Abstract
A novel cost-effective, rapid, and eco-friendly method was described for the removal of carbon dioxide (CO2) from the gaseous emissions of gasoline engines. This involved the use of a sandwich filter (~10 cm diameter) made of a nonwoven poly (m-phenylene isophthalamide) (Nomex) fabric loaded with a thin layer of activated carbon. The optimized filter, with an activated carbon mass of 2.89 mg/cm2, a thickness of 4.8 mm, and an air permeability of 0.5 cm3/cm2/s, was tested. A simple homemade sampling device equipped with solid-state electrochemical sensors to monitor the concentration levels of CO2 before and after filtration of the emissions was utilized. The data were transmitted via a General Packet Radio Service (GPRS) link to an Internet of Things (IoT)-based gas monitoring system for remote management, and real-time data visualization. The proposed device achieved a 70 ± 3.4% CO2-removal efficiency within 7 min of operation. Characterization of the filter was conducted using a high-resolution scanning electron microscopy (SEM), energy dispersive X-ray analysis (EDX) and Brunauer–Emmett–Teller (BET) analysis. The effects of loaded activated carbon mass, fabric type, filter porosity, gaseous removal time, and adsorption kinetics were also examined. The proposed filter displayed several advantages, including simplicity, compactness, dry design, ease of regeneration, scalability, durability, low cost, and good efficiency. Heat resistance, fire retardancy, mechanical stability, and the ability to remove other gasoline combustion products such as CO, SOx, NOx, VOCs, and particulates were also offered. The filtration system enabled both in situ and on-line CO2 real-time continuous emission monitoring.
1. Introduction
Carbon dioxide is a major component of the greenhouse gas family (GHG), emitted by human activities. It contributes more than 60% of global warming due to its increasing concentration in the atmosphere due to burning fossil fuels in industrial sites and in engines used to generate energy [1]. The increase in CO2 emissions due to the large consumption of fossil fuels has put the world at great risk if emissions continue to accumulate unabated in the atmosphere. This accumulation disturbs the global carbon cycle, leading to a global warming impact and climate change [2]. The effects of global warming range from ecological and physical to health impacts, including extreme weather events such as storms, crop growth, floods, heat waves, sea-level rise, and disrupted water systems [3,4]. Nowadays, many parts of the world severely suffer from all of these problems and need an urgent solution to stop the increase in atmospheric greenhouse gas (GHG) levels and their devastating impacts on climate change. Carbon dioxide removal (CDR) is now recognized as a critical component for achieving ambitious climate goals [5,6].
However, over the past two centuries, humankind has increased the concentration of CO2 in the atmosphere from 280 to more than 380 parts per million by volume, and it is still increasing every day [5]. Global carbon dioxide emissions increased from 0 in the year 1850 to 32,500 million metric tons in 2010, and they are expected to reach 39,000 million metric tons by 2030 [7]. According to the statistics, almost a quarter of carbon emissions are produced by the transportation sector due to emissions from combustion engines [8]. The international treaties concerned by legally binding targets to cut emissions of carbon dioxide and other greenhouse gases are those in the “Kyoto Protocol of 1997” [9] and the “Paris Agreement of 2016” [10]. According to the Intergovernmental Panel on Climate Change (IPCC), GHG emissions must be reduced by 50 to 80 percent by 2050 to avoid or reduce the dramatic dangerous consequences [11].
These treaties create a strong interest and stimulate worldwide efforts to control and reduce the emission of carbon dioxide from their sources. Many methods have been developed for capturing (CDC) and reducing or removing (CDR) carbon dioxide to meet the global goal established by these treaties and other UN regulations. Various treatment methods have been suggested for mitigation, including adsorption, absorption, condensation, membrane processes, cryogenic distillation, and catalytic oxidation. The advantages and limitations of these methods have been reviewed [12,13,14]. However, amine liquid scrubbing technology is the most widely used technique for removing CO2 from flue gases and industrial gas streams [15,16,17]. This technique involves the use of columns containing hazardous corrosive chemicals and requires continuous absorbent regeneration [18].
The dispersion and grafting of organic amines onto solid supports such as activated carbon, zeolites, polymeric resins, and metal–organic frameworks (MOFs) have shown a promising CO2 sorption performance due to the formation of carbamate or bicarbonate complexes with CO2 [19,20]. Moreover, recent studies have demonstrated that amine-functionalized polymers and amide-containing materials, including those based on cellulose and other porous supports, provide enhanced chemisorption efficiency and regeneration potential [21,22]. Given that Nomex is a poly (m-phenylene isophthalamide), its inherent amide groups may contribute to CO2 affinity through similar interactions.
The present investigation is undertaken to develop, examine, and evaluate the viability and effectiveness of a novel, simple, and dry system for CO2 removal from gasoline engine emissions. This study introduces a uniquely designed device composed of a nonwoven sandwich fabric structure made of poly (m-phenylene isophthalamide) (Nomex) combined with a thin activated carbon (AC) layer. Unlike conventional approaches, our design integrates a solid-phase amine-rich polymer with activated carbon, leveraging both physical adsorption and chemisorption mechanisms for enhanced CO2 capture. The Nomex component, containing approximately 12% nitrogen from secondary amine functional groups, interacts synergistically with AC to target acidic gaseous pollutants. This filter material is unique due to its mechanical robustness, thermal stability, flexibility, compactness, scalability, eco-friendliness, and cost-effectiveness, making it suitable for use under harsh operational conditions. Notably, the system enables both in situ and on-line CO2 monitoring, a practical advantage over conventional methods. These features mark a significant advancement compared to traditional amine scrubbing and adsorption-based techniques, highlighting the novelty and application potential of this study.
2. Experimental
2.1. Materials and Instruments
Poly (m-phenylene isophthalamide) (Nomex)-based nonwoven fabrics were obtained from DuPont (Wilmington, DE, USA) through Egypt-Tex Company (Cairo, Egypt) and were characterized in accordance with international standards. The molecular structure of Nomex, shown in Figure 1, illustrated its composition as poly (m-phenylene isophthalamide), which contributes to its exceptional thermal stability and mechanical properties. The mass per unit area (weight) of the textile fabric materials was measured using the Standard Test Method ASTM D3776/D3776M-20 [23]. Fabric thickness was evaluated according to the Standard Test Method ASTM D1777-96(2019) [24]. The air permeability of the fabric was determined using the Standard Test Method ASTM D737-04 [25] with a Toyoseiki (JIKA) air permeability tester (Toyoseiki Co., Ltd., Tokyo, Japan). The general characteristics of the used Nomex fabric were as follows: weight: 926–1083 g/m2, thickness: 4.40–4.80 mm, and air permeability: 0.498–7.680 L/m2.s.
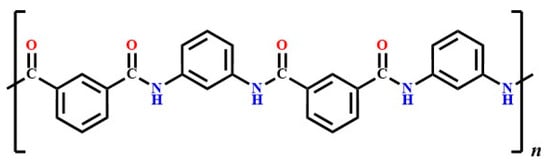
Figure 1.
Structure of Nomex (poly (m-phenylene isophthalamide)).
Activated carbon (AC), obtained from Laboratories Rasayan (LR) (Mumbai, Maharashtra, India), preheated at 60 °C for 24 h and stored in a desiccator until use. The activated carbon used had the following characteristics: particle size < 300 mesh (~50 μm), greater than 60%; acid-soluble fraction: 2.5%; water-soluble fraction: 1.5%; moisture content: 5%; ash content: 2.5%; pH value: ranging between 6.0 and 7.5; methylene blue adsorption using a 0.15% solution: more than 18 mL/0.1 g.
Scanning electron microscopy–energy dispersive X-ray analysis (HSEM-EDX) of the treated fabric was conducted using a high-resolution scanning electron microscope (SEM Quanta FEG 250 with a field emission gun, FEI Company, Hillsboro, OR, USA). Additionally, porosity and surface area assessments of the fabric were carried out using Brunauer–Emmett–Teller (BET) analysis by adsorption and desorption of nitrogen gas using a BELSORP MINI X automatic surface and pore size analyzer (MicrotracBEL Corp., Osaka, Japan).
2.2. Preparation of Nomex/Activated Carbon Filters
The preparation process of the Nomex/AC filters is illustrated in Figure 2. Different masses of activated carbon (250, 500, 750, and 1000 mg) were evenly distributed between two layers of nonwoven Nomex fabrics, each with a diameter of 10 cm. The sandwich filter was heated for 15 min at a temperature of 120–130 °C and a pressure of 520.5 kPa using a hydraulic press.
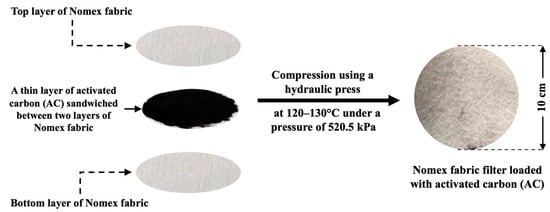
Figure 2.
Schematic diagram of the preparation steps for the Nomex/activated carbon sandwich filter.
2.3. Equipment for Sampling and Measuring Combustion Emissions
A low-cost, homemade apparatus with a Nomex/AC filter was designed to evaluate the filtration efficiency. The device was assembled and used for the sampling and evaluation of the gas emissions. The apparatus consisted of an air sampler unit connected to a suction pump and a pressure meter to maintain a consistent gas flow rate of 10 L/min during the sampling process. The examined sandwich filter was placed in the filter-holder located in the vacuum chamber, which received combustion exhaust gases emitted from the generator muffler. The apparatus components are shown schematically in Figure 3.
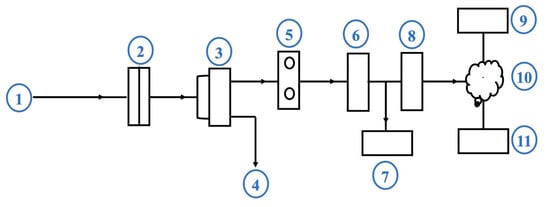
Figure 3.
Schematic diagram of the apparatus for sampling and measuring combustion emissions. Components: (1) gas inlet; (2) filter sample holder; (3) differential pressure gauge/vacuum pump; (4) gas outlet; (5) CO2 sensor module; (6) sensor interface; (7) digital detector; (8) gateway (Ethernet/GPRS/WIFI); (9) local server; (10) cloud; (11) mobile phone.
2.4. Emissions Monitoring
The concentrations of CO2 gas passing from the emission source through the filter were monitored using solid-state potentiometric and electrical–thermal gas sensors (Alphasense Ltd., Great Notley, UK), with a measurement range of 0 to 2000 ppm for CO2. These sensors were integrated into a custom gas monitoring system (GMS) built on an Internet of Things (IoT) cloud platform. The GMS was fully embedded within the filter apparatus to allow the seamless, real-time tracking of CO2 concentrations across the filtration process.
Data collected from the sensors were transmitted wirelessly via a General Packet Radio Service (GPRS) link using the Global System for Mobile Communications (GSM) network. Secure transmission was ensured using HTTPS POST requests and validated with security keys. The validated data were stored in a cloud-based database, allowing for remote access, processing, and visualization through mobile applications. The scalability of the IoT platform enabled efficient handling for large datasets and extended monitoring sessions. These measures were implemented to ensure cybersecurity and data integrity of the transmitted data, which is particularly critical for reliable operation in mobile and vehicular applications.
2.5. Adsorption Technique
The adsorption capabilities and removal effectiveness of the proposed sandwich filter were investigated at the adsorption equilibrium conditions. The concentrations of CO2 emissions before and after filtration were measured using a gas monitoring system (GMS) connected to a cloud-based Internet of Things (IoT) platform. The concentrations of CO2 gas passing from the emission source through the sandwich Nomex filter (10 cm diameter) containing a thin layer of activated carbon (1.0 g) as an adsorbent were measured. After reaching the equilibrium state, the adsorption capacity (mg/g) and removal efficiency (%) of the adsorbent were estimated using Equations (1) and (2) [17,26]:
where Co was the initial concentration of the gas pollutant (ppm), Ce the concentration of the gas pollutant at equilibrium (ppm), V flow rate of gas emission (L/min) and M the mass of activated carbon adsorbent (g).
Qe = (Co − Ce) × V/M
Removal efficiency of CO2 (%) = [(Co − Ce)/Co] × 100
2.6. Kinetic Modeling
The prepared Nomex/AC filter was exposed to CO2 emissions from a gasoline engine at different time intervals, and the kinetic studies of CO2 gas adsorption were conducted. The adsorption rates were analyzed and compared with pseudo-first-order and pseudo-second-order models, as described by Equations (3) and (4), respectively [27,28].
where qe and qt are the adsorption capacities (mg·g−1) of the adsorbent at equilibrium and at any time, respectively. t represents the contact time (min), while k1 (min−1) and k2 (g·mg−1·min−1) are the rate constants of the pseudo-first-order and pseudo-second-order adsorption models, respectively.
log(qe − qt) = log(qe) − k1 t/(2.303)
t/qt = 1/(k2 qe2) + t/qe
3. Results and Discussion
Different sandwich filters consisting of two Nomex layers containing activated carbon in-between were tested for the removal of CO2 from the emissions of gasoline combustion engines. Filters with a surface area of 78.54 cm2, loaded with 250, 500, 750, and 1000 mg of activated carbon per 78.54 cm2 were prepared, characterized, and tested.
3.1. Scanning Electron Microscopy (SEM) and Energy Dispersive X-Ray (EDX)
The scanning electron microscopy–energy dispersive X-ray analysis (SEM-EDX) technique was used to provide a non-destructive determination of the elemental identification and quantitative composition of the adsorbent sample. EDX analysis was used to confirm the existence of the elements in the engine exhaust, the adsorbed CO2, and other species from the engine exhaust on the filter. EDX spectra (elemental mapping patterns) of the Nomex fabric before and after the addition of activated carbon were measured and re-examined after passing the emissions from the gasoline engines.
The morphology of the nonwoven fabrics before the addition of activated carbon displayed a smooth fiber surface with a normal structure (Figure 4a). The morphological structure of the filter fabric after the addition of activated carbon showed a uniform distribution of activated carbon granules within all parts of the fabric filter (Figure 4b). However, a few irregular activated carbon granules were detected on the filter surface after filtration probably due to agglomerated carbon particles attached to the fibers during the filtration process.
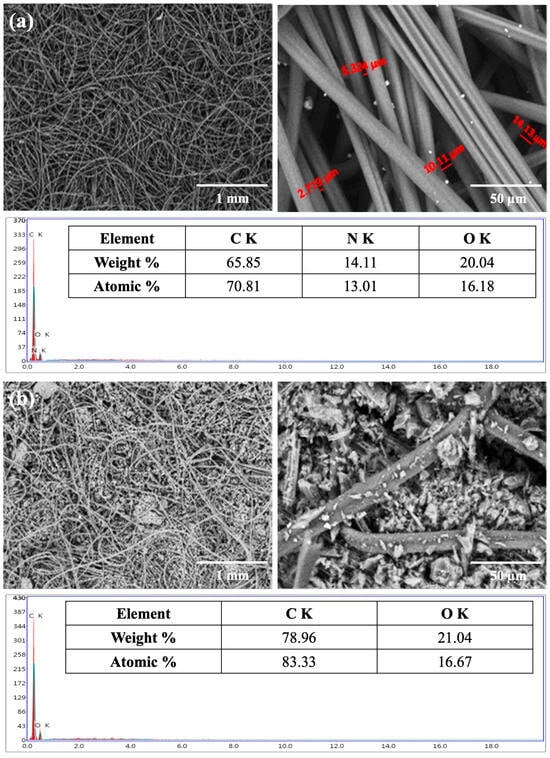
Figure 4.
SEM and EDX analyses of Nomex filters: (a) blank filter before loading with activated carbon, and (b) filter loaded with activated carbon prior to emission filtration.
EDX elemental examination revealed that the dominant elemental composition of the surface of the Nomex fabric (the blank) before the addition of activated carbon (Figure 4a) was carbon (70.81%), nitrogen (13.01%), and oxygen (16.18%), which was close to the constituents of Nomex: carbon (70.58%), nitrogen (11.76%), and oxygen (13.44%). After the addition of activated carbon to the Nomex filter, EDX showed (Figure 4b) the presence of only carbon with a higher percentage (83.33%) and oxygen (16.67%). The absence of nitrogen was attributed to the complete and homogeneous coverage of the fabric surface of the filter with the activated carbon.
Comparing the surface composition of the sandwich filter before and after CO2 filtration revealed an increase in carbon and the appearance of nitrogen and sulfur peaks. EDX showed (Figure 5) the presence of carbon (86.49%), oxygen (10.61%), nitrogen (2.71%), and sulfur (0.19%). Nitrogen and sulfur originated from nitrogen and sulfur oxides released during gasoline combustion and their subsequent retention on the carbon filter. The white patches visible in the SEM image are attributed to these retained combustion products, particularly NOx and SOx species, as well as possible particulate matter that agglomerates on the filter surface. These species tend to form localized accumulations at high-adsorption sites, appearing as bright regions due to their different atomic contrast in SEM imaging. The accompanying EDX spectrum and composition table confirm this interpretation, and the presence of nitrogen and sulfur, not originally detected in the pre-filtration filter, further supports their origin from engine exhaust gases.

Figure 5.
SEM and EDX analyses of Nomex filters after being loaded with activated carbon and following emission filtration.
3.2. Surface Area and Pore Size Distribution of the Filter
The Brunauer–Emmett–Teller (BET) method [29,30] was used to measure the surface area and porosity of the activated carbon (AC) sample used in the filter. Figure 6a,b depict the N2 adsorption–desorption isotherm and the pore size distribution of the sample, respectively. As shown in Figure 4a, the isotherm of the prepared sample displayed a hysteresis loop at relative pressures between 0.005 and 0.99, caused by the adsorption and desorption of N2 during the capillary condensation. According to the IUPAC classification system for adsorption isotherms, Figure 6a reveals a type-II isotherm, indicating that nanopores or macropores were filled with N2 gas to form a monolayer, followed by multilayer adsorption [31]. The measured surface area, total pore volume, and average pore diameter of the AC sample were 1010.5 m2/g, 0.5834 cm3/g, and 2.3096 nm, respectively.
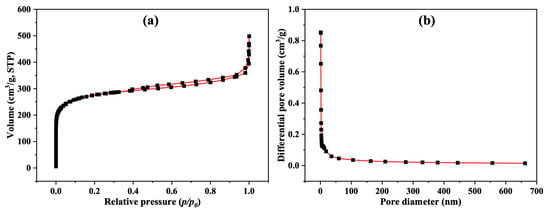
Figure 6.
(a) N2 adsorption–desorption isotherms of AC; (b) the corresponding pore size distribution.
3.3. Adsorption Kinetics
The relationship between the adsorbed carbon dioxide concentration and the mass of activated carbon adsorbent as a function of contact time was investigated. It was observed that the removal of CO2 gas increased gradually with time and reached a state of equilibrium after 7 min with a 70% removal efficiency. As illustrated in Figure 7a,b, pseudo-first-order and pseudo-second-order models were used to fit the obtained practical kinetics data. Since the pseudo-second order model displayed a higher correlation coefficient (R2 = 0.9919) than the pseudo-first-order model (R2 = 0.8207), and due to closer approximations between the practical and calculated data (qe and qeexp) (Table 1), it was concluded that the current adsorption process followed the second-order model. Since the present study primarily focused on adsorption kinetics and filter performance under ambient conditions, thermodynamic parameters such as the heat of sorption would not add practical useful information and were not determined.
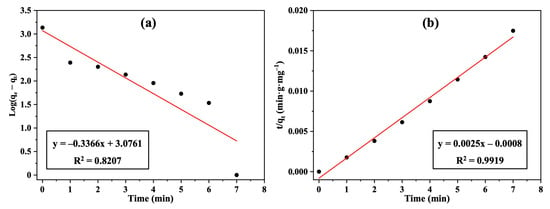
Figure 7.
Plots of (a) pseudo-first-order kinetics, and (b) pseudo-second-order kinetics for CO2 removal using the Nomex/AC filter.

Table 1.
Adsorption kinetic parameters for CO2 removal using pseudo-first-order and pseudo-second-order models.
3.4. Effect of Fabric Type
Fabrics made of polyester and polyamide (Nomex), with and without activated carbon (AC), were examined and compared for the removal of carbon dioxide as well as other fuel combustion products (e.g., carbon monoxide, sulfur dioxide, and nitrogen oxides). The results revealed that Nomex fabric without AC exhibited a noticeable affinity for removing acidic gases (CO2, SO2, and NOx), likely due to its basic nature, as well as for removing neutral gases (CO, VOC) owing to its inherent adsorption capacity. This affinity was significantly enhanced in the presence of AC, showing a synergistic effect. Furthermore, Nomex fabric demonstrated some significant advantages over polyester fabric, such as thermal stability, resistance to shrinkage and stretching, and superior overall efficiency.
The adsorption capacity observed in the Nomex/AC filter was attributed to both physical and chemical characteristics. The activated carbon provided a high surface area and porous structure, as confirmed by BET analysis, which promoted effective physisorption of CO2 molecules. Additionally, the Nomex fabric itself contained nitrogen-based functional groups, which facilitated chemical interactions with acidic gas species such as CO2. This combination of microporosity and chemical reactivity enhanced the overall adsorption performance of the filter.
3.5. Effect of the Mass of Activated Carbon (AC)
The effect of the mass of activated carbon (mg) per filter unit area (cm2) was tested. Four Nomex filters, each with a diameter of 10 cm, were loaded with 250, 500, 750, and 1000 mg of activated carbon (corresponding to 0.72, 1.44, 2.17, and 2.89 mg/cm2 of carbon per fabric area, respectively) and used for CO2 filtration. The emission removal efficiencies were 8.8 ± 0.4%, 43.8 ± 2.2%, 57 ± 2.9%, and 70 ± 3.4%, respectively, based on triplicate measurements (n = 3), as shown in Figure 8. This improvement in efficiency is attributed to the increased specific filtration surface area (SFSA) of the adsorbing material, which enhances the filtration efficiency.
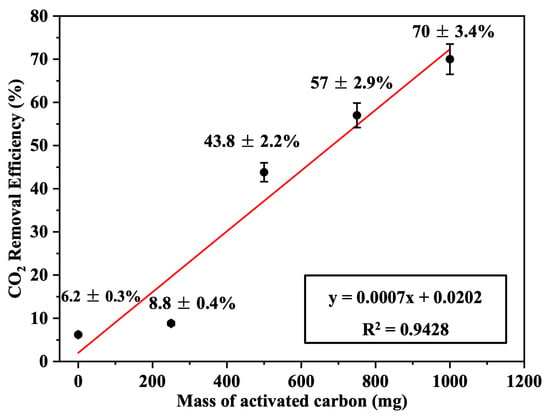
Figure 8.
CO2-removal efficiency as a function of the loaded mass of activated carbon (AC) in the Nomex/AC filter (n = 3, error bars represent standard deviation).
These results were compared with CO2-removal efficiencies obtained by activated carbon-based filters reported in the literature under practical or semi-commercial conditions. Although detailed specifications from commercial manufacturers are often unavailable, previous studies provide relevant performance benchmarks. For example, Maniarasu et al. [32] reported a maximum CO2-removal efficiency of approximately 50% using activated carbon derived from coconut shell in a compression ignition (CI) engine exhaust system. Similarly, Chue et al. [33] examined CO2 recovery from flue gas using commercial BPL activated carbon in a pressure swing adsorption (PSA) system and reported removal efficiencies around 53%. These systems were generally operated with larger filter masses or more complex configurations than those evaluated in the present study. In contrast, our compact, dry-type Nomex/AC sandwich filter achieved a higher removal efficiency of 70 ± 3.4% using only 2.89 mg/cm2 of activated carbon under ambient conditions. This highlights the material synergy, optimized structure, and enhanced adsorption efficiency of the proposed filter, suggesting practical advantages over comparable systems in terms of both performance and adsorbent utilization.
3.6. Effect of Filter Thickness
The thickness of the Nomex filters loaded with 250, 500, 750, and 1000 mg of activated carbon was measured and found to be 4.47 ± 0.02, 4.60 ± 0.02, 4.68 ± 0.02, and 4.80 ± 0.02 mm, respectively, based on triplicate measurements (n = 3). The thickness of the blank filter (0 mg carbon) was 4.40 mm. Figure 9 displays a linear increase in the filter thickness as a function of the loaded activated carbon mass, with a good positive correlation (R2 = 0.9923). This indicates a uniform and homogeneous distribution of activated carbon on the surface of the fabric filter under the applied temperature and pressure used during the preparation process.
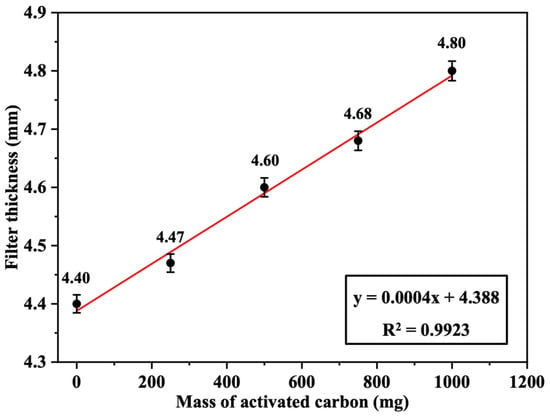
Figure 9.
Filter thickness as a function of the loaded mass of activated carbon (AC) (n = 3, error bars represent standard deviation).
3.7. Effect of Filter Air Permeability
The air permeability of the Nomex sandwich filters loaded with 0, 250, 500, 750, and 1000 mg of activated carbon per filter was measured and found to be 7.62 ± 0.21, 6.61 ± 0.19, 2.57 ± 0.15, 0.93 ± 0.09, and 0.50 ± 0.08 cm3/cm2/s, respectively, based on triplicate measurements (n = 3). As shown in Figure 10, this demonstrated a linear decrease in filter air permeability as the mass of activated carbon increased. The negative correlation was high (R2 = 0.9226) and probably attributed to the high density of carbon, which reduced the airflow across the filter fabric.
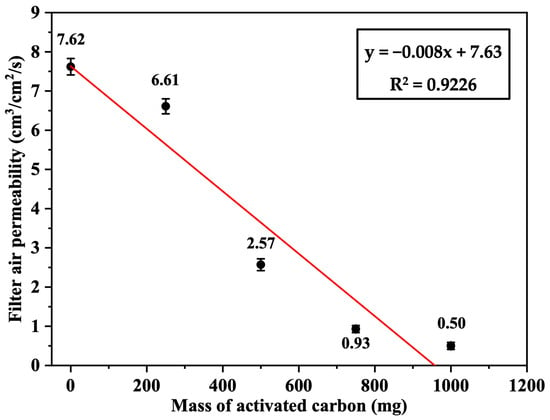
Figure 10.
Air permeability of the Nomex/AC filter as a function of the loaded mass of activated carbon (AC) (n = 3, error bars represent standard deviation).
3.8. Effect of Time and Regeneration on CO2 Removal Efficiency
The removal efficiency of CO2 as a function of time over a 15 min period was examined using the Internet of Things (IoT)-based gas monitoring system described in Section 2.4. Engine emissions were passed through the proposed Nomex/AC filter loaded with 1.0 g of activated carbon, and the average concentration of CO2 (mg/m3) was continuously monitored in real time using an embedded solid-state CO2 gas sensor.
The system transmitted data every 5 s to a secured cloud server, enabling the real-time monitoring and analysis of filter performance. This monitoring system allowed for immediate feedback during operation, making it highly suitable for adaptive control and practical deployment in mobile or remote environments. Sensor calibration was performed using certified CO2 gas mixtures to ensure accurate and reliable readings within the 0–2000 ppm range. The results showed that CO2 concentration reached a steady state after 7 min, with a removal efficiency of 70 ± 3.4%. These findings confirm the effective integration and real-time functionality of the IoT-based system for continuous and accurate emission monitoring.
To evaluate the operational stability and regeneration potential of the Nomex/AC filter under conditions simulating prolonged exposure to gasoline combustion emissions, seven consecutive CO2 adsorption–desorption cycles were conducted. Each cycle involved 15 min of exposure to engine exhaust, followed by thermal regeneration at 120 °C for 1 h. The filter maintained a consistent CO2-removal efficiency of approximately 70 ± 3.4% throughout the first four cycles (equivalent to 60 min of total filtration time), after which a gradual performance deterioration was observed. This behavior suggested partial saturation of the adsorption sites or the irreversible binding of combustion byproducts over time. Although the elevated regeneration temperature restores short-term performance, it may not fully desorb all of the retained CO2 under repeated thermal and chemical stress. Overall, the filter demonstrated stable performance under continuous operation for at least four successive cycles, confirming its durability and reusability for practical emission control applications.
3.9. Comparison of Some Methods Used for CO2 Emission Removal
A comparison of the general characteristics of some previously suggested CO2-removal methods and the present proposed method is presented in Table 2. It can be seen that many of the methods used suffered from some significant disadvantages, such as time consumption, the use of corrosive or difficult-to-prepare adsorbent/absorbing materials, operation under non-standard temperature and pressure conditions, and a complicated setup for application.

Table 2.
Comparison of the efficiency of various methods used for the removal of CO2 emissions.
However, the proposed method is eco-friendly, cost-effective, durable, scalable, and easily processable. The simple proposed compact dry design required minimal manipulation while maintaining good efficiency. The filter material (Nomex) had several advantages such as being heat-resistant, fire-retardant, un-stretchable, non-shrinkable, mechanically stable, chemically inert, and having the ability to remove other gasoline combustion products (e.g., CO, SO2, NOx, VOCs, and particulates).
It can be seen that the use of the proposed Nomex/activated carbon sandwich filter system for CO2 removal offered clear advantages over many conventional systems such as amine-based scrubbers [34], PSA units [33], and MOFs [44]. It provided a 70 ± 3.4% CO2-removal efficiency under ambient conditions. These traditional systems often require high energy input, chemical regeneration, or complex infrastructure. However, the present filtration system involved the use of much lower carbon loading compared with 2.89 mg/cm2 for the present dry, compact, and eco-friendly design, which also minimized the environmental impact and made it more suitable for mobile or decentralized applications.
4. Conclusions
A simple, compact, and portable filtration system consisting of a sandwich of Nomex fabric containing a thin layer of activated carbon was described for the removal of CO2 from the exhaust emissions of gasoline engines. The influence of various experimental parameters was examined and optimized to achieve about a 70 ± 3.4% CO2-removal efficiency within 7 min. The proposed solid dry filter system displayed many advantages in terms of simplicity, compact design, scalability, durability, cost-effectiveness, and stability under harsh conditions. Additionally, the filter effectively removed other gaseous combustion products, such as SOx, NOx, CO, VOCs, and particulates, with 50–95% removal efficiencies. In situ and on-line emission monitoring and data management were conducted through an Internet of Things (IoT)-based gas monitoring system, using mobile internet via the Global System for Mobile Communications (GSM) network and General Packet Radio Service (GPRS) links for secure cloud storage, real-time observation, and remote management. The scalability of the system will permit its application in industrial zones, transportation networks, and urban settings, by adapting the technology to higher-volume filtration and integrating it into existing infrastructures. Its widespread adoption could lead to significant societal benefits, including enhanced public health by reducing respiratory diseases caused by air pollution and contributing to global efforts to meet emission reduction targets under the Paris Agreement.
Author Contributions
Conceptualization, S.S.M.H. and M.A.F.; Methodology, N.R.G.M. and M.A.F.; Investigation, N.R.G.M., M.M.A.S., Y.H.I. and M.A.F.; Formal analysis, M.A.F.; Data curation, M.A.F.; Resources, M.M.A.S., Y.H.I. and A.A.E.; Writing—original draft preparation, N.R.G.M. and M.A.F.; Writing—review and editing, S.S.M.H. and M.A.F.; Supervision, S.S.M.H., M.M.A.S., Y.H.I. and A.A.E.; Visualization, M.A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Ethical Committee of the Faculty of Science, Ain Shams University, Cairo, Egypt (Code: ASU-SCI/CHEM/2024/3/2).
Data Availability Statement
The raw data supporting the findings of this study are available from the corresponding authors, S.S.M. Hassan or M.A. Fathy, upon reasonable request.
Conflicts of Interest
The authors declare no competing interests.
References
- Jawad, Z.A. Membrane Technology for CO2 Sequestration; CRC Press: Boca Raton, FL, USA, 2019; Available online: https://books.google.com.eg/books?id=lQaQDwAAQBAJ (accessed on 1 November 2022).
- Moussa, R.R. Reducing carbon emissions in Egyptian roads through improving the streets quality. Environ. Dev. Sustain. 2023, 25, 4765–4786. [Google Scholar] [CrossRef]
- IEA. Global Energy & CO2 Status Report 2018; International Energy Agency: Paris, France, 2019; Available online: https://www.iea.org/reports/global-energy-co2-status-report-2019 (accessed on 31 August 2022).
- Ritchie, H.; Roser, M.; Rosado, P. CO2 and Greenhouse Gas Emissions. Our World Data. 2020. Available online: https://ourworldindata.org/greenhouse-gas-emissions (accessed on 8 March 2021).
- Morrow, D.R.; Thompson, M.S.; Anderson, A.; Batres, M.; Buck, H.J.; Dooley, K.; Geden, O.; Ghosh, A.; Low, S.; Njamnshi, A.; et al. Principles for thinking about carbon dioxide removal in just climate policy. One Earth 2020, 3, 150–153. [Google Scholar] [CrossRef]
- Smith, S.; Geden, O.; Gidden, M.; Lamb, W.; Nemet, G.; Minx, J.; Buck, H.; Burke, J.; Cox, E.; Edwards, M. The State of Carbon Dioxide Removal. 2024. Available online: https://pure.iiasa.ac.at/id/eprint/19787/1/The-State-of-Carbon-Dioxide-Removal-2Edition.pdf (accessed on 8 March 2025).
- Gilfillan, D.; Marland, G. CDIAC-FF: Global and national CO2 emissions from fossil fuel combustion and cement manufacture: 1751–2017. Earth Syst. Sci. Data 2021, 13, 1667–1680. [Google Scholar] [CrossRef]
- Moussa, R.R. The Effect of Piezo-Bumps on Energy Generation and Reduction of the Global Carbon Emissions. 2019. Available online: https://buescholar.bue.edu.eg/cgi/viewcontent.cgi?article=1005&context=arch_eng (accessed on 6 October 2022).
- Nations, U. Kyoto Protocol to the United Nations Framework Convention on Climate Change. 1998. Available online: https://unfccc.int/resource/docs/convkp/kpeng.pdf (accessed on 16 May 2023).
- Bodansky, D.; Brunnée, J.; Rajamani, L. Paris Agreement. United Nations Audiovisual Library of International Law. 2015. Available online: https://legal.un.org/avl/pdf/ha/pa/pa_e.pdf (accessed on 15 February 2021).
- da Graça Carvalho, M. EU energy and climate change strategy. Energy 2012, 40, 19–22. [Google Scholar] [CrossRef]
- Songolzadeh, M.; Soleimani, M.; Takht Ravanchi, M.; Songolzadeh, R. Carbon dioxide separation from flue gases: A technological review emphasizing reduction in greenhouse gas emissions. Sci. World J. 2014, 2014, 828131. [Google Scholar] [CrossRef] [PubMed]
- Raganati, F.; Miccio, F.; Ammendola, P. Adsorption of carbon dioxide for post-combustion capture: A review. Energy Fuels 2021, 35, 12845–12868. [Google Scholar] [CrossRef]
- Ramezani, R.; Di Felice, L.; Gallucci, F. A review on hollow fiber membrane contactors for carbon capture: Recent advances and future challenges. Processes 2022, 10, 2103. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, B.; Li, L. Advance in post-combustion CO2 capture with alkaline solution: A brief review. Energy Procedia 2012, 14, 1515–1522. [Google Scholar] [CrossRef]
- Rao, A.B.; Rubin, E.S. A technical, economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control. Environ. Sci. Technol. 2002, 36, 4467–4475. [Google Scholar] [CrossRef]
- Veawab, A.; Tontiwachwuthikul, P.; Chakma, A. Corrosion behavior of carbon steel in the CO2 absorption process using aqueous amine solutions. Ind. Eng. Chem. Res. 1999, 38, 3917–3924. [Google Scholar] [CrossRef]
- Hassan, H.M.; Alruwaili, M.S.; Alsohaimi, I.H.; El-Hashemy, M.A.; Alqadami, A.A.; Alshammari, K.; Alali, I.K.; Alanazi, S.J.; El-Aassar, M. Electrospun polyacrylonitrile nanofiber composites integrated with Al-MOF/mesoporous carbon for superior CO2 capture and VOC removal. Diam. Relat. Mater. 2024, 149, 111649. [Google Scholar] [CrossRef]
- Ünveren, E.E.; Monkul, B.Ö.; Sarıoğlan, Ş.; Karademir, N.; Alper, E. Solid amine sorbents for CO2 capture by chemical adsorption: A review. Petroleum 2017, 3, 37–50. [Google Scholar] [CrossRef]
- Akaya, H.; Lamnini, S.; Sehaqui, H.; Jacquemin, J. Amine-Functionalized Cellulose as Promising Materials for Direct CO2 Capture: A Review. ACS Appl. Mater. Interfaces 2025, 17, 16380–16395. [Google Scholar] [CrossRef]
- Drzeżdżon, J.; Dai, S.; Fan, H.; Sikorski, A.; Baluk, M.A.; Datta, J.; Ranskiy, A.; Jacewicz, D. Synthesis of nanoporous poly (2-chloro-2-propen-1-ol) and its modification via ethylenediamine: Vanadyl-catalyzed process, structural characterization, and CO2 sorption. React. Chem. Eng. 2025. [Google Scholar] [CrossRef]
- ASTM D3776/D3776M-20; Standard Test Methods for Mass Per Unit Area (Weight) of Fabric. ASTM International: West Conshohocken, PA, USA, 2020. [CrossRef]
- ASTM D1777-96(2019); Standard Test Method for Thickness of Textile Materials. ASTM International: West Con-shohocken, PA, USA, 2019. [CrossRef]
- ASTM D737-04; Standard Test Method for Air Permeability of Textile Fabrics. ASTM International: West Conshohocken, PA, USA, 2004. [CrossRef]
- Hassan, S.S.M.; El-Shalakany, H.H.; Fathy, M.A.; Kamel, A.H. A magnetic macroporous α-Fe2O3/Mn2O3 nanocomposite as an efficient adsorbent for simple and rapid removal of Pb (II) from wastewater and electronic waste leachate. Environ. Sci. Pollut. Res. 2024, 31, 65648–65660. [Google Scholar] [CrossRef]
- Fathy, M.A.; Kamel, A.H.; Hassan, S.S.M. Novel magnetic nickel ferrite nanoparticles modified with poly (aniline-co-o-toluidine) for the removal of hazardous 2, 4-dichlorophenol pollutant from aqueous solutions. RSC Adv. 2022, 12, 7433–7445. [Google Scholar] [CrossRef]
- Simonin, J.-P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Sinha, P.; Datar, A.; Jeong, C.; Deng, X.; Chung, Y.G.; Lin, L.-C. Surface area determination of porous materials using the Brunauer–Emmett–Teller (BET) method: Limitations and improvements. J. Phys. Chem. C 2019, 123, 20195–20209. [Google Scholar] [CrossRef]
- Saberi, M.; Rouhi, P. Extension of the Brunauer-Emmett-Teller (BET) model for sorption of gas mixtures on the solid substances. Fluid Phase Equilibria 2021, 534, 112968. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Maniarasu, R.; Rathore, S.K.; Murugan, S. Preparation, characterization, and performance of activated carbon for CO2 adsorption from CI engine exhaust. Greenh. Gases Sci. Technol. 2022, 12, 284–304. [Google Scholar] [CrossRef]
- Chue, K.; Kim, J.; Yoo, Y.; Cho, S.; Yang, R. Comparison of activated carbon and zeolite 13X for CO2 recovery from flue gas by pressure swing adsorption. Ind. Eng. Chem. Res. 1995, 34, 591–598. [Google Scholar] [CrossRef]
- Sun, W.; Shao, Y.; Zhao, L.; Wang, Q. Co-removal of CO2 and particulate matter from industrial flue gas by connecting an ammonia scrubber and a granular bed filter. J. Clean. Prod. 2020, 257, 120511. [Google Scholar] [CrossRef]
- Sepahvand, S.; Jonoobi, M.; Ashori, A.; Gauvin, F.; Brouwers, H.; Oksman, K.; Yu, Q. A promising process to modify cellulose nanofibers for carbon dioxide (CO2) adsorption. Carbohydr. Polym. 2020, 230, 115571. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Tsai, M.T.; Kao, C.Y.; Ong, S.C.; Lin, C.S. The air-lift photobioreactors with flow patterning for high-density cultures of microalgae and carbon dioxide removal. Eng. Life Sci. 2009, 9, 254–260. [Google Scholar] [CrossRef]
- Qi, G.; Fu, L.; Choi, B.H.; Giannelis, E.P. Efficient CO2 sorbents based on silica foam with ultra-large mesopores. Energy Environ. Sci. 2012, 5, 7368–7375. [Google Scholar] [CrossRef]
- Romero-Guzmán, L.; Reyes-Gutiérrez, L.R.; Romero-Guzmán, E.T.; Savedra-Labastida, E. Carbon nanotube filters for removal of air pollutants from mobile sources. J. Miner. Mater. Charact. Eng. 2017, 6, 105–118. [Google Scholar] [CrossRef][Green Version]
- Mulukutla, T.; Obuskovic, G.; Sirkar, K.K. Novel scrubbing system for post-combustion CO2 capture and recovery: Experimental studies. J. Membr. Sci. 2014, 471, 16–26. [Google Scholar] [CrossRef]
- Zareie-kordshouli, F.; Lashani-zadehgan, A.; Darvishi, P. Comparative evaluation of CO2 capture from flue gas by [Emim][Ac] ionic liquid, aqueous potassium carbonate (without activator) and MEA solutions in a packed column. Int. J. Greenh. Gas Control 2016, 52, 305–318. [Google Scholar] [CrossRef]
- Shirazian, S.; Ashrafizadeh, S. Mass transfer simulation of carbon dioxide absorption in a hollow-fiber membrane contactor. Sep. Sci. Technol. 2010, 45, 515–524. [Google Scholar] [CrossRef]
- Durán-Muñoz, F.; Romero-Ibarra, I.C.; Pfeiffer, H. Analysis of the CO2 chemisorption reaction mechanism in lithium oxosilicate (Li8SiO6): A new option for high-temperature CO2 capture. J. Mater. Chem. A 2013, 1, 3919–3925. [Google Scholar] [CrossRef]
- Nur, M.; Sumariyah, S.; Suseno, A. Removal of emission gas COx, NOx and SOx from automobile using non-thermal plasma. In Proceedings of the 13th Joint Conference on Chemistry (13th JCC), Semarang, Indonesia, 7–8 September 2018; p. 012085. [Google Scholar] [CrossRef]
- Xiang, Z.; Hu, Z.; Cao, D.; Yang, W.; Lu, J.; Han, B.; Wang, W. Metal–organic frameworks with incorporated carbon nanotubes: Improving carbon dioxide and methane storage capacities by lithium doping. Angew. Chem. Int. Ed. 2011, 50, 491–494. [Google Scholar] [CrossRef]
- Lee, Z.H.; Lee, K.T.; Bhatia, S.; Mohamed, A.R. Post-combustion carbon dioxide capture: Evolution towards utilization of nanomaterials. Renew. Sustain. Energy Rev. 2012, 16, 2599–2609. [Google Scholar] [CrossRef]
- Choi, W.-K.; Kwon, T.-I.; Yeo, Y.-K.; Lee, H.; Song, H.K.; Na, B.-K. Optimal operation of the pressure swing adsorption (PSA) process for CO2 recovery. Korean J. Chem. Eng. 2003, 20, 617–623. [Google Scholar] [CrossRef]
- Tlili, N.; Grévillot, G.; Vallières, C. Carbon dioxide capture and recovery by means of TSA and/or VSA. Int. J. Greenh. Gas Control 2009, 3, 519–527. [Google Scholar] [CrossRef]
- Zhang, J.; Webley, P.A.; Xiao, P. Effect of process parameters on power requirements of vacuum swing adsorption technology for CO2 capture from flue gas. Energy Convers. Manag. 2008, 49, 346–356. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).