Accelerated Degradation of Poly-ε-caprolactone Composite Scaffolds for Large Bone Defects
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bone Bricks and Scaffolds Production
2.3. Degradation Procedure
2.4. Morphological Characterization
2.5. Thermal Gravimetric Analysis
2.6. Differential Scanning Calorimetry
2.7. Mechanical Testing
2.8. Data Analysis
3. Results and Discussion
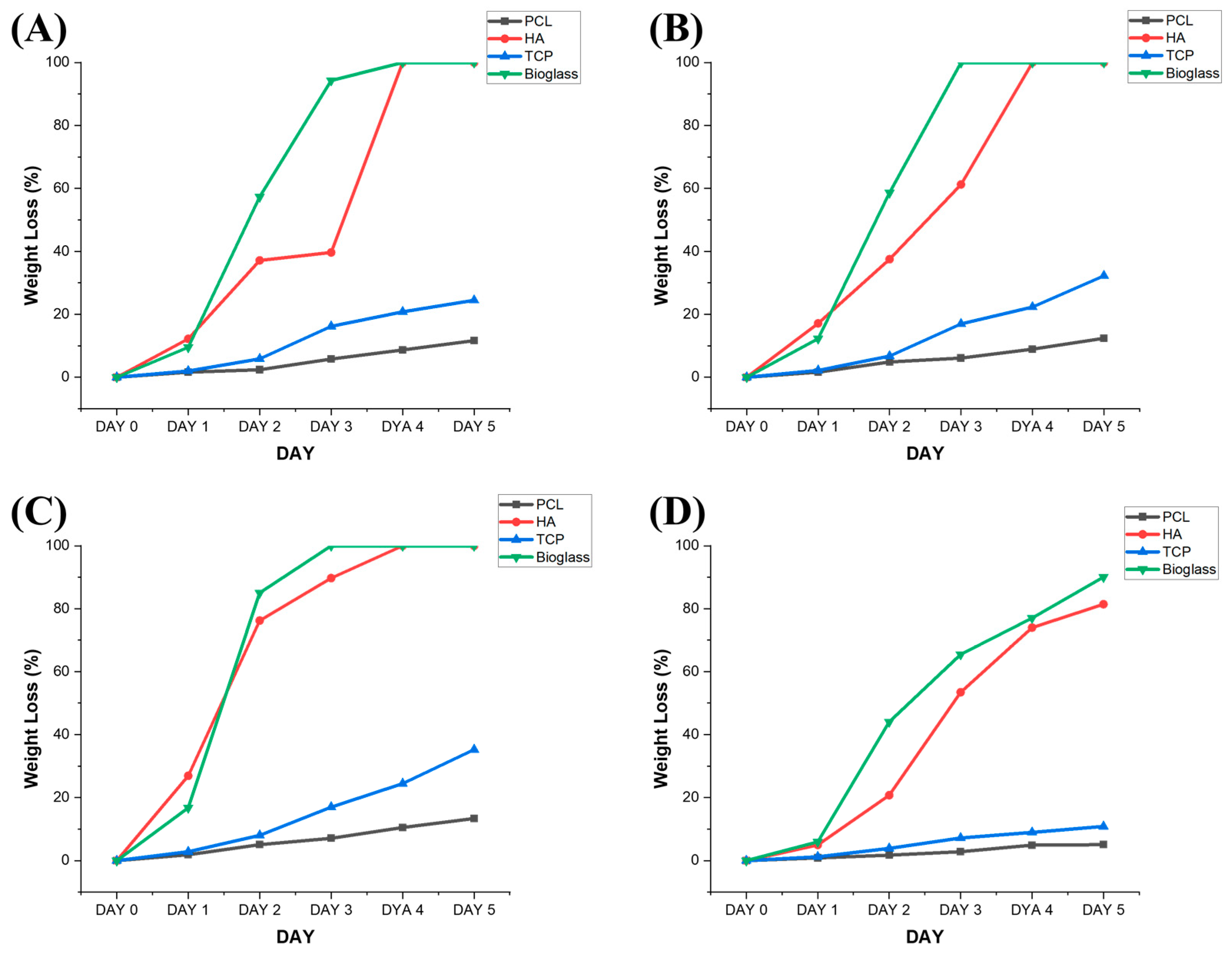
3.1. Degradation Analysis
3.2. Thermal Gravimetric Analysis
3.3. Chemical Analysis
3.4. Mechanical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruyas, A.; Lou, F.; Stahl, A.; Gardner, M.; Maloney, W.; Goodman, S.; Yang, Y. Systematic characterization of 3D-printed PCL/β-TCP scaffolds for biomedical devices and bone tissue engineering: Influence of composition and porosity. J. Mater. Res. 2018, 33, 1948–1959. [Google Scholar] [CrossRef]
- Chlanda, A.; Kijeńska-Gawrońska, E.; Zdunek, J.; Swieszkowski, W. Internal nanocrystalline structure and stiffness alterations of electrospun polycaprolactone-based mats after six months of in vitro degradation. An atomic force microscopy assay. J. Mech. Behav. Biomed. Mater. 2020, 101, 103437. [Google Scholar] [CrossRef] [PubMed]
- Díaz, E.; Aresti, J.; León, J. Evaluation of physicochemical and mechanical properties with the in vitro degradation of PCL/nHA/MWCNT composite scaffolds. J. Reinf. Plast. Compos. 2020, 40, 134–142. [Google Scholar] [CrossRef]
- Pogorielov, M.; Hapchenko, A.; Deineka, V.; Rogulska, L.; Oleshko, O.; Vodseďálková, K.; Berezkinová, L.; Vysloužilová, L.; Klápšťová, A.; Erben, J. In vitro degradation and in vivotoxicity of NanoMatrix3D® polycaprolactone and poly(lactic acid) nanofibrous scaffolds. J. Biomed. Mater Res. Part A 2018, 106, 2200–2212. [Google Scholar] [CrossRef] [PubMed]
- Melnik, E.; Shkarina, S.; Ivlev, S.; Weinhardt, V.; Baumbach, T.; Chaikina, M.; Surmeneva, M.; Surmenev, R. In vitro degradation behaviour of hybrid electrospun scaffolds of polycaprolactone and strontium-containing hydroxyapatite microparticles. Polym. Degrad. Stab. 2019, 167, 21–32. [Google Scholar] [CrossRef]
- Motealleh, A.; Eqtesadi, S.; Pajares, A.; Miranda, P. Enhancing the mechanical and in vitro performance of robocast bioglass scaffolds by polymeric coatings: Effect of polymer composition. J. Mech. Behav. Biomed. Mater. 2018, 84, 35–45. [Google Scholar] [CrossRef]
- Conoscenti, G.; Carfì Pavia, F.; Ciraldo, F.; Liverani, L.; Brucato, V.; La Carrubba, V.; Boccaccini, A. In vitro degradation and bioactivity of composite poly-l-lactic (PLLA)/bioactive glass (BG) scaffolds: Comparison of 45S5 and 1393BG compositions. J. Mater. Sci. 2017, 53, 2362–2374. [Google Scholar] [CrossRef]
- Gaikwad, A.; Lu, W.; Dey, S.; Bhattarai, P.; Chia, C.; Larby, J.; Haug, G.; Myers, S.; Jaffar, J.; Westall, G.; et al. Vascular remodelling in IPF patients and its detrimental effect on lung physiology: Potential role of endothelial to mesenchymal transition (EndMT). ERJ Open Res. 2022, 8, 00571-2021. [Google Scholar] [CrossRef]
- Mousovich-Neto, F.; Matos, M.; Costa, A.; Melo Reis, R.; Atella, G.; Miranda-Alves, L.; Carvalho, D.; Ketzer, L.; Corrêa da Costa, V. Brown adipose tissue remodelling induced by corticosterone in male Wistar rats. Exp. Physiol. 2019, 104, 514–528. [Google Scholar] [CrossRef]
- Wang, G.; Mohammadtursun, N.; Lv, Y.; Zhang, H.; Sun, J.; Dong, J. Baicalin Exerts Anti-Airway Inflammation and Anti-Remodelling Effects in Severe Stage Rat Model of Chronic Obstructive Pulmonary Disease. Evid.-Based Complement. Altern. Med. 2018, 2018, 7591348. [Google Scholar] [CrossRef]
- Rijsbergen, M.; van Rietbergen, B.; Barthelemy, V.; Eltes, P.; Lazáry, Á.; Lacroix, D.; Noailly, J.; Ho Ba Tho, M.; Wilson, W.; Ito, K. Comparison of patient-specific computational models vs. clinical follow-up, for adjacent segment disc degeneration and bone remodelling after spinal fusion. PLoS ONE 2018, 13, e0200899. [Google Scholar] [CrossRef]
- Wong, S.; Tamatam, C.; Cho, I.; Toth, P.; Bargi, R.; Belvitch, P.; Lee, J.; Rehman, J.; Reddy, S.; Shin, J. Inhibition of aberrant tissue remodelling by mesenchymal stromal cells singly coated with soft gels presenting defined chemomechanical cues. Nat. Biomed. Eng. 2021, 6, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Contessotto, P.; Pandit, A. Therapies to prevent post-infarction remodelling: From repair to regeneration. Biomaterials 2021, 275, 120906. [Google Scholar] [CrossRef]
- Kubota, S.I.; Takahashi, K.; Mano, T.; Matsumoto, K.; Katsumata, T.; Shi, S.; Tainaka, K.; Ueda, H.R.; Ehata, S.; Miyazono, K. Whole-organ analysis of TGF-β-mediated remodelling of the tumour microenvironment by tissue clearing. Commun. Biol. 2021, 4, 294. [Google Scholar] [CrossRef] [PubMed]
- Maes, L.; Cloet, A.S.; Fourneau, I.; Famaey, N. A homogenized constrained mixture model of restenosis and vascular remodelling after balloon angioplasty. J. R. Soc. Interface 2021, 18, 20210068. [Google Scholar] [CrossRef] [PubMed]
- Żółtowska, S.; Koltsov, I.; Alejski, K.; Ehrlich, H.; Ciałkowski, M.; Jesionowski, T. Thermal decomposition behaviour and numerical fitting for the pyrolysis kinetics of 3D spongin-based scaffolds. The classic approach. Polym. Test. 2021, 97, 107148. [Google Scholar] [CrossRef]
- He, Y.; Santana, M.; Staneviciute, A.; Pimentel, M.; Yang, F.; Goes, J.; Kawaji, K.; Vaicik, M.; Abdulhadi, R.; Hibino, N.; et al. Hydrogel Scaffolds: Cell-Laden Gradient Hydrogel Scaffolds for Neovascularization of Engineered Tissues (Adv. Healthcare Mater. 7/2021). Adv. Healthcare Mater. 2021, 10, 2170030. [Google Scholar] [CrossRef]
- Bose, S.; Bhattacharjee, A.; Banerjee, D.; Boccaccini, A.; Bandyopadhyay, A. Influence of random and designed porosities on 3D printed tricalcium phosphate-bioactive glass scaffolds. Addit. Manuf. 2021, 40, 101895. [Google Scholar] [CrossRef]
- González-Pérez, F.; Ibáñez-Fonseca, A.; Alonso, M.; Rodríguez-Cabello, J. Combining tunable proteolytic sequences and a VEGF-mimetic peptide for the spatiotemporal control of angiogenesis within Elastin-Like Recombinamer scaffolds. Acta Biomater. 2021, 130, 149–160. [Google Scholar] [CrossRef]
- Fernandes, J.; Soek, R.; do Nascimento, T.; Menezes, L.; Sassaki, G.; Campos, R. Degradation of Organophosphates Promoted by 1,2,4-Triazole Anion: Exploring Scaffolds for Efficient Catalytic Systems. J. Org. Chem. 2021, 86, 4027–4034. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Abdal-hay, A.; Bartnikowski, N.; Kyoung Kim, Y.; Ivanovski, S. A comprehensive study of acid and base treatment of 3D printed poly(ε-caprolactone) scaffolds to tailor surface characteristics. Appl. Surf. Sci. 2021, 555, 149602. [Google Scholar] [CrossRef]
- Dong, Q.; Zhang, M.; Zhou, X.; Shao, Y.; Li, J.; Wang, L.; Chu, C.; Xue, F.; Yao, Q.; Bai, J. 3D-printed Mg-incorporated PCL-based scaffolds: A promising approach for bone healing. Mater. Sci. Eng. C. 2021, 129, 112372. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, S.; Tang, D.; Jiang, L.; Shi, J.; Wang, S. Mechanical Properties and Degradability of Electrospun PCL/PLGA Blended Scaffolds as Vascular Grafts. Trans. Tianjin Univ. 2018, 25, 152–160. [Google Scholar] [CrossRef]
- Sánchez-González, S.; Diban, N.; Urtiaga, A. Hydrolytic Degradation and Mechanical Stability of Poly(ε-caprolactone)/Reduced Graphene Oxide Membranes as Scaffolds for In Vitro Neural Tissue Regeneration. Membranes 2018, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Woodard, L.; Grunlan, M. Hydrolytic Degradation of PCL–PLLA Semi-IPNs Exhibiting Rapid, Tunable Degradation. ACS Biomater. Sci. Eng. 2018, 5, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Woodard, L.; Grunlan, M. Hydrolytic Degradation and Erosion of Polyester Biomaterials. ACS Macro Lett. 2018, 7, 976–982. [Google Scholar] [CrossRef]
- Farzan, A.; Borandeh, S.; Zanjanizadeh Ezazi, N.; Lipponen, S.; Santos, H.; Seppälä, J. 3D scaffolding of fast photocurable polyurethane for soft tissue engineering by stereolithography: Influence of materials and geometry on growth of fibroblast cells. Eur. Polym. J. 2020, 139, 109988. [Google Scholar] [CrossRef]
- Silva, M.; Ferreira, F.N.; Alves, N.M.; Paiva, M.C. Biodegradable polymer nanocomposites for ligament/tendon tissue engineering. J. Nanobiotechnol. 2020, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hou, P.; Liu, S.; Qi, J.; Feng, S.; Zhang, L.; Ma, P.; Bai, W. Effect of poly(lactic-co-glycolic acid) blend ratios on the hydrolytic degradation of poly(para-dioxanone). J. Polym. Res. 2021, 28, 166. [Google Scholar] [CrossRef]
- Anju, S.; Prajitha, N.; Sukanya, V.; Mohanan, P. Complicity of degradable polymers in health-care applications. Mater. Today Chem. 2020, 16, 100236. [Google Scholar] [CrossRef]
- Ben Abdallah, A.; Kallel, A.; Gamaoun, F.; Tcharkhtchi, A. Enzymatic Hydrolysis of Poly (Caprolactone) and its Blend with Styrene–Butadiene–Styrene (40% PCL/60% SBS). J. Polym. Environ. 2019, 27, 2341–2351. [Google Scholar] [CrossRef]
- Leroux, A.; Ngoc Nguyen, T.; Rangel, A.; Cacciapuoti, I.; Duprez, D.; Castner, D.; Migonney, V. Long-term hydrolytic degradation study of polycaprolactone films and fibers grafted with poly(sodium styrene sulfonate): Mechanism study and cell response. Biointerphases 2020, 15, 061006. [Google Scholar] [CrossRef] [PubMed]
- Germain, L.; Fuentes, C.; van Vuure, A.; des Rieux, A.; Dupont-Gillain, C. 3D-printed biodegradable gyroid scaffolds for tissue engineering applications. Mater. Des. 2018, 151, 113–122. [Google Scholar] [CrossRef]
- Prabhath, A.; Vernekar, V.; Vasu, V.; Badon, M.; Avochinou, J.; Asandei, A.; Kumbar, S.; Weber, E.; Laurencin, C. Kinetic degradation and biocompatibility evaluation of polycaprolactone-based biologics delivery matrices for regenerative engineering of the rotator cuff. J. Biomed. Mater Res. Part A 2021, 109, 2137–2153. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Erdal, N.; Lando, G.; Yadav, A.; Srivastava, R.; Hakkarainen, M. Hydrolytic Degradation of Porous Crosslinked Poly(ε-Caprolactone) Synthesized by High Internal Phase Emulsion Templating. Polymers 2020, 12, 1849. [Google Scholar] [CrossRef]
- Sattary, M.; Khorasani, M.; Rafienia, M.; Rozve, H. Incorporation of nanohydroxyapatite and vitamin D3 into electrospun PCL/Gelatin scaffolds: The influence on the physical and chemical properties and cell behavior for bone tissue engineering. Polym. Adv. Technol. 2017, 29, 451–462. [Google Scholar] [CrossRef]
- Ogueri, K.; Jafari, T.; Escobar Ivirico, J.; Laurencin, C. Polymeric Biomaterials for Scaffold-Based Bone Regenerative Engineering. Regener. Eng. Transl. Med. 2018, 5, 128–154. [Google Scholar] [CrossRef] [PubMed]
- Saudi, A.; Zebarjad, S.; Salehi, H.; Katoueizadeh, E.; Alizadeh, A. Assessing physicochemical, mechanical, and in vitro biological properties of polycaprolactone/poly(glycerol sebacate)/hydroxyapatite composite scaffold for nerve tissue engineering. Mater. Chem. Phys. 2022, 275, 125224. [Google Scholar] [CrossRef]
- Li, R.; Song, Y.; Fouladian, P.; Arafat, M.; Chung, R.; Kohlhagen, J.; Garg, S. Three-Dimensional Printing of Curcumin-Loaded Biodegradable and Flexible Scaffold for Intracranial Therapy of Glioblastoma Multiforme. Pharmaceutics 2021, 13, 471. [Google Scholar] [CrossRef]
- Bazgir, M.; Zhang, W.; Zhang, X.; Elies, J.; Saeinasab, M.; Coates, P.; Youseffi, M.; Sefat, F. Degradation and Characterisation of Electrospun Polycaprolactone (PCL) and Poly(lactic-co-glycolic acid) (PLGA) Scaffolds for Vascular Tissue Engineering. Materials 2021, 14, 4773. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, H.; Jia, G.; Zeng, H.; Yuan, G. Fatigue and dynamic biodegradation behavior of additively manufactured Mg scaffolds. Acta Biomater. 2021, 135, 705–722. [Google Scholar] [CrossRef] [PubMed]
- Saranti, A.; Tiron-Stathopoulos, A.; Papaioannou, L.; Gioti, C.; Ioannou, A.; Karakassides, M.; Avgoustakis, K.; Koutselas, I.; Dimos, K. 3D-printed bioactive scaffolds for bone regeneration bearing carbon dots for bioimaging purposes. Smart Mater. Med. 2022, 3, 12–19. [Google Scholar] [CrossRef]
- He, J.; Lin, Z.; Hu, X.; Xing, L.; Liang, G.; Chen, D.; An, J.; Xiong, C.; Zhang, X.; Zhang, L. Biocompatible and biodegradable scaffold based on polytrimethylene carbonate-tricalcium phosphate microspheres for tissue engineering. Colloids Surf. B Biointerfaces 2021, 204, 111808. [Google Scholar] [CrossRef]
- Kleer-Reiter, N.; Julmi, S.; Feichtner, F.; Waselau, A.; Klose, C.; Wriggers, P.; Maier, H.; Meyer-Lindenberg, A. Biocompatibility and degradation of the open-pored magnesium scaffolds LAE442 and La2. Biomed. Mater. 2021, 16, 035037. [Google Scholar] [CrossRef]
- Krieghoff, J.; Kascholke, C.; Loth, R.; Starke, A.; Koenig, A.; Schulz-Siegmund, M.; Hacker, M. Composition-controlled degradation behavior of macroporous scaffolds from three-armed biodegradable macromers. Polym. Degrad. Stab. 2022, 195, 109775. [Google Scholar] [CrossRef]
- Kowalewicz, K.; Vorndran, E.; Feichtner, F.; Waselau, A.; Brueckner, M.; Meyer-Lindenberg, A. In-Vivo Degradation Behavior and Osseointegration of 3D Powder-Printed Calcium Magnesium Phosphate Cement Scaffolds. Materials 2021, 14, 946. [Google Scholar] [CrossRef]
- Daskalakis, E.; Huang, B.; Vyas, C.; Acar, A.; Liu, F.; Fallah, A.; Cooper, G.; Weightman, A.; Blunn, G.; Koç, B.; et al. Bone Bricks: The Effect of Architecture and Material Composition on the Mechanical and Biological Performance of Bone Scaffolds. ACS Omega 2022, 7, 7515–7530. [Google Scholar] [CrossRef]
- Daskalakis, E.; Huang, B.; Vyas, C.; Acar, A.; Fallah, A.; Cooper, G.; Weightman, A.; Koc, B.; Blunn, G.; Bartolo, P. Novel 3D Bioglass Scaffolds for Bone Tissue Regeneration. Polymers 2022, 14, 445. [Google Scholar] [CrossRef]
- Huang, B.; Wang, Y.; Vyas, C.; Bartolo, P. Crystal Growth of 3D Poly(ε-caprolactone) Based Bone Scaffolds and Its Effects on the Physical Properties and Cellular Interactions. Adv. Sci. 2022, 10, 2203183. [Google Scholar] [CrossRef]
- Guo, L.; Du, Z.; Wang, Y.; Cai, Q.; Yang, X. Degradation behaviors of three-dimensional hydroxyapatite fibrous scaffolds stabilized by different biodegradable polymers. Ceram. Int. 2020, 46, 14124–14133. [Google Scholar] [CrossRef]
- Tarik Arafat, M.; Lam, C.; Ekaputra, A.; Wong, S.; He, C.; Hutmacher, D.; Li, X.; Gibson, I. High performance additive manufactured scaffolds for bone tissue engineering application. Soft Matter 2011, 7, 8013. [Google Scholar] [CrossRef]
- Lam, C.; Hutmacher, D.; Schantz, J.; Woodruff, M.; Teoh, S. Evaluation of polycaprolactone scaffold degradation for 6 months in vitro and in vivo. J. Biomed. Mater Res. Part A 2009, 90A, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Suryani, L.; Zhou, X.; Muthukumaran, P.; Rakshit, M.; Yang, F.; Wen, F.; Hassanbhai, A.; Parida, K.; Simon, D.; et al. Synergistic Effect of PVDF-Coated PCL-TCP Scaffolds and Pulsed Electromagnetic Field on Osteogenesis. Int. J. Mol. Sci. 2021, 22, 6438. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.; Savalani, M.; Teoh, S.; Hutmacher, D. Dynamics of in vitro polymer degradation of polycaprolactone-based scaffolds: Accelerated versus simulated physiological conditions. Biomed. Mater. 2008, 3, 034108. [Google Scholar] [CrossRef]
- Siqueira, D.; Luna, C.; Araújo, E.; Barros, A.; Wellen, R. Approaches on PCL/macaíba biocomposites—Mechanical, thermal, morphological properties and crystallization kinetics. Polym. Adv. Technol. 2021, 32, 3572–3587. [Google Scholar] [CrossRef]
- Przybysz-Romatowska, M.; Barczewski, M.; Mania, S.; Tercjak, A.; Haponiuk, J.; Formela, K. Morphology, Thermo-Mechanical Properties and Biodegradibility of PCL/PLA Blends Reactively Compatibilized by Different Organic Peroxides. Materials 2021, 14, 4205. [Google Scholar] [CrossRef]
- Lam, C.; Teoh, S.; Hutmacher, D. Comparison of the degradation of polycaprolactone and polycaprolactone–(β-tricalcium phosphate) scaffolds in alkaline medium. Polym. Int. 2007, 56, 718–728. [Google Scholar] [CrossRef]
- Salerno, A.; Domingo, C. Pore structure properties of scaffolds constituted by aggregated microparticles of PCL and PCL-HA processed by phase separation. J. Porous Mater. 2015, 22, 425–435. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Song, P.; Pei, X.; Sun, H.; Wu, L.; Zhou, C.; Wang, K.; Fan, Y.; Zhang, X. 3D printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations. Mater. Des. 2021, 201, 109490. [Google Scholar] [CrossRef]
- Luo, K.; Wang, L.; Wang, Y.; Zhou, S.; Zhang, P.; Li, J. Porous 3D hydroxyapatite/polyurethane composite scaffold for bone tissue engineering and its in vitro degradation behavior. Ferroelectrics 2020, 566, 104–115. [Google Scholar] [CrossRef]
- Fanovich, M.A.; Ivanovic, J.; Zizovic, I.; Misic, D.; Jaeger, P. Functionalization of polycaprolactone/hydroxyapatite scaffolds with Usnea lethariiformis extract by using supercritical CO2. Mater. Sci. Eng. C 2016, 58, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Dziadek, M.; Pawlik, J.; Menaszek, E.; Stodolak-Zych, E.; Cholewa-Kowalska, K. Effect of the preparation methods on architecture, crystallinity, hydrolytic degradation, bioactivity, and biocompatibility of PCL/bioglass composite scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 103, 1580–1593. [Google Scholar] [CrossRef]
- Wang, Z.; Lim, J.; Ho, Y.; Zhang, Q.; Chong, M.; Tang, M.; Hong, M.; Chan, J.; Teoh, S.; Thian, E. Biomimetic three-dimensional anisotropic geometries by uniaxial stretching of poly(ε-caprolactone) films: Degradation and mesenchymal stem cell responses. J. Biomed. Mater. Res. Part A 2013, 102, 2197–2207. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, Y.; Zhang, Y.; Ye, Z.; Tan, W.; Lang, M. Effect of porosity on long-term degradation of poly(ε-caprolactone) scaffolds and their cellular response. Polym. Degrad. Stab. 2013, 98, 209–218. [Google Scholar] [CrossRef]
- Kirillova, A.; Yeazel, T.; Asheghali, D.; Petersen, S.; Dort, S.; Gall, K.; Becker, M. Fabrication of Biomedical Scaffolds Using Biodegradable Polymers. Chem. Rev. 2021, 121, 11238–11304. [Google Scholar] [CrossRef] [PubMed]
- Hajebi, S.; Mohammadi Nasr, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Tahriri, M.; Tayebi, L.; Hamblin, M. Bioresorbable composite polymeric materials for tissue engineering applications. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 926–940. [Google Scholar] [CrossRef]
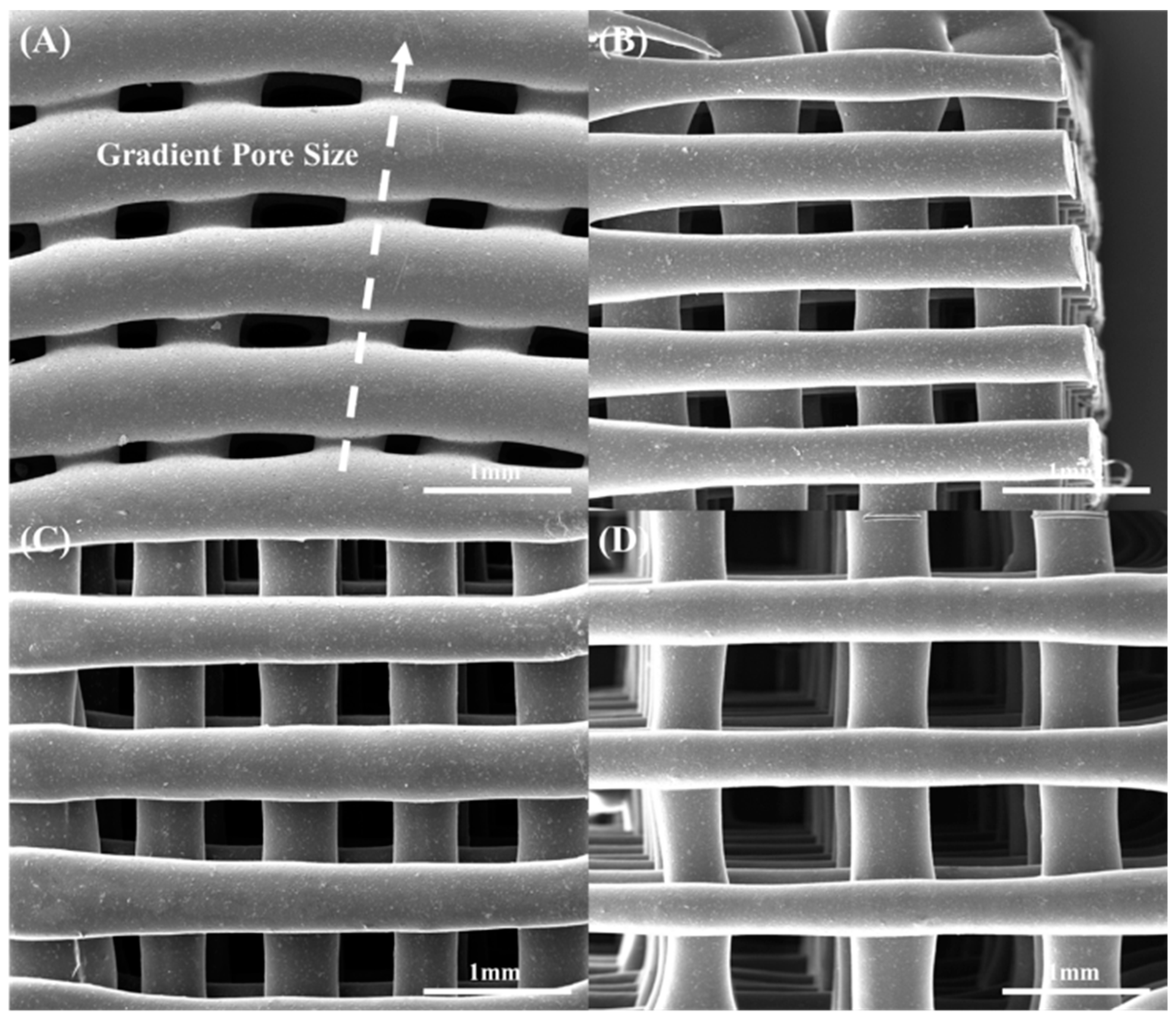

| Materials | Pore Size (μm) | Porosity (%) |
|---|---|---|
| Day 0 | ||
| PCL | 333 ± 90 | 52 |
| HA | 336 ± 10 | 54 |
| TCP | 377 ± 64 | 55 |
| Bioglass | 389 ± 47 | 56 |
| Day 1 | ||
| PCL | 400 ± 56 | 58 |
| HA | 398 ± 31 | 58 |
| TCP | 412 ± 94 | 60 |
| Bioglass | 430 ± 29 | 62 |
| Day 2 | ||
| PCL | 402 ± 63 | 58 |
| HA | 400 ± 31 | 58 |
| TCP | 420 ± 61 | 61 |
| Bioglass | 599 ± 81 | 70 |
| Day 3 | ||
| PCL | 428 ± 77 | 62 |
| HA | 657 ± 52 | 75 |
| TCP | 443 ± 26 | 64 |
| Bioglass | 678 ± 41 | 77 |
| Day 4 | ||
| PCL | 452 ± 42 | 65 |
| HA | 851 ± 69 | 84 |
| TCP | 463 ± 52 | 66 |
| Bioglass | 876 ± 42 | 86 |
| Day 5 | ||
| PCL | 479 ± 36 | 67 |
| HA | 505 ± 8 | 90 |
| TCP | 510 ± 47 | 69 |
| Bioglass | 616 ± 6 | 92 |
| Materials | Pore Size (μm) | Porosity (%) |
|---|---|---|
| Day 0 | ||
| PCL | 205 ± 1 | 42 |
| HA | 207 ± 4 | 42 |
| TCP | 214 ± 3 | 43 |
| Bioglass | 217 ± 8 | 43 |
| Day 1 | ||
| PCL | 214 ± 5 | 43 |
| HA | 252 ± 3 | 45 |
| TCP | 220 ± 4 | 43 |
| Bioglass | 233 ± 4 | 44 |
| Day 2 | ||
| PCL | 246 ± 2 | 45 |
| HA | 443 ± 53 | 64 |
| TCP | 249 ± 1 | 45 |
| Bioglass | 333 ± 15 | 52 |
| Day 3 | ||
| PCL | 249 ± 2 | 45 |
| HA | 505 ± 18 | 68 |
| TCP | 263 ± 4 | 46 |
| Bioglass | 0 | 100 |
| Day 4 | ||
| PCL | 257 ± 2 | 45 |
| HA | 0 | 100 |
| TCP | 281 ± 6 | 47 |
| Bioglass | 0 | 100 |
| Day 5 | ||
| PCL | 257 ± 2 | 45 |
| HA | 0 | 100 |
| TCP | 281 ± 6 | 47 |
| Bioglass | 0 | 100 |
| Materials | Pore Size (μm) | Porosity (%) |
|---|---|---|
| Day 0 | ||
| PCL | 306 ± 3 | 50 |
| HA | 310 ± 5 | 50 |
| TCP | 312 ± 3 | 50 |
| Bioglass | 315 ± 7 | 51 |
| Day 1 | ||
| PCL | 314 ± 3 | 50 |
| HA | 394 ± 1 | 57 |
| TCP | 312 ± 3 | 50 |
| Bioglass | 315 ± 7 | 50 |
| Day 2 | ||
| PCL | 331 ± 2 | 51 |
| HA | 420 ± 3 | 61 |
| TCP | 345 ± 1 | 53 |
| Bioglass | 462 ± 4 | 66 |
| Day 3 | ||
| PCL | 340 ± 3 | 52 |
| HA | 630 ± 7 | 73 |
| TCP | 354 ± 1 | 53 |
| Bioglass | 0 | 100 |
| Day 4 | ||
| PCL | 345 ± 2 | 53 |
| HA | 0 | 100 |
| TCP | 363 ± 3 | 54 |
| Bioglass | 0 | 100 |
| Day 5 | ||
| PCL | 360 ± 2 | 54 |
| HA | 0 | 100 |
| TCP | 364 ± 1 | 54 |
| Bioglass | 0 | 100 |
| Materials | Pore Size (μm) | Porosity (%) |
|---|---|---|
| Day 0 | ||
| PCL | 502 ± 3 | 68 |
| HA | 506 ± 4 | 68 |
| TCP | 510 ± 2 | 68 |
| Bioglass | 508 ± 4 | 68 |
| Day 1 | ||
| PCL | 508 ± 5 | 68 |
| HA | 608 ± 1 | 70 |
| TCP | 515 ± 6 | 68 |
| Bioglass | 544 ± 3 | 69 |
| Day 2 | ||
| PCL | 538 ± 6 | 68 |
| HA | 616 ± 5 | 70 |
| TCP | 552 ± 4 | 69 |
| Bioglass | 617 ± 4 | 70 |
| Day 3 | ||
| PCL | 550 ± 5 | 69 |
| HA | 837 ± 4 | 84 |
| TCP | 561 ± 5 | 69 |
| Bioglass | 0 | 100 |
| Day 4 | ||
| PCL | 556 ± 6 | 69 |
| HA | 0 | 100 |
| TCP | 570 ± 7 | 70 |
| Bioglass | 0 | 100 |
| Day 5 | ||
| PCL | 568 ± 8 | 69 |
| HA | 0 | 100 |
| TCP | 583 ± 1 | 70 |
| Bioglass | 0 | 100 |
| Bone Bricks | Designed Concentration of Inorganic Material (wt%) | Measured Concentration of Inorganic Material (wt%) | Inorganic Material that Remained (wt%) | Degradation Temperature (°C) |
|---|---|---|---|---|
| Day 0 | ||||
| PCL | 0 | 0 | 0 | 437.11 ± 0.21 |
| HA | 20 | 20.27 ± 0.001 | 100 | 425.55 ± 0.38 |
| TCP | 20 | 20.38 ± 0.05 | 100 | 424.04 ± 0.23 |
| Bioglass | 20 | 20.68 ± 0.05 | 100 | 412.67 ± 0.18 |
| Day 1 | ||||
| PCL | - | 0 | 0 | 436.95 ± 1.07 |
| HA | - | 21.64 ± 0.54 | 19.26 | 392.71 ± 1.42 |
| TCP | - | 19.45 ± 0.43 | 20.14 | 414.27 ± 0.18 |
| Bioglass | - | 20.73 ± 0.04 | 19.45 | 343.03 ± 0.12 |
| Day 2 | ||||
| PCL | - | 0 | 0 | 435.79 ± 4.43 |
| HA | - | 20.77 ± 0.71 | 16.08 | 389.37 ± 2.51 |
| TCP | - | 19.41 ± 0.41 | 19.59 | 413.97 ± 0.86 |
| Bioglass | - | 21.71 ± 0.32 | 11.57 | 325.56 ± 2.26 |
| Day 3 | ||||
| PCL | - | 0 | 0 | 431.83 ± 2.67 |
| HA | - | 22.44 ± 1.4 | 9.44 | 381.22 ± 2.63 |
| TCP | - | 18.92 ± 0.44 | 18.92 | 411.85 ± 0.12 |
| Bioglass | - | 22.14 ± 0.76 | 7.14 | 317.15 ± 2.16 |
| Day 4 | ||||
| PCL | - | 0 | 0 | 431.83 ± 2.67 |
| HA | - | 21.11 ± 0.55 | 5.27 | 372.81 ± 1.64 |
| TCP | - | 18.69 ± 0.14 | 18.55 | 410.1 ± 1.21 |
| Bioglass | - | 18.12 ± 0.01 | 4.75 | 312.83 ± 3.41 |
| Day 5 | ||||
| PCL | - | 0 | 0 | 427.87 ± 0.13 |
| HA | - | 21.34 ± 0.04 | 3.77 | 367.33 ± 9.5 |
| TCP | - | 18.17 ± 0.22 | 18.17 | 409.93 ± 0.01 |
| Bioglass | - | 19.14 ± 0.25 | 2.01 | 304.12 ± 5.78 |
| Material | Day | Tg (°C) | Tm (°C) | ΔHm (J/g) | χc (%) |
|---|---|---|---|---|---|
| PCL | Day 0 | −59.19 | 64.65 | 89.89 | 86.91 |
| Day 1 | −58.09 | 61.48 | 83.92 | 81.13 | |
| Day 2 | −58.03 | 60.83 | 81.23 | 78.54 | |
| Day 3 | −57.64 | 60.29 | 80.69 | 78.02 | |
| Day 4 | −55.81 | 55.06 | 79.2 | 76.58 | |
| Day 5 | −54.46 | 54.85 | 74.67 | 72.2 | |
| HA | Day 0 | −59.22 | 60.98 | 65.06 | 61.08 |
| Day 1 | −57.76 | 59.19 | 64.98 | 61.86 | |
| Day 2 | −56.74 | 58.85 | 64.98 | 51.98 | |
| Day 3 | −57 | 58 | 64.21 | 55.12 | |
| Day 4 | −56.45 | 57.63 | 63.92 | 15.94 | |
| Day 5 | 0 | 0 | 0 | 0 | |
| TCP | Day 0 | −59.11 | 59.82 | 66.28 | 64.08 |
| Day 1 | −58.58 | 59.73 | 66.16 | 63.96 | |
| Day 2 | −56.64 | 58.03 | 65.64 | 62.97 | |
| Day 3 | −58.57 | 57.8 | 64.58 | 60.82 | |
| Day 4 | −58.47 | 57.53 | 65.32 | 60.53 | |
| Day 5 | −58.41 | 57.42 | 65.22 | 59.37 | |
| Bioglass | Day 0 | −59.1 | 59.94 | 68.48 | 66.21 |
| Day 1 | −58.36 | 59.27 | 67.97 | 65.72 | |
| Day 2 | −57.82 | 57.33 | 64.34 | 48.4 | |
| Day 3 | −57.78 | 57.2 | 64.03 | 34.51 | |
| Day 4 | −57.69 | 56.01 | 63.91 | 25.76 | |
| Day 5 | −59.1 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daskalakis, E.; Hassan, M.H.; Omar, A.M.; Acar, A.A.; Fallah, A.; Cooper, G.; Weightman, A.; Blunn, G.; Koc, B.; Bartolo, P. Accelerated Degradation of Poly-ε-caprolactone Composite Scaffolds for Large Bone Defects. Polymers 2023, 15, 670. https://doi.org/10.3390/polym15030670
Daskalakis E, Hassan MH, Omar AM, Acar AA, Fallah A, Cooper G, Weightman A, Blunn G, Koc B, Bartolo P. Accelerated Degradation of Poly-ε-caprolactone Composite Scaffolds for Large Bone Defects. Polymers. 2023; 15(3):670. https://doi.org/10.3390/polym15030670
Chicago/Turabian StyleDaskalakis, Evangelos, Mohamed H. Hassan, Abdalla M. Omar, Anil A. Acar, Ali Fallah, Glen Cooper, Andrew Weightman, Gordon Blunn, Bahattin Koc, and Paulo Bartolo. 2023. "Accelerated Degradation of Poly-ε-caprolactone Composite Scaffolds for Large Bone Defects" Polymers 15, no. 3: 670. https://doi.org/10.3390/polym15030670
APA StyleDaskalakis, E., Hassan, M. H., Omar, A. M., Acar, A. A., Fallah, A., Cooper, G., Weightman, A., Blunn, G., Koc, B., & Bartolo, P. (2023). Accelerated Degradation of Poly-ε-caprolactone Composite Scaffolds for Large Bone Defects. Polymers, 15(3), 670. https://doi.org/10.3390/polym15030670













