Mixed Matrix Membranes Using Porous Organic Polymers (POPs)—Influence of Textural Properties on CO2/CH4 Separation
Abstract
:1. Introduction
| Porous Organic Filler | Polymer Matrix | Filler Loading (wt.%) | P(CO2) (Barrer) | CO2/CH4 Selectivity | Other Characterization |
|---|---|---|---|---|---|
| Pillar [5] arene, SOF [24] | Matrimid 5218™ | 0 | 73 ± 2 | 27 ± 5 | Single gas permeation, 20 °C, 1 atm PXRD, SEM |
| 10 | 63 ± 4 | 31 ± 7 | |||
| 50 | 75 ± 4 | 25 ± 4 | |||
| POP2 [29] | Matrimid 5218 | 20 | 26.9 ± 1.0 | 35.86 | Pure gas permeability in the absence and presence of H2S in CH4 and/or N2 |
| POCs [21] | Matrimid 9725 PEEK-WC | 0 | 10.8 | 31.1 | Single gas permeation 1H NMR, SEM, PXRD, SXRD, ATR-FTIR, TGA, gas sorption at 273 K and 25 °C, BET |
| 20 | 16.7 | 41.7 | |||
| 0 | 6.04 | 23.9 | |||
| 20 | 6.15 | 25.7 | |||
| SNW-1 [36] | Polysulfone (PSf) | 0 12 | 8.00 22.4 | 17.5 34 | Mixed gas CO2:CH4 (1:1) Permeation, 298 K 13C CP/MAS NMR, 15N CP/MS NMR, TGA, mechanical properties, SEM, FTIR, BET |
| HOF-21 [25] | Pebax MH 1657 | 0 | 240 | 8 | FTIR, 13C NMR, SEM-EDX, TGA, PXRD, DFT |
| 3 | 780 | 40 |
2. Materials and Methods
2.1. Materials
2.2. Synthesis Procedures
Synthesis of Polymer Membranes
2.3. Characterization
2.4. Gas Transport and Separation
3. Results
3.1. Characterization of Materials and Membranes
3.1.1. Physico-Chemical and Morphological Characterization
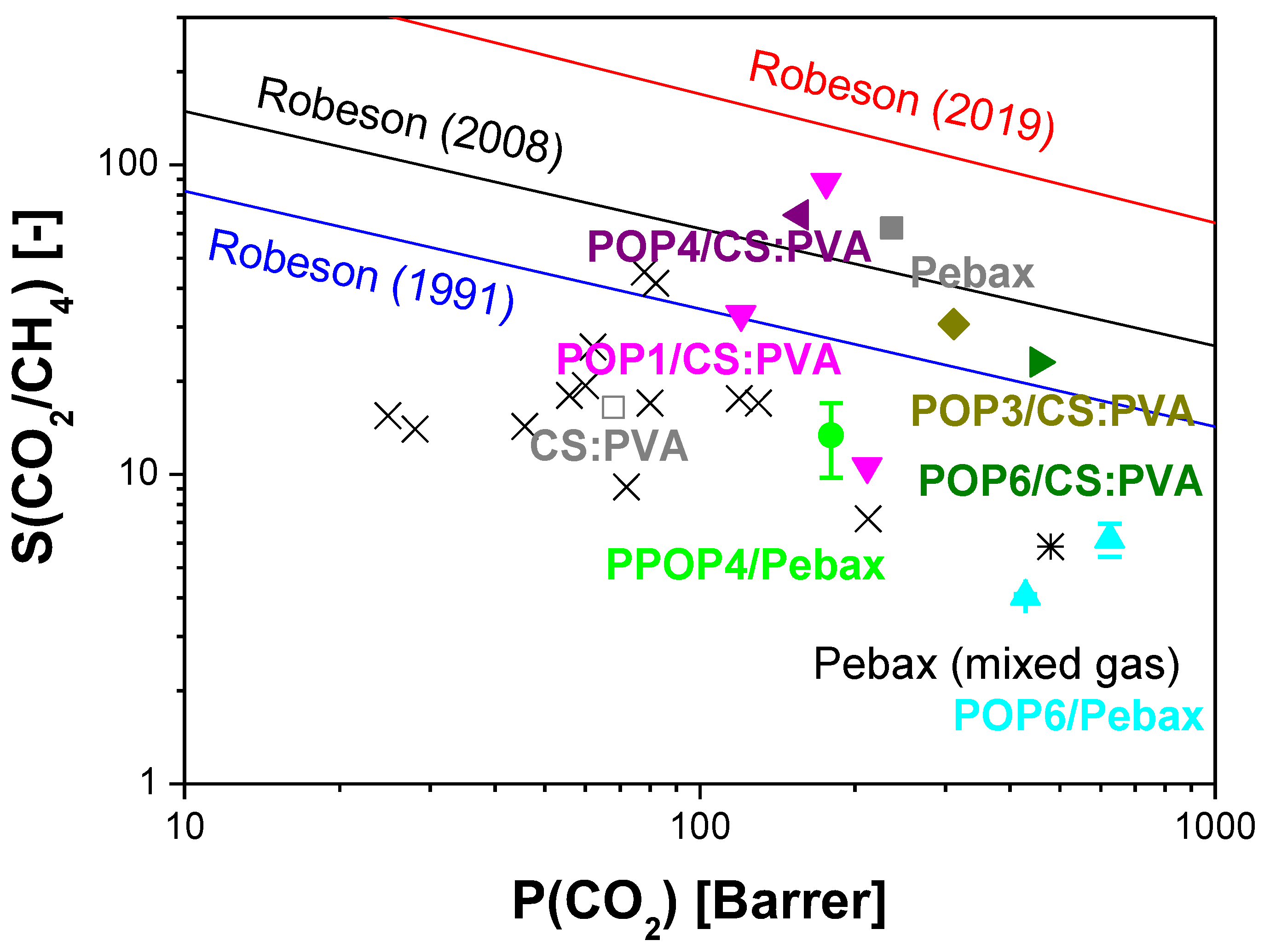
3.1.2. Gas Separation and Separation Characterization
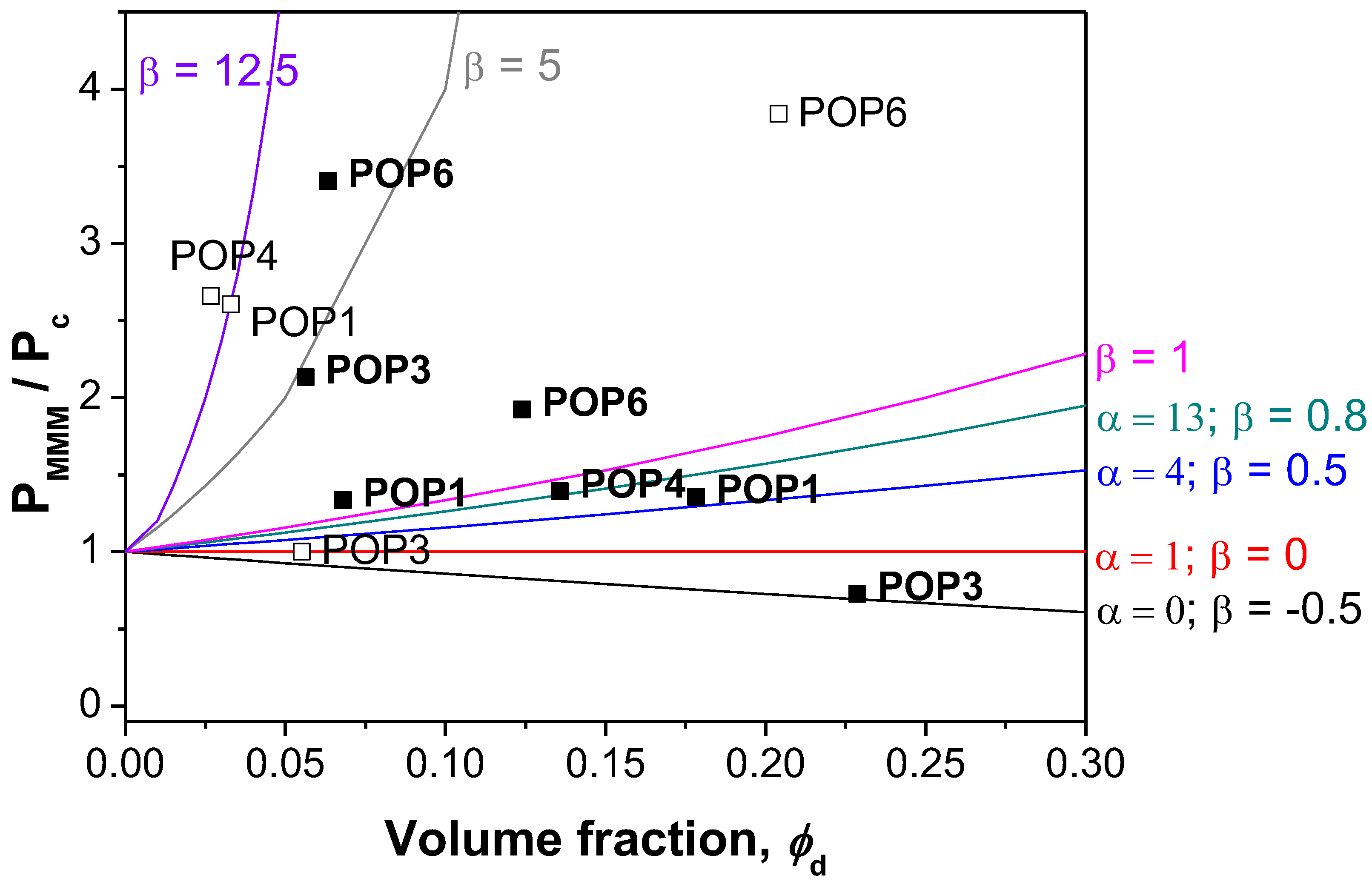
3.2. Mechanism of Transport through MMMs
- Case 1 corresponds to an ideal behavior or perfect contact between the polymer matrix and the filler.
- Cases II and III are characterized by voids at the interface, causing an increase in permeability without large changes in selectivity, in comparison with pure polymer membranes. In Case II, the effective void thickness is of the order of magnitude of the gas penetrant molecules. Most of the Pebax-based MMMs belong to this range.
- Cases IV and V, where a rigidified polymer region is estimated around the filler causing reductions in permeability and a slight increase in the selectivity of the MMMs in comparison with the pure polymer membrane. Unsurprisingly, the Matrimid MMMs fall into these categories, and are attributed to the rigidified polyimide structure of Matrimid.
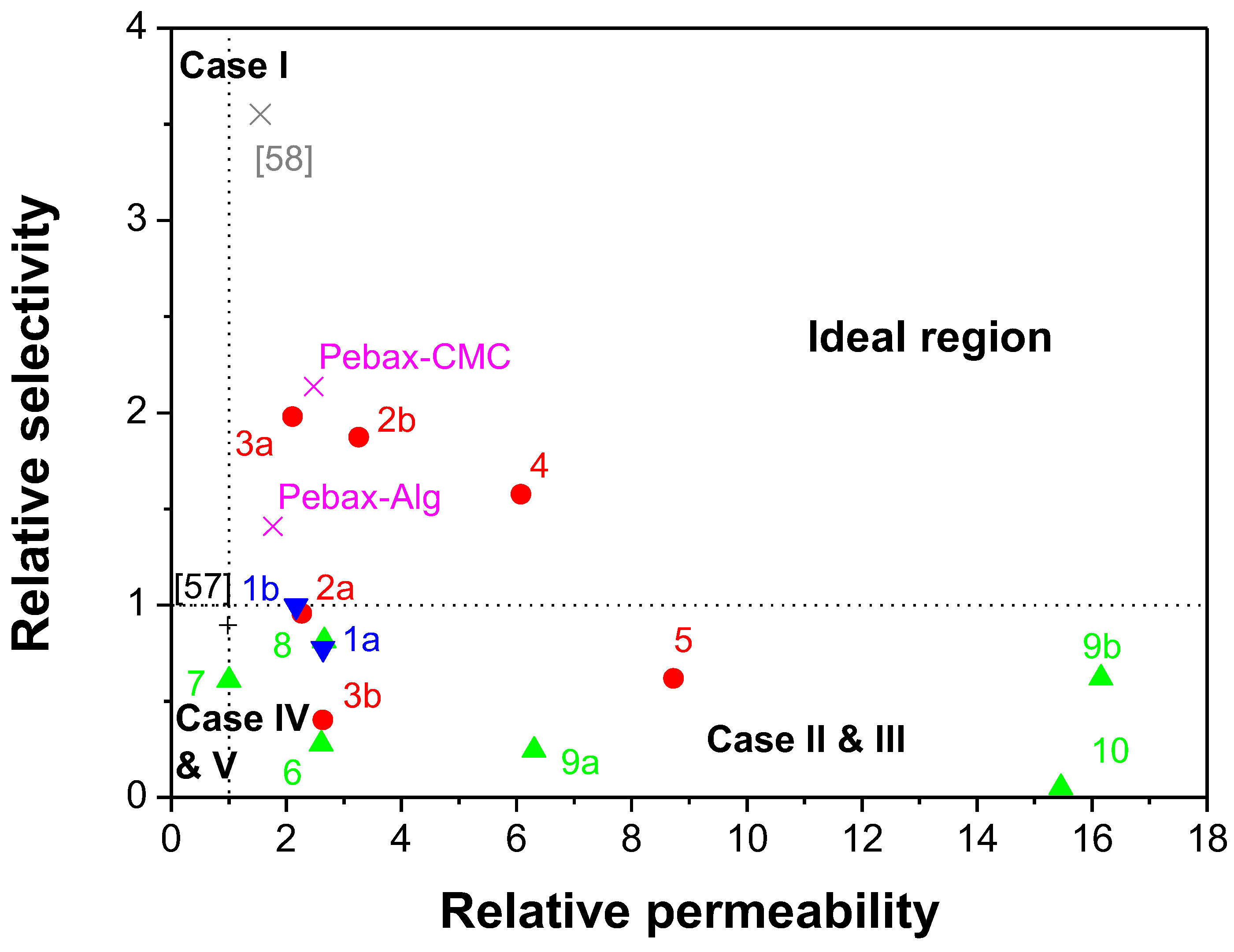
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brémond, U.; Bertrandias, A.; Steyer, J.P.; Bernet, N.; Carrere, H. A Vision of European Biogas Sector Development towards 2030: Trends and Challenges. J. Clean. Prod. 2021, 287, 125065. [Google Scholar] [CrossRef]
- Tao, J.; Wang, J.; Zhu, L.; Chen, X. Integrated Design of Multi-Stage Membrane Separation for Landfill Gas with Uncertain Feed. J. Memb. Sci. 2019, 590, 117260. [Google Scholar] [CrossRef]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas Upgrading and Utilization: Current Status and Perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef]
- Torre-Celeizabal, A.; Casado-Coterillo, C.; Abejón, R.; Garea, A. Simultaneous Production of High-Quality CO2 and CH4 via Multistage Process Using Chitosan-Based Membranes. Sep. Purif. Technol. 2023, 320, 124050. [Google Scholar] [CrossRef]
- Evonik Industries. SEPURAN® Green Membrane Technology for Upgrading Biogas Efficiently; Evonik Industries: Essen, Germany, 2020. [Google Scholar]
- Schuldt, K.; Pohlmann, J.; Shishatskiy, S.; Brinkmann, T. Applicability of PolyactiveTM Thin Film Composite Membranes for CO2 Separation from C2H4 Containing Multi-Component Gas Mixtures at Pressures up to 30 Bar. Membranes 2018, 8, 27. [Google Scholar] [CrossRef]
- Lin, H.; He, Z.; Sun, Z.; Vu, J.; Ng, A.; Mohammed, M.; Kniep, J.; Merkel, T.C.; Wu, T.; Lambrecht, R.C. CO2-Selective Membranes for Hydrogen Production and CO2 Capture—Part I: Membrane Development. J. Memb. Sci. 2014, 457, 149–161. [Google Scholar] [CrossRef]
- Jeon, Y.W.; Lee, D.H. Gas Membranes for CO2/CH4 (Biogas) Separation: A Review. Environ. Eng. Sci. 2015, 32, 71–85. [Google Scholar] [CrossRef]
- Lin, H.; Yavari, M. Upper Bound of Polymeric Membranes for Mixed-Gas CO2/CH4 Separations. J. Memb. Sci. 2015, 475, 101–109. [Google Scholar] [CrossRef]
- Comesaña-Gándara, B.; Chen, J.; Bezzu, C.G.; Carta, M.; Rose, I.; Ferrari, M.C.; Esposito, E.; Fuoco, A.; Jansen, J.C.; McKeown, N.B. Redefining the Robeson Upper Bounds for CO2/CH4 and CO2/N2 Separations Using a Series of Ultrapermeable Benzotriptycene-Based Polymers of Intrinsic Microporosity. Energy Environ. Sci. 2019, 12, 2733–2740. [Google Scholar] [CrossRef]
- Powell, C.E.; Qiao, G.G. Polymeric CO2/N2 Gas Separation Membranes for the Capture of Carbon Dioxide from Power Plant Flue Gases. J. Memb. Sci. 2006, 279, 1–49. [Google Scholar] [CrossRef]
- Buonomenna, M.G.; Yave, W.; Golemme, G. Some Approaches for High Performance Polymer Based Membranes for Gas Separation: Block Copolymers, Carbon Molecular Sieves and Mixed Matrix Membranes. RSC Adv. 2012, 2, 10745–10773. [Google Scholar] [CrossRef]
- Russo, F.; Galiano, F.; Iulianelli, A.; Basile, A.; Figoli, A. Biopolymers for Sustainable Membranes in CO2 Separation: A Review. Fuel Process. Technol. 2021, 213, 106643. [Google Scholar] [CrossRef]
- Vatanpour, V.; Yavuzturk Gul, B.; Zeytuncu, B.; Korkut, S.; İlyasoğlu, G.; Turken, T.; Badawi, M.; Koyuncu, I.; Saeb, M.R. Polysaccharides in Fabrication of Membranes: A Review. Carbohydr. Polym. 2022, 281, 119041. [Google Scholar] [CrossRef] [PubMed]
- Borgohain, R.; Pattnaik, U.; Prasad, B.; Mandal, B. A Review on Chitosan-Based Membranes for Sustainable CO2 Separation Applications: Mechanism, Issues, and the Way Forward. Carbohydr. Polym. 2021, 267, 118178. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed Matrix Membranes (MMMs) Comprising Organic Polymers with Dispersed Inorganic Fillers for Gas Separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Xiang, Z.; Cao, D. Porous Covalent-Organic Materials: Synthesis, Clean Energy Application and Design. J. Mater. Chem. A Mater. 2013, 1, 2691–2718. [Google Scholar] [CrossRef]
- Lopez-Iglesias, B.; Suárez-García, F.; Aguilar-Lugo, C.; González Ortega, A.; Bartolomé, C.; Martínez-Ilarduya, J.M.; De La Campa, J.G.; Lozano, Á.E.; Álvarez, C. Microporous Polymer Networks for Carbon Capture Applications. ACS Appl. Mater. Interfaces 2018, 10, 26195–26205. [Google Scholar] [CrossRef]
- Venna, S.R.; Carreon, M.A. Metal Organic Framework Membranes for Carbon Dioxide Separation. Chem. Eng. Sci. 2015, 124, 3–19. [Google Scholar] [CrossRef]
- Shan, M.; Seoane, B.; Andres-Garcia, E.; Kapteijn, F.; Gascon, J. Mixed-Matrix Membranes Containing an Azine-Linked Covalent Organic Framework: Influence of the Polymeric Matrix on Post-Combustion CO2-Capture. J. Memb. Sci. 2018, 549, 377–384. [Google Scholar] [CrossRef]
- La Cognata, S.; Mobili, R.; Milanese, C.; Boiocchi, M.; Gaboardi, M.; Armentano, D.; Jansen, J.C.; Monteleone, M.; Antonangelo, A.R.; Carta, M.; et al. CO2 Separation by Imide/Imine Organic Cages. Chem. Eur. J. 2022, 28, e202201631. [Google Scholar] [CrossRef]
- Rivera, M.P.; Lively, R.P. Analysis of Gas Transport in Molecularly-Mixed Composite Membranes. J. Memb. Sci. 2022, 661, 120880. [Google Scholar] [CrossRef]
- Gao, X.; Zou, X.; Ma, H.; Meng, S.; Zhu, G. Highly Selective and Permeable Porous Organic Framework Membrane for CO2 Capture. Adv. Mater. 2014, 26, 3644–3648. [Google Scholar] [CrossRef]
- Singh, Z.V.; Tan, L.L.; Cowan, M.G.; Yang, Y.W.; Zhang, W.; Gin, D.L.; Noble, R.D. Pillar [5]Arene/MatrimidTM Materials for High-Performance Methane Purification Membranes. J. Memb. Sci. 2017, 539, 224–228. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, Y.; Cao, Y.; Liang, X.; He, G.; Ma, H.; Dong, H.; Fang, X.; Pan, F.; Jiang, Z. Engineering HOF-Based Mixed Matrix Membranes for Efficient CO2 Separation. Nanomicro Lett. 2023, 15, 50. [Google Scholar] [CrossRef]
- Vinh-Thang, H.; Kaliaguine, S. Predictive Models for Mixed-Matrix Membrane Performance: A Review. Chem. Rev. 2013, 113, 4980–5028. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Huang, S.; Wu, H.; Li, Y.; Tian, Z.; Jiang, Z. Pebax-PEG-MWCNT Hybrid Membranes with Enhanced CO2 Capture Properties. J. Memb. Sci. 2014, 460, 62–70. [Google Scholar] [CrossRef]
- Rodríguez-Jardón, L.; López-González, M.; Iglesias, M.; Maya, E.M. Effect of Porous Organic Polymers in Gas Separation Properties of Polycarbonate Based Mixed Matrix Membranes. J. Memb. Sci. 2021, 619, 118795. [Google Scholar] [CrossRef]
- Kanehashi, S.; Aguiar, A.; Lu, H.T.; Chen, G.Q.; Kentish, S.E. Effects of Industrial Gas Impurities on the Performance of Mixed Matrix Membranes. J. Memb. Sci. 2018, 549, 686–692. [Google Scholar] [CrossRef]
- Minelli, M.; Doghieri, F.; Papadokostaki, K.G.; Petropoulos, J.H. A Fundamental Study of the Extent of Meaningful Application of Maxwell’s and Wiener’s Equations to the Permeability of Binary Composite Materials. Part I: A Numerical Computation Approach. Chem. Eng. Sci. 2013, 104, 630–637. [Google Scholar] [CrossRef]
- Vu, D.Q.; Koros, W.J.; Miller, S.J. Mixed Matrix Membranes Using Carbon Molecular Sieves: II. Modeling Permeation Behavior. J. Memb. Sci. 2003, 211, 335–348. [Google Scholar] [CrossRef]
- Klepić, M.; Fuoco, A.; Monteleone, M.; Esposito, E.; Friess, K.; Izák, P.; Jansen, J.C. Effect of the CO2-Philic Ionic Liquid [BMIM][Tf2N] on the Single and Mixed Gas Transport in PolyActiveTM Membranes. Sep. Purif. Technol. 2021, 256, 117813. [Google Scholar] [CrossRef]
- Rahman, M.M.; Filiz, V.; Shishatskiy, S.; Abetz, C.; Georgopanos, P.; Khan, M.M.; Neumann, S.; Abetz, V. Influence of Poly(Ethylene Glycol) Segment Length on CO2 Permeation and Stability of Polyactive Membranres and Their Nanocomposites with PEG POSS. ACS Appl. Mater. Interfaces 2015, 7, 12289–12298. [Google Scholar] [CrossRef] [PubMed]
- Galiano, F.; Briceño, K.; Marino, T.; Molino, A.; Christensen, K.V.; Figoli, A. Advances in Biopolymer-Based Membrane Preparation and Applications. J. Memb. Sci. 2018, 564, 562–586. [Google Scholar] [CrossRef]
- Tomietto, P.; Russo, F.; Galiano, F.; Loulergue, P.; Salerno, S.; Paugam, L.; Audic, J.L.; De Bartolo, L.; Figoli, A. Sustainable Fabrication and Pervaporation Application of Bio-Based Membranes: Combining a Polyhydroxyalkanoate (PHA) as Biopolymer and CyreneTM as Green Solvent. J. Memb. Sci. 2022, 643, 120061. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, C.; Zhu, M.; Fu, Y.; Huang, H.; Liu, Y.; Kang, Z. Highly Selective and Efficient Electroreduction of Carbon Dioxide to Carbon Monoxide with Phosphate Silver-Derived Coral-like Silver. ACS Sustain. Chem. Eng. 2019, 7, 3536–3543. [Google Scholar] [CrossRef]
- Rico-Martínez, S.; Álvarez, C.; Hernández, A.; Miguel, J.A.; Lozano, Á.E. Mixed Matrix Membranes Loaded with a Porous Organic Polymer Having Bipyridine Moieties. Membranes 2022, 12, 547. [Google Scholar] [CrossRef] [PubMed]
- Hashemifard, S.A.; Ismail, A.F.; Matsuura, T. A New Theoretical Gas Permeability Model Using Resistance Modeling for Mixed Matrix Membrane Systems. J. Memb. Sci. 2010, 350, 259–268. [Google Scholar] [CrossRef]
- Wang, Y.; Goh, S.H.; Chung, T.S. Miscibility Study of Torlon® Polyamide-Imide with Matrimid® 5218 Polyimide and Polybenzimidazole. Polymer 2007, 48, 2901–2909. [Google Scholar] [CrossRef]
- Boroglu, M.-S.; Ugur, M.; Boz, I. Enhanced Gas Transport Properties of Mixed Matrix Membranes Consisting of Matrimid and RHO Type ZIF-12 Particles. Chem. Eng. Res. Des. 2017, 123, 201–213. [Google Scholar] [CrossRef]
- Sakurai, K.; Maegawa, T.; Takahashi, T. Glass Transition Temperature of Chitosan and Miscibility of Chitosan/Poly(N-Vinyl Pyrrolidone) Blends. Polymer 2000, 41, 7051–7056. [Google Scholar] [CrossRef]
- Chung, T.S.; Chan, S.S.; Wang, R.; Lu, Z.; He, C. Characterization of Permeability and Sorption in Matrimid/C60 Mixed Matrix Membranes. J. Memb. Sci. 2003, 211, 91–99. [Google Scholar] [CrossRef]
- Mi, F.L.; Shyu, S.S.; Wu, Y.B.; Lee, S.T.; Shyong, J.Y.; Huang, R.N. Fabrication and Characterization of a Sponge-like Asymmetric Chitosan Membrane as a Wound Dressing. Biomaterials 2001, 22, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Xue, R.; He, L.; Liu, Y.; Xiao, Q. The Structure and Properties of Chitosan/Polyethylene Glycol/Silica Ternary Hybrid Organic-Inorganic Films. Chin. J. Polym. Sci. 2008, 26, 621–630. [Google Scholar] [CrossRef]
- Chen, G.Q.; Scholes, C.A.; Doherty, C.M.; Hill, A.J.; Qiao, G.G.; Kentish, S.E. The Thickness Dependence of Matrimid Films in Water Vapor Permeation. Chem. Eng. J. 2012, 209, 301–312. [Google Scholar] [CrossRef]
- Pardo, F.; Zarca, G.; Urtiaga, A. Separation of Refrigerant Gas Mixtures Containing R32, R134a, and R1234yf through Poly(Ether-Block-Amide) Membranes. ACS Sustain. Chem. Eng. 2020, 8, 2548–2556. [Google Scholar] [CrossRef]
- Casado-Coterillo, C.; del Mar López-Guerrero, M.; Irabien, Á. Synthesis and Characterisation of ETS-10/Acetate-Based Ionic Liquid/Chitosan Mixed Matrix Membranes for CO2/N2 Permeation. Membranes 2014, 4, 287–301. [Google Scholar] [CrossRef]
- Marcos-Madrazo, A.; Casado-Coterillo, C.; García-Cruz, L.; Iniesta, J.; Simonelli, L.; Sebastián, V.; Encabo-Berzosa, M.d.M.; Arruebo, M.; Irabien, Á. Preparation and Identification of Optimal Synthesis Conditions for a Novel Alkaline Anion-Exchange Membrane. Polymers 2018, 10, 913–922. [Google Scholar] [CrossRef]
- Santos, E.; Rodríguez-Fernández, E.; Casado-Coterillo, C.; Irabien, Á. Hybrid Ionic Liquid-Chitosan Membranes for CO2 Separation: Mechanical and Thermal Behavior. Int. J. Chem. React. Eng. 2016, 14, 713–718. [Google Scholar] [CrossRef]
- Casado-Coterillo, C.; Fernández-Barquín, A.; Zornoza, B.; Téllez, C.; Coronas, J.; Irabien, Á. Synthesis and Characterisation of MOF/Ionic Liquid/Chitosan Mixed Matrix Membranes for CO2/N2 Separation. RSC Adv. 2015, 5, 102350–102361. [Google Scholar] [CrossRef]
- Abarca, J.A.; Díaz-Sainz, G.; Merino-Garcia, I.; Beobide, G.; Albo, J.; Irabien, A. Optimized Manufacturing of Gas Diffusion Electrodes for CO2 Electroreduction with Automatic Spray Pyrolysis. J. Environ. Chem. Eng. 2023, 11, 109724. [Google Scholar] [CrossRef]
- Franck-Lacaze, L.; Sistat, P.; Huguet, P. Determination of the PKa of Poly (4-Vinylpyridine)-Based Weak Anion Exchange Membranes for the Investigation of the Side Proton Leakage. J. Memb. Sci. 2009, 326, 650–658. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. The Solution-Diffusion Model: A Review. J. Memb. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Torre-Celeizabal, A.; Casado-Coterillo, C.; Garea, A. Biopolymer-Based Mixed Matrix Membranes (MMMs) for CO2/CH4 Separation: Experimental and Modeling Evaluation. Membranes 2022, 12, 561–583. [Google Scholar] [CrossRef] [PubMed]
- Kanehashi, S.; Chen, G.Q.; Scholes, C.A.; Ozcelik, B.; Hua, C.; Ciddor, L.; Southon, P.D.; D’Alessandro, D.M.; Kentish, S.E. Enhancing Gas Permeability in Mixed Matrix Membranes through Tuning the Nanoparticle Properties. J. Memb. Sci. 2015, 482, 49–55. [Google Scholar] [CrossRef]
- Hua, C.; Rawal, A.; Faust, T.B.; Southon, P.D.; Babarao, R.; Hook, J.M.; D’Alessandro, D.M. Exploiting Stable Radical States for Multifunctional Properties in Triarylamine-Based Porous Organic Polymers. J. Mater. Chem. A Mater. 2014, 2, 12466–12474. [Google Scholar] [CrossRef]
- El-Azzami, L.A.; Grulke, E.A. Parametric Study of CO2 Fixed Carrier Facilitated Transport through Swollen Chitosan Membranes. Ind. Eng. Chem. Res. 2009, 48, 894–902. [Google Scholar] [CrossRef]
- Dhawade, P.P.; Jagtap, R.N. Characterization of the Glass Transition Temperature of Chitosan and Its Oligomers by Temperature Modulated Differential Scanning Calorimetry. Pelagia Res. Libr. Adv. Appl. Sci. Res. 2012, 3, 1372–1382. [Google Scholar]
- Dai, Y.; Ruan, X.; Yan, Z.; Yang, K.; Yu, M.; Li, H.; Zhao, W.; He, G. Imidazole Functionalized Graphene Oxide/PEBAX Mixed Matrix Membranes for Efficient CO2 Capture. Sep. Purif. Technol. 2016, 166, 171–180. [Google Scholar] [CrossRef]
- Kanehashi, S.; Chen, G.Q.; Danaci, D.; Webley, P.A.; Kentish, S.E. Can the Addition of Carbon Nanoparticles to a Polyimide Membrane Reduce Plasticization? Sep. Purif. Technol. 2017, 183, 333–340. [Google Scholar] [CrossRef]
- Tan, P.C.; Jawad, Z.A.; Ooi, B.S.; Ahmad, A.L.; Low, S.C. Correlation between Polymer Packing and Gas Transport Properties for CO2/N2 Separation in Glassy Fluorinated Polyimde Membrane. J. Eng. Sci. Technol. 2016, 11, 935–946. [Google Scholar]
- Robeson, L.M. Correlation of Separation Factor versus Permeability for Polymeric Membranes. J. Memb. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The Upper Bound Revisited. J. Memb. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Luna, A.D.; de León, G.C.; Rodríguez, S.P.; López, N.C.; Camacho, O.P.; Mercado, Y.A. Na+/Ca2+ Aqueous Ion Exchange in Natural Clinoptilolite Zeolite for Polymer-Zeolite Composite Membranes Production and Their CH4/CO2/N2 Separation Performance. J. Nat. Gas. Sci. Eng. 2018, 54, 47–53. [Google Scholar] [CrossRef]
- Minelli, M.; Sarti, G.C. Permeability and Diffusivity of CO2 in Glassy Polymers with and without Plasticization. J. Memb. Sci. 2013, 435, 176–185. [Google Scholar] [CrossRef]
- Xiao, S.; Feng, X.; Huang, R.Y.M. Trimesoyl Chloride Crosslinked Chitosan Membranes for CO2/N2 Separation and Pervaporation Dehydration of Isopropanol. J. Memb. Sci. 2007, 306, 36–46. [Google Scholar] [CrossRef]
- Ito, A.; Sato, M.; Anma, T. Permeability of CO2 through Chitosan Membrane Swollen by Water Vapor in Feed Gas. Angew. Makromol. Chem. 1997, 248, 85–94. [Google Scholar] [CrossRef]
- Salestan, S.K.; Rahimpour, A.; Abedini, R. Experimental and Theoretical Studies of Biopolymers on the Efficient CO2/CH4 Separation of Thin-Film Pebax®1657 Membrane. Chem. Eng. Process.-Process Intensif. 2021, 163, 108366. [Google Scholar] [CrossRef]
- Casado-Coterillo, C.; Fernández-Barquín, A.; Valencia, S.; Irabien, Á. Estimating CO2/N2 Permselectivity through Si/Al = 5 Small-Pore Zeolites/PTMSP Mixed Matrix Membranes: Influence of Temperature and Topology. Membranes 2018, 8, 32. [Google Scholar] [CrossRef]
- Hao, L.; Li, P.; Yang, T.; Chung, T.S. Room Temperature Ionic Liquid/ZIF-8 Mixed-Matrix Membranes for Natural Gas Sweetening and Post-Combustion CO2 Capture. J. Memb. Sci. 2013, 436, 221–231. [Google Scholar] [CrossRef]
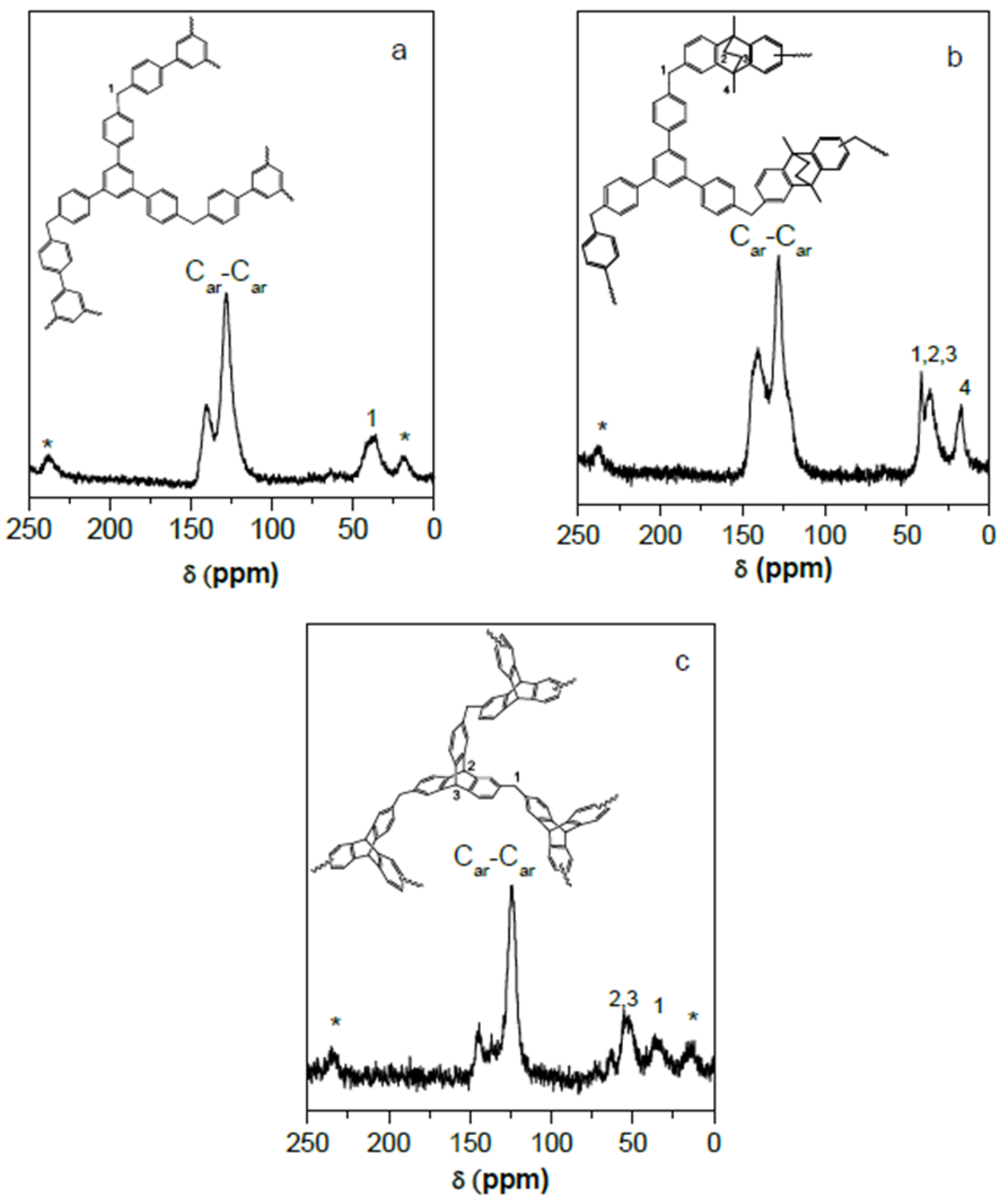

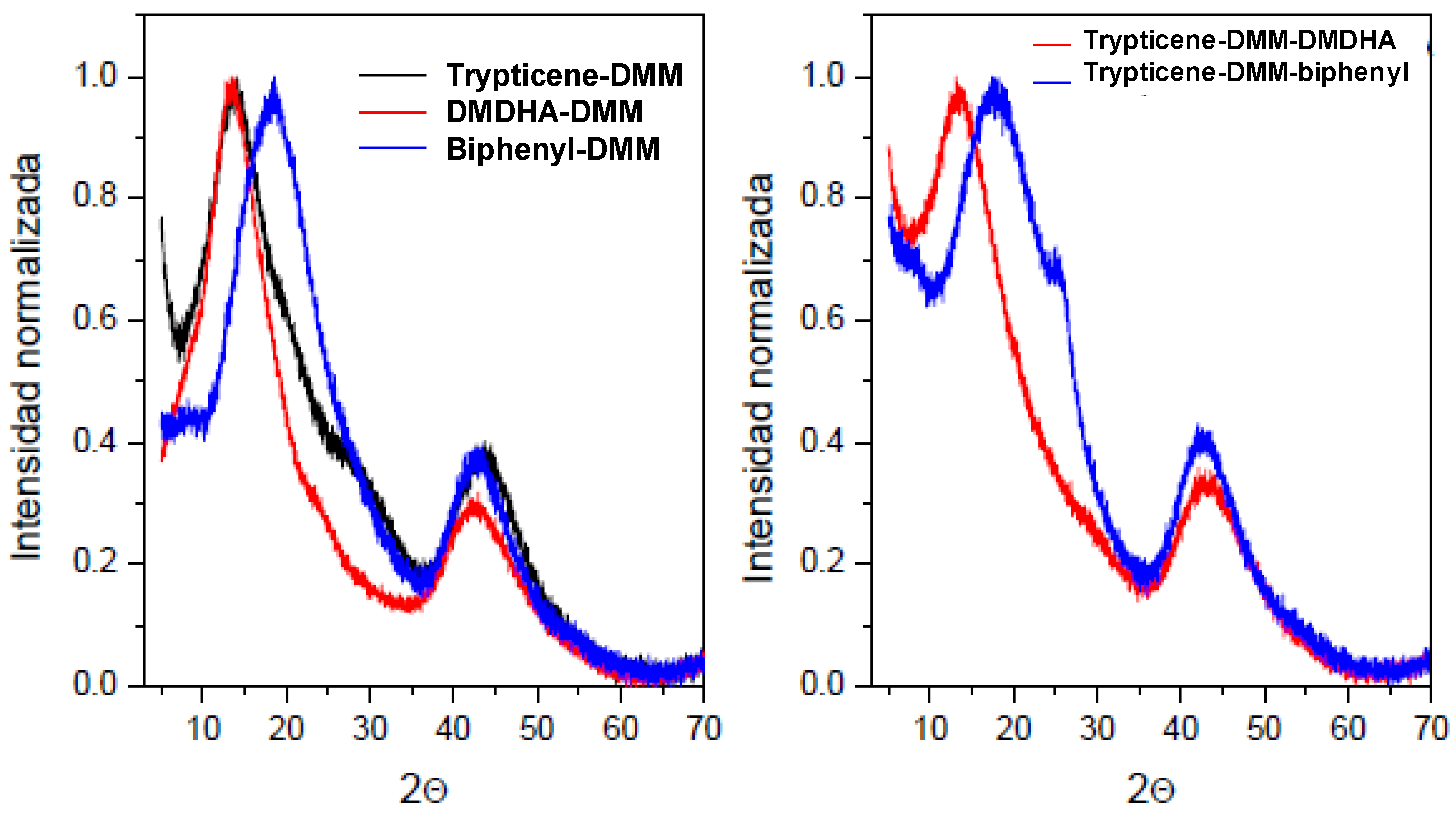
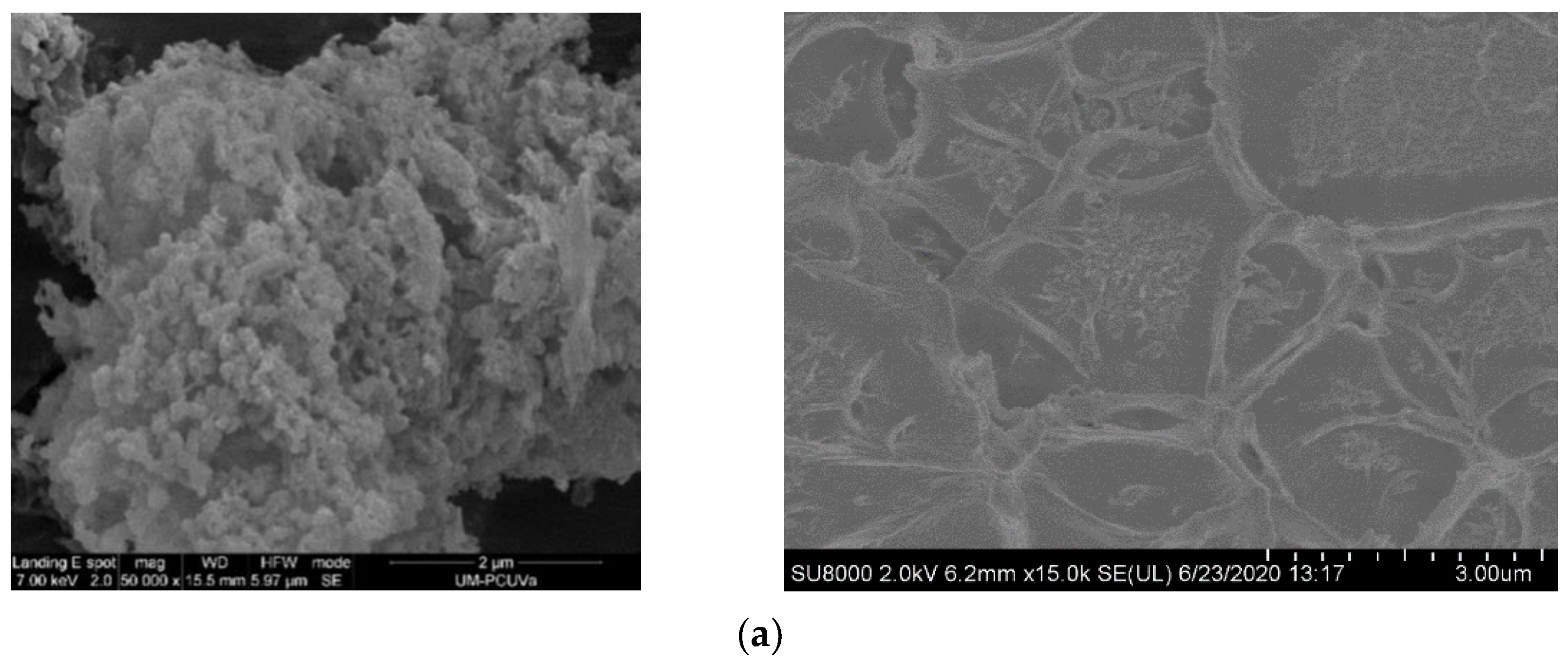
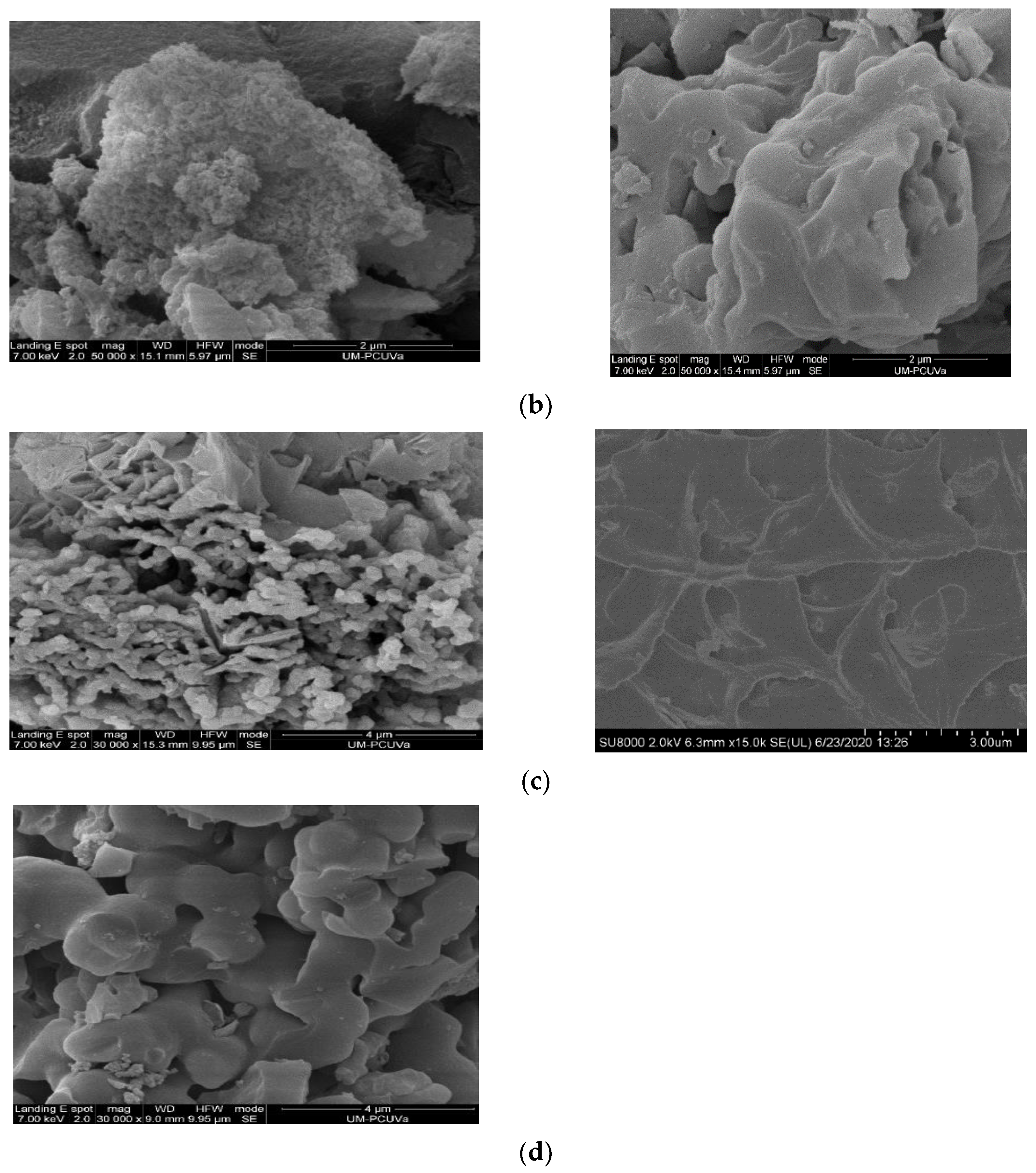
| POP | Triptycene | 135-TPB 1 | Biphenyl | DMDHA 2 | DMM 3 | FeCl3 |
|---|---|---|---|---|---|---|
| POP1 | 1 | - | - | - | 3 | 3 |
| POP3 | 1 | - | - | 0.67 | 4.33 | 4.33 |
| POP4 | 1 | - | 0.67 | - | 4.33 | 4.33 |
| POP6 | - | 1 | - | - | 3 | 3 |
| POP9 | - | 1 | - | 0.67 | 4.33 | 4.33 |
| Property | Matrimid 5218 | Pebax MH 1657 | Chitosan |
|---|---|---|---|
| Chemical structure |  | 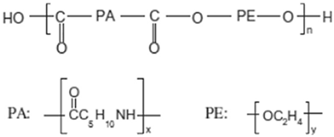 | 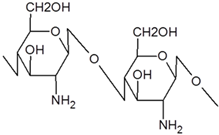 |
| Tg (°C) | 317 [39]; 308 [40] | −53 | 203 [41] |
| Density (g cm−3) | 1.225 [42] | 1.14 | 0.942 [43] |
| Melting point (°C) | >300 [40] | 204 1 | 270 [44] |
| Estimated fractional free volume | 0.21 [45] | 0.143 [46] | 0.228 [47] |
| Type of POP | Skeletal Density (g/cm3) | SBET (m2/g) | VTOTAL (cm3/g) | VMICRO (cm3/g) * | Reference |
|---|---|---|---|---|---|
| POP1 | 1.2624 | 1538 | 1.281 | 0.314 | This work |
| POP3 | 1.2051 | 1596 | 1.394 | 0.293 | This work |
| POP4 | 1.1994 | 1318 | 0.727 | 0.368 | This work |
| POP6 | 1.2014 | 1638 | 0.964 | 0.450 | This work |
| POP9 | 1.2288 | 1525 | 1.606 | 0.325 | This work |
| POP2 | 1.33 | 781 | 0.554 | NA | [55] |
| KAP (2Ph-NO2) | 1.618 | 605 | 0.313 | NA | [28] |
| KAP (2Ph-CH2NH2) | 1.459 | 617 | 0.282 | NA | |
| SNW-1 | NA | 821 | NA | 0.26 | [36] |
| TRPI (135TRP-DAFO) | 1.113 | 806 | 0.42 | 0.24 | [37] |
| Membrane | Filler wt. Fraction | Thickness (cm) | Density (g/cm3) | WU (%) | WC (%) | Td (°C) | Porosity (%) | Volume Fraction, ød |
|---|---|---|---|---|---|---|---|---|
| Matrimid [37] | 0 | 0.005 | 1.223 [60] | NA | NA | NA | 16.7 [60] | 0 |
| POP1/Matrimid | 0.20 | 0.005 | 1.232 | NA | 0.195 | |||
| POP4/Matrimid | 0.20 | 0.005 | NA | 0.203 | ||||
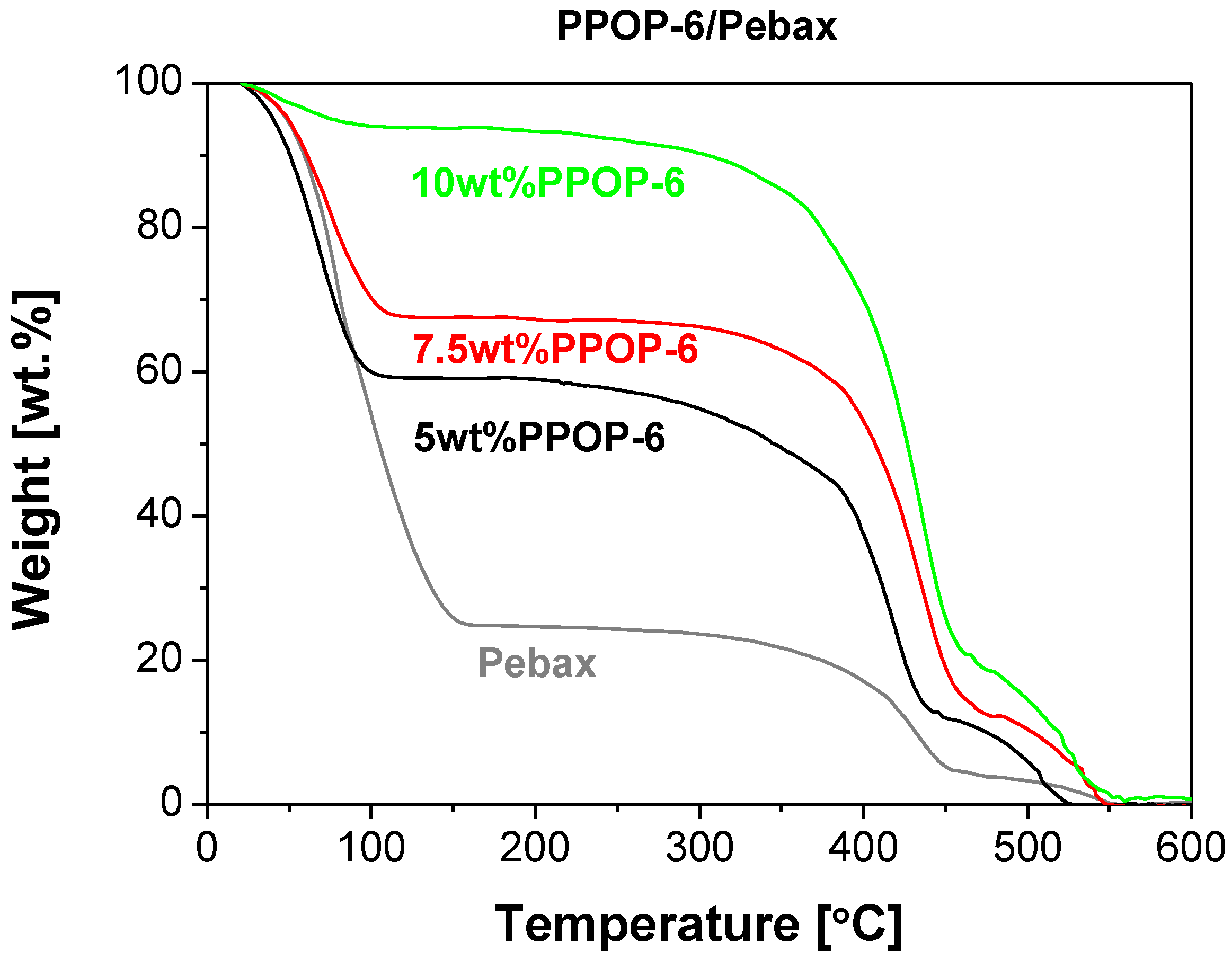
| Pebax | 0 | 0.01102 | - | (*) | 58 | 290 | - | 0 |
| POP1/Pebax | 0.05 | 0.00772 | 1.124 | (*) | 34 | 172 | 27 | 0.033 |
| POP3/Pebax | 0.05 | 0.00785 | 1.225 | (*) | 59 | 226 | 42 | 0.028 |
| POP4/Pebax | 0.05 | 0.00818 | 1.289 | (*) | 60 | 222 | 44 | 0.027 |
| POP6/Pebax | 0.16 | 0.0189 | 1.009 | 50 | 53.7 | 377 | 40 | 0.091 |
| 0.32 | 0.0250 | 1.240 | 64 | 219 | 34 | 0.204 | ||
| POP9/Pebax | 0.10 | 0.0934 | 1.240 | 64 | 50.8 | 219 | 40 | 0.052 |
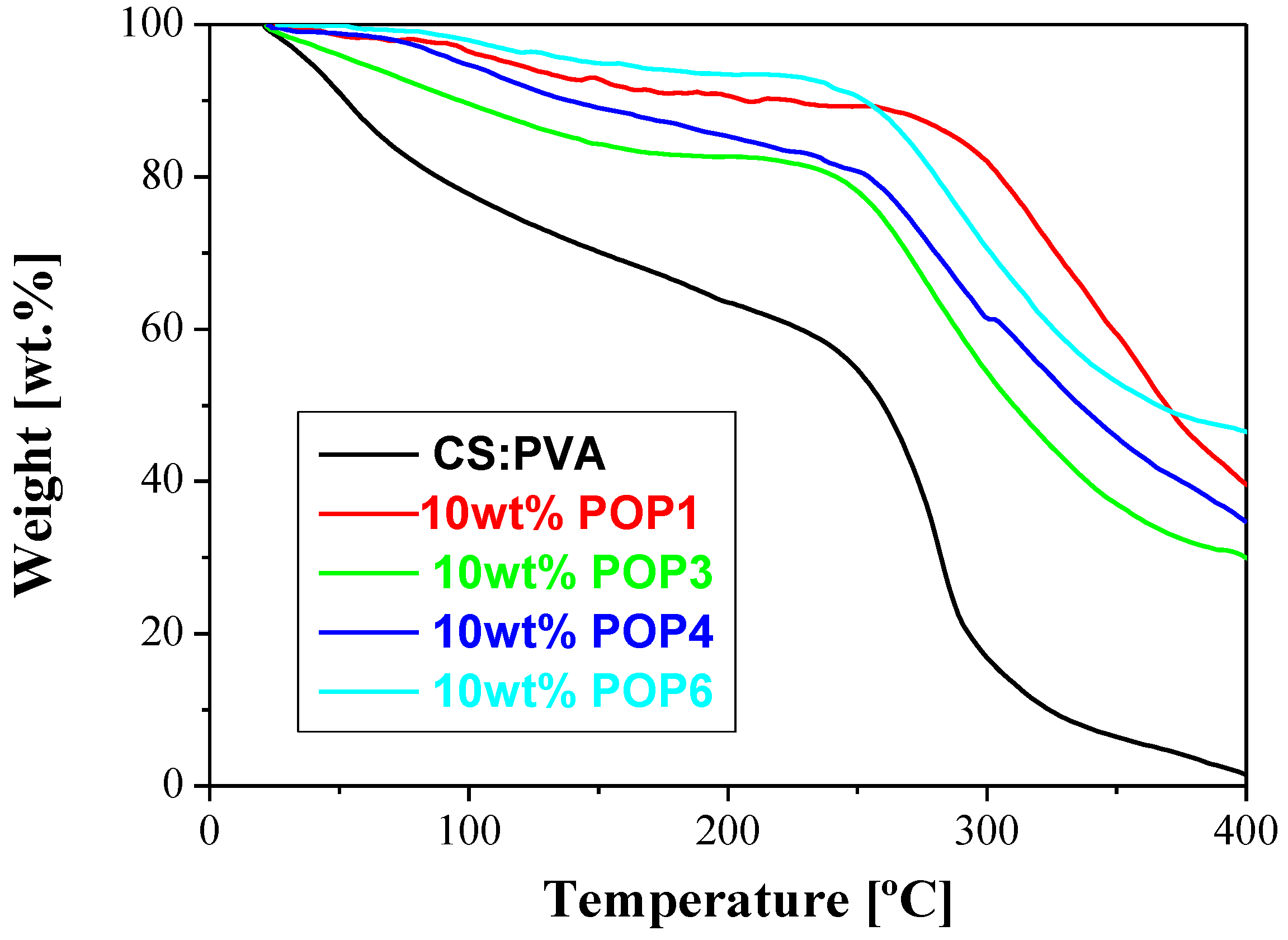
| CS:PVA | 0 | 0.016 | 1.749 | 39.80 ± 1.26 | 131 | 41 | 0 | |
| POP1/CS:PVA | 0.05 | 0.0147 | 1.349 | 47.88 | 33 | 171 | 39 | 0.041 |
| 0.10 | 0.0098 | 1.782 | 32 | 226 | 48 | 0.070 | ||
| POP3/CS:PVA | 0.05 | 0.0097 | 2.147 | 37.2 | 40 | 171 | 44 | 0.039 |
| 0.10 | 0.0136 | 1.305 | 23 | 172 | 18 | 0.111 | ||
| POP4/CS:PVA | 0.10 | 0.01185 | 1.389 | 18 | 23 | 172 | 20 | 0.111 |
| POP6/CS:PVA | 0.10 | 0.0133 | 0.850 | 14.5 | 17 | 242 | 11 | 0.124 |
| Polymer Matrix | POP, Filler Loading | Thickness (cm) | P(CO2) (Barrer) (a) | P(CH4) (Barrer) (a) | α(CO2/CH4) | S.F. (CO2/CH4) |
|---|---|---|---|---|---|---|
| Matrimid (b) | 0 | 0.005 | 7.84 | 0.186 | 42 | - |
| POP1, 20 wt.% | 0.005 | 20.68 | 0.632 | 37 | - | |
| POP4, 20 wt.% | 0.005 | 17.0 | 0.400 | 42 | - | |
| Pebax (c) | 0 | 0.011 | 67.95 ± 13.51 | 4.132 | 16.44 ±0.31 | 11.78 |
| POP1, 5 wt.% | 0.077 | 176.95 ± 5.17 | 38.73 | 4.57 ± 0.32 | 3.98 | |
| POP3, 5 wt.% | 0.0078 | 67.95 ± 13.50 | 6.79 | 10.01 ± 3.25 | - | |
| POP4, 5 wt.% | 0.0082 | 180.68 ± 90.54 | 13.50 | 13.38 ± 3.64 | 12.0 | |
| POP6, 16 wt.% | 0.0189 | 1098 | 107.82 | 10.18 | 7.88 | |
| POP6, 32 wt.% | 0.0255 | 428 ± 15.5 | 106.20 | 4.034 ± 0.02 | 3.21 ± 0.04 | |
| POP9, 8.3 wt.% | 0.00632 | 1050 | 1282 | 0.82 | 0.85 | |
| CS:PVA (c) | 0 | 0.01605 | 51.99 | 1.55 | 33.64 | 31.19 |
| POP1, 5 wt%. | 0.0147 | 66.15 | 2.06 | 32.14 | 31.43 | |
| POP3, 5 wt.% | 0.0097 | 109.80 | 1.65 | 66.59 | 27.50 | |
| POP3, 10 wt.% | 0.0136 | 36.86 | 2.73 | 13.50 | 13.00 | |
| POP4, 10 wt.% | 0.0118 | 62.81 | 1.19 | 53.00 | 62.5 | |
| POP6, 10 wt.% | 0.0133 | 453.80 | 20.75 | 21.80 | 17.30 |
| Continuous Matrix | Dispersed Phase | Parallel | Series | Maxwell |
|---|---|---|---|---|
| Matrimid | POP1 | 13.11 | 15.26 | 11.18 |
| POP4 | 5.12 | 22.26 | 17.12 | |
| Pebax | POP1 | 31.02 | 31.13 | 31.05 |
| POP3 | 0.48 | 0.74 | 4.40 | |
| POP4 | 31.37 | 31.46 | 31.39 | |
| POP6 | 42.6 | 42.8 | 6.42 | |
| POP9 | - | - | - | |
| CS:PVA | POP1 | 10.96 | 10.99 | 10.97 |
| POP3 | 26.47 | 26.50 | 27.38 | |
| POP4 | 51.09 | 9.43 | 43.55 | |
| POP6 | 44.38 | 44.39 | 44.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matesanz-Niño, L.; Moranchel-Pérez, J.; Álvarez, C.; Lozano, Á.E.; Casado-Coterillo, C. Mixed Matrix Membranes Using Porous Organic Polymers (POPs)—Influence of Textural Properties on CO2/CH4 Separation. Polymers 2023, 15, 4135. https://doi.org/10.3390/polym15204135
Matesanz-Niño L, Moranchel-Pérez J, Álvarez C, Lozano ÁE, Casado-Coterillo C. Mixed Matrix Membranes Using Porous Organic Polymers (POPs)—Influence of Textural Properties on CO2/CH4 Separation. Polymers. 2023; 15(20):4135. https://doi.org/10.3390/polym15204135
Chicago/Turabian StyleMatesanz-Niño, Laura, Jorge Moranchel-Pérez, Cristina Álvarez, Ángel E. Lozano, and Clara Casado-Coterillo. 2023. "Mixed Matrix Membranes Using Porous Organic Polymers (POPs)—Influence of Textural Properties on CO2/CH4 Separation" Polymers 15, no. 20: 4135. https://doi.org/10.3390/polym15204135
APA StyleMatesanz-Niño, L., Moranchel-Pérez, J., Álvarez, C., Lozano, Á. E., & Casado-Coterillo, C. (2023). Mixed Matrix Membranes Using Porous Organic Polymers (POPs)—Influence of Textural Properties on CO2/CH4 Separation. Polymers, 15(20), 4135. https://doi.org/10.3390/polym15204135








