Development and Evaluation of a Novel Anti-Ageing Cream Based on Hyaluronic Acid and Other Innovative Cosmetic Actives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cosmetic Ingredients and Actives Selection for the Development of an Anti-Ageing Formulation
2.2. Development and Formulation of the Anti-Ageing Cream—Preparation Procedure
2.3. Evaluation of the PhysicoChemical and Microbiological Characteristics of the Developed Formulation
2.3.1. Stability Testing of the Cosmetic Formulation
2.3.2. Quality Control of the Cosmetic Formulation
2.3.3. Microbiological Control and Assessment of the Effectiveness of the Preservation of the Cosmetic Formulation
2.4. In Silico Approaches for Safety Evaluation of Cosmetic-Related Substances and Risk Assessment of the Cosmetic Formulation
2.5. Safety Evaluation (Skin Tolerance) and Efficacy Assessment of the Cosmetic Formulation
2.5.1. Dermatological Semi-Open Test
2.5.2. Use Test and Instrumental Test under Dermatological Control
In Use test with dermatological control
Instrumental Test for Wrinkle Length and Depth
In Vivo Determination of the Sun Protection Factor (SPF)
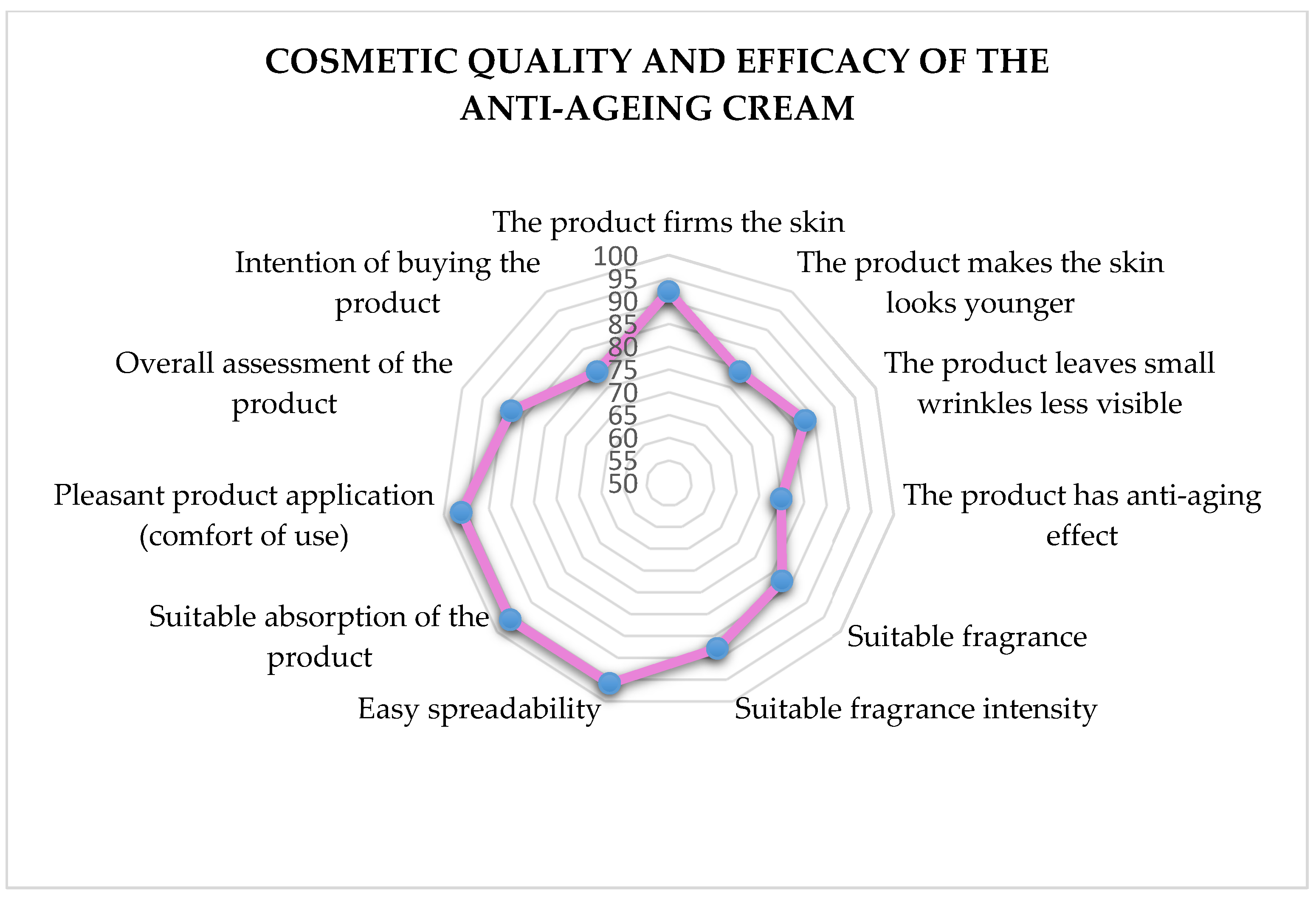
Assessment of the Effect Claimed for the Cosmetic Product
3. Results
3.1. Development and Formulation of the Anti-Ageing Cream
3.2. Quality Control of the Anti-Ageing Cream—Stability Testing, Physicochemical and Microbiological Assessment
3.3. In Silico Approaches for the Safety Evaluation of Cosmetic-Related Substances and Risk Assessment of the Cosmetic Formulation
3.4. Safety Evaluation (Skin Tolerance) and Efficacy Assessment of the Cosmetic Formulation
3.4.1. Dermatological Semi-Open Test
3.4.2. Use Test and Instrumental Test under Dermatological Control
In Use Test with Dermatological Control
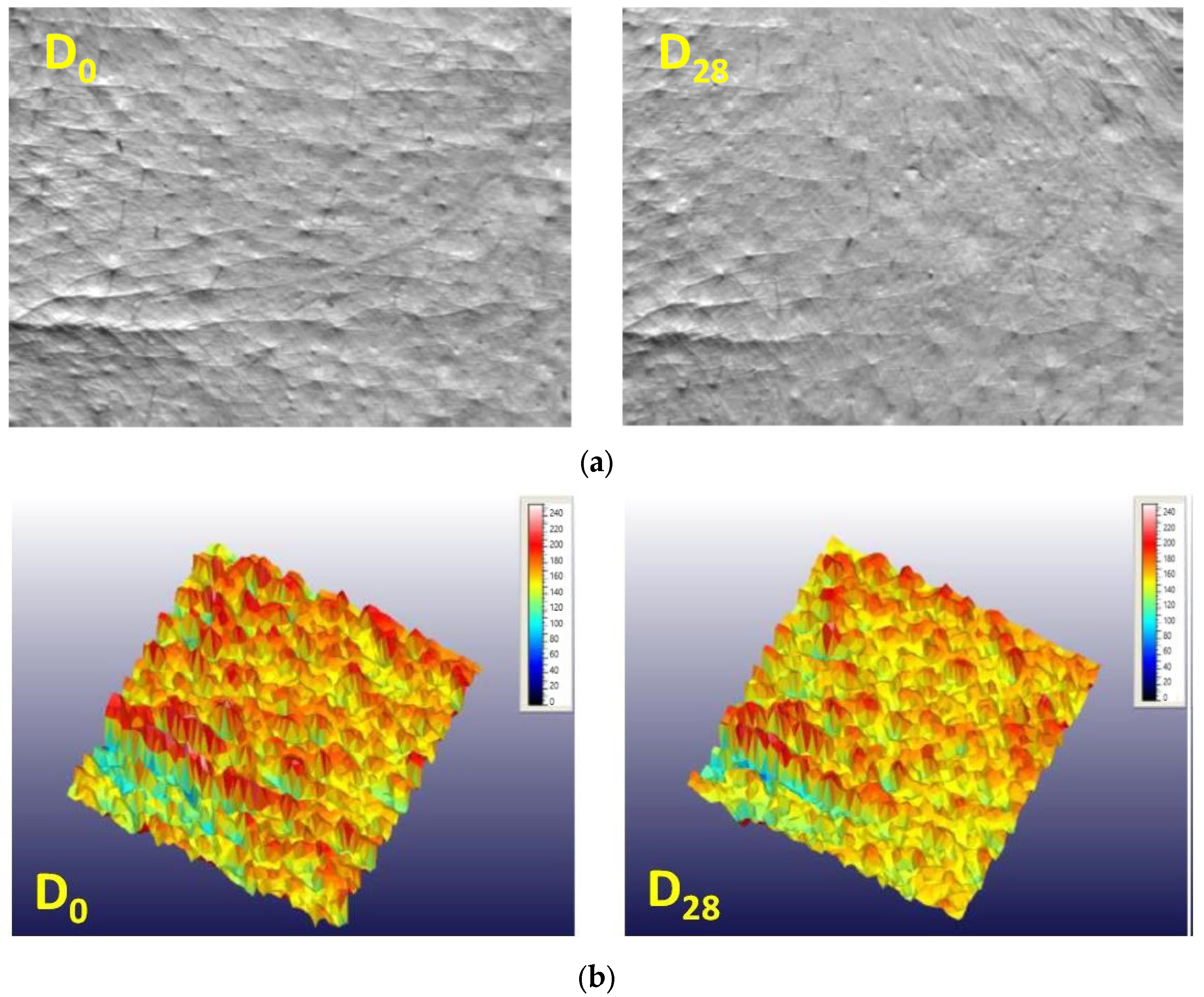
Instrumental Test Result for Wrinkle Length and Depth
In Vivo Evaluation of the SPF Factor
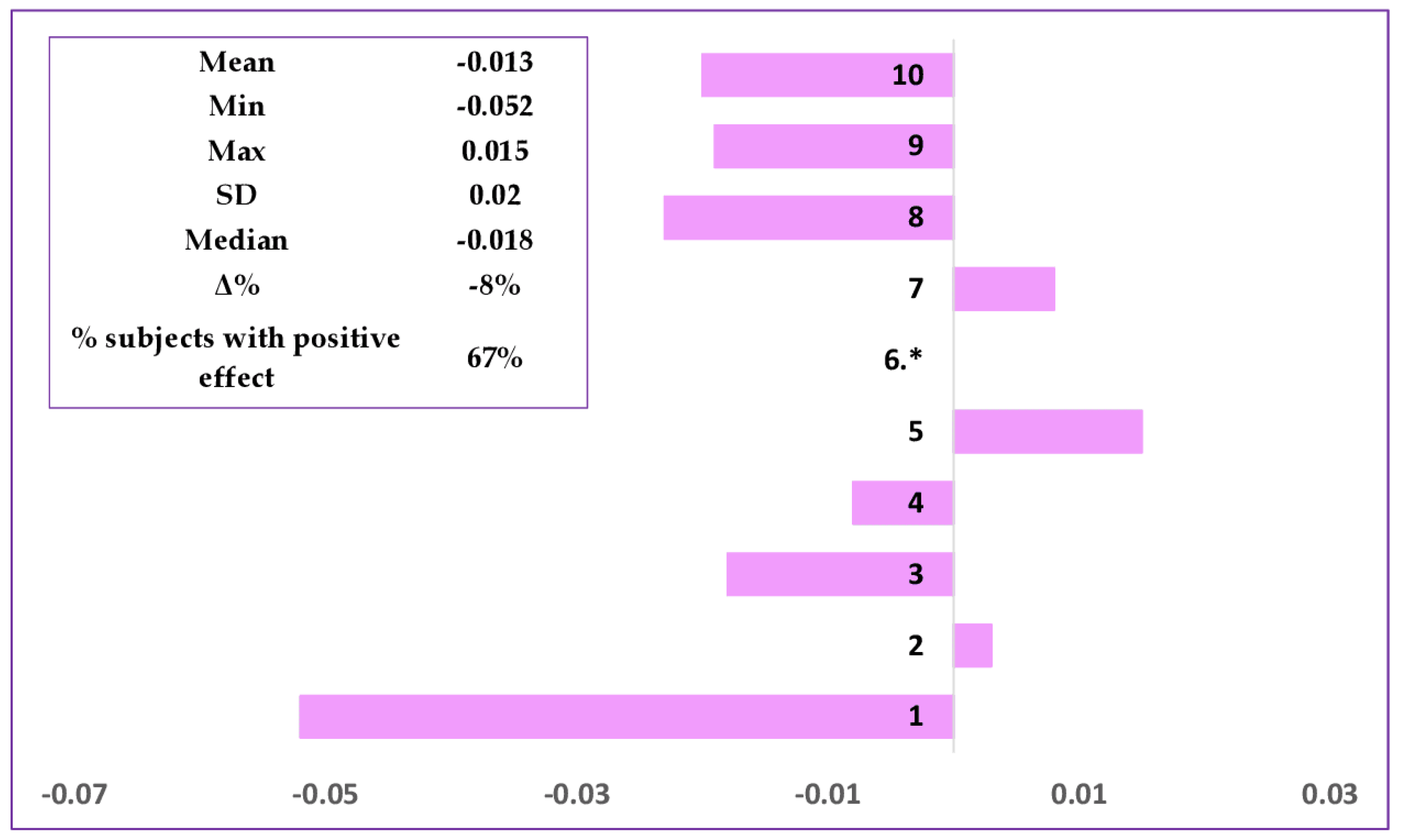
Assessment of the Claimed Effect for the Cosmetic Formulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elsner, P.; Fluhr, J.W.; Gehring, W.; Kerscher, M.J.; Krutmann, J.; Lademann, J.; Makrantonaki, E.; Wilhelm, K.P.; Zouboulis, C.C. Anti-Aging Data and Support Claims—Consensus Statement. J. Ger. Soc. Dermatol. JDDG 2011, 9 (Suppl. 3), S1–S32. [Google Scholar] [CrossRef]
- Hettwer, S.; Gyenge, E.B.; Obermayer, B. Analysis of ‘The Trilogy of Lifting’. Personal Care, 12 March 2020; pp. 37–42. [Google Scholar]
- Ayer, J.; Griffiths, C.E.M. Photoaging in Caucasians. In Cutaneous Photoaging; Rhodes, L., Sage, E., Trotta, M., Eds.; The Royal Society of Chemistry: London, UK, 2019; pp. 1–30. [Google Scholar]
- Oliveira, R.; Ferreira, J.; Azevedo, L.F.; Almeida, I.F. An Overview of Methods to Characterize Skin Type: Focus on Visual Rating Scales and Self-Report Instruments. Cosmetics 2023, 10, 14. [Google Scholar] [CrossRef]
- Naylor, E.C.; Watson, R.E.B.; Sherratt, M.J. Molecular Aspects of Skin Ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Maibach, H.I. Textbook of Aging Skin; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–18. ISBN 9783642278143. [Google Scholar]
- Draelos, Z.D. Cosmetics, Categories, and the Future. Dermatol. Ther. 2012, 25, 223–228. [Google Scholar] [CrossRef]
- Havas, F.; Krispin, S.; Borenstein-Auerbach, N.; Loing, E. Narcissus Tazetta Delays Telomere Shortening, Imparts Anti-Aging Effects. Cosmet. Toilet. 2020, 135, 49–57. [Google Scholar]
- Anti-Aging and Anti-Wrinkle Products Market. Available online: https://www.factmr.com/report/4337/anti-aging-and-anti-wrinkle-products-market (accessed on 13 February 2023).
- Mondon, P.; Doridot, E.; Ringenbach, C.; Gracioso, O. Hyaluronic Acid: History and Future Potential. Personal Care, 9 June 2015; pp. 27–30. [Google Scholar]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef]
- Essendoubi, M.; Gobinet, C.; Reynaud, R.; Angiboust, J.F.; Manfait, M.; Piot, O. Human Skin Penetration of Hyaluronic Acid of Different Molecular Weights as Probed by Raman Spectroscopy. Ski. Res. Technol. 2016, 22, 55–62. [Google Scholar] [CrossRef]
- Juncan, A.M.; Moisă, D.G.; Santini, A.; Morgovan, C.; Rus, L.L.; Vonica-Țincu, A.L.; Loghin, F. Advantages of Hyaluronic Acid and Its Combination with Other Bioactive Ingredients in Cosmeceuticals. Molecules 2021, 26, 4429. [Google Scholar] [CrossRef]
- Jojoba Esters (and) Helianthus Annuus (Sunflower) Seed Wax (and) Acacia Decurrens Flower Wax (and) Polyglycerin-3 ACTICIRE MB. Available online: https://www.ulprospector.com/documents/1520629.pdf?bs=3983&b=719008&st=1&sl=149104681&crit=a2V5d29yZDpbQUNUSUNJUkVd&k=ACTICIRE&r=eu&ind=personalcare (accessed on 16 February 2023).
- Octyldodecyl Myristate MOD MB. Available online: https://www.ulprospector.com/documents/1395526.pdf?bs=3983&b=588277&st=1&sl=149105263&crit=a2V5d29yZDpbTU9EIE1CXQ%3d%3d&k=MOD|MB&r=eu&ind=personalcare (accessed on 16 February 2023).
- Caprylic/Capric Triglyceride LABRAFAC CC. Available online: https://www.ulprospector.com/documents/1395523.pdf?bs=3983&b=588276&st=1&sl=149105203&crit=a2V5d29yZDpbTGFicmFmYWMgQ0Nd&k=Labrafac|CC&r=eu&ind=personalcare (accessed on 16 February 2023).
- Cetyl Alcohol (and) Glyceryl Stearate (and) PEG-75 Stearate (and) Ceteth-20 (and) Steareth-20 EMULIUM DELTA MB. Available online: https://www.ulprospector.com/documents/1520632.pdf?bs=3983&b=719010&st=1&sl=149105458&crit=a2V5d29yZDpbRW11bGl1bcKuIERlbHRhIE1CXQ%3d%3d&k=Emulium%c2%ae|Delta|MB&r=eu&ind=personalcare (accessed on 16 February 2023).
- Hydrogenated Ethylhexyl Olivate (and) Hydrogenated Olive Oil Unsaponifiables SOFTOLIVE. Available online: https://www.ulprospector.com/documents/1597710.pdf?bs=830&b=724179&st=1&sl=149105673&crit=a2V5d29yZDpbU29mdG9saXZlXQ%3d%3d&k=Softolive&r=eu&ind=personalcare (accessed on 16 February 2023).
- Phenoxyethanol (and) Ethylhexylglycerin Euxyl PE 9010 Preservative. Available online: https://www.ashland.com/industries/personal-and-home-care/hair-care/euxyl-pe-9010-preservative (accessed on 16 February 2023).
- Carbomer Carbopol® ETD 2050. Available online: https://www.ulprospector.com/documents/1172655.pdf?bs=77&b=3755&st=1&sl=149106076&crit=a2V5d29yZDpbQ2FyYm9wb2zCriBFVEQgMjA1MF0%3d&k=Carbopol%c2%ae|ETD|2050&r=eu&ind=personalcare (accessed on 16 February 2023).
- Sodium Polyacrylate (and) Hydrogenated Polydecene (and) Trideceth-6 RapiThix. Available online: https://www.ulprospector.com/documents/1189975.pdf?bs=4989&b=13946&st=1&sl=149109631&crit=a2V5d29yZDpbUmFwaXRoaXggQS02MF0%3d&k=Rapithix|A-60&r=eu&ind=personalcare (accessed on 16 February 2023).
- Butyl Methoxydibenzoylmethane Eusolex® 9020 UV Filter. Available online: https://www.ulprospector.com/documents/56532.pdf?bs=824&b=34416&st=1&sl=149109973&crit=a2V5d29yZDpbRXVzb2xleCA5MDIwXQ%3d%3d&k=Eusolex|9020&r=eu&ind=personalcare (accessed on 16 February 2023).
- Octocrylene Eusolex® OCR UV Filter. Available online: https://www.ulprospector.com/documents/56540.pdf?bs=824&b=34419&st=1&sl=149110066&crit=a2V5d29yZDpbRXVzb2xleCBPQ1Jd&k=Eusolex|OCR&r=eu&ind=personalcare (accessed on 16 February 2023).
- Hydrolyzed hyaluronic acid PRIMALHYAL 500. Available online: https://www.ulprospector.com/documents/1597694.pdf?bs=830&b=724175&st=1&sl=149148604&crit=a2V5d29yZDpbUFJJTUFMSFlBTCA1MF0%3d&k=PRIMALHYAL|50&r=eu&ind=personalcare (accessed on 17 February 2023).
- Hydrolyzed hyaluronic acid PRIMALHYAL 300. Available online: https://www.ulprospector.com/documents/1597712.pdf?bs=830&b=724176&st=1&sl=149148732&crit=a2V5d29yZDpbUHJpbWFsSHlhbOKEoiAzMDBd&k=PrimalHyal%e2%84%a2|300&r=eu&ind=personalcare (accessed on 17 February 2023).
- Sodium hyaluronate HYALUSPHERE PF. Available online: https://www.ulprospector.com/documents/1597711.pdf?bs=830&b=724168&st=1&sl=149110383&crit=a2V5d29yZDpbSFlBTFVTUEhFUkVd&k=HYALUSPHERE&r=eu&ind=personalcare (accessed on 17 February 2023).
- Mannitol Cellulose Calcium Sodium Borosilicate CI 77492 (US: Iron Oxides) Silica CI 77891 (US: Titanium Dioxide) CI 77480 (Gold) Tin Oxide Hydroxypropyl Methylcellulose HYALUSPHERE PF. Available online: https://www.givaudan.com/fragrance-beauty/active-beauty/products/unispheres-original (accessed on 17 February 2023).
- Kienemund, J.; Glik, E.; Schmitz, R. Natural Ectoin: Protecting against Air Pollution. Personal Care, 6 May 2022; pp. 29–32. [Google Scholar]
- Ectoin RonaCare® Ectoin. Available online: https://www.ulprospector.com/documents/56627.pdf?bs=824&b=34445&st=20&r=eu&ind=personalcare (accessed on 17 February 2023).
- Silica; CI 77891 (Titanium Dioxide); CI 77491 (Iron Oxides) RonaFlair® Flawless. Available online: https://www.ulprospector.com/documents/1330438.pdf?bs=824&b=435710&st=1&sl=149150045&crit=a2V5d29yZDpbUm9uYUZsYWlywq4gRmxhd2xlc3Nd&k=RonaFlair%c2%ae|Flawless&r=eu&ind=personalcare (accessed on 17 February 2023).
- Fructose (and) Glycerin (and) Water (and) Aesculus Hippocastanum (Horse Chestnut) Extract GATULINE LINK N LIFT. Available online: https://www.ulprospector.com/documents/1520619.pdf?bs=3983&b=718995&st=1&sl=149149406&crit=a2V5d29yZDpbR2F0dWxpbmXCriBMaW5rIG4gTGlmdF0%3d&k=Gatuline%c2%ae|Link|n|Lift&r=eu&ind=personalcare (accessed on 17 February 2023).
- Clairet, A.; Bardin, V.; Trevisan, S.; Jomier, M. Horse Chestnut Flower Extract Redesigns Eye Contour. Personal Care, 9 May 2018; pp. 37–40. [Google Scholar]
- Cosmetics Europe. Guidelines on Stability Testing of Cosmetic Products. 2004. Available online: https://www.cosmeticseurope.eu/files/5914/6407/8121/Guidelines_on_Stability_Testing_of_Cosmetics_CE-CTFA_-_2004.pdf (accessed on 21 February 2023).
- Juncan, A.M. Packaging Evaluation and Safety Assessment of a Cosmetic Product. Mater. Plast. 2018, 55, 644–647. [Google Scholar] [CrossRef]
- Juncan, A.M.; Rus, L.L. Influence of Packaging and Stability Test Assessment of an Anti-Aging Cosmetic Cream. Mater. Plast. 2018, 55, 426–430. [Google Scholar] [CrossRef]
- Montenegro, L.; Rapisarda, L.; Ministeri, C.; Puglisi, G. Effects of Lipids and Emulsifiers on the Physicochemical and Sensory Properties of Cosmetic Emulsions Containing Vitamin E. Cosmetics 2015, 2, 35–47. [Google Scholar] [CrossRef]
- Olejnik, A.; Kapuscinska, A.; Schroeder, G.; Nowak, I. Physico-Chemical Characterization of Formulations Containing Endomorphin-2 Derivatives. Amino Acids 2017, 49, 1719–1731. [Google Scholar] [CrossRef]
- Bashir, A.; Lambert, P. Microbiological Study of Used Cosmetic Products: Highlighting Possible Impact on Consumer Health. J. Appl. Microbiol. 2020, 128, 598–605. [Google Scholar] [CrossRef]
- Devlieghere, F.; De Loy-Hendrickx, A.; Rademaker, M.; Pipelers, P.; Crozier, A.; De Baets, B.; Joly, L.; Keromen, S. A New Protocol for Evaluating the Efficacy of Some Dispensing Systems of a Packaging in the Microbial Protection of Water-Based Preservative-Free Cosmetic Products. Int. J. Cosmet. Sci. 2015, 37, 627–635. [Google Scholar] [CrossRef]
- Fiorentino, F.A.M.; Chorilli, M.; Salgado, H.R.N. The Use of the Challenge Test to Analyse Preservative Efficiency in Non-Sterile Cosmetic and Health Products: Applications and Critical Points. Anal. Methods 2011, 3, 790–798. [Google Scholar] [CrossRef]
- Siegert, W. ISO 11930—A Comparison to Other Methods to Evaluate the Efficacy of Antimicrobial Preservation. SOFW J. 2012, 138, 44–53. [Google Scholar]
- Siegert, W. Comparison of Microbial Challenge Testing Methods for Cosmetics. H PC Today 2013, 8, 32–39. [Google Scholar]
- ISO 6658:2005; Sensory analysis—Methodology—General guidance. ISO: Geneva, Switzerland, 2005.
- ISO 21149:2017; Cosmetics—Microbiology—Enumeration and Detection of Aerobic Mesophilic bacteria. ISO: Geneva, Switzerland, 2017.
- ISO 16212:2017; Cosmetics—Microbiology—Enumeration of Yeast and Mould. ISO: Geneva, Switzerland, 2017.
- ISO 22718:2016; Cosmetics—Microbiology—Detection of Staphylococcus aureus. ISO: Geneva, Switzerland, 2016.
- ISO 18416:2016; Cosmetics—Microbiology—Detection of Candida albicans. ISO: Geneva, Switzerland, 2016.
- ISO 21150:2016; Cosmetics—Microbiology—Detection of Escherichia coli. ISO: Geneva, Switzerland, 2016.
- ISO 22717:2016; Cosmetics—Microbiology—Detection of Pseudomonas aeruginosa. ISO: Geneva, Switzerland, 2016.
- ISO 11930:2012; Cosmetics—Microbiology—Evaluation of the Antimicrobial Protection of a Cosmetic Product. ISO: Geneva, Switzerland, 2012.
- SpheraCosmolife: New Software Specific for Risk Assessment of Cosmetic Products. Available online: https://www.vegahub.eu/portfolio-item/vermeer-cosmolife/ (accessed on 21 February 2023).
- Selvestrel, G.; Robino, F.; Baderna, D.; Manganelli, S.; Asturiol, D.; Manganaro, A.; Russo, M.Z.; Lavado, G.; Toma, C.; Roncaglioni, A.; et al. SpheraCosmolife: A New Tool for the Risk Assessment of Cosmetic Products. ALTEX 2021, 38, 565–579. [Google Scholar] [CrossRef]
- Rogiers, V. Animal-Free Cosmetics in Europe. In The History of Alternative Test Methods in Toxicology; Balls, M., Combes, R., Worth, A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 157–166. ISBN 9780128136980. [Google Scholar]
- Cosmetics Europe. Guidelines for the Assessment of Skin Tolerance of Potentially Irritant Cosmetic Ingredients. 1997. Available online: https://cosmeticseurope.eu/files/2314/6407/8977/Guidelines_for_Assessment_of_Skin_Tolerance_of_Potentially_Irritant_Cosmetic_Ingredients_-_1997.pdf (accessed on 21 February 2023).
- Goossens, A.E. Semi-Open (or Semi-Occlusive) Tests. In Patch Testing Tips; Lachapelle, J.-M., Bruze, M., Elsner, P.U., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 123–127. ISBN 978-3-642-45394-6. [Google Scholar]
- Lazzarini, R.; Duarte, I.; Ferreira, A.L. Patch Tests. An. Bras. Dermatol. 2013, 88, 879–888. [Google Scholar] [CrossRef]
- Cosmetics Europe. Product Test Guidelines for the Assessment of Human Skin Compatibility. 1997. Available online: https://www.cosmeticseurope.eu/files/6014/6407/8875/Product_Test_Guidelines_for_the_Assessment_of_Human_Skin_Compatibility_-_1997.pdf (accessed on 21 February 2023).
- Walker, A.P.; Basketter, D.A.; Baverel, M.; Diembeck, W.; Matthies, W.; Mougin, D.; Paye, M.; Rothlisberger, R.; Dupuis, J. Regulatory Test Guidelines for Assessment of Skin Compatibility of Cosmetic Finished Products in Man. Food Chem. Toxicol. 1996, 34, 651–660. [Google Scholar] [CrossRef]
- Ennen, J.; Degwert, J.; Duttiné, M.; Gerlach, N.; Jassoy, C.; Jünger, M.; Matthies, W.; Mehling, A.; Merk, H.; Rossow, U.; et al. Studies on the Skin Compatibility of Cosmetics Involving Human Subjects-Study Portfolio and Test Strategy. IFSCC Mag. 2014, 17, 19–23. [Google Scholar]
- Jackson, E.M.; Robillard, N.F. The controlled use test in a cosmetic product safety substantiation program. J. Toxicol.-Cutan. Ocul. Toxicol. 1982, 1, 117–132. [Google Scholar] [CrossRef]
- Houser, T.; Zerweck, C.; Grove, G.; Wickett, R. Shadow Analysis via the C+K Visioline: A Technical Note. Ski. Res. Technol. 2017, 23, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Garre, A.; Martinez-Masana, G.; Piquero-Casals, J.; Granger, C. Redefining Face Contour with a Novel Anti-Aging Cosmetic Product: An Open-Label, Prospective Clinical Study. Clin. Cosmet. Investig. Dermatol. 2017, 10, 473–482. [Google Scholar] [CrossRef]
- Leveque, J.L. EEMCO Guidance for the Assessment of Skin Topography. J. Eur. Acad. Dermatol. Venereol. 1999, 12, 103–114. [Google Scholar] [CrossRef]
- Berardesca, E.; Farinelli, N.; Rabbiosi, G.; Maibach, H.I. Skin Bioengineering in the Noninvasive Assessment of Cutaneous Aging. Dermatology 1991, 182, 1–6. [Google Scholar] [CrossRef]
- Corcuff, P.; Lévêque, J.L. Skin Surface Replica Image Analysis of Furrows and Wrinkles. In Handbook of Non-Invasive Methods and the Skin; Jorgen Serup, J., Jemec, G., Grove, G.L., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 155–162. ISBN 10:0-8493-1437-2. [Google Scholar]
- Matts, P.J.; Alard, V.; Brown, M.W.; Ferrero, L.; Gers-Barlag, H.; Issachar, N.; Moyal, D.; Wolber, R. The COLIPA in Vitro UVA Method: A Standard and Reproducible Measure of Sunscreen UVA Protection. Int. J. Cosmet. Sci. 2010, 32, 35–46. [Google Scholar] [CrossRef]
- Miksa, S.; Lutz, D.; Guy, C.; Delamour, E. Sunscreen Sun Protection Factor Claim Based on in Vivo Interlaboratory Variability. Int. J. Cosmet. Sci. 2016, 38, 541–549. [Google Scholar] [CrossRef]
- Commission Recommendation of 22 September 2006 on the Efficacy of Sunscreen Products and the Claims Made Relating Thereto. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006H0647&from=EN (accessed on 8 March 2023).
- Lionetti, N.; Rigano, L. The New Sunscreens among Formulation Strategy, Stability Issues, Changing Norms, Safety and Efficacy Evaluations. Cosmetics 2017, 4, 15. [Google Scholar] [CrossRef]
- Cosmetics Europe. International Sun Protection Factor (Spf) Test Method. 2006. Available online: https://downloads.regulations.gov/FDA-1978-N-0018-0698/attachment_65.pdf (accessed on 1 March 2023).
- World Health Organization. Active Ageing: A Policy Framework; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Martin, P.; Kelly, N.; Kahana, B.; Kahana, E.; Willcox, B.J.; Willcox, D.C.; Poon, L.W. Defining Successful Aging: A Tangible or Elusive Concept? Gerontologist 2015, 55, 14–25. [Google Scholar] [CrossRef]
- Aubert, G.; Lansdorp, P.M. Telomeres and Aging. Physiol. Rev. 2008, 88, 557–579. [Google Scholar] [CrossRef]
- Gutermuth, J. Fundamental Concepts in Skin Aging. Intensive Course in Dermato-Cosmetic Sciences; Vrije Universiteit Brussel: Ixelles, Belgium, 2022; September. [Google Scholar]
- Gorcea, M.; Sona, P. Face and Neck Firming care with Teff Natural Peptides. Personal Care, 19 February 2021; pp. 59–62. [Google Scholar]
- Salvador, A.; Pascual-Martı, M.C. COSMETICS AND TOILETRIES. In Encyclopedia of Analytical Science; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2005; pp. 226–233. ISBN 0127641009. [Google Scholar]
- Draelos, Z.D. The Science behind Skin Care: Moisturizers. J. Cosmet. Dermatol. 2018, 17, 138–144. [Google Scholar] [CrossRef]
- Petry, T.; Bury, D.; Fautz, R.; Hauser, M.; Huber, B.; Markowetz, A.; Mishra, S.; Rettinger, K.; Schuh, W.; Teichert, T. Review of Data on the Dermal Penetration of Mineral Oils and Waxes Used in Cosmetic Applications. Toxicol. Lett. 2017, 280, 70–78. [Google Scholar] [CrossRef]
- Scott, R. Ingredient Focus: Waxes and Butters. Personal Care, 13 June 2017; pp. 71–73. [Google Scholar]
- Juncan, A.M.; Morgovan, C.; Rus, L.L. Selection and Application of Synthetic versus Natural Emollients in the Formulation of Skin Care Products. Rev. Chim. 2019, 70, 2764–2768. [Google Scholar] [CrossRef]
- Draelos, Z.D. Revisiting the Skin Health and Beauty Pyramid: A Clinically Based Guide to Selecting Topical Skincare Products. J. Drugs Dermatol. 2021, 20, 695–699. [Google Scholar] [CrossRef] [PubMed]
- ISO/TR:18811; Cosmetics-Guidelines on the Stability Testing of Cosmetic Products. ISO: Geneva, Switzerland, 2018.
- Vieira, R.P.; Fernandes, A.R.; Kaneko, T.M.; Consiglieri, V.O.; Pinto, C.A.S.O.; Pereira, C.S.C.; Baby, A.R.; Velasco, M.V.R. Physical and Physicochemical Stability Evaluation of Cosmetic Formulations Containing Soybean Extract Fermented by Bifidobacterium Animalis. Braz. J. Pharm. Sci. 2009, 45, 515–524. [Google Scholar] [CrossRef]
- Dréno, B.; Zuberbier, T.; Gelmetti, C.; Gontijo, G.; Marinovich, M. Safety Review of Phenoxyethanol When Used as a Preservative in Cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 15–24. [Google Scholar] [CrossRef]
- Lilienblum, W. Opinion of the Scientific Committee on Consumer Safety (SCCS)—Final Version of the Opinion on Phenoxyethanol in Cosmetic Products. Regul. Toxicol. Pharmacol. 2016, 82, 156. [Google Scholar] [CrossRef]
- Nowak, K.; Jabłońska, E.; Ratajczak-Wrona, W. Controversy around Parabens: Alternative Strategies for Preservative Use in Cosmetics and Personal Care Products. Environ. Res. 2021, 198, 110488. [Google Scholar] [CrossRef] [PubMed]
- Orus, P.; Leranoz, S. Current Trends in Cosmetic Microbiology Microorganisms and Cosmetics. Int. Microbiol. 2005, 8, 77–79. [Google Scholar] [PubMed]
- Kim, K.B.; Kwack, S.J.; Lee, J.Y.; Kacew, S.; Lee, B.M. Current Opinion on Risk Assessment of Cosmetics. J. Toxicol. Environ. Health B Crit. Rev. 2021, 24, 137–161. [Google Scholar] [CrossRef] [PubMed]
- SCCS. The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation, 10th Revision. 2018. Available online: https://health.ec.europa.eu/system/files/2019-02/sccs_o_224_0.pdf (accessed on 1 March 2023).
- Cronin, M.T.D.; Enoch, S.J.; Madden, J.C.; Rathman, J.F.; Richarz, A.N.; Yang, C. A Review of in Silico Toxicology Approaches to Support the Safety Assessment of Cosmetics-Related Materials. Comput. Toxicol. 2022, 21, 1–67. [Google Scholar] [CrossRef]
- Gellatly, N.; Sewell, F. Regulatory Acceptance of in Silico Approaches for the Safety Assessment of Cosmetic-Related Substances. Comput. Toxicol. 2019, 11, 82–89. [Google Scholar] [CrossRef]
- Rogiers, V.; Benfenati, E.; Bernauer, U.; Bodin, L.; Carmichael, P.; Chaudhry, Q.; Coenraads, P.J.; Cronin, M.T.D.; Dent, M.; Dusinska, M.; et al. The Way Forward for Assessing the Human Health Safety of Cosmetics in the EU—Workshop Proceedings. Toxicology 2020, 436, 152421. [Google Scholar] [CrossRef] [PubMed]
- Santander Ballestín, S.; Luesma Bartolomé, M.J. Toxicity of Different Chemical Components in Sun Cream Filters and Their Impact on Human Health: A Review. Appl. Sci. 2023, 13, 712. [Google Scholar] [CrossRef]
- Montenegro, L.; Turnaturi, R.; Parenti, C.; Pasquinucci, L. In Vitro Evaluation of Sunscreen Safety: Effects of the Vehicle and Repeated Applications on Skin Permeation from Topical Formulations. Pharmaceutics 2018, 10, 27. [Google Scholar] [CrossRef]
- Nash, J.F. Human Safety and Efficacy of Ultraviolet Filters and Sunscreen Products. Dermatol. Clin. 2006, 24, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Registration Dossier—ECHA 1-[4-(1,1-Dimethylethyl)phenyl]-3-(4-methoxyphenyl)propane-1,3-dione. Available online: https://echa.europa.eu/ro/registration-dossier/-/registered-dossier/14835/7/6/2 (accessed on 14 April 2023).
- Matta, M.K.; Zusterzeel, R.; Pilli, N.R.; Patel, V.; Volpe, D.A.; Florian, J.; Oh, L.; Bashaw, E.; Zineh, I.; Sanabria, C.; et al. Effect of Sunscreen Application under Maximal Use Conditions on Plasma Concentration of Sunscreen Active Ingredients: A Randomized Clinical Trial. JAMA—J. Am. Med. Assoc. 2019, 321, 2082–2091. [Google Scholar] [CrossRef]


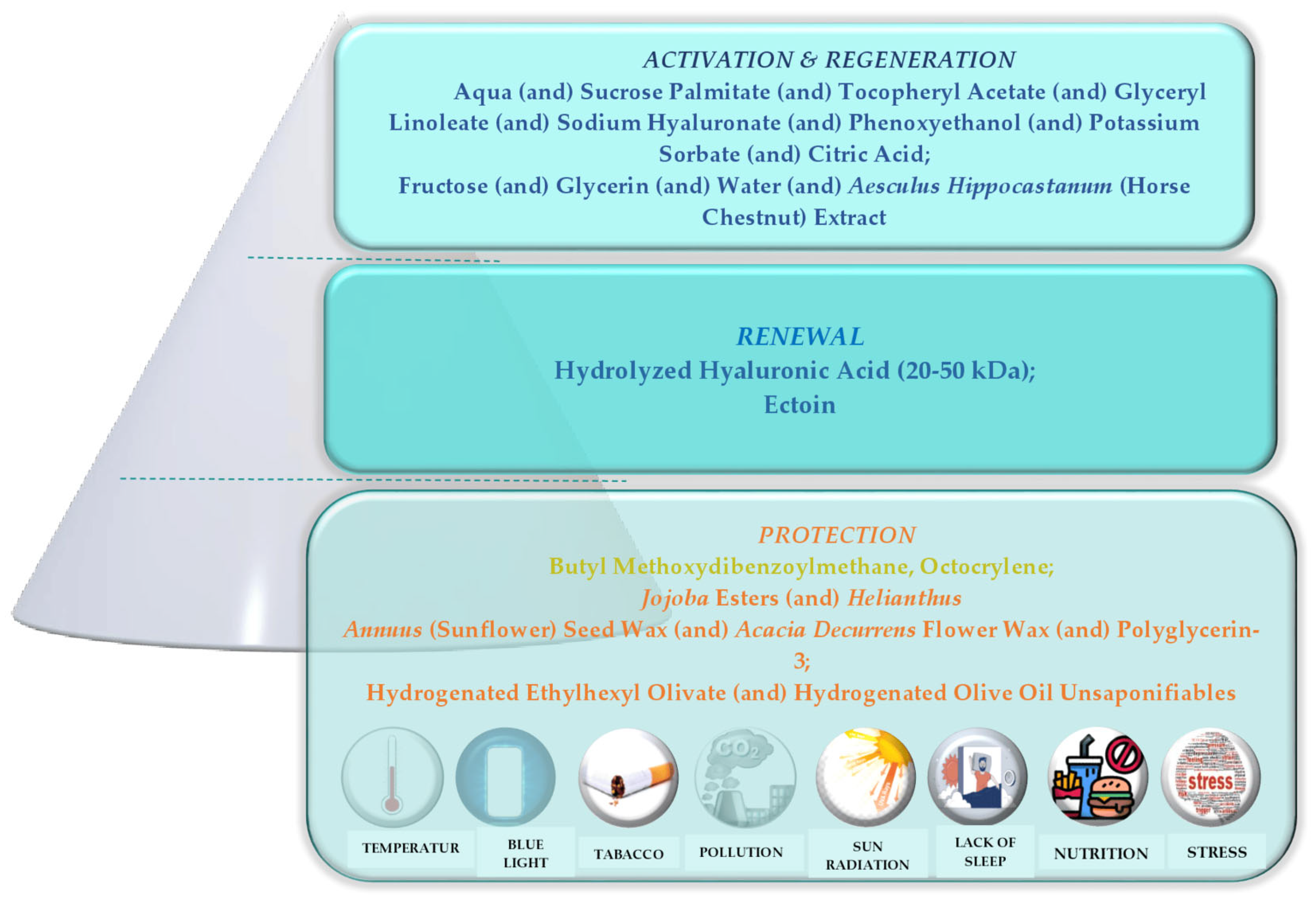
| Commercial Name | INCI Name | Molecular Weight | Cosmetic Claim |
|---|---|---|---|
| Primalhyal 50 | Hydrolysed Hyaluronic Acid | 20–50 kDa | firming, anti-ageing |
| Hyalusphere PF | Aqua, Sucrose Palmitate, Tocopheryl Acetate, Glyceryl Linoleate, Sodium Hyaluronate, Phenoxyethanol, Potassium Sorbate, and Citric Acid | / | anti-ageing |
| Commercial Name | INCI | Function | Supplier | INCI-KEY * (%) |
|---|---|---|---|---|
| Acticire MB | Jojoba Esters, Helianthus Annuus (Sunflower) Seed Wax (and) Acacia Decurrens Flower Wax, and Polyglycerin-3 | emollient | Gattefossé | E |
| MOD MB | Octyldodecyl Myristate | emollient | Gattefossé | E |
| Labrafac CC | Caprylic/Capric Triglyceride | emollient | Gattefossé | E |
| Softolive | Hydrogenated Ethylhexyl Olivate (and) Hydrogenated Olive Oil Unsaponifiables | emollient | Givaudan Active Beauty | D |
| Emulium Delta MB | Cetyl Alcohol (and) Glyceryl Stearate (and) PEG-75 Stearate (and) Ceteth-20 (and) Steareth-2 | emulsifier | Gattefossé | E |
| Eusolex 9020 | Butyl Methoxydibenzoyl methane | UVA filter | Merck | E |
| Eusolex OCR | Octocrylene | UVB filter | Merck | D |
| Aqua | Water | solvent | A | |
| Glycerol | Glycerin | denaturant/humectant/solvent | ELTON | E |
| Carbopol ETD 2050 | Carbomer | emulsion stabilising/viscosity controlling/gel forming | Lubrizol | F |
| Euxyl PE 9010 | Phenoxyethanol and Ethylhexylglycerin | preservative | Schülke & Mayr GmbH | F |
| TEA | Triethanolamine | buffering agent | ELTON | G |
| Rapithix A-60 | Sodium Polyacrylate, Hydrogenated Polydecene, and Trideceth-6 | viscosity controlling/binding/film forming | Barentz | F |
| PrimalHyal™ 50 | Hydrolysed Hyaluronic Acid | antistatic/humectant/skin conditioning/moisturising | Givaudan Active Beauty | F |
| Hyalusphere PF | Aqua, Sucrose Palmitate, Tocopheryl Acetate, Glyceryl Linoleate, Sodium Hyaluronate, Phenoxyethanol, Potassium Sorbate, and Citric Acid | active ingredient/anti-wrinkle | Givaudan Active Beauty | E |
| Luxury Unispheres Gold | Mannitol Cellulose Calcium Sodium Borosilicate CI 77492 (US: Iron Oxides) Silica CI 77891 (US: Titanium Dioxide) CI 77480 (Gold) Tin Oxide Hydroxypropyl Methylcellulose | active ingredient/shimmer effect | Givaudan Active Beauty | E |
| Gatuline Link n Lift | Fructose (and) Glycerin (and) Water (and) Aesculus Hippocastanum (Horse Chestnut) Extract | active ingredient/anti-ageing | Gattefossé | E |
| Rona Flair Flawless | Silica (and) CI 77891 (and) CI 77491 | functional filler/anti-wrinkle/anti-ageing agent | Merck | E |
| Luminating Skin Care Eco-Boost HICC MOD | Perfume | deodorant/masking | CPL | F |
| Test | Unit | Result |
|---|---|---|
| Viscosity at 20 °C (Brookfield DV-III Ultra) | mPa·s | 412.2 × 103 ± 9 × 103 |
| Density at 20 °C (PB-155 ed.I of 2 May 2012) | g/cm3 | 1.002 ± 0.003 |
| Organoleptic testing (ISO 6658:2005 p. 5.4.2) | ||
| Appearance | Homogeneous emulsion * | |
| Color | Light beige (with gold microcapsules) | |
| Odor | Specific | |
| Consistency | Specific of emulsion | |
| pH (PB-234 ed. I of 03.10.2013r.) | 5.9 ± 0.2 |
| Parameter | ISO Standard | Result (CFU/g) | Permissible Limits (CFU/g) | Concordance |
|---|---|---|---|---|
| Enumeration and detection of aerobic mesophilic bacteria | 21149:2017 | <10 | <100 | √ |
| Yeast and mould count | 16212:2017 | <10 | <10 | √ |
| Staphylococcus aureus detection | 22718:2016 | 0 | 0 | √ |
| Candida albicans detection | 18416:2016 | 0 | 0 | √ |
| Escherichia coli detection | 21150:2016 | 0 | 0 | √ |
| Pseudomonas aeruginosa detection | 22717:2016 | 0 | 0 | √ |
| Ingredient Id | CAS | INCI | Conc. % | Annex | Mutagenicity | Skin Sensitization | Dermal Abs. | MoS | TTC |
|---|---|---|---|---|---|---|---|---|---|
| Jojoba Esters | 68953 | Jojoba Esters | 3.75 | - | - | - | - | - | |
| Helianthus Annuus (Sunflower) Seed Wax | 8001-21-6 | Helianthus Annuus (Sunflower) Seed Wax | 0.5 | - | - | - | - | - | |
| Acacia Decurrens Flower Wax | 98903-76-5 | Acacia Decurrens Flower Wax | 0.05 | - | - | - | - | - | |
| Polyglycerin-3 | 25618-55-7 | Polyglycerin-3 | 0.05 | - | NM (e.v.) | NS (e.v.) | 80% | 26,725.35 | 0.046 mg/kg bw/day |
| Octyldodecyl Myristate | 22766-83-2 | Octyldodecyl Myristate | 1.0 | - | NM (+++) | S (+) | 80% | 1345.12 | 0.046 mg/kg bw/day |
| Caprylic/Capric Triglyceride | 65381-09-1 | Caprylic/Capric Triglyceride | 3.0 | - | - | - | - | - | |
| Hydrogenated Ethylhexyl Olivate | 22047-49-0 | Hydrogenated Ethylhexyl Olivate | 5.0 | - | - | - | - | - | |
| Hydrogenated Olive Oil Unsaponifiables | 111-01-3 | Hydrogenated Olive Oil Unsaponifiables | 2.5 | - | - | - | - | - | |
| Cetyl Alcohol | 36653-82-4 | Cetyl Alcohol | 0.5 | - | NM (+++) | S (++) | 40% | 20,712.51 | 0.046 mg/kg bw/day |
| Glyceryl Stearate | 31566-31-1 | Glyceryl Stearate | 0.5 | - | NM (+++) | S (+++) | 40% | 8983.43 | 0.046 mg/kg bw/day |
| PEG-75 Stearate | 9004-99-3 | PEG-75 Stearate | 0.25 | - | NM (+++) | S (+++) | 40% | 10,347.97 | 0.046 mg/kg bw/day |
| Ceteth-20/Steareth-20 | 68439-49-6 | Ceteth-20/Steareth-20 | 0.25 | - | - | - | - | - | |
| Butyl Methoxydibenzoylmethane | 70356-09-1 | Butyl Methoxydibenzoylmethane | 2.0 | VI | NM (+++) | S (+) | 40% | 51.11 | 0.0023 mg/kg bw/day |
| Octocrylene | 6197-30-4 | Octocrylene | 10.0 | VI | NM (e.v.) | S (+) | 10% | 675.23 | 0.0023 mg/kg bw/day |
| Deionized Water | 7732-18-5 | Aqua | 59.2 | - | - | - | - | - | |
| Glycerine | 56-81-5 | Glycerine | 3.0 | - | NM (e.v.) | NS (e.v.) | 80% | 445.42 | 0.046 mg/kg bw/day |
| Triethanolamine | 102-71-6 | Triethanolamine | 0.45 | III | NM (e.v.) | S (+) | 80% | 5753.48 | 0.046 mg/kg bw/day |
| Carbomer | 9007-20-9 | Carbomer | 0.35 | - | NM (e.v.) | S (++) | 80% | 1597.82 | 0.0023 mg/kg bw/day |
| Phenoxyethanol | 122-99-6 | Phenoxyethanol | 0.9 | V | NM (e.v.) | NS (e.v.) | 80% | 460.28 | 0.046 mg/kg bw/day |
| Ethylhexylglycerin | 70445-33-9 | Ethylhexylglycerin | 0.1 | - | NM (+++) | NS (+) | 80% | 9737.47 | 0.0023 mg/kg bw/day |
| Sodium Polyacrylate | 9003-04-7 | Sodium Polyacrylate | 0.65 | - | - | - | - | - | |
| Hydrogenated Polydecene | 68037-01-4 | Hydrogenated Polydecene | 0.45 | - | NM (e.v.) | NS (+) | 40% | 682.82 | 0.046 mg/kg bw/day |
| Trideceth-6 | 78330-21-9 | Trideceth-6 | 0.06 | - | NM (+++) | S (++) | 40% | 24,235.36 | 0.046 mg/kg bw/day |
| Hydrolysed Hyaluronic Acid | 9004-61-9 | Hydrolysed Hyaluronic Acid | 0.5 | - | NM (++) | S (+) | 10% | 13,598,771.33 | 0.0023 mg/kg bw/day |
| Sucrose Palmitate | 26446-38-8 | Sucrose Palmitate | 0.25 | - | NM (+++) | S (+) | 10% | 724,425.85 | 0.0023 mg/kg bw/day |
| Tocopheryl Acetate | 7695-91-2 | Tocopheryl Acetate | 0.09 | - | NM (+++) | S (++) | 40% | 9153.78 | 0.0023 mg/kg bw/day |
| Glyceryl Linoleate | 2277-28-3 | Glyceryl Linoleate | 0.09 | - | NM (+++) | S (+++) | 40% | 49,907.94 | 0.046 mg/kg bw/day |
| Sodium Hyaluronate | 9067-32-7 | Sodium Hyaluronate | 0.009 | - | - | - | - | - | |
| Potassium Sorbate | 24634-61-5 | Potassium Sorbate | 0.005 | V | NM (e.v.) | S (+++) | 40% | 1,035,625.52 | 0.046 mg/kg bw/day |
| Citric Acid | 77-92-9 | Citric Acid | 0.009 | - | NM (e.v.) | NS (++) | 80% | 752,796.19 | 0.046 mg/kg bw/day |
| Fructose | 57-48-7 | Fructose | 2.5 | - | NM (+++) | NS (++) | 80% | 2120.28 | 0.046 mg/kg bw/day |
| Aesculus Hippocastanum (Horse Chestnut) Extract | 8053-39-2 | Aesculus Hippocastanum (Horse Chestnut) Extract | 0.5 | - | - | - | - | - | |
| Silica | 7631-86-9 | Silica | 0.9 | - | NM (++) | S (+) | 40% | 3365.21 | 0.0023 mg/kg bw/day |
| CI 77891 | 13463-67-7 | CI 77891 | 0.24 | VI | NM (e.v.) | S (+) | 10% | 4263.33 | 0.0023 mg/kg bw/day |
| CI 77491 | 1309-37-1 | CI 77491 | 0.02 | IV | - | - | - | - | |
| Ectoin | 96702-03-3 | Ectoin | 0.3 | - | M (++) | S (+) | 40% | 3167.98 | 0.0023 mg/kg bw/day |
| Mannitol | 69-65-8 | Mannitol | 0.75 | - | NM (e.v.) | S (+) | 40% | 18,652.44 | 0.046 mg/kg bw/day |
| Cellulose | 9004-34-6 | Cellulose | 0.3 | - | - | - | - | - | |
| Calcium Sodium Borosilicate | 65997-17-3 | Calcium Sodium Borosilicate | 0.25 | - | - | - | - | - | |
| CI 77492 | 51274-00-1 | CI 77492 | 0.05 | IV | - | - | - | - | |
| CI 77891 | 13463-67-7 | CI 77891 | 0.05 | VI | NM (e.v.) | S (+) | 10% | 20,463.96 | 0.0023 mg/kg bw/day |
| Gold | 7440-57-5 | Gold | 0.01 | IV | NM (++) | - | 10% | 107,207.95 | 0.0023 mg/kg bw/day |
| Tin Oxide | 18282-10-5 | Tin Oxide | 0.01 | - | NM (++) | S (+) | 40% | 14,415.91 | 0.0023 mg/kg bw/day |
| Hydroxypropyl Methylcellulose | 9004-65-3 | Hydroxypropyl Methylcellulose | 0.001 | - | NM (++) | S (+) | 80% | 2,614,954.43 | 0.046 mg/kg bw/day |
| Parfum | Parfum | 0.1 | - | - | - | - | - |
| Non-mutagen: experimental value | Non-mutagen: good (+++) / moderate reliability (++) | Mutagen: moderate reliabity (++) | MoS > 100 | MoS < 100 | |||||
| Non-sensitizer: experimental value | Non-sensitizer: low reliability (+) | Sensitizer: good relibility (+++) | Sensitizer: moderate reliability (++) | Sensitizer: low reliability (++) |
| T1 (48 h after Cosmetic Formulation Application) | T2 (72 h after Cosmetic Formulation Application) | |
|---|---|---|
| Erythema | 0 | 0 |
| Oedema | 0 | 0 |
| Xav * | 0 | 0 |
| Subject’s Characteristics * | MEDu (mJ/cm2) | MEDs (mJ/cm2) | SPFs (MEDs/MEDu) | MEDp (mJ/cm2) | SPFi p (MEDp/MEDu) |
|---|---|---|---|---|---|
| S1 (38, M, I) | 21 | 380 | 18.1 | 336 | 16.0 |
| S2 (47, M, III) | 25 | 425 | 17.0 | 375 | 15.0 |
| S3 (64, W, III) | 26 | 468 | 18.0 | 436.8 | 16.8 |
| S4 (30, W, II) | 19 | 342 | 18.0 | 340.5 | 17.9 |
| S5 (54, W, II) | 18 | 306 | 17.0 | 302.4 | 16.8 |
| S6 (58, W, I) | 16 | 288 | 18.0 | 256 | 16.0 |
| S7 (48, W, I) | 15 | 270 | 18.0 | 225 | 15.0 |
| S8 (51, W, I) | 16 | 288 | 18.0 | 268.8 | 16.8 |
| S9 (42, W, II) | 20 | 360 | 18.0 | 300 | 15.0 |
| S10 (56, W, III) | 27 | 432 | 16.0 | 405 | 15.0 |
| Average value ± standard deviation | 17.6 ± 0.7 | 16.0 ± 1.0 | |||
| Parameter | Value |
|---|---|
| Amount of product applied daily (SCCS/1628/21 Table 3A) | 24.14 mg/kg bw/day |
| Ingredient Concentration in finished product | 2% |
| Typical body weight of human (bw) | 60 kg |
| Absorption of active ingredient (DAp) (dermal absorption not known, considered as 50%) | 50% |
| Systemic exposure dose (SED) | 0.2414 mg/kg bw/day |
| NOAEL (considering sub chronic oral repeated dose toxicity study, rats) | 450 mg/kg bw/day |
| MoS | NOAEL/SED = 932 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juncan, A.M.; Morgovan, C.; Rus, L.-L.; Loghin, F. Development and Evaluation of a Novel Anti-Ageing Cream Based on Hyaluronic Acid and Other Innovative Cosmetic Actives. Polymers 2023, 15, 4134. https://doi.org/10.3390/polym15204134
Juncan AM, Morgovan C, Rus L-L, Loghin F. Development and Evaluation of a Novel Anti-Ageing Cream Based on Hyaluronic Acid and Other Innovative Cosmetic Actives. Polymers. 2023; 15(20):4134. https://doi.org/10.3390/polym15204134
Chicago/Turabian StyleJuncan, Anca Maria, Claudiu Morgovan, Luca-Liviu Rus, and Felicia Loghin. 2023. "Development and Evaluation of a Novel Anti-Ageing Cream Based on Hyaluronic Acid and Other Innovative Cosmetic Actives" Polymers 15, no. 20: 4134. https://doi.org/10.3390/polym15204134
APA StyleJuncan, A. M., Morgovan, C., Rus, L.-L., & Loghin, F. (2023). Development and Evaluation of a Novel Anti-Ageing Cream Based on Hyaluronic Acid and Other Innovative Cosmetic Actives. Polymers, 15(20), 4134. https://doi.org/10.3390/polym15204134