in millable stalks/ha (Table 5), and the best performance was observed in the U3 × B9 combination.
3.6. Cane and Sugar Yield
As shown in Table 6, the cane yield generally showed U1 < U2, U3 for different N application levels. The U2 and U3 treatments significantly increased the mean of cane yield by 49.3% and 50.9%, respectively, compared with U1. Higher cane yield was obtained in the plant crop (2015) and first ratoon crop (2016), and then decreased sharply in 2017, which showed no significant difference form 2018. The sugar yield also showed the same trend as the cane yield, and the U2 and U3 treatments significantly increased it by 51.9% and 50.4%, respectively, compared with U1 (Table 6).

Table 6.
The effects of N application level and cultivar on cane and sugar yield in sugarcane.
For different sugarcane cultivars, the cane yield significantly increased by 27.3% and 25.8%, respectively, and the sugar yield significantly increased by 38.1% and 32.6%, respectively, in ROC22 and B9 than GT11 (Table 6).
There was no interaction between N fertilizer application level and sugarcane cultivar in cane yield and sugar yield (Table 6). The highest cane yield was observed in the combination U3 × ROC22, and the highest sugar yield was found in the combination U2 × ROC22. However, there was an interaction between N fertilizer application level and sugarcane cultivar in cane yield and sugar yield in the first ratoon crop (Table 6), the highest cane yield was observed in the combination U3 × ROC22, and the highest sugar yield in the combination U3 × B9.
3.7. Field Brix and Milled Juice Brix
It was found that the field brix was significantly lower in U3 than U1 and U2 for different N levels in the plant crop but the same in the mean and the ratoon crops (Table 7). The milled juice brix also showed U3 < U1, U2 significantly for different N levels. For different sugarcane cultivars, both the means of field brix and milled juice brix showed significantly lower in GT11 than B9 and ROC22, but B9 > ROC22 > GT11 significantly in the plant crop. There was no interaction between N fertilizer application level and cultivar in the field brix and milled juice brix. The highest field brix and milled juice brix was found in the combination U2 × B9.

Table 7.
The effects of N application level and cultivar on field and milled juice brix in sugarcane.
3.8. Juice Gravity Purity
The data in Table 8 show that there is no significant difference on juice gravity purity for different N application levels. For sugarcane cultivars, the juice gravity purity showed ROC22 > B9 > GT11 significantly in the ratoon crops but not in the plant crop. There was no interaction between N fertilizer application level and sugarcane cultivar in juice gravity purity. The combination U3 × ROC22 recorded the highest juice gravity purity.

Table 8.
The effects of N application level and cultivar on juice gravity purity in sugarcane.
3.9. Juice Rate and Sucrose % Cane
As presented in Table 9, the mean of sugarcane juice rate showed U1 < U2, U3 for different N application levels. For different cultivars, the juice rate showed ROC22 > GT11 > B9. There was an interaction between N fertilizer application level and sugarcane cultivar in juice rate. The combination with highest juice rate was U3 × ROC22.

Table 9.
The effects of N application level and cultivar on juice rate and sucrose % cane in sugarcane.
The sucrose % cane was the highest in U1, which was significantly higher than U3 in the plant crop. In the ratoon crops, however, that in U2 was the highest among the three N level treatments, and significantly higher than the other two in the first ratoon crop and higher than U1 in the third ratoon crop (Table 9). For different cultivars, the sucrose % cane showed B9 > ROC22 > GT11 significantly in the plant crop, but ROC22 > B9 > GT11 in the ratoon crops and total mean. There was no interaction between N fertilizer and sugarcane cultivar on sucrose % cane except for the year 2017 (Table 9), and the combination with highest sucrose % cane was U2 × ROC22.
3.10. GPT, GOT, and Nitrate N in Leaves
The data in Figure 3 show that, in general, the GPT activity in leaves of different sugarcane cultivars under different N levels is stronger at the late tillering stage and great elongation stage than great tillering stage and late elongation stage. For the three sugarcane cultivars, the GPT activity was lower in GT11 than B9 and ROC22 at tillering stage under low N conditions. Consistent with this, GT11 showed lower tillering rate than B9 and ROC22 (Table 3). Obviously, the effects of different N levels and sugarcane cultivars on GPT activity varied significantly at different growth stages.

Figure 3.
The glutamic pyruvic transaminase (GPT) activity in leaves of sugarcane at different N levels. Data with different letters in the same column are significantly different at LSR 0.05 using Duncan′s multiple range test (DMRT).
The GOT activity in leaves was found lower in GT11 than B9 and ROC22 under different N levels. In general, that was higher in B9 than ROC22 at U1 and U2 levels except for late elongation stage (Figure 4).

Figure 4.
The glutamic oxaloacetic transaminase (GOT) activity in leaves of sugarcane at different N levels. Data with different letters in the same column are significantly different at LSR 0.05 using Duncan′s multiple range test (DMRT).
As shown in Figure 5, the nitrate N content in leaves of different sugarcane cultivars under different N levels had an increasing trend with plant growth. Generally, the nitrate N content showed the highest in ROC22 while the lowest in GT11 under different N levels. The nitrate N content generally showed U3 > U2 > U1 for the same sugarcane cultivar.
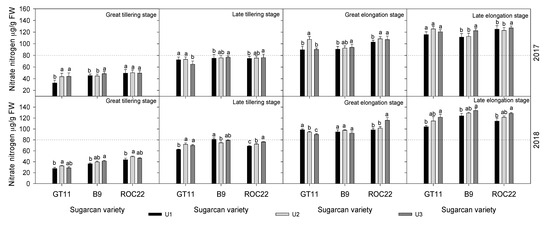
Figure 5.
The Nitrate N content in leaves of sugarcane at different N levels. Data with different letters in the same column are significantly different at LSR 0.05 using Duncan′s multiple range test (DMRT).
3.11. Dry Matter Distribution
It can be seen from Figure 6 that the N levels had a significant effect on the dry matter distribution in different sugarcane cultivars. The ratio of dry matter of stalks to leaves at U1 level was the lowest in GT11 at the tillering stage, which indicated that the application of N fertilizer at the tillering stage significantly promoted the dry matter distribution to the stalks in this cultivar. The ratio showed the highest in B9 among the three cultivars under different N levels, so the N application at the tillering stage was not conducive to dry matter distribution to the stalks in this cultivar. For ROC22, the ratio basically showed higher at U3 than U2 and U1 levels. During the elongation stage, the ratio in GT11 generally showed U1 > U2 > U3 for different N levels, and that in B9 was significantly higher at U3 than U2 and U1 levels. At the mature stage, the ratio generally showed U2 > U3 > U1 in GT11 and ROC22 while U3 > U2 > U1 in B9 for different N levels.
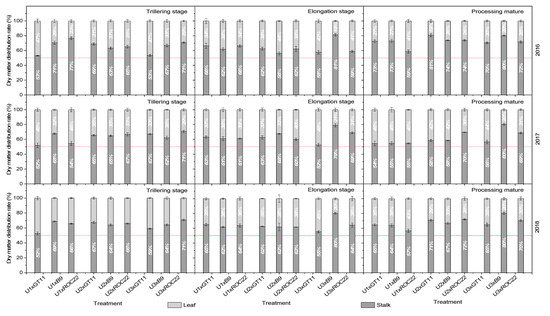
Figure 6.
Dry matter distribution in sugarcane in different combinations of nitrogen application level and sugarcane cultivar.
3.12. NUE
As presented in Table 10, the NUE showed generally as U3 > U2 > U1 for different N levels, and B9 > GT11 > ROC22 among the sugarcane cultivars. From tillering to mature stages, the NUE in GT11 increased first and then decreased, while that in B9 and ROC22 increased continuously. From the perspective of difference analysis, it was found that B9 showed higher NUE than GT11 and ROC22 at the tillering and elongation stages. There was an interaction between N application level and sugarcane cultivar in NUE. The NUE was much higher in leaves than stalks under different N application levels in the three cultivars (Table 11). The NUE in leaves increased from tillering to mature stage at U2 and U3 levels while that in stalks increased first and then decreased, and that in stalks at U3 level showed the highest. The NUE was higher in B9 than GT11 and ROC22, and showed an increase with growth in different sugarcane cultivars. N application level and sugarcane cultivar interacted significantly each other in NUE in both leaves and stalks of sugarcane.

Table 10.
The effects of N application level and cultivar on NUE in sugarcane during 2016 to 2018.

Table 11.
The NUE in sugarcane leaves and stalks in 2016–2018.
4. Discussion
Sugar yield per hectare is determined by cane yield and sucrose % cane, while millable stalks/ha and stalk weight are the components of cane yield in sugarcane. Millable stalks/ha is mainly determined by seedcane rate, emergence rate, shooting rate, and tillering rate, while stalk weight is mainly determined by plant height and stalk diameter. The quality of sugarcane is determined by sucrose % cane, juice rate, and juice gravity purity [36]. Nitrogen, phosphorus, and potassium are three essential factors for plant growth. They not only affect plant growth and cane yield but also affect the cane quality. Meanwhile, cultivation methods and pests are also important factors affecting sugar content, yield, and quality of sugarcane [1].
4.1. Effects of Different N Application Levels on Agronomic Traits in Sugarcane
It was found in this study that an appropriate N application can reduce the withered heart incidence rate in sugarcane. It is speculated that N application may improve the tillering rate and increase the number of shoots, thereby reduce the withered heart rate indirectly. Tillering rate basically increases with the increase of N application level, different cultivars show different responses to N fertilization, and an N-efficient cultivar shows a particularly prominent effect. In this study, the tillering rate was found significantly higher in B9 than ROC22 and GT11 at the same N level. Smut is one of the most serious fungal diseases causing a great economic loss in sugarcane production, especially in ratoon crops of susceptible cultivars [37]. GT11 had the highest smut incidence rate among the three cultivars, causing considerable loss of shoots, and resulting in the lowest millable stalks/ha and cane yield at the same N level. In this study, it was found that different N fertilization levels affected the emergence rate and shooting rate in sugarcane, low N, or no N application was more conducive to the emergence in the plant crop, while high N fertilizer treatment reduced the germination and shooting rates significantly. The shooting rate also varied from cultivar to cultivar, and the cultivar B9 showed the best ratoon ability among the three cultivars at the same N level.
There is a strong correlation between chlorophyll content and N content in leaves, so the N content in leaves can be indirectly determined by measuring the SPAD value of leaves [38]. In this study, the application of N fertilizer significantly increased the SPAD value in sugarcane leaves and decreased with plant growth. Previous studies indicated leaves are the largest N sink organ at the early growth stage, and N content in green leaves showed a downward trend with growth, and then the stalk became the largest N pool in sugarcane [39,40]. In this study, the SPAD values in sugarcane leaves were higher in May and June than August and September in the two treatments with N application. From August to September, the SPAD values decreased, which may be mainly related to the transfer of N from leaf to stalk. In 2017, the SPAD value was lower in July than August and September, which may be due to drought. Chlorophyll content in leaves is closely related to photosynthesis and then affects the plant’s normal growth and yield [41,42]. In this study, the SPAD values in different sugarcane cultivars were different. The cultivars with higher SPAD values had higher cane yield, sugar yield, sucrose % cane, and juice gravity purity. In addition, there was a significant positive correlation between the SPAD value and the juice gravity purity, which was consistent with the research results of Zhao et al. [43], and similar to the results in wheat and other crops [44,45].
4.2. Effects of Different N Application Levels on Yield and Quality in Sugarcane
The components of cane yield include stalk length, stalk diameter, and millable stalks/ha in sugarcane. The millable stalks/ha is mainly determined by the plant population at the early stage, while the growth of individuals depends on the nutrient competitiveness at the later stage. Therefore, N application mainly affects millable stalks/ha by affecting the emergence rate and tillering rate and then jointly determines the cane yield by affecting the stalk length, stalk diameter, and stalk weight of sugarcane at the later stage. In this study, with the increase of N levels, the stalk length and stalk diameter increased to a certain extent, but they did not show a significant difference between the treatments with 150 and 300 kg urea/kg application levels. In general, all three sugarcane cultivars obtained the highest cane and sugar yields at the U3 level from 2015 to 2016 and at the U2 level from 2017 to 2018. In total, for the four years, the highest sugar yield was obtained in the treatment with urea 150 kg/ha application.
Stalk is the most economically valuable part of sugarcane, and carbohydrate is the most important organic components in stalk. N level affects the cane quality by regulating the balance of carbohydrates in plants. Sucrose content is the most important quality index for sugar milling, and juice gravity purity is an important index reflecting the sugarcane maturity, so the higher the better. Under the conditions of the present study, the juice gravity purity tended to increase with the increase of N application levels, although the difference was not significant between U2 and U3, and the juice gravity purity in ROC22 was the highest at the same N level among the three sugarcane cultivars. Brix is another indicator of sugarcane maturity and quality. The brix at U2 level was the highest in this study. For different cultivars, B9 presented the highest brix during the whole 4-year planting period under the same N level. Brix is highly correlated with sucrose % cane in sugarcane. Obviously, the treatment with 150 kg/ha urea produced the best cane quality and the highest sugar yield, and further increase in the level of N fertilizer had a negative effect on the cane brix and sucrose content. These results are consistent with those of previous studies [46]. It was reported that the high N supply level will lead to the decrease of sucrose content in cane stalks, thus reducing the economic value of sugarcane stalks [47], which is consistent with the results of this study.
4.3. Effects of Different N Application Levels on N Metabolism in Sugarcane
N metabolism is the most basic physiological metabolism in sugarcane, and its strength affects the cane yield and quality. Transaminase is essential in sugarcane N metabolism. The activity of GOT in rice leaves increased significantly with the increase of N levels within a certain range [48]. Ge et al. [49] showed that N treatment could effectively improve the activities of GOT and GPT in tomato, and similar results were also found in wheat [50]. In this study, the activities of GPT and GOT in different sugarcane cultivars under different N levels were stronger at the late tillering and great elongation stages than at the great tillering stage. From the late tillering stage to great elongation stage of sugarcane growth, the temperature in the environment is high, sunlight and rain are abundant, so sugarcane crop grows vigorously, and the N metabolism is active. The results of the present study indicated that, at the beginning of the great tillering stage, the temperature had just started to rise, there was less rainfall, and the activity of N metabolism was relatively low. The direction of N metabolism in sugarcane leaves before the late tillering stage is dominated by assimilation and absorption of N, and the conversion and utilization of N gradually increased from late tillering stage to great elongation stage, which is similar to previous studies [11]. However, it was found that the GOT activity in sugarcane at the end of the elongation stage still increased to a certain extent while the GPT activity decreased significantly. This may be because the temperature and rainfall had gradually decreased at the end of the elongation stage. Previous studies have shown that GPT activity is very sensitive to temperature, and the reaction rate of GTPase is the highest at 35 °C [51]. From the great tillering stage to the late elongation stage, the GOT activity was higher in B9 and ROC22 than GT11. Nitrate N is an important N source for sugarcane. In previous studies, the nitrate N content in rice leaves was significantly increased by increasing N application during the rice filling stage [52], which is consistent with the results of this study. The present study shows that the nitrate N content in different sugarcane cultivars under different N levels basically increased with growth, showing an increasing ability to absorb N nutrients. Moreover, the result of a previous study indicated that the dynamic change of nitrate N content in leaves of spring maize was restricted by the N levels and the growth period, and nitrate N in the leaves was mainly accumulated during the early growth stage and reused at the late growth stage [53].
N levels affect the accumulation and distribution of dry matter during plant growth and development. In this study, the distribution of dry matter in stalks and leaves of sugarcane varied with sugarcane cultivars at different growth stages and different N levels, which is consistent with previous studies [54]. The ratio of dry matter of stalks to leaves was higher than 1 in different sugarcane cultivars at different growth stages at different N levels. The sugarcane cultivar GT11 distributed more dry matter to stalks at U2 than U1 and U3 levels at tillering and mature stages, but less at U2 level at elongation stage. This may be one of the reasons why GT11 showed larger stalk diameter but lower plant height than ROC22 and B9, which resulted in lower single stalk weight. The ratio in B9 was the highest at U1 level at the tillering stage and the lowest at U1 level at both elongation and mature stages. The ratio in ROC22 was relatively higher at U3 level from tillering to mature stages, so it is speculated that, for ROC22, the dry matter is contributed more to the stalk part at a high N level. It has been reported that the distribution rate of dry matter in stalk decreased with the increase of N application [54,55]. However, it was not the same in this study; this may be caused by different application rates of nitrogen fertilizer and different experimental conditions. The results showed that the accumulation and distribution of dry matter varied with sugarcane cultivars, and changed with the growth stage and the level of N application. The dry matter distribution was a complex process, and more cultivars and fertilization experiments were needed for further explanation.
The N loss in soil increases significantly with the increase of N levels when excessive N is applied [56,57]. The NUE at different growth stages increased with the increase of N application level in this study, which may be because the sugarcane crop had not reached the turning point from high to low in NUE at U3 level. Therefore, the N levels set for this study is reasonable and right. The sugarcane cultivars showed the greatest difference in NUE at the tillering stage under different N levels, and the NUE was higher at U3 level for all three cultivars. In general, the cultivar B9 showed the highest NUE among the cultivars. Interestingly, B9 also presented the highest emergence rate, tillering rate, and millable stalks/ha. Similar to the results in maize [58], the cultivars with higher N metabolism related enzyme activity could have higher NUE and yield. The NUE in GT11 showed higher compared with ROC22 and similar in withered heart and smut incidence rate performance, which had a great impact on the total number of plants in both cultivars. However, there was no positive correlation between the tillering rate and millable stalks and NUE in GT11 and ROC22. It was also found that the cane yield in a sugarcane cultivar with higher NUE at different N levels was not necessarily higher than that in those with lower NUE. At the mature stage, the NUE showed a positive correlation with sucrose % cane and sugar yield in sugarcane.
4.4. Interaction between N Application Level and Sugarcane Cultivar
In this study, there were significant interactions between N application level and sugarcane cultivar in withered heart incidence rate, millable stalks/ha, and juice rate. Besides, there were significant interactions between N application level and sugarcane cultivar in smut incidence rate, tillering rate, cane yield and sugar yield in 2016, and in stalk diameter and plant height in 2016 and 2017. At the same time, the elongation rate was found fluctuated greatly in 2015 and 2016, while relatively more stable in 2015 and 2018. The stalk diameter, stalk weight, cane yield, and sugar yield were the highest in 2016 compared to the other years. The cane yield declined sharply in 2017, which was similar to 2018. This may be due to the shallow distribution of sugarcane shoots from the upper buds in the ratoon crops, which could reduce the nutrient uptake. The compaction of soil was increased while soil permeability decreased in the ratoon crops, which not only hindered the growth of the root system and the nutrient absorption but also lost fertilizer more easily.
5. Conclusions
Appropriate application of nitrogen fertilizer promotes the tillering, growth, nitrogen metabolism, dry matter distribution, and sugar accumulation, and so significantly improves nitrogen use efficiency and cane and sugar productivity in sugarcane, but there are significant interactions between N application level and sugarcane cultivar. Under the condition of the present study, 300 kg/ha urea is recommended for plant and the first ratoon crops, and 150 kg/ha urea for the second and third ratoon crops, which could produce the highest sugar yield.
Author Contributions
L.-T.Y. and Y.-R.L. designed the study. X.-P.Z., K.Z., J.-M.L., Y.J., and Y.-X.X. conducted the experiments. X.-P.Z. and K.Z. analyzed data. K.Z., X.-P.Z., Y.-X.X. and Y.-R.L. wrote the manuscript. Y.-X.X. and Y.-R.L. revised and finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported financially in part by the National High-tech Research and Development Program (863) (2013AA102604-02), Guangxi Natural Science Foundation (2011GXNSFF018002), Guangxi Special Fund for Scientific Base and Talent (GKAD17195100), Guangxi Sugarcane Innovation Team of National Agricultural Industry Technology System (gjnytxgxcxtd-03-01), and Guangxi Academy of Agricultural Sciences (2015YT02).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviation
| N | Nitrogen |
| NUE | Nitrogen use efficiency |
| DMRT | Duncan’s multiple range test |
References
- Li, Y.R.; Yang, L.T. Sugarcane agriculture and sugar industry in China. Sugar Technol. 2015, 17, 1–8. [Google Scholar] [CrossRef]
- Li, Y.R. On sugarcane breeding in Guangxi. Guangxi Sugar Ind. 2019, 3, 3–9. [Google Scholar]
- Li, Y.R.; Yang, L.T. Research and development priorities for sugar industry of China: Recent Research Highlights. Sugar Technol. 2015, 17, 9–12. [Google Scholar] [CrossRef]
- Wei, C.L. Analysis and countermeasures of the degradation status of sugarcane cultivar ROC22 in Guangxi. J. South. Agric. 2012, 43, 2113–2117. [Google Scholar]
- Abayomi, A.Y. Growth, yield and crop quality performance of sugarcane cultivar Co957 under different rates of application of nitrogen and potassium fertilizers. J. Agric. Sci. 1987, 109, 285–292. [Google Scholar] [CrossRef]
- Ladha, J.K.; Pathak, H.; Krupnik, T.J.; Six, J.; Kessel, C.V. Efficiency of fertilizer nitrogen in cereal production: Retrospects and prospects. Adv. Agron. 2005, 87, 85–156. [Google Scholar]
- Harmel, D.; Qian, S.; Reckhow, K.; Casebolt, P. The manage database: Nutrient load and site characteristic updates and runoff concentration data. J. Environ. Qual. 2008, 37, 2403–2406. [Google Scholar] [CrossRef]
- Li, Y.R. Modern Sugarcane Science; China Agriculture Press: Beijing, China, 2010; pp. 85–102. [Google Scholar]
- Oenema, O.; Liere, L.V.; Schoumans, O. Effects of lowering nitrogen and phosphorus surpluses in agriculture on the quality of groundwater and surface water in the Netherlands. J. Hydrol. 2005, 304, 289–301. [Google Scholar] [CrossRef]
- Randall, G.W.; Delgado, J.A.; Schepers, J.S. Nitrogen management to protect water resources. In Nitrogen in Agricultural Systems; Schepers, J.S., Raun, W.R., Eds.; American Society of Agronomy: Madison, WI, USA, 2008; pp. 907–940. [Google Scholar]
- Zhang, Y.M.; Yang, L.T.; Li, X.; Li, Y.R. Effects of different nitrogen levels on key enzymes of nitrogen metabolism and contents of related active substances for three sugarcane varieties. J. South. Agric. 2015, 35, 556–563. [Google Scholar]
- Wang, X.C.; Wang, X.H.; Xiong, S.P.; Ma, X.M.; Ding, S.J.; Wu, K.Y.; Guo, J.B. Differences in nitrogen efficiency and nitrogen metabolism of wheat varieties under different nitrogen levels. Sci. Agric. Sin. 2015, 48, 2569–2579. [Google Scholar]
- Vitale, L.; Arena, C.; Carillo, P.; Di Tommasi, P.; Mesolella, B.; Nacca, F.; De Santo, A.V.; Fuggi, A.; Magliulo, V. Gas exchange and leaf metabolism of irrigated maize at different growth stages. Plant Biosyst. 2011, 145, 485–494. [Google Scholar] [CrossRef]
- Gascho, G.J.; Anderson, D.L.; Ozaki, H.Y. Cultivar dependent sugarcane response to nitrogen. Agron. J. 1986, 78, 1064–1069. [Google Scholar] [CrossRef]
- Lofton, J.; Tubaña, B. Effect of nitrogen rates and application time on sugarcane yield and quality. J. Plant Nutr. 2015, 38, 161–176. [Google Scholar] [CrossRef]
- Allen, D.E.; Kingston, G.; Rennenberg, H.; Dalal, R.C.; Schmidt, S. Effect of nitrogen fertilizer management and waterlogging on nitrous oxide emission from subtropical sugarcane soils. Agric. Ecosyst. Environ. 2010, 136, 209–217. [Google Scholar] [CrossRef]
- Barker, A.V.; Pilbeam, D.J. Handbook of Plant Nutrition; CRC Press: Boca Raton, FL, USA, 2007; pp. 21–120. [Google Scholar]
- Guo, J.W.; Zhang, Y.B.; Cui, X.W.; Liu, S.C.; Dao, J.M.; Fan, X. Accumulation of NPK and their effects on sugarcane yield and quality. Soils 2012, 44, 977–981. [Google Scholar]
- Robinson, N.; Fletcher, A.; Whan, A.; Vinall, K.; Bracken, R.; Lakshmanan, P.; Schmidt, S. Sustainable sugarcane production systems: Reducing plant nitrogen demand. Proc. Aust. Soc. Sugar Cane Technol. 2008, 30, 212–219. [Google Scholar]
- Stanturf, J.A.; Stone, E.L., Jr.; McKittrick, R.C. Effects of added nitrogen on growth of hardwood trees in southern New York. Can. J. For. Res. 1989, 19, 279–284. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, J.; Qu, Z.; Zou, D.; Sha, H.; Liu, H.; Sun, J.; Zheng, H.; Wang, J.; Yang, L.; et al. Effects of low water temperature during reproductive growth on photosynthetic production and nitrogen accumulation in rice. Field Crops Res. 2019, 242, 107587. [Google Scholar] [CrossRef]
- Liang, K.; Jiang, Y.F.; Nyiraneza, J.; Fuller, K.; Murnaghan, D.; Meng, F.R. Nitrogen dynamics and leaching potential under conventional and alternative potato rotations in Atlantic Canada. Field Crops Res. 2019, 242, 107603. [Google Scholar] [CrossRef]
- Fan, X.; Dao, J.M.; Shi, L.M.; Liu, S.C.; Gao, X.X.; Zhang, Y.B. Effects of different levels of nitrogen application on yield and quality of sugarcane Yunzhe 03-194. Sugar Crops China 2013, 2, 44–47. [Google Scholar]
- Paungfoolonhienne, C.; Yun, K.Y.; Kasinadhuni, N.R.P.; Lonhienne, T.G.A.; Robinson, N.; Hugenholtz, P.; Ragan, M.A.; Schmidt, S. Nitrogen fertilizer dose alters fungal communities in sugarcane soil and rhizosphere. Sci. Rep. 2015, 5, 8678. [Google Scholar] [CrossRef] [PubMed]
- Weiske, A.; Benckiser, G.; Ottow, J.C.G. Effect of the new nitrification inhibitor DMPP in comparison to DCD on nitrous oxide (N2O) emissions and methane (CH4) oxidation during 3 years of repeated applications in field experiments. Nutr. Cycl. Agroecosyst. 2001, 60, 57–64. [Google Scholar] [CrossRef]
- Wang, L.W.; Li, Y.R.; Yang, R.Z.; Li, X.; Huang, J.Y.; Fang, F.X. Response of low nitrogen stress in different sugarcane genotypes. Southwest China J. Agric. Sci. 2010, 23, 508–514. [Google Scholar]
- Zhao, D.; Glaz, B.; Comstock, J.C. Physiological and growth responses of sugarcane genotypes to nitrogen rate on a sand soil. J. Agron. Crop Sci. 2014, 200, 290–301. [Google Scholar] [CrossRef]
- Xie, J.L.; Wang, W.Z.; Zhu, Q.Z.; Liu, X.Y.; Liang, Q.; Li, Y.J.; Luo, Y.W.; Liang, T. Effects of nitrogen fertilizer application mode on sugarcane yield and soil nutrient change. Agric. Sci. Technol. 2014, 15, 115–122. [Google Scholar]
- Schultz, N.; Pereira, W.; Silva, P.D.A.; Baldani, J.I.; Boddey, R.M.; Alves, B.J.R.; Urquiaga, S.; Reis, V.M. Yield of sugarcane varieties and their sugar quality grown in different soil types and inoculated with a diazotrophic bacteria consortium. Plant Prod. Sci. 2017, 20, 1–9. [Google Scholar] [CrossRef]
- Shekinah, D.E.; Rakkiyappan, P. Relative significance of n nutrition on yield, quality and ethanol in sugarcane (Saccharum species hybrid) plant: Ratoon system. Sugar Technol. 2012, 14, 134–137. [Google Scholar] [CrossRef]
- Thorburn, P.J.; Biggs, J.S.; Attard, S.J.; Kemei, J. Environmental impacts of irrigated sugarcane production: Nitrogen lost through runoff and leaching. Agric. Ecosyst. Environ. 2011, 144, 1–12. [Google Scholar] [CrossRef]
- Wang, L.W.; Li, Y.R.; Tan, Y.M.; He, H. Performances and application prospects of several Brazilian sugarcane varieties in Guangxi. Sugarcane Canesugar 2005, 5, 1–7. [Google Scholar]
- Xing, Y.X.; Yang, L.T.; Li, Y.R. The comparison of different nitrogen metabolism between sugarcane varieties introduced from Brazil and cultivated in Guangxi. J. Anhui Agric. Sci. 2008, 36, 9003–9007. [Google Scholar]
- Yuan, D.; Zhu, k.; Li, J.H.; Yang, L.T.; Nong, Y.Y.; Li, Y.R. Effects of nitrogen application rate on chloroplast ultrastructure and photosynthetic rate in sugarcane. J. South. Agric. 2017, 48, 1190–1195. [Google Scholar]
- Yang, X.L.; Tong, Y.N.; Yong, L.; Ma, H.Y. Research advances in the calculating method of nitrogen use efficiency (NUE) in cultivated lands. Chin. J. Appl. Ecosyst. 2015, 26, 2203–2212. [Google Scholar]
- Anderson, D.L. A review: Soils, nutrition, and fertility practices of the Florida sugarcane industry. Proc. Soil Crop Sci. Soc. Fla. 1990, 49, 78–87. [Google Scholar]
- Su, Y.C.; Xu, L.P.; Wang, Z.Q.; Peng, Q.; Yang, Y.T.; Chen, Y.; Que, Y.X. Comparative proteomics reveals that central metabolism changes are associated with resistance against Sporisorium scitamineum in sugarcane. BMC Genom. 2016, 17, 800. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, J.; Yang, J.; Wang, H.; Zou, J.; He, J. Effects of nitrogen application rate and leaf age on the distribution pattern of leaf SPAD readings in the rice canopy. PLoS ONE 2014, 9, e88421. [Google Scholar] [CrossRef]
- Allison, J.C.S.; Williams, H.T.; Pammenter, N.W. Effect of specific leaf nitrogen content on photosynthesis of sugarcane. Ann. Appl. Biol. 1997, 131, 339–350. [Google Scholar] [CrossRef]
- Robertson, M.J.; Wood, A.W.; Muchow, R.C. Growth of sugarcane under high input conditions in tropical Australia. I. Radiation use, biomass accumulation and partitioning. Field Crops Res. 1996, 48, 11–25. [Google Scholar] [CrossRef]
- Osborne, B.A.; Raven, J.A. Light absorption by plants and its implications for photosynthesis. Biol. Rev. 1986, 61, 1–60. [Google Scholar] [CrossRef]
- Acreche, M.M.; Briceño-Félix, G.; Sánchez, J.A.M.; Slafer, G.A. Radiation interception and use efficiency as affected by breeding in Mediterranean wheat. Field Crops Res. 2009, 110, 91–97. [Google Scholar] [CrossRef]
- Zhao, Y.; Ying, X.M.; Yang, K.; Luo, Z.M.; Zan, F.G.; Li, F.Q.; Zhu, J.R. Variation and correlation analysis of chlorophyll SPAD values of different sugarcane varieties. J. Zhejiang Agric. Sci. 2019, 60, 569–572. [Google Scholar]
- Wang, G.; Bronson, K.F.; Thorp, K.R.; Mon, J.; Badaruddin, M. Multiple leaf measurements improve effectiveness of chlorophyll meter for durum wheat nitrogen management. Crop Sci. 2014, 54, 817–826. [Google Scholar] [CrossRef]
- Monostori, I.; Árendás, T.; Hoffman, B.; Galiba, G.; Gierczik, K.; Szira, F.; Vágújfalvi, A. Relationship between SPAD value and grain yield can be affected by cultivar, environment and soil nitrogen content in wheat. Euphytica 2016, 211, 103–112. [Google Scholar] [CrossRef]
- Dao, J.M.; Guo, J.W.; Cui, X.W.; Fan, X.; Liu, S.C.; Zhang, Y.B. Effects of different nitrogen application on yield and quality of sugarcane. Sugarcane Canesugar 2011, 2, 22–23. [Google Scholar]
- Muchow, R.C.; Robertson, M.J.; Wood, A.W.; Keating, B.A. Effect of nitrogen on the time-course of sucrose accumulation in sugarcane. Field Crops Res. 1996, 47, 143–153. [Google Scholar] [CrossRef]
- Wang, Q.L.; Wu, L.S.; Zhao, Z.Q.; Zhao, L.P. Effects of nitrogen rate on nitrogen loss from rice plant tissue. Plant Nutr. Fertil. Sci. 2010, 16, 14–19. [Google Scholar]
- Ge, T.D.; Song, S.W.; Jiang, W.; Tang, D.M.; Huang, D.F. Influence of glycine-N concentration on the growth and nitrogen metabolism of tomato seedings under sterile hydroponics cultivation. Acta Ecol. Sin. 2009, 29, 1994–2002. [Google Scholar]
- Shao, Q.Q. Studies on Uniconazole Applied to Seed on the Effects of Wheat Quality Mechanism under Different Nitrogen Levels. Master’s Thesis, Sichuan Agricultural University, Ya’an, China, 2004. [Google Scholar]
- Liu, W.Y.; Zhu, G.Y.; Cao, J.; Li, L.W.; Li, W.X. The optimization of conditions for assaying activity of GPT from Oncomelania hupensis in vitro by orthogonal matrix method. Acta Hydrobiol. Sin. 2010, 34, 463–466. [Google Scholar]
- Zhu, F.X.; Guo, X.D.; Tong, L.G.; Zhang, Y.L.; Pan, D.; Li, M.Y.; Li, D.; Zhang, Z.C.; Jin, Z.X. Expression response of Rubisco and GS isoform gene to the ratio of tillering and heading nitrogen fertilization at rice filling stage. J. Plant Nutr. Fertil. 2016, 23, 324–332. [Google Scholar]
- Juan, Y.H.; Sun, W.T.; Han, X.R.; Xing, Y.H.; Wang, L.H.; Xie, J.G. Response of spring maize to nitrogen application in physiological characteristics of functional leaves and grain yield. J. Nucl. Agric. Sci. 2015, 29, 391–396. [Google Scholar]
- Wei, J.F.; Liang, Q.X.; Chen, C.J.; Lan, L.B.; Liang, H. Effect of application amount of nitrogen fertilizer (15N) on nitrogen absorption and utilization in sugarcane. Guangdong Agric. Sci. 2012, 6, 609–614. [Google Scholar]
- Yang, L.; Ou, H.P.; Liang, Y.J.; Liu, X.H.; Yang, L.T.; Wang, M.; Huang, D.L.; Li, Y.R. The research progress of nitrogen physiology in sugarcane. Soil Fert. Sci. China 2014, 6, 1–7. [Google Scholar]
- Otto, R.; Castro, S.A.Q.; Mariano, E.; Castro, S.G.Q.; Franco, H.C.J.; Trivelin, P.C.O. Nitrogen use efficiency for sugarcane-biofuel production: What is next? Bioenergy Res. 2016, 9, 1272–1289. [Google Scholar] [CrossRef]
- Vale, D.W.D.; Prado, R.D.M.; Moraes, J.R.D.S.C.D.; Cruz, F.J.R. Nitrogen efficiency in production, nutrient accumulation and nitrogen efficiency use of second ratoon sugarcane harvested without straw burning. Aust. J. Crop Sci. 2017, 11, 616–623. [Google Scholar] [CrossRef]
- Li, W.L.; Lv, Y.J.; Liu, X.M.; Tong, T.; Cao, X.B.; Gu, W.R.; Wei, S. Effect of nitrogen fertilizer on nitrogen metabolism enzymes, nitrogen use and yield of maize with different nitrogen efficiency. Southwest China J. Agric. Sci. 2018, 31, 1829–1835. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).