Abstract
The peculiarities of viscosity data treatment for two series of polymer systems exhibiting associative properties: brush-like amphiphilic copolymers—charged alkylated N-methyl-N-vinylacetamide and N-methyl-N-vinylamine copolymer (MVAA-co-MVACnH2n+1) and charged chains of sodium polystyrene-4-sulfonate (PSSNa) in large-scale molecular masses (MM) and in extreme-scale of the ionic strength of solutions were considered in this study. The interest in amphiphilic macromolecular systems is explained by the fact that they are considered as micellar-forming structures in aqueous solutions, and these structures are able to carry hydrophobic biologically active compounds. In the case of appearing the hydrophobic interactions, attention was paid to discussing convenient ways to extract the correct value of intrinsic viscosity from the combined analysis of Kraemer and Huggins plots, which were considered as twin plots. Systems and situations were demonstrated where intrachain hydrophobic interactions occurred. The obtained data were discussed in terms of vs. plots as well as in terms of normalized scaling relationships where was the relative viscosity of the polymer solution. The first plot allowed for the detection and calibration of hydrophobic interactions in polymer chains, while the second plot allowed for the monitoring of the change in the size of charged chains depending on the ionic strength of solutions.
1. Introduction
Amphiphilic polymers have unique properties that are provided by the association of hydrophobic groups and their micro segregation with hydrophilic fragments in aqueous solutions. The association of such polymers in solutions has been studied extensively [1,2,3,4,5]. Amphiphilic polymers are synthesized in the form of various types of block copolymers or molecular brushes when hydrophobic side chains are attached to the hydrophilic main chain of a polymer. Such macromolecules often contain charged groups. In solutions, they exhibit associative properties, which open up possibilities for creating structures that respond to various stimuli and exhibit reversible properties. With an increase in the concentration of solutions, such polymers generate a network structure, where, due to non-covalent interactions, a system of points of non-covalent bonds between different chains (stickers) is formed, which allow for a labile material. Due to the presence of non-covalent interactions (hydrogen bonds, host–guest interactions, electrostatic interactions), amphiphilic polymers acquire new functions that significantly expand their applications.
Such amphiphilic polymers are based on synthetic structures [6,7,8,9,10,11], as well as on natural polymers [12,13,14,15]. They can be used for enzyme immobilization, controlled drug release [16,17,18], microencapsulation, production of membranes for separation processes, viscosity modifiers for oil production [19,20,21], and as emulsifiers, dispersants, foaming agents, thickeners, etc. The structures formed in solutions of associating polymers depend on the concentration of the polymer, the number of attracting groups in the chains, the strength of the physical bonds they form, and the degree of affinity between the polymer and the solvent. In dilute solutions of amphiphilic polymers, intramolecular self-association (i.e., the formation of physical bonds between attracting groups of the same chain) can occur which can lead to a decrease in the size of an individual macromolecule and the formation of loops in it or the formation of a uni-macro-molecular micelle. At high concentrations, the aggregation of several macromolecules into an intermolecular micelle and then to the formation of a physical gel of polymer chains can be observed. Interchain aggregation in a physical network leads to a sharp increase in the viscosity of the solution and the appearance of its high elasticity. These complex dynamic processes in the solutions of associating polymers are extensively studied by suggesting theoretical models of the system of interest [22,23,24,25,26,27] and rheological methods [28,29,30,31,32].
Rheology studies both the linear viscoelastic behavior of associative polymers, taking into account the density of stickers and the strength of associations with stickers, and the dynamics of associative polymers, determined by the structure of sticker clusters/aggregates and their dissociation [27,33].
In dilute polymer solutions, viscometry serves to determine the characteristics of an isolated macromolecule, following Staudinger [34]. The macromolecules of amphiphilic polymers manifest their peculiar behavior already in dilute solutions, and, as a rule, demonstrate high values of the Huggins parameter [35,36,37,38].
The study of the viscous flow of dilute solutions of natural and synthetic polymers has been one of the cornerstones in establishing their macromolecular nature and today is a rather routine but indispensable means to start any polymer investigation. The intrinsic viscosity is one of the most important and easily accessible quantities that characterizes the size and conformation of linear polymer molecules. The physical meaning of the value of linear polymer molecules is revealed by the Flory–Fox relation: , where is the mean square distance between the ends of the macromolecular chain, is the hydrodynamic Flory parameter [39].
The intrinsic viscosity was defined by Staudinger as [34] and by Kraemer as [40], where is relative viscosity, is specific viscosity, and is the concentration of the dissolved substance in g/cm3. This definition emphasizes that the value characterizes a friction of the isolated macromolecule surrounded by solvent molecules. The method of extrapolating the and values to was not indicated by the authors of [34,40]. Various extrapolation plots have been proposed and are still being proposed to determine the intrinsic viscosity of polymers [41,42,43,44,45,46].
For a long time, and even now, in polymer science the Huggins [47] plot is overwhelmingly used to determine the of polymer molecules:
Sometimes the Mead–Fuoss plot [46,48] is used. It is also known as the Kraemer plot [40]:
Intrinsic viscosity values determined by Equations (1) and (2) are indicated in the text as and , respectively. At , these plots should lead to the equivalent estimates of value because can be expanded into a sign-alternating series in when . The equality of the values is an axiomatic condition, i.e., must be performed with a highest level of accuracy. In addition, the mathematical result of this expansion into a mathematical series is: . As Garcia de la Torre et al. aptly noted [49], the last correlation is just a mathematical consequence that does not correlate with the chemical and physical features of polymer solutions, i.e., the validity of the latter relationship does not depend on either the nature of the solvent or polymer–solvent interactions. Note that the ratio is not fulfilled in many cases. Unfortunately, in the literature there are only few works with the simultaneous presence of the two above-mentioned methods ((1) and (2)). In rare cases, the authors only mention the Kraemer plot, as a rule, without comparing the results obtained. For example, The Polymer Handbook [50] contains extensive tables of the Huggins parameter, but there are no tables for .
In this work, the viscometry data were thoroughly analyzed from a methodological and metrological point of view. We demonstrated the obvious need to use the Huggins–Kraemer twin plot in the study of dilute solutions of amphiphilic polymers, as well as neutral linear polymers in thermodynamically poor solvents. First, we demonstrated the evolution of the twin plot for linear polymers as the solvent quality worsening. Then we analyzed the viscometric behavior of the strong linear polyelectrolyte (sodium polystyrene-4-sulfonate (PSSNa)) over wide ranges of both molecular masses (MM) and ionic strengths. In addition, the behavior of brush-like amphiphilic copolymers strong polyelectrolyte (alkylated copolymer of N-methyl-N-vinylacetamide and N-methyl-N-vinylamine (MVAA-co-MVACnH2n+1)) with hydrophobic groups of different lengths in wide ranges of MM and ionic strengths were considered, as well as some data from the literature [51,52]. Finally, the results were discussed in terms of the concept of the normalized Kuhn-Mark-Houwink-Sakurada (KMHS) relationship, which made it possible to compare the results with the full spectrum of possible conformational states of linear macromolecules.
2. Materials and Methods
The synthesis of polymer systems under discussion was described in works [53,54,55,56]. The structures of polymers are shown in Scheme 1.
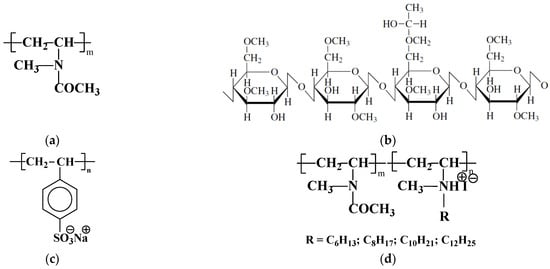
Scheme 1.
The structural formula of: (a)—poly-N-methyl-N-vinylacetamide homopolymer (PMVA); (b)—hydroxypropylmethylcellulose (HPMC); (c)—sodium polystyrene-4-sulfonate (PSSNa); and (d)—alkylated amphiphilic copolymer of N-methyl-N-vinylacetamide and N-methyl-N-vinylamine (MVAA-co-MVACnH2n+1).
The polymers were investigated earlier in water and salt-water solutions by the methods of molecular hydrodynamics: viscometry, sedimentation velocity, and translational diffusion. The results for poly-N-methyl-N-vinylacetamide (PMVA) are described in [57]; for hydroxypropylmethylcellulose (HPMC) in [58]; for PSSNa in [54,59]; for MVAA-co-MVACnH2n+1 in [55,60,61]. The copolymer bears charges at the junction of the main and each side chain. The composition of the copolymer is 15 mol.% side chains.
All investigations were carried out at 25 °C. The viscous flow of dilute solutions was investigated in Ostwald capillary viscometer with solvent flow times: 83.5 s (H2O), 84.1 s (aqueous 0.1 M NaCl), 84.3 s (aqueous 0.2 M NaCl), 120 s (aqueous 4.17 M NaCl). The molecular masses were determined through the Svedberg relationship , where is the intrinsic sedimentation coefficient, is the intrinsic diffusion coefficient, and is the gas constant. Translational diffusion was studied with a Tsvetkov polarizing diffusometer [48] in a cell [62,63] with optical path 30 mm by classical method of forming a boundary between solvent and solution. The velocity sedimentation was investigated with a Proteomelab XLI (Beckman) ultracentrifuge in a 12-mm two-sector cell at a rotor speed of 40,000 rpm. Sedimentation interference curves were processed with the Sedfit software using general scaling law approach [64,65]. The buoyancy factor was measured with a DMA-4000 densitometer (Anton Paar).
3. Results and Discussion
3.1. From Linear Polymers in Thermodynamically Good Solvent through Marginal One and θ-Solvent to Amphiphilic Graft Copolymers
3.1.1. Hydrophylic Flexible Noncharged Nonassociating Poly-N-Methyl-N-Vinylacetamide (PMVA)
For flexible-chain polymers in thermodynamically good solvents, the parameter is negative and smaller in modulus than the parameter. Therefore, the dependence vs. changes more slowly with concentration than the dependence vs. . For this reason, as Flory noted, extrapolation is more preferable than [66] (page 325). However, Flory’s note was not accepted by the polymer community, and the vast majority of information published up to date on the determination of the value is derived only from Huggins plots. (See, for example, [50]).
The Huggins and Kraemer classical twin plot for flexible noncharged PMVA in water is shown in Figure 1. In the range of relative viscosities the following results were obtained: from the Huggins plot and from the Kraemer plot (Sample 4, Table 1). These results testify both the equivalence of the assessment of the main quantity—intrinsic viscosity—by Huggins and Kraemer plots and the validity of the relation . The data for other PMVA samples [57] are shown in Table 1.
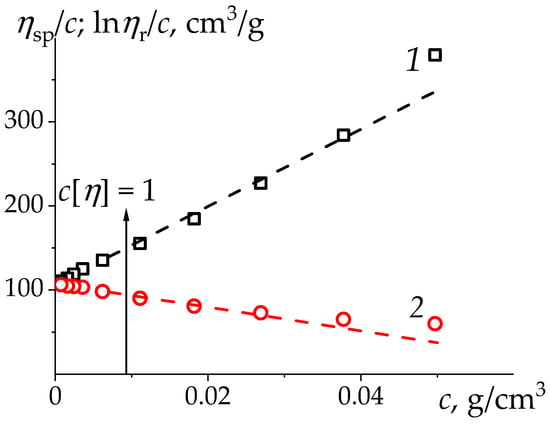
Figure 1.
Huggins (1) and Kraemer (2) plots for poly-N-methyl-N-vinylacetamide (PMVA) (sample 4 in Table 1) in H2O at 25 °C. The values of intrinsic viscosity, Huggins and Kraemer parameters were determined in the interval of relative viscosity: by extrapolation to . Dashed lines are drawn over the entire range of concentrations and relative viscosities .

Table 1.
The molecular masses , the values of intrinsic viscosities obtained from Huggins ( ) and Kraemer ( ) plots and Huggins ( ) and Kraemer ( ) parameters for PMVA in H2O at 25 °C.
The average deviation between the and values is 0.7%, and over the entire Table 1 data array. It should be noted that for flexible-chain polymers in thermodynamically good solvents, relations (1) and (2) usually lead to virtually the same value , and the ratio is also practically fulfilled. This kind of accuracy is common for the viscosity measurements of flexible macromolecules.
This led to the fact that in the most studies, the use of the Kraemer plot was considered an unnecessary waste of time and the process of determining value has been optimized as much as possible, i.e., only the Huggins plot was used.
3.1.2. Peculiarities of Viscosity Behavior of Associating Systems
As the thermodynamic quality of the solvent worsens, the absolute value of the parameter decreases to zero, and then becomes positive and continues to increase. The parameter continuously increases in this case.
The slope of vs. (Equation (2)) is the quantity . When , this dependence in the region of dilute solutions looks like some “fluctuation” of values depending on the concentration. Apparently, it is this range of values that induces doubt among experimenters about the reliability of intrinsic viscosity determining from Kraemer plot. Indeed, in this case, this dependence may have a small or extremely small linear correlation coefficient. However, this does not mean that in this case the main sought value becomes less certain when using the Kraemer plot compared to the Huggins plot. When the absolute error in determining is greater than its value. But nevertheless, the absolute error in determining is practically the same as that of for the same data set. The is typical for thermodynamically poor or marginal solvents, i.e., solvents approaching the θ-solvent in terms of their thermodynamic quality.
An example of such a rare system is shown in Figure 2 with a treatment of our viscometric data for a water-soluble hydroxypropylmethylcellulose (HPMC) sample with the degrees of substitution of 1.90 for methoxy and 0.26 for hydroxypropyl groups and with a MM of 207,000 g/mol. MM was calculated from the KMHS ratio established for HPMC samples studied in water [58].
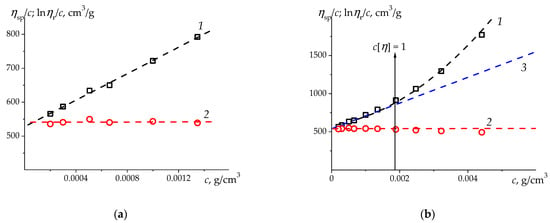
Figure 2.
The Huggins (1) and Kraemer (2) plots for a sample of hydroxypropylmethylcellulose (HPMC) () in H2O at 25 °C [58]: (a)—in the range of relative viscosities , where extrapolation to was carried out and the values of intrinsic viscosity and Huggins and Kraemer parameters were determined , , ;, , ; ); (b)—in the entire range of concentrations (Line 3 is drawn as a tangent to the 2-nd degree polynomial fit in the initial range of values which corresponds to following values: , . Overall parabola (1) demonstrates the curvature of dependence of ηsp/c in contrast with one of lnηr/c in the entire interval ).
The difference between the estimates of from the two plots (Figure 2a) is , which is usually not considered significant. Obviously, in this case, there is a good agreement between the and values. However, this is 3.5 times greater than the deviation observed for the flexible chain PMVA (Table 1). It is noteworthy that the coefficient of linear correlation of the Huggins plot is , whereas this coefficient for the Kraemer plot is , which means that the slope of the Kraemer plot is practically zero. At the same time, the absolute errors of the value for the two plots practically coincide .
The value of the Kraemer parameter indicates that water is not a thermodynamically good solvent for hydroxypropylmethylcellulose. It can be expected that as the concentration of solutions increases, the dependences will clearly show the hydrophobicity of this polymer. Indeed, Figure 2b shows a noticeable difference in the behavior of the Kraemer and Huggins dependences in the interval . The dependence of on increases sharply and can be approximated by a parabola, while the dependence of changes slightly. In this regard, we have carried out a correction of the in the low concentrations. For this, we formed a data system consisting of experimental values at the and add to them a point (, ), taking into account an axiom . We processed this data with a second degree polynomial function and draw the tangent to it at . The tangent equation is , i.e., , . Thus, a slight increase in by 2.3% leads to a twofold decrease in the Huggins parameter. In a similar way, we processed the data of in the region of extremely dilute solutions for the amphiphilic polymer systems.
Note that Garcia de la Torre et al. had already developed the program for linear least-square fits with a common intercept [67] for systems with a negative value of the Kraemer parameter. It would be useful to adapt the program for joint processing of the Huggins and Kraemer plots for the associating polymer systems with a positive value of the Kraemer parameter, as suggested above and below.
With a further decrease in the polymer-solvent affinity and an increase in the role of polymer-polymer interactions compared to polymer-solvent interactions, the parameter changes sign to positive. The relation is no more valid. However, in all cases, the parameter is less in absolute value than the parameter , therefore, the dependence will remain linear in a larger concentration range compared to the dependence .
This situation is illustrated by an charged brush-like alkylated amphiphilic copolymer of N-methyl-N-vinylacetamide and N-methyl-N-vinylamine, which combines a hydrophilic charged base and hydrophobic C12H25 side groups (MVAA-co-MVAC12H25) [55,61] (Figure 3) and linear homopolymer PSSNa, which exhibits hydrophobic interactions in θ-solvent [54,59,68] (Figure 4).
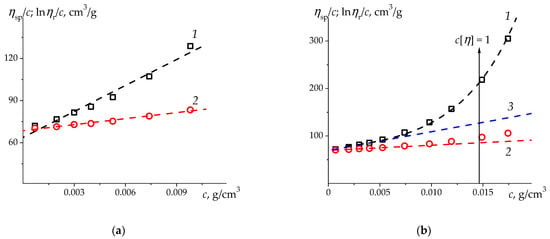
Figure 3.
The Huggins (1) and Kraemer (2) plots for alkylated copolymer of N-methyl-N-vinylacetamide and N-methyl-N-vinylamine (MVAA-co-MVAC12H25) [55,61] (Sample 4 in Table 4) in aqueous 0.1 M NaCl solution at 25 °C: (a)—in the range of relative viscosities (, , ;, , ); and (b)—in the entire range of concentrations (Line 3 is drawn as a tangent to the 2-nd degree polynomial fit in the initial range of values , its values: , . Overall parabola (1) demonstrates the curvature of dependence of in contrast with one of in the entire interval ).
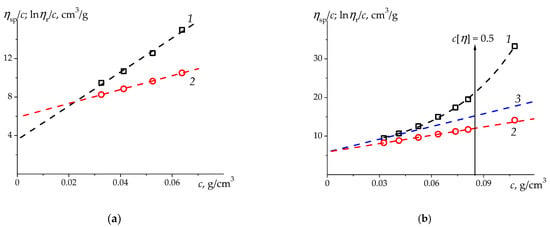
Figure 4.
The Huggins (1) and Kraemer (2) plots for sodium polystyrene-4-sulfonate (PSSNa) (sample PSSNa-7 in Table 2) in aqueous 4.17 M NaCl (θ-solvent at 25 °C) [54,59] with different data approximation: (a)—linear fits (lines 1 and 2) in the range of relative viscosities (, , (1); , , (2)); and (b)—overall parabola (1) in the full measured range , Kraemer linear fit (line 2), and tangent (line 3) to the 2-nd degree polynomial fit in the range (which (3) leads to the values , ). Overall parabola (1) is drawn for clarity in order to emphasize the curvature of the dependence in the interval ).
The results obtained in this way for all investigated PSSNa fractions in aqueous 4.17 M NaCl, are presented in Table 2. The values obtained by direct linear extrapolation of (let’s indicate it ) in the entire dataset are lower than values, and the error in determining is noticeably greater than that for . After the values are converged to a single value corresponding to , the corrected values of the Huggins parameter are reduced by 2–6 times compared to the values obtained by simple linear treatment (Figure 3a, line 1, Figure 4a, line 1). A consistent estimate of will lead to a change in both parameters of the Kuhn-Mark-Houwink-Sakurada ratio and, accordingly, to a refinement of the conformational status of PSSNa macromolecules in aqueous 4.17 M NaCl compared to estimates based on [54].

Table 2.
The molecular masses , the values of intrinsic viscosities, obtained from Huggins ( ) and Kraemer ( ) plots and Kraemer ( ) and Huggins (, ) parameters for PSSNa in aqueous 4.17 M NaCl solutions at 25 °C.
The most important postulate when using the Huggins and Kraemer plots is the statement that , i.e., the dependencies must converge at one point on the y-axis at . However, for many polymer systems the intercept on the y-axis at on Huggins plot is less than that on Kraemer one. That is, from the Huggins plot, we get a somewhat underestimated value of the intrinsic viscosity, while the brutto values of the Huggins parameter will be quite large, the larger is the value of . In the considered Figure 2, Figure 3 and Figure 4, the initial values of are less than <!-- MathType@Translator@5@5@MathML2 (no namespace).tdl@MathML 2.0 (no namespace)@ -->
by 2.3, 7.1 and 34%, respectively. When is corrected to , the Huggins parameter decreases by factors of 2.0, 1.7, and 2.8, respectively, compared to its brutto values. The correlation between and over the entire range of their values requires more detailed study for a wide set of polymer systems.
3.2. Viscometric Data at Different Ionic Strengths of Solutions
3.2.1. Sodium Poly(Styrene-4-Sulfonate)
Equation (2) can be represented as the dependence of on the Debye parameter ():
The Debye parameter characterizes the dilution degree of the solution.
This is a well-known plot in rheology which allows one to compare viscosity data for polymers with various molecular masses and chemical structures, and in different solvents.
Over a wide concentration range the dependence on is described by a second-degree polynomial:
According to the Kraemer definition of , its initial slope (at ) is equal to 1. The second derivative , determined in the region of dilute solutions, is the Kraemer parameter . Its sign determines the trend of the dependence. Thus, at with , , and the relationship (4) is transformed into (3).
The plot for PSSNa in aqueous 4.17 M NaCl (a), aqueous 0.2 M NaCl (b), and in salt-free water (e) solutions at 25 °C as well as the data for two polystyrene (PS) samples with MM 146,000 and 600,000 g/mol in toluene at 30 °C (points c and d) [52] are shown in Figure 5.
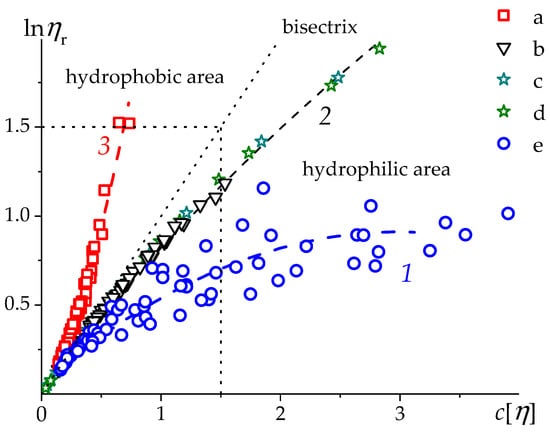
Figure 5.
The dependence of natural logarithm of relative viscosity vs. resolved for PSSNa in aqueous solutions of different ionic strength [54,59] (a—4.17 M NaCl, ; b—0.2 M NaCl, , and e—H2O, ) and polystyrene (PS) in toluene, [52] (c and d—samples with 146,000 and 600,000 g/mol, correspondingly). Dashed line represents the bisectrix (i.e., line ) of current coordinates, 1—is extrapolation of PSSNa data in H2O (e), 2—PSSNa in aqueous 0.2 M NaCl (b), PS in toluene (c and d), and 3—PSSNa in aqueous 4.17 M NaCl (a).
The points for PSSNa in aqueous 0.2 M NaCl solutions (b in Figure 5) for all fractions fit into virtualy single convex dependence, i.e., characterized by a negative second derivative (), which corresponds to a negative value of the Kraemer parameter (Table 3). Note that the data on the toluene-soluble fractions of PS (c, d in Figure 5) [52], fit well into a single dependence with PSSNa in aqueous 0.2 M NaCl. This means that these macromolecules are in virtually identical thermodynamic conditions. The PSSNa data in aqueous 4.17 M NaCl solution (a θ-solvent at 25 °C [54,69], form a single concave curve, which corresponds to positive values of both and . The space defined by coordinates can be divided into two parts by a straight line (bisecrix) corresponding to the condition (). To the right is an area of hydrophilic systems and/or systems that are in thermodynamically good conditions; to the left is an area of hydrophobic and/or systems near θ-conditions. Note that, both in aqueous 0.2 M and 4.17 M NaCl solutions, the system of PSSNa fractions manifests itself as a molecular homologues system. In salt-free solutions (points e in Figure 5), the fractions of charged PSSNa macromolecules do not form a single dependence. This situation will be discussed below.

Table 3.
The molecular masses , the values of intrinsic viscosities, obtained from Huggins ( ) and Kraemer ( ) plots, and Huggins ( ) and Kraemer ( ) parameters for PSSNa in aqueous 0.2 M NaCl at 25 °C.
In contrast with data in Table 2 (PSSNa in 4.17 M NaCl) the average deviation between the and values is 0.8%, and over the entire Table 3 data array in 0.2 M NaCl. This is similar to statistic of Table 1 for noncharged PMVA in H2O.
Note that despite the demonstration of associative properties in 4.17 M NaCl, PSSNa macromolecules behave both in 0.2 M and 4.17 M NaCl as a homologous series (Figure 5).
3.2.2. Alkylated Random Copolymers of N-Methyl-N-Vinylacetamide and N-Methyl-N-Vinylamine (MVAA-co-MVACnH2n+1)
For brush-like amphiphilic copolymers with hydrophobic side chains and hydrophilic backbone an important structural parameter is the ratio of the average distance between two neighboring alkyl side chains () to contour length of their side chain (). This parameter () characterizes the ratio between hydrophilic and hydrophobic parts in a copolymer molecule. The MVAA-co-MVAC12H;25 random copolymer has such a composition of the side aliphatic groups (15 mol.%) when the average distance between adjacent side groups is almost equal to the contour length of the C12H25 side group (). These copolymers, being strong polyelectrolytes, are readily soluble in salt-free water, and being flexible-chain in nature, they strongly react to changes in the ionic strength of the solution.
As follows from the viscometric plots, the primary polyelectrolyte effects are suppressed in aqueous 0.1 M NaCl solutions. The viscometric data for MVAA-co-MVACnH2n+1 are given in Figure 6 and Table 4. The data in Figure 6 are presented in vs. coordinates with the division of space into two regions: hydrophobic and hydrophilic.
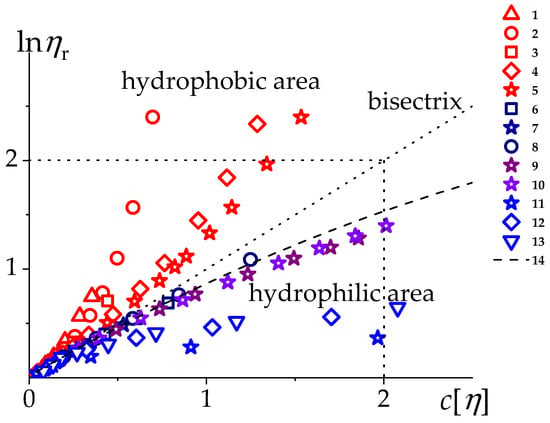
Figure 6.
The dependence of the on the degree of dilution for MVAA-co-MVAC12H25 with different molecular masses in aqueous 0.1 M NaCl (1–5), DMFA + 0.1 M LiCl (6–8), in H2O (11–13), MVAA-co-MVAC6H13 (9) and MVAA-co-MVAC8H17 (10) in aqueous 0.1 M NaCl solution and PMVA in H2O (14). The dashed line separates two regions: the region of polymers with hydrophobic interactions and the region of polymers with hydrophilic interactions.

Table 4.
The molecular masses , the values of intrinsic viscosities, obtained from Huggins () and Kraemer () plots and Huggins () and Kraemer () parameters, for MVAA-co-MVAC12H25 in aqueous 0.1 M NaCl (Samples 1–5) and DMFA + 0.1 M LiCl (Samples 6–8); MVAA-co-MVAC6H13 (Sample 9) and MVAA-co-MVAC8H17 (Sample 10) in aqueous 0.1 M NaCl.
Despite the fact that the samples have the same chemical composition, the viscometric results do not fit into a single curve but represent a set of curves with different values of the positive second derivative . Thus, the samples can be differentiated by the level of hydrophobicity: the more , the more the sample is hydrophobic. As a result, the homology of the studied series is violated, which is associated evidently with their irregular, random distribution of C12H25 side groups along the chain.
In DMF + 0.1 M LiCl solutions of MVAA-co-MVAC12H;25 the hydrophobicity effect “turns off” and the viscometric results (points 6–8, Figure 6) are in good agreement with the data obtained for the parent uncharged homopolymer PMVA (dashed curve, Figure 6). In organic solvents, where hydrophobic interactions are absent, these copolymers behave as polymer homologues.
Note that samples with short aliphatic groups C6H13 and C8H17 (points 9 and 10, Figure 6) do not exhibit hydrophobic behavior in 0.1 M NaCl solutions at all, being located near the data corresponding to the homopolymer. For these copolymers (with the composition mol. 15%), the contour length of the side radicals is much less than the average distance between them along the main chain. In this case, the total share of hydrophobicity of the macromolecule is less than the share of hydrophilicity.
For the studied copolymers the ratio changes within the limits: . When , intramolecular hydrophobic interactions occur in the copolymer; when they are not observed. The latter is shown in Figure 6 for MVAA-co-MVAC6H13 (points 9) and MVAA-co-MVAC8H17 (points 10) in aqueous 0.1 M NaCl solutions.
3.2.3. The Behaviour of Polymers in Salt-Free Solutions
The two studied systems PSSNa and MVAA-co-MVAC12H25 are strong polyelectrolytes. The value was determined from initial slope of the dependence of on according to the Kraemer definition [40,56,70].
The results on PSSNa are presented at Figure 7 and Figure 8, and in Table 5. For comparison, the data from the unique viscometric study of PSSNa in H2O [51] were added to Figure 8.
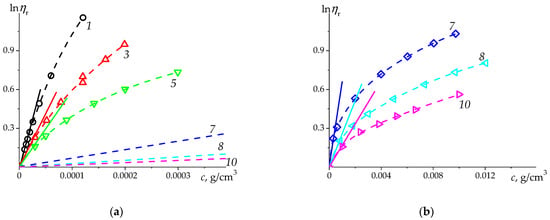
Figure 7.
The natural logarithm of relative viscosity vs. concentration for PSSNa data in H2O at 25 °C. The dashed curves are the 2-nd degree polynomial fit of the presented data sets, the solid lines correspond to the initial slope of the dependences representing the intrinsic viscosity value, as at . The numbers next to the lines correspond to the sample numbering in the Table 5. The lines 7, 8 and 10 on the (a) represent the slopes from the (b).
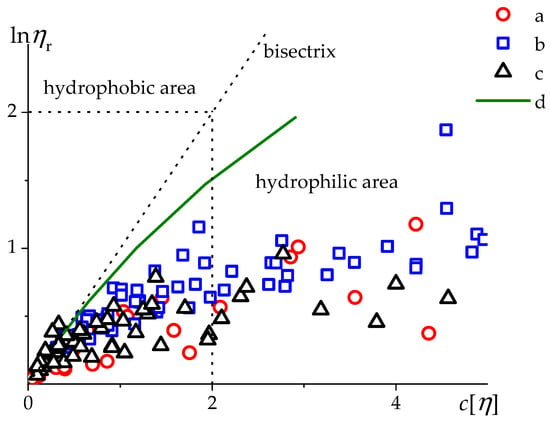
Figure 8.
The dependence of natural logarithm of relative viscosity on dilution degree resolved in H2O solution for PSSNa (a—[51], b—[54]), MVAA-co-MVAC12H25 (c—[55], and PMVA [57] (d, sample 4 in Table 1). Dashed line is the bisectrix of current coordinates (i.e., line ).

Table 5.
The molecular masses , the values of intrinsic viscosities and parameters (Equation 4) for PSSNa in H2O at 25 °C.
In salt-free solutions of highly charged linear chains, electrostatic interactions are dominant.
Irregular re-condensation of counterions onto a charged chain leads to a statistical distribution of charges along the chain and loss of homology of such polymer series in salt-free solutions.
One can see that the points for all charged polymers (a, b, c in Figure 8) lie below the line d for a neutral water-soluble homopolymer PMVA. It is explained by the fact that with an increase in the concentration of the polyelectrolyte in pure water, the ionic strength of the solution increases due to an increase in the number of counterions in the solution, and the coils shrink. As c[η] increases, the interaction of hydrophobic C12 side groups slightly appears. The dependence at Figure 8 demonstrates a weak manifestation of the hydrophobicity of MVAA-co-MVAC12H25 in comparison with the PSSNa. The MVAA-co-MVAC12H25 data (points c in Figure 8) lie a little bit lower in the region than that for PSSNa (points a, b in Figure 8).
At very high values of polymer charge density and low ionic strength, the level of hydrophobicity practically does not affect the size of polyelectrolyte chains.
3.3. Normalized Scaling Relationships: Comparison of Viscometric Data
Intrinsic viscosity is the most sensitive characteristic to the size, shape, and their change for linear macromolecules in solutions. This is why the Kuhn-Mark-Houwink-Sakurada relationship () is so popular in polymer science. To date, an extensive library of these relationships for polymers of various structures and in various solvents has been formed and is being replenished [71]. If we put all these data on the vs. plot, we get a rather chaotic filling of this two-dimensional space. However, it turned out that this “pointillist picture” is transformed into a fairly clear ordered structure of the viscometric spectrum of linear macromolecules of different nature and structure, unfolded in size, if we take into account on this plot the mass of a linear chain length unit (), which characterizes the linear density of the macromolecule [72,73,74]. This transformation follows from the fundamental Flory–Fox relation that , where is the volume occupied by the macromolecule in solution. Consequently, the value characterizes the volume occupied by the chain segment corresponding to the unit contour length of the macromolecule (which is conveniently chosen as a repeating link). This value will be the greater, the greater is the equilibrium rigidity of the macromolecule and the better is the thermodynamic quality of the solvent. Thus, by transforming the standard plot used to establish the classical KMHS relation, we obtained the plot (, which represents a sweep by their sizes of the entire spectrum of linear macromolecules so far studied. Linear macromolecules were divided into the following classes: extra-rigid, rigid, flexible in thermodynamically good solvents, flexible under θ-conditions, and globular. This plot is the result of an analysis of a large number of data in the literature and covers the area of macromolecules contour lengths changing by three orders of magnitude and the area of equilibrium rigidities of linear chains changing by approximately 500 times. Thus, by applying the experimental data to the obtained template/sweep, one can judge whether the studied polymer belongs to one or another class of linear polymers and get an idea of the length of the statistical segment or the persistent length of the chain. Let us analyze the results discussed in this paper in the coordinates vs. (Figure 9).
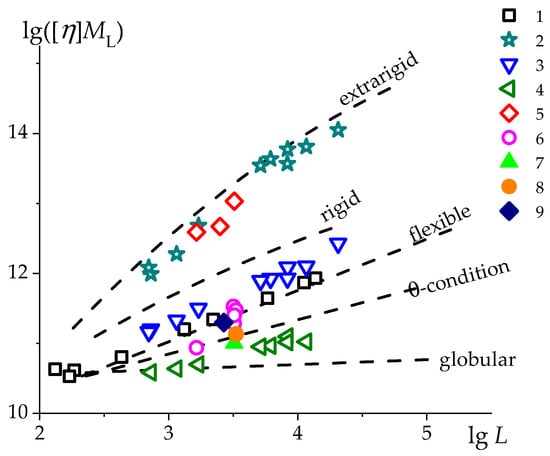
Figure 9.
Normalized Kuhn-Mark-Houwink-Sakurada dependencies, (i.e., dependences of characterizing the volume occupied by a chain segment corresponding to unit contour length of the macromolecule on the contour length of the macromolecule in a double-logarithmic scale) for PMVA ( 393 g/mol nm) [57] in H2O (1); PSSNa ( 750 g/mol nm) [54,59] in different aqueous compositions: H2O (2), 0.2 M NaCl (3), 4.17 M NaCl (4); MVAA-co-MVAC12H25 ( 468 g/mol nm) [55] in H2O (5), in aqueous 0.1 M NaCl (6), 1 M NaCl (7), and 1.6 M NaCl (8) solutions; MVAA-co-MVAC10H21 ( 452 g/mol nm) in aqueous 2 M NaCl solution (9). Dashed lines correspond to different conformations of linear chains: extra-rigid, rigid, flexible, globular, and θ-conditions [72,73].
The joint manifestation of electrostatic long-range effects and short-range interaction leads to the maximum sizes of charged chains in salt-free solutions of both PSSNa chains and MVAA-co-MVAC12H25 chains (points 2 and 5). In the salt-free solutions, the hydrophobicity of the chains does not manifest in any way. Despite the different composition (PSSNa—100 mol.% and MVAA-co-MVACnH2n+1—15 mol.%), these chains are strong polyelectrolytes [61,75]. As the ionic strength of the solutions is increased to 0.1–0.2 M NaCl, the polyelectrolyte effects are largely suppressed, as indicated by the linear viscometric dependences (Equations 1 and 2). The data system shifts to the region of flexible chain macromolecules (points 3 and 6). Here, in MVAA-co-MVAC12H25 chains, hydrophobic interactions begin to manifest to a greater extent than in linear PSSNa chains. As the ionic strength increases the MVAA-co-MVAC12H25 [η] value decreases insignificantly (points 7, 8, Figure 9). With a further increase in the ionic strength of solutions, MVAA-co-MVAC12H25 samples cease to dissolve molecularly. The mobility of -C12H25 side groups and their interaction in aqueous solutions are facilitated in comparison with the interactions of the elements of the same linear chain, as occurs in PSSNa chains. This is due to the fact that the side chains of graft copolymer chains are richer in entropy than linear chains of PSSNa. It is the copolymer side chains that cause hydrophobic interactions in these copolymers. Therefore, the manifestation of nonmolecular solutions and the onset of gelation occur earlier for MVAA-co-MVAC12H25 copolymers than for PSSNa.
Under the θ-condition (4.17 M NaCl, 25 °C), the PSSNa chains are additionally compressed (points 4) and their sizes fall between the θ-conditions for conventional flexible-chain polymers and the globular state. Under these conditions, the standard relation for PSSNa chains has the form: , where the scaling index that is, it becomes less than the minimum value allowed for linear macromolecules. Thus, the macromolecules of the strong polyelectrolyte PSSNa, going from top to the bottom, change their conformation from a slightly bent rod to a coil, and then approach the globular conformation, practically realizing the entire conformational spectrum of linear chain macromolecules.
4. Conclusions
The study of the viscous flow of dilute solutions of associating polymers allows to detect the presence of hydrophobic interactions in solutions already in the range of their sufficient dilution at . Obtaining such information will require the comparative use of both the Huggins plot ( on ) and the Kraemer plot ( on ), in a method we call the twin plot. It should be noted that this technique was quite well known in the early 50 s of the 20th century, but then forgotten. Indeed, new is well-forgotten old!
When interpreting the results of studying the viscous flow of polymer solutions, it should be taken into account that both and should converge at the same point. In this case, the concentration intervals used for linear extrapolation of the and to can/should be different. Since always , the interval used for extrapolation of to can be much shorter than that for dependence. Recall that the only results/part of the results are obtained in the region of dilute polymer solutions when and , are the subject to analysis. The final measurement results must contain a single intrinsic viscosity value and correctly determined Kraemer and, especially, Huggins parameters. The values of the Huggins parameter exceeding 1, given anywhere in the literature, are overestimated. In turn, this leads to some underestimated values.
The parameter is a sign-changing parameter, changing its sign from negative to positive for polymer systems exhibiting significant polymer–polymer interactions in solutions. Such systems include polymers in θ-solvents and amphiphilic copolymers of various topologies. Thus, the parameter is a source of additional information about the manifestation of polymer–polymer interactions in solutions of macromolecular compounds.
The plot vs. is very informative when comparing homologous series of different kind of polymers or the same series in different solution conditions. This plot allows the series under study to be separated into hydrophilic or hydrophobic systems, depending on the sign of the curvature of the second-degree polynomial describing evolution of in function of the degree of dilution. A positive sign corresponds to hydrophobic systems, and a negative one—to hydrophilic ones. A similar separation will occur between polymers in thermodynamically good solvents and polymer systems near θ-conditions.
A special situation arose in the study of amphiphilic polyelectrolytes in salt-free solutions. The results of the viscous flow of such solutions demonstrated the breach of homology, which also sometimes manifested under suppressed polyelectrolyte effects (compare sodium polystyrene-4-sulfonate and alkylated statistical copolymer N-methyl-N-vinyl acetamide and N-methyl-N-vinyl amine, Figure 5 and Figure 6). Apparently, this can be associated with the irregular re-condensation of some of the counterions on the charged chain, which will lead to a random distribution of the charges remaining on the chain. This means a loss of homology and appears in the irregularity of ln ηr vs. c[η] dependence. An informative and powerful tool in interpreting the molecular dependence of intrinsic viscosity is the use of the Kuhn-Mark-Houwink-Sakurada plot normalized to the mass per unit length of a linear polymer chain. Using the pattern established on the basis of the analysis of the literature data bank, one can semi-quantitatively establish the belonging of the studied polymer chains to one or another class of polymers. These possibilities were demonstrated on two series of polymers studied in various solvents.
Author Contributions
Conceptualization, G.M.P.; methodology, G.M.P.; validation, A.G., A.S.G. and O.D.; formal analysis, A.G., A.S.G., O.D. and O.O.; investigation, A.G., A.S.G., O.O. and O.D.; data curation, G.M.P.; writing—original draft preparation, A.G., O.D. and O.O.; writing—review and editing, G.M.P.; visualization, A.G., A.S.G. and O.D.; supervision, G.M.P.; project administration, G.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors would like to thank the Corresponding Member of the Russian Academy of Sciences Evgenii Panarin and scientific researcher Irina Gavrilova for provided materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Winnik, M.A.; Yekta, A. Associative polymers in aqueous solution. Curr. Opin. Colloid Interface Sci. 1997, 2, 424–436. [Google Scholar] [CrossRef]
- Hamley, I.W. Block Copolymers in Solution: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Liu, K.; Kang, Y.; Wang, Z.; Zhang, X. 25th Anniversary Article: Reversible and Adaptive Functional Supramolecular Materials: “Noncovalent Interaction” Matters. Adv. Mater. 2013, 25, 5530–5548. [Google Scholar] [CrossRef]
- Krieg, E.; Bastings, M.M.; Besenius, P.; Rybtchinski, B. Supramolecular polymers in aqueous media. Chem. Rev. 2016, 116, 2414–2477. [Google Scholar] [CrossRef]
- van der Tol, J.J.B.; Vantomme, G.; Palmans, A.R.A.; Meijer, E.W. Controlling the Processability and Stability of Supramolecular Polymers Using the Interplay of Intra- and Intermolecular Interactions. Macromolecules 2022, 55, 6820–6829. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Bronich, T.K.; Kabanov, V.A.; Yu, K.; Eisenberg, A. Soluble stoichiometric complexes from poly (N-ethyl-4-vinylpyridinium) cations and poly (ethylene oxide)-block-polymethacrylate anions. Macromolecules 1996, 29, 6797–6802. [Google Scholar] [CrossRef]
- Burova, T.V.; Grinberg, N.V.; Grinberg, V.Y.; Tang, Y.; Zhang, G.; Khokhlov, A.R. Order-Disorder Conformational Transitions of N-Isopropylacrylamide−Sodium Styrene Sulfonate Copolymers in Aqueous Solutions. Macromolecules 2008, 41, 5981–5984. [Google Scholar] [CrossRef]
- Nicolas, M.; Beyou, E.; Fumagalli, M. Two-step synthesis of polystyrene sulfonate based copolymers bearing pendant primary amines. Eur. Polym. J. 2021, 152, 110455. [Google Scholar] [CrossRef]
- Piogé, S.; Fontaine, L.; Gaillard, C.; Nicol, E.; Pascual, S. Self-Assembling Properties of Well-Defined Poly(ethylene oxide)-b-poly(ethyl acrylate) Diblock Copolymers. Macromolecules 2009, 42, 4262–4272. [Google Scholar] [CrossRef]
- Kawata, T.; Hashidzume, A.; Sato, T. Micellar structure of amphiphilic statistical copolymers bearing dodecyl hydrophobes in aqueous media. Macromolecules 2007, 40, 1174–1180. [Google Scholar] [CrossRef]
- Shashkina, Y.A.; Zaroslov, Y.D.; Smirnov, V.A.; Philippova, O.E.; Khokhlov, A.R.; Pryakhina, T.A.; Churochkina, N.A. Hydrophobic aggregation in aqueous solutions of hydrophobically modified polyacrylamide in the vicinity of overlap concentration. Polymer 2003, 44, 2289–2293. [Google Scholar] [CrossRef]
- Krayukhina, M.A.; Samoilova, N.A.; Yamskov, I.A. Polyelectrolyte complexes of chitosan: Formation, properties and applications. Russ. Chem. Rev. 2008, 77, 799. [Google Scholar] [CrossRef]
- Ortona, O.; D’Errico, G.; Mangiapia, G.; Ciccarelli, D. The aggregative behavior of hydrophobically modified chitosans with high substitution degree in aqueous solution. Carbohydr. Polym. 2008, 74, 16–22. [Google Scholar] [CrossRef]
- Lopez, C.G.; Colby, R.H.; Cabral, J.T. Electrostatic and Hydrophobic Interactions in NaCMC Aqueous Solutions: Effect of Degree of Substitution. Macromolecules 2018, 51, 3165–3175. [Google Scholar] [CrossRef]
- Lu, A.; Wang, J.; Najarro, M.C.; Li, S.; Deratani, A. Synthesis and self-assembly of AB2-type amphiphilic copolymers from biobased hydroxypropyl methyl cellulose and poly (L-lactide). Carbohydr. Polym. 2019, 211, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Burova, T.V.; Grinberg, N.V.; Tur, D.R.; Papkov, V.S.; Dubovik, A.S.; Shibanova, E.D.; Bairamashvili, D.I.; Grinberg, V.Y.; Khokhlov, A.R. Ternary interpolyelectrolyte complexes insulin-poly (methylaminophosphazene)-dextran sulfate for oral delivery of insulin. Langmuir 2013, 29, 2273–2281. [Google Scholar] [CrossRef]
- Adams, M.L.; Lavasanifar, A.; Kwon, G.S. Amphiphilic block copolymers for drug delivery. J. Pharm. Sci. 2003, 92, 1343–1355. [Google Scholar] [CrossRef]
- Kedracki, D.; Abraham, J.N.; Prado, E.; Nardin, C. Self-Assembly of Biohybrid Polymers. In Macromolecular Self-Assembly; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 193–229. [Google Scholar]
- Moradi-Araghi, A. A review of thermally stable gels for fluid diversion in petroleum production. J. Pet. Sci. Eng. 2000, 26, 1–10. [Google Scholar] [CrossRef]
- Abirov, R.; Ivakhnenko, A.P.; Abirov, Z.; Eremin, N.A. The associative polymer flooding: An experimental study. J. Pet. Explor. Prod. Technol. 2020, 10, 447–454. [Google Scholar] [CrossRef]
- Gogoi, S.; Gogoi, S.B. Review on microfluidic studies for EOR application. J. Pet. Explor. Prod. Technol. 2019, 9, 2263–2277. [Google Scholar] [CrossRef]
- Green, M.S.; Tobolsky, A.V. A New Approach to the Theory of Relaxing Polymeric Media. J. Chem. Phys. 1946, 14, 80–92. [Google Scholar] [CrossRef]
- Rubinstein, M.; Dobrynin, A.V. Associations leading to formation of reversible networks and gels. Curr. Opin. Colloid Interface Sci. 1999, 4, 83–87. [Google Scholar] [CrossRef]
- Rubinstein, M.; Dobrynin, A.V. Solutions of associative polymers. Trends Polym. Sci. 1997, 5, 181–186. [Google Scholar]
- Semenov, A.N.; Rubinstein, M. Thermoreversible Gelation in Solutions of Associative Polymers. 1. Statics. Macromolecules 1998, 31, 1373–1385. [Google Scholar] [CrossRef]
- Rubinstein, M.; Semenov, A.N. Thermoreversible gelation in solutions of associating polymers. 2. Linear dynamics. Macromolecules 1998, 31, 1386–1397. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Q.; Colby, R.H. Dynamics of associative polymers. Soft Matter 2018, 14, 2961–2977. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, P.; Audibert-Hayet, A.; Selb, J.; Candau, F. Rheological properties of multisticker associative polyelectrolytes in semidilute aqueous solutions. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 1640–1655. [Google Scholar] [CrossRef]
- Kujawa, P.; Audibert-Hayet, A.; Selb, J.; Candau, F. Effect of Ionic Strength on the Rheological Properties of Multisticker Associative Polyelectrolytes. Macromolecules 2006, 39, 384–392. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, C.; Weiss, R.A.; Chen, Q. Association energy in strongly associative polymers. J. Rheol. 2017, 61, 1199–1207. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, H.; Tang, P.; Yang, Y. Linear Viscoelasticity of Associative Polymers: Sticky Rouse Model and the Role of Bridges. Macromolecules 2020, 53, 3438–3451. [Google Scholar] [CrossRef]
- Chassenieux, C.; Nicolai, T.; Benyahia, L. Rheology of associative polymer solutions. Curr. Opin. Colloid Interface Sci. 2011, 16, 18–26. [Google Scholar] [CrossRef]
- Middleton, L.R.; Winey, K.I. Nanoscale aggregation in acid-and ion-containing polymers. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 499–523. [Google Scholar] [CrossRef] [PubMed]
- Staudinger, H.; Heuer, W. Über hochpolymere Verbindungen, 33. Mitteilung: Beziehungen zwischen Viscosität und Molekulargewicht bei Poly-styrolen. Ber. Dtsch. Chem. Ges. A B Ser. 1930, 63, 222–234. [Google Scholar] [CrossRef]
- Wolff, C.; Silberberg, A.; Priel, Z.; Layec-Raphalen, M. Influence of the association of macromolecules in dilute solution on their reduced viscosity. Polymer 1979, 20, 281–287. [Google Scholar] [CrossRef]
- Abdala, A.A.; Olesen, K.; Khan, S.A. Solution rheology of hydrophobically modified associative polymers: Solvent quality and hydrophobic interactions. J. Rheol. 2003, 47, 497–511. [Google Scholar] [CrossRef]
- Pathak, J.A.; Nugent, S.; Bender, M.F.; Roberts, C.J.; Curtis, R.J.; Douglas, J.F. Comparison of Huggins Coefficients and Osmotic Second Virial Coefficients of Buffered Solutions of Monoclonal Antibodies. Polymers 2021, 13, 601. [Google Scholar] [CrossRef]
- Roche, A.; Gentiluomo, L.; Sibanda, N.; Roessner, D.; Friess, W.; Trainoff, S.P.; Curtis, R. Towards an improved prediction of concentrated antibody solution viscosity using the Huggins coefficient. J. Colloid Interface Sci. 2022, 607, 1813–1824. [Google Scholar] [CrossRef]
- Flory, P.J.; Fox, T.G. Treatment of Intrinsic Viscosities. J. Am. Chem. Soc. 1951, 73, 1904–1908. [Google Scholar] [CrossRef]
- Kraemer, E.O. Molecular Weights of Celluloses and Cellulose Derivates. Ind. Eng. Chem. 1938, 30, 1200–1203. [Google Scholar] [CrossRef]
- Rutgers, I.R. Relative viscosity and concentration. Rheol. Acta 1962, 2, 305–348. [Google Scholar] [CrossRef]
- Budtov, V.P. Physical Chemistry of Polymer Solutions; Khimiya: Saint Petersburg, Russia, 1992; p. 381. [Google Scholar]
- Lu, Y.; An, L.; Wang, Z.-G. Intrinsic Viscosity of Polymers: General Theory Based on a Partially Permeable Sphere Model. Macromolecules 2013, 46, 5731–5740. [Google Scholar] [CrossRef]
- Lu, Y.; Shi, T.; An, L.; Jin, L.; Wang, Z.-G. A simple model for the anomalous intrinsic viscosity of dendrimers. Soft Matter 2010, 6, 2619–2622. [Google Scholar] [CrossRef]
- Yan, D. Intrinsic viscosity of polymer solutions: Fresh ideas to an old problem. Sci. China Chem. 2015, 58, 835–838. [Google Scholar] [CrossRef]
- Mead, D.J.; Fuoss, R.M. Viscosities of Solutions of Polyvinyl Chloride. J. Am. Chem. Soc. 1942, 64, 277–282. [Google Scholar] [CrossRef]
- Huggins, M.L. The viscosity of dilute solutions of long-chain molecules. IV. Dependence on concentration. J. Am. Chem. Soc. 1942, 64, 2716–2718. [Google Scholar] [CrossRef]
- Tsvetkov, V.N.; Eskin, V.E.; Frenkel, S.Y. Structure of Macromolecules in Solution; The National Lending Library for Science and Technology: London, UK, 1971. [Google Scholar]
- Pamies, R.; Hernández Cifre, J.G.; del Carmen López Martínez, M.; García de la Torre, J. Determination of intrinsic viscosities of macromolecules and nanoparticles. Comparison of single-point and dilution procedures. Colloid Polym. Sci. 2008, 286, 1223–1231. [Google Scholar] [CrossRef]
- Schoff, C. Concentration Dependence of the Viscosity of Dilute Polymer Solutions: Huggins and Schulz-Blaschke Constants. In The Wiley Database of Polymer Properties; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Cohen, J.; Priel, Z.; Rabin, Y. Viscosity of dilute polyelectrolyte solutions. J. Chem. Phys. 1988, 88, 7111–7116. [Google Scholar] [CrossRef]
- Weissberg, S.; Simha, R.; Rothman, S. Viscosity of dilute and moderately concentrated polymer solutions. J. Res. Natl. Bur. Stand. 1951, 47, 298–314. [Google Scholar] [CrossRef]
- Gavrilova, I.I.; Panarin, E.F.; Nesterova, N.A. Homopolymerization of N-vinylamides in the presence of water-soluble initiators and preparation of polyelectrolytes from the polymerization products. Russ. J. Appl. Chem. 2012, 85, 413–416. [Google Scholar] [CrossRef]
- Pavlov, G.M.; Okatova, O.V.; Gubarev, A.S.; Gavrilova, I.I.; Panarin, E.F. Strong Linear Polyelectrolytes in Solutions of Extreme Concentrations of One–One Valent Salt. Hydrodynamic Study. Macromolecules 2014, 47, 2748–2758. [Google Scholar] [CrossRef]
- Pavlov, G.M.; Gosteva, A.A.; Okatova, O.V.; Dommes, O.A.; Gavrilova, I.I.; Panarin, E.F. Detection and evaluation of polymer–polymer interactions in dilute solutions of associating polymers. Polym. Chem. 2021, 12, 2325–2334. [Google Scholar] [CrossRef]
- Pavlov, G.M.; Zaitseva, I.I.; Gubarev, A.S.; Gavrilova, I.I.; Panarin, E.F. Diffusion-viscometric analysis and conformational characteristics of sodium polystyrenesulfonate molecules. Russ. J. Appl. Chem. 2006, 79, 1490–1493. [Google Scholar] [CrossRef]
- Pavlov, G.M.; Okatova, O.V.; Mikhailova, A.V.; Ulyanova, N.N.; Gavrilova, I.I.; Panarin, E.F. Conformational Parameters of Poly(N-methyl-N-vinylacetamide) Molecules Through the Hydrodynamic Characteristics Studies. Macromol. Biosci. 2010, 10, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, G.; Zaitseva, I.I.; Mikhailova, N.A. Hydrodynamic and molecular characteristics of hydroxypropylmethyl cellulose and rheology of its aqueous solutions. Polym. Sci. Ser. A 2004, 46, 1068–1071. [Google Scholar]
- Pavlov, G.M.; Gubarev, A.S.; Gavrilova, I.I.; Panarin, E.F. Conformations of sodium poly(styrene-4-sulfonate) macromolecules in solutions with different ionic strengths. Polym. Sci. Ser. A 2011, 53, 1003–1011. [Google Scholar] [CrossRef]
- Pavlov, G.M.; Kolbina, G.F.; Okatova, O.V.; Gavrilova, I.I.; Panarin, E.F. Unimolecular micelles based on amphiphilic of N-methyl-N-vinylacetamide copolymers. Dokl. Chem. 2015, 463, 181–184. [Google Scholar] [CrossRef]
- Dommes, O.A.; Gosteva, A.A.; Okatova, O.V.; Pavlov, G.M. Sizes Monitoring of Polyelectrolyte Flexible Chains over the Entire Range of Ionic Strength through Viscometry of Dilute Solutions. Rev. Adv. Chem. 2021, 11, 134–144. [Google Scholar] [CrossRef]
- Lavrenko, P.N.; Okatova, O.V. New cell and methods of forming boundaries when studying macromolecular diffusion in solutions. Polym. Sci. USSR 1977, 19, 3049–3054. [Google Scholar] [CrossRef]
- Tsvetkov, V.N. Rigid-Chain Polymers: Hydrodynamic and Optical Properties in Solution; Springer: New York, NY, USA, 1989; p. 490. [Google Scholar]
- Schuck, P. Size-Distribution Analysis of Macromolecules by Sedimentation Velocity Ultracentrifugation and Lamm Equation Modeling. Biophys. J. 2000, 78, 1606–1619. [Google Scholar] [CrossRef]
- Brown, P.H.; Schuck, P. A new adaptive grid-size algorithm for the simulation of sedimentation velocity profiles in analytical ultracentrifugation. Comput. Phys. Commun. 2008, 178, 105–120. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- López Martínez, M.C.; Díaz Baños, F.G.; Ortega Retuerta, A.; García de la Torre, J. Multiple Linear Least-Squares Fits with a Common Intercept: Determination of the Intrinsic Viscosity of Macromolecules in Solution. J. Chem. Educ. 2003, 80, 1036. [Google Scholar] [CrossRef]
- Gubarev, A.S. Conformations of Water-Soluble Aromatic Macromolecules in Solutions of Various Ionic Strengths and Orientational Order in Films Prepared on Their Basis; Saint Petersburg State University: Saint Petersburg, Russia, 2010. [Google Scholar]
- Takahashi, A.; Kato, T.; Nagasawa, M. The Second Virial Coefficient of Polyelectrolytes. J. Phys. Chem. 1967, 71, 2001–2010. [Google Scholar] [CrossRef]
- Pavlov, G.M.; Gubarev, A.S. Intrinsic viscosity of strong linear polyelectrolytes in solutions of low ionic strength and its interpretation. In Advances in Physicochemical Properties of Biopolymers: Part 1; Bentham Science Publishers BV: Sharjah, United Arab Emirates, 2017; pp. 433–460. [Google Scholar]
- Kurata, M.; Tsunashima, Y. Viscosity—Molecular Weight Relationships and Unperturbed Dimensions of Linear Chain Molecules. In The Wiley Database of Polymer Properties; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Pavlov, G.; Harding, S.; Rowe, A. Normalized scaling relations as a natural classification of linear macromolecules according to size. In Analytical Ultracentrifugation V; Springer: Berlin/Heidelberg, Germany, 1999; pp. 76–80. [Google Scholar]
- Pavlov, G.M. Size and average density spectra of macromolecules obtained from hydrodynamic data. Eur. Phys. J. E 2007, 22, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, G.M. Hydrodynamics of Macromolecules: Conformation Zoning for General Macromolecules. In Encyclopedia of Biophysics; Roberts, G., Watts, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–13. [Google Scholar]
- Pavlov, G.M.; Dommes, O.A.; Okatova, O.V.; Gavrilova, I.I.; Panarin, E.F. Spectrum of hydrodynamic volumes and sizes of macromolecules of linear polyelectrolytes versus their charge density in salt-free aqueous solutions. Phys. Chem. Chem. Phys. 2018, 20, 9975–9983. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).