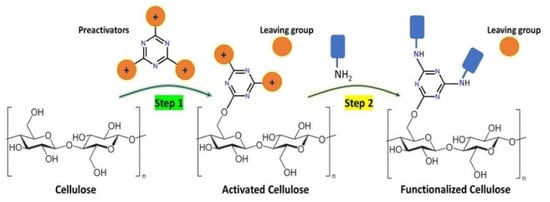
Cellulose Functionalization Using N-Heterocyclic-Based Leaving Group Chemistry
Abstract
1. Introduction
2. Materials and Methods
2.1. General Materials and Methods
2.1.1. Chemicals and Instruments
2.1.2. Chromatography
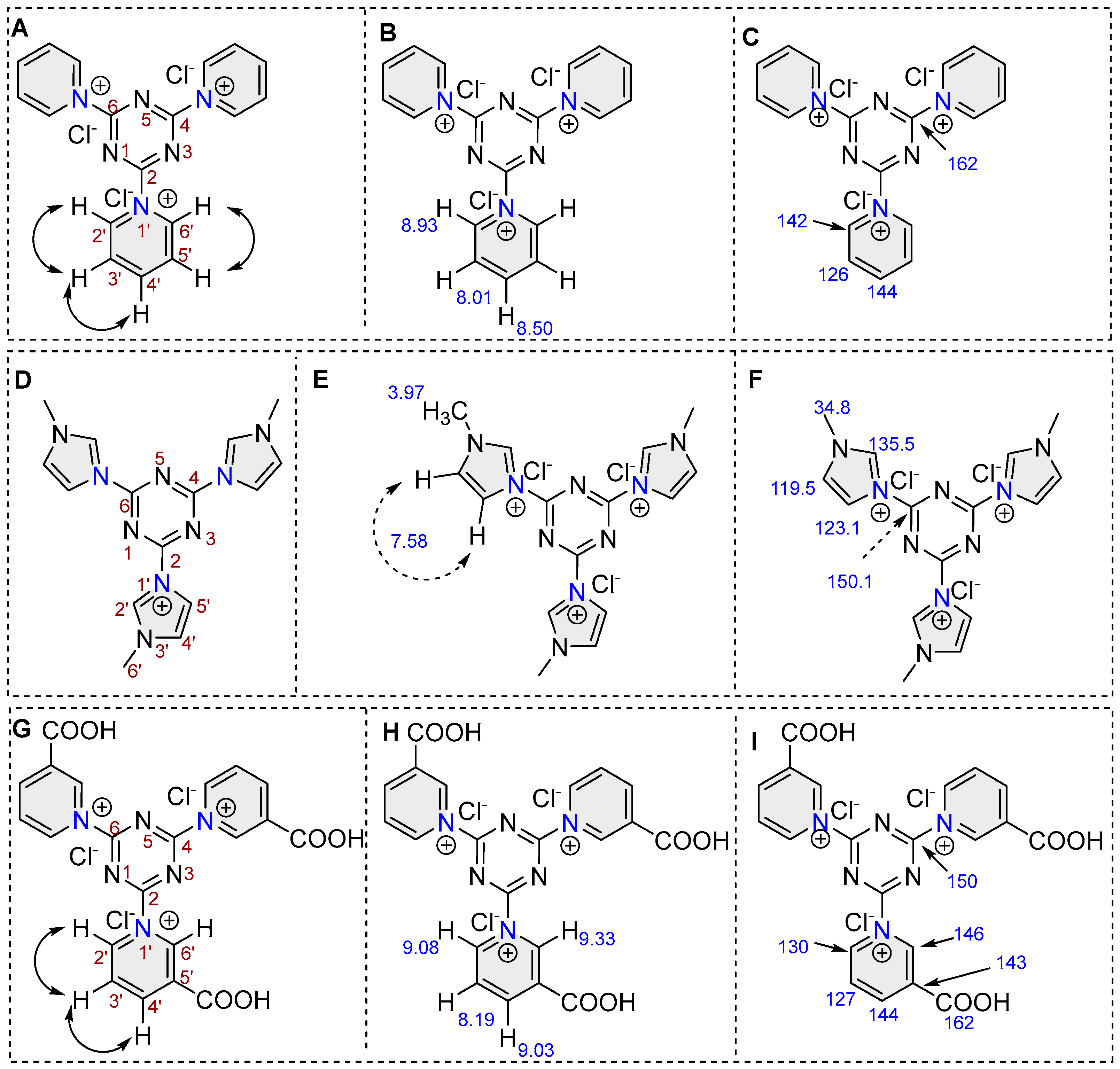
2.1.3. NMR Spectroscopy
2.1.4. Zeta Potential Measurements
2.1.5. Reflectance Spectroscopy
2.1.6. Fourier-Transform Infrared Spectroscopy
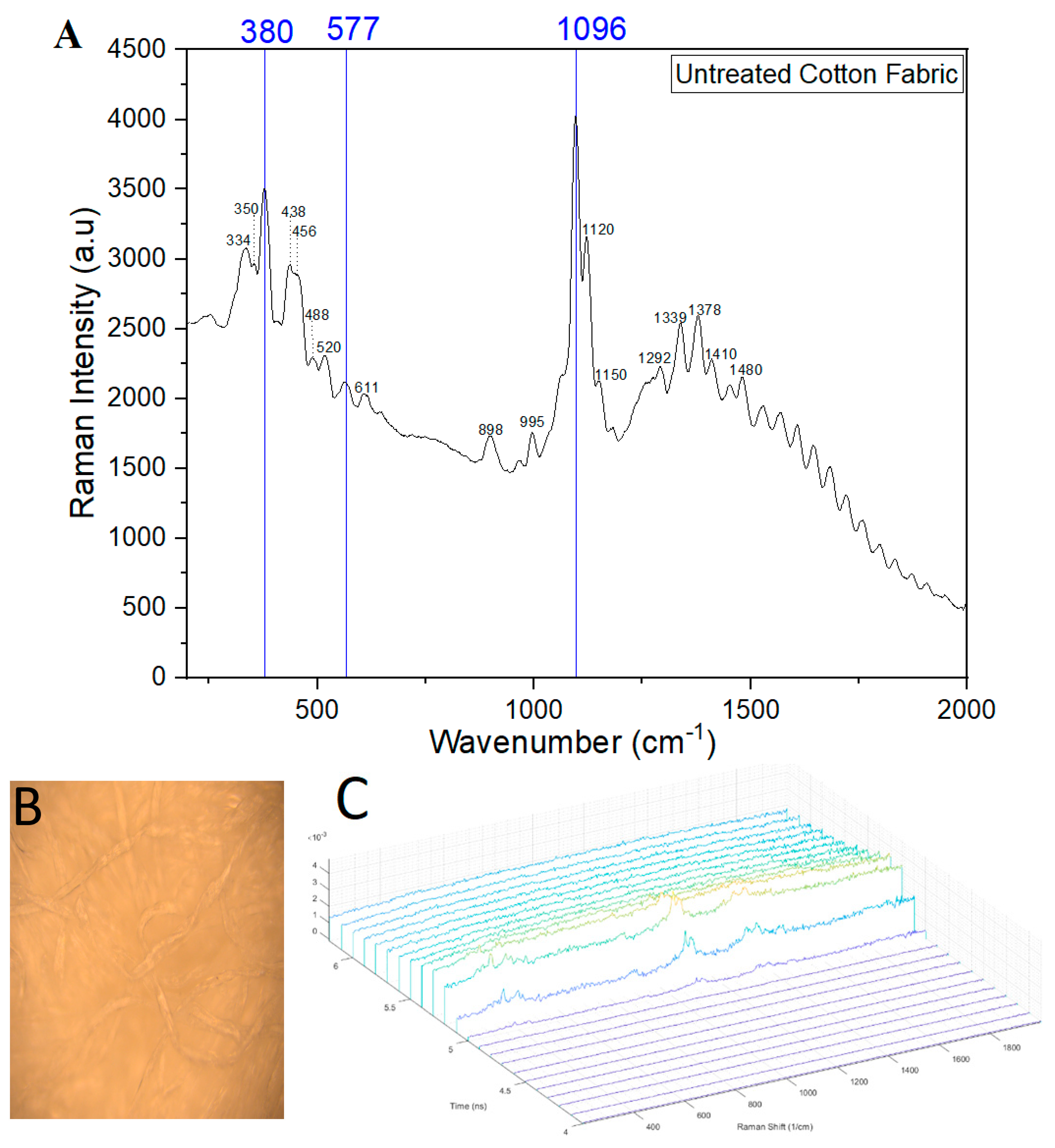
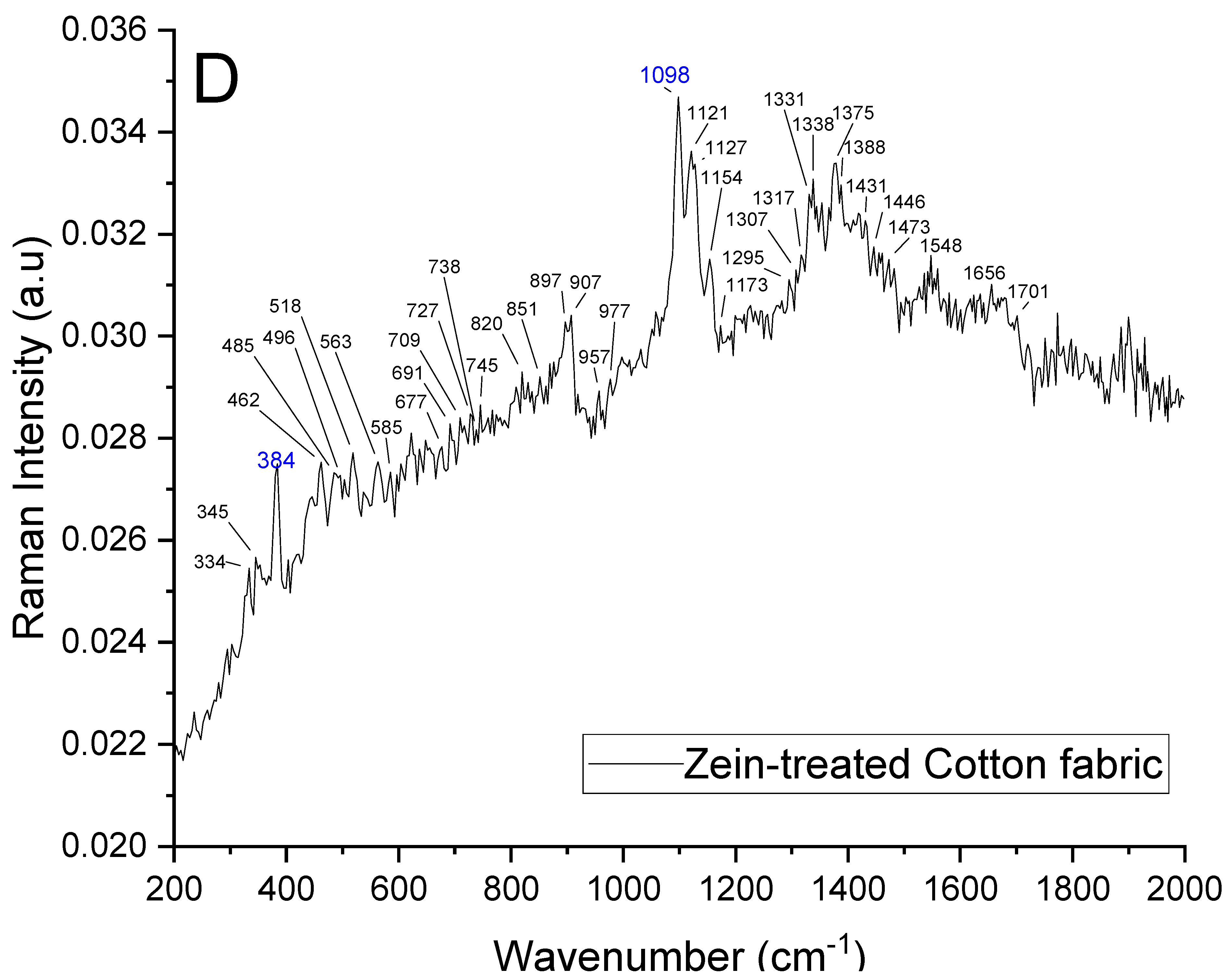
2.1.7. Time-Gated Raman Spectroscopy
2.2. Synthesis, Purification, and Characterization
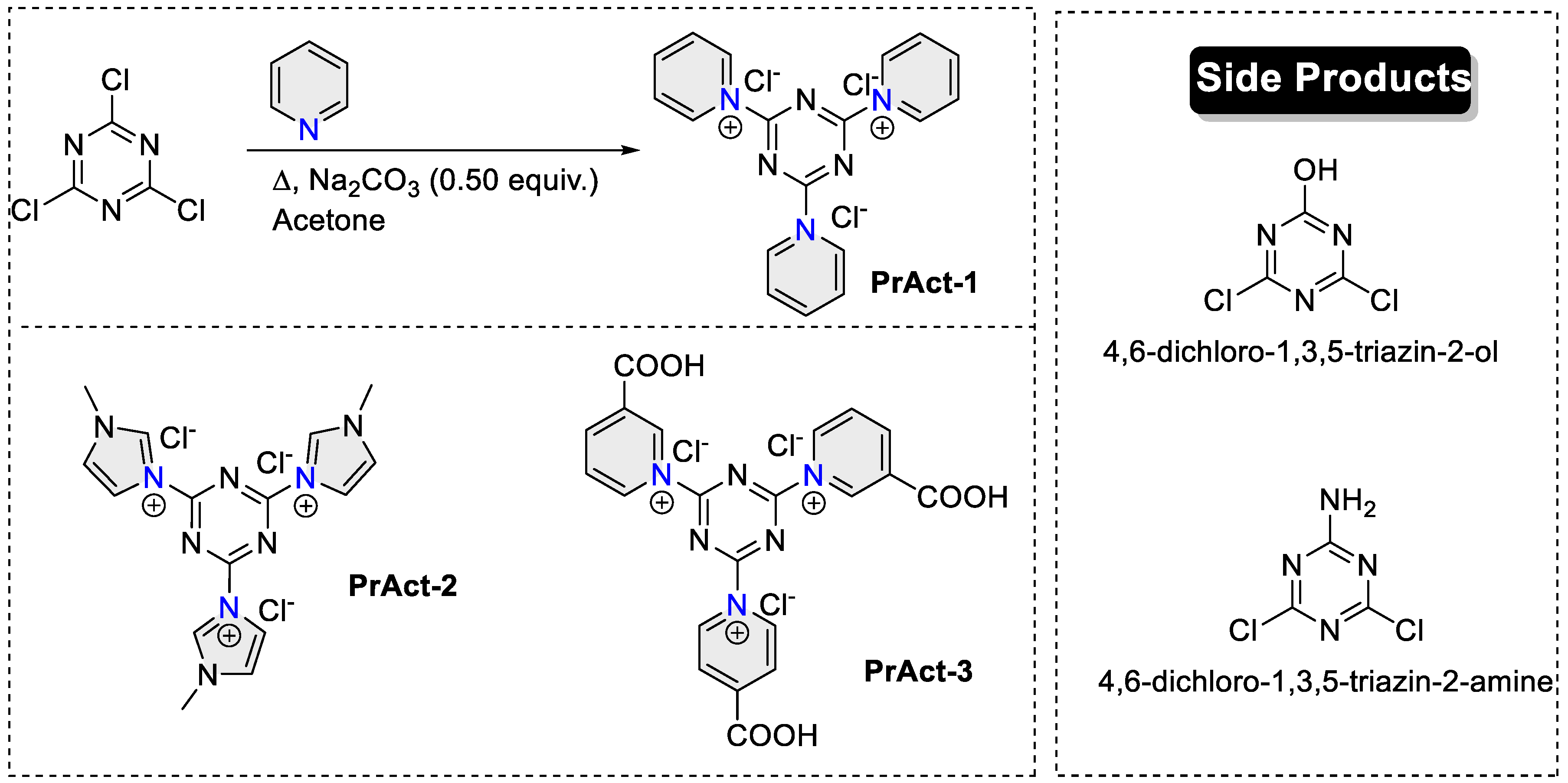
2.2.1. 1,1′,1″-(1,3,5-Triazine-2,4,6-triyl)tris(pyridin-1-ium) (PrAct-1)
2.2.2. 3,3′,3″-(1,3,5-Triazine-2,4,6-triyl)tris(1-methyl-1H-imidazol-3-ium) (PrAct-2)
2.2.3. 1,1′,1″-(1,3,5-Triazine-2,4,6-triyl)tris(3-carboxypyridin-1-ium) (PrAct-3)
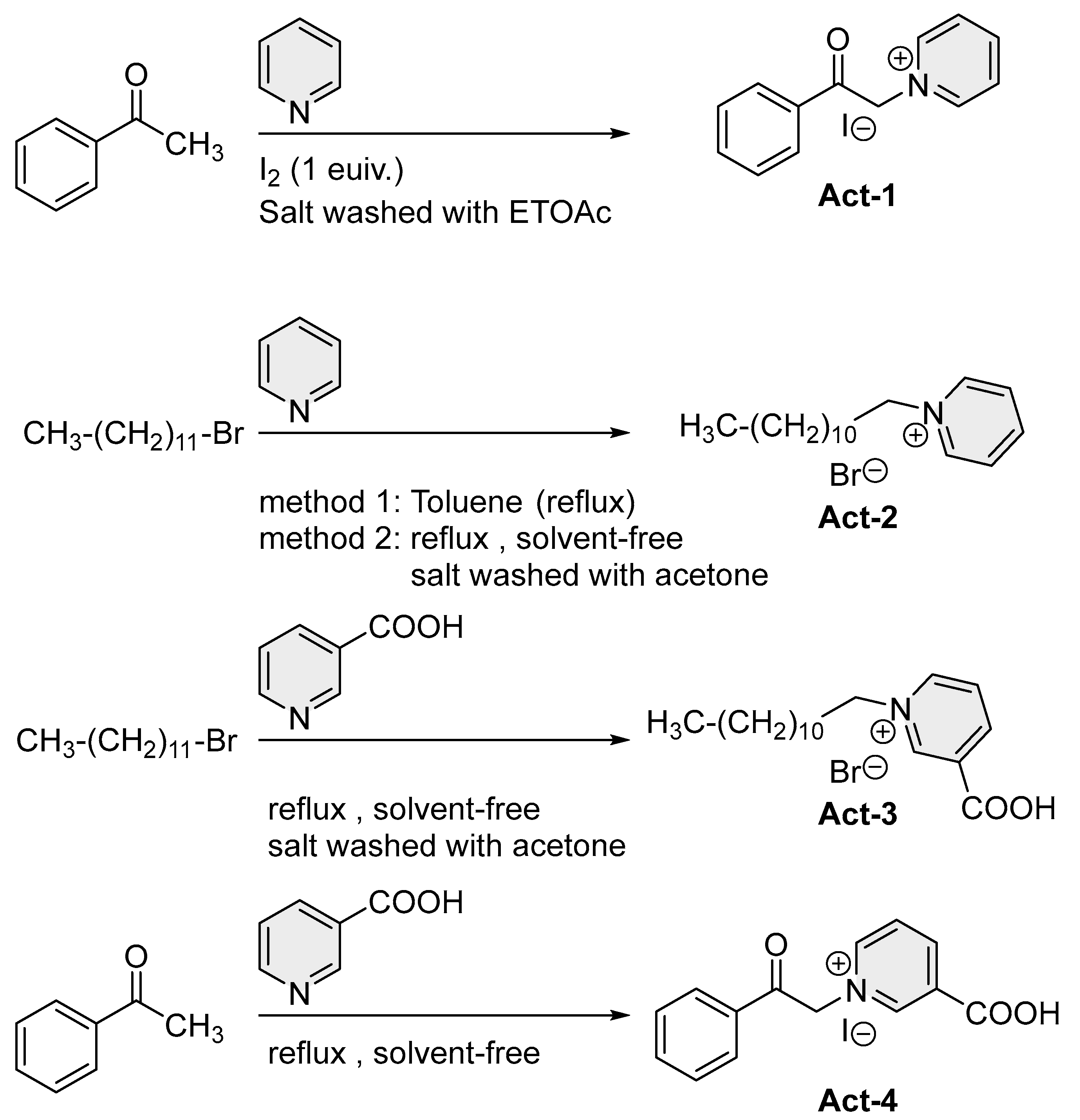
2.2.4. 2-Pyridinium Methyl Phenyl Ketone (Act-1)
2.2.5. 2-Pyridinium Methyl Decanyl Ketone (Act-2)
2.2.6. 3-Carboxy-1-Dodecylpyridin-1-Ium (Act-3)
2.2.7. 2-Nicotinyl Methyl Phenyl ketone (Act-4)
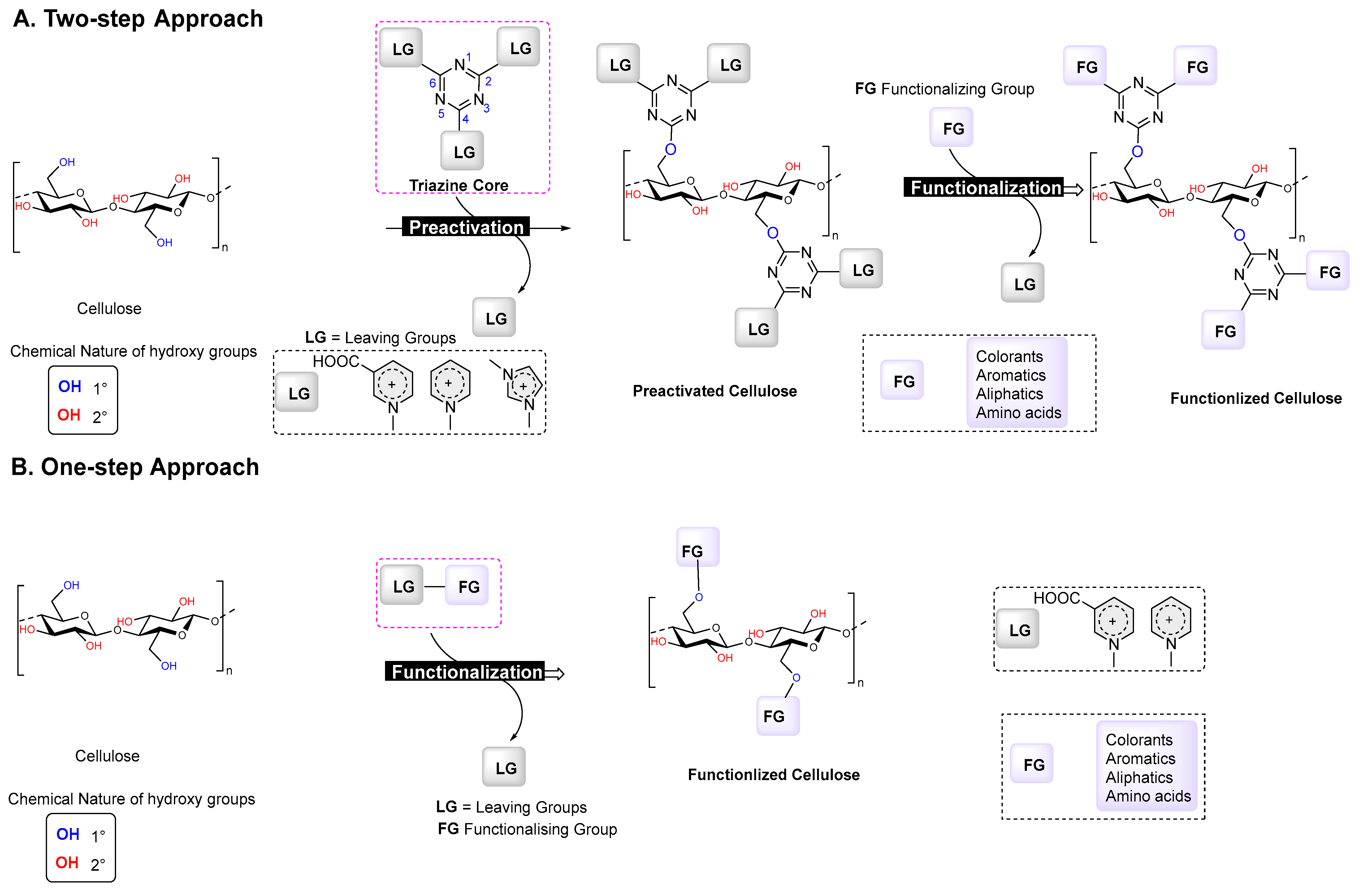
2.3. Two-Step Functionalization of Cellulose
2.3.1. Cellulose Preactivation (Step 1)
2.3.2. Functionalization of the Preactivated Cellulose Material (Step 2)
Functionalization with Choline
Functionalization with Colorants
Functionalization with Amino Acids and Peptides
Functionalization with Aromatic and Aliphatic Moieties
2.4. One-Step Functionalization of Cellulose
3. Results
3.1. Chemistry
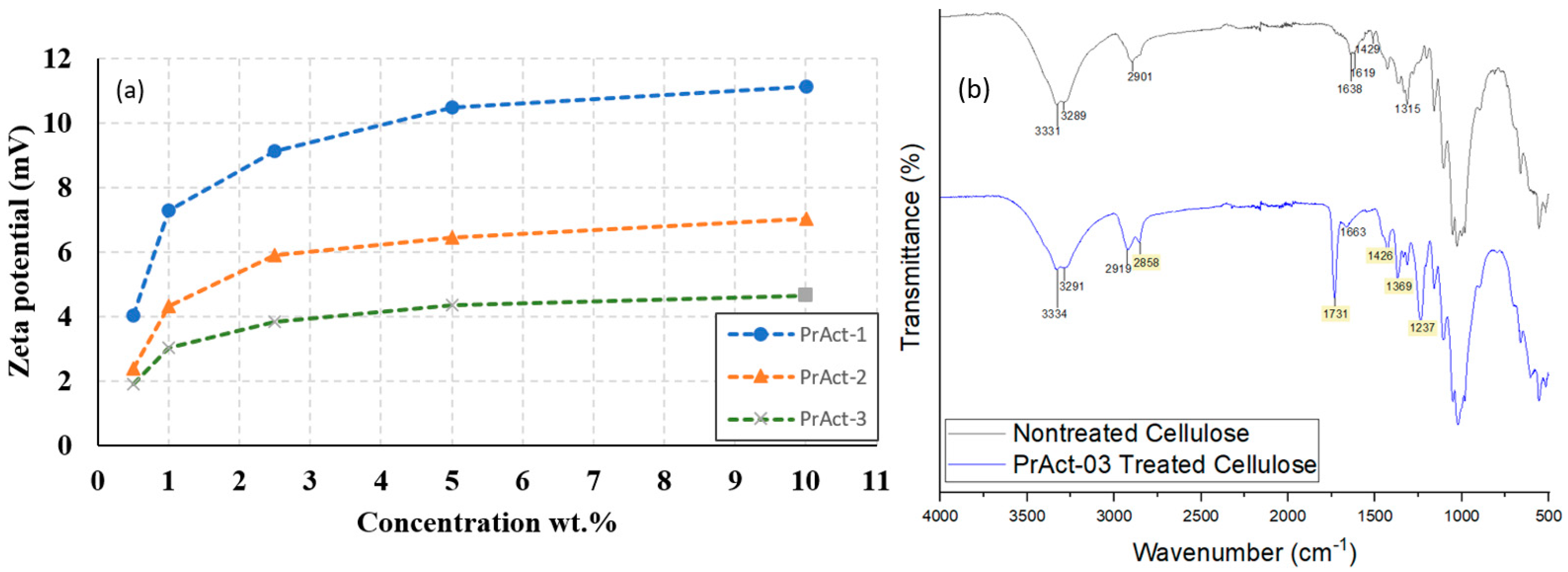
3.2. Cellulose Treatment with Preactivators
3.3. Optimization of Preactivation-Treated Cellulose with Functionalizing Agents
3.4. Functionalization of Preactivated Cellulose with Amine-Containing Colorants
3.5. Functionalization of Preactivated Cellulose with Amino Acids and Peptides
3.6. Functionalization of Preactivated Cellulose with Hydrocarbons
3.7. One-Step Cellulose Functionalization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mokhena, T.C.; Sadiku, E.R.; Mochane, M.J.; Ray, S.S.; John, M.J.; Mtibe, A. Mechanical properties of cellulose nanofibril papers and their bionanocomposites: A review. Carbohydr. Polym. 2021, 273, 118507. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, V.; Muthukumaran, G.; Sudhagar, P.E.; Rashedi, A.; Norrrahim, M.N.F.; Ilyas, R.A.; Goh, K.L.; Jawaid, M.; Naveen, J. Mechanical properties of cellulose-based multiscale composites: A review. Polym. Compos. 2023, 44, 734–756. [Google Scholar] [CrossRef]
- Li, T.; Chen, C.; Brozena, A.H.; Zhu, J.; Xu, L.; Driemeier, C.; Dai, J.; Rojas, O.J.; Isogai, A.; Wågberg, L. Developing fibrillated cellulose as a sustainable technological material. Nature 2021, 590, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. Synthetic textile fibers: Regenerated cellulose fibers. In Textiles and Fashion; Elsevier: Amsterdam, The Netherlands, 2015; pp. 79–95. [Google Scholar]
- McKelvey, J.B.; Webre, B.G.; Klein, E. Reaction of epoxides with cotton cellulose in the presence of sodium hydroxide. Text. Res. J. 1959, 29, 918–925. [Google Scholar] [CrossRef]
- Nechifor, A.C.; Pîrțac, A.; Albu, P.C.; Grosu, A.R.; Dumitru, F.; Dimulescu, I.A.; Oprea, O.; Pașcu, D.; Nechifor, G.; Bungău, S.G. Recuperative amino acids separation through cellulose derivative membranes with microporous polypropylene fiber matrix. Membranes 2021, 11, 429. [Google Scholar] [CrossRef]
- Mujtaba, M.; Negi, A.; King, A.W.; Zare, M.; Kuncova-Kallio, J. Surface modifications of nanocellulose for drug delivery applications; a critical review. Curr. Opin. Biomed. Eng. 2023, 28, 100475. [Google Scholar] [CrossRef]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R. A review on the modification of cellulose and its applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef]
- Yan, S.; Wang, C.; Hu, H.; Gu, W.; Wang, Q.; Jiang, L.; Zhang, Q. Mechanochemical preparation of a H3PO4-based solid catalyst for heterogeneous hydrolysis of cellulose. ACS Omega 2020, 5, 29971–29977. [Google Scholar] [CrossRef]
- Vu, A.N.; Le, H.N.T.; Phan, T.B.; Le, H.V. Facile Hydrothermal Synthesis of Ag/Fe3O4/Cellulose Nanocomposite as Highly Active Catalyst for 4-Nitrophenol and Organic Dye Reduction. Polymers 2023, 15, 3373. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Du, Y.; Shan, Y.; Duan, P.; Ramzan, N. Hydrothermal Carbonization of Cellulose with Ammonium Sulfate and Thiourea for the Production of Supercapacitor Carbon. Polymers 2023, 15, 4478. [Google Scholar] [CrossRef]
- Paksung, N.; Pfersich, J.; Arauzo, P.J.; Jung, D.; Kruse, A. Structural effects of cellulose on hydrolysis and carbonization behavior during hydrothermal treatment. ACS Omega 2020, 5, 12210–12223. [Google Scholar] [CrossRef] [PubMed]
- Negi, A.; Kesari, K.K. Light-Driven Depolymerization of Cellulosic Biomass into Hydrocarbons. Polymers 2023, 15, 3671. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.K.; Frollini, E.; Thakur, V.K. Cellulose nanocrystals: Pretreatments, preparation strategies, and surface functionalization. Int. J. Biol. Macromol. 2021, 182, 1554–1581. [Google Scholar] [CrossRef]
- Jedvert, K.; Heinze, T. Cellulose modification and shaping—A review. J. Polym. Eng. 2017, 37, 845–860. [Google Scholar] [CrossRef]
- Varshney, V.; Naithani, S. Chemical functionalization of cellulose derived from nonconventional sources. In Cellulose Fibers: Bio-and Nano-Polymer Composites; Springer: Berlin/Heidelberg, Germany, 2011; pp. 43–60. [Google Scholar]
- Jaturapiree, A.; Ehrhardt, A.; Groner, S.; Öztürk, H.B.; Siroka, B.; Bechtold, T. Treatment in swelling solutions modifying cellulose fiber reactivity—Part 1: Accessibility and sorption. In Proceedings of the Macromolecular Symposia; Wiley: Hoboken, NJ, USA, 2008; pp. 39–49. [Google Scholar]
- Moral, A.; Aguado, R.; Tijero, A. Alkalization and cationization of cellulose: Effects on intrinsic viscosity. Fibers Polym. 2016, 17, 857–861. [Google Scholar] [CrossRef]
- Nambela, L.; Haule, L.V.; Mgani, Q. A review on source, chemistry, green synthesis and application of textile colorants. J. Clean. Prod. 2020, 246, 119036. [Google Scholar] [CrossRef]
- Correia, J.; Rainert, K.T.; Oliveira, F.R.; de Cássia Siqueira Curto Valle, R.; Valle, J.A.B. Cationization of cotton fiber: An integrated view of cationic agents, processes variables, properties, market and future prospects. Cellulose 2020, 27, 8527–8550. [Google Scholar] [CrossRef]
- Kasavan, S.; Yusoff, S.; Guan, N.C.; Zaman, N.S.K.; Fakri, M.F.R. Global trends of textile waste research from 2005 to 2020 using bibliometric analysis. Environ. Sci. Pollut. Res. 2021, 28, 44780–44794. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W. Clean dyeing of cotton fiber using a novel nicotinic acid quaternary triazine cationic reactive dye: Salt-free, alkali-free, and non-toxic by-product. Clean Environ. Policy 2015, 17, 563–569. [Google Scholar] [CrossRef]
- Broadbent, A.D.; Mir, Y.; Lhachimi, M.; Bissou Billong, J.; Capistran, S. Continuous dyeing of cotton/polyester and polyester fabrics with reactive and disperse dyes using infrared heat. Ind. Eng. Chem. Res. 2007, 46, 2710–2714. [Google Scholar] [CrossRef]
- Clark, M. Handbook of Textile and Industrial Dyeing: Principles, Processes and Types of Dyes; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Hashem, M.; Hauser, P.; Smith, B. Reaction efficiency for cellulose cationization using 3-chloro-2-hydroxypropyl trimethyl ammonium chloride. Text. Res. J. 2003, 73, 1017–1023. [Google Scholar] [CrossRef]
- Zhai, S.; Li, Y.; Dong, W.; Zhao, H.; Ma, K.; Zhang, H.; Wang, H.; Zhao, Y.; Li, X.; Cai, Z. Cationic cotton modified by 3-chloro-2-hydroxypropyl trimethyl ammonium chloride for salt-free dyeing with high levelling performance. Cellulose 2022, 29, 633–646. [Google Scholar] [CrossRef]
- Yang, J.; Chen, S.; Liu, G. Eco-friendly dual-chromophore functionalized polyvinylamine derivatives for clean and sustainable coloration of cotton fabric. Dye. Pigment. 2022, 207, 110792. [Google Scholar] [CrossRef]
- He, X.; Li, R.; Choy, P.Y.; Liu, T.; Wang, J.; Yuen, O.Y.; Leung, M.P.; Shang, Y.; Kwong, F.Y. DMAP-catalyzed annulation approach for modular assembly of furan-fused chromenes. Org. Lett. 2020, 22, 9444–9449. [Google Scholar] [CrossRef]
- Jiang, J.; MacLachlan, M.J. Unsymmetrical triangular Schiff base macrocycles with cone conformations. Org. Lett. 2010, 12, 1020–1023. [Google Scholar] [CrossRef] [PubMed]
- Pervez, H.; Onyiriuka, S.O.; Rees, L.; Rooney, J.R.; Suckling, C.J. Selective functionalisatlon: Part 10. The nitration of phenols by pyridine derivatives carrying a transferable nitro group. Tetrahedron 1988, 44, 4555–4568. [Google Scholar] [CrossRef]
- Almutaleb, A.A.; Alabbasi, A.A. Synthesis, characterization and computational studies for (2′S*,3R*,3′S*,8a′R*)-2′,3′-bis (ethoxycarbonyl)-2-oxo-2′,3′-dihydro-8a′H-spiro [indoline-3,1′-indolizine]-6′-carboxylic acid and some derivatives. J. Phys. Org. Chem. 2023, 36, e4452. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Wang, Q.; Zhang, K.; Luo, Y.; Liu, Y.; Lyu, Y.; Huang, B. Porphyrin-based covalent triazine framework and its carbonized derivative as catalyst scaffold of Au and Ag nanoparticles for 4-nitrophenol reduction. Microporous Mesoporous Mater. 2022, 330, 111611. [Google Scholar] [CrossRef]
- Lau, V.W.h.; Lotsch, B.V. A Tour-Guide through Carbon Nitride-Land: Structure-and Dimensionality-Dependent Properties for Photo (Electro) Chemical Energy Conversion and Storage. Adv. Energy Mater. 2022, 12, 2101078. [Google Scholar] [CrossRef]
- Liang, H.; Li, G.; Zhang, L.; Wang, G.; Song, M.; Li, H.; Yuan, B. Scalable Synthetic Strategy for Unsymmetrical Trisubstituted s-Triazines. Org. Lett. 2021, 23, 5821–5825. [Google Scholar] [CrossRef]
- Cheng, B.; Li, Y.; Wang, T.; Zhang, X.; Li, H.; He, Y.; Li, Y.; Zhai, H. Application of pyridinium 1, 4-zwitterionic thiolates: Synthesis of benzopyridothiazepines and benzothiophenes. J. Org. Chem. 2020, 85, 6794–6802. [Google Scholar] [CrossRef] [PubMed]
- Deady, L.W.; Finlayson, W.L.; Potts, C. Alkylation of salts of pyridinols, quinolinols and isoquinolinols. Aust. J. Chem. 1977, 30, 1349–1352. [Google Scholar] [CrossRef]
- Binks, J.; Szwarc, M. Effect of Conjugation, Hyperconjugation, and Steric Hindrance on Methyl Affinities. J. Chem. Phys. 1959, 30, 1494–1501. [Google Scholar] [CrossRef]
- Hestekin, J.; Bachas, L.; Bhattacharyya, D. Poly (amino acid)-functionalized cellulosic membranes: Metal sorption mechanisms and results. Ind. Eng. Chem. Res. 2001, 40, 2668–2678. [Google Scholar] [CrossRef]
- Miao, J.; Pangule, R.C.; Paskaleva, E.E.; Hwang, E.E.; Kane, R.S.; Linhardt, R.J.; Dordick, J.S. Lysostaphin-functionalized cellulose fibers with antistaphylococcal activity for wound healing applications. Biomaterials 2011, 32, 9557–9567. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Zhang, D.; Shao, Z.; Wang, J.; Mu, K.; Zhao, L. Short-chain amino acids functionalized cellulose nanofibers composite ultrafiltration membrane with enhanced properties. RSC Adv. 2016, 6, 76336–76343. [Google Scholar] [CrossRef]
- Brotzel, F.; Mayr, H. Nucleophilicities of amino acids and peptides. Org. Biomol. Chem. 2007, 5, 3814–3820. [Google Scholar] [CrossRef]
- Lim, S.-H.; Hudson, S.M. Synthesis and antimicrobial activity of a water-soluble chitosan derivative with a fiber-reactive group. Carbohydr. Res. 2004, 339, 313–319. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Ralph, S.A.; Baez, C.; Reiner, R.S. Detection and quantitation of cellulose II by Raman spectroscopy. Cellulose 2021, 28, 9069–9079. [Google Scholar] [CrossRef]
- Wiley, J.H.; Atalla, R.H. Band assignments in the Raman spectra of celluloses. Carbohydr. Res. 1987, 160, 113–129. [Google Scholar] [CrossRef]
- Edwards, H.; Farwell, D.; Webster, D. FT Raman microscopy of untreated natural plant fibres. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1997, 53, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Righetti, P.; Gianazza, E.; Viotti, A.; Soave, C. Heterogeneity of storage proteins in maize. Planta 1977, 136, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.-L.; Weng, Y.-M.; Liao, Y.-H.; Chen, W. Structural investigation of edible zein films/coatings and directly determining their thickness by FT-Raman spectroscopy. J. Agric. Food Chem. 2005, 53, 5089–5095. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Rakotonirainy, A.M.; Padua, G.W. Thermal behavior of zein-based biodegradable films. Starch-Stärke 2003, 55, 25–29. [Google Scholar] [CrossRef]
- Lai, H.M.; Geil, P.; Padua, G. X-ray diffraction characterization of the structure of zein–Oleic acid films. J. Appl. Polym. Sci. 1999, 71, 1267–1281. [Google Scholar] [CrossRef]
- Tatham, A.; Field, J.; Morris, V.; I’Anson, K.; Cardle, L.; Dufton, M.; Shewry, P. Solution conformational analysis of the alpha-zein proteins of maize. J. Biol. Chem. 1993, 268, 26253–26259. [Google Scholar] [CrossRef]
- Siamwiza, M.N.; Lord, R.C.; Chen, M.C.; Takamatsu, T.; Harada, I.; Matsuura, H.; Shimanouchi, T. Interpretation of the doublet at 850 and 830 cm−1 in the Raman spectra of tyrosyl residues in proteins and certain model compounds. Biochemistry 1975, 14, 4870–4876. [Google Scholar] [CrossRef]
- Khanjanzadeh, H.; Behrooz, R.; Bahramifar, N.; Gindl-Altmutter, W.; Bacher, M.; Edler, M.; Griesser, T. Surface chemical functionalization of cellulose nanocrystals by 3-aminopropyltriethoxysilane. Int. J. Biol. Macromol. 2018, 106, 1288–1296. [Google Scholar] [CrossRef]
- Shahriari-Khalaji, M.; Hu, G.; Chen, L.; Cao, Z.; Andreeva, T.; Xiong, X.; Krastev, R.; Hong, F.F. Functionalization of aminoalkylsilane-grafted bacterial nanocellulose with ZnO-NPs-doped pullulan electrospun nanofibers for multifunctional wound dressing. ACS Biomater. Sci. Eng. 2021, 7, 3933–3946. [Google Scholar] [CrossRef]
- Voicu, S.I.; Thakur, V.K. Aminopropyltriethoxysilane as a linker for cellulose-based functional materials: New horizons and future challenges. Curr. Opin. Green Sustain. Chem. 2021, 30, 100480. [Google Scholar] [CrossRef]
- Neves, R.M.; Ornaghi, H.L., Jr.; Zattera, A.J.; Amico, S.C. The influence of silane surface modification on microcrystalline cellulose characteristics. Carbohydr. Polym. 2020, 230, 115595. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Belgacem, M.N.; Bras, J. Effect of variable aminoalkyl chains on chemical grafting of cellulose nanofiber and their antimicrobial activity. Mater. Sci. Eng. C 2017, 75, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, S.; Rabl, T.; McGloin, D.; Kiefer, J. Intermediate phases during solid to liquid transitions in long-chain n-alkanes. Phys. Chem. Chem. Phys. 2017, 19, 13941–13950. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Y.W.; Zhao, Y. Characterization of polycyclic aromatic hydrocarbons using Raman and surface-enhanced Raman spectroscopy. J. Raman Spectrosc. 2015, 46, 64–69. [Google Scholar] [CrossRef]
- Andronie, L.; Pop, I.; Mireşan, V.; Coroian, A.; Răducu, C.; Cocan, D.; Coroian, C.O. Adsorption behavior of 1-and 2-Naphthol species on Ag colloidal nanoparticles. Hum. Vet. Med. 2014, 6, 210–213. [Google Scholar]
- Nogueira, H.I.; Quintal, S.M. Surface-enhanced Raman scattering (SERS) studies on 1,1′-bi-2-naphthol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2000, 56, 959–964. [Google Scholar] [CrossRef]
- Negi, A.; Mirallai, S.I.; Konda, S.; Murphy, P.V. An improved method for synthesis of non-symmetric triarylpyridines. Tetrahedron 2022, 121, 132930. [Google Scholar] [CrossRef]



| Solvent | Additives (mol%) | N-Heterocycle | Temp. | Time (h) | Yields |
|---|---|---|---|---|---|
| Diethyl ether | - | Pyridine | r.t | 24 | Unreacted mixture |
| Ethyl acetate | - | Pyridine | Reflux | 6 | 24 |
| Cyclohexane | - | Pyridine | Reflux | 4 | Unreacted mixture |
| Acetone | - | Pyridine | Reflux | 2 | 41 |
| MeOH | - | Pyridine | Reflux | 3 | NR |
| CH3CN | - | Pyridine | Reflux | 2 | Inseparable mixture |
| THF | - | Pyridine | Reflux | 3 | 36 |
| DMF | - | Pyridine | Reflux | 1 | Inseparable mixture |
| DCM | - | Pyridine | r.t | 8 | ni |
| Ethyl acetate | Na2CO3 (0.5) | Pyridine | Reflux | 4 | 43 |
| Cyclohexane | Na2CO3(0.5) | Pyridine | Reflux | 2 | 79 |
| Acetone | K2CO3(0.5) | Pyridine | Reflux | 2 | 88 |
| Acetone | K2CO3(0.1) | Pyridine | Reflux | 2 | 51 |
| Acetone | K2CO3(1.0) | Pyridine | Reflux | 2 | 85 |
| Acetone | K2CO3(0.5) | Pyridine | r.t | 2 | 42 |
| Acetone | NaHCO3(0.5) | Pyridine | r.t | 5 | 70 |
| Acetone | NH3–MeOH (1.1) | Pyridine | reflux | 1 | Inseparable mixture |
| Acetone | NH3–MeOH (2.0) | Pyridine | r.t | 4 | Inseparable mixture |
| Type of Preactivators | Concentration (%w/w) | Solvent | Functionalizing Agent (10% w/w) | ζ-Potential (mV) |
|---|---|---|---|---|
| - | - | Acetone | - | −9.63 |
| - | - | Acetone | choline chloride | −7.90 |
| PrAct-1 | 10 | Acetone | choline chloride | +15.53 |
| PrAct-1 | 10 | THF | choline chloride | +12.60 |
| PrAct-1 | 10 | DCM | choline chloride | +13.42 |
| PrAct-1 | 10 | CHCl3 | choline chloride | +13.87 |
| PrAct-1 | 10 | Ethyl acetate | choline chloride | +11.55 |
| PrAct-2 | 0.5 | Acetone | choline chloride | +4.98 |
| PrAct-2 | 1 | Acetone | choline chloride | +7.25 |
| PrAct-2 | 2.5 | Acetone | choline chloride | +9.17 |
| PrAct-2 | 5 | Acetone | choline chloride | +10.04 |
| PrAct-2 | 10 | Acetone | choline chloride | +10.78 |
| PrAct-3 | 0.5 | Acetone | choline chloride | +4.43 |
| PrAct-3 | 1 | Acetone | choline chloride | +6.96 |
| PrAct-3 | 2.5 | Acetone | choline chloride | +8.79 |
| PrAct-3 | 5 | Acetone | choline chloride | +9.36 |
| PrAct-3 | 10 | Acetone | choline chloride | +10.02 |
| Cellulose with Preactivators (10 wt.%) | ζ-Potential (mV) | ||
|---|---|---|---|
| Before Treating with Choline Chloride | After Treating with Choline Chloride (10% w/w) | ||
| r.t | T = 50° | ||
| PrAct-1 | +11.38 | +14.97 | +18.19 |
| PrAct-2 | +7.60 | +10.51 | +12.70 |
| PrAct-3 | +3.95 | +10.19 | +11.57 |
| Colorant | PrAct-1 | Sample Picture | Color Strength (K/S Values) | Color Fixation (%) | ||
|---|---|---|---|---|---|---|
| Before Washing | After Washing | Before Washing | After Washing | |||
| 1 | Yes |  |  | 6.99 | 4.46 | 63.8 |
| 1 | No |  |  | 0.34 | 0.16 | No coloration |
| 2 | Yes |  |  | 4.46 | 3.92 | 87.9 |
| 2 | No |  |  | 0.34 | 0.16 | No coloration |
| Long-Chain Hydrocarbon | Modified Cellulose Form | FTIR Characterization |
|---|---|---|
| 1,5-pentan-diol | O-tethering Pentoyl group | C-H stretching visible at 2913 cm−1, O-H stretching at 3341 cm−1 |
| 1-nonanol | O-tethering nonanyl group | C-H stretching visible at 2892 cm−1 |
| Hexamine | NH-tethering Hexyl group | C-H stretching visible at 2952 cm−1, C-H stretching visible at 2952 cm−1, -H stretching at 3419 cm−1 |
| Octamine | NH-tethering Octyl group | Sharp C-H stretching visible at 2968 cm−1, N-H stretching at 3408 cm−1 |
| Aromatic hydrocarbons | ||
| 1-napthol | O-tethering naptholyl group | Aromatic region stretching visible 1340–1480 cm−1 |
| Benzylamine | NH-tethering Benzyl group | C-H stretching visible at 2952 cm−1 N-H stretching at 3419 cm−1 |
| Benzyl alcohol | O-tethering Benzyl group | Aromatic region stretching visible at 1390–1460 cm−1 O-H stretching at 3341 cm−1 |
| Phenol | O-tethering Phenyl group | Aromatic region stretching visible at 1380–1450 cm−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negi, A.; Tehrani-Bagha, A.R. Cellulose Functionalization Using N-Heterocyclic-Based Leaving Group Chemistry. Polymers 2024, 16, 149. https://doi.org/10.3390/polym16010149
Negi A, Tehrani-Bagha AR. Cellulose Functionalization Using N-Heterocyclic-Based Leaving Group Chemistry. Polymers. 2024; 16(1):149. https://doi.org/10.3390/polym16010149
Chicago/Turabian StyleNegi, Arvind, and Ali R. Tehrani-Bagha. 2024. "Cellulose Functionalization Using N-Heterocyclic-Based Leaving Group Chemistry" Polymers 16, no. 1: 149. https://doi.org/10.3390/polym16010149
APA StyleNegi, A., & Tehrani-Bagha, A. R. (2024). Cellulose Functionalization Using N-Heterocyclic-Based Leaving Group Chemistry. Polymers, 16(1), 149. https://doi.org/10.3390/polym16010149