Abstract
The photochemical degradation of natural rubber (NR) is a prevalent method used to modify its inherent properties. Natural rubber, predominantly derived from the Hevea Brasiliensis tree, exhibits an exceptionally high molecular weight (MW), often reaching a million daltons (Da). This high MW restricts its solubility in various solvents and its reactivity with polar compounds, thereby constraining its versatile applications. In our previous work, we employed TiO2 in its powdered form as a photocatalyst for the functionalization of NR latex. However, the post-process separation and reuse of this powder present substantial challenges. In this present study, we aimed to functionalize deproteinized NR (DPNR) latex. We systematically reduced its MW via photochemical degradation under UVA irradiation facilitated by H2O2. To enhance the efficiency of the degradation process, we introduced TiO2-coated hollow glass beads (TiO2-HGBs) as photocatalysts. This approach offers the advantage of easy collection and repeated reuse. The modified DPNR showed a reduction in its number-average MW from 9.48 × 105 to 0.28 × 105 Da and incorporated functional groups, including hydroxyl, carbonyl, and epoxide. Remarkably, the TiO2-HGBs maintained their performance over seven cycles of reuse. Due to their superior efficacy, TiO2-HGBs stand out as promising photocatalysts for the advanced functionalization of NR across various practical applications.
1. Introduction
Natural rubber (NR) is a hydrocarbon polymer harvested from the Hevea brasiliensis tree. It comprises 94% cis-1,4-polyisoprene and 6% non-rubber components such as lipids, proteins, and inorganic constituents []. Due to its high molecular weight, NR exhibits excellent physical properties in resilience, strength, and fatigue resistance, making it desirable for various applications. However, NR is composed of hydrocarbons with very high molecular weights; therefore, it is difficult to process and obtain uniform composites due to its high viscosity and incompatibility with polar fillers and other molecules. The modification of NR latex, which involves reducing its molecular weight and imparting reactive functional groups, has attracted attention as a potential alternative for overcoming these disadvantages. Degraded and shorter NR chains have proven to be beneficial in a variety of applications, including as plasticizers [], compatibilizers [,], adhesives [], chain extension promoters [,,], and grafting reaction linkers []. Approaches such as redox [], oxidation [], and photochemical degradation [] can generate free radicals for the further preparation of low-molecular-weight NR (LNR). However, photochemical degradation is more promising than the alternatives because it is clean, low-energy, and non-toxic. Ravindran et al. [] produced hydroxyl-terminated liquid NR in a rubber solution in the presence of hydrogen peroxide (H2O2) under ultraviolet (UV) irradiation from a medium-pressure mercury vapor lamp and sunlight.
It is well known that numerous semiconductors have been applied as photocatalysts, such as TiO2, CdS, ZnO, WO3, etc. [,,,]. ZnO and TiO2 have attracted attention as photocatalysts with wide applications due to their suitable morphologies, desired band gaps, and high surface areas, as well as their stability and reusability. However, ZnO has several drawbacks including an unsuitable band gap energy (Eg = 3.7 eV) and a low surface area or large particle size []. Therefore, TiO2 seems to be an interesting material with great properties, such as high stability, low cost, good biocompatibility, and environmental friendliness. It can be synthesized using a simple method.
Furthermore, our group is continuously studying the application of TiO2 photocatalyst powder, and we found that it shows good results for our purpose. However, the powdery form is impractical because it is difficult to collect and reuse after the process. Thus, we developed the TiO2-based photocatalyst in this work by coating hollow glass beads for facile collection and repetitive reuse.
TiO2 is a non-toxic and insoluble semiconductor powder used in various applications such as paints, cosmetics, and toothpaste []. Additionally, it is a well-known photocatalyst. TiO2 exists as three naturally occurring polymorphs with distinct characteristics: anatase, rutile, and brookite. Anatase permanently transforms into rutile at high temperatures. Due to a large band gap (3.23–3.59 eV) between the valence and conduction bands, the anatase form is recognized as the superior photocatalyst []. The photocatalytic activity of TiO2 is applicable in important technological areas [], such as self-cleaning coatings [,], water splitting [], water treatment [,], dye-sensitized solar cells [,], air purification [], and self-sterilizing coatings []. The photocatalytic reaction on the TiO2 surface occurs when an electron (e−) in the valence band is excited by UV light (290–380 nm), thereby generating a positively charged hole (h+) in the conduction band. These excited h+ and e− then transfer to the reactive species (i.e., O2 or H2O) adsorbed onto the catalyst surface, thus generating reactive free radicals that are crucial for the breakdown of organic molecules. A large surface area is essential for utilizing the photocatalytic capabilities of TiO2 effectively. Consequently, TiO2 powders for achieving high photocatalytic efficiencies have been explored. P-25 (Degussa) powder is a typical material with anatase and rutile phases with a considerably large surface area []. It is highly active in various photocatalytic processes, including destroying microcystin-LR, a cyanobacterial toxin in drinking water sources [].
Due to its high photocatalytic efficiency, TiO2 has been used as a photocatalyst in the photodegradation of NR latex to achieve LNR. Fine TiO2 powder is advantageous for promoting the reaction; however, separating the powder after use is difficult. Our group previously employed TiO2-coated glass and quartz substrates as containers for NR latex to manufacture telechelic liquid NR [,]. The reaction was conducted for 5 h in the presence of H2O2 under UV irradiation. The glass substrate was reusable; however, the container size constrained the degradation process.
Moreover, functionalized styrene–butadiene rubber and skim NR latex were manufactured via photocatalysis using a TiO2-coated petri dish as the photocatalyst []. The hydroxyl group (-OH) amount increased with increasing UV-irradiation time but decreased after 5 h. In addition, a small amount of H2O2, 5% w/w of dry rubber, was sufficient for the photocatalysis of skim latex.
Recently, TiO2-coated Pyrex glass beads were employed as a photocatalyst to suppress algal growth in eutrophic water under UV illumination []. These beads considerably accelerated photocatalysis. In addition, they were easily isolated from the algae.
Therefore, in this study, TiO2-coated hollow borosilicate glass beads (TiO2-HGBs) were fabricated and utilized as photocatalysts to produce functionalized LNR (FLNR) latex in the presence of H2O2 under UV irradiation. The TiO2-HGBs were proposed to facilitate separation after usage by flotation and promote UV contact on the TiO2 surface during the reaction, thereby increasing the photocatalytic activity. Typically, an NR particle is surrounded by a layer of mixed protein and phospholipid domains []; the polyisoprene molecules subsequently form a hydrophobic core, resulting in a core–shell-like NR particle, as shown in Figure 1. The mixed layer may obscure NR particles making it difficult for them to react. This study used deproteinized NR (DPNR) latex as the starting material.
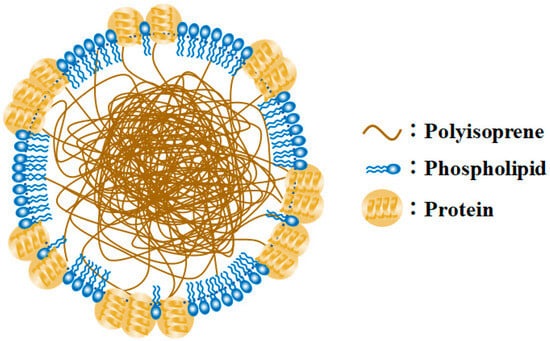
Figure 1.
Model of latex particle surrounded by a mixture of proteins and phospholipids (Modified from []).
2. Materials and Methods
2.1. Materials
Fresh NR latex was generously provided by Thai Rubber Latex Corporation (Bangphli, Samutprakan, Thailand) Public Company Limited. Hollow borosilicate glass beads (HGBs) with an average diameter of 7 mm were made by the glassmaker shop. H2O2 (30% w/w), urea, and sodium dodecyl sulfate (SDS) were purchased from British Drug Houses (BDH Chemicals). Tetrapropyl orthotitanate was purchased from Fluka. Toluene, methanol, acetone, and tetrahydrofuran were purchased from RCI Labscan Limited. All chemicals were used without further purification.
2.2. Preparation of TiO2-Coated HGBs
First, a mixture of tetrapropyl orthotitanate (9.0 mL), distilled water (100.0 mL), and 70% w/w HNO3 (1.0 mL) was stirred for 24 h at 25 °C. After further stirring of the slurry for 6 h at 55 °C, a milky-looking TiO2 precursor sol was obtained.
Second, the HGBs were washed in acetone via ultrasonication for 1 h. The dried HGBs were then submerged in the synthesized TiO2 precursor sol, followed by dehydration and calcination at 550 °C for 1 h at a rate of 5 °C/min to eliminate the solvent and crystallize the TiO2 precursor layer.
2.3. Characterization of TiO2-HGBs
X-ray diffractometry with Cu-Kα1 radiation (λ = 0.15405980 nm) over the range of 20° < 2θ < 60° was used to analyze the crystallinity of the TiO2 films deposited on the HGBs. The step and time sizes were 0.02° and 30 s, respectively. We determined the mass percentages of the anatase and rutile phases using the following formulae, which are based on the intensity of the peak at 2θ values of 25.3° (101-plane) for anatase and 27.4° (110-plane) for rutile, respectively [,].
A (mass %) = 100/[1 + 1.265(IR/IA)]
R (mass %) = 100 − A = 100 × 1.265 IR/[IA + 1.265 IR]
Here, A and R are the mass percentages of the anatase and rutile phases, respectively; IA and IR are the intensities of the (101) anatase and (110) rutile peaks.
Scanning electron microscope (SEM, JSM5410LV, JEOL®, Tokyo, Japan) equipped with energy dispersive X-ray spectrometer (EDS, Link ISIS-300, Oxford®, Abingdon, UK) was employed to analyze the surface morphologies of the HGBs before and after the formation of the TiO2 coating, and to determine the thickness of the TiO2 layer. Palladium sputtering was used in advance to impart conductivity to the samples, and further analysis was carried out by operating at an accelerating voltage of 20 kV under high vacuum mode while maintaining a working distance of 20 mm.
2.4. Preparation of FLNR Latex
In this study, DPNR latex was preliminarily prepared as the starting NR latex by incubating fresh NR latex (preserved with 0.3% v/v NH3) with 0.1% w/v urea and 1% w/v SDS at RT for 2 h while stirring. Then, the treated latex was centrifuged at 13,000 rpm and 25 °C for 30 min. The cream fraction was redispersed in 1% w/v SDS solution. After a second centrifugation under the same conditions, the cream fraction was redispersed in distilled water to obtain DPNR latex with a dry rubber content (DRC) of 30% [].
A mixture of DPNR latex, H2O2, and TiO2-HGBs in a round-bottom flask was exposed to a 1000 W UVA lamp in a chamber. After irradiation, the FLNR was coagulated with acetone and dried at 40 °C under vacuum until a constant weight was reached. This procedure is the standard method used for preparing FLNR in subsequent investigations. The impact of H2O2 concentration in units of parts per hundred rubber (phr), TiO2-HGB quantity (number of beads), UVA-irradiation time, and the reusability of TiO2-HGBs were examined.
2.5. Characterization of FLNR Latex
FLNR and DPNR were re-precipitated in toluene/methanol before further analysis. The deproteinization was confirmed by investigating the nitrogen content of DPNR using a nitrogen analyzer (LECO FP-258) based on the combustion of oxygen gas. About 0.25 g of rubber sample was weighed and subjected to a nitrogen analyzer. The combustion of the rubber sample changed the nitrogen in the compounds to nitrogen gas which was detected as nitrogen content (% w/w). The results were obtained from the triplicate analysis. The weight-average molecular weight () and number-average molecular weight ( of the prepared samples were determined using gel permeation chromatography (GPC−Waters® 2414, Milford, CT, USA) using polystyrene standards and a refractive index (RI) detector equipped with a Styragel HR5E 7.8 × 300 mm column. The column was eluted with tetrahydrofuran (THF) at a 1.0 mL/min flow rate at 40 °C. The functional groups were investigated via Fourier-transform infrared spectroscopy (FTIR, FTIR-460 Plus, JASCO®, Tokyo, Japan) at RT at a resolution of 4 cm−1 and 16 scans per spectrum. The chemical microstructures were analyzed using 1H- and 13C-Nuclear Magnetic Resonance (NMR) spectroscopy at 50 °C using chloroform-d as the solvent. The measured frequency, pulse delay time, and number of scans for 1H- and 13C-NMR were 500 and 125 MHz, 4 and 5 s, and 500 and 12,000 scans, respectively.
Additionally, the gel contents of the samples were examined for side reactions by immersing the dried samples in toluene and storing them in the dark at RT for 7 days. The gel fraction was recovered by centrifugation, coagulated with methanol, and dried in an air-flow oven at 70 °C. The gel contents of the samples were estimated using the following formula:
3. Results
3.1. Characterization of TiO2-Coated HGBs
The XRD pattern exhibits characteristic reflections of the anatase and rutile phases (Figure 2). The anatase phase of TiO2 shows diffraction peaks at approximately 25.3°, 37.8°, 38.5°, and 48.0°, corresponding to the (101), (004), (112), and (200) reflections, respectively. In addition, the rutile phase is identified by the peaks at 27.2°, 36.0°, 41.2°, and 54.3°, which are assigned to (110), (101), (111), and (211), respectively. Using Equations (1) and (2), the phase composition was estimated to be 68% anatase and 32% rutile. The mixed crystalline phases may have resulted from some anatase transitioning to the rutile phase during calcination. Recent reports show that the mixed phase of TiO2 (i.e., anatase and rutile) exhibits higher photocatalytic activity than either pure phase alone []. This result implies that the current procedure is suitable for synthesizing TiO2-HGBs to yield a mixture of anatase and rutile phases that may provide effective photoactivity for the photochemical degradation of organic compounds.
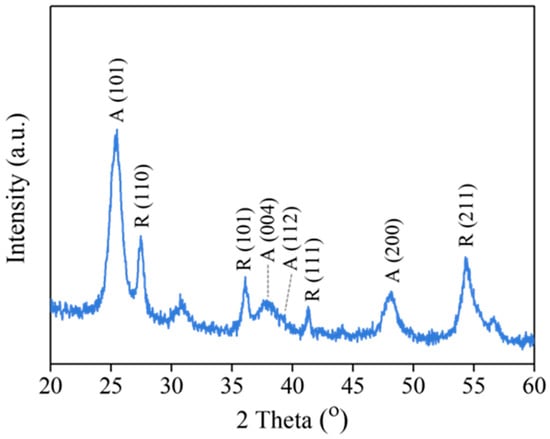
Figure 2.
XRD pattern of TiO2 film coated on HGBs.
The morphology of the TiO2-HGBs was determined using SEM. The HGB surface is transparent and smooth before TiO2 coating (Figure 3a). Meanwhile, a uniformly dispersed porous TiO2 film is present on the HGB surface after TiO2 coating (Figure 3b), which is consistent with the report by Kim et al. []. These porous structures may have originated from the thermal decomposition of the components within the TiO2 precursor during calcination, which increases the porosity and specific surface area, causing the TiO2 film to exhibit significant photocatalytic efficiency []. SEM was also used to observe the cross-section of the TiO2-HGBs, and EDS analysis was performed. The thickness of the deposited TiO2 film is 297 nm (Figure 3c). The SEM-EDS analysis of Ti within the TiO2-HGBs using the mapping mode indicates the presence of a TiO2 layer on the HGB surface (Figure 3d). Additionally, the EDS spectrum of the TiO2-HGBs presented in Figure 3e confirms the successful coating of TiO2. It exhibits three additional Ti peaks in addition to the signals of oxygen, sodium, aluminum, and silicon, which are the primary components of the HGBs.
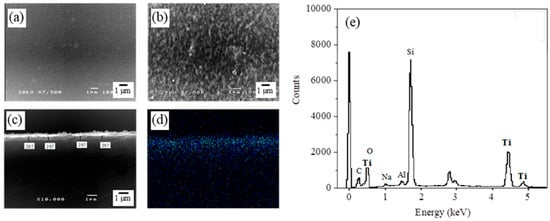
Figure 3.
SEM images of the HGB surface (a) before and (b) after TiO2 coating at 7500X. The cross-sectional SEM images of the TiO2-HGBs in (c) secondary electron image (SEI) and (d) Ti element mapping. (e) The EDS spectrum of elements on the TiO2-HGBs.
3.2. Preparation of FLNR Latex
3.2.1. Effect of H2O2 Concentration
Ravindran T. et al. [] discovered that H2O2 was essential for the formation of the hydroxyl radical (•OH). Herein, the optimal concentration of H2O2 was investigated, and GPC analysis was used to evaluate the degradation of the DPNR latex based on the reduction of its molecular weight. Figure 4a,b show the and of the product obtained using 20 g of DPNR latex at 10% DRC irradiated for 0 to 40 min under a 1000 W UVA lamp. The and of the samples gradually decrease as the H2O2 concentration increases, indicating that the •OH produced by the dissociation of H2O2 under UVA irradiation plays an important role in DPNR degradation. Moreover, the and also decrease with increasing irradiation time up to 20 min, after which they are unaffected by exposure time up to 30 min. During this irradiation period, the and of the samples treated with 30 phr of H2O2 are comparable to those treated with 40 phr of H2O2. Therefore, the H2O2 concentration of 30 phr was considered adequate for breaking NR chains from DPNR latex.
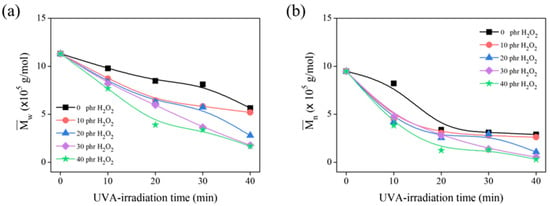
Figure 4.
The (a) and (b) of DPNR latex in the presence of 0, 10, 20, 30, and 40 phr of H2O2 after several UVA-irradiation time intervals.
3.2.2. Effect of TiO2-HGB Quantity
The impact of TiO2-HGB quantity was investigated by measuring the molecular weight of the FLNR produced using varying amounts of TiO2-HGBs (0–35 pieces) in the presence of 30 phr of H2O2. The reaction was conducted for 30 min under UVA irradiation. The and of the FLNR samples decreased with the increasing amount of TiO2-HGBs and reached a minimum when 25 pieces of TiO2-HGB were utilized (equal to 25 mmol of TiO2 calculated using the difference in HGB weight before and after TiO2 coating) (Figure 5a). Therefore, 25 pieces of TiO2-HGB were regarded as the optimal quantity for FLNR preparation, obtaining a and of 1.43 × 105 and 0.28 × 105 Da, respectively. However, when 30 and 35 pieces of TiO2-HGB were employed, the and of the FLNR increased. This trend was expected because excess HGBs may disable the UVA light from penetrating the photochemical degradation cycle.
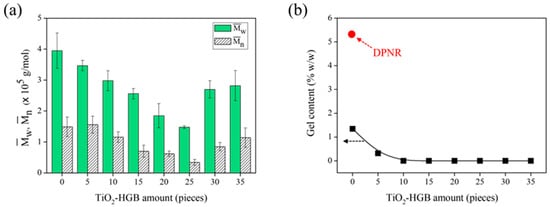
Figure 5.
(a) The and and (b) gel content of DPNR and FLNR samples prepared via UVA irradiation for 30 min in the presence of 30 phr of H2O2 using various amounts of TiO2-HGBs.
Under UV irradiation, cross-linking normally occurs as a competitive reaction in the presence of reactive radical species, that is, •OH. The gel content of the FLNR samples was also assessed to explore cross-links during the photochemical degradation process. Figure 5b shows the gel content of the samples with varying amounts of TiO2-HGBs. NR gel typically results from the interactions between non-rubber components (i.e., proteins and phospholipids) []. The gel content dropped from 5.31 to 1.35% w/w with the addition of H2O2 under UVA irradiation. This reduction in gel content suggests that the gel fraction of DPNR degrades owing to the chain scission generated by photooxidation in the H2O2/UVA system []. The gel fraction decreased to approximately 0.32% w/w with the addition of 5 pieces of TiO2-HGB and became almost zero with the addition of 10 pieces of TiO2-HGB. Therefore, an increase in TiO2-HGB quantity might expedite the chain scission of rubber in DPNR latex without any cross-linking reaction being detected.
Figure 6 illustrates the FTIR spectra of DPNR (control) and FLNR produced using varying amounts of TiO2-HGBs. For the DPNR spectrum, the peaks at 3280 cm−1 and 1548 cm−1, characteristic bands of NR proteins, disappeared []. Moreover, the nitrogen content of DPNR was also determined and found to be only 0.04 % w/w. These results confirm the reduction in proteins after the deproteinization of NR latex, which is possibly due to the weak attractive forces between proteins and the NR particle surface. After centrifugation, most proteins detach themselves from the rubber particles by forming hydrogen bonds with urea.
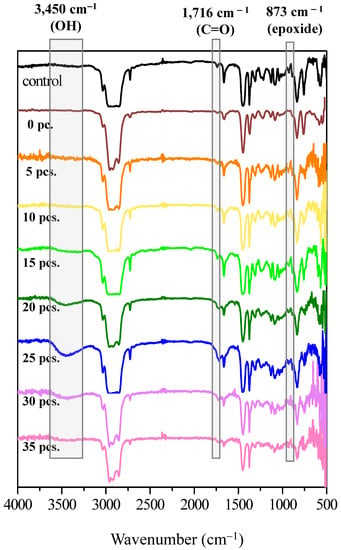
Figure 6.
FTIR spectra of DPNR (0 piece) and FLNR samples prepared via UVA irradiation for 30 min in the presence of 30 phr of H2O2 using various quantities of TiO2-HGBs.
In the comparison of the FTIR spectra of DPNR and FLNR prepared using various TiO2-HGB quantities, all spectra reveal the distinctive characteristic peaks of NR at 1664, 1375, and 836 cm−1, which are attributed to C=C stretching, –CH3 asymmetric, and =C–H deformations, respectively. New broad bands corresponding to the hydroxyl (-OH), carbonyl (C=O), and epoxide groups were observed in the FLNR spectrum at 3450, 1716, and 873 cm−1, respectively. Interestingly, the intensity of the –OH and C=O groups increased as the amount of TiO2-HGBs increased to 25 pieces. This result indicates that a larger quantity of HGBs promotes a more active site on the TiO2 film, thereby increasing •OH generation. Thus, DPNR particles had a high opportunity of being attacked by •OH, leading to their depolymerization and functionalization. However, the intensities decreased when more than 25 pieces of TiO2-HGB were utilized. It was hypothesized that the high concentration of TiO2-HGBs might absorb and conceal the UV light, resulting in reduced energy interaction between the TiO2-HGBs, rubber particles, and H2O2 molecules. These results aligned with those of the molecular weight results.
3.2.3. Effect of UVA-Irradiation Time
Isa et al. reported that temperature and reaction time can influence the degradation of NR latex []. Here, the effect of the irradiation period was evaluated by observing the decrease in the and of the FLNR samples (Figure 7a). After 30 min of irradiation, the decreased from 1.13 × 106 to 1.48 × 105 Da similar to the that decreased from 9.48 × 105 to 0.34 × 105 Da. A longer irradiation period facilitated the degradation of more NR chains into shorter segments. However, a prolonged reaction time can accelerate radical formation, leading to cross-linking. Figure 7b shows the gel content of the FLNR samples after various irradiation periods. The gel content decreases considerably after 10 min of irradiation. It disappears almost completely after 30 min, suggesting that no cross-linking occurs after 30 min of UVA exposure.
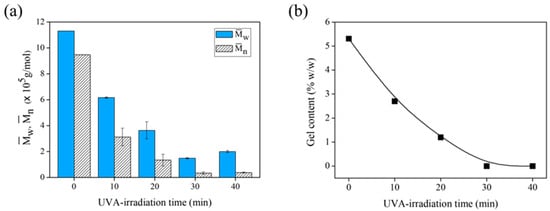
Figure 7.
(a) The and and (b) gel content of DPNR (no TiO2-HGB) and FLNR samples prepared in the presence of 30 phr of H2O2 using 25 pieces of TiO2-HGB under different UVA-irradiation time intervals.
Figure 8 shows the FTIR spectra of the DPNR (control) and FLNR samples. The intensities of the –OH and C=O groups increased steadily with increasing exposure duration, reaching their maximum ratios after 30 min of UVA irradiation. Nevertheless, the –OH and C=O contents decreased after 40 min of exposure. This result may be because a long irradiation time results in a high production rate of •OH, which can oxidize the functional groups on the NR chains and form free radicals in the system.
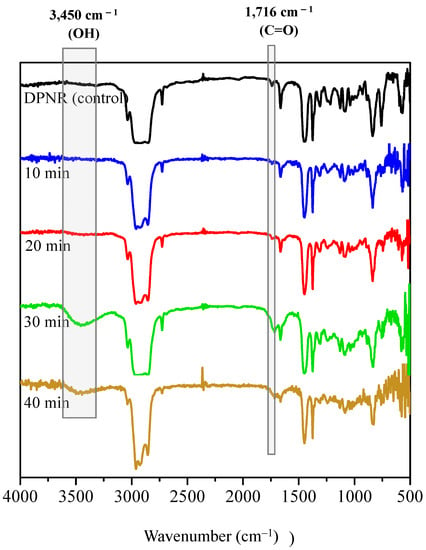
Figure 8.
FTIR spectra of DPNR (control) and FLNR samples in the presence of 30 phr of H2O2 and 25 pieces of TiO2-HGB at various irradiation time intervals.
Moreover, the structure of the produced samples was analyzed using 1H- and 13C-NMR spectroscopies. Figure 9 shows the 1H-NMR spectra of DPNR and FLNR. The characteristic signals of cis-1,4-polyisoprene units are detected in both samples at 1.70, 2.07, and 5.15 ppm, corresponding to the methyl (–CH3), methylene (–CH2–), and methine (=CH–) protons, respectively []. Additional signals between the chemical shifts of 3.4 and 4.2 ppm assigned to the –OH groups on the NR chains were detected in FLNR (Figure 9b) []. In addition, signals at 2.71 and 1.28 ppm suggest the presence of methylenic and methylic protons in the epoxide ring, respectively. Furthermore, the presence of C=O groups is indicated by an additional signal at 2.20 ppm and two small signals at 9.41 and 9.83 ppm. The signal at 2.20 ppm corresponds to the protons on the carbon adjacent to the C=O. However, the signals at 9.41 and 9.83 ppm are assigned to the aldehydic protons that have shifted far downfield due to the anisotropy of the C=O groups.
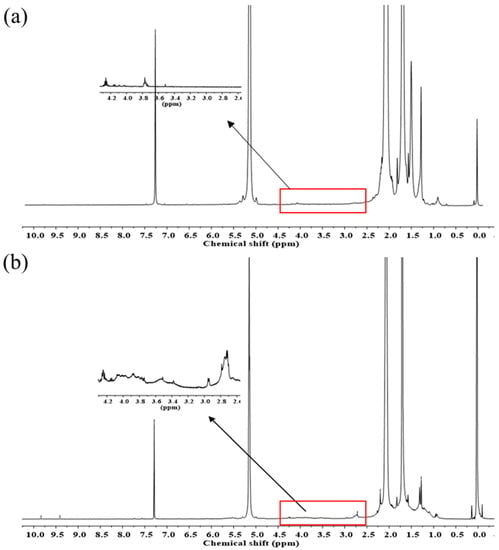
Figure 9.
1H-NMR spectra of purified (a) DPNR and (b) FLNR.
The 13C-NMR spectra of the purified DPNR and FLNR are shown in Figure 10. The signals at 23.37, 26.40, 32.21, 125.03, and 135.17 ppm are the characteristic signals of NR. The small signal at 68.17 ppm corresponding to the carbon attached to the –OH groups was detected in FLNR (Figure 10b). Additional signals at 60.75 and 64.49 in the FLNR spectrum correspond to the carbon of the epoxide ring []. A carbon signal close to that of C=O was observed at 38.81. The thermal degradation of NR can form C=O and epoxide groups once a high temperature is reached during irradiation.
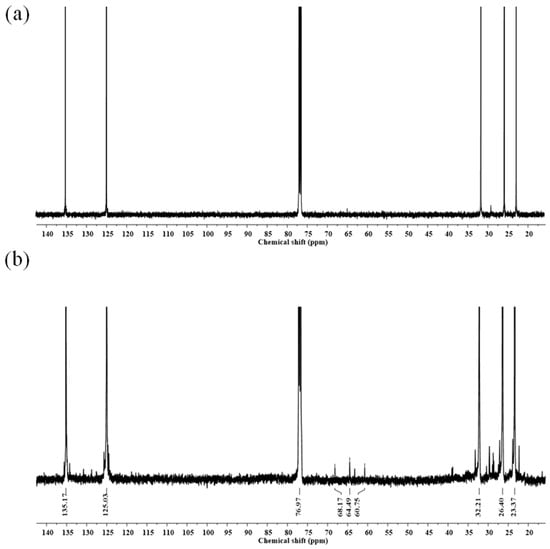
Figure 10.
13C-NMR spectra of purified (a) DPNR and (b) FLNR.
These results are consistent with the molecular weight results that suggest that the breakage of the NR chain results in a decrease in the molecular weight and the introduction of a functional group at the point of chain scission. The proposed mechanism is illustrated in Figure 11.
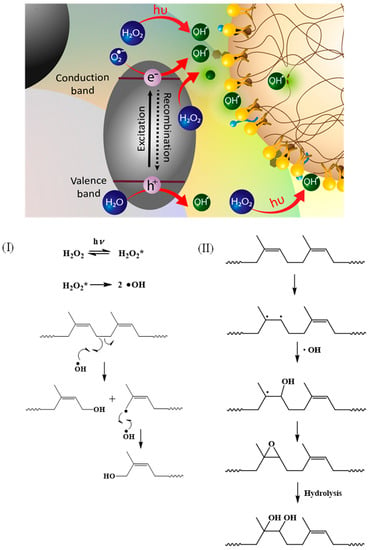
Figure 11.
Possible proposed mechanisms of the photochemical degradation process of DPNR latex. Path (I): Reaction at single bond of DPNR chain and Path (II): reaction at double bond of DPNR chain.
When •OH is generated via the dissociation of H2O2 under UV irradiation, it attacks the broken single bond and forms a hydroxyl-terminated chain (Path I). In another case, the double bond of the cis-1,4-polyisoprene chain breaks first, and then the •OH attacks the broken points, forming a hydroxyl group that transforms into an epoxide (Path II). However, the epoxide group can be hydrolyzed, forming hydroxyl groups attached to the chain.
3.3. Reusability of TiO2-HGBs
Reusability was a major advantage of the TiO2-HGBs used in the current study. The effectiveness of the TiO2-HGBs was measured by reusing them nine times. Figure 12I presents the SEM images of the TiO2-HGB surface and the cross-sectional SEM images of the TiO2-HGBs after the photochemical degradation of DPNR latex. After using the TiO2-HGBs four times (Figure 12(Ib)), the TiO2 layer and some components suspected to be degraded NR are still visible on the HGB surface. However, the TiO2 film thins out and is damaged during photochemical degradation, as confirmed by the SEM images of the cross-section in Figure 12(Ie). The thickness of the TiO2 film decreases from approximately 297 to 246 nm. After nine uses, the TiO2 film is covered by layers that could be leftover NR (Figure 12(Ic)). The TiO2 film becomes thinner, to approximately 111 nm, as shown in Figure 12(If).
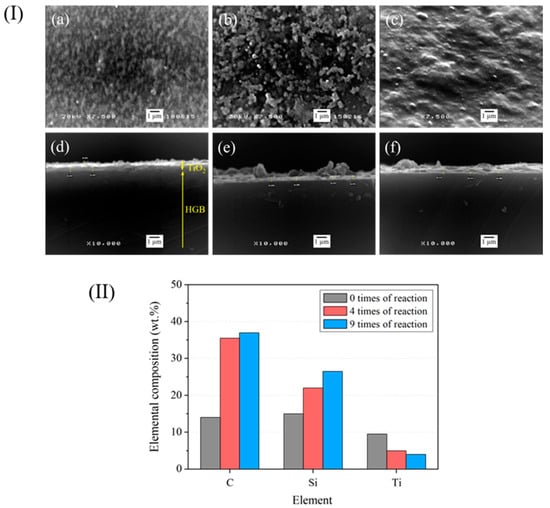
Figure 12.
(I) SEM images of the TiO2-HGB surface (a–c) and the cross-section (d–f). (II) Quantity of elements on the TiO2-HGB surface after using for 0, 4, and 9 times, respectively.
Figure 12II shows the elemental composition obtained from the EDS analysis of the TiO2-HGB surface. The amounts of C and Si increase slightly after using FLNR nine times. The increased C content may be attributed to the numerous carbon atoms in the remaining NR. Contrastingly, the decrease in the quantity of Ti after four and nine uses corresponds to the reduced thickness of the TiO2 layer, as shown in the SEM images of the TiO2-HGB cross-section.
Figure 13 shows the of FLNR samples at different recycling times. FLNR was prepared using the same 25 pieces of TiO2-HGB in the presence of 30 phr of H2O2 for 30 min under UVA irradiation. The of the samples did not change much after using the TiO2-HGBs up to four times. After seven uses, the slightly increases, but it is acceptable for FLNR preparation. However, after eight and nine applications of the TiO2-HGBs, the of FLNR dramatically increases. The of the FLNR samples increased to approximately 2.20 × 105 Da after using the TiO2-HGBs nine times, suggesting that the effectiveness of the TiO2-HGBs weakened after seven cycles. From all evidence, despite the destruction of a few TiO2 films during the photochemical degradation, TiO2-HGBs can be reused with excellent performance at least seven times.
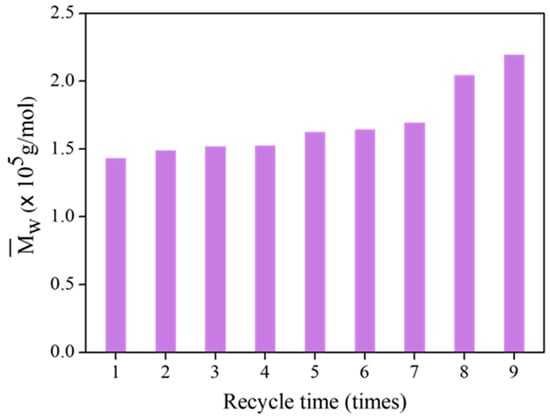
Figure 13.
The of FLNR samples at various recycling times.
4. Conclusions
This study elucidates the preparation and comprehensive characterization of TiO2-coated hollow glass beads (TiO2-HGBs) employed to functionalize deproteinized natural rubber (DPNR) latex via photocatalytic degradation. This process was facilitated under UVA irradiation, utilizing H2O2 as the oxidizing agent. The TiO2 film, comprising anatase and rutile phases, manifested uniform coatings on the HGBs, with a thickness of approximating 297 nm. Upon exposure to 1000 W of UVA irradiation for 30 min, and in the simultaneous presence of 30 phr of H2O2 and 25 individual TiO2-HGBs, the number-average molecular weight () of functionalized low-molecular-weight NR (FLNR) notably diminished to 0.28 × 105 Da without any discernible gel formation. The truncated DPNR chains incorporated hydroxyl, carbonyl, and epoxide groups attributed to the •OH radicals binding at their rupture points. Notably, the TiO2-HGBs demonstrated consistent efficacy over seven cycles of reuse. Given these findings, TiO2-HGBs are proposed as potent photocatalysts for the photochemical degradation of DPNR in latex form, underpinned by their distinguished physical and structural attributes, minimal toxicity, and robust chemical stability. This approach not only indicates potential for optimization, but also seems suitable for practical applications and potential commercialization.
Author Contributions
Conceptualization, J.S. and A.N.; methodology, J.S., A.N. and S.N.; validation, J.S., A.N., S.N., P.R. and T.H.; formal analysis, S.N. and P.R.; investigation, J.S., S.N. and P.R.; data curation, A.N. and S.N.; writing—original draft preparation, J.S. and S.N.; writing—review and editing, J.S., A.N. and P.R.; visualization, J.S., S.N., T.H., R.I. and J.-H.P.; supervision, J.S.; project administration, J.S.; funding acquisition, J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Mahidol University and the National Research Council of Thailand (NRCT), grant number NRCT5-RSA63015-09.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by Mahidol University and the National Research Council of Thailand (NRCT), grant number NRCT5-RSA63015-09. Sincere gratitude is expressed to Thai Rubber Latex Group Public Co., Ltd. for providing NR latex.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Sansatsadeekul, J.; Sakdapipanich, J.; Rojruthai, P. Characterization of associated proteins and phospholipids in natural rubber latex. J. Biosci. Bioeng. 2011, 111, 628–634. [Google Scholar] [CrossRef]
- Srilathakutty, R.; John, N.; Joseph, R.; George, K.E. Use of amine terminated liquid natural rubber as a plasticiser in filled NR and NBR compounds. Int. J. Polym. Mater. Polym. Biomater. 1996, 32, 235–246. [Google Scholar] [CrossRef]
- Dahlan, H.M.; Zaman, M.D.K.; Ibrahim, A. Liquid natural rubber (LNR) as a compatibiliser in NR/LLDPE blends-II: The effects of electron-beam (EB) irradiation. Radiat. Phys. Chem. 2002, 64, 429–436. [Google Scholar] [CrossRef]
- Mounir, A.; Darwish, N.A.; Shehata, A. Effect of maleic anhydride and liquid natural rubber as compatibilizers on the mechanical properties and impact resistance of the NR-NBR blend. Polym. Adv. Technol. 2004, 15, 209–213. [Google Scholar] [CrossRef]
- Wayakron, P.C.; Bumee, R.; Namahoot, J.; Ruamcharoen, J.; Ruamcharoen, P. Polyurethane polyester elastomer: Innovative environmental friendly wood adhesive from modified PETs and hydroxyl liquid natural rubber polyols. Int. J. Adhes. Adhes. 2013, 41, 127–131. [Google Scholar] [CrossRef]
- Kébir, N.; Campistron, I.; Laguerre, A.; Pilard, J.F.; Bunel, C. New cross-linked polyurethane elastomers with various physical properties from natural rubber derivatives. J. Appl. Polym. Sci. 2011, 122, 1677–1687. [Google Scholar] [CrossRef]
- Saetung, A.; Kaenhin, L.; Klinpitukda, P.; Rungvichaniwat, A.; Tulyapitak, T.; Munleh, S.; Campistron, I.; Pilard, J.F. Synthesis, characteristic, and properties of waterborne polyurethane based on natural rubber. J. Appl. Polym. Sci. 2012, 124, 2742–2752. [Google Scholar] [CrossRef]
- Pilard, K.F. Waterborne Polyurethane: Effect of functional groups in aromatic isocyanate and the chain length of hydroxyl terminated natural rubber. Adv. Mater. Res. 2012, 415–417, 2032–2035. [Google Scholar]
- Ly, P.H. Reinforcement of natural rubber from hydroxyl-terminated liquid natural rubber grafted carbon black. I. Grafting of acyl chloride capped liquid natural rubber onto carbon black. J. Macromol. Sci. A. 1996, 33, 1931–1937. [Google Scholar] [CrossRef]
- Bac, N.V.; Terlemezyan, L.; Mihailov, M. Epoxidation of natural rubber in latex in the presence of a reducing agent. J. Appl. Polym. Sci. 1993, 50, 845–849. [Google Scholar] [CrossRef]
- Ibrahim, S.; Daik, R.; Abdullah, I. Functionalization of liquid natural rubber via oxidative degradation of natural rubber. Polymers 2014, 6, 2928. [Google Scholar] [CrossRef]
- Ravindran, T.; Nayar, M.R.G.; Francis, D.J. Production of hydroxyl-terminated liquid natural rubber-mechanism of photochemical depolymerization and hydroxylation. J. Appl. Polym. Sci. 1988, 35, 1227–1239. [Google Scholar] [CrossRef]
- Shamraiz, U.; Hussain, R.A.; Badshah, A.; Raza, B.; Saba, S. Functional metal sulfides and selenides for the removal of hazardous dyes from Water. J. Photochem. Photobiol. B Biol. 2016, 159, 33–41. [Google Scholar] [CrossRef]
- Juabrum, S.; Chankhanittha, T.; Nanan, S. Hydrothermally grown SDS-capped ZnO photocatalyst for degradation of RR141 azo dye. Mater. Lett. 2019, 245, 1–5. [Google Scholar] [CrossRef]
- Moon, J.; Yun, C.Y.; Chung, K.W.; Kang, M.S.; Yi, J. Photocatalytic activation of TiO2 under visible light using Acid Red 44. Catal. Today 2003, 87, 77–86. [Google Scholar] [CrossRef]
- Dong, P.; Hou, G.; Xi, X.; Shao, R.; Dong, F. WO3-based photocatalysts: Morphology control, activity enhancement and multifunctional applications. Environ. Sci. Nano 2017, 4, 539–557. [Google Scholar] [CrossRef]
- Van Cuong, N. Bifunctionalcore–shell nanocomposite Mn-doped ZnO/Fe3O4 for photodegradation of reactive blue 198 dye. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 035014. [Google Scholar]
- Fisher, J.; Egerton, T.A. Titanium Compounds, Inorganic. Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: New York, NY, USA, 2000; pp. 1–76. [Google Scholar]
- Reddy, M.K.; Manorama, S.V.; Reddy, A.R. Band gap studies on anatase titanium oxide nanoparticles. Mater. Chem. Phys. 2003, 78, 239–245. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Franch, M.I.; Peral, J.; Domenech, X.; Ayllon, J.A. Aluminium(iii) adsorption: A soft and simple method to prevent TiO2 deactivation during salicylic acid photodegradation. Chem. Commun. 2005, 14, 1851–1853. [Google Scholar] [CrossRef]
- Haick, H.; Paz, Y. Remote photocatalytic activity as probed by measuring the degradation of self-assembled monolayers anchored near microdomains of titanium oxide. J. Phys. Chem. B. 2001, 105, 3045–3051. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Pozzo, R.L.; Baltanás, M.A.; Cassano, A.E. Supported titanium oxide as photocatalyst in water decontamination: State of the art. Catal. Today 1997, 39, 219–231. [Google Scholar] [CrossRef]
- Baram, N.; Starosvetsky, D.; Starosvetsky, J.; Epshtein, M.; Armon, R.; Ein, E.Y. Enhanced photo-efficiency of immobilized TiO2 catalyst via intense anodic bias. Electrochem. Commun. 2007, 9, 1684–1688. [Google Scholar] [CrossRef]
- O’Regan, B.; Gratzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature. 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Huang, S.Y.; Schlichthörl, G.; Nozik, A.J.; Grätzel, M.; Frank, A.J. Charge recombination in dye-sensitized nanocrystalline TiO2 solar cells. J. Phys. Chem. B. 1997, 101, 2576–2582. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Kisch, H.; Burgeth, G.; Macyk, W. Visible light photocatalysis by a titania transition metal complex. In Advances in Inorganic Chemistry; Van Eldik, R., Ed.; Academic Press: London, UK, 2004; Volume 56, pp. 241–259. [Google Scholar]
- Ohno, T.; Sarukawa, K.; Tokieda, K.; Matsumura, M. Morphology of a TiO2 photocatalyst (Degussa, P-25) consisting of anatase and rutile crystalline phases. J. Catal. 2001, 203, 82–86. [Google Scholar] [CrossRef]
- Cornish, B.J.P.A.; Lawton, L.A.; Robertson, P.K.J. Hydrogen peroxide enhanced photocatalytic oxidation of microcystin-LR using titanium oxide. Appl. Catal. B Environ. 2000, 25, 59–67. [Google Scholar] [CrossRef]
- Sakdapipanich, J.; Suksawad, P.; Insom, K.; Kawahara, S. Preparation of functionalized low molecular weight natural rubber latex using solid nanometric TiO2 film as a photocatalyst. Rubber Chem. Technol. 2005, 78, 597–605. [Google Scholar] [CrossRef]
- Sillapasuwan, A.; Saekhow, P.; Rojruthai, P.; Sakdapipanich, J. The preparation of hydroxyl-terminated deproteinized natural rubber latex by photochemical reaction utilizing nanometric TiO2 depositing on quartz substrate as a photocatalyst. Polymers 2022, 14, 2877. [Google Scholar] [CrossRef]
- Nijpanich, S.; Nimpaiboon, A.; Sakdapipanich, J. Functionalization of styrene-butadiene rubber and skim latex by photocatalytic reaction using nanometric TiO2 film as a photocatalyst. Key Eng. Mater. 2015, 659, 409–413. [Google Scholar] [CrossRef]
- Kim, S.C.; Lee, D.K. Preparation of TiO2-coated hollow glass beads and their application to the control of algal growth in eutrophic water. Microchem. J. 2005, 80, 227–232. [Google Scholar] [CrossRef]
- Nawamawat, K.; Sakdapipanich, J.T.; Ho, C.C.; Ma, Y.; Song, J.; Vancso, J.G. Surface nanostructure of Hevea brasiliensis natural rubber latex particles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 390, 157–166. [Google Scholar] [CrossRef]
- Karunakaran, C.; Gomathisankar, P.; Manikandan, G. Solar photocatalytic detoxification of cyanide by different forms of TiO2. Korean J. Chem. Eng. 2011, 28, 1214–1220. [Google Scholar] [CrossRef]
- Yu, J.; Yu, H.; Cheng, B.; Zhou, M.; Zhao, X. Enhanced photocatalytic activity of TiO2 powder (P25) by hydrothermal treatment. J. Mol. Catal. A Chem. 2006, 253, 112–118. [Google Scholar] [CrossRef]
- Klinklai, W.; Saito, T.; Kawahara, S.; Tashiro, K.; Suzuki, Y.; Sakdapipanich, J.T.; Isono, Y. Hyperdeproteinized natural rubber prepared with urea. J. Appl. Polym. Sci. 2004, 93, 555–559. [Google Scholar] [CrossRef]
- Addamo, M.; Augugliaro, V.; Di, P.A.; García, L.E.; Loddo, V.; Marci, G.; Molinari, R.; Palmisano, L.; Schiavello, M. Preparation, characterization, and photoactivity of polycrystalline nanostructured TiO2 Catalysts. J. Phys. Chem. B. 2004, 108, 3303–3310. [Google Scholar] [CrossRef]
- Guo, B.; Liu, Z.; Hong, L.; Jiang, H.; Lee, J.Y. Photocatalytic effect of the sol-gel derived nanoporous TiO2 transparent thin films. Thin Solid Films 2005, 479, 310–315. [Google Scholar] [CrossRef]
- Nimpaiboon, A.; Amnuaypornsri, S.; Sakdapipanich, J. Influence of gel content on the physical properties of unfilled and carbon black filled natural rubber vulcanizates. Polym. Test. 2013, 32, 1135–1144. [Google Scholar] [CrossRef]
- De, A.K.; Chaudhuri, B.; Bhattacharjee, S.; Dutta, B.K. Estimation of •OH radical reaction rate constants for phenol and chlorinated phenols using UV/H2O2 photooxidation. J. Hazard. Mater. 1999, 64, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Rippel, M.M.; Lee, L.-T.; Leite, C.A.P.; Galembeck, F. Skim and cream natural rubber particles: Colloidal properties, coalescence and film formation. J. Colloid Interface Sci. 2003, 268, 330–340. [Google Scholar] [CrossRef]
- Isa, S.Z.; Yahya, R.; Hassan, A.; Tahir, M. The influence of temperature and reaction time in the degradation of natural rubber latex. Malays. J. Anal. Sci. 2007, 11, 42–47. [Google Scholar]
- Vilar, W.D.; Menezes, S.M.C.; Akcelrud, L. Characterization of hydroxyl-terminated polybutadiene. Polym. Bull. 1994, 33, 557–561. [Google Scholar] [CrossRef]
- Anancharoenwong, E. Synthesis and Characterization of cis-1, 4-Polyisoprene-Based Polyurethane Coatings; Study of Their Adhesive Properties on Metal Surface. Ph.D. Thesis, Université du Maine, Le Mans, France, 21 September 2011. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).