Utilization of Hydrolyzed Agro-Industrial Waste from Arti-Chokes to Obtain Structurally Functional Bacterial Cellulose by Komagataeibacter rhaeticus QK23
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining and Characterizing Agroindustrial Waste from Artichoke Bracts (AWAB)
2.2. Hydrolysis of AWAB
2.3. Evaluation of BC Production Efficiency from AWAB
2.4. BC Extraction and Purification
2.5. Analytical Measurements
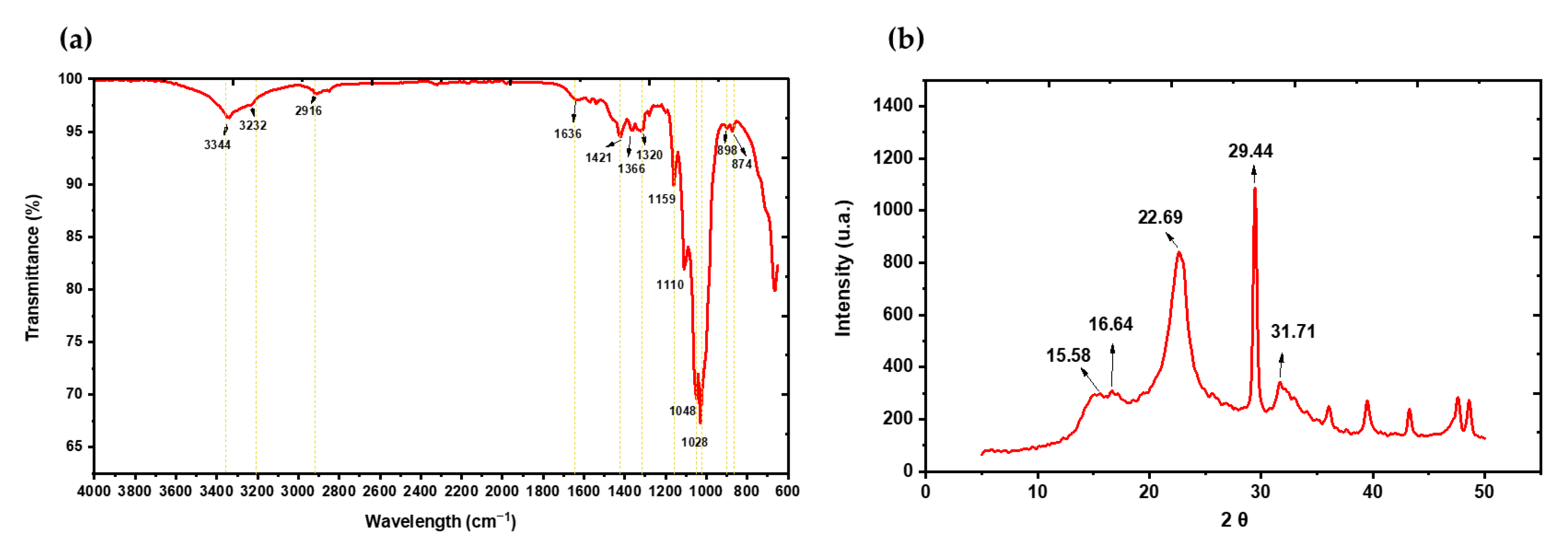
2.6. Characterization of BC
2.6.1. FTIR
2.6.2. XRD
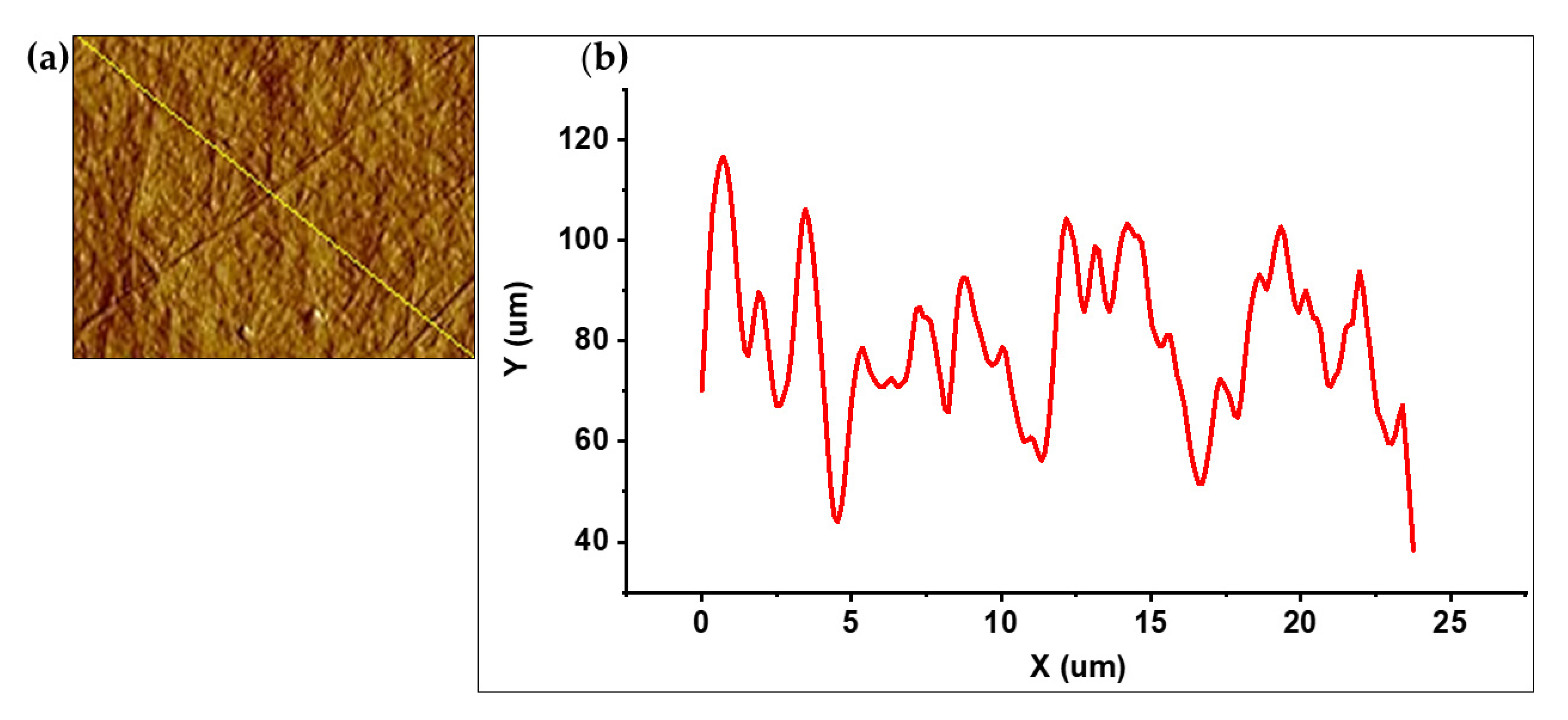
2.6.3. AFM
2.7. Statistical Analysis
3. Results and Discussion
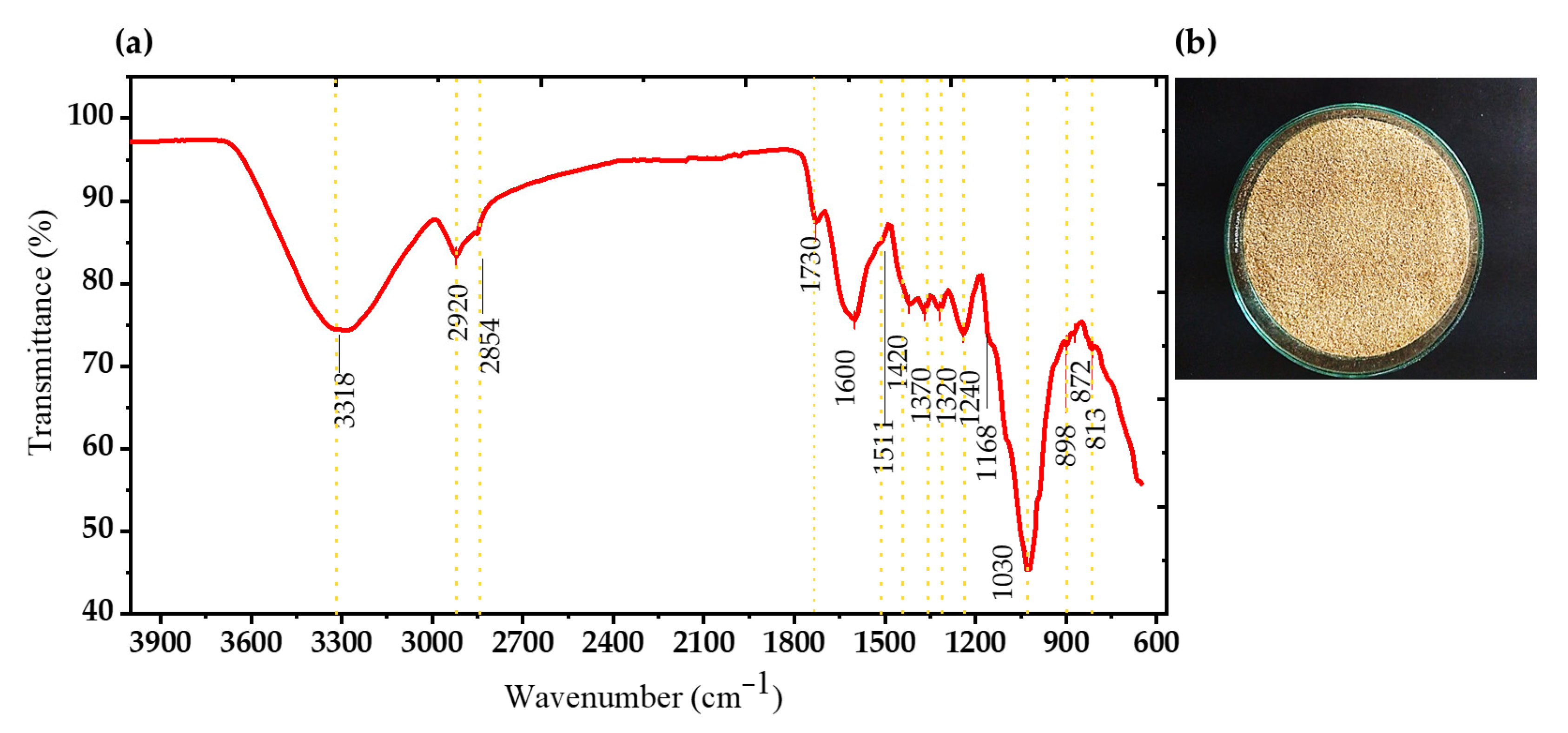
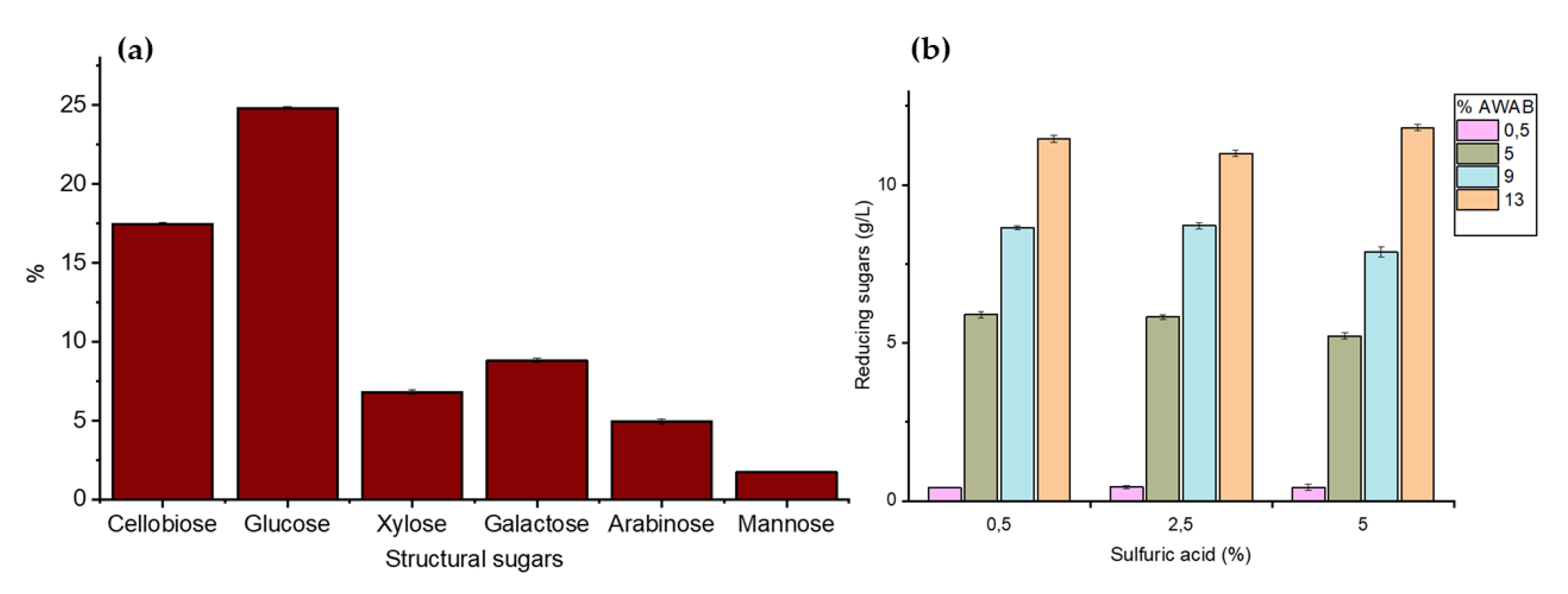
3.1. Characterization of Agroindustrial Waste
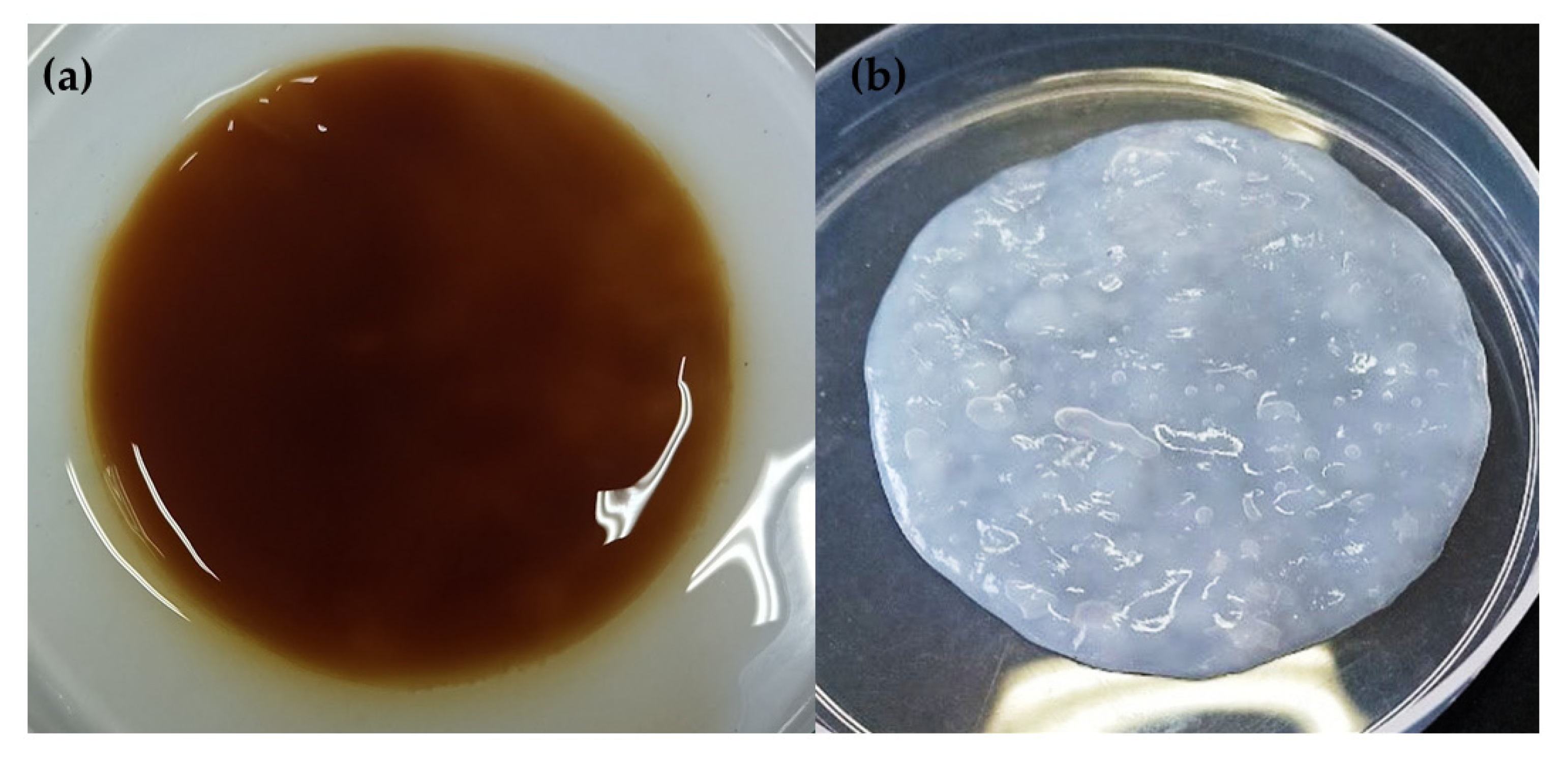
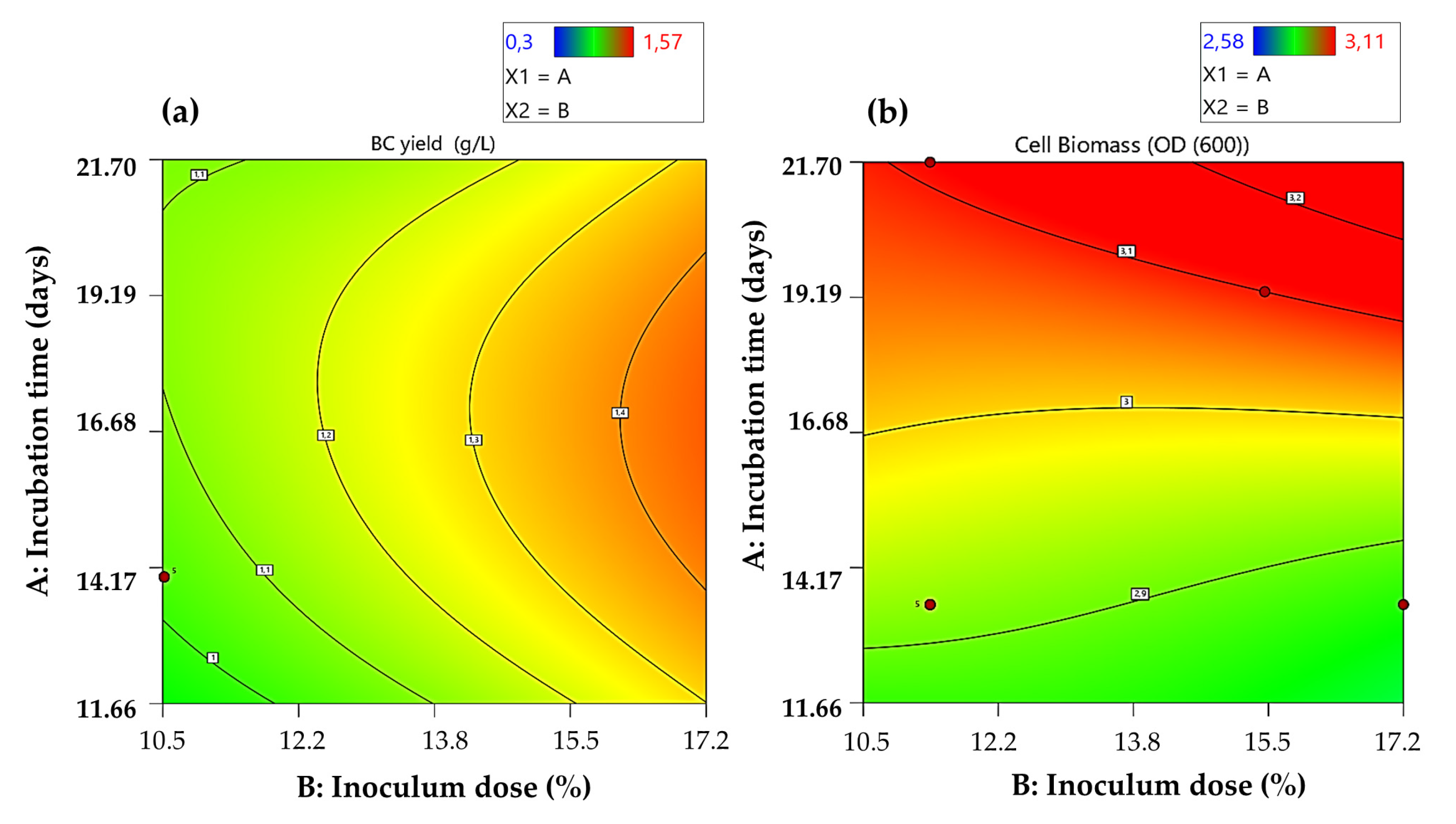
3.2. BC Production from Agroindustrial Waste
3.3. BC Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jittaut, P.; Hongsachart, P.; Audtarat, S.; Dasri, T. Production and characterization of bacterial cellulose produced by Gluconacetobacter xylinus BNKC 19 using agricultural waste products as nutrient source. Arab. J. Basic Appl. Sci. 2023, 30, 221–230. [Google Scholar] [CrossRef]
- Hamed, D.A.; Maghrawy, H.H.; Kareem, H.A. Biosynthesis of bacterial cellulose nanofibrils in black tea media by a symbiotic culture of bacteria and yeast isolated from commercial kombucha beverage. World J. Microbiol. Biotechnol. 2023, 39, 48. [Google Scholar] [CrossRef] [PubMed]
- Potočnik, V.; Gorgieva, S.; Trček, J. From Nature to Lab: Sustainable Bacterial Cellulose Production and Modification with Synthetic Biology. Polymers 2023, 15, 3466. [Google Scholar] [CrossRef] [PubMed]
- Fatima, A.; Ortiz-Albo, P.; Neves, L.A.; Nascimento, F.X.; Crespo, J.G. Biosynthesis and characterization of bacterial cellulose membranes presenting relevant characteristics for air/gas filtration. J. Membr. Sci. 2023, 674, 121509. [Google Scholar] [CrossRef]
- Bagewadi, Z.K.; Dsouza, V.; Yaraguppi, D.A.; Mulla, S.I.; Deshpande, S.H.; Shettar, S.S. Low cost production of bacterial cellulose through statistical optimization and developing its composites for multipurpose applications. Process Biochem. 2023, 125, 47–60. [Google Scholar] [CrossRef]
- Hussain, Z.; Sajjad, W.; Khan, T.; Wahid, F. Production of bacterial cellulose from industrial wastes: A review. Cellulose 2019, 26, 2895–2911. [Google Scholar] [CrossRef]
- Heydorn, R.L.; Lammers, D.; Gottschling, M.; Dohnt, K. Effect of food industry by-products on bacterial cellulose production and its structural properties. Cellulose 2023, 30, 4159–4179. [Google Scholar] [CrossRef]
- Płoska, J.; Garbowska, M.; Klempová, S.; Stasiak-Różańska, L. Obtaining Bacterial Cellulose through Selected Strains of Acetic Acid Bacteria in Classical and Waste Media. Appl. Sci. 2023, 13, 6429. [Google Scholar] [CrossRef]
- Tsouko, E.; Pilafidis, S.; Kourmentza, K.; Gomes, H.I.; Sarris, G.; Koralli, P.; Papagiannopoulos, A.; Pispas, S.; Sarris, D. A sustainable bioprocess to produce bacterial cellulose (BC) using waste streams from wine distilleries and the biodiesel industry: Evaluation of BC for adsorption of phenolic compounds, dyes and metals. Biotechnol. Biofuels Bioprod. 2024, 17, 40. [Google Scholar] [CrossRef]
- Ramirez-Hernandez, A.; Galagarza, O.A.; Rodriguez, M.V.Á.; Vera, E.P.; Ortiz, M.d.C.V.; Deering, A.J.; Oliver, H.F. Food safety in Peru: A review of fresh produce production and challenges in the public health system. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3323–3342. [Google Scholar] [CrossRef]
- Monge, C.; Nuñez, N.; Velez, F. Aprovechamiento de Residuos de Alcachofa (Cynara scolymus L.) Para la Fabricación de Harina Utilizada en el Enriquecimiento Con Fibra de Un Yogurt Frutado Con Probióticos, Repositorio Institucional de la Universidad San Ignacio de Loyola. 2019. Available online: https://repositorio.usil.edu.pe/entities/publication/b1c751dd-f00b-4bb2-ac53-0e9f0c236399 (accessed on 12 April 2025).
- Kammoun, M.; Ayeb, H.; Bettaieb, T.; Richel, A. Chemical characterization and technical assessment of agri-food residues, marine matrices, and wild grasses in the South Mediterranean area: A considerable inflow for biorefineries. Waste Manag. 2020, 118, 247–257. [Google Scholar] [CrossRef]
- Zeaiter, Z.; Regonesi, M.E.; Cavini, S.; Labra, M.; Sello, G.; Di Gennaro, P. Extraction and characterization of inulin-type fructans from artichoke wastes and their effect on the growth of intestinal bacteria associated with health. Biomed. Res. Int. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Pérez-Contreras, S.; Hernández-Rosas, F.; Lizardi-Jiménez, M.A.; Herrera-Corredor, J.A.; Baltazar-Bernal, O.; la Cruz, D.A.A.-D.; Hernández-Martínez, R. Sugarcane Industry By-Products: A Decade of Research Using Biotechnological Approaches. Recycling 2025, 10, 154–184. [Google Scholar] [CrossRef]
- Asuquo, A.J.; Zhang, X.; Lin, L.; Li, J. Green heterogeneous catalysts derived from fermented kola nut pod husk for sustainable biodiesel production. Int. J. Green Energy 2024, 21, 2218–2227. [Google Scholar] [CrossRef]
- Woo, W.X.; Nasoha, N.Z.; Luthfi, A.A.; Yeap, S.K.; Hui, Y.W.; Bukhari, N.A.; Manaf, S.F.; Tan, J.P. Bio-based succinic acid production from durian husk: A rising Southeast Asia agricultural waste. Ind. Crops Prod. 2023, 206, 117624. [Google Scholar] [CrossRef]
- Trilokesh, C.; Uppuluri, K.B. Isolation and characterization of cellulose nanocrystals from jackfruit peel. Sci. Rep. 2019, 9, 6709. [Google Scholar] [CrossRef]
- Kleawkla, A.; Chuenkruth, P. Reducing sugar production from agricultural wastes by acid hydrolysis. Key Eng. Mater. 2016, 675–676, 31–34. [Google Scholar] [CrossRef]
- Quiñones-Cerna, C.; Rodríguez-Soto, J.C.; Barraza-Jáuregui, G.; Huanes-Carranza, J.; Cruz-Monzón, J.A.; Ugarte-López, W.; Hurtado-Butrón, F.; Samanamud-Moreno, F.; Haro-Carranza, D.; Valdivieso-Moreno, S.; et al. Bioconversion of Agroindustrial Asparagus Waste into Bacterial Cellulose by Komagataeibacter rhaeticus. Sustainability 2024, 16, 736. [Google Scholar] [CrossRef]
- Feng, X.; Ullah, N.; Wang, X.; Sun, X.; Li, C.; Bai, Y.; Chen, L.; Li, Z. Characterization of Bacterial Cellulose by Gluconacetobacter hansenii CGMCC 3917. J. Food Sci. 2015, 80, E2217–E2227. [Google Scholar] [CrossRef]
- Volova, T.G.; Prudnikova, S.V.; Sukovatyi, A.G.; Shishatskaya, E.I. Production and properties of bacterial cellulose by the strain Komagataeibacter xylinus B-12068. Appl. Microbiol. Biotechnol. 2018, 102, 7417–7428. [Google Scholar] [CrossRef] [PubMed]
- Mardawati, E.; Rahmah, D.M.; Rachmadona, N.; Saharina, E.; Pertiwi, T.Y.; Zahrad, S.A.; Ramdhani, W.; Srikandace, Y.; Ratnaningrum, D.; Endah, E.S.; et al. Pineapple core from the canning industrial waste for bacterial cellulose production by Komagataeibacter xylinus. Heliyon 2023, 9, e22010. [Google Scholar] [CrossRef]
- Al-Ramlawee, K.H.; Alkalifawi, E.J. Production and characterization of bacterial cellulose utilizing Iraqi vinegar’s mother pellicles. J. Appl. Nat. Sci. 2023, 15, 1619–1626. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, X.; Huo, M.; Zhai, X.; Li, F.; Zhong, C. Preparation and characterization of a novel bacterial cellulose/chitosan bio-hydrogel. Nanomater. Nanotechnol. 2017, 7, 1847980417707172. [Google Scholar] [CrossRef]
- Chinga, G.; Johnsen, P.O.; Dougherty, R.; Berli, E.L.; Walter, J. Quantification of the 3D microstructure of SC surfaces. J. Microsc. 2007, 227, 254–265. [Google Scholar] [CrossRef]
- Rodríguez, N.A.T.; Quiñones-Cerna, C.E.; Castillo, H.M.R.; Cruz-Monzon, J.A.; Butrón, F.J.H.; Soto, J.C.R. Optimization of Total Carotenoid Production by Rhodotorula mucilaginosa from Artichoke Agroindustrial Waste Using Response Surface Methodology. Environ. Res. Eng. Manag. 2023, 79, 111–121. [Google Scholar] [CrossRef]
- Al Kamzari, S.M.A.; Rao, L.N.; Lakavat, M.; Gandi, S.; P, S.R.; Sri, G.K. Extraction and characterization of cellulose from agricultural waste materials. Mater. Today Proc. 2023, 80, 2740–2743. [Google Scholar] [CrossRef]
- Biswas, S.; Rahaman, T.; Gupta, P.; Mitra, R.; Dutta, S.; Kharlyngdoh, E.; Guha, S.; Ganguly, J.; Pal, A.; Das, M. Cellulose and lignin profiling in seven, economically important bamboo species of India by anatomical, biochemical, FTIR spectroscopy and thermogravimetric analysis. Biomass Bioenergy 2022, 158, 106362. [Google Scholar] [CrossRef]
- Thongsomboon, W.; Baimark, Y.; Srihanam, P. Valorization of Cellulose-Based Materials from Agricultural Waste: Comparison between Sugarcane Bagasse and Rice Straw. Polymers 2023, 15, 3190. [Google Scholar] [CrossRef]
- Thandavamoorthy, R.; Devarajan, Y.; Kaliappan, N. Antimicrobial, function, and crystalline analysis on the cellulose fiber extracted from the banana tree trunks. Sci. Rep. 2023, 13, 15301. [Google Scholar] [CrossRef]
- Akram, W.; Garud, N. Optimization of inulin production process parameters using response surface methodology. Futur. J. Pharm. Sci. 2020, 6, 68. [Google Scholar] [CrossRef]
- Francavilla, M.; Marone, M.; Marasco, P.; Contillo, F.; Monteleone, M. Artichoke biorefinery: From food to advanced technological applications. Foods 2021, 10, 112. [Google Scholar] [CrossRef]
- Nalaeram, S.; Senthil-Muthu, K.T.; Sivakumar, P.; Srinivasan, M.; Surya-Narayana, B.Y.; Ebrahimnezhad-Khaljiri, H.; Meena, M.; Sanjay-Mavinkere, R.; Suchart, S. Isolation and characterization of agro-waste biomass sapodilla seeds as reinforcement in potential polymer composite applications. Heliyon 2023, 9, e17760. [Google Scholar] [CrossRef] [PubMed]
- Bekiaris, G.; Tagkouli, D.; Koutrotsios, G.; Kalogeropoulos, N.; Zervakis, G.I. Pleurotus Mushrooms Content in Glucans and Ergosterol Assessed by ATR-FTIR Spectroscopy and Multivariate Analysis. Foods 2020, 9, 535. [Google Scholar] [CrossRef]
- Cebin, A.V.; Komes, D.; Ralet, M.C. Development and Validation of HPLC-DAD Method with Pre-Column PMP Derivatization for Monomeric Profile Analysis of Polysaccharides from Agro-Industrial Wastes. Polymers 2022, 14, 544. [Google Scholar] [CrossRef]
- Yadav, A.; Pandey, S.K.; Agrawal, D.C.; Mishra, H.; Srivastava, A.; Kayastha, A.M. Carbon nanotubes molybdenum disulfide 3D nanocomposite as novel nanoscaffolds to immobilize Lens culinaris β-galactosidase (Lsbgal): Robust stability, reusability, and effective bioconversion of lactose in whey. Food Chem. 2019, 297, 125005. [Google Scholar] [CrossRef]
- Tesfaw, A.A.; Tizazu, B.Z. Reducing Sugar Production from Teff Straw Biomass Using Dilute Sulfuric Acid Hydrolysis: Characterization and Optimization Using Response Surface Methodology. Int. J. Biomater. 2021, 2021, 2857764. [Google Scholar] [CrossRef] [PubMed]
- Shangdiar, S.; Lin, Y.C.; Ponnusamy, V.K.; Wu, T.Y. Pretreatment of lignocellulosic biomass from sugar bagasse under microwave assisted dilute acid hydrolysis for biobutanol production. Bioresour. Technol. 2022, 361, 127724. [Google Scholar] [CrossRef]
- Cardona, V.F.M.; Avila, I.G.; Vanegas, A.L.; Buitrago, J.R. Assessment of reducing sugars production from agro-industrial wastes by batch and semicontinuous subcritical water hydrolysis. CTyF 2021, 11, 55–63. [Google Scholar] [CrossRef]
- Akintunde, M.O.; Adebayo-Tayo, B.C.; Ishola, M.M.; Zamani, A.; Horváth, I.S. Bacterial Cellulose Production from agricultural Residues by two Komagataeibacter sp. Strains. Bioengineered 2022, 13, 10010–10025. [Google Scholar] [CrossRef]
- Huang, C.; Yang, X.; Xiong, L.; Guo, H.; Luo, J.; Wang, B.; Zhang, H.; Lin, X.; Chen, X. Utilization of Corncob Acid Hydrolysate for Bacterial Cellulose Production by Gluconacetobacter xylinus. Appl. Biochem. Biotechnol. 2015, 175, 1678–1688. [Google Scholar] [CrossRef]
- Distler, T.; Huemer, K.; Leitner, V.; Bischof, R.H.; Groiss, H.; Guebitz, G.M. Production of bacterial cellulose by Komagataeibacter intermedius from spent sulfite liquor. Bioresour. Technol. Rep. 2023, 24, 101655. [Google Scholar] [CrossRef]
- Ngo, T.T.N.; Phan, T.H.; Le, T.M.T.; Le, T.N.T.; Huynh, Q.; Phan, T.P.T.; Hoang, M.; Vo, T.P.; Nguyen, D.Q. Producing bacterial cellulose from industrial recycling paper waste sludge. Heliyon 2023, 9, e17663. [Google Scholar] [CrossRef]
- Meng, Q.; Jiang, Y.; Rogiewicz, A.; Hu, J. A sustainable strategy for biosynthesis of bacterial cellulose using a microbial symbiotic culture from hemp (Cannabis sativa L.) waste hydrolysate. Ind. Crops Prod. 2025, 234, 121509. [Google Scholar] [CrossRef]
- Kim, H.; Son, J.; Lee, J.; Yoo, H.Y.; Lee, T.; Jang, M.; Oh, J.M.; Park, C. Improved production of bacterial cellulose through investigation of effects of inhibitory compounds from lignocellulosic hydrolysates. GCB Bioenergy 2021, 13, 436–444. [Google Scholar] [CrossRef]
- El-Gendi, H.; Taha, T.H.; Ray, J.B.; Saleh, A.K. Recent advances in bacterial cellulose: A low-cost effective production media, optimization strategies and applications. Cellulose 2022, 29, 7495–7533. [Google Scholar] [CrossRef]
- Yanti, N.A.; Ahmad, S.W.; Muhiddin, N.H. Evaluation of inoculum size and fermentation period for bacterial cellulose production from sago liquid waste. J. Phys. Conf. Ser. 2018, 1116, 052076. [Google Scholar] [CrossRef]
- Aswini, K.; Gopal, N.O.; Uthandi, S. Optimized culture conditions for bacterial cellulose production by Acetobacter senegalensis MA1. BMC Biotechnol. 2020, 20, 46. [Google Scholar] [CrossRef] [PubMed]
- Uğurel, C.; Öğüt, H. Optimization of Bacterial Cellulose Production by Komagataeibacter rhaeticus K23. Fibers 2024, 12, 29. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, H.; Wang, X.; Cai, K.; Chen, J.; Xu, Y.; Yu, F.; Nie, S.; Wang, S.; Liu, X. Anti-moisture, anti-bacterial cellulosic triboelectric materials enabled by hydroxyl coordination effect. Nano Energy 2024, 124, 109472. [Google Scholar] [CrossRef]
- Parida, C.; Dash, S.K.; Pradhan, C. FTIR and Raman Studies of Cellulose Fibers of Luffa cylindrica. Open J. Compos. Mater. 2015, 5, 5–10. [Google Scholar] [CrossRef]
- Alekseeva, O.V.; Noskov, A.V.; Agafonov, A.V. Structure, physicochemical properties, and adsorption performance of the ethyl cellulose/bentonite composite films. Cellulose 2022, 29, 3947–3961. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, V.U.; Almehjani, T.M.; Sapuan, S.; Jamal, T.; Ilyas, R.; Eldin, S.M.; Khan, A.; Jameel, Y. Isolation and Characterization of Cellulose from Pomegranate (Punica granatum) Peel. J. Nat. Fibers 2024, 21, 2299943. [Google Scholar] [CrossRef]
- Raza, M.; Mustafa, J.; Al-Marzouqi, A.H.; Abu-Jdayil, B. Isolation and characterization of cellulose from date palm waste using rejected brine solution. Int. J. Thermofluids 2024, 21, 100548. [Google Scholar] [CrossRef]
- Mohite, B.V.; Patil, S.V. Physical, structural, mechanical and thermal characterization of bacterial cellulose by G. hansenii NCIM 2529. Carbohydr. Polym. 2014, 106, 132–141. [Google Scholar] [CrossRef]
- Schroeder, L.R.; Gentile, V.M.; Atalla, R.H. Nondegradative preparation of amorphous cellulose. Biotechnol. Biofuels 2010, 6, 1–14. [Google Scholar] [CrossRef]
- Mouro, C.; Gomes, A.; Gomes, A.P.; Gouveia, I.C. Sustainable Bacterial Cellulose Production Using Low-Cost Fruit Wastewater Feedstocks. Nanomaterials 2025, 15, 271. [Google Scholar] [CrossRef]
- Liu, Z.; Siddique, F.; Wei, Y.; Haque, A.; Na, L.; Yang, X.; Lin, C.S.K. Efficient Production of Bacterial Cellulose Using Komagataeibacter sucrofermentans on Sustainable Feedstocks. Chem. Sus. Chem. 2024, 18, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yang, F.; Dai, Y.; Tao, F.; Shen, Y.; Duan, W.; Zhou, X.; Ma, H.; Tang, L.; Li, J. Exploring crystalline structural variations of cellulose during pulp beating of tobacco stems. Carbohydr. Polym. 2017, 174, 146–153. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, A.; Singh, M.K. Novel low-cost green method for production bacterial cellulose. Polym. Bull 2023, 81, 6721–6741. [Google Scholar] [CrossRef]
- Avcioglu, N.H.; Birben, M.; Bilkay, I.S. Optimization and physicochemical characterization of enhanced microbial cellulose production with a new Kombucha consortium. Process. Biochem. 2021, 108, 60–68. [Google Scholar] [CrossRef]
- Guhados, G.; Wan, W.; Hutter, J.L. Measurement of the elastic modulus of single bacterial cellulose fibers using atomic force microscopy. Langmuir 2005, 21, 6642–6646. [Google Scholar] [CrossRef]
- Revin, V.V.; Dolganov, A.V.; Liyaskina, E.V.; Nazarova, N.B.; Balandina, A.V.; Devyataeva, A.A.; Revin, V.D. Characterizing bacterial cellulose produced by Komagataeibacter sucrofermentans h-110 on molasses medium and obtaining a biocomposite based on it for the adsorption of fluoride. Polymers 2021, 13, 1422. [Google Scholar] [CrossRef] [PubMed]
- Hamzah, N.A.; Razak, N.A.; Karim, M.S.; Salleh, S.Z. Validation of a roughness parameters for defining surface roughness of prosthetic polyethylene Pe-Lite liner. Sci. Rep. 2022, 12, 2636. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.D.; Riegel-Vidotti, I.C.; Grein, A.; Tischer, C.A.; Faria-Tischer, P.C. Bacterial cellulose and hyaluronic acid hybrid membranes: Production and characterization. Int. J. Biol. Macromol. 2014, 67, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Yanti, N.A.; Ahmad, S.W.; Ramadhan, L.O.A.N.; Jamili; Muzuni; Walhidayah, T.; Mamangkey, J. Properties and application of edible modified bacterial cellulose film based sago liquid waste as food packaging. Polymers 2021, 13, 3570. [Google Scholar] [CrossRef]
- Aditya, T.; Allain, J.P.; Jaramillo, C.; Restrepo, A.M. Surface Modification of Bacterial Cellulose for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 610. [Google Scholar] [CrossRef]
- Mir, I.S.; Riaz, A.; Fréchette, J.; Roy, J.S.; Mcelhinney, J.; Pu, S.; Balakrishnan, H.K.; Greener, J.; Dumée, L.F.; Messaddeq, Y. Bacterial cellulose-graphene oxide composite membranes with enhanced fouling resistance for bio-effluents management. Npj Clean Water 2024, 7, 111. [Google Scholar] [CrossRef]
| Coded Levels | |||||
|---|---|---|---|---|---|
| −1.4 | −1 | 0 | 1 | +1.4 | |
| Incubation Time (days) | 3 | 6 | 14 | 17 | 25 |
| Inoculum Dose (%) | 1.0 | 3.8 | 10.5 | 17.2 | 20.0 |
| Treatment (T) | Inoculum Dose (%) | Incubation Time (Days) | Cell Biomass (OD600) | BC Yield (g/L) |
|---|---|---|---|---|
| 1 | 3.8 | 6 | 2.58 ± 0.011 | 1.27 ± 0.05 |
| 2 | 17.2 | 6 | 2.81 ± 0.012 | 0.80 ± 0.02 |
| 3 | 3.8 | 22 | 3.07 ± 0.030 | 1.23 ± 0.21 |
| 4 | 17.2 | 22 | 3.10 ± 0.013 | 1.32 ± 0.02 |
| 5 | 1 | 14 | 2.93 ± 0.026 | 0.48 ± 0.05 |
| 6 | 20 | 14 | 2.86 ± 0.021 | 1.57 ± 0.09 |
| 7 | 10.5 | 3 | 2.70 ± 0.017 | 0.30 ± 0.02 |
| 8 | 10.5 | 25 | 3.11 ± 0.044 | 1.00 ± 0.08 |
| 9 | 10.5 | 14 | 2.90 ± 0.018 | 0.97 ± 0.19 |
| 10 | 10.5 | 14 | 2.94 ± 0.007 | 1.02 ± 0.05 |
| 11 | 10.5 | 14 | 2.98 ± 0.015 | 1.05 ± 0.13 |
| 12 | 10.5 | 14 | 2.82 ± 0.008 | 1.02 ± 0.07 |
| 13 | 10.5 | 14 | 2.95 ± 0.012 | 1.08 ± 0.27 |
| Response | BC (g/L) | Cell Biomass (OD600) | ||||
|---|---|---|---|---|---|---|
| Source | Sum of squares | F | p | Sum of squares | F | p |
| Model | 1.35 | 5.33 | 0.0245 | 0.2674 | 8.75 | 0.02 |
| A-Inoculum dose | 0.5868 | 310.66 | <0.0001 | 0.0025 | 0.6414 | 0.47 |
| B-Incubation Time | 0.2473 | 130.94 | 0.0003 | 0.084 | 22 | 0.009 |
| AB | 0.0784 | 46.95 | 0.0024 | 0.01 | 2.62 | 0.18 |
| Pure Error | 0.0067 | 0.01 | ||||
| Cor Total | 1.36 | 0.28 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiñones-Cerna, C.E.; Barraza-Jáuregui, G.; Cruz-Monzón, J.A.; Hurtado-Butrón, F.; Soriano-Bernilla, B.S.; Gutiérrez-Rodríguez, D.M.; Huanes-Carranza, J.; Ugarte-López, W.; Rodríguez-Soto, J.C.; Robles-Castillo, H.M.; et al. Utilization of Hydrolyzed Agro-Industrial Waste from Arti-Chokes to Obtain Structurally Functional Bacterial Cellulose by Komagataeibacter rhaeticus QK23. Polymers 2025, 17, 2783. https://doi.org/10.3390/polym17202783
Quiñones-Cerna CE, Barraza-Jáuregui G, Cruz-Monzón JA, Hurtado-Butrón F, Soriano-Bernilla BS, Gutiérrez-Rodríguez DM, Huanes-Carranza J, Ugarte-López W, Rodríguez-Soto JC, Robles-Castillo HM, et al. Utilization of Hydrolyzed Agro-Industrial Waste from Arti-Chokes to Obtain Structurally Functional Bacterial Cellulose by Komagataeibacter rhaeticus QK23. Polymers. 2025; 17(20):2783. https://doi.org/10.3390/polym17202783
Chicago/Turabian StyleQuiñones-Cerna, Claudio Eduardo, Gabriela Barraza-Jáuregui, José Alfredo Cruz-Monzón, Fernando Hurtado-Butrón, Bertha Soledad Soriano-Bernilla, Diego Miguel Gutiérrez-Rodríguez, Johnny Huanes-Carranza, Wilmer Ugarte-López, Juan Carlos Rodríguez-Soto, Heber Max Robles-Castillo, and et al. 2025. "Utilization of Hydrolyzed Agro-Industrial Waste from Arti-Chokes to Obtain Structurally Functional Bacterial Cellulose by Komagataeibacter rhaeticus QK23" Polymers 17, no. 20: 2783. https://doi.org/10.3390/polym17202783
APA StyleQuiñones-Cerna, C. E., Barraza-Jáuregui, G., Cruz-Monzón, J. A., Hurtado-Butrón, F., Soriano-Bernilla, B. S., Gutiérrez-Rodríguez, D. M., Huanes-Carranza, J., Ugarte-López, W., Rodríguez-Soto, J. C., Robles-Castillo, H. M., López-Quiroz, E., & De La Cruz-Noriega, M. (2025). Utilization of Hydrolyzed Agro-Industrial Waste from Arti-Chokes to Obtain Structurally Functional Bacterial Cellulose by Komagataeibacter rhaeticus QK23. Polymers, 17(20), 2783. https://doi.org/10.3390/polym17202783









