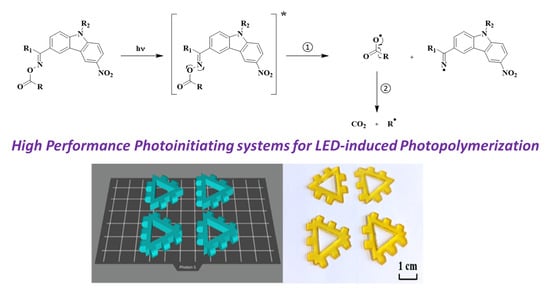
High-Performance Photoinitiating Systems for LED-Induced Photopolymerization
Abstract
1. Introduction
2. Dye-Based PISs
2.1. Carbazole-Based Photoinitiators
| Chemical Structures | Absorption Properties (λ/nm, εmax/M−1 cm−1) | Refs. |
|---|---|---|
 C1 | λmax ~ 364 εmax ~ 11,750 ε405nm ~ 2600 | [59] |
 C2 | λmax ~ 374 εmax ~ 11,180 ε405nm ~ 5200 | [59] |
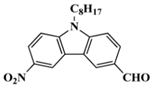 C3 | λmax ~ 364 εmax ~ 14,000 ε405nm ~ 2450 | [59] |
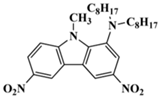 C4 | λmax ~ 388 εmax ~ 6000 ε405nm ~ 5200 | [59] |
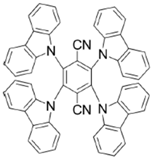 A1 | λmax ~ 330 εmax ~ 8800 ε405nm ~ 1350 | [60] |
 A2 | λmax ~ 340 εmax ~ 40,000 ε405nm ~ 7800 | [60] |
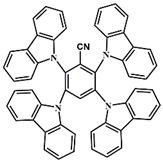 A3 | λmax ~ 333 εmax ~ 33,000 ε405nm ~ 5700 | [60] |
 A4 | λmax ~ 349 εmax ~ 18,000 ε405nm ~ 3300 | [60] |
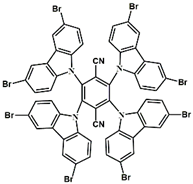
 A3–1 | λmax ~ 350 εmax ~ 49,000 | [61] |
 A3–2 | λmax ~ 360 εmax ~ 53,000 | [61] |
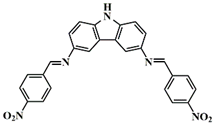 A3–3 | λmax ~ 415 εmax ~ 29,000 | [61] |
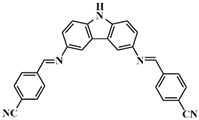 A3–4 | λmax ~ 394 εmax ~ 29,000 | [61] |
2.2. Triphenylamine-Based Photoinitiators
| Chemical Structures | Absorption Properties (λ/nm, εmax/M−1 cm−1) | Refs. |
|---|---|---|
 CDM2 | λmax ~ 467 εmax ~ 77,190 ε460nm ~ 75,490 ε520nm ~ 12,000 | [62] |
 Dye3 | λmax ~ 352 εmax ~ 26,610 ε405nm ~ 4110 | [63] |
 Dye4 | λmax ~ 357 εmax ~ 17,700 ε405nm ~ 4740 | [63] |
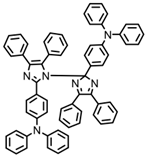 HABI1 | λmax ~ 383 εmax ~ 6600 | [64] |
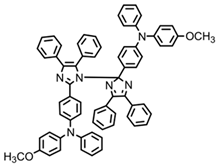 HABI2 | λmax ~ 385 εmax ~ 12,100 | [64] |
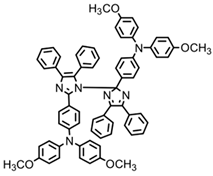 HABI3 | λmax ~ 384 εmax ~ 14,800 | [64] |
 PI-PAG | λmax ~ 381 εmax ~ 23,200 ε365nm ~ 19,200 ε405nm ~ 14,900 | [65] |
2.3. Naphthalimide-Based Photoinitiators
| Chemical Structures | Absorption Properties (λ/nm, εmax/M−1 cm−1) | Refs. |
|---|---|---|
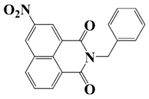 NDP1 | λmax ~ 421 εmax ~ 620 ε405nm ~560 | [71] |
 NDP2 | λmax ~ 417 εmax ~ 5600 ε405nm ~ 5100 | [71] |
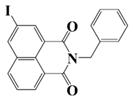 NDP3 | λmax ~ 334 εmax ~ 13,100 | [71] |
 NDP4 | λmax ~ 426 εmax ~ 9800 ε405nm ~ 8200 | [71] |
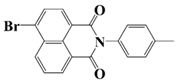 NDP5 | λmax ~ 340 εmax ~ 17,800 | [71] |
 NDP6 | λmax ~ 431 εmax ~ 17,400 ε405nm ~ 12,100 | [71] |
 NDP7 | λmax ~ 440 εmax ~ 11,300 ε405nm ~ 8800 | [71] |
 NDA1 | λmax ~ 416 εmax ~ 4600 ε405nm ~ 4300 ε455nm ~ 1200 | [72] |
 NDA2 | λmax ~ 431 εmax ~ 14,600 ε405nm ~ 10,300 | [72] |
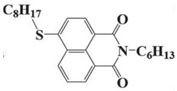 NDA3 | λmax ~ 387 εmax ~ 18,000 ε405nm ~ 13,000 ε455nm ~ 1000 | [72] |
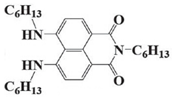 NDA4 | λmax ~ 439 εmax ~ 16,300 ε405nm ~ 9300 | [72] |
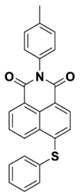 NAS1 | λmax ~ 389 ε405nm ~ 10,100 | [73] |
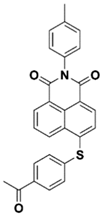 NAS2 | λmax ~ 385 ε405nm ~ 9300 | [73] |
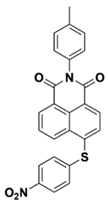 NAS3 | λmax ~ 340 ε405nm ~ 6100 | [73] |
 NAS4 | λmax ~ 387 ε405nm ~ 12,800 | [73] |
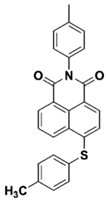 NAS5 | λmax ~ 391 ε405nm ~ 12,600 | [73] |
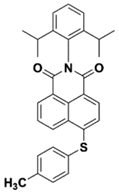 NAS6 | λmax ~ 395 ε405nm ~ 11,700 | [73] |
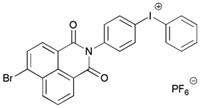 Naphth-Iod | λmax ~ 340 | [75] |
2.4. Coumarine-Based Photoinitiators
| Chemical Structures | Absorption Properties (λ/nm, εmax/M−1 cm−1) | Refs. |
|---|---|---|
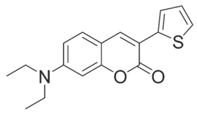 CoumA | λmax ~ 421 εmax ~ 35,200 ε405nm ~ 30,600 | [77] |
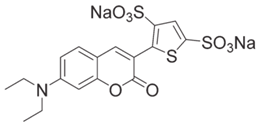 CoumB | λmax ~ 405 εmax ~ 28,100 ε405nm ~ 28,100 | [77] |
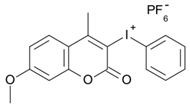 7M-P | λmax ~ 350 εmax ~ 20,440 ε365nm ~ 16,660 ε405nm ~ 280 | [78] |
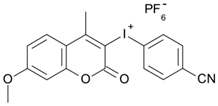 7M-CN-P | λmax ~ 352 εmax ~ 18,930 ε365nm ~ 16,800 ε405nm ~ 360 | [78] |
 7M-NO2-P | λmax ~ 351 εmax ~ 19,100 ε365nm ~ 16,770 ε405nm ~ 460 | [78] |
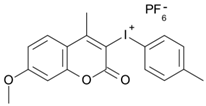 7M-Me-P | λmax ~ 349 εmax ~ 22,700 ε365nm ~ 18,200 ε405nm ~ 240 | [78] |
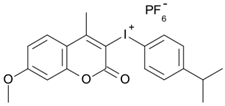 7M-iPr-P | λmax ~ 350 εmax ~ 18,440 ε365nm ~ 14,960 ε405nm ~ 220 | [78] |
2.5. Chalcone-Based Photoinitiators
| Chemical Structures | Absorption Properties (λ/nm, εmax/M−1 cm−1) | Refs. |
|---|---|---|
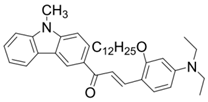 Chalcone 1 | λmax ~ 425 εmax ~ 8930 ε405nm ~ 7450 | [85] |
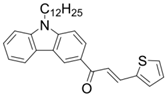 Chalcone 2 | λmax ~ 369 εmax ~ 20,520 ε405nm ~ 4830 | [85] |
 Chalcone 3 | λmax ~ 369 εmax ~ 17,740 ε405nm ~ 5960 | [85] |
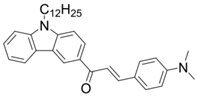 Chalcone 4 | λmax ~ 408 εmax ~ 23,900 ε405nm ~ 23,580 | [85] |
 Chalcone 5 | λmax ~ 370 εmax ~ 21,100 ε405nm ~ 7020 | [85] |
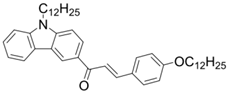 Chalcone 6 | λmax ~ 360 εmax ~ 24,900 ε405nm ~ 3530 | [85] |
 Chalcone 7 | λmax ~ 405 εmax ~ 18,740 ε405nm ~ 18,740 | [85] |
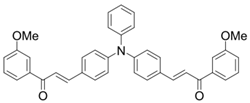 Chalcone 8 | λmax ~ 430 εmax ~ 7990 ε405nm ~ 6760 | [85] |
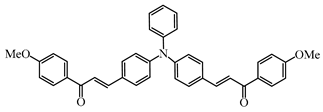 Chalcone 9 | λmax ~ 428 εmax ~ 8540 ε405nm ~ 7200 | [85] |
 Chalcone 10 | λmax ~ 430 εmax ~ 10,500 ε405nm ~ 9020 | [85] |
 Bis-chalcone 1 | λmax ~ 370 εmax ~ 21,900 ε405nm ~ 12,740 | [86] |
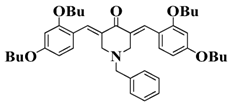 Bis-chalcone 2 | λmax ~ 380 εmax ~ 28,200 ε405nm ~ 19,730 | [86] |
 Bis-chalcone 3 | λmax ~ 332 εmax ~ 18,200 ε405nm ~ 4420 | [86] |
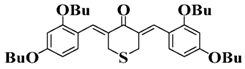 Bis-chalcone 4 | λmax ~ 369 εmax ~ 4980 ε405nm ~ 2550 | [86] |
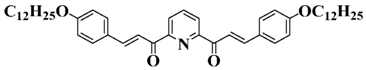 Bis-chalcone 5 | λmax ~ 347 εmax ~ 23,100 ε405nm ~ 6800 | [86] |
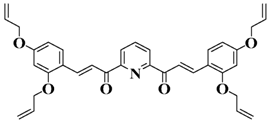 Bis-chalcone 6 | λmax ~ 364 εmax ~ 22,700 ε405nm ~ 10,070 | [86] |
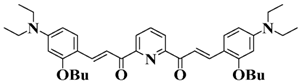 Bis-chalcone 7 | λmax ~ 430 εmax ~ 38,900 ε405nm ~ 26,420 | [86] |
 Bis-chalcone8 | λmax ~ 330 εmax ~ 19,800 ε405nm ~ 2960 | [86] |
 Bis-chalcone 9 | λmax ~ 370 εmax ~ 24,600 ε405nm ~ 13,670 | [86] |
 Bis-chalcone 10 | λmax ~ 350 εmax ~ 49,300 ε405nm ~ 2220 | [86] |
3. Type Ⅱ Photoinitiators
3.1. Benzophenone Photoinitiators
| Chemical Structures | Absorption Properties (λ/nm, εmax/M−1 cm−1) | Refs. |
|---|---|---|
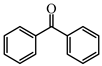 BP | λmax ~ 253 εmax ~ 22,000 ε363nm ~250 | [104,107] |
 BPB | λmax ~ 290 εmax ~ 14,610 | [100] |
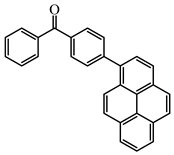 Py_BP | λmax ~ 348 εmax ~ 26,000 ε405nm ~1400 | [103] |
 Py_BP5 | λmax ~ 395 εmax ~ 4400 | [104] |
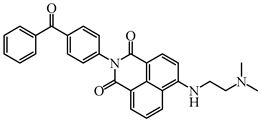 BPND | λmax ~ 431 εmax ~ 15,700 ε405nm ~ 10,900 ε470nm ~ 5700 | [106] |
 BP1 | λmax ~ 369 εmax ~ 22,300 | [107] |
 BP2 | λmax ~ 341 εmax ~ 13,300 | [107] |
 BP3 | λmax ~ 368 εmax ~ 20,200 | [107] |
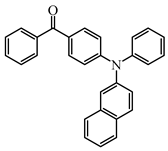 BP4 | λmax ~ 369 εmax ~ 42,900 | [107] |
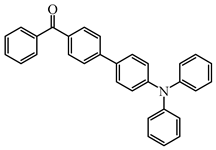 BP5 | λmax ~ 374 εmax ~ 27,800 | [107] |
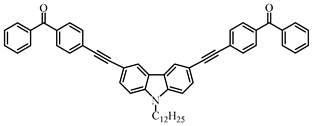 A4 | λmax ~ 349 εmax ~ 18,000 | [108] |
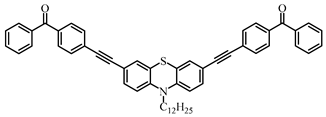 A3–1 | λmax ~ 350 εmax ~ 49,000 | [108] |
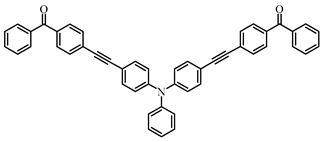 A3–2 | λmax ~ 360 εmax ~ 53,000 | [108] |
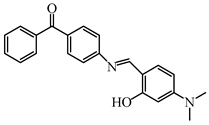 BPN | λmax ~ 400 εmax ~ 43,700 ε405nm ~ 42,400 | [109] |
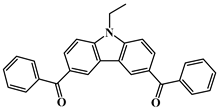 BPC | λmax ~ 342 εmax ~ 18,600 ε365nm ~ 6000 | [110] |
 BPC1 | λmax ~ 334 εmax ~ 13,910 ε365nm ~ 3270 | [111] |
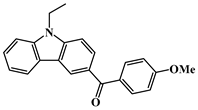 BPC2 | λmax ~ 325 εmax ~ 13,900 ε365nm ~ 2210 | [111] |
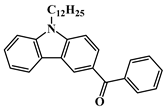 BPC3 | λmax ~ 334 εmax ~ 13,350 ε365nm ~ 3460 | [111] |
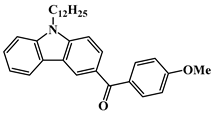 BPC4 | λmax ~ 325 εmax ~ 12,400 ε365nm ~ 2170 | [111] |
 BT1 | λmax ~ 359 εmax ~ 21,000 ε405nm ~ 1800 | [112] |
 BT2 | λmax ~ 373 εmax ~ 27,200 ε405nm ~ 5000 | [112] |
 BT3 | λmax ~ 370 εmax ~ 41,600 ε405nm ~ 6100 | [112] |
 BT4 | λmax ~ 377 εmax ~ 21,700 ε405nm ~ 6700 | [112] |
3.2. Thioxanthone Photoinitiators
| Chemical Structures | Absorption Properties (λ/nm, εmax/M−1 cm−1) | Refs. |
|---|---|---|
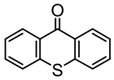 TX | λmax ~ 380 εmax ~ 5300 | [131] |
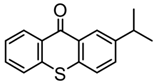 ITX | λmax ~ 386 εmax ~ 6500 ε395nm ~ 3900 | [132] |
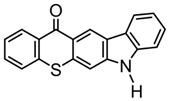 TX-C | λmax ~ 434 εmax ~ 2010 | [118,119] |
 TX-A | λmax ~ 368 εmax ~ 14,000 | [120,121] |
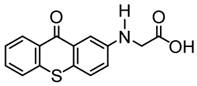 TX-NPG | λmax ~ 392,583 ε392nm ~ 1670 ε583nm ~ 440 | [124] |
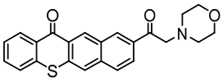 TX-MPM | λmax ~ 410 εmax ~ 4390 | [126] |
 TX-1 | λmax ~ 438 εmax ~ 4400 | [127] |
 TX-2 | λmax ~ 438 εmax ~ 4800 | [127] |
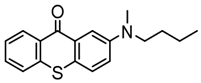 TX-3 | λmax ~ 444 εmax ~ 4100 | [127] |
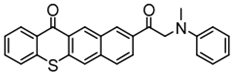 TX-MPA | λmax ~ 407 εmax ~ 3610 | [128] |
 TX-2DPA | λmax ~ 478 εmax ~ 3400 ε405nm ~ 2000 | [129] |
 TX-2CBZ | λmax ~ 396 εmax ~ 7900 ε405nm ~ 5900 | [129] |
 TX-2PTZ | λmax ~ 305,415 ε305nm ~ 2400 ε405nm ~ 2600 | [129] |
4. Type Ⅰ Photoinitiators
4.1. Oxime Ester Photoinitiators
| Structure | Absorption Properties (λ/nm, εmax/M−1 cm−1) | Refs. |
|---|---|---|
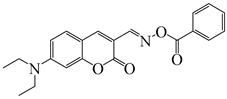 O-3 | λmax ~ 436 εmax ~ 41,690 ε450nm ~ 37,450 | [136] |
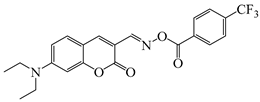 O-3F | λmax ~ 436 εmax ~ 29,930 ε450nm ~ 26,630 | [136] |
 O-3O | λmax ~ 436 εmax ~ 29,950 ε450nm ~ 26,620 | [136] |
 O-4 | λmax ~ 433 εmax ~ 7680 ε450nm ~ 6790 | [136] |
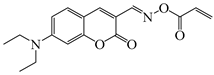 DCCA | λmax ~ 436 εmax ~ 51,000 ε450nm ~ 45,000 | [138] |
 OEC3–1 | λmax ~ 406 εmax ~ 51,000 ε450nm ~ 21,000 | [139] |
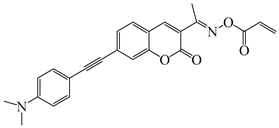 OEC3–2 | λmax ~ 405 εmax ~ 42,000 ε450nm ~ 17,000 | [139] |
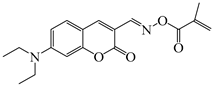 OXE-B | λmax ~ 431 εmax ~ 33,000 ε405nm ~ 22,000 | [140] |
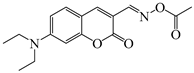 OXE-D | λmax ~ 431 εmax ~ 34,000 ε405nm ~ 22,500 | [140] |
 OXE-E | λmax ~ 437 εmax ~ 28,500 ε405nm ~ 16,500 | [140] |
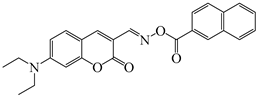 OXE-F | λmax ~ 437 εmax ~ 36,000 ε405nm ~ 21,000 | [140] |
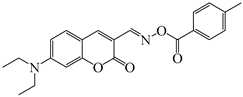 OXE-G | λmax ~ 436 εmax ~ 31,500 ε405nm ~ 18,000 | [140] |
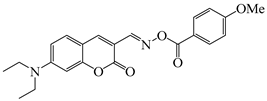 OXE-H | λmax ~ 435 εmax ~ 26,500 ε405nm ~ 17,000 | [140] |
 OXE-I | λmax ~ 437 | [140] |
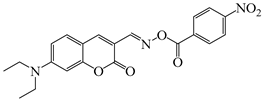 OXE-J | λmax ~ 441 εmax ~ 50,000 ε405nm ~ 25,000 | [140] |
 OXE-K | λmax ~ 435 εmax ~ 31,000 ε405nm ~ 18,000 | [140] |
 OXE1 | λmax ~ 350 εmax ~ 46,900 ε405nm ~ 1100 | [141] |
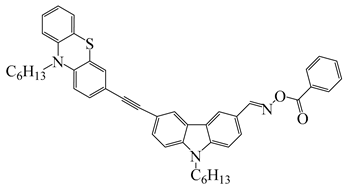 OXE2 | λmax ~ 357 εmax ~ 29,300 ε405nm ~ 4410 | [141] |
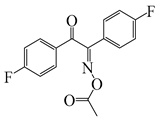 E-FBOXE-Me | λmax ~ 260 ε260nm ~ 17,980 ε395nm ~ 20 | [142] |
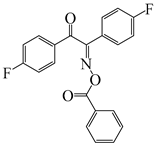 E-FBOXE-Ph | λmax ~ 262 ε260nm ~ 51,410 ε395nm ~ 50 | [142] |
 OXE-EM | λmax ~ 374 εmax ~ 16,400 ε355nm ~ 12,500 | [143] |
 OXE-IM | λmax ~ 374 εmax ~ 16,000 ε355nm ~ 12,800 | [143] |
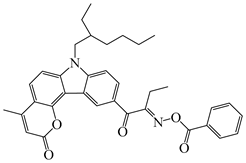 OXE-EP | λmax ~ 374 εmax ~ 17,000 ε355nm ~ 13,200 | [143] |
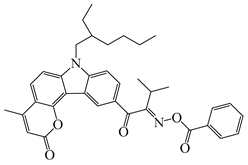 OXE-IP | λmax ~ 374 εmax ~ 16,600 ε355nm ~ 13,000 | [143] |
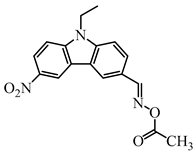 OXE-M | λmax ~ 369 εmax ~ 13,000 ε405nm ~ 4100 | [147] |
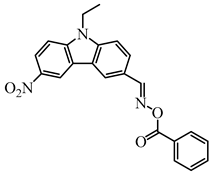 OXE-P | λmax ~ 368 εmax ~ 13,800 ε405nm ~ 4100 | [147] |
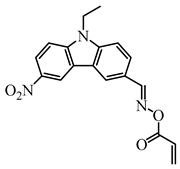 OXE-V | λmax ~ 369 εmax ~ 12,400 ε405nm ~ 3900 | [147] |
 D1 | λmax ~ 372 εmax ~ 13,000 ε405nm ~ 5200 | [148] |
 D2 | λmax ~ 372 εmax ~ 14,000 ε405nm ~ 5400 | [148] |
4.2. Acylphosphine Oxide Photoinitiators
| Structure | Absorption Properties (λ/nm, εmax/M−1 cm−1) | Refs. |
|---|---|---|
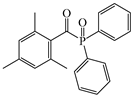 TPO | λmax ~ 380 εmax ~ 570 ε385nm ~ 510 ε420nm ~ 20 | [150] |
 TPO-L | λmax ~ 383 ε395nm ~ 130 | [149] |
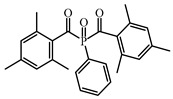 BAPO | λmax ~ 384 ε395nm ~ 660 | [149] |
 ADPO-1 | λmax ~ 373 ε395nm ~ 300 | [149] |
 ADPO-2 | λmax ~ 379 ε395nm ~ 390 | [149] |
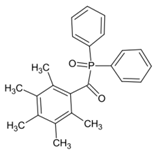 ADPO-3 | λmax ~ 382 ε395nm ~ 1060 | [149] |
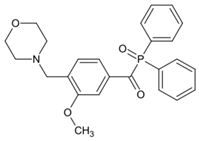 ADPO-4 | λmax ~ 386 ε395nm ~ 160 | [149] |
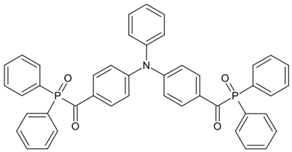 ADPO-5 | λmax ~ 405 ε395nm ~ 26,600 | [149] |
 ADPO-6 | λmax ~ 405 | [149] |
 ADPO-7 | λmax ~ 384 | [149] |
 DEAPO | λmax ~ 386 εmax ~ 43,810 ε385nm ~ 43,800 ε420nm ~ 5950 | [150] |
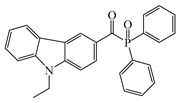 ETPO | λmax ~ 366 εmax ~ 13,830 ε395nm ~ 4530 ε405nm ~ 2270 | [151] |
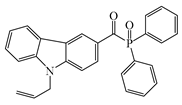 ALPO | λmax ~ 362 εmax ~ 12,200 ε395nm ~ 2890 ε405nm ~ 1300 | [151] |
4.3. Other Type I photoinitiators
| Structure | Absorption Properties (λ/nm, εmax/M−1 cm−1) | Refs. |
|---|---|---|
 Ivocerin® | λmax ~ 408 εmax ~ 711 ε385nm ~ 505 | [157] |
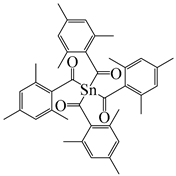 tetrakis(2,4,6-trimethylbenzoyl)stannane | [152] | |
 NPIE1 | λmax ~ 397 εmax ~ 16,000 ε405nm ~ 15,000 | [156] |
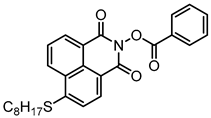 NPIE2 | λmax ~ 398 εmax ~ 15,200 ε405nm ~ 14,400 | [156] |
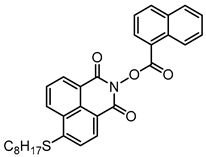 NPIE3 | λmax ~ 398 εmax ~ 15,600 ε405nm ~ 14,900 | [156] |
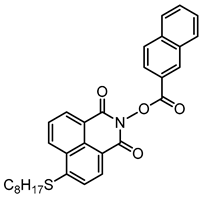 NPIE4 | λmax ~ 397 εmax ~ 14,100 ε405nm ~ 13,100 | [156] |
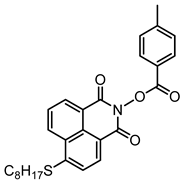 NPIE5 | λmax ~ 397 εmax ~ 15,300 ε405nm ~ 14,500 | [156] |
 NPIE6 | λmax ~ 397 εmax ~ 14,700 ε405nm ~ 13,800 | [156] |
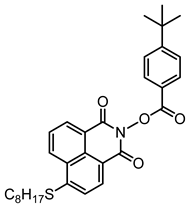 NPIE7 | λmax ~ 397 εmax ~ 13,500 ε405nm ~ 12,800 | [156] |
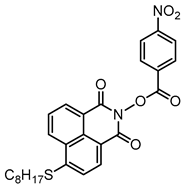 NPIE8 | λmax ~ 397 εmax ~ 12,900 ε405nm ~ 12,000 | [156] |
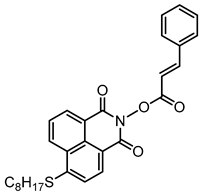 NPIE9 | λmax ~ 397 εmax ~ 14,900 ε405nm ~ 14,000 | [156] |
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corrigan, N.; Yeow, J.; Judzewitsch, P.; Xu, J.; Boyer, C. Seeing the Light: Advancing Materials Chemistry through Photopolymerization. Angew. Chem. Int. Ed. 2019, 58, 5170–5189. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, E. Photopolymerization kinetics of multifunctional monomers. Prog. Polym. Sci. 2001, 26, 605–665. [Google Scholar] [CrossRef]
- Chen, M.; Zhong, M.; Johnson, J.A. Light-Controlled Radical Polymerization: Mechanisms, Methods, and Applications. Chem. Rev. 2016, 116, 10167–10211. [Google Scholar] [CrossRef] [PubMed]
- Dumur, F. Recent advances on benzylidene ketones as photoinitiators of polymerization. Eur. Polym. J. 2022, 178, 111500. [Google Scholar] [CrossRef]
- Yilmaz, G.; Yagci, Y. Light-induced step-growth polymerization. Prog. Polym. Sci. 2020, 100, 101178. [Google Scholar] [CrossRef]
- Khudyakov, I.V. Fast photopolymerization of acrylate coatings: Achievements and problems. Prog. Org. Coat. 2018, 121, 151–159. [Google Scholar] [CrossRef]
- Sangermano, M.; Pegel, S.; Pötschke, P.; Voit, B. Antistatic Epoxy Coatings With Carbon Nanotubes Obtained by Cationic Photopolymerization. Macromol. Rapid Commun. 2008, 29, 396–400. [Google Scholar] [CrossRef]
- Gam-Derouich, S.; Carbonnier, B.; Turmine, M.; Lang, P.; Jouini, M.; Hassen-Chehimi, D.B.; Chehimi, M.M. Electrografted aryl diazonium initiators for surface-confined photopolymerization: a new approach to designing functional polymer coatings. Langmuir 2010, 26, 11830–11840. [Google Scholar] [CrossRef] [PubMed]
- Nunes, T.G.; Ceballos, L.; Osorio, R.; Toledano, M. Spatially resolved photopolymerization kinetics and oxygen inhibition in dental adhesives. Biomaterials 2005, 26, 1809–1817. [Google Scholar] [CrossRef]
- Daniloska, V.; Carretero, P.; Tomovska, R.; Asua, J.M. High performance pressure sensitive adhesives by miniemulsion photopolymerization in a continuous tubular reactor. Polymer 2014, 55, 5050–5056. [Google Scholar] [CrossRef]
- Besse, V.; Derbanne, M.A.; Pham, T.N.; Cook, W.D.; Le Pluart, L. Photopolymerization study and adhesive properties of self-etch adhesives containing bis(acyl)phosphine oxide initiator. Dent. Mater. 2016, 32, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef]
- Layani, M.; Wang, X.; Magdassi, S. Novel Materials for 3D Printing by Photopolymerization. Adv. Mater. 2018, 30, 1706344. [Google Scholar] [CrossRef] [PubMed]
- Aduba, D.C.; Margaretta, E.D.; Marnot, A.E.C.; Heifferon, K.V.; Surbey, W.R.; Chartrain, N.A.; Whittington, A.R.; Long, T.E.; Williams, C.B. Vat photopolymerization 3D printing of acid-cleavable PEG-methacrylate networks for biomaterial applications. Mater. Today Commun. 2019, 19, 204–211. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, Y.; Li, M.D.; Li, Z.; Peng, H.; Xie, T.; Xie, X. Efficient 3D printing via photooxidation of ketocoumarin based photopolymerization. Nat. Commun. 2021, 12, 2873. [Google Scholar] [CrossRef]
- Jandt, K.D.; Mills, R.W. A brief history of LED photopolymerization. Dent. Mater. 2013, 29, 605–617. [Google Scholar] [CrossRef]
- Weems, A.C.; Chiaie, K.R.D.; Yee, R.; Dove, A.P. Selective Reactivity of Myrcene for Vat Photopolymerization 3D Printing and Postfabrication Surface Modification. Biomacromolecules 2020, 21, 163–170. [Google Scholar] [CrossRef]
- Yang, H.; Li, G.; Stansbury, J.W.; Zhu, X.; Wang, X.; Nie, J. Smart Antibacterial Surface Made by Photopolymerization. ACS Appl. Mater. Interfaces 2016, 8, 28047–28054. [Google Scholar] [CrossRef] [PubMed]
- Gibson, I.; Rosen, D.; Stucker, B. Vat Photopolymerization Processes. Additive Manufacturing Technologies; Springer: New York, NY, USA, 2015; pp. 63–106. [Google Scholar]
- Vitale, A.; Priola, A.; Tonelli, C.; Bongiovanni, R. Nanoheterogeneous networks by photopolymerization of perfluoropolyethers and acrylic co-monomers. Polym. Int. 2013, 62, 1395–1401. [Google Scholar] [CrossRef]
- Crivello, J.V.; Reichmanis, E. Photopolymer Materials and Processes for Advanced Technologies. Chem. Mater. 2013, 26, 533–548. [Google Scholar] [CrossRef]
- Zhou, J.; Allonas, X.; Ibrahim, A.; Liu, X. Progress in the development of polymeric and multifunctional photoinitiators. Prog. Polym. Sci. 2019, 99, 101165. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Doran, S.; Yagci, Y. Photoinduced Electron Transfer Reactions for Macromolecular Syntheses. Chem. Rev. 2016, 116, 10212–10275. [Google Scholar] [CrossRef] [PubMed]
- Christmann, J.; Ley, C.; Allonas, X.; Ibrahim, A.; Croutxé-Barghorn, C. Experimental and theoretical investigations of free radical photopolymerization: Inhibition and termination reactions. Polymer 2019, 160, 254–264. [Google Scholar] [CrossRef]
- Lin, J.T.; Cheng, D.C.; Chen, K.T.; Chiu, Y.C.; Liu, H.W. Enhancing UV Photopolymerization by a Red-light Preirradiation: Kinetics and Modeling Strategies for Reduced Oxygen Inhibition. J. Polym. Sci. 2020, 58, 683–691. [Google Scholar] [CrossRef]
- Courtecuisse, F.; Karasu, F.; Allonas, X.; Croutxé-Barghorn, C.; van der Ven, L. Confocal Raman microscopy study of several factors known to influence the oxygen inhibition of acrylate photopolymerization under LED. Prog. Org. Coat. 2016, 92, 1–7. [Google Scholar] [CrossRef]
- Ma, Q.; Song, J.; Zhang, X.; Jiang, Y.; Ji, L.; Liao, S. Metal-free atom transfer radical polymerization with ppm catalyst loading under sunlight. Nat. Commun. 2021, 12, 429. [Google Scholar] [CrossRef]
- Lecamp, L.; Lebaudy, P.; Youssef, B.; Bunel, C. Influence of UV radiation wavelength on conversion and temperature distribution profiles within dimethacrylate thick material during photopolymerization. Polymer 2001, 42, 8541–8547. [Google Scholar] [CrossRef]
- Li, Z.; Shen, W.; Liu, X.; Liu, R. Efficient unimolecular photoinitiators for simultaneous hybrid thiol–yne–epoxy photopolymerization under visible LED light irradiation. Polym. Chem. 2017, 8, 1579–1588. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Simon-Masseron, A.; Lalevee, J. Radical photoinitiation with LEDs and applications in the 3D printing of composites. Chem. Soc. Rev. 2021, 50, 3824–3841. [Google Scholar] [CrossRef] [PubMed]
- Christmann, J.; Allonas, X.; Ley, C.; Ibrahim, A.; Croutxé-Barghorn, C. Triazine-Based Type-II Photoinitiating System for Free Radical Photopolymerization: Mechanism, Efficiency, and Modeling. Macromol. Chem. Phys. 2017, 218, 1600597. [Google Scholar] [CrossRef]
- Dietlin, C.; Schweizer, S.; Xiao, P.; Zhang, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P.; Lalevée, J. Photopolymerization upon LEDs: new photoinitiating systems and strategies. Polym. Chem. 2015, 6, 3895–3912. [Google Scholar] [CrossRef]
- Kocaarslan, A.; Kütahya, C.; Keil, D.; Yagci, Y.; Strehmel, B. Near-IR and UV-LED Sensitized Photopolymerization with Onium Salts Comprising Anions of Different Nucleophilicities. ChemPhotoChem 2019, 3, 1127–1132. [Google Scholar] [CrossRef]
- Zuo, X.; Morlet-Savary, F.; Schmitt, M.; Le Nouën, D.; Blanchard, N.; Goddard, J.-P.; Lalevée, J. Novel applications of fluorescent brighteners in aqueous visible-light photopolymerization: high performance water-based coating and LED-assisted hydrogel synthesis. Polym. Chem. 2018, 9, 3952–3958. [Google Scholar] [CrossRef]
- Schmitt, M. Method to analyse energy and intensity dependent photo-curing of acrylic esters in bulk. RSC Adv. 2015, 5, 67284–67298. [Google Scholar] [CrossRef]
- Crivello, J.V. A new visible light sensitive photoinitiator system for the cationic polymerization of epoxides. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 866–875. [Google Scholar] [CrossRef]
- Oprych, D.; Schmitz, C.; Ley, C.; Allonas, X.; Ermilov, E.; Erdmann, R.; Strehmel, B. Photophysics of Up-Conversion Nanoparticles: Radical Photopolymerization of Multifunctional Methacrylates Comprising Blue- and UV-Sensitive Photoinitiators. ChemPhotoChem 2019, 3, 1119–1126. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Poriel, C.; Dumur, F.; Toufaily, J.; Hamieh, T.; Fouassier, J.P.; Lalevée, J. Zinc Tetraphenylporphyrin as High Performance Visible Light Photoinitiator of Cationic Photosensitive Resins for LED Projector 3D Printing Applications. Macromolecules 2017, 50, 746–753. [Google Scholar] [CrossRef]
- Li, J.; Hao, Y.; Zhong, M.; Tang, L.; Nie, J.; Zhu, X. Synthesis of furan derivative as LED light photoinitiator: One-pot, low usage, photobleaching for light color 3D printing. Dye. Pigment. 2019, 165, 467–473. [Google Scholar] [CrossRef]
- Zhang, J.; Frigoli, M.; Dumur, F.; Xiao, P.; Ronchi, L.; Graff, B.; Morlet-Savary, F.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Design of Novel Photoinitiators for Radical and Cationic Photopolymerizations under Near UV and Visible LEDs (385, 395, and 405 nm). Macromolecules 2014, 47, 2811–2819. [Google Scholar] [CrossRef]
- Yang, J.; Liao, W.; Xiong, Y.; Wang, X.; Li, Z.; Tang, H. A multifunctionalized macromolecular silicone-naphthalimide visible photoinitiator for free radical polymerization. Prog. Org. Coat. 2018, 115, 151–158. [Google Scholar] [CrossRef]
- Garra, P.; Fouassier, J.P.; Lakhdar, S.; Yagci, Y.; Lalevée, J. Visible light photoinitiating systems by charge transfer complexes: Photochemistry without dyes. Prog. Polym. Sci. 2020, 107, 101277. [Google Scholar] [CrossRef]
- Shi, S.; Croutxé-Barghorn, C.; Allonas, X. Photoinitiating systems for cationic photopolymerization: Ongoing push toward long wavelengths and low light intensities. Prog. Polym. Sci. 2017, 65, 1–41. [Google Scholar] [CrossRef]
- Xue, T.; Huang, B.; Li, Y.; Li, X.; Nie, J.; Zhu, X. Enone dyes as visible photoinitiator in radical polymerization: The influence of peripheral N-alkylated (hetero)aromatic amine group. J. Photochem. Photobiol. A Chem. 2021, 419, 113449. [Google Scholar] [CrossRef]
- Li, F.; Song, Y.; Yao, M.; Nie, J.; He, Y. Design and properties of novel photothermal initiators for photoinduced thermal frontal polymerization. Polym. Chem. 2020, 11, 3980–3986. [Google Scholar] [CrossRef]
- Yao, M.; Liu, S.; Huang, C.; Nie, J.; He, Y. Significantly improve the photoinitiation ability of hydroxyalkyl-derived polymerizable α-hydroxyalkylacetophenone photoinitiators by blocking hyperconjugation. J. Photochem. Photobiol. A Chem. 2021, 419, 113451. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Blue-to-Red Light Sensitive Push–Pull Structured Photoinitiators: Indanedione Derivatives for Radical and Cationic Photopolymerization Reactions. Macromolecules 2013, 46, 3332–3341. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.-A.; Peter, M.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.P. Efficient dual radical/cationic photoinitiator under visible light: a new concept. Polym. Chem. 2011, 2, 1986–1991. [Google Scholar] [CrossRef]
- Breloy, L.; Brezová, V.; Blacha-Grzechnik, A.; Presset, M.; Yildirim, M.S.; Yilmaz, I.; Yagci, Y.; Versace, D.-L. Visible Light Anthraquinone Functional Phthalocyanine Photoinitiator for Free-Radical and Cationic Polymerizations. Macromolecules 2019, 53, 112–124. [Google Scholar] [CrossRef]
- Liao, W.; Liao, Q.; Xiong, Y.; Li, Z.; Tang, H. Design, synthesis and properties of carbazole-indenedione based photobleachable photoinitiators for photopolymerization. J. Photochem. Photobiol. A Chem. 2023, 435, 114297. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, J.; Dumur, F.; Tehfe, M.A.; Morlet-Savary, F.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Visible light sensitive photoinitiating systems: Recent progress in cationic and radical photopolymerization reactions under soft conditions. Prog. Polym. Sci. 2015, 41, 32–66. [Google Scholar] [CrossRef]
- Allushi, A.; Kutahya, C.; Aydogan, C.; Kreutzer, J.; Yilmaz, G.; Yagci, Y. Conventional Type II photoinitiators as activators for photoinduced metal-free atom transfer radical polymerization. Polym. Chem. 2017, 8, 1972–1977. [Google Scholar] [CrossRef]
- Schmitt, M.; Dietlin, C.; Lalevée, J. Towards Visible LED Illumination: ZnO-ZnS Nanocomposite Particles. ChemistrySelect 2020, 5, 985–987. [Google Scholar] [CrossRef]
- Schmitt, M.; Garra, P.; Lalevée, J. Bulk Polymerization Photo-Initiator ZnO: Increasing of the Benzoyl Formic Acid Concentration and LED Illumination. Macromol. Chem. Phys. 2018, 219, 1800208. [Google Scholar] [CrossRef]
- Tasdelen, M.A.; Lalevée, J.; Yagci, Y. Photoinduced free radical promoted cationic polymerization 40 years after its discovery. Polym. Chem. 2020, 11, 1111–1121. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances on carbazole-based photoinitiators of polymerization. Eur. Polym. J. 2020, 125, 109503. [Google Scholar] [CrossRef]
- Abdallah, M.; Bui, T.-T.; Goubard, F.; Theodosopoulou, D.; Dumur, F.; Hijazi, A.; Fouassier, J.-P.; Lalevée, J. Phenothiazine derivatives as photoredox catalysts for cationic and radical photosensitive resins for 3D printing technology and photocomposite synthesis. Polym. Chem. 2019, 10, 6145–6156. [Google Scholar] [CrossRef]
- Sun, K.; Liu, S.; Chen, H.; Morlet-Savary, F.; Graff, B.; Pigot, C.; Nechab, M.; Xiao, P.; Dumur, F.; Lalevée, J. N-ethyl carbazole-1-allylidene-based push-pull dyes as efficient light harvesting photoinitiators for sunlight induced polymerization. Eur. Polym. J. 2021, 147, 110331. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Dumur, F.; Garra, P.; Toufaily, J.; Hamieh, T.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Carbazole Scaffold Based Photoinitiator/Photoredox Catalysts: Toward New High Performance Photoinitiating Systems and Application in LED Projector 3D Printing Resins. Macromolecules 2017, 50, 2747–2758. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Lara, D.M.; Noirbent, G.; Dumur, F.; Toufaily, J.; Hamieh, T.; Bui, T.-T.; Goubard, F.; Graff, B.; Gigmes, D.; et al. Carbazole Derivatives with Thermally Activated Delayed Fluorescence Property as Photoinitiators/Photoredox Catalysts for LED 3D Printing Technology. Macromolecules 2017, 50, 4913–4926. [Google Scholar] [CrossRef]
- Li, Z.; Hu, P.; Zhu, J.; Gao, Y.; Xiong, X.; Liu, R. Conjugated Carbazole-Based Schiff Bases as Photoinitiators: From Facile Synthesis to Efficient Two-Photon Polymerization. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 2692–2700. [Google Scholar] [CrossRef]
- Han, W.; Fu, H.; Xue, T.; Liu, T.; Wang, Y.; Wang, T. Facilely prepared blue-green light sensitive curcuminoids with excellent bleaching properties as high performance photosensitizers in cationic and free radical photopolymerization. Polym. Chem. 2018, 9, 1787–1798. [Google Scholar] [CrossRef]
- Abdallah, M.; Dumur, F.; Graff, B.; Hijazi, A.; Lalevée, J. High performance dyes based on triphenylamine, cinnamaldehyde and indane-1,3-dione derivatives for blue light induced polymerization for 3D printing and photocomposites. Dye. Pigment. 2020, 182, 108580. [Google Scholar] [CrossRef]
- Li, Y.-H.; Chen, Y.-C. Triphenylamine-hexaarylbiimidazole derivatives as hydrogen-acceptor photoinitiators for free radical photopolymerization under UV and LED light. Polym. Chem. 2020, 11, 1504–1513. [Google Scholar] [CrossRef]
- Jin, M.; Wu, X.; Malval, J.P.; Wan, D.; Pu, H. Dual roles for promoting monomers to polymers: A conjugated sulfonium salt photoacid generator as photoinitiator and photosensitizer in cationic photopolymerization. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2722–2730. [Google Scholar] [CrossRef]
- Wang, C.; Meng, X.; Li, Z.; Li, M.; Jin, M.; Liu, R.; Yagci, Y. Chemiluminescence Induced Cationic Photopolymerization Using Sulfonium Salt. ACS Macro Lett. 2020, 9, 471–475. [Google Scholar] [CrossRef]
- Noirbent, G.; Dumur, F. Recent advances on naphthalic anhydrides and 1,8-naphthalimide-based photoinitiators of polymerization. Eur. Polym. J. 2020, 132, 109702. [Google Scholar] [CrossRef]
- Zivic, N.; Kuroishi, P.K.; Dumur, F.; Gigmes, D.; Dove, A.P.; Sardon, H. Recent Advances and Challenges in the Design of Organic Photoacid and Photobase Generators for Polymerizations. Angew. Chem. Int. Ed. 2019, 58, 10410–10422. [Google Scholar] [CrossRef]
- Yang, J.; Xu, C.; Xiong, Y.; Wang, X.; Xie, Y.; Li, Z.; Tang, H. A Green and Highly Efficient Naphthalimide Visible Photoinitiator with an Ability Initiating Free Radical Polymerization under Air. Macromol. Chem. Phys. 2018, 219, 1800256. [Google Scholar] [CrossRef]
- Kanji, S.; Masamitsu, S. Photobase generators: Recent progress and application trend in polymer systems. Prog. Polym. Sci. 2009, 34, 194–209. [Google Scholar]
- Zhang, J.; Dumur, F.; Xiao, P.; Graff, B.; Bardelang, D.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Structure Design of Naphthalimide Derivatives: Toward Versatile Photoinitiators for Near-UV/Visible LEDs, 3D Printing, and Water-Soluble Photoinitiating Systems. Macromolecules 2015, 48, 2054–2063. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Naphthalimide Derivatives: Substituent Effects on the Photoinitiating Ability in Polymerizations under Near UV, Purple, White and Blue LEDs (385, 395, 405, 455, or 470 nm). Macromol. Chem. Phys. 2015, 216, 1782–1790. [Google Scholar] [CrossRef]
- Yu, J.; Gao, Y.; Jiang, S.; Sun, F. Naphthalimide Aryl Sulfide Derivative Norrish Type I Photoinitiators with Excellent Stability to Sunlight under Near-UV LED. Macromolecules 2019, 52, 1707–1717. [Google Scholar] [CrossRef]
- Suga, T.; Shimazu, S.; Ukaji, Y. Low-Valent Titanium-Mediated Radical Conjugate Addition Using Benzyl Alcohols as Benzyl Radical Sources. Org. Lett. 2018, 20, 5389–5392. [Google Scholar] [CrossRef] [PubMed]
- Zivic, N.; Bouzrati-Zerrelli, M.; Villotte, S.; Morlet-Savary, F.; Dietlin, C.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. A novel naphthalimide scaffold based iodonium salt as a one-component photoacid/photoinitiator for cationic and radical polymerization under LED exposure. Polym. Chem. 2016, 7, 5873–5879. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances on coumarin-based photoinitiators of polymerization. Eur. Polym. J. 2022, 163, 110962. [Google Scholar] [CrossRef]
- Abdallah, M.; Hijazi, A.; Graff, B.; Fouassier, J.-P.; Rodeghiero, G.; Gualandi, A.; Dumur, F.; Cozzi, P.G.; Lalevée, J. Coumarin derivatives as versatile photoinitiators for 3D printing, polymerization in water and photocomposite synthesis. Polym. Chem. 2019, 10, 872–884. [Google Scholar] [CrossRef]
- Topa, M.; Hola, E.; Galek, M.; Petko, F.; Pilch, M.; Popielarz, R.; Morlet-Savary, F.; Graff, B.; Lalevée, J.; Ortyl, J. One-component cationic photoinitiators based on coumarin scaffold iodonium salts as highly sensitive photoacid generators for 3D printing IPN photopolymers under visible LED sources. Polym. Chem. 2020, 11, 5261–5278. [Google Scholar] [CrossRef]
- Giacoletto, N.; Dumur, F. Recent Advances in bis-Chalcone-Based Photoinitiators of Polymerization: From Mechanistic Investigations to Applications. Molecules 2021, 26, 3192. [Google Scholar] [CrossRef]
- Sharma, V.S.; Sharma, A.S.; Agarwal, N.K.; Shah, P.A.; Shrivastav, P.S. Self-assembled blue-light emitting materials for their liquid crystalline and OLED applications: from a simple molecular design to supramolecular materials. Mol. Syst. Des. Eng. 2020, 5, 1691–1705. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Wang, B.-X.; Yang, Y.-S.; Liang, C.; Yang, C.; Chai, H.-L. Synthesis and self-assembly of chalcone-based organogels. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 449–455. [Google Scholar] [CrossRef]
- Sun, K.; Xiao, P.; Dumur, F.; Lalevée, J. Organic dye-based photoinitiating systems for visible-light-induced photopolymerization. J. Polym. Sci. 2021, 59, 1338–1389. [Google Scholar] [CrossRef]
- Ibrahim-Ouali, M.; Dumur, F. Recent advances on chalcone-based photoinitiators of polymerization. Eur. Polym. J. 2021, 158, 110688. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Sun, K.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. Design of photoinitiating systems based on the chalcone-anthracene scaffold for LED cationic photopolymerization and application in 3D printing. Eur. Polym. J. 2021, 147, 110300. [Google Scholar] [CrossRef]
- Chen, H.; Noirbent, G.; Zhang, Y.; Brunel, D.; Gigmes, D.; Morlet-Savary, F.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. Novel D–π-A and A–π-D–π-A three-component photoinitiating systems based on carbazole/triphenylamino based chalcones and application in 3D and 4D printing. Polym. Chem. 2020, 11, 6512–6528. [Google Scholar] [CrossRef]
- Chen, H.; Noirbent, G.; Liu, S.; Brunel, D.; Graff, B.; Gigmes, D.; Zhang, Y.; Sun, K.; Morlet-Savary, F.; Xiao, P.; et al. Bis-chalcone derivatives derived from natural products as near-UV/visible light sensitive photoinitiators for 3D/4D printing. Mater. Chem. Front. 2021, 5, 901–916. [Google Scholar] [CrossRef]
- Chen, H.; Noirbent, G.; Sun, K.; Brunel, D.; Gigmes, D.; Morlet-Savary, F.; Zhang, Y.; Liu, S.; Xiao, P.; Dumur, F.; et al. Photoinitiators derived from natural product scaffolds: monochalcones in three-component photoinitiating systems and their applications in 3D printing. Polym. Chem. 2020, 11, 4647–4659. [Google Scholar] [CrossRef]
- Chen, H.; Noirbent, G.; Zhang, Y.; Sun, K.; Liu, S.; Brunel, D.; Gigmes, D.; Graff, B.; Morlet-Savary, F.; Xiao, P.; et al. Photopolymerization and 3D/4D applications using newly developed dyes: Search around the natural chalcone scaffold in photoinitiating systems. Dye. Pigment. 2021, 188, 109213. [Google Scholar] [CrossRef]
- Arsu, N.; Balta, D.K.; Yagci, Y.; Jockusch, S.; Turro, N.J. Mechanistic Study of Photoinitiated Free Radical Polymerization Using Thioxanthone Thioacetic Acid as One-Component Type II Photoinitiator. Macromolecules 2005, 38, 4133–4138. [Google Scholar]
- Kork, S.; Yilmaz, G.; Yagci, Y. Poly(vinyl alcohol)-Thioxanthone as One-Component Type II Photoinitiator for Free Radical Polymerization in Organic and Aqueous Media. Macromol. Rapid Commun. 2015, 36, 923–928. [Google Scholar] [CrossRef]
- Arsu, N.; Cokbaglan, L.; Yagci, Y.; Jockusch, S.; Turro, N.J. 2-Mercaptothioxanthone as a Novel Photoinitiator for Free Radical Polymerization. Macromolecules 2003, 36, 2649–2653. [Google Scholar]
- Griesser, M.; Rosspeintner, A.; Dworak, C.; Hofer, M.; Grabner, G.; Liska, R.; Gescheidt, G. Initiators Based on Benzaldoximes: Bimolecular and Covalently Bound Systems. Macromolecules 2012, 45, 8648–8657. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.S.; Lam, E.; Howells, E.M.; Green, P.N.; Green, A.; Catalina, F.; Peinado, C. Photochemistry and photopolymerization activity of novel 4-alkylamino benzophenone initiators-synthesis, characterization, spectroscopic and photopolymerization activity. Eur. Polym. J. 1990, 26, 1345–1353. [Google Scholar] [CrossRef]
- Temel, G.; Enginol, B.; Aydin, M.; Balta, D.K.; Arsu, N. Photopolymerization and photophysical properties of amine linked benzophenone photoinitiator for free radical polymerization. J. Photochem. Photobiol. A Chem. 2011, 219, 26–31. [Google Scholar] [CrossRef]
- Jauk, S.; Liska, R. Photoinitiators with Functional Groups, 8. Macromol. Rapid Commun. 2005, 26, 1687–1692. [Google Scholar] [CrossRef]
- Jauk, S.; Liska, R. Photoinitiators with Functional Groups 9: New Derivatives of Covalently Linked Benzophenone-amine Based Photoinitiators. J. Macromol. Sci. Part A 2008, 45, 804–810. [Google Scholar] [CrossRef]
- Pietrzak, M.; Wrzyszczyński, A. Novel sulfur-containing benzophenone derivative as radical photoinitiator for photopolymerization. J. Appl. Polym. Sci. 2011, 122, 2604–2608. [Google Scholar] [CrossRef]
- Xiao, P.; Lalevée, J.; Allonas, X.; Fouassier, J.P.; Ley, C.; El-Roz, M.; Shi, S.Q.; Nie, J. Photoinitiation mechanism of free radical photopolymerization in the presence of cyclic acetals and related compounds. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 5758–5766. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, X.; Yin, J. Novel polymeric photoinitiators comprising of side-chain benzophenone and coinitiator amine: Photochemical and photopolymerization behaviors. Eur. Polym. J. 2009, 45, 437–447. [Google Scholar] [CrossRef]
- Temel, G.; Esen, D.S.; Arsu, N. One-component benzoxazine type photoinitiator for free radical polymerization. Polym. Eng. Sci. 2012, 52, 133–138. [Google Scholar] [CrossRef]
- Yang, J.; Shi, S.; Xu, F.; Nie, J. Synthesis and photopolymerization kinetics of benzophenone sesamol one-component photoinitiator. Photochem. Photobiol. Sci. 2013, 12, 323–329. [Google Scholar] [CrossRef]
- Balta, D.K.; Karahan, Ö.; Avci, D.; Arsu, N. Synthesis, photophysical and photochemical studies of benzophenone based novel monomeric and polymeric photoinitiators. Prog. Org. Coat. 2015, 78, 200–207. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Design of new Type I and Type II photoinitiators possessing highly coupled pyrene–ketone moieties. Polym. Chem. 2013, 4, 2313–2324. [Google Scholar] [CrossRef]
- Telitel, S.; Dumur, F.; Gigmes, D.; Graff, B.; Fouassier, J.P.; Lalevée, J. New functionalized aromatic ketones as photoinitiating systems for near visible and visible light induced polymerizations. Polymer 2013, 54, 2857–2864. [Google Scholar] [CrossRef]
- Lalevee, J.; Tehfe, M.A.; Dumur, F.; Gigmes, D.; Graff, B.; Morlet-Savary, F.; Fouassier, J.P. Light-harvesting organic photoinitiators of polymerization. Macromol. Rapid Commun. 2013, 34, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zivic, N.; Dumur, F.; Xiao, P.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. A benzophenone-naphthalimide derivative as versatile photoinitiator of polymerization under near UV and visible lights. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 445–451. [Google Scholar] [CrossRef]
- Huang, T.L.; Li, Y.-H.; Chen, Y.-C. Benzophenone derivatives as novel organosoluble visible light Type II photoinitiators for UV and LED photoinitiating systems. J. Polym. Sci. 2020, 58, 2914–2925. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, D.; You, J.; Hao, T.; Li, X.; Nie, J.; Wang, T. Acetylene bridged D-(π-A)2 type dyes containing benzophenone moieties: Photophysical properties, and the potential application as photoinitiators. Dye. Pigment. 2021, 184, 108583. [Google Scholar] [CrossRef]
- Xue, T.; Li, Y.; Zhao, X.; Nie, J.; Zhu, X. A facile synthesized benzophenone Schiff-base ligand as efficient type II visible light photoinitiator. Prog. Org. Coat. 2021, 157, 106329. [Google Scholar] [CrossRef]
- Liu, S.; Brunel, D.; Sun, K.; Xu, Y.; Morlet-Savary, F.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. A monocomponent bifunctional benzophenone–carbazole type II photoinitiator for LED photoinitiating systems. Polym. Chem. 2020, 11, 3551–3556. [Google Scholar] [CrossRef]
- Liu, S.; Chen, H.; Zhang, Y.; Sun, K.; Xu, Y.; Morlet-Savary, F.; Graff, B.; Noirbent, G.; Pigot, C.; Brunel, D.; et al. Monocomponent Photoinitiators based on Benzophenone-Carbazole Structure for LED Photoinitiating Systems and Application on 3D Printing. Polymers 2020, 12, 1394. [Google Scholar] [CrossRef]
- Liu, S.; Brunel, D.; Noirbent, G.; Mau, A.; Chen, H.; Morlet-Savary, F.; Graff, B.; Gigmes, D.; Xiao, P.; Dumur, F.; et al. New multifunctional benzophenone-based photoinitiators with high migration stability and their applications in 3D printing. Mater. Chem. Front. 2021, 5, 1982–1994. [Google Scholar] [CrossRef]
- Liu, S.; Brunel, D.; Sun, K.; Zhang, Y.; Chen, H.; Xiao, P.; Dumur, F.; Lalevée, J. Novel Photoinitiators Based on Benzophenone-Triphenylamine Hybrid Structure for LED Photopolymerization. Macromol. Rapid Commun. 2020, 41, 2000460. [Google Scholar] [CrossRef] [PubMed]
- Dadashi-Silab, S.; Aydogan, C.; Yagci, Y. Shining a light on an adaptable photoinitiator: advances in photopolymerizations initiated by thioxanthones. Polym. Chem. 2015, 6, 6595–6615. [Google Scholar] [CrossRef]
- Yilmaz, G.; Aydogan, B.; Temel, G.; Arsu, N.; Moszner, N.; Yagci, Y. Thioxanthone−Fluorenes as Visible Light Photoinitiators for Free Radical Polymerization. Macromolecules 2010, 43, 4520–4526. [Google Scholar] [CrossRef]
- Esen, D.S.; Temel, G.; Balta, D.K.; Allonas, X.; Arsu, N. One-component thioxanthone acetic acid derivative photoinitiator for free radical polymerization. Photochem. Photobiol. 2014, 90, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, G.; Tuzun, A.; Yagci, Y. Thioxanthone-carbazole as a visible light photoinitiator for free radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 5120–5125. [Google Scholar] [CrossRef]
- Yilmaz, G.; Beyazit, S.; Yagci, Y. Visible light induced free radical promoted cationic polymerization using thioxanthone derivatives. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 1591–1596. [Google Scholar] [CrossRef]
- Karaca, N.; Balta, D.K.; Ocal, N.; Arsu, N. Mechanistic studies of thioxanthone–carbazole as a one-component type II photoinitiator. J. Lumin. 2014, 146, 424–429. [Google Scholar] [CrossRef]
- Arsu, N.; Balta, D.K.; Yagci, Y.; Jockusch, S.; Turro, N.J. Thioxanthone-Anthracene: A New Photoinitiator for Free Radical Polymerization in the Presence of Oxygen. Macromolecules 2007, 40, 4138–4141. [Google Scholar]
- Balta, D.K.; Arsu, N.; Yagci, Y.; Sundaresan, A.K.; Jockusch, S.; Turro, N.J. Mechanism of Photoinitiated Free Radical Polymerization by Thioxanthone−Anthracene in the Presence of Air. Macromolecules 2011, 44, 2531–2535. [Google Scholar] [CrossRef]
- Balta, D.K.; Temel, G.; Goksu, G.; Ocal, N.; Arsu, N. Thioxanthone–Diphenyl Anthracene: Visible Light Photoinitiator. Macromolecules 2011, 45, 119–125. [Google Scholar] [CrossRef]
- Balta, D.K.; Arsu, N. Thioxanthone-ethyl anthracene. J. Photochem. Photobiol. A Chem. 2013, 257, 54–59. [Google Scholar] [CrossRef]
- Tar, H.; Esen, D.S.; Aydin, M.; Ley, C.; Arsu, N.; Allonas, X. Panchromatic Type II Photoinitiator for Free Radical Polymerization Based on Thioxanthone Derivative. Macromolecules 2013, 46, 3266–3272. [Google Scholar] [CrossRef]
- Yilmaz, G.; Acik, G.; Yagci, Y. Counteranion Sensitization Approach to Photoinitiated Free Radical Polymerization. Macromolecules 2012, 45, 2219–2224. [Google Scholar] [CrossRef]
- Dogruyol, S.K.; Dogruyol, Z.; Arsu, N. A thioxanthone-based visible photoinitiator. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4037–4043. [Google Scholar] [CrossRef]
- Wu, Q.; Xiong, Y.; Liang, Q.; Tang, H. Developing thioxanthone based visible photoinitiators for radical polymerization. RSC Adv. 2014, 4, 52324–52331. [Google Scholar] [CrossRef]
- Doğruyol, S.K.; Doğruyol, Z.; Arsu, N. Thioxanthone based 9-[2-(methyl-phenyl-amino)-acetyl]-thia-naphthacene-12-one as a visible photoinitiator. J. Lumin. 2013, 138, 98–104. [Google Scholar] [CrossRef]
- Mau, A.; Le, T.H.; Dietlin, C.; Bui, T.-T.; Graff, B.; Dumur, F.; Goubard, F.; Lalevee, J. Donor–acceptor–donor structured thioxanthone derivatives as visible photoinitiators. Polym. Chem. 2020, 11, 7221–7234. [Google Scholar] [CrossRef]
- Breloy, L.; Losantos, R.; Sampedro, D.; Marazzi, M.; Malval, J.-P.; Heo, Y.; Akimoto, J.; Ito, Y.; Brezová, V.; Versace, D.-L. Allyl amino-thioxanthone derivatives as highly efficient visible light H-donors and co-polymerizable photoinitiators. Polym. Chem. 2020, 11, 4297–4312. [Google Scholar] [CrossRef]
- Rodrigues, M.R.; Neumann, M.G. Mechanistic Study of Tetrahydrofuran Polymerization Photoinitiated by a Sulfonium Salt/Thioxanthone System. Macromol. Chem. Phys. 2001, 202, 2776–2782. [Google Scholar] [CrossRef]
- Guo, X.D.; Zhou, H.Y.; Wang, J.X. A novel thioxanthone-hydroxyalkylphenone bifunctional photoinitiator: Synthesis, characterization and mechanism of photopolymerization. Prog. Org. Coat. 2021, 154, 106214. [Google Scholar] [CrossRef]
- Dworak, C.; Liska, R.J. Alternative initiators for bimolecular photoinitiating systems. Polym. Sci. Part A Polym. Chem. 2010, 48, 5865–5871. [Google Scholar] [CrossRef]
- Dietliker, K.; Hüsler, R.; Birbaum, J.L.; Ilg, S.; Villeneuve, S.; Studer, K.; Jung, T.; Benkhoff, J.; Kura, H.; Matsumoto, A.; et al. Advancements in photoinitiators—Opening up new applications for radiation curing. Prog. Org. Coat. 2007, 58, 146–157. [Google Scholar] [CrossRef]
- Fast, D.E.; Lauer, A.; Menzel, J.P.; Kelterer, A.-M.; Gescheidt, G.; Barner-Kowollik, C. Wavelength-Dependent Photochemistry of Oxime Ester Photoinitiators. Macromolecules 2017, 50, 1815–1823. [Google Scholar] [CrossRef]
- Li, Z.; Zou, X.; Zhu, G.; Liu, X.; Liu, R. Coumarin-Based Oxime Esters: Photobleachable and Versatile Unimolecular Initiators for Acrylate and Thiol-Based Click Photopolymerization under Visible Light-Emitting Diode Light Irradiation. ACS Appl. Mater. Interfaces 2018, 10, 16113–16123. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhao, Y.; Wu, F.; Fang, D.-C. Effect of Bridging Position on the Two-Photon Polymerization Initiating Efficiencies of Novel Coumarin/Benzylidene Cyclopentanone Dyes. J. Phys. Chem. A 2010, 114, 5171–5179. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Zhu, J.; Dietliker, K.; Li, Z. Polymerizable Oxime Esters: An Efficient Photoinitiator with Low Migration Ability for 3D Printing to Fabricate Luminescent Devices. ChemPhotoChem 2020, 4, 5296–5303. [Google Scholar] [CrossRef]
- Qiu, W.; Hu, P.; Zhu, J.; Liu, R.; Li, Z.; Hu, Z.; Chen, Q.; Dietliker, K.; Liska, R. Cleavable Unimolecular Photoinitiators Based on Oxime-Ester Chemistry for Two-Photon Three-Dimensional Printing. ChemPhotoChem 2019, 3, 1090–1094. [Google Scholar] [CrossRef]
- Hammoud, F.; Giacoletto, N.; Noirbent, G.; Graff, B.; Hijazi, A.; Nechab, M.; Gigmes, D.; Dumur, F.; Lalevée, J. Substituent effects on the photoinitiation ability of coumarin-based oxime-ester photoinitiators for free radical photopolymerization. Mater. Chem. Front. 2021, 5, 8361–8370. [Google Scholar] [CrossRef]
- Ma, X.; Cao, D.; Fu, H.; You, J.; Gu, R.; Fan, B.; Nie, J.; Wang, T. Multicomponent photoinitiating systems containing arylamino oxime ester for visible light photopolymerization. Prog. Org. Coat. 2019, 135, 517–524. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, S.; Gao, Y.; Nie, J.; Du, H.; Sun, F. Photochromic Polymers Based on Fluorophenyl Oxime Ester Photoinitiators as Photoswitchable Molecules. Macromolecules 2020, 53, 5701–5710. [Google Scholar] [CrossRef]
- Zhou, R.; Sun, X.; Mhanna, R.; Malval, J.-P.; Jin, M.; Pan, H.; Wan, D.; Morlet-Savary, F.; Chaumeil, H.; Joyeux, C. Wavelength-Dependent, Large-Amplitude Photoinitiating Reactivity within a Carbazole-Coumarin Fused Oxime Esters Series. ACS Appl. Polym. Mater. 2020, 2, 2077–2085. [Google Scholar] [CrossRef]
- Chen, S.; Jin, M.; Malval, J.-P.; Fu, J.; Morlet-Savary, F.; Pan, H.; Wan, D. Substituted stilbene-based oxime esters used as highly reactive wavelength-dependent photoinitiators for LED photopolymerization. Polym. Chem. 2019, 10, 6609–6621. [Google Scholar] [CrossRef]
- Wang, W.; Jin, M.; Pan, H.; Wan, D. Remote effect of substituents on the properties of phenyl thienyl thioether-based oxime esters as LED-sensitive photoinitiators. Dye. Pigment. 2021, 192, 109435. [Google Scholar] [CrossRef]
- Zhou, R.; Pan, H.; Wan, D.; Malval, J.-P.; Jin, M. Bicarbazole-based oxime esters as novel efficient photoinitiators for photopolymerization under UV-Vis LEDs. Prog. Org. Coat. 2021, 157, 106306. [Google Scholar] [CrossRef]
- Liu, S.; Graff, B.; Xiao, P.; Dumur, F.; Lalevee, J. Nitro-Carbazole Based Oxime Esters as Dual Photo/Thermal Initiators for 3D Printing and Composite Preparation. Macromol. Rapid Commun. 2021, 42, 2100207. [Google Scholar] [CrossRef]
- Liu, S.; Giacoletto, N.; Schmitt, M.; Nechab, M.; Graff, B.; Morlet-Savary, F.; Xiao, P.; Dumur, F.; Lalevée, J. Effect of Decarboxylation on the Photoinitiation Behavior of Nitrocarbazole-Based Oxime Esters. Macromolecules 2022, 55, 2475–2485. [Google Scholar] [CrossRef]
- Dietlin, C.; Trinh, T.T.; Schweizer, S.; Graff, B.; Morlet-Savary, F.; Noirot, P.-A.; Lalevée, J. Rational Design of Acyldiphenylphosphine Oxides as Photoinitiators of Radical Polymerization. Macromolecules 2019, 52, 7886–7893. [Google Scholar] [CrossRef]
- Xie, C.; Wang, Z.; Liu, Y.; Song, L.; Liu, L.; Wang, Z.; Yu, Q. A novel acyl phosphine compound as difunctional photoinitiator for free radical polymerization. Prog. Org. Coat. 2019, 135, 34–40. [Google Scholar] [CrossRef]
- Wu, Y.; Li, R.; Wang, J.; Situ, Y.; Huang, H. A new carbazolyl-basedacylphosphine oxide photoinitiator with high performance and low migration. J. Polym. Sci. 2022, 60, 52–61. [Google Scholar] [CrossRef]
- Mitterbauer, M.; Knaack, P.; Naumov, S.; Markovic, M.; Ovsianikov, A.; Moszner, N.; Liska, R. Acylstannanes: Cleavable and Highly Reactive Photoinitiators for Radical Photopolymerization at Wavelengths above 500 nm with Excellent Photobleaching Behavior. Angew. Chem. Int. Ed. Engl. 2018, 57, 12146–12150. [Google Scholar] [CrossRef]
- Radebner, J.; Eibel, A.; Leypold, M.; Gorsche, C.; Schuh, L.; Fischer, R.; Torvisco, A.; Neshchadin, D.; Geier, R.; Moszner, N.; et al. Tetraacylgermanes: Highly Efficient Photoinitiators for Visible-Light-Induced Free-Radical Polymerization. Angew. Chem. Int. Ed. Engl. 2017, 56, 3103–3107. [Google Scholar] [CrossRef] [PubMed]
- Neshchadin, D.; Rosspeintner, A.; Griesser, M.; Lang, B.; Mosquera-Vazquez, S.; Vauthey, E.; Gorelik, V.; Liska, R.; Hametner, C.; Ganster, B.; et al. Acylgermanes: photoinitiators and sources for Ge-centered radicals. insights into their reactivity. J. Am. Chem. Soc. 2013, 135, 17314–17321. [Google Scholar] [CrossRef] [PubMed]
- Ganster, B.; Fischer, U.K.; Moszner, N.; Liska, R. New Photocleavable Structures. Diacylgermane-Based Photoinitiators for Visible Light Curing. Macromolecules 2008, 41, 2394–2400. [Google Scholar] [CrossRef]
- Liu, S.; Giacoletto, N.; Graff, B.; Morlet-Savary, F.; Nechab, M.; Xiao, P.; Dumur, F.; Lalevée, J. N-naphthalimide ester derivatives as Type Ⅰ photoinitiators for LED photopolymerization. Mater. Today Chem. 2022, 26, 101137. [Google Scholar] [CrossRef]
- Haslinger, C.; Leutgeb, L.P.; Haas, M.; Baudis, S.; Liska, R. Synthesis and Photochemical Investigation of Tetraacylgermanes. ChemPhotoChem 2022, 6, e202200108. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Borjigin, T.; Schmitt, M.; Morlet-Savary, F.; Xiao, P.; Lalevée, J. High-Performance Photoinitiating Systems for LED-Induced Photopolymerization. Polymers 2023, 15, 342. https://doi.org/10.3390/polym15020342
Liu S, Borjigin T, Schmitt M, Morlet-Savary F, Xiao P, Lalevée J. High-Performance Photoinitiating Systems for LED-Induced Photopolymerization. Polymers. 2023; 15(2):342. https://doi.org/10.3390/polym15020342
Chicago/Turabian StyleLiu, Shaohui, Timur Borjigin, Michael Schmitt, Fabrice Morlet-Savary, Pu Xiao, and Jacques Lalevée. 2023. "High-Performance Photoinitiating Systems for LED-Induced Photopolymerization" Polymers 15, no. 2: 342. https://doi.org/10.3390/polym15020342
APA StyleLiu, S., Borjigin, T., Schmitt, M., Morlet-Savary, F., Xiao, P., & Lalevée, J. (2023). High-Performance Photoinitiating Systems for LED-Induced Photopolymerization. Polymers, 15(2), 342. https://doi.org/10.3390/polym15020342